Submitted:
19 May 2023
Posted:
19 May 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Fundamentals of Producing H2 by Photocatalytic Water Splitting over TiO2
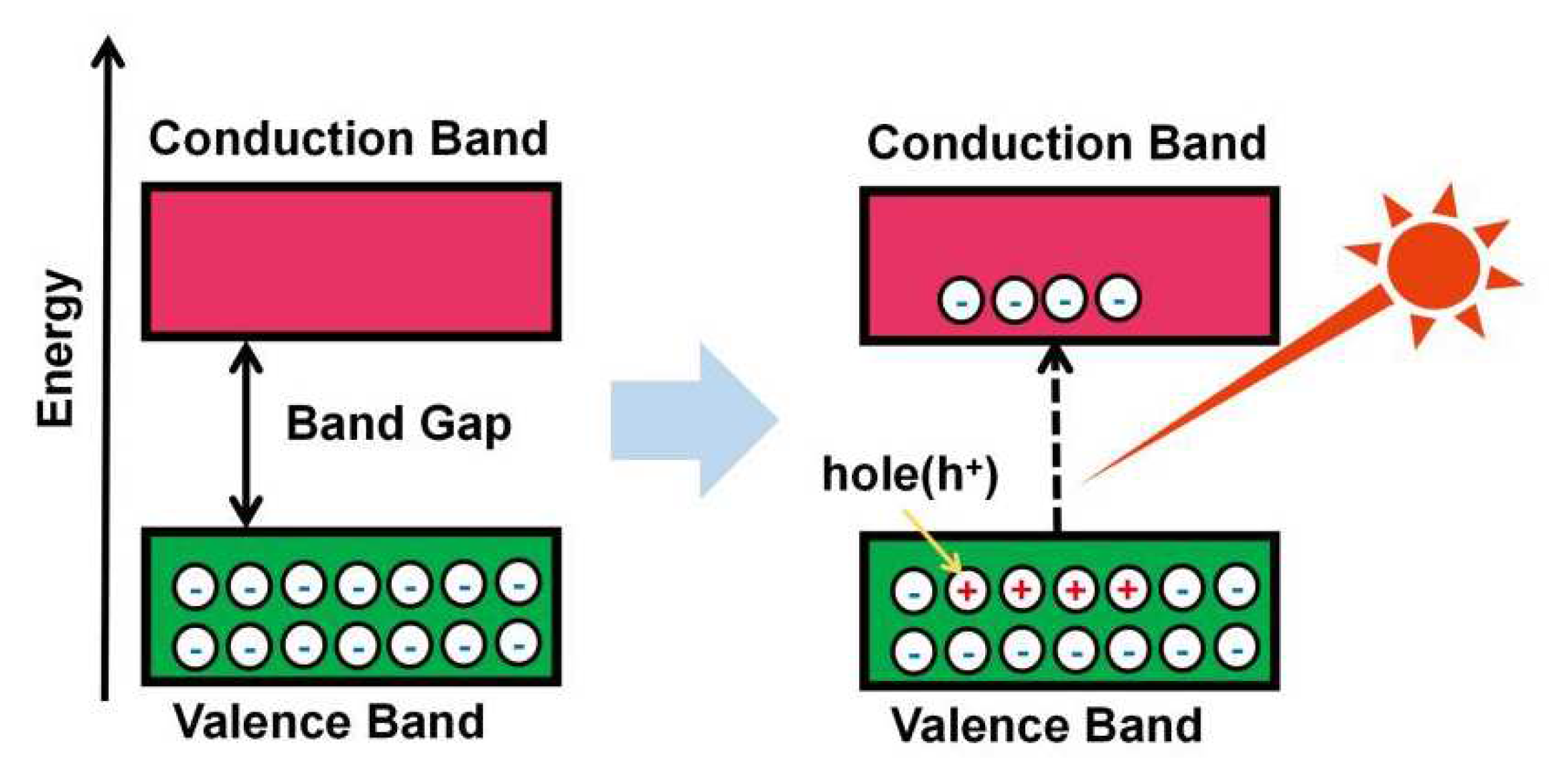
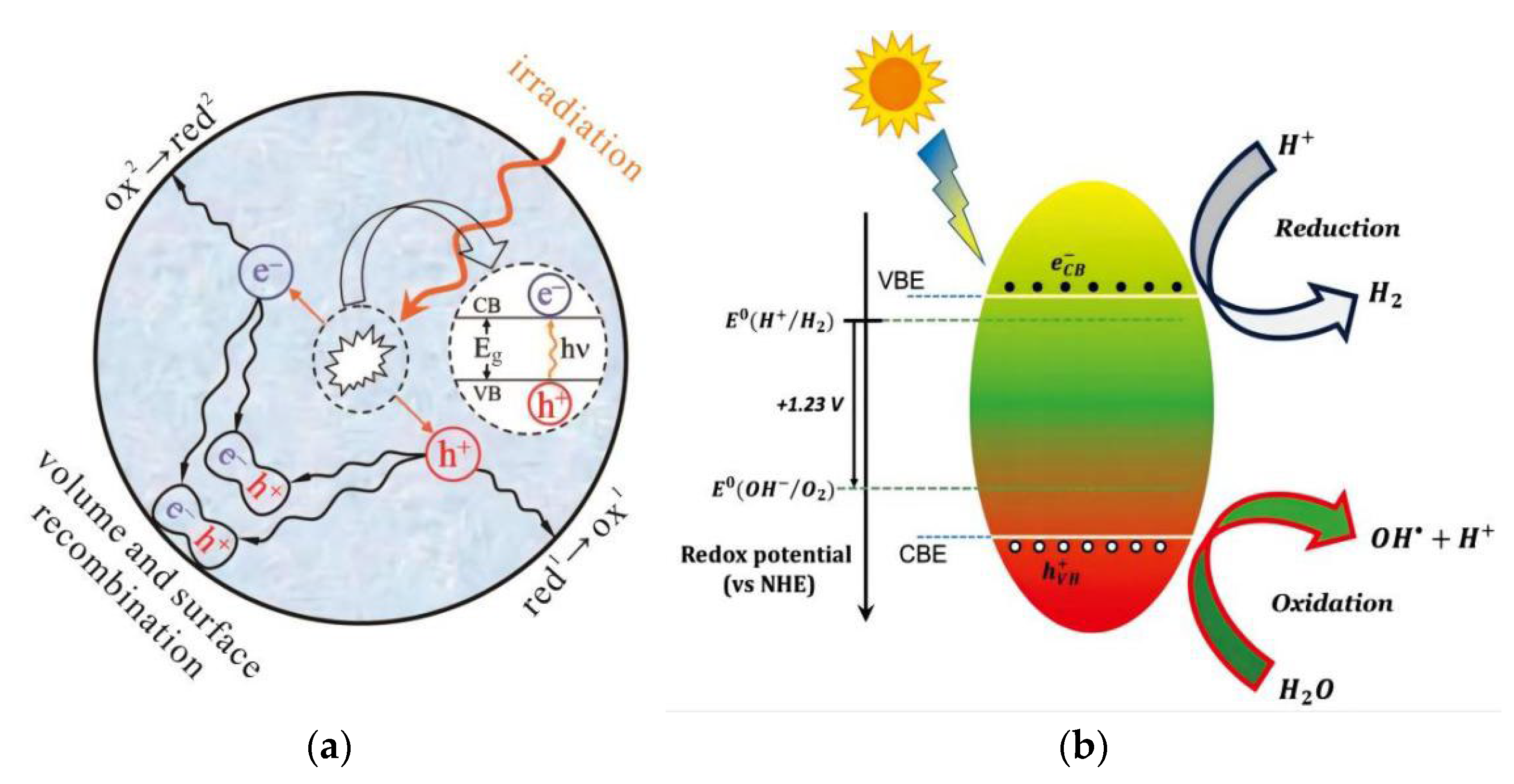
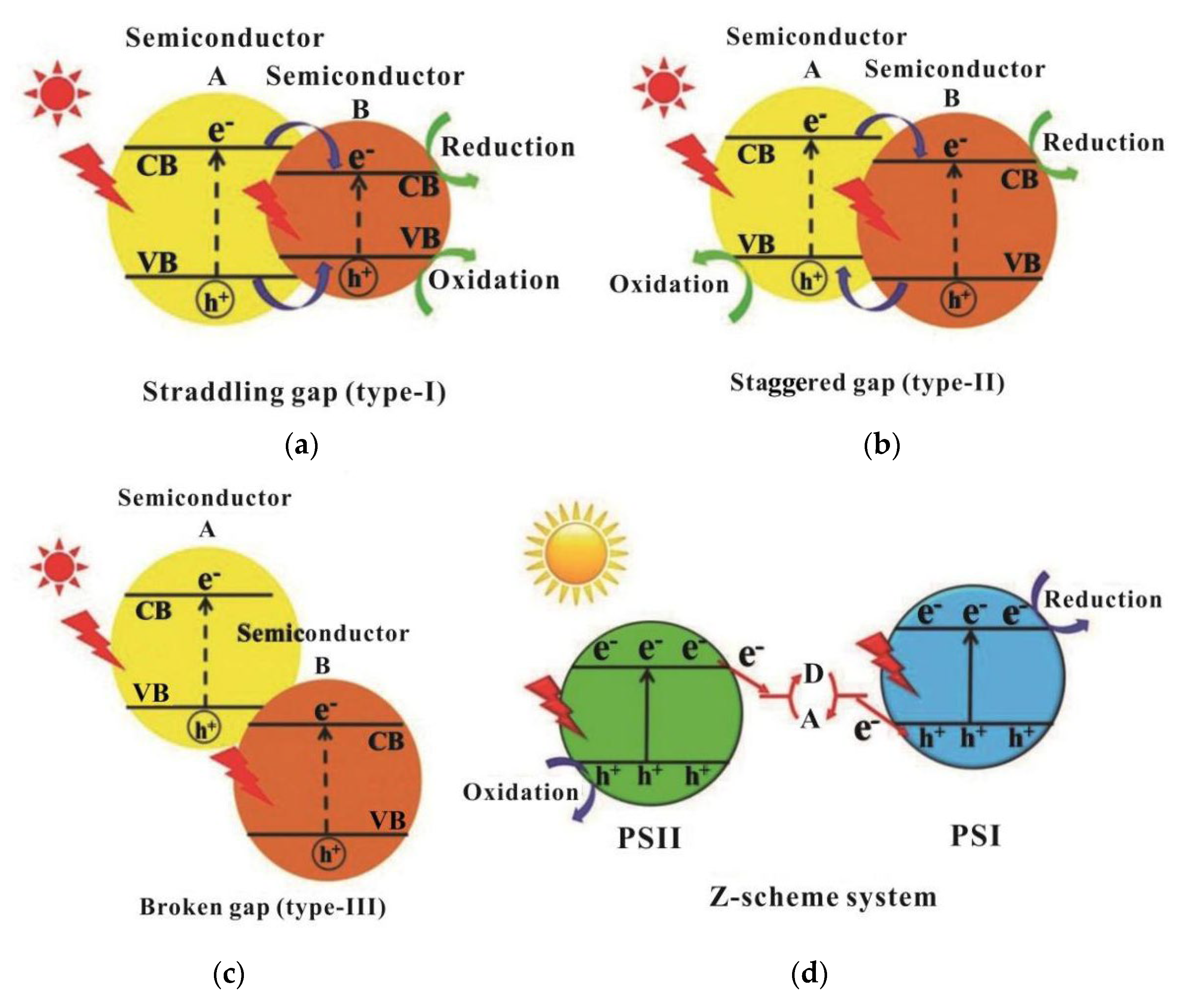
2.1. Mechanism of Photocatalytic Water Splitting to Generate H2
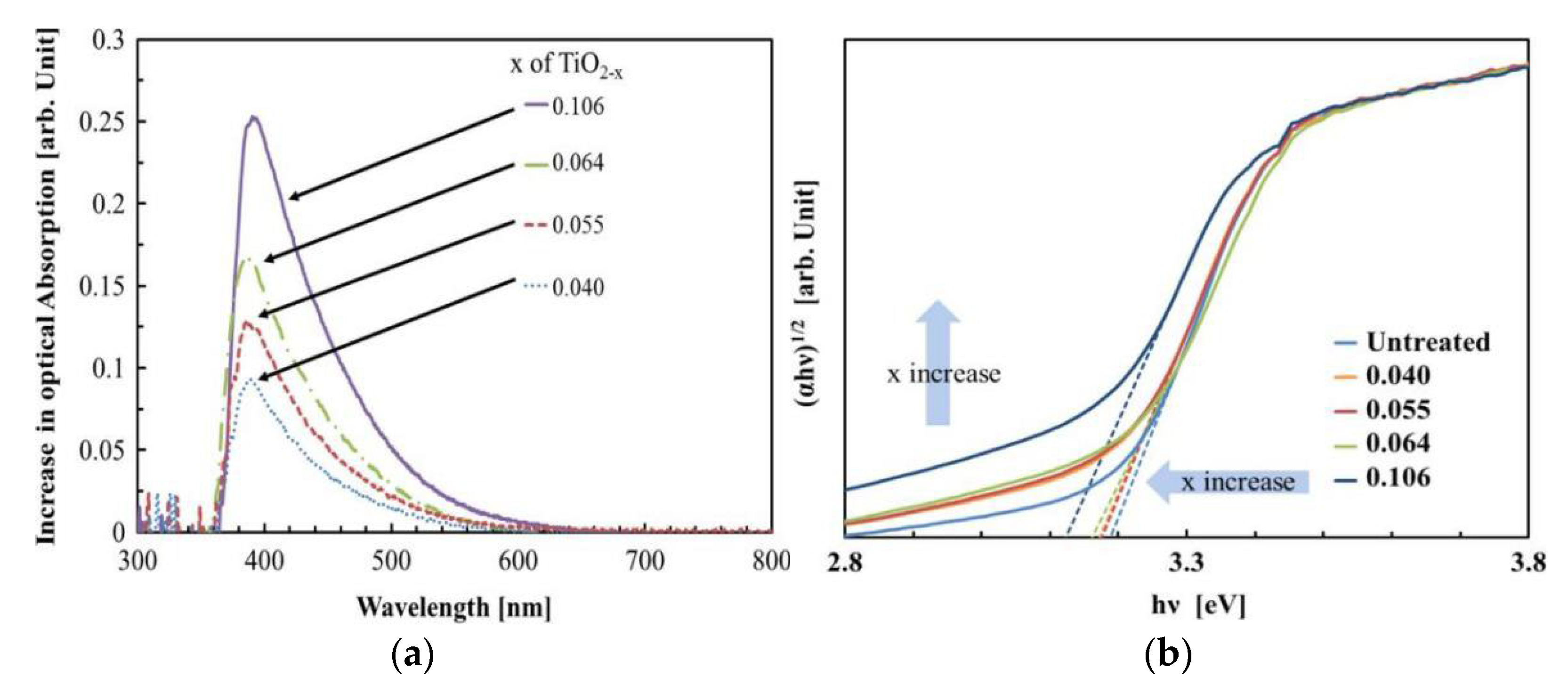
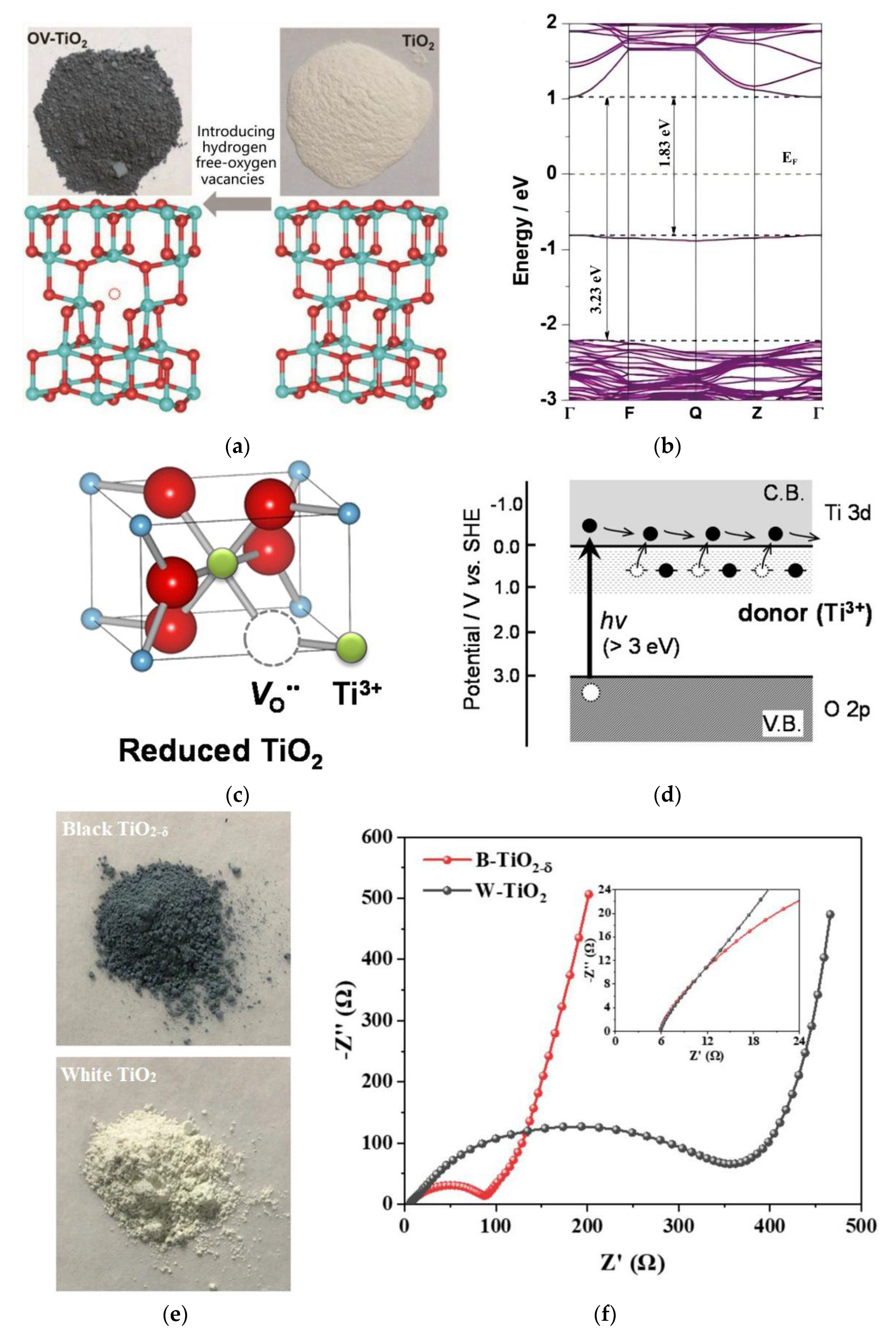
2.2. Impact of Oxygen Defects on the Photocatalytic Activity of TiO2
2.3. Brief Overview on Photocatalytic Water Splitting to Generate H2 over TiO2-δ
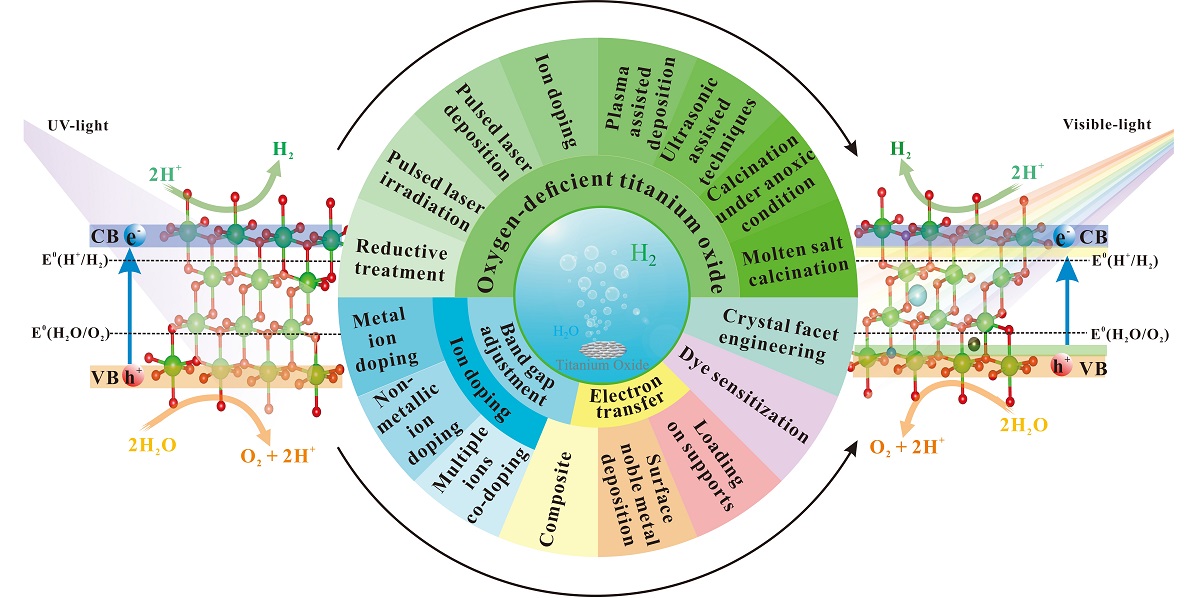
3. Methods of Introducing Oxygen Defects in TiO2
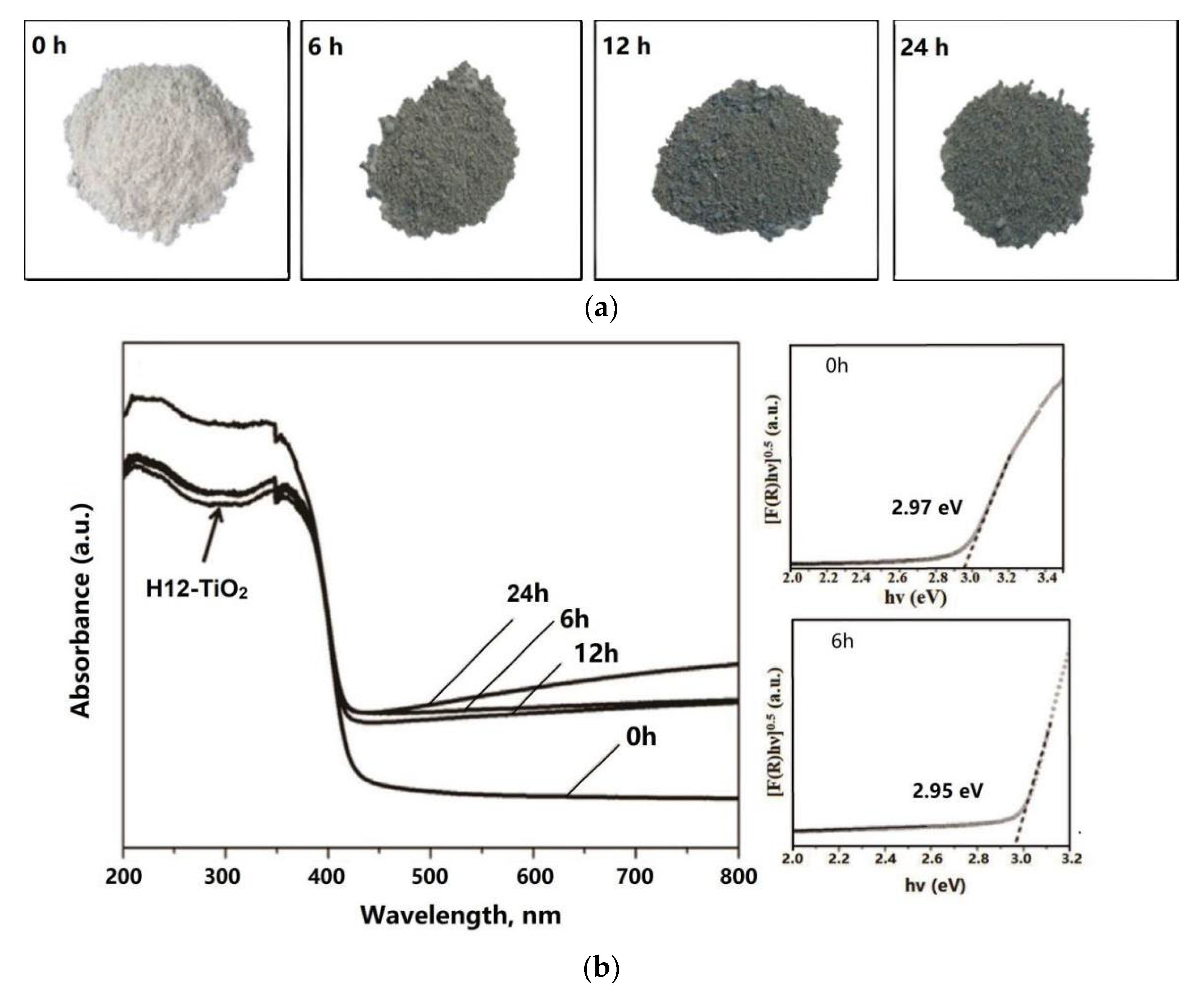
3.1. Reductive Treatment
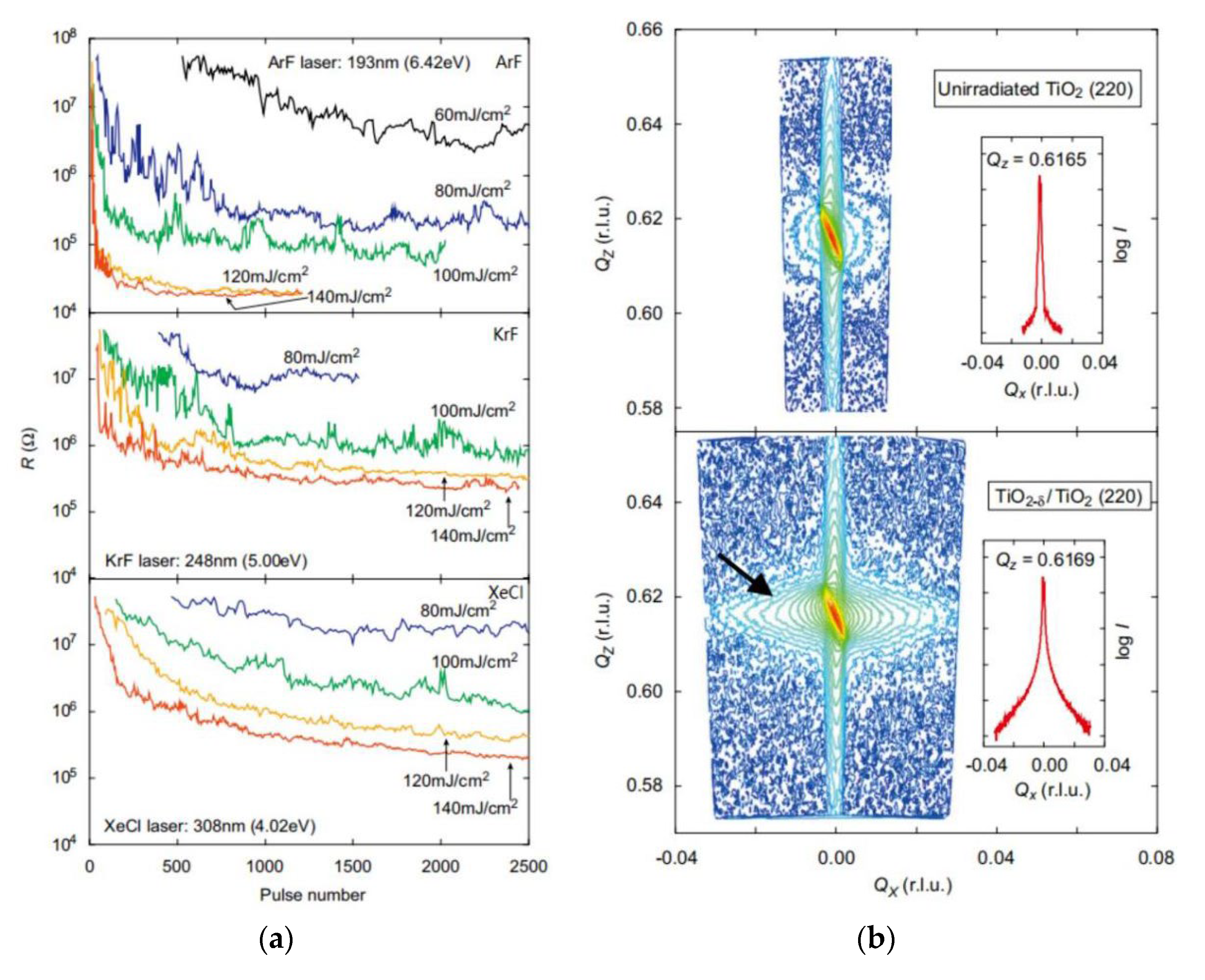
3.2. Pulsed Laser Irradiation
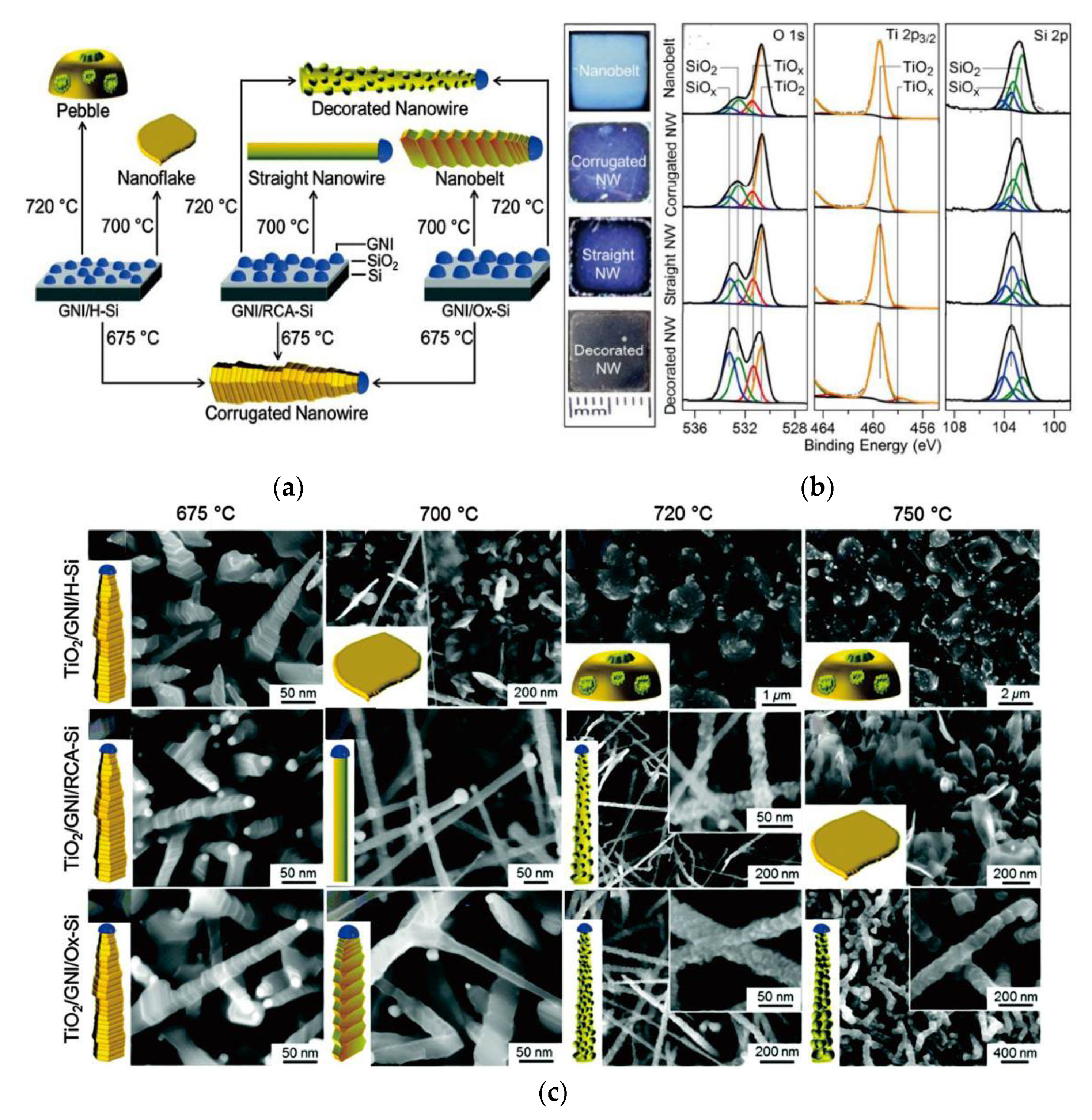
3.3. Pulsed Laser Deposition
3.4. Ion Doping
3.5. Plasma-Assisted Deposition
3.6. Ultrasonic Assisted Techniques
3.7. Calcination under Anoxic Condition
3.8. Molten Salt Calcination
4. Modification Methods of TiO2-δ Photocatalysts
4.1. Ion Doping
4.1.1. Metal Ion Doping
4.1.2. Nonmetallic Ion Doping
4.1.3. Multiple Ions Co-Doping
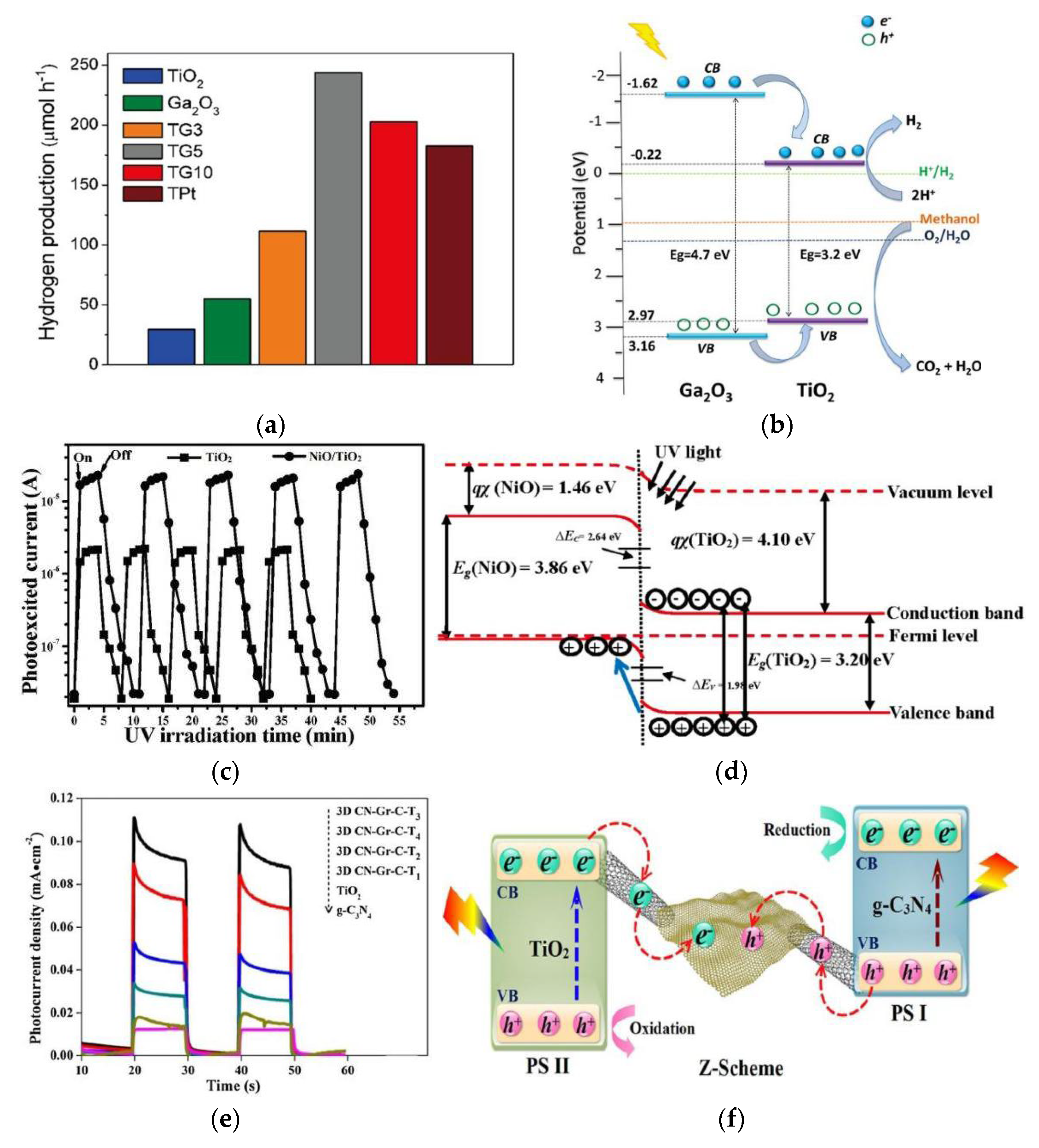
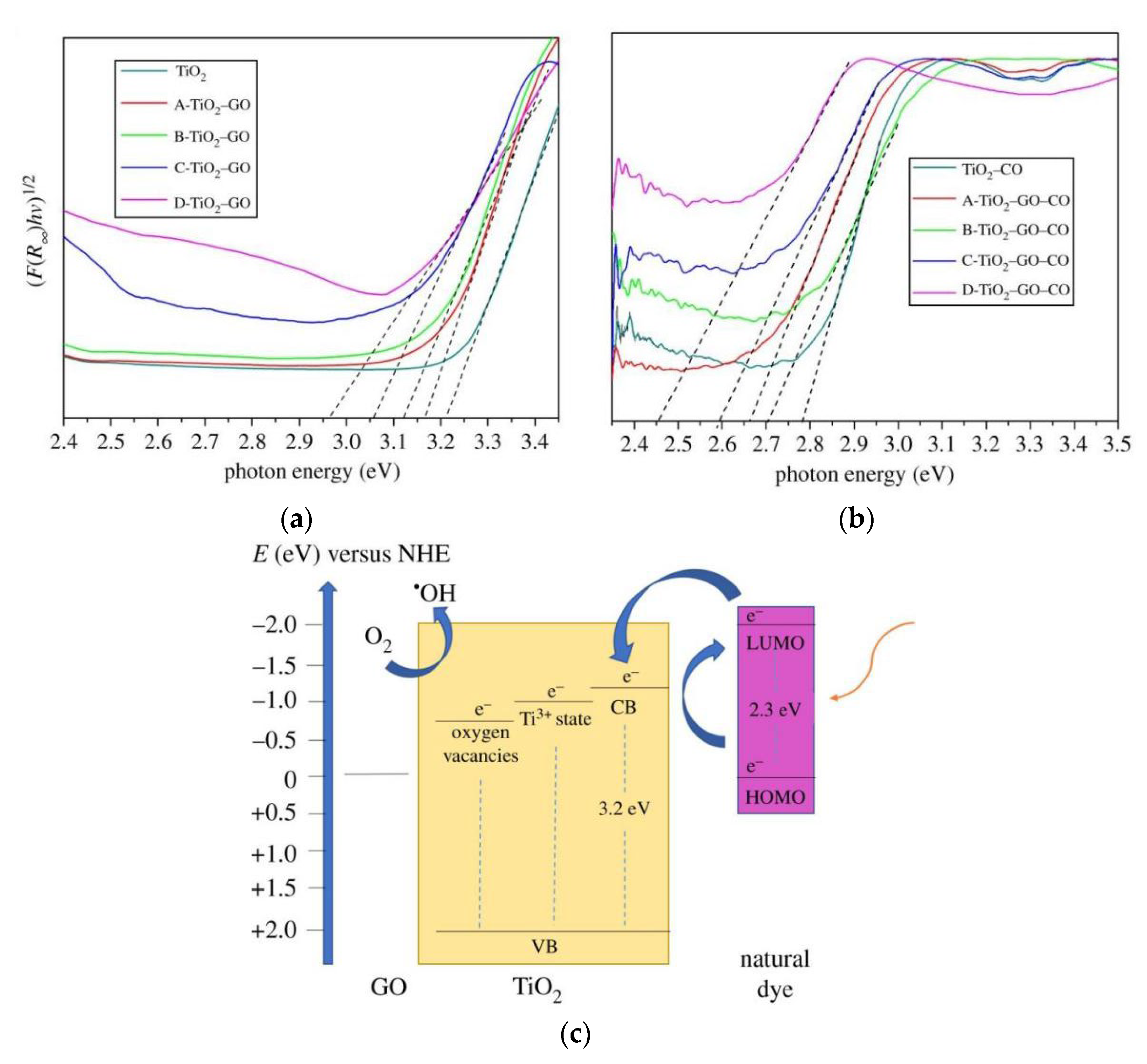
4.2. Composite
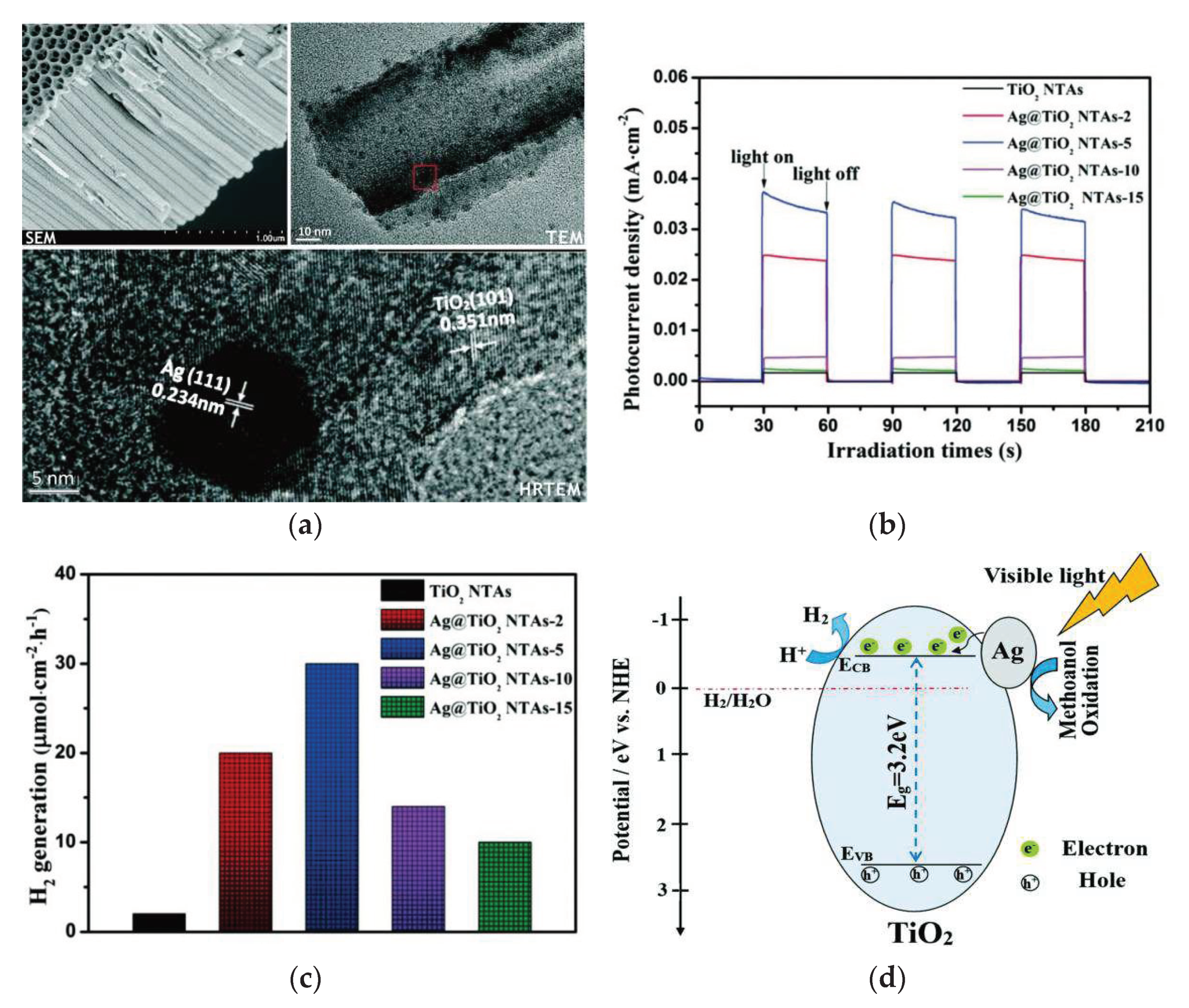
4.3. Surface Noble Metal Deposition
4.4. Dye Sensitization
4.5. Loading on Supports
4.6. Crystal Facet Engineering
5. Conclusions and Outlooks
Author Contributions
Funding
Conflicts of Interest
References
- Akorede, M.F.; Hizam, H.; Pouresmaeil, E. Distributed energy resources and benefits to the environment. Renewable & Sustainable Energy Reviews 2010, 14, 724–734. [Google Scholar]
- Aslan, M.; Isik, H. Green energy for the battlefield. International Journal of Green Energy 2017, 14, 1020–1026. [Google Scholar] [CrossRef]
- Dorian, J.P.; Franssen, H.T.; Simbeck, D.R. Global challenges in energy. Energy Policy 2006, 34, 1984–1991. [Google Scholar] [CrossRef]
- Koroneos, C.; Spachos, T.; Moussiopoulos, N. Exergy analysis of renewable energy sources. Renewable Energy 2003, 28, 295–310. [Google Scholar] [CrossRef]
- Kumar, G.; Kim, S.H.; Lay, C.H.; Ponnusamy, V.K. Recent developments on alternative fuels, energy and environment for sustainability Preface. Bioresource Technology 2020, 317, 124010. [Google Scholar] [CrossRef] [PubMed]
- Mulligan, S. Energy, Environment, and Security: Critical Links in a Post-Peak World. Global Environmental Politics 2010, 10, 79–100. [Google Scholar] [CrossRef]
- Veziroglu, T.N.; Sahin, S. 21st Century's energy: Hydrogen energy system. Energy Conversion and Management 2008, 49, 1820–1831. [Google Scholar] [CrossRef]
- Momirlan, M.; Veziroglu, T.N. The properties of hydrogen as fuel tomorrow in sustainable energy system for a cleaner planet. International Journal of Hydrogen Energy 2005, 30, 795–802. [Google Scholar] [CrossRef]
- Sartbaeva, A.; Kuznetsov, V.L.; Wells, S.A.; Edwards, P.P. Hydrogen nexus in a sustainable energy future. Energy & Environmental Science 2008, 1, 79–85. [Google Scholar]
- Midilli, A.; Dincer, I. Hydrogen as a renewable and sustainable solution in reducing global fossil fuel consumption. International Journal of Hydrogen Energy 2008, 33, 4209–4222. [Google Scholar] [CrossRef]
- Muradov, N. Low to near-zero CO2 production of hydrogen from fossil fuels: Status and perspectives. International Journal of Hydrogen Energy 2017, 42, 14058–14088. [Google Scholar] [CrossRef]
- Rosen, M.A. Combating global warming via non-fossil fuel energy options. International Journal of Global Warming 2009, 1, 2–28. [Google Scholar] [CrossRef]
- Wang, Z.; Naterer, G.F. Integrated fossil fuel and solar thermal systems for hydrogen production and CO2 mitigation. International Journal of Hydrogen Energy 2014, 39, 14227–14233. [Google Scholar] [CrossRef]
- Sapountzi, F.M.; Gracia, J.M.; Weststrate, C.J.; Fredriksson, H.O.A.; Niemantsverdriet, J.W. Electrocatalysts for the generation of hydrogen, oxygen and synthesis gas. Progress in Energy and Combustion Science 2017, 58, 1–35. [Google Scholar] [CrossRef]
- Tee, S.Y.; Win, K.Y.; Teo, W.S.; Koh, L.D.; Liu, S.; Teng, C.P.; Han, M.Y. Recent Progress in Energy-Driven Water Splitting. Advanced Science 2017, 4, 1600337. [Google Scholar] [CrossRef] [PubMed]
- Fujishima, A.; Honda, K. Electrochemical photolysis of water at a semiconductor electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef] [PubMed]
- Faraji, M.; Yousefi, M.; Yousefzadeh, S.; Zirak, M.; Naseri, N.; Jeon, T.H.; Choi, W.; Moshfegh, A.Z. Two-dimensional materials in semiconductor photoelectrocatalytic systems for water splitting. Energy & Environmental Science 2019, 12, 59–95. [Google Scholar]
- Gholipour, M.R.; Cao-Thang, D.; Beland, F.; Trong-On, D. Nanocomposite heterojunctions as sunlight-driven photocatalysts for hydrogen production from water splitting. Nanoscale 2015, 7, 8187–8208. [Google Scholar] [CrossRef]
- Ismail, A.A.; Bahnemann, D.W. Photochemical splitting of water for hydrogen production by photocatalysis: A review. Solar Energy Materials and Solar Cells 2014, 128, 85–101. [Google Scholar] [CrossRef]
- Bich Ha, N.; Van Hieu, N.; Dinh Lam, V. Photocatalytic composites based on titania nanoparticles and carbon nanomaterials. Advances in Natural Sciences-Nanoscience and Nanotechnology 2015, 6, 033001. [Google Scholar]
- Chan, S.H.S.; Wu, T.Y.; Juan, J.C.; Teh, C.Y. Recent developments of metal oxide semiconductors as photocatalysts in advanced oxidation processes (AOPs) for treatment of dye waste-water. Journal of Chemical Technology and Biotechnology 2011, 86, 1130–1158. [Google Scholar] [CrossRef]
- Djurisic, A.B.; He, Y.; Ng, A.M.C. Visible-light photocatalysts: Prospects and challenges. APL Materials 2020, 8, 030903. [Google Scholar] [CrossRef]
- Yang, Y.; Yin, L.C.; Gong, Y.; Niu, P.; Wang, J.Q.; Gu, L.; Chen, X.; Liu, G.; Wang, L.; Cheng, H.M. An Unusual Strong Visible-Light Absorption Band in Red Anatase TiO2 Photocatalyst Induced by Atomic Hydrogen-Occupied Oxygen Vacancies. Advanced Materials 2018, 30, 1704479. [Google Scholar] [CrossRef] [PubMed]
- Cushing, S.K.; Meng, F.K.; Zhang, J.Y.; Ding, B.F.; Chen, C.K.; Chen, C.J.; Liu, R.S.; Bristow, A.D.; Bright, J.; Zheng, P.; Wu, N.Q. Effects of defects on photocatalytic activity of hydrogen-treated titanium oxide nanobelts. ACS Catal 2017, 7, 1742–1748. [Google Scholar] [CrossRef]
- Tian, M.; Mahjouri-Samani, M.; Eres, G.; Sachan, R.; Yoon, M.; Chisholm, M.F.; Wang, K.; Puretzky, A.A.; Rouleau, C.M.; Geohegan, D.B.; Duscher, G. Structure and Formation Mechanism of Black TiO2 Nanoparticles. ACS Nano 2015, 9, 10482–10488. [Google Scholar] [CrossRef]
- Hong, R.J.; Deng, C.; Jing, M.; Lin, H.; Tao, C.X.; Zhang, D.W. Oxygen flows-dependent photocatalytic performance in Ti3+ doped TiO2 thin films. Optical Materials 2019, 95, 109224. [Google Scholar] [CrossRef]
- Nakajima, T.; Nakamura, T.; Shinoda, K.; Tsuchiya, T. Rapid formation of black titania photoanodes: pulsed laser-induced oxygen release and enhanced solar water splitting efficiency. Journal of Materials Chemistry A 2014, 2, 6762–6771. [Google Scholar] [CrossRef]
