1. Introduction
Corneal biomechanics [
1,
2] depends on the distribution of collagen fibers in the stroma. These corneal type-I fibrillary collagen arrangement allows maintaining the three-dimensional structure and transparency of the corneal stroma [
4].The knowledge of BMPs allows to ensure the success of refractive corneal surgery or the diagnosis and follow-up of corneal pathologies such us keratoconus, a progressive degeneration that can lead to corneal transplantation.
The clinical relevance of BMP reached special interest with the development of the surgery techniques to modify the refractive power of the cornea by laser ablation [
5] or lenticular extraction [
6]. These techniques consist on modify the lamellar structure of cornea causing redistribution of mechanical stress. The biomechanical response is expected to provide the correct corneal curvature [
7,
8] and normal vision.
Various methods based on different inherent principles have been developed to characterize corneal biomechanics
in vivo. The most commonly used approach involves the use of air-puff tonometry, such as the Ocular Response Analyzer (ORA), which is a non-invasive device that measures IOP and corneal biomechanics, including corneal hysteresis (CH) and corneal resistance factor (CRF) parameters [
9].
The development of optical techniques, such as the Scheimpflug camera or optical coherence tomography, allows for the direct monitoring of corneal deformation following air perturbations (e.g., CORVIS ST) [
10,
11]. In recent years, new technologies such as Brillouin microscopy [
12] or optical coherence elastography [
13] have been developed. Moreover, the finite element method (an in silico method) has been extensively used in several studies to evaluate corneal biomechanical properties [
14].
Due to its inherent elastic properties, the cornea the cornea can behave as a biomechanical resonance system under the action of certain mechanical vibrations [
15], then the cornea can be considered a biomechanical resonant oscillator when external perturbations are applied [
16,
17].
In that sense, sound-induced corneal vibrometry has previously been reported to explore the corneal vibrational modes. Akca et al., [
