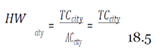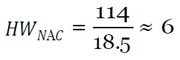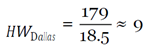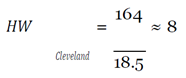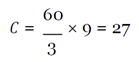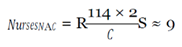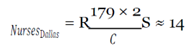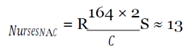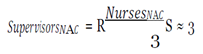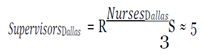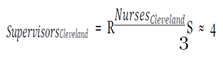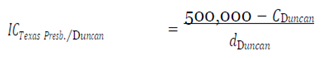Introduction
The economics of emerging infectious diseases are becoming increasingly relevant in global policymaking [
1,
2]. With a rising burden of emerging infectious diseases in the past two decades, as evidenced by the appearance of epidemics like SARS, H1N1, dengue fever, the Zika virus and the Ebola Virus Disease (EVD), the cost implications of these outbreaks extend far beyond traditional health sectors [
3]. In a world recognizing these trends, efforts have emphasized the need for a better integrated global approach to health security by targeting identification and mitigation of newly-emerging infectious disease threats before they reach human populations and responding rapidly to diseases once detected to curb their spread [
4,
5].
Yet, at this stage, global health models are based on episodic, acute care models primarily concentrated on treating sick populations [
5,
6]. This was particularly evident during the 11 cases of EVD in the United States. Declared a public health event of international concern by the World Health Organization (WHO) in July 2014, the outbreak was primarily concentrated in the West African nations of Guinea, Liberia and Sierra Leone, where it manifested in 28,616 confirmed cases[
7]. Partially due to poor health system capacity, skilled international healthcare workers were deployed to help mitigate the crisis. Amongst the American staff to participate, several were repatriated home after contracting EVD. Others were only identified as ill after arriving within the country, hence opening the possibility of a domestic EVD outbreak [
8].
With the first confirmed case of Ebola identified in the United States in late September 2014, the nation engaged in preparative efforts to mitigate the threat of an epidemic. The Department of Health and Human Services (HHS), along with the Office of the Assistant Secretary for Preparedness and Response (ASPR), and the Office of Emergency Management’s Division of National Healthcare Preparedness Programs (OEMDNHPP), identified a cohesive response framework [
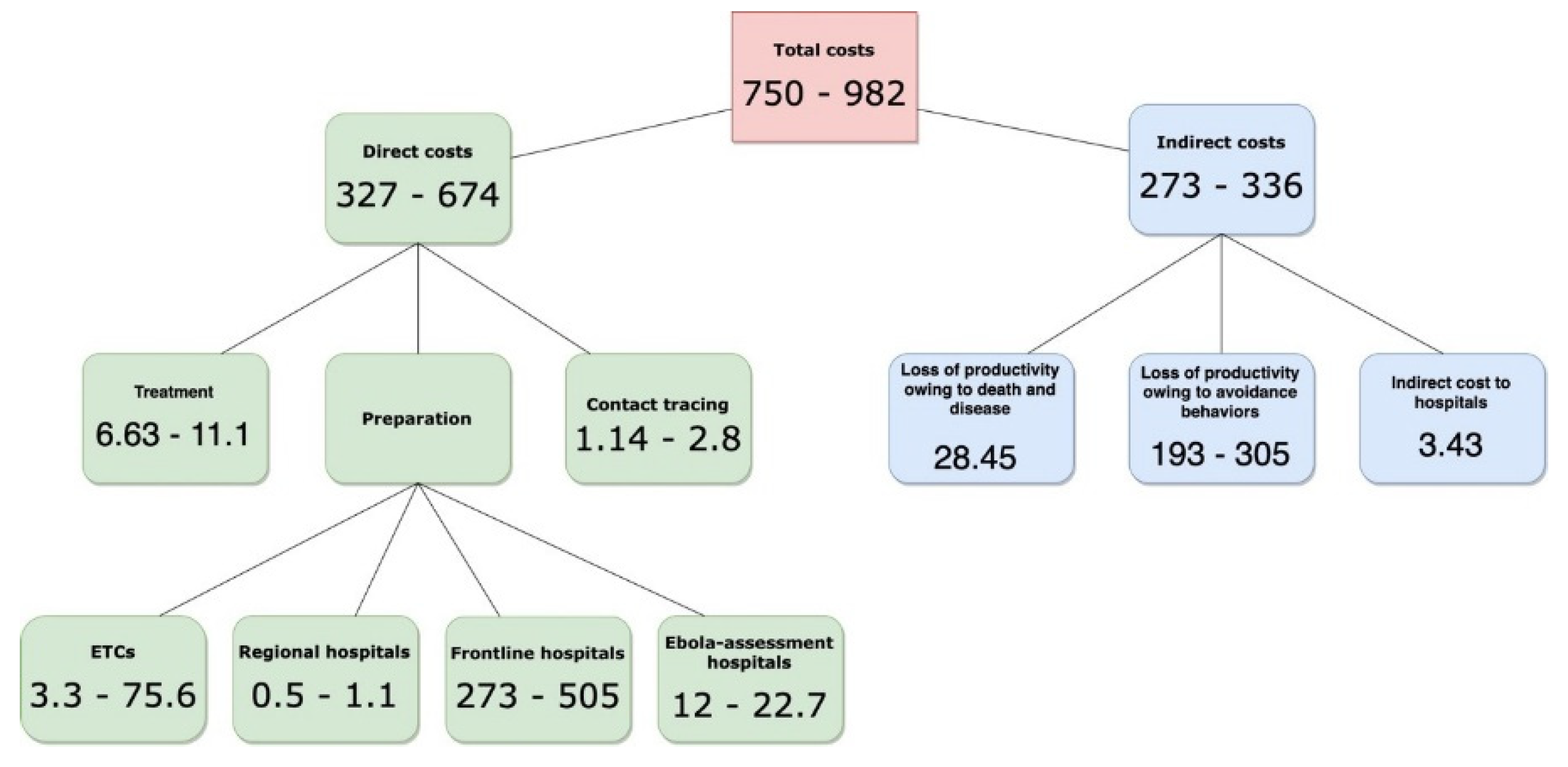