- Yuan, K.; Cao, Q.; Lu, H.L.; Zhong, M.; Zheng, X.; Chen, H.Y.; Wang, T.; Delaunay, J.J.; Luo, W.; Zhang, L.; Wang, Y.Y.; Deng, Y.; Ding, S.J.; Zhang, D.W. Oxygen-deficient WO3-x@TiO2-x core-shell nanosheets for efficient photoelectrochemical oxidation of neutral water solutions. Journal of Materials Chemistry A 2017, 5, 14697–14706. [Google Scholar] [CrossRef]
- Zhao, Z.; Tan, H.; Zhao, H. F.; Lv, Y.; Zhou, L. J.; Song, Y. J.; Sun, Z. C. Reduced TiO2 rutile nanorods with well-defined facets and their visible-light photocatalytic activity. Chemical Communications 2014, 50, 2755–2757. [Google Scholar] [CrossRef]
- Wang, Y.Q.; Yu, X.J.; Sun, D.Z. Synthesis, characterization, and photocatalytic activity of TiO2-xNx nanocatalyst. Journal of Hazardous Materials 2007, 144, 328–333. [Google Scholar] [CrossRef]
- Yuan, J.; Liu, Y.Y.; Bo, T.T.; Zhou, W. Activated HER performance of defected single layered TiO2 nanosheet via transition metal doping. International Journal of Hydrogen Energy 2020, 45, 2681–2688. [Google Scholar] [CrossRef]
- Liu, G.; Yang, H.G.; Wang, X.; Cheng, L.; Lu, H.; Wang, L.; Lu, G.Q.; Cheng, H.M. Enhanced photoactivity of oxygen-deficient anatase TiO2 sheets with dominant {001} facets. Journal of Physical Chemistry C 2009, 113, 21784–21788. [Google Scholar] [CrossRef]
- Weng, X.L.; Zhang, Y.L.; Dong, F.; Wu, Z.B.; Darr, J.A. Thermocatalytic syntheses of highly defective hybrid nano-catalysts for photocatalytic hydrogen evolution. Journal of Materials Chemistry A 2017, 5, 23766–23775. [Google Scholar] [CrossRef]
- Han, C.; Wang, Y.D.; Lei, Y.P.; Wang, B.; Wu, N.; Shi, Q.; Li, Q. In situ synthesis of graphitic-C3N4 nanosheet hybridized N-doped TiO2 nanofibers for efficient photocatalytic H2 production and degradation. Nano Research 2015, 8, 1199–1209. [Google Scholar] [CrossRef]
- Rajeshwar, K. Hydrogen generation at irradiated oxide semiconductor-solution interfaces. Journal of Applied Electrochemistry 2007, 37, 765–787. [Google Scholar] [CrossRef]
- Palmisano, G.; Augugliaro, V.; Pagliaro, M.; Palmisano, L. Photocatalysis: a promising route for 21st century organic chemistry. Chemical Communications 2007, (33), 3425–3437. [Google Scholar] [CrossRef] [PubMed]
- Lettieri, S.; Pavone, M.; Fioravanti, A.; Santamaria Amato, L.; Maddalena, P. Charge carrier processes and optical properties in TiO2 and TiO2-based heterojunction photocatalysts: A review. Materials 2021, 14, 1645. [Google Scholar] [CrossRef] [PubMed]
- Nakano, T.; Ito, R.; Kogoshi, S.; Katayama, N. Optimal levels of oxygen deficiency in the visible light photocatalyst TiO2-x and long-term stability of catalytic performance. Journal of Physics and Chemistry of Solids 2016, 98, 136–142. [Google Scholar] [CrossRef]
- Wu, H.; Wang, Z.; Jin, S.; Cao, X.; Ren, F.; Wu, L.; Xing, Z.; Wang, X.; Cai, G.; Jiang, C. Jiang. Enhanced photoelectrochemical performance of TiO2 through controlled Ar+ ion irradiation: A combined experimental and theoretical study. International Journal of Hydrogen Energy 2018, 43, 6936–6944. [Google Scholar] [CrossRef]
- Amano, F.; Nakata, M.; Yamamoto, A.; Tanaka, T. Effect of Ti3+ ions and conduction band electrons on photocatalytic and photoelectrochemical activity of rutile titania for water oxidation. Journal of Physical Chemistry C 2016, 120, 6467–6474. [Google Scholar] [CrossRef]
- Singh, A.P.; Kodan, N.; Mehta, B.R. Enhancing the photoelectrochemical properties of titanium dioxide by thermal treatment in oxygen deficient environment. Applied Surface Science 2016, 372, 63–69. [Google Scholar] [CrossRef]
- Li, Y.; Cooper, J.K.; Liu, W.; Sutter-Fella, C.M.; Amani, M.; Beeman, J.W.; Javey, A.; Ager, J.W.; Liu, Y.; Toma, F.M.; Sharp, I.D. Defective TiO2 with high photoconductive gain for efficient and stable planar heterojunction perovskite solar cells. Nature Communications 2016, 7, 12446. [Google Scholar] [CrossRef] [PubMed]
- Hao, Z.; Chen, Q.; Dai, W.; Ren, Y.; Zhou, Y.; Yang, J.; Xie, S.; Shen, Y.; Wu, J.; Chen, W.; Xu, G.Q. Oxygen-deficient blue TiO2 for ultrastable and fast lithium storage. Advanced Energy Materials 2020, 10, 1903107. [Google Scholar] [CrossRef]
- Wendt, S.; Sprunger, P.T.; Lira, E.; Madsen, G.K.H.; Li, Z.; Hansen, J.O.; Matthiesen, J.; Blekinge-Rasmussen, A.; Laegsgaard, E.; Hammer, B.; Besenbacher, F. The role of interstitial sites in the Ti3d defect state in the band gap of Titania. Science 2008, 320, 1755–1759. [Google Scholar] [CrossRef] [PubMed]
- Long, Z.; Li, Q.; Wei, T.; Zhang, G.; Ren, Z. Historical development and prospects of photocatalysts for pollutant removal in water. Journal of Hazardous Materials 2020, 395, 122599. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Sallet, D.; Lemaire, J. Photochemistry of polyundecanamides 2. TiO2-photocatalyzed and zno-photocatalyzed oxidation. Macromolecules 1982, 15, 1437–1441. [Google Scholar] [CrossRef]
- Baba, R.; Nakabayashi, S.; Fujishima, A.; Honda, K. Investigation of the mechanism of hydrogen evolution during photocatalytic water decomposition on metal-loaded semiconductor powders. Journal of Physical Chemistry 1985, 89, 1902–1905. [Google Scholar] [CrossRef]
- Matthews, R.W. Photooxidation of organic impurities in water using thin-films of titanium-dioxide. Journal of Physical Chemistry 1987, 91, 3328–3333. [Google Scholar] [CrossRef]
- Wagner, N.; Brummer, O.; Sauer, N. Photoemission-studies of titanium-oxides. Crystal Research and Technology 1982, 17, 1151–1158. [Google Scholar] [CrossRef]
- Sasikala, R.; Sudarsan, V.; Sudakar, C.; Naik, R.; Sakuntala, T.; Bharadwaj, S.R. Enhanced photocatalytic hydrogen evolution over nanometer sized Sn and Eu doped titanium oxide. International Journal of Hydrogen Energy 2008, 33, 4966–4973. [Google Scholar] [CrossRef]
- Amano, F.; Nakata, M. High-temperature calcination and hydrogen reduction of rutile TiO2: A method to improve the photocatalytic activity for water oxidation. Applied Catalysis B-Environmental 2014, 158, 202–208. [Google Scholar] [CrossRef]
- Mali, M.G.; Yoon, H.; An, S.; Choi, J.Y.; Kim, H.Y.; Lee, B.C.; Kim, B.N.; Park, J.H.; Al-Deyab, S.S.; Yoon, S.S. Enhanced solar water splitting of electron beam irradiated titania photoanode by electrostatic spray deposition. Applied Surface Science 2014, 319, 205–210. [Google Scholar] [CrossRef]
- Pitchaimuthu, S.; Honda, K.; Suzuki, S.; Naito, A.; Suzuki, N.; Katsumata, K.i.; Nakata, K.; Ishida, N.; Kitamura, N.; Idemoto, Y.; Kondo, T.; Yuasa, M.; Takai, O.; Ueno, T.; Saito, N.; Fujishima, A.; Terashima, C. Solution plasma process-derived defect-induced heterophase anatase/brookite TiO2 nanocrystals for enhanced gaseous photocatalytic performance. ACS Omega 2018, 3, 898–905. [Google Scholar] [CrossRef] [PubMed]
- Ennaceri, H.; Boujnah, M.; Taleb, A.; Khaldoun, A.; Saez-Araoz, R.; Ennaoui, A.; El Kenz, A.; Benyoussef, A. Thickness effect on the optical properties of TiO2-anatase thin films prepared by ultrasonic spray pyrolysis: Experimental and ab initio study. International Journal of Hydrogen Energy 2017, 42, 19467–19480. [Google Scholar] [CrossRef]