18] reported the visualization of the resonance modes of ex-vivo corneas by low-power sound waves and optical coherent tomography imaging. In addition, these vibrational resonance modes of the cornea are sensitive to the IOP [
19].
Therefore, corneal resonance induced by acoustic waves seems to be sensitive to both the structure and the biomechanics of the cornea. This work presents an acoustic wave generator to induce subtle modifications in corneal structure that can alters corneal biomechanics within the physiological range. We show the first in vivo observations of corneal biomechanics and structural changes due to acoustic pressure within the range 50-350 Hz at a maximum pressure level of 90 dB.
2. Materials and Methods
2.1. Experimental corneal acoustic wave generator
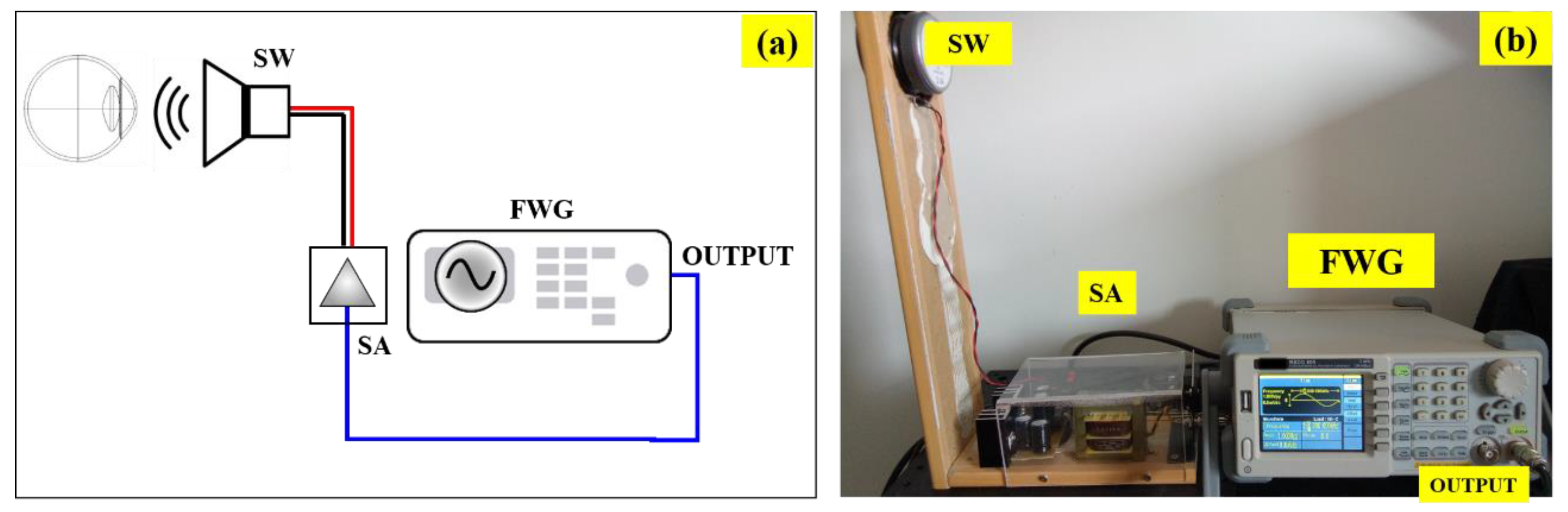
Figure 1a shows a schematic diagram of the custom-built instrument. The acoustic waves are generated by a function/arbitrary waveform (FWG) generator (RSGD 805, RS PRO) with maximum output frequency of 5MHz, 125MSa/s sample rate and resolution frequency of 1μHz (14bits vertical resolution). The output wave can be generated as a sine, square, ramp, pulse, Gaussian noise or arbitrary forms. The output is the connected to a sound amplifier (SA) which drives a full-range subwoofer (SW) (FR8, VISATON, Germany) to produce acoustic pressure at the corneal plane.
Figure 1 shows a real picture of the set up, vertically oriented for clinical measurements.
2.2. Instrument calibration
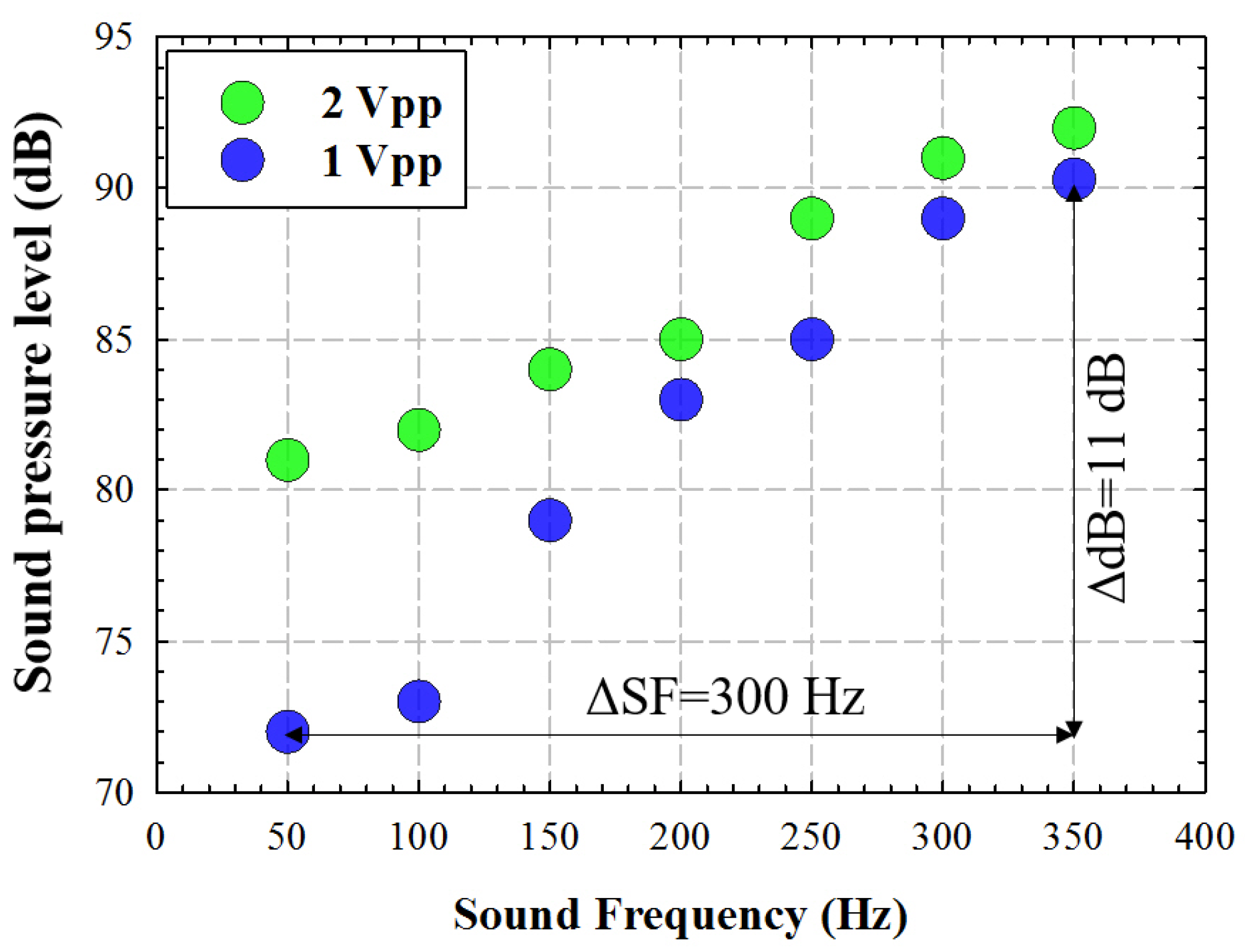
A sinusoidal waveform signal was generated at the FWG for two different amplitudes (peak to peak voltage): 1 and 2 Vpp. The sound pressure level was measured in dB using a digital sound meter placed at 100 mm from the SW, as a function of the signal frequency.
Figure 2 shows the measured acoustic pressure (dB) for a frequency range between 50 and 350 Hz (with a step size of 50 Hz) for two different amplitude values. For in vivo corneal testing, we chose the lower amplitude that provided a maximum pressure of 90 dB at the maximum frequency (350 Hz), with a modulation of 11 dB within the range of tested frequencies.
2.3. Experimental measurements