9]. A collaboration between the private health care system, federal officials and public institutions culminated in the division of national health facilities into four primary response units. 4,845 frontline health care facilities were identified for first-response duties, isolation and diagnosis and eventual discharge or transfer. 217 Ebola assessment hospitals and 63 Ebola treatment centers (ETCs), specialized as buffer zones, had the isolative capacity to treat high volumes of patients. Lastly, 10 regional EVD hospitals were organized to be ready to receive domestically diagnosed patients with confirmed Ebola from their region, or a patient medically repatriated, within 8 hours. In total, 11 patients were diagnosed with Ebola in the U.S., including domestic healthcare-acquired infections. The cost implications of implementing the response framework and domestic transmission has not been estimated to date.
As such, this paper offers an analysis of domestic costs of EVD borne by the United States. Firstly, I estimated the costs of preparation, treatment, and contact tracing efforts as a response to the 11 domestic cases of EVD. Secondly, I explored the indirect costs of the disease associated with the societal behaviors of Americans during the domestic crisis.
Literature Review
Several studies examined the socio-economic impacts of the Ebola crisis in West Africa. The World Bank estimates
$2.8 billion in GDP growth projection was lost in Sierra Leone, Guinea and Liberia in 2015 [
10], from lower investments and decrease in private sector growth, increased food insecurity and rising commodity prices. The United Nations assessed it cost the three West-African nations roughly
$290.6 million in direct costs to control the epidemic [
11,
12]. A study calculating the costs of responding to an Ebola cluster varied from
$113,000 to
$1.8 million, primarily driven by travel and personnel costs [
12].
Compared to estimates of costs incurred for response in West Africa, the scientific literature on the costs of EVD preparedness and response for domestic cases within the United States is limited. Deploying a phone survey with 222 hospitals, a study finds the mean costs of overtime, training and supply combined was circa
$80,000 per acute care hospital [
13]. For Ebola Treatment Centers (ETCs), a hospital spent roughly 1.2 million dollars [
14], which includes initial training of staff, construction and modifications. A recent study states the United States spent
$3.3 billion in direct costs, contributing to a cost amounting to
$53 billion incurred globally [
11].
The costs of potentially producing 27 million vaccinations in the United States was estimated at
$73 million [
15]. Depending on the relative transmissibility of isolated individuals, the cost and incidence of the EVD outbreak yielded between 4 and 5 billion dollars. In the “best case” scenario, the forecast still amounted to 1-2 billion dollars.