- Amano, F.; Yasumoto, T.; Prieto-Mahaney, O.O.; Uchida, S.; Shibayama, T.; Ohtani, B. Photocatalytic activity of octahedral single-crystalline mesoparticles of anatase titanium(IV) oxide. Chemical Communications. 2009, 2311–2313. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Cao, X.; Lin, B.; Gao, B. Origin of the visible-light photoactivity of NH3-treated TiO2: Effect of nitrogen doping and oxygen vacancies. Applied Surface Science 2013, 264, 845–852. [Google Scholar] [CrossRef]
- Leichtweiss, T.; Henning, R.A.; Koettgen, J.; Schmidt, R.M.; Hollaender, B.; Martin, M.; Wuttig, M.; Janek, J. Amorphous and highly nonstoichiometric titania (TiOx) thin films close to metal-like conductivity. Journal of Materials Chemistry A 2014, 2, 6631–6640. [Google Scholar] [CrossRef]
- Zhang, Y.X.; Wu, S.M.; Tian, G.; Zhao, X.F.; Wang, L.Y.; Yin, Y.X.; Wu, L.; Li, Q.N.; Zhang, Y.X.; Wu, J.S.; Janiak, C.; Ozoemena, K.I.; Shalom, M.; Yang, X.Y. Titanium vacancies in TiO2 nanofibers enable highly efficient photodriven seawater splitting. Chemistry-A European Journal 2021, 27, 14202–14208. [Google Scholar] [CrossRef]
- An, X.; Hu, C.; Liu, H.; Qu, J. Oxygen vacancy mediated construction of anatase/brookite heterophase junctions for high-efficiency photocatalytic hydrogen evolution. Journal of Materials Chemistry A 2017, 5, 24989–24994. [Google Scholar] [CrossRef]
- Zhang, K.; Zhou, W.; Chi, L.; Zhang, X.; Hu, W.; Jiang, B.; Pan, K.; Tian, G.; Jiang, Z. Black N/H-TiO2 nanoplates with a flower-like hierarchical architecture for photocatalytic hydrogen evolution. Chemsuschem 2016, 9, 2841–2848. [Google Scholar] [CrossRef]
- Qiu, J.Y.; Feng, H.Z.; Chen, Z.H.; Ruan, S.H.; Chen, Y.P.; Xu, T.T.; Su, J.Y.; Ha, E.N.; Wang, L.Y. Selective introduction of surface defects in anatase TiO2 nanosheets for highly efficient photocatalytic hydrogen generation. Rare Metals 2022, 41, 2074–2083. [Google Scholar] [CrossRef]
- Yuan, D.; Jiao, Y.; Li, Z.; Chen, X.; Ding, J.; Dai, W.L.; Wan, H.; Guan, G. TiN bridged all-Solid Z-Scheme CNNS/TiN/TiO2-x heterojunction by a facile in situ reduction strategy for enhanced photocatalytic hydrogen evolution. Advanced Materials Interfaces 2021, 8, 2100695. [Google Scholar] [CrossRef]
- Yamazaki, Y.; Mori, K.; Kuwahara, Y.; Kobayashi, H.; Yamashita, H. Defect engineering of Pt/TiO2-x photocatalysts via reduction treatment assisted by hydrogen spillover. ACS Applied Materials & Interfaces 2021, 13, 48669–48678. [Google Scholar]
- Jia, G.R.; Wang, Y.; Cui, X.Q.; Zhang, H.Z.; Zhao, J.X.; Li, L.H.; Gu, L.; Zhang, Q.H.; Zheng, L.R.; Wu, J.D.; Wu, Q.; Singh, D.J.; Li, W.W.; Zhang, L.; Zheng, W.T. Wet-chemistry hydrogen doped TiO2 with switchable defects control for photocatalytic hydrogen evolution. Matter 2022, 5, 206–218. [Google Scholar] [CrossRef]
- Park, E.; Patil, S.S.; Lee, H.; Kumbhar, V.S.; Lee, K. Photoelectrochemical H2 evolution on WO3/BiVO4 enabled by single-crystalline TiO2 overlayer modulations. Nanoscale 2021, 13, 16932–16941. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Song, J.; Luo, J.; Zhang, H.; Sun, Z.; Li, C.; Zheng, S.; Liu, Q. Single-atomic Pt sites anchored on defective TiO2 nanosheets as a superior photocatalyst for hydrogen evolution. Journal of Energy Chemistry 2021, 62, 1–10. [Google Scholar] [CrossRef]
- Mo, L.B.; Bai, Y.; Xiang, Q.Y.; Li, Q.; Wang, J.O.; Ibrahim, K.; Cao, J.L. Band gap engineering of TiO2 through hydrogenation. Applied Physics Letters 2014, 105, 202114. [Google Scholar] [CrossRef]
- Wang, H.; Wang, G.; Ling, Y.; Lepert, M.; Wang, C.; Zhang, J.Z.; Li, Y. Photoelectrochemical study of oxygen deficient TiO2 nanowire arrays with CdS quantum dot sensitization. Nanoscale 2012, 4, 1463–1466. [Google Scholar] [CrossRef]
- Liu, N.; Zhou, X.M.; Nguyen, N.T.; Peters, K.; Zoller, F.; Hwang, I.; Schneider, C.; Miehlich, M.E.; Freitag, D.; Meyer, K.; Fattakhova-Rohlfing, D.; Schmuki, P. Black magic in gray titania: noble-metal-free photocatalytic H2 evolution from hydrogenated anatase. ChemSusChem 2017, 10, 62–67. [Google Scholar] [CrossRef]
- Xu, Y.F.; Zhang, C.; Zhang, L.X.; Zhang, X.H.; Yao, H.L.; Shi, J.L. Pd-catalyzed instant hydrogenation of TiO2 with enhanced photocatalytic performance. Energy & Environmental Science 2016, 9, 2410–2417. [Google Scholar]
- Zhang, J.W.; Wang, S.; Liu, F.S.; Fu, X.J.; Ma, G.Q.; Hou, M.S.; Tang, Z. Preparation of defective TiO2-x hollow microspheres for photocatalytic degradation of methylene blue. Acta Physico-Chimica Sinica 2019, 35, 8–885. [Google Scholar] [CrossRef]
- Wierzbicka, E.; Altomare, M.; Wu, M.J.; Liu, N.; Yokosawa, T.; Fehn, D.; Qin, S.S.; Meyer, K.; Unruh, T.; Spiecker, E.; Palmisano, L.; Bellardita, M.; Will, J.; Schmuki, P. Reduced grey brookite for noble metal free photocatalytic H2 evolution. Journal of Materials Chemistry A 2021, 9, 1168–1179. [Google Scholar] [CrossRef]
- Samsudin, E.M.; Hamid, S.B.A.; Juan, J.C.; Basirun, W.J.; Kandjani, A.E. Surface modification of mixed-phase hydrogenated TiO2 and corresponding photocatalytic response. Applied Surface Science 2015, 359, 883–896. [Google Scholar] [CrossRef]
- Ihara, T.; Miyoshi, M.; Iriyama, Y.; Matsumoto, O.; Sugihara, S. Visible-light-active titanium oxide photocatalyst realized by an oxygen-deficient structure and by nitrogen doping. Applied Catalysis B-Environmental 2003, 42, 403–409. [Google Scholar] [CrossRef]
- Guan, S.; Hao, L.; Lu, Y.; Yoshida, H.; Pan, F.; Asanuma, H. Fabrication of oxygen-deficient TiO2 coatings with nano-fiber morphology for visible-light photocatalysis. Materials Science in Semiconductor Processing 2016, 41, 358–363. [Google Scholar] [CrossRef]
- Zhao, H.; Chen, J.; Rao, G.; Deng, W.; Li, Y. Enhancing photocatalytic CO2 reduction by coating an ultrathin Al2O3 layer on oxygen deficient TiO2 nanorods through atomic layer deposition. Applied Surface Science 2017, 404, 49–56. [Google Scholar] [CrossRef]
- Martinez-Oviedo, A.; Ray, S.K.; Hoang Phuc, N.; Lee, S.W. Efficient photo-oxidation of NOx by Sn doped blue TiO2 nanoparticles. Journal of Photochemistry and Photobiology A-Chemistry 2019, 370, 18–25. [Google Scholar] [CrossRef]
- Pereira, A.L.J.; Lisboa Filho, P.N.; Acuna, J.; Brandt, I.S.; Pasa, A.A.; Zanatta, A.R.; Vilcarromero, J.; Beltran, A.; Dias da Silva, J.H. Enhancement of optical absorption by modulation of the oxygen flow of TiO2 films deposited by reactive sputtering. Journal of Applied Physics 2012, 111, 113513. [Google Scholar] [CrossRef]
- Dhumal, S.Y.; Daulton, T.L.; Jiang, J.; Khomami, B.; Biswas, P. Synthesis of visible light-active nanostructured TiOx (x < 2) photocatalysts in a flame aerosol reactor. Applied Catalysis B-Environmental 2009, 86, 145–151. [Google Scholar]
- Xiao, P.; Liu, D.W.; Garcia, B.B.; Sepehri, S.; Zhang, Y.H.; Cao, G.Z. Electrochemical and photoelectrical properties of titania nanotube arrays annealed in different gases. Sensors and Actuators B-Chemical 2008, 134, 367–372. [Google Scholar] [CrossRef]