6 participants (33 ± 9 years old) of our research group were included in the study. The measurements were carried out at the Visual Science and Instrumentation Lab of the University of Zaragoza (Spain). The participants did not have any ocular disease, glaucoma, or corneal complications that could affect the measurements. Dual Placido-Scheimpflug imaging (Galilei G2) and air-puff tonometry (ORA) commercial devices were employed to obtain morphometric data for structural characterization and biomechanical assessment, respectively, as shown in
Table 1.
2.3.1. Galilei Dual Scheimpflug Analyzer
The Galilei Dual Scheimpflug Analyzer (Galilei G2; Ziemer Ophthalmic Systems AG, Port, Switzerland),is an advanced clinical optical system that combines Placido Disk imaging with a revolving Scheimpflug camera [
10]. This integration allows for the simultaneous capture of corneal topography information from both the internal and external surfaces of the cornea. The device captures two corneal images for each analyzed meridian, and the Galilei G2 software overlays these images to enhance the accuracy of corneal parameter estimation. Central corneal thickness (CCT) and eccentricity (e) were extracted for each participant and sound frequency. Three measurements of good quality, as indicated by the Galilei G2 software, were captured at each session to assess repeatability, using the 16-picture (i.e., 16 corneal meridians) scan mode.
2.3.2. Ocular Response Analyzer
Ocular Response Analyzer (ORA, Reichert Instruments, Depew, NY, USA) is a non-contact air puff applanation tonometer that provides corneal hysteresis (CH) and corneal resistance factor (CRF) measurements [
9]. Corneal hysteresis refers to the dissipation of energy when an external stress is applied to the cornea. Unlike purely elastic materials that immediately return to their initial state once the stress is removed, the cornea exhibits time-dependent stress. On the other hand, the CRF measures the resistance of the cornea, encompassing aspects of rigidity and/or elasticity. Corneal hysteresis (CH) and Corneal Compensated IOP were extracted for each participant and frequency. Four measurements of good quality were captured at each session.