Internationally, the costs of preparedness and response to EVD in the Dutch health system equaled €12.6 million (
$13.9 million), with a range of €6.7 to €22.5 million (
$7.3 –
$24.7 million) for 13 evaluated and 1 confirmed case [
16]. This includes the amount of time dedicated to preparation and treatment of Dutch healthcare professionals, the cost of equipment, cleaning, patient hospitalization and monitoring. Using a cost of illness method to calculate future non-health gross domestic product (NHGDP) losses associated with EVD deaths in five West-African nations, a study found that for 11,234 deaths, the discounted value of future NHGDP loss amounted to
$155.6 million, a cost borne primarily by Guinea, Sierra Leone and Liberia [
17]. In Nigeria
, where 20 cases were seen, the costs of running awareness campaigns, purchasing prevention materials and training staff about EVD over a four months period amounted to 1.2 billion naira, or approximately US
$2.85 million [
18]. These efforts extended to 4,000 schools, 253 hospitals and 451 hotels, from July 20 to November 20, 2014.
In the U.S, former President Barack Obama made a 6.2 billion dollars funding request named FY2015 Emergency Ebola Appropriation, later approved by Congress for 5.4 billion dollars [
19]. As no literature details the exact distribution of these funds to domestic uses, versus international response efforts, I was unable to determine whether the costs found in our study related to U.S.- based cases had been covered by the FY2015 EEA (i.e., whether hospitals and public health departments were fully reimbursed by federal funding). In addition to real-time outbreak response in West Africa and screening at ports of entry, a portion of the Ebola Appropriation funds supported post-Ebola recovery in the form of laboratory and other public health system strengthening abroad; additionally, a portion of the funds was redirected to Zika virus response in Latin America. In order to avoid double-counting of costs, I elected to omit this funding package from our calculations. I recognize the importance, however, of factoring this budget into the broader analysis of the total economic impact of EVD globally and, more generally, the economics of newly-emerging infectious diseases.
Methods
The calculations of direct and indirect costs in the U.S. reflect the period January – December 2014, during which 11 cases were diagnosed, with three of those infected within the U.S. (i.e., localized/domestic transmission) from imported cases (infections acquired abroad). Direct costs are understood in this paper as the monetary amount applied to prevention, treatment and response. Indirect costs, meanwhile, refer to the cascading costs resulting from societal behavior. In other words, indirect costs embody the market opportunity costs of populations reacting to the news of disease and adopting specific risk avoidance behaviors.
Health care facility costs
As the mean cost per ETC was already calculated by a previous study [
14], including construction/facility modification, personal protective equipment (PPE) supplies, staff training, unit planning, laboratory equipment and non-PPE laboratory equipment, I scaled the figure to match the number of ETCs and find the cost-estimate of preparation for these facilities.
To find the costs of preparation for acute-care hospitals, I extrapolated the findings in the study by Smit
et al. [
13] at the national level by considering the
mean average costs of preparation and multiplying it by the
number of facility type (excluding ETCs). I assumed all hospitals in the nation were classified in a tier as per the response framework detailed above and had prepared in some capacity for the potential eventuality of an EVD epidemic. This gave a representative figure of the costs of preparation for non-specialized hospitals.
Treatment costs
I used grey literature to estimate the treatment costs for the 11 cases. Chancellor Jeffrey Gold of the University of Nebraska Medical Center stated in a testimony that it cost
$30,000 a day to treat an Ebola patient[
20]. I contrasted this figure with the
$18 to
$24 thousand per day cost stated by Professor Anderson at John Hopkins University’s Bloomberg School of Public Health to estimate a range of cost of treatment [
21]. Using these figures, I calculated the cost per patient based on the number of days in hospital for each person using 24 thousand as the mean estimate, 18 as minimum and 30 as maximum costs.