- Kushwaha, S.; Nagarajan, R. Black TiO2-graphitic carbon nanocomposite from a single source precursor and its interaction with colored and colorless contaminants under visible radiation. Materials Research Bulletin 2020, 132, 110983. [Google Scholar] [CrossRef]
- Starbova, K.; Yordanova, V.; Nihtianova, D.; Hintz, W.; Tomas, J.; Starbov, N. Excimer laser processing as a tool for photocatalytic design of sol-gel TiO2 thin films. Applied Surface Science 2008, 254, 4044–4051. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, C.Y.; Lin, T.Q.; Yin, H.; Chen, P.; Wan, D.Y.; Xu, F.F.; Huang, F.Q.; Lin, J.H.; Xie, X.M.; Jiang, M.H. H-doped black titania with very high solar absorption and excellent photocatalysis enhanced by localized surface plasmon resonance. Advanced Functional Materials 2013, 23, 5444–5450. [Google Scholar] [CrossRef]
- Filice, S.; Fiorenza, R.; Reitano, R.; Scalese, S.; Scire, S.; Fisicaro, G.; Deretzis, I.; La Magna, A.; Bongiorno, C.; Compagnini, G. TiO2 colloids laser-treated in ethanol for photocatalytic H2 production. ACS Applied Nano Materials 2020, 3, 9127–9140. [Google Scholar] [CrossRef]
- Fiorenza, R.; Scire, S.; D'Urso, L.; Compagnini, G.; Bellardita, M.; Palmisano, L. Efficient H2 production by photocatalytic water splitting under UV or solar light over variously modified TiO2-based catalysts. International Journal of Hydrogen Energy 2019, 44, 14796–14807. [Google Scholar] [CrossRef]
- Nakajima, T.; Tsuchiya, T.; Kumagai, T. Pulsed laser-induced oxygen deficiency at TiO2 surface: Anomalous structure and electrical transport properties. Journal of Solid State Chemistry 2009, 182, 2560–2565. [Google Scholar] [CrossRef]
- Kunti, A.K.; Chowdhury, M.; Sharma, S.K.; Gupta, M.; Chaudhary, R.J. Influence of O2 pressure on structural, morphological and optical properties of TiO2-SiO2 composite thin films prepared by pulsed laser deposition. Thin Solid Films 2017, 629, 79–89. [Google Scholar] [CrossRef]
- Ali, N.; Bashir, S.; Umm-i-Kalsoom, *!!! REPLACE !!!*; Akram, M.; Mahmood, K. Effect of dry and wet ambient environment on the pulsed laser ablation of titanium. Applied Surface Science 2013, 270, 49–57. [Google Scholar] [CrossRef]
- Davila, Y.; Petitmangin, A.; Hebert, C.; Perriere, J.; Seiler, W. Oxygen deficiency in oxide films grown by PLD. Applied Surface Science 2011, 257, 5354–5357. [Google Scholar] [CrossRef]
- Socol, G.; Gnatyuk, Y.; Stefan, N.; Smirnova, N.; Djokic, V.; Sutan, C.; Malinovschi, V.; Stanculescu, A.; Korduban, O.; Mihailescu, I.N. Photocatalytic activity of pulsed laser deposited TiO2 thin films in N2, O2 and CH4. Thin Solid Films 2010, 518, 4648–4653. [Google Scholar] [CrossRef]
- Nath, A.; Laha, S.S.; Khare, A. Effect of focusing conditions on synthesis of titanium oxide nanoparticles via laser ablation in titanium-water interface. Applied Surface Science 2011, 257, 3118–3122. [Google Scholar] [CrossRef]
- Rahman, M.A.; Bazargan, S.; Srivastava, S.; Wang, X.; Abd-Ellah, M.; Thomas, J.P.; Heinig, N.F.; Pradhan, D.; Leung, K.T. Defect-rich decorated TiO2 nanowires for super-efficient photoelectrochemical water splitting driven by visible light. Energy & Environmental Science 2015, 8, 3363–3373. [Google Scholar]
- Bellardita, M.; Garlisi, C.; Ozer, L.Y.; Venezia, A.M.; Sa, J.; Mamedov, F.; Palmisano, L.; Palmisano, G. Highly stable defective TiO2-x with tuned exposed facets induced by fluorine: Impact of surface and bulk properties on selective UV/visible alcohol photo-oxidation. Applied Surface Science 2020, 510, 145419. [Google Scholar] [CrossRef]
- Lo, H.H.; Gopal, N.O.; Ke, S.C. Origin of photoactivity of oxygen-deficient TiO2 under visible light. Applied Physics Letters 2009, 95, 083126. [Google Scholar] [CrossRef]
- Pu, X.; Hu, Y.; Cui, S.; Cheng, L.; Jiao, Z. Preparation of N-doped and oxygen-deficient TiO2 microspheres via a novel electron beam-assisted method. Solid State Sciences 2017, 70, 66–73. [Google Scholar] [CrossRef]
- Wang, M.; Xu, X.Y.; Lin, L.; He, D.N. Gd-La codoped TiO2 nanoparticles as solar photocatalysts. Progress in Natural Science-Materials International 2015, 25, 6–11. [Google Scholar] [CrossRef]
- Kunti, A.K.; Sharma, S.K. Structural and spectral properties of red light emitting Eu3+ activated TiO2 nanophosphor for white LED application. Ceramics International 2017, 43, 9838–9845. [Google Scholar] [CrossRef]
- Zhang, J.W.; Zhang, J.W.; Ren, H.H.; Yu, L.G.; Wu, Z.S.; Zhang, Z.J. High rate capability and long cycle stability of TiO2-delta-La composite nanotubes as anode material for lithium ion batteries. Journal of Alloys and Compounds 2014, 609, 178–184. [Google Scholar] [CrossRef]
- Zhang, J.Y.; Zhao, Z.Y.; Wang, X.Y.; Yu, T.; Guan, J.; Yu, Z.T.; Li, Z.S.; Zou, Z.G. Increasing the oxygen vacancy density on the TiO2 surface by la-doping for dye-sensitized solar cells. Journal of Physical Chemistry C 2010, 114, 18396–18400. [Google Scholar] [CrossRef]
- Hatanaka, Y.; Naito, H.; Itou, S.; Kando, M. Photocatalytic characteristics of hydro-oxygenated amorphous titanium oxide films prepared using remote plasma enhanced chemical vapor deposition. Applied Surface Science 2005, 244, 554–557. [Google Scholar] [CrossRef]
- Sakai, T.; Kuniyoshi, Y.; Aoki, W.; Ezoe, S.; Endo, T.; Hoshi, Y. High-rate deposition of photocatalytic TiO2 films by oxygen plasma assist reactive evaporation method. Thin Solid Films 2008, 516, 5860–5863. [Google Scholar] [CrossRef]
- Li, Y.; Wang, W.; Wang, F.; Di, L.; Yang, S.; Zhu, S.; Yao, Y.; Ma, C.; Dai, B.; Yu, F. Enhanced photocatalytic degradation of organic dyes via defect-rich TiO2 prepared by dielectric barrier discharge plasma. Nanomaterials 2019, 9, 720. [Google Scholar] [CrossRef] [PubMed]
- Hojo, M.; Okimura, K. Effect of annealing with ar plasma irradiation for transparent conductive Nb-doped TiO2 films on glass substrate. Japanese Journal of Applied Physics 2009, 48, 08HK06. [Google Scholar] [CrossRef]
- Kawakami, R.; Mimoto, Y.; Yanagiya, S.i.; Shirai, A.; Niibe, M.; Nakano, Y.; Mukai, T. Photocatalytic activity enhancement of anatase/rutile-mixed phase TiO2 nanoparticles annealed with low-temperature O2 plasma. Physica Status Solidi a-Applications and Materials Science 2021, 218, 2100536. [Google Scholar] [CrossRef]
- An, H.R.; Hong, Y.C.; Kim, H.; Huh, J.Y.; Park, E.C.; Park, S.Y.; Jeong, Y.; Park, J.I.; Kim, J.P.; Lee, Y.C.; Hong, W.K.; Oh, Y.K.; Kim, Y.J.; Yang, M.; Lee, H.U. Studies on mass production and highly solar light photocatalytic properties of gray hydrogenated-TiO2 sphere photocatalysts. Journal of Hazardous Materials 2018, 358, 222–233. [Google Scholar] [CrossRef] [PubMed]
- Mizukoshi, Y.; Ohwada, M.; Seino, S.; Horibed, H.; Nishimura, Y.; Terashima, C. Synthesis of oxygen-deficient blue titanium oxide by discharge plasma generated in aqueous ammonia solution. Applied Surface Science 2019, 489, 489,255–261. [Google Scholar] [CrossRef]
- Ji, M.; Choa, Y.H.; Lee, Y.I. One-step synthesis of black TiO2-x microspheres by ultrasonic spray pyrolysis process and their visible-light-driven photocatalytic activities. Ultrasonics Sonochemistry 2021, 74, 105557. [Google Scholar] [CrossRef] [PubMed]