3. Results
3.1. Corneal structure changes as a function of the sound frequency
Understanding the acoustic sinusoidal wave reaching the corneal surface as an external sound pressure perturbation applied to a viscoelastic material, an observed deformation or structural change is expected in relation to the relaxed state (i.e. FWG off). As described in
Section 2.3, the structural changes in the corneal tissue were quantified by computing the corneal central thickness (CCT) and eccentricity (e) parameters from Scheimpflug imaging.
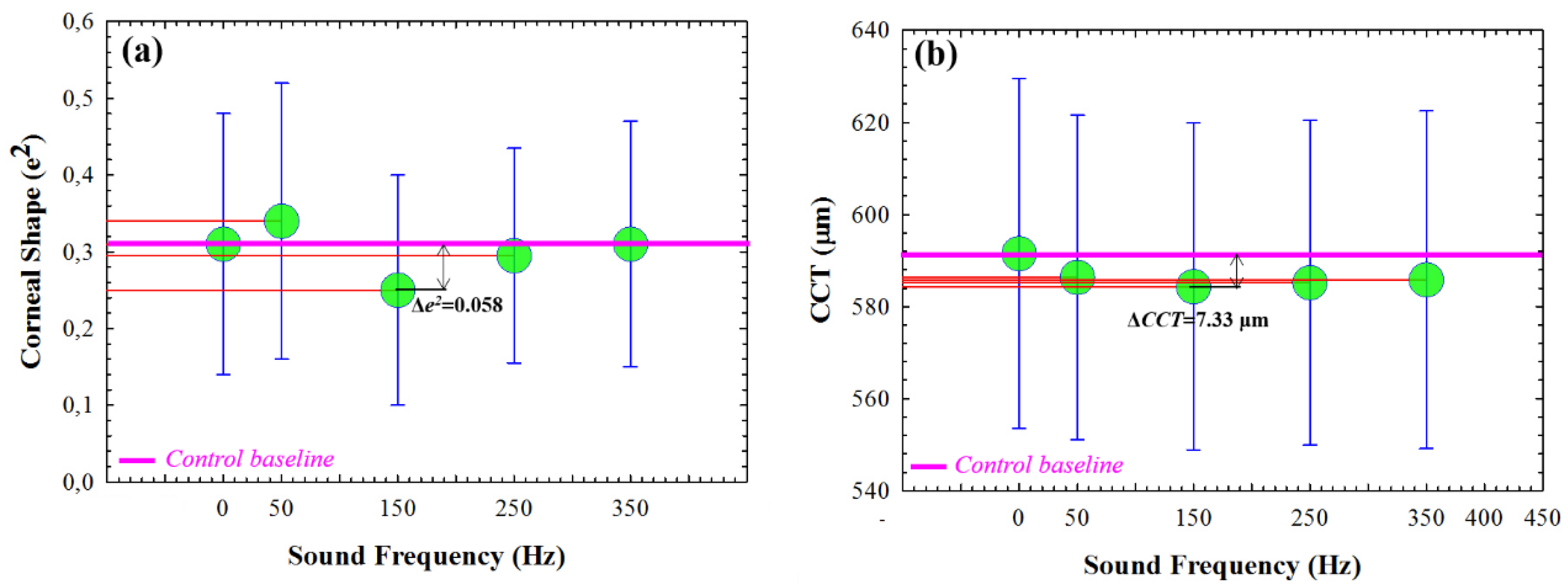
Figure 3 shows the mean values for 6 volunteers as a function of the sound frequency compared to the control baseline mean values (FGW off). Results showed that, at a given sound frequency the corneal tissue reaches the lowest values of eccentricity and central thickness. In particular, the most sensitive sound frequency was 150 Hz, which resulted in an average reduction of 0.058 in shape (
Figure 3a) and 7.33 μm in thickness (
Figure 3b) compared to the relaxed state.