Productivity Losses
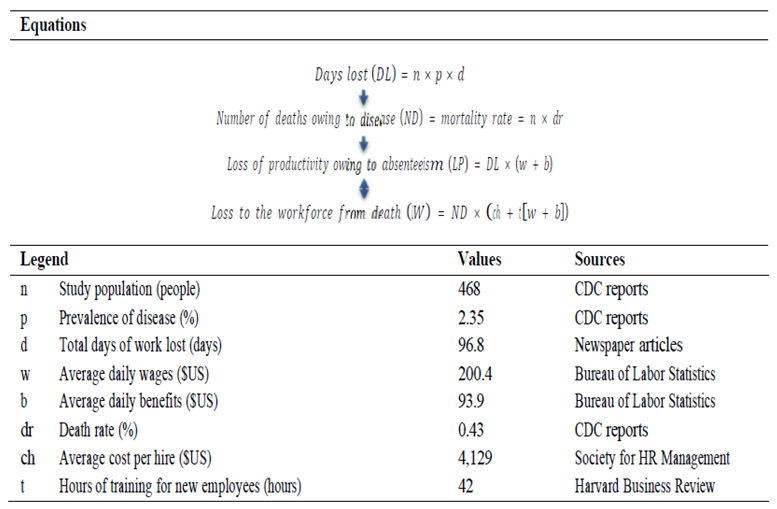
The loss of productivity was calculated using the loss of productivity owing to EVD, both to cases (including for the two deaths that ensued) and to the general public that enacted disease avoidance measures, derived from existing literature using Weintraub’s equations for disease impacts and can be found in
Table 3 [
26].
The variables averaging daily wage, daily benefits and average cost per hire were pulled from the Bureau of Labor Statistics. The number of cases and contacts, prevalence, morbidity and mortality rates, and days lost were taken from CDC case studies. Lastly, the staff numbers were extracted from the findings.
With a population sample consisting of infected patients and contacts traced, the sample is unrepresentative of the entire American population. However, the findings can still be interpreted as a starting point for further research. As the two EVD deaths in the U.S. were of people in the medical profession, I assumed the two replacements did not require work- specific training and would be ‘productive from day one’. However, I considered that there may be environment-specific training and workshops for context familiarization and team-building. I assumed both replacements would receive at a minimum the equivalent of one week of familiarization that had to be discounted in our analysis (42 hours, in 5 days a week full-time).
The avoidance behavior analysis was based on a study performed by the analytics firm Gallup [
27,
28]. They compared Americans’ avoidance behavior tendencies by contrasting the fear Americans had of contracting H1N1 at the heart of the epidemic in 2009 and the fear they had of being infected with EVD in 2014, relative to the total number of domestic cases. The results show 22% of Americans were afraid to contract EVD, an identical figure to Americans’ fear of getting H1N1 in 2009, despite a disproportionate difference in number of domestic cases (11 EVD cases compared to 60 million influenza cases domestically [
29]). In comparison, 90% of Americans thought they were unlikely to contract Zika virus [
30]. From these findings, we reasonably inferred that Americans had
at a minimum employed similar avoidance behavior to H1N1 during the EVD outbreak and therefore used previous avoidance findings for H1N1 in the U.S. to inform our calculations.
A study found that on average people spent an additional 22 minutes per day at home during the peak period of H1N1 [
31]. I extrapolated this number to match EVD’s peak period of 65 days, ranging from the first to the last reported domestic case of EVD and two incubation periods [
32].
With this figure, I used the mean hourly wage [
24] for NYC, Dallas and Cleveland’s metropolitan areas and their average populations [
33] to determine the loss of productivity from avoidance behaviors, assuming they affected work activities (e.g. late or missed work because of panic, whether rational or irrational, of coming into contact with the virus) in the three cities over EVD’s peak period.
I was able to estimate the indirect costs from having to isolate EVD patients incurred by the four hospitals by subtracting the calculated cost of treatment for the first case of Ebola introduction into the U.S. from the total costs incurred by Texas Presbyterian as estimated by the hospital’s CEO at
$500,000 [
21]. The patient, Thomas Eric Duncan, was stated to be uninsured.
From this, I then estimated the individual indirect costs of hospitals for the 11 cases.
Discussion
The calculations of this study estimate that the mean cost per Ebola patient diagnosed in the United States (i.e., total costs divided by 11 patients) ranges from $47.5 to 80 million (direct and indirect costs) depending on severity of cases, sample population size for behavioral computations and magnitude of investment for response interventions. As expected, the major driver of cost per case was preparation and treatment, but estimated losses from behavioral adaptation to avoid disease were much more significant than anticipated as a portion of total costs.