- Nakaruk, A.; Reece, P.J.; Ragazzon, D.; Sorrell, C.C. TiO2 films prepared by ultrasonic spray pyrolysis. Materials Science and Technology 2010, 26, 469–472. [Google Scholar] [CrossRef]
- Osorio-Vargas, P.A.; Pulgarin, C.; Sienkiewicz, A.; Pizzio, L.R.; Blanco, M.N.; Torres-Palma, R.A.; Petrier, C.; Rengifo-Herrera, J.A. Low-frequency ultrasound induces oxygen vacancies formation and visible light absorption in TiO2 P-25 nanoparticles. Ultrasonics Sonochemistry 2012, 19, 383–386. [Google Scholar] [CrossRef]
- Bellardita, M.; El Nazer, H.A.; Loddo, V.; Parrino, F.; Venezia, A.M.; Palmisano, L. Photoactivity under visible light of metal loaded TiO2 catalysts prepared by low frequency ultrasound treatment. Catalysis Today 2017, 284, 92–99. [Google Scholar] [CrossRef]
- Langhammer, D.; Thyr, J.; Osterlund, L. Surface properties of reduced and stoichiometric TiO2 as probed by SO2 adsorption. Journal of Physical Chemistry C 2019, 123, 24549–24557. [Google Scholar] [CrossRef]
- Xu, M.; Chen, Y.; Qin, J.; Feng, Y.; Li, W.; Chen, W.; Zhu, J.; Li, H.; Bian, Z. Unveiling the role of defects on oxygen activation and photodegradation of organic pollutants. Environmental Science & Technology 2018, 52, 13879–13886. [Google Scholar]
- Albetran, H.; O'Connor, B.H.; Low, I.M. Effect of calcination on band gaps for electrospun titania nanofibers heated in air-argon mixtures. Materials & Design 2016, 92, 480–485. [Google Scholar]
- Sang, L.X.; Zhang, Z.Y.; Ma, C.F. Photoelectrical and charge transfer properties of hydrogen-evolving TiO2 nanotube arrays electrodes annealed in different gases. International Journal of Hydrogen Energy 2011, 36, 4732–4738. [Google Scholar] [CrossRef]
- Qi, W.; Zhang, F.; An, X.; Liu, H.; Qu, J. Oxygen vacancy modulation of {010}-dominated TiO2 for enhanced photodegradation of Sulfamethoxazole. Catalysis Communications 2019, 118, 35–38. [Google Scholar] [CrossRef]
- Li, Y.; Ye, X.; Cao, S.; Yang, C.; Wang, Y.; Ye, J. Oxygen-Deficient Dumbbell-Shaped Anatase TiO2-x Mesocrystals with Nearly 100% Exposed {101} Facets: Synthesis, Growth Mechanism, and Photocatalytic Performance. Chemistry-a European Journal 2019, 25, 3032–3041. [Google Scholar] [CrossRef]
- Du, M.; Chen, Q.; Wang, Y.; Hu, J.; Meng, X. Synchronous construction of oxygen vacancies and phase junction in TiO2 hierarchical structure for enhancement of visible light photocatalytic activity. Journal of Alloys and Compounds 2020, 830, 154649. [Google Scholar] [CrossRef]
- Sheng, Z.; Song, S.; Wang, H.; Wu, Z.; Liu, Y. One-step hydrothermal synthesis of Pd-modified TiO2 with high photocatalytic activity for nitric oxide oxidation in gas phase. Environmental Engineering Science 2012, 29, 972–978. [Google Scholar] [CrossRef]
- Sasirekha, N.; Basha, S.J.S.; Shanthi, K. Photocatalytic performance of Ru doped anatase mounted on silica for reduction of carbon dioxide. Applied Catalysis B-Environmental 2006, 62, 169–180. [Google Scholar] [CrossRef]
- Gao, P.; Yang, L.; Xiao, S.; Wang, L.; Guo, W.; Lu, J. Effect of Ru, Rh, Mo, and Pd adsorption on the electronic and optical properties of anatase TiO2 (101): A DFT Investigation. Materials 2019, 12, 814. [Google Scholar] [CrossRef]
- Thalgaspitiya, W.R.K.; Kapuge, T.K.; He, J.; Deljoo, B.; Meguerdichian, A.G.; Aindow, M.; Suib, S.L. Multifunctional transition metal doped titanium dioxide reduced graphene oxide composites as highly efficient adsorbents and photocatalysts. Microporous and Mesoporous Materials 2020, 307, 110521. [Google Scholar] [CrossRef]
- Wang, T.; Li, B.R.; Wu, L.G.; Yin, Y.B.; Jiang, B.Q.; Lou, J.Q. Enhanced performance of TiO2/reduced graphene oxide doped by rare-earth ions for degrading phenol in seawater excited by weak visible light. Advanced Powder Technology 2019, 30, 1920–1931. [Google Scholar] [CrossRef]
- Stengl, V.; Bakardjieva, S.; Murafa, N. Preparation and photocatalytic activity of rare earth doped TiO2 nanoparticles. Materials Chemistry and Physics 2009, 114, 217–226. [Google Scholar] [CrossRef]
- Fang, X.L.; Chen, X.H.; Zhu, Z.S. Optical and photocatalytic properties of Er3+ and/or Yb3+ doped TiO2 photocatalysts. Journal of Materials Science-Materials in Electronics 2017, 28, 474–479. [Google Scholar] [CrossRef]
- Setiawati, E.; Kawano, K. Stabilization of anatase phase in the rare earth; Eu and Sm ion doped nanoparticle TiO2. Journal of Alloys and Compounds 2008, 451, 293–296. [Google Scholar] [CrossRef]
- Yu, Y.G.; Chen, G.; Zhou, Y.S.; Han, Z.H. Recent advances in rare-earth elements modification of inorganic semiconductor-based photocatalysts for efficient solar energy conversion: A review. Journal of Rare Earths 2015, 33, 453–462. [Google Scholar] [CrossRef]
- Liu, S.Y.; Zuo, C.G.; Xia, J. Solid-state synthesis and photodegradation property of anatase TiO2 micro-nanopowder by sodium replacement. Solid State Sciences 2021, 115, 106589. [Google Scholar] [CrossRef]
- Lv, C.; Lan, X.; Wang, L.; Yu, Q.; Zhang, M.; Sun, H.; Shi, J. Alkaline-earth-metal-doped TiO2 for enhanced photodegradation and H2 evolution: insights into the mechanisms. Catalysis Science & Technology 2019, 9, 6124–6135. [Google Scholar]
- Li, H.; Hao, Y.B.; Lu, H.Q.; Liang, L.; Wang, Y.; Qiu, J.H.; Shi, X.; Wang, Y.Y.; Yao, J.F. systematic study on visible-light N-doped TiO2 photocatalyst obtained from ethylenediamine by sol-gel method. Applied Surface Science 2015, 344, 112–118. [Google Scholar] [CrossRef]
- Chaudhari, N.S.; Warule, S.S.; Dhanmane, S.A.; Kulkarni, M.V.; Valant, M.; Kale, B.B. Nanostructured N-doped TiO2 marigold flowers for an efficient solar hydrogen production from H2S. Nanoscale 2013, 5, 9383–9390. [Google Scholar] [CrossRef]
- Wang, Y.W.; Huang, Y.; Ho, W.K.; Zhang, L.; Zou, Z.G.; Lee, S.C. Biomolecule-controlled hydrothermal synthesis of C-N-S-tridoped TiO2 nanocrystalline photocatalysts for NO removal under simulated solar light irradiation. Journal of Hazardous Materials 2009, 169, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.X.; Ma, G.F.; Peng, S.; Lu, G.; Li, S.B. Boron and nitrogen co-doped titania with enhanced visible-light photocatalytic activity for hydrogen evolution. Applied Surface Science 2008, 254, 6831–6836. [Google Scholar] [CrossRef]
- Yuan, J.; Chen, M.X.; Shi, J.W.; Shangguan, W.F. Preparations and photocatalytic hydrogen evolution of N-doped TiO2 from urea and titanium tetrachloride. International Journal of Hydrogen Energy 2006, 31, 1326–1331. [Google Scholar] [CrossRef]
- Momeni, M.M.; Ghayeb, Y.; Ghonchegi, Z. Visible light activity of sulfur-doped TiO2 nanostructure photoelectrodes prepared by single-step electrochemical anodizing process. Journal of Solid State Electrochemistry 2015, 19, 1359–1366. [Google Scholar] [CrossRef]
- Carmichael, P.; Hazafy, D.; Bhachu, D.S.; Mills, A.; Darr, J.A.; Parkin, I.P. Atmospheric pressure chemical vapour deposition of boron doped titanium dioxide for photocatalytic water reduction and oxidation. Physical Chemistry Chemical Physics 2013, 15, 16788–16794. [Google Scholar] [CrossRef]
- Wu, G.S.; Chen, A. Direct growth of F-doped TiO2 particulate thin films with high photocatalytic activity for environmental applications. Journal of Photochemistry and Photobiology a-Chemistry 2008, 195, 47–53. [Google Scholar] [CrossRef]