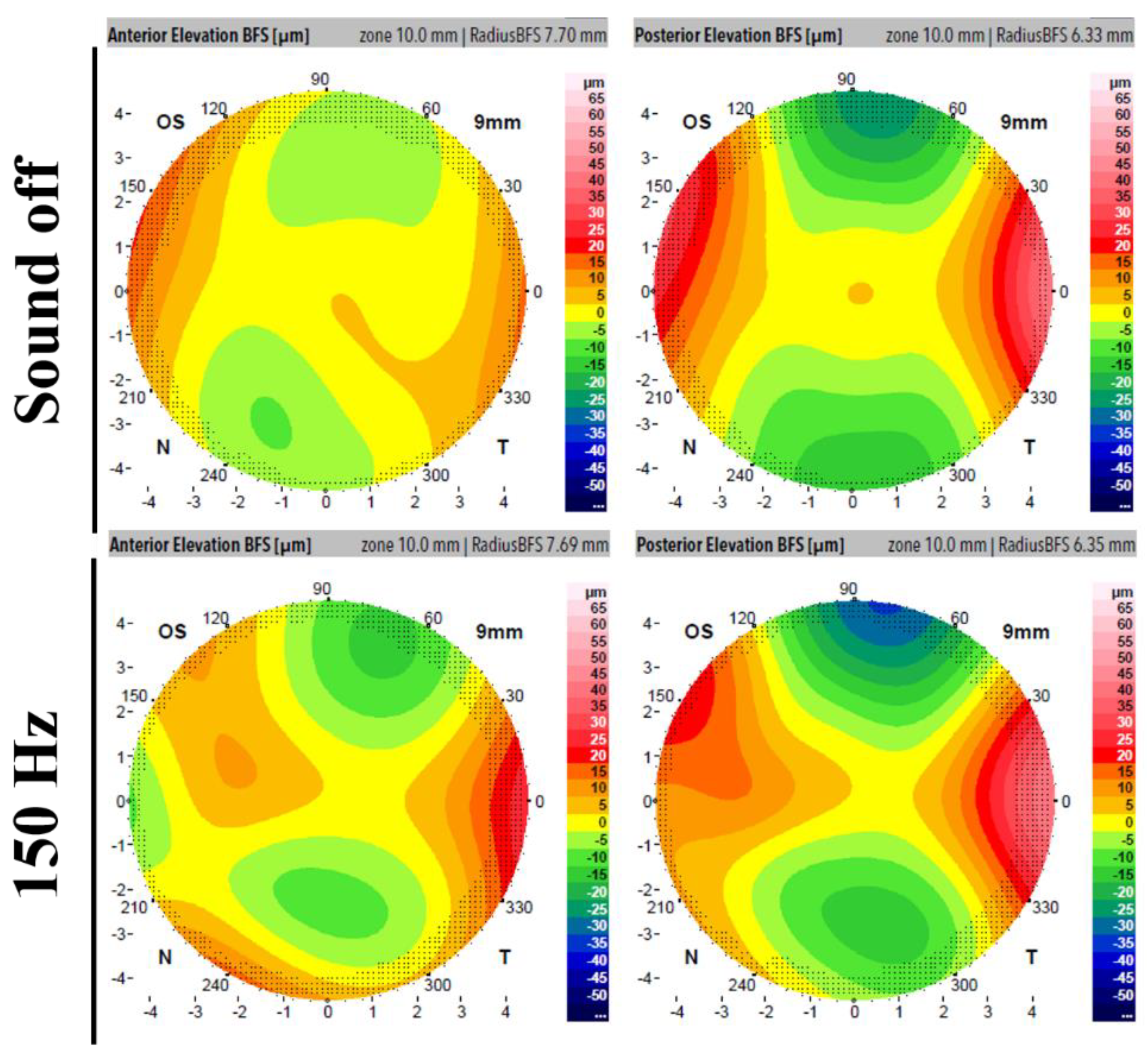
Figure 4 displays the Scheimpflug output elevation maps for a regular measurement (upper