A key challenge of comparing these findings to existing literature is the difference in methodologies and locations used to calculate costs-per-case. A study estimated a single Ebola case in West Africa cost between
$480 and
$18,929, but their calculations exclude indirect costs borne by hospitals[
34]. Estimates from the United Nations claim direct costs (to control the epidemic) for Sierra Leone, Guinea and Liberia to have amounted to
$290.6 million [
11]. These estimates are significantly lower than our findings and may be due to differences in level of economic development, cost of healthcare, technological and regulatory differences and extensiveness of preparation.
The total costs of
$522 to
$880 million are significantly less than estimated by a study that found EVD to have generated costs of at least
$53.2 billion globally, with the United States spending at least
$3.32 billion in direct funding [
11]. Differences in findings may be explained by methodological variance (e.g. using standardized costs scales across counties that are less precise than our national and sub-national estimates), and the likely inclusion of the Congressional budget for Ebola for response costs abroad in their estimations. Additionally, their study used broad-scale predictive modeling to determine the costs of Ebola sequelae, the cost of death of healthcare workers on non-EVD illnesses, and long-term social factors (death of both parents, food insecurity, education) - calculations I omitted due to a lack of long-term validated data on these circumstances and their relevance to the U.S. The World Bank’s reported cost of the outbreak on the three West African nations was
$2.8 billion (
$600 million for Guinea,
$300 million for Liberia and
$1.9 billion for Sierra Leone) although a significant part of these results is declines in economic growth, investment, production and consumption as well as a severe drop in commodity prices[
10].
The findings appear at odds with other single-case cost estimates for existing infectious diseases (as opposed to newly-emerging infectious diseases). The average cost of a single measles case was estimated at
$369-
$451 [
35], although the study does not include loss of productivity. An outbreak of malaria among children in Ghana, Tanzania, and Kenya ranged from
$6 to
$334 per patient [
36] (in 2014 USD). The average cost per person with cholera in the WHO African region is a reported as
$771 (discounted to 2014 USD) [
37]. The drastic differences in costs could be explained by the location of the studies (African nations versus the United States) and the well-documented scientific literature around the treatment processes of these diseases, including vaccinations or effective medication. Additionally, I believe investments to treat these diseases may have been incorporated within national public health policies to develop the entire healthcare system and thus are not reflected in these calculations. Additionally, most disease impact accounting to date has focused solely on direct medical costs, rather than the wider societal impacts incurred that our study incorporated via indirect costs.
The results are also inconsistent with several cost estimates for other recent emerging infectious diseases, namely H1N1, Zika and dengue fever. A study determined H1N1 cost China roughly
$3.84 billion [
38], but their findings include investments in the educational system, self-medication expenses and medical expenses for non-hospitalized self-diagnosed patients. Adjusting for the calculations not included in our own study, the total amounts to
$875 million for 128,000 confirmed cases, or roughly
$6,836 per case. In Mexico, the World Bank calculated the Zika epidemic cost roughly
$744 million for approximately 60,000 cases [
39], including
$80 million in foregone income from reductions economic growth, or roughly
$12,400 per confirmed case. A study on the economic burden of dengue fever in Colombia, Vietnam and Thailand determined the cost per case ranged from
$141 to
$385 for inpatient and
$40 to
$158 for outpatient [
40]. Different income per capita leading to drastic differences in loss of productivity estimates, severity of the diseases and differing levels of investment in healthcare prior to the outbreaks could potentially explain the high discrepancies with our findings. However, our findings match trends seen with Severe Acute Respiratory Disease (approximately
$30-45 billion for ~9,000 cases globally).
In general, this study aims to approximate conservative costs of EVD in the United States. The total costs appear insignificant when placed within the broader national context (
$880 million would only be 0.025% of the federal U.S. government spending on healthcare in 2017 [
41]). However, the disproportionate cost-per-case when contrasted to other recent outbreaks globally suggests two hypotheses. Firstly, that both the American government and people reacted disproportionately to the threat of an EVD epidemic. It is difficult to determine whether the federal response was excessive, as the threat was averted successfully and comparisons with previous case studies would require significant data and resources. Furthermore, it is likely that some investments into specialized equipment will have trickle-down benefits into research and development, rather than solely ad-hoc and limited-term utility, and will reduce costs of response to future potential outbreaks (however, certain resources, such as stockpiled PPE, may have a short window of use or not be allocated for optimal preparedness for future). Lastly, certain benefits extracted from such costs (e.g. training, experience) are unquantifiable yet may have long-lasting impacts.