- Zhu, H.X.; Liu, J.M. First principles calculations of electronic and optical properties of Mo and C co-doped anatase TiO2. Applied Physics a-Materials Science & Processing 2014, 117, 831–839. [Google Scholar]
- Diao, W.Y.; He, J.; Wang, Q.; Rao, X.; Zhang, Y.P. K, Na and Cl co-doped TiO2 nanorod arrays on carbon cloth for efficient photocatalytic degradation of formaldehyde under UV/visible LED irradiation. Catalysis Science & Technology 2021, 11, 230–238. [Google Scholar]
- Barakat, N.A.M.; Zaki, A.H.; Ahmed, E.; Farghali, A.A.; Al-Mubaddel, F.S. FexCo1-x-doped titanium oxide nanotubes as effective photocatalysts for hydrogen extraction from ammonium phosphate. International Journal of Hydrogen Energy 2018, 43, 7990–7997. [Google Scholar] [CrossRef]
- Filippatos, P.P.; Soultati, A.; Kelaidis, N.; Petaroudis, C.; Alivisatou, A.A.; Drivas, C.; Kennou, S.; Agapaki, E.; Charalampidis, G.; Yusoff, A.R.b.M.; Lathiotakis, N.N.; Coutsolelos, A.G.; Davazoglou, D.; Vasilopoulou, M.; Chroneos, A. Preparation of hydrogen, fluorine and chlorine doped and co-doped titanium dioxide photocatalysts: a theoretical and experimental approach. Scientific Reports 2021, 11, 5700. [Google Scholar] [CrossRef]
- Meng, S.G.; Zhang, J.F.; Chen, S.F.; Zhang, S.J.; Huang, W.X. Perspective on construction of heterojunction photocatalysts and the complete utilization of photogenerated charge carriers. Applied Surface Science 2019, 476, 982–992. [Google Scholar] [CrossRef]
- Low, J.X.; Yu, J.G.; Jaroniec, M.; Wageh, S.; Al-Ghamdi, A.A. Heterojunction photocatalysts. Advanced Materials 2017, 29, 1601694. [Google Scholar] [CrossRef] [PubMed]
- Smith, Y.R.; Sarma, B.; Mohanty, S.K.; Misra, M. Formation of TiO2-WO3 nanotubular composite via single-step anodization and its application in photoelectrochemical hydrogen generation. Electrochemistry Communications 2012, 19, 131–134. [Google Scholar] [CrossRef]
- Choudhury, S.; Sasikala, R.; Saxena, V.; Aswal, D.K.; Bhattacharya, D. A new route for the fabrication of an ultrathin film of a PdO-TiO2 composite photocatalyst. Dalton Transactions 2012, 41, 12090–12095. [Google Scholar] [CrossRef] [PubMed]
- Navarrete, M.; Cipagauta-Diaz, S.; Gomez, R. Ga2O3/TiO2 semiconductors free of noble metals for the photocatalytic hydrogen production in a water/methanol mixture. Journal of Chemical Technology and Biotechnology 2019, 94, 3457–3465. [Google Scholar] [CrossRef]
- Gholami, M.; Shirzad-Siboni, M.; Farzadkia, M.; Yang, J.K. Synthesis, characterization, and application of ZnO/TiO2 nanocomposite for photocatalysis of a herbicide (Bentazon). Desalination and Water Treatment 2016, 57, 13632–13644. [Google Scholar] [CrossRef]
- Chen, J.Z.; Chen, T.H.; Lai, L.W.; Li, P.Y.; Liu, H.W.; Hong, Y.Y.; Liu, D.S. Preparation and characterization of surface photocatalytic activity with NiO/TiO2 nanocomposite structure. Materials 2015, 8, 8,4273–4286. [Google Scholar] [CrossRef]
- Wang, W.T.; Wu, Z.Q.; Eftekhari, E.; Huo, Z.Y.; Li, X.M.; Tade, M.O.; Yan, C.; Yan, Z.F.; Li, C.H.; Li, Q.; Zhao, D.Y. High performance heterojunction photocatalytic membranes formed by embedding Cu2O and TiO2 nanowires in reduced graphene oxide. Catalysis Science & Technology 2018, 8, 1704–1711. [Google Scholar]
- Morales-Torres, S.; Pastrana-Martinez, L.M.; Figueiredo, J.L.; Faria, J.L.; Silva, A.M.T. Design of graphene-based TiO2 photocatalysts-a review. Environmental Science and Pollution Research 2012, 19, 3676–3687. [Google Scholar] [CrossRef]
- Gao, P.; Sun, D.D. ultrasonic preparation of hierarchical graphene-oxide/TiO2 composite microspheres for efficient photocatalytic hydrogen production. Chemistry-an Asian Journal 2013, 8, 2779–2786. [Google Scholar] [CrossRef]
- Kong, D.W.; Zhao, M.; Li, S.K.; Huang, F.; Song, J.M.; Yuan, Y.P.; Shen, Y.H.; Xie, A.J. Synthesis of TiO2/rGO nanocomposites with enhanced photoelectrochemical performance and photocatalytic activity. Nano 2016, 11, 1650007. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Li, H.P.; Cui, X.L. Preparation and photocatalytic activity for hydrogen evolution of TiO2/graphene sheets composite. Chinese Journal of Inorganic Chemistry 2009, 25, 1903–1907. [Google Scholar]
- Shen, J.F; Shi, M.; Yan, B.; Ma, H.W.; Li, N.; Ye, M.X. Ionic liquid-assisted one-step hydrothermal synthesis of TiO2-reduced graphene oxide composites. Nano Research 2011, 4, 795–806. [Google Scholar] [CrossRef]
- Fu, Z.; Wang, H.; Wang, Y.N.; Wang, S.H.; Li, Z.L.; Sun, Q. Construction of three-dimensional g-C3N4/Gr-CNTs/TiO2 Z-scheme catalyst with enhanced photocatalytic activity. Applied Surface Science 2020, 510, 145494. [Google Scholar] [CrossRef]
- Chiang, H.H.; Wang, S.H.; Chou, H.Y.; Huang, C.C.; Tsai, T.L.; Yang, Y.C.; Lee, J.W.; Lin, T.Y.; Wu, Y.J.; Chen, C.C. . Surface modification of ato photocatalyst on its bactericidal effect against escherichia coli. Journal of Marine Science and Technology-Taiwan 2014, 22, 269–276. [Google Scholar]
- Zhou, X.M. TiO2-supported single-atom catalysts for photocatalytic reactions. Acta Physico-Chimica Sinica 2021, 37, 2008064. [Google Scholar]
- Bernareggi, M.; Chiarello, G.L.; West, G.; Ratova, M.; Ferretti, A.M.; Kelly, P.; Selli, E. Cu and Pt clusters deposition on TiO2 powders by DC magnetron sputtering for photocatalytic hydrogen production. Catalysis Today 2019, 326, 15–21. [Google Scholar] [CrossRef]
- Dozzi, M.V.; Brocato, S.; Marra, G.; Tozzola, G.; Meda, L.; Selli, E. Aqueous ammonia abatement on Pt- and Ru-modified TiO2: Selectivity effects of the metal nanoparticles deposition method. Catalysis Today 2017, 287, 148–154. [Google Scholar] [CrossRef]
- Zheng, Z.; Murakamia, N.; Liu, J.J.; Teng, Z.; Zhang, Q.T.; Cao, Y.; Cheng, H.H.; Ohno, T. Development of plasmonic photocatalyst by site-selective loading of bimetallic nanoparticles of Au and Ag on titanium(IV) oxide. Chemcatchem 2020, 12, 3783–3792. [Google Scholar] [CrossRef]
- Luo, J.; Li, D.L.; Yang, Y.; Liu, H.Q.; Chen, J.Y.; Wang, H.Y. Preparation of Au/reduced graphene oxide/hydrogenated TiO2 nanotube arrays ternary composites for visible-light-driven photoelectrochemical water splitting. Journal of Alloys and Compounds 2016, 661, 380–388. [Google Scholar] [CrossRef]
- Ge, M.Z.; Cao, C.Y.; Li, S.H.; Tang, Y.X.; Wang, L.N.; Qi, N.; Huang, J.Y.; Zhang, K.Q.; Al-Deyab, S.S.; Lai, Y.K. In situ plasmonic Ag nanoparticle anchored TiO2 nanotube arrays as visible-light-driven photocatalysts for enhanced water splitting. Nanoscale 2016, 8, 5226–5234. [Google Scholar] [CrossRef] [PubMed]
- Liu, E.Z.; Kang, L.M.; Yang, Y.; Sun, T.; Hu, X.Y.; Zhu, C.; Liu, H.C.; Wang, Q.P.; Li, X.H.; Fan, J. Plasmonic Ag deposited TiO2 nano-sheet film for enhanced photocatalytic hydrogen production by water splitting. Nanotechnology 2014, 25, 165401. [Google Scholar] [CrossRef] [PubMed]
- Barrios, C.E.; Albiter, E.; Jimenez, J.; Tiznado, H.; Romo-Herrera, J.; Zanella, R. Photocatalytic hydrogen production over titania modified by gold - Metal (palladium, nickel and cobalt) catalysts. International Journal of Hydrogen Energy 2016, 41, 23287–23300. [Google Scholar] [CrossRef]
- Monamary, A.; Vijayalakshmi, K. Substantial effect of palladium overlayer deposition on the H2 sensing performance of TiO2/ITO nanocomposite. Ceramics International 2018, 44, 22957–22962. [Google Scholar] [CrossRef]
- Shi, J.W.; Guan, X.J.; Zhou, Z.H.; Liu, H.P.; Guo, L.J. Eosin Y-sensitized nanosheet-stacked hollow-sphere TiO2 for efficient photocatalytic H2 production under visible-light irradiation. Journal of Nanoparticle Research 2015, 17, 252. [Google Scholar] [CrossRef]
- Murcia, J.J.; Avila-Martinez, E.G.; Rojas, H.; Cubillos, J.; Ivanova, S.; Penkova, A.; Laguna, O.H. Powder and nanotubes titania modified by dye sensitization as photocatalysts for the organic pollutants elimination. Nanomaterials 2019, 9, 517. [Google Scholar] [CrossRef]
- Vallejo, W.; Rueda, A.; Diaz-Uribe, C.; Grande, C.; Quintana, P. Photocatalytic activity of graphene oxide-TiO2 thin films sensitized by natural dyes extracted from Bactris guineensis. Royal Society Open Science 2019, 6, 181824. [Google Scholar] [CrossRef]
- Rodriguez, H.B.; Di Iorio, Y.; Meichtry, J.M.; Grela, M.A.; Litter, M.I.; San Roman, E. Evidence on dye clustering in the sensitization of TiO2 by aluminum phthalocyanine. Photochemical & Photobiological Sciences 2013, 12, 1984–1990. [Google Scholar]
- Ding, H.M.; Sun, H.; Shan, Y.K. Preparation and characterization of mesoporous SBA-15 supported dye-sensitized TiO2 photocatalyst. Journal of Photochemistry and Photobiology a-Chemistry 2005, 169, 101–107. [Google Scholar] [CrossRef]
- Wahyuningsih, S.; Purnawan, C.; Kartikasari, P.A.; Praistia, N. Visible light photoelectrocatalytic degradation of rhodamine B using a dye-sensitised TiO2 electrode. Chemical Papers 2014, 68, 1248–1256. [Google Scholar] [CrossRef]
- Li, Y.; Li, H.M.; Lu, X.L.; Yu, X.; Kong, M.H.; Duan, X.D.; Qin, G.; Zhao, Y.H.; Wang, Z.L.; Dionysiou, D.D. Molybdenum disulfide nanosheets vertically grown on self-supported titanium dioxide/nitrogen-doped carbon nanofiber film for effective hydrogen peroxide decomposition and "memory catalysis". Journal of Colloid and Interface Science 2021, 596, 384–395. [Google Scholar] [CrossRef] [PubMed]
- Ikeue, K.; Yamashita, H.; Anpo, M. Photocatalytic reduction of CO2 with H2O on titanium oxides prepared within zeolites and mesoporous molecular sieves. Electrochemistry 2002, 70, 402–408. [Google Scholar] [CrossRef]
- Rasalingam, S.; Kibombo, H.S.; Wu, C.M.; Budhi, S.; Peng, R.; Baltrusaitis, J.; Koodali, R.T. Influence of Ti-O-Si hetero-linkages in the photocatalytic degradation of Rhodamine B. Catalysis Communications 2013, 31, 66–70. [Google Scholar] [CrossRef]
- Liu, B.; Zeng, H.C. Carbon nanotubes supported mesoporous mesocrystals of anatase TiO2. Chemistry of Materials 2008, 20, 2711–2718. [Google Scholar] [CrossRef]
- Najafabadi, A.T.; Taghipour, F. Physicochemical impact of zeolites as the support for photocatalytic hydrogen production using solar-activated TiO2-based nanoparticles. Energy Conversion and Management 2014, 82, 106–113. [Google Scholar] [CrossRef]
- Xu, Y.M.; Langford, C.H. Enhanced photoactivity of a titanium(iv) oxide-supported on ZSM5 and zeolite-a at low-coverage. Journal of Physical Chemistry 1995, 99, 11501–11507. [Google Scholar] [CrossRef]
- Kim, H.J.; Shul, Y.G.; Han, H.S. Photocatalytic properties of silica-supported TiO2. Topics in Catalysis 2005, 35, 287–293. [Google Scholar] [CrossRef]
- Yin, J.W.; Xing, Z.P.; Kuang, J.Y.; Li, Z.Z.; Li, M.; Jiang, J.J.; Tan, S.Y.; Zhu, Q.; Zhou, W. Bi plasmon-enhanced mesoporous Bi2MoO6/Ti3+ self-doped TiO2 microsphere heterojunctions as efficient visible-light-driven photocatalysts. Journal of Alloys and Compounds 2018, 750, 659–668. [Google Scholar] [CrossRef]
- Xing, M.Y.; Zhang, J.L.; Qiu, B.C.; Tian, B.Z.; Anpo, M.; Che, M. A brown mesoporous TiO2-x/MCF Composite with an extremely high quantum yield of solar energy photocatalysis for H2 evolution. Small 2015, 11, 1920–1929. [Google Scholar] [CrossRef]
- Wen, C.Z.; Jiang, H.B.; Qiao, S.Z.; Yang, H.G.; Lu, G.Q. Synthesis of high-reactive facets dominated anatase TiO2. Journal of Materials Chemistry 2011, 21, 7052–7061. [Google Scholar] [CrossRef]
- Li, H.M.; Zeng, Y.S.; Huang, T.C.; Piao, L.; Liu, M. Controlled synthesis of anatase TiO2 single crystals with dominant {001} facets from TiO2 powders. Chempluschem 2012, 77, 1017–1021. [Google Scholar] [CrossRef]
- Wang, W.; Lu, C.H.; Ni, Y.R.; Song, J.B.; Su, M.X.; Xu, Z.Z. Enhanced visible-light photoactivity of {001} facets dominated TiO2 nanosheets with even distributed bulk oxygen vacancy and Ti3+. Catalysis Communications 2012, 22, 19–23. [Google Scholar] [CrossRef]
- Shang, Q.Q.; Tan, X.; Yu, T.; Zhang, Z.Y.; Zou, Y.; Wang, S.Y. Efficient gaseous toluene photoconversion on graphene-titanium dioxide nanocomposites with dominate exposed {001} facets. Journal of Colloid and Interface Science 2015, 455, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Lv, Z.G.; Cheng, X.; Liu, B.Q.; Guo, Z.M.; Jin, M.M.; Zhang, C. Enhanced photoredox water splitting of Sb-N donor-acceptor pairs in TiO2. Inorganic Chemistry Frontiers 2019, 6, 2404–2411. [Google Scholar] [CrossRef]
- Koci, K.; Troppova, I.; Edelmannova, M.; Starostka, J.; Matejova, L.; Lang, J.; Reli, M.; Drobna, H.; Rokicinska, A.; Kustrowski, P.; Capek, L. Photocatalytic decomposition of methanol over La/TiO2 materials. Environmental Science and Pollution Research 2018, 25, 34818–34825. [Google Scholar] [CrossRef] [PubMed]
- Bharatvaj, J.; Preethi, V.; Kanmani, S. Hydrogen production from sulphide wastewater using Ce3+-TiO2 photocatalysis. International Journal of Hydrogen Energy 2018, 43, 3935–3945. [Google Scholar] [CrossRef]
- Agegnehu, A.K.; Pan, C.J.; Tsai, M.C.; Rick, J.; Su, W.N.; Lee, J.F.; Hwang, B.J. Visible light responsive noble metal-free nanocomposite of V-doped TiO2 nanorod with highly reduced graphene oxide for enhanced solar H2 production. International Journal of Hydrogen Energy 2016, 41, 6752–6762. [Google Scholar] [CrossRef]
- Barakat, N.A.M.; Ahmed, E.; Amen, M.T.; Abdelkareem, M.A.; Farghali, A.A. N-doped Ni/C/TiO2 nanocomposite as effective photocatalyst for water splitting. Materials Letters 2018, 210, 317–320. [Google Scholar] [CrossRef]
- Gao, L.S.; Zhang, S.N.; Zou, X.X.; Wang, J.F.; Su, J.; Chen, J.S. Oxygen vacancy engineering of titania-induced by Sr2+ dopants for visible-light-driven hydrogen evolution. Inorganic Chemistry 2021, 60, 32–36. [Google Scholar] [CrossRef]
- Xu, J.C.; Zhang, J.J.; Cai, Z.Y.; Huang, H.; Huang, T.H.; Wang, P.; Wang, X.Y. Facile and large-scale synthesis of defective black TiO2-x(B) nanosheets for efficient visible-light-driven photocatalytic hydrogen evolution. Catalysts 2019, 9, 1048. [Google Scholar] [CrossRef]
- Barakat, N.A.M.; Erfan, N.A.; Mohammed, A.A.; Mohamed, S.E.I. Ag-decorated TiO2 nanofibers as Arrhenius equation-incompatible and effective photocatalyst for water splitting under visible light irradiation. Colloids and Surfaces a-Physicochemical and Engineering Aspects 2020, 604, 125307. [Google Scholar] [CrossRef]