The findings on indirect costs, including the magnitude of avoidance behaviors which are not routinely estimated for disease impacts, are remarkable and contributed significantly to overall costs. One could argue losses in productivity due to risk avoidance behaviors were disproportionately high (especially within the scope of our conservative estimates), or at least could have been substantially reduced through better management. I suspect the sensationalist media coverage [
42,
43] – including the strong presence of misleading information about the outbreak circulated on social media [
44,
45] – contributed to the promotion of reactionary, panic-fueled attitudes geared towards avoiding other people. More research on the psychology of graphic images and online news portrayal of disease risk on society is needed to better understand their role as cost drivers.
Secondly, there is a lack of a global integrated response to emerging infectious diseases that leads to high country-borne costs of preparation and response. The findings suggest the American health system was inadequately prepared to mitigate an emerging pandemic threat introduced into the U.S. from over 6,000 miles away and was constrained to invest in large-scale renovation projects in a short period of time. Better collaboration not only between governments but also between health sectors (e.g. human, animal and environmental health professionals) could perhaps have had an impact on reducing singular preparative costs related to initial emergence of the disease; similarly, preventive measures for reducing spread are needed for a suite of known and unknown emerging infections. To-date, efforts largely remain concentrated around reactionary practices taken after determination of a risk as opposed to identification and prevention occurring further up the process of transmission [
5], as highlighted by the drafting of a Congressional budget after confirmation of a case on U.S. soil. The Global Health Security Agenda, which the U.S. is part of and has expanded investments in following the West Africa Ebola epidemic, recognizes the health and financial risks of emerging diseases and the benefits of stopping diseases at their source. Despite a complex global health system relying on formal and informal networks [
4], recent outbreaks of Ebola, Zika, dengue, H1N1, and Middle East Respiratory Syndrome (MERS) and looming threat of anti- microbial resistance illustrate a necessity to act in a more synergetic fashion and to create better linkages between the various levels of government, the traditional health sector and its contemporary subsidiaries to mitigate economic impacts of disease events.
This study has several limitations. Due to a lack of primary data, literature and methodology, several key assumptions were made that may not fully capture the reality of costs of EVD for the United States. The composition of the contact tracing teams may be inaccurate - a lack of sufficient documentation did not allow for a more accurate estimate. Similarly, estimated wages were taken from the Bureau of Labor Statistics, but reflect a mean and may not be precise for the actual populations affected. Contact tracing was calculated for the three areas, but there may have been significantly more efforts around other parts of the country. The analysis assumes that the contact tracing staff was pulled away from their day-to-day activities to lead the tracing efforts - the opportunity cost of their absences was not factored into the calculations.
The direct costs were primarily extracted from existing literature. Whilst they encompass a significant portion of the costs, they remain estimates and it is likely other costs were omitted, such as national and state public health system alerts, rumor investigation, and possibly testing costs if additional suspected cases were screened. Treatment and indirect costs to hospitals were drawn directly from interviews. More data would have allowed for more precise and validated calculations. The loss of productivity and loss of workforce owing to the disease for indirect costs were extracted from pre-existing equations that I adapted to in this study – drafting my own equations may have been more representative of our sample of interest and the specific disease context for EVD. The behavioral avoidance cost calculations only display staying an additional 22 minutes at home for the entire duration of the local outbreak. They do not include the costs borne by the entertainment industry (such as avoiding going to the movies or restaurants), the public transportation companies, possible avoidance of healthcare treatment for other diseases as seen in West Africa due to concern of being infected with EVD, or the subsequent loss of productivity to companies from taking days off from work. Due to a lack of literature, the behavioral analysis is based on the American population’s behavioral reactions to the H1N1 virus - there is a strong possibility that people reacted significantly differently to EVD; the two are spread via different transmission routes (airborne vs. bodily fluids), with influenza being far more transmissible; however, lack of familiarity with Ebola and its depiction in the media may have resulted in disproportionate and irrational fear.