- Dang, H.F.; Dong, X.F.; Dong, Y.C.; Zhang, Y.; Hampshire, S. TiO2 nanotubes coupled with nano-Cu(OH)2 for highly efficient photocatalytic hydrogen production. International Journal of Hydrogen Energy 2013, 38, 2126–2135. [Google Scholar] [CrossRef]
- Diaz, L.; Rodriguez, V.D.; Gonzalez-Rodriguez, M.; Rodriguez-Castellon, E.; Algarra, M.; Nunez, P.; Moretti, E. M/TiO2 (M = Fe, Co, Ni, Cu, Zn) catalysts for photocatalytic hydrogen production under UV and visible light irradiation. Inorganic Chemistry Frontiers 2021, 8, 3491–3500. [Google Scholar] [CrossRef]
- El-Bery, H.M.; Abdelhamid, H.N. Abdelhamid. Photocatalytic hydrogen generation via water splitting using ZIF-67 derived Co3O4@C/TiO2. Journal of Environmental Chemical Engineering 2021, 9, 105702. [Google Scholar] [CrossRef]
- Fujita, S.; Kawamori, H.; Honda, D.; Yoshida, H.; Arai, M. Photocatalytic hydrogen production from aqueous glycerol solution using NiO/TiO2 catalysts: Effects of preparation and reaction conditions. Applied Catalysis B-Environmental 2016, 181, 818–824. [Google Scholar] [CrossRef]
- Kokporka, L.; Onsuratoom, S.; Puangpetch, T.; Chavadej, S. Sol-gel-synthesized mesoporous-assembled TiO2-ZrO2 mixed oxide nanocrystals and their photocatalytic sensitized H2 production activity under visible light irradiation. Materials Science in Semiconductor Processing 2013, 16, 667–678. [Google Scholar] [CrossRef]
- Mou, Z.G.; Wu, Y.J.; Sun, J.H.; Yang, P.; Du, Y.K.; Lu, C. TiO2 Nanoparticles-functionalized N-doped graphene with superior interfacial contact and enhanced charge separation for photocatalytic hydrogen generation. ACS Applied Materials & Interfaces 2014, 6, 13798–13806. [Google Scholar]
- Sharma, A.; Thuan, D.V.; Pham, T.D.; Tung, M.H.T.; Truc, N.T.T.; Vo, D.V.N. Advanced surface of fibrous activated carbon immobilized with FeO/TiO2 for photocatalytic evolution of hydrogen under visible light. Chemical Engineering & Technology 2020, 43, 752–761. [Google Scholar]
- Wang, C.; Hu, Q.Q.; Huang, J.Q.; Zhu, C.; Deng, Z.H.; Shi, H.L.; Wu, L.; Liu, Z.G.; Cao, Y.G. Enhanced hydrogen production by water splitting using Cu-doped TiO2 film with preferred (001) orientation. Applied Surface Science 2014, 292, 161–164. [Google Scholar] [CrossRef]
- Ma, J.; Tan, X.L.; Yu, T.; Li, X. Fabrication of g-C3N4/TiO2 hierarchical spheres with reactive {001} TiO2 crystal facets and its visible-light photocatalytic activity. International Journal of Hydrogen Energy 2016, 41, 3877–3887. [Google Scholar] [CrossRef]
- Xiang, Q.J.; Yu, J.G.; Jaroniec, M. Enhanced photocatalytic H2 production activity of graphene-modified titania nanosheets. Nanoscale 2011, 3, 3670–3678. [Google Scholar] [CrossRef] [PubMed]
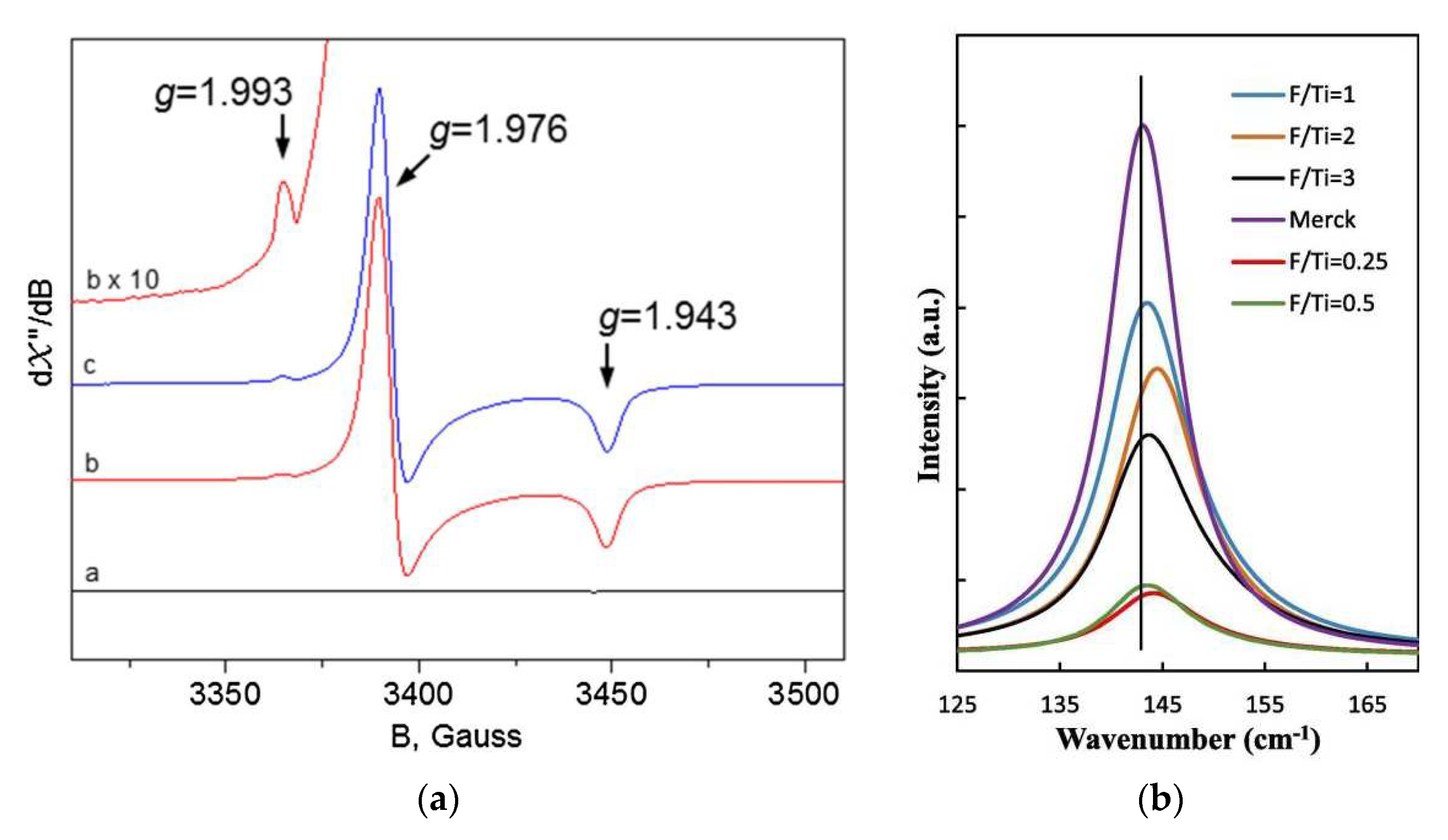
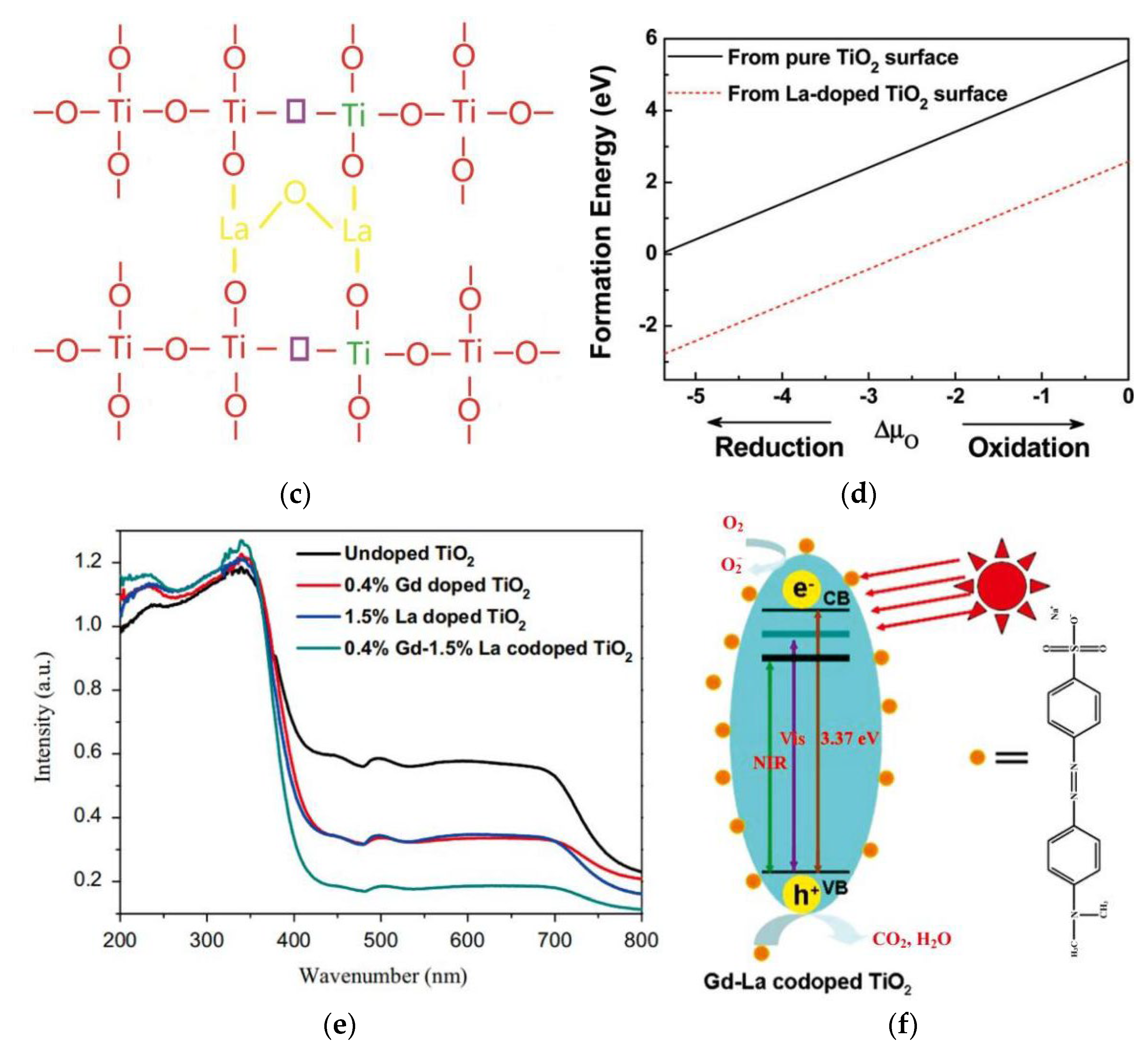
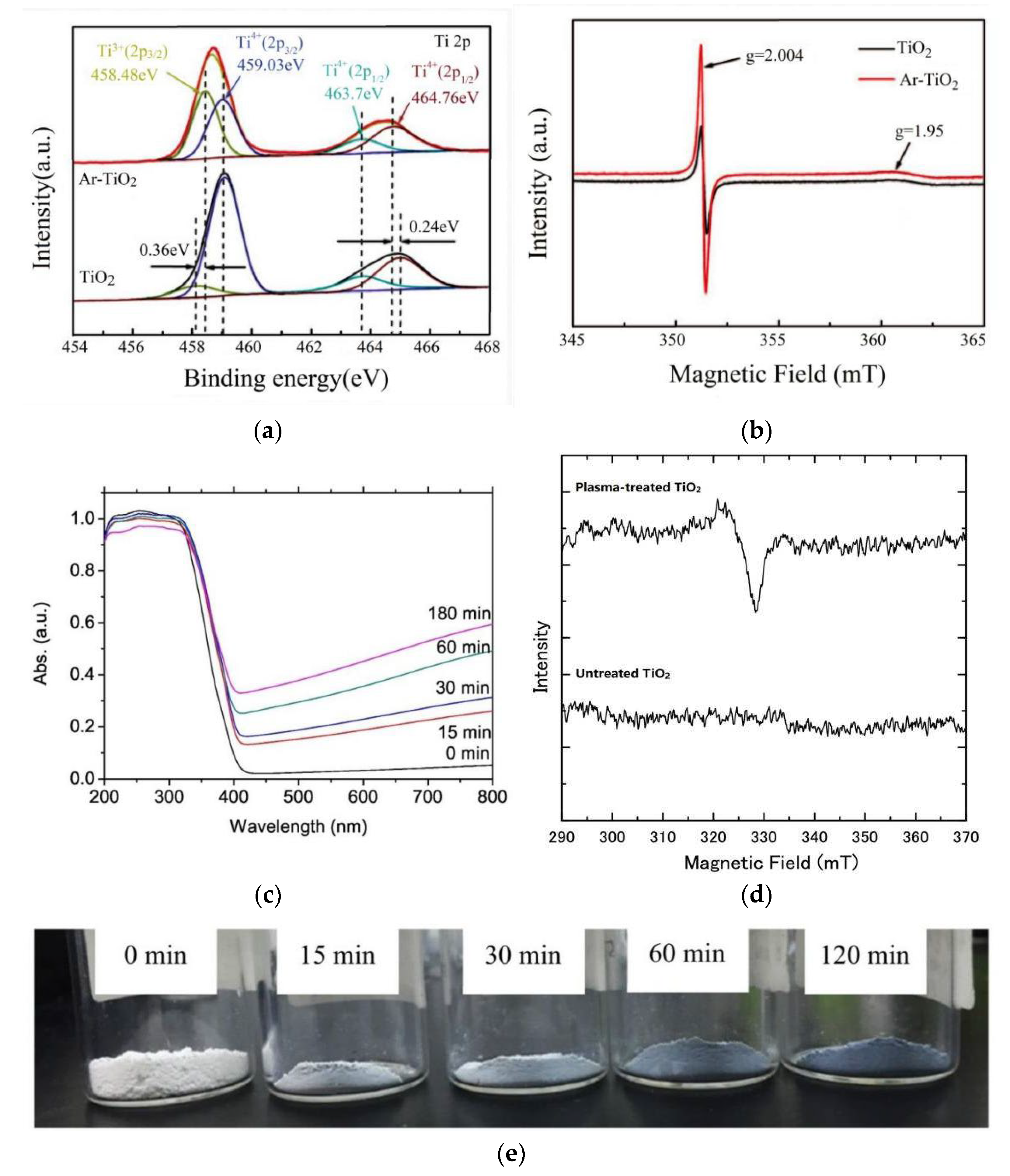
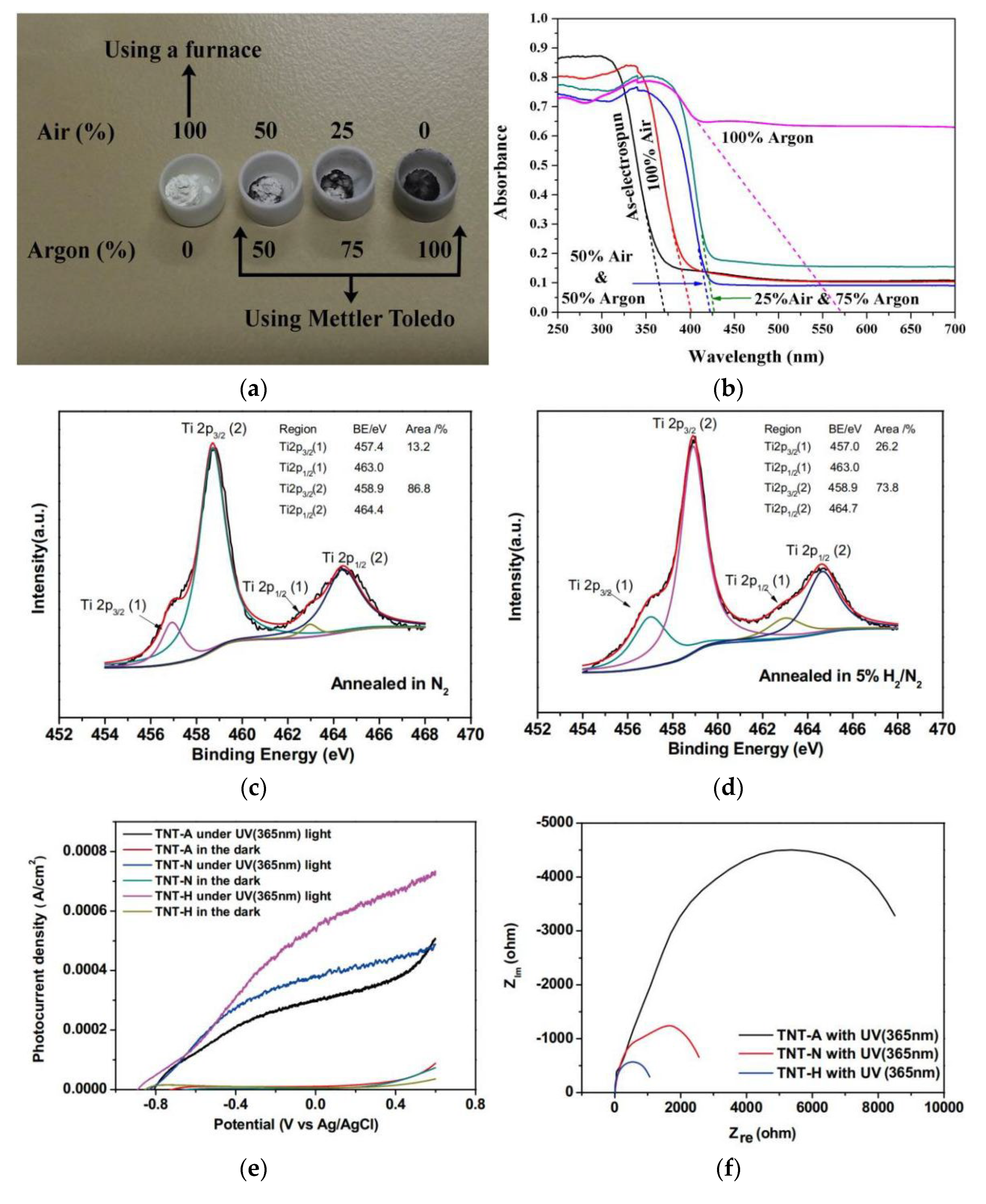
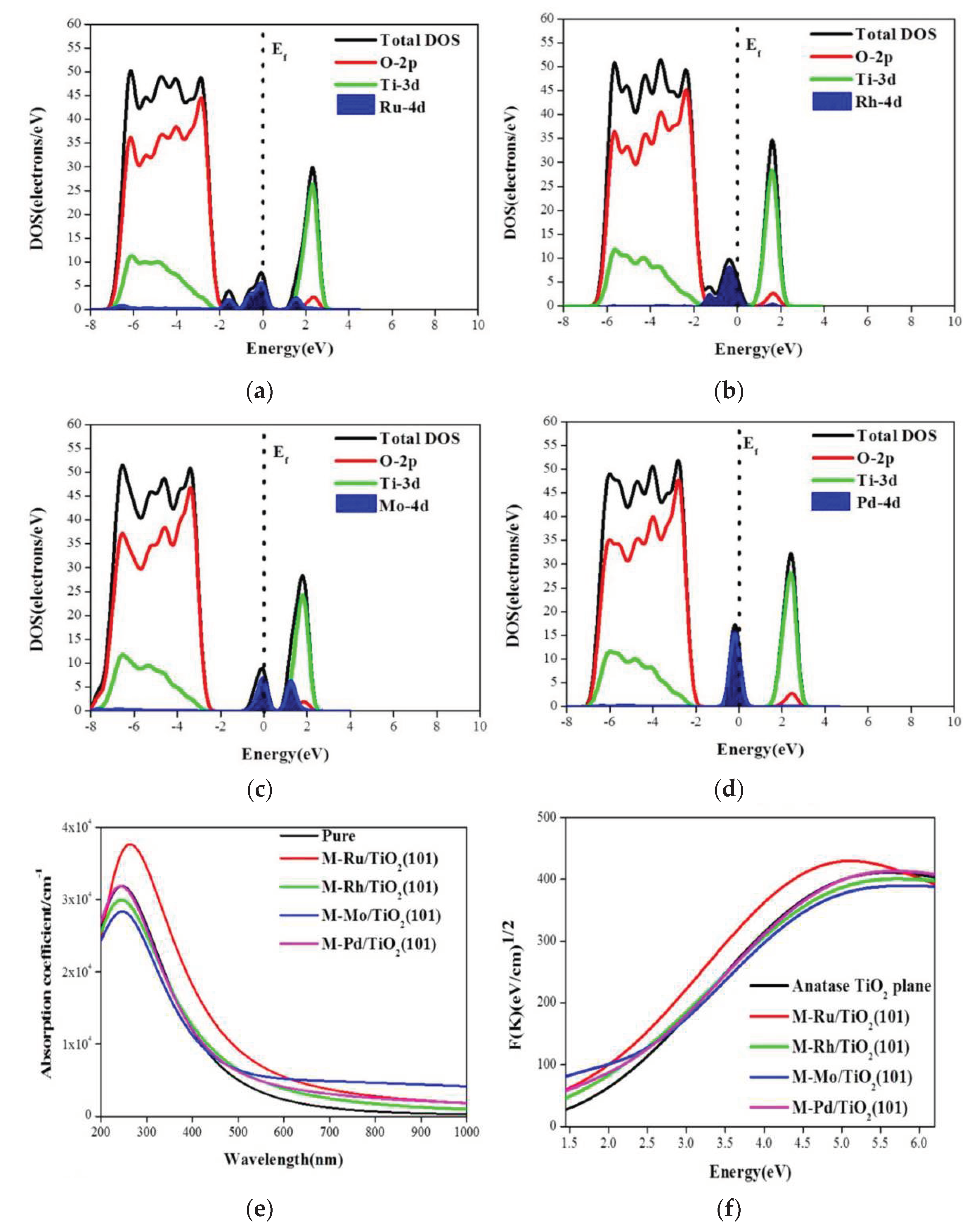
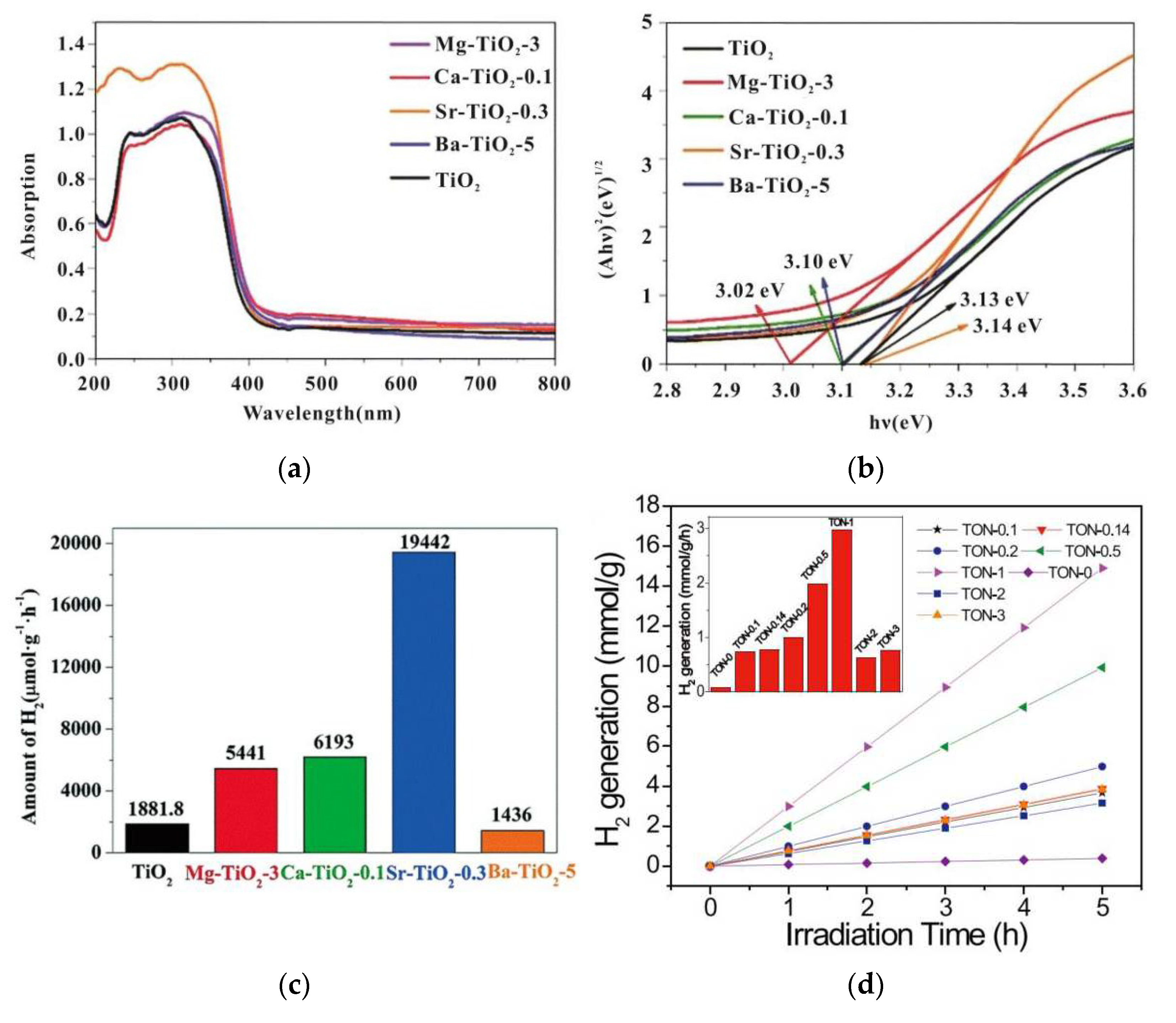
| Fuels | Heat of Combustion (kJ·mol-1) |
Heat of Combustion (kJ·kg-1) |
Ignition Point (℃) |
|---|---|---|---|
| hydrogen | 285.8 | 1.42 × 105 | 585 |
| coal | -- | 8.36 × 103 ~ 3.06 × 104 | 300 ~ 700 |
| gasoline | -- | 4.31 × 104 | 427 |
| diesel | -- | 4.26 × 104 | 220 |
| kerosene | -- | 4.31 × 104 | 80 |
| natural gas | -- | 3.89 × 104 kJ·m-3 | 650 |
| wood | -- | 1.2 × 104 | 200 ~ 290 |
| ethanol | 1366.8 | 2.97 × 104 | 12 |
| methane | 890.3 | 5.55 × 104 | 538 |
| butane | 2653 | 4.56 × 104 | 365 |
| acetone | 1788.7 | 3.08 × 104 | 465 |
| graphite | 393.7 | 3.28 × 104 | ~ 650 |
| Catalyst | Light Source | Reaction Condition | H2 Production (mmol h-1) |
Ref. |
|---|---|---|---|---|
| N-doped TiO2 | >400 nm | Water | 0.315 | [133] |
| N-doped TiO2 | >420 nm | EDTA-2Na solution | 2.21 | [132] |
| (B,N)-co-doped TiO2 | >420 nm | EDTA-2Na solution | 10.45 | [132] |
| (Sb,N)-co-doped TiO2 | Xe lamp | 10% aqueous TEOA solution | 2.33 | [184] |
| B-doped TiO2 | 365 nm | 0.2 M HCl and absolute ethanol aqueous solution (1:1) | 0.099 | [135] |
| N-doped TiO2 | visible light | H2S/0.25 M KOH solution | 8.8 | [130] |
| N-doped TiO2 | Xe lamp | 20% aqueous methanol solution | 2.98 | [129] |
| S-doped TiO2 | Xe lamp | 1 M NaOH aqueous solution | 0.17 | [134] |
| Fe-doped TiO2 | solar light radiation | triammonium phosphate aqueous solution | 4.01 | [139] |
| Co-doped TiO2 | solar light radiation | triammonium phosphate aqueous solution | 9.82 | [139] |
| (Fe,Co)-co-doped TiO2 | solar light radiation | triammonium phosphate aqueous solution | 17.41 | [139] |
| La-doped TiO2 | Hg UVA lamp | 12 M aqueous methanol solution | 80 | [185] |
| Ce-doped TiO2 | visible light | sulphide wastewater from refinery | 6.789 | [186] |
| H-doped TiO2 | 365 nm | 25% aqueous methanol solution | 0.286 | [140] |
| F-doped TiO2 | 365 nm | 25% aqueous methanol solution | 0.0928 | [140] |
| Cl-doped TiO2 | 365 nm | 25% aqueous methanol solution | 0.336 | [140] |
| V-doped TiO2/rGO | Xe lamp | 20% aqueous methanol solution | 0.12 | [187] |
| N-doped Ni/C/TiO2 | Hg lamp | 30% aqueous methanol solution | 0.383 | [188] |
| Sr-doped TiO2-δ | >400 nm | water | 1.092 | [189] |
| TiO2-δ | >420 nm | 30% aqueous methanol solution | 0.00058 | [190] |
| Pt/TiO2-δ | visible light | 50% aqueous methanol solution | 4.9 | [63] |
| Ag-decorated TiO2 | Hg lamp | water | 120 | [191] |
| Au-decorated TiO2 | 254 nm | aqueous methanol solution | 106 | [163] |
| Au,Pd-decorated TiO2 | 254 nm | aqueous methanol solution | 266 | [163] |
| Au,Ni-decorated TiO2 | 254 nm | aqueous methanol solution | 256 | [163] |
| Au,Co-decorated TiO2 | 254 nm | aqueous methanol solution | 171 | [163] |
| Pd-decorated TiO2 | 254 nm | aqueous methanol solution | 59 | [163] |
| Ni-decorated TiO2 | 254 nm | aqueous methanol solution | 20 | [163] |
| Co-decorated TiO2 | 254 nm | aqueous methanol solution | 10 | [163] |
| Cu(OH)2/TiO2 | ultraviolet light | 10% aqueous methanol solution | 14.94 | [192] |
| Cu/TiO2 | UV lamp | 25% aqueous methanol solution | 5 | [193] |
| Cu/TiO2 | visible light | 25% aqueous methanol solution | 0.22 | [193] |
| Co3O4@C/TiO2 | 365 nm | 25% aqueous methanol solution | 11.4 | [194] |
| NiO/TiO2 | Hg lamp | glycerol and distilled water | 1.2 | [195] |
| g-C3N4/N-TiO2 | Xe lamp | 20% aqueous methanol solution | 8.931 | [34] |
| EosinY-sensitized TiO2/ZrO2 | Xe arc lamp | 15% DEA aqueous solution | 1.87 | [196] |
| β-Ga2O3/TiO2 | 254 nm | 50% aqueous methanol solution | 0.244 | [145] |
| N-doped TiO2/N-doped graphene | Xe lamp | 10% aqueous TEOA solution | 0.039 | [197] |
| FeO-TiO2/ACF | visible light | 20% aqueous methanol solution | 6.178 | [198] |
| TiO2/ACF | visible light | 20% aqueous methanol solution | 1.672 | [198] |
| Cu-doped TiO2 with preferred (001) orientation | Xe lamp | 10% aqueous methanol solution | 0.81 | [199] |
| g-C3N4/TiO2 with preferred (001) orientation | >420 nm | 10% aqueous TEOA solution | 0.033 | [200] |
| TiO2/graphene with exposed (001) facets | Xe lamp | 25% aqueous methanol solution | 0.736 | [201] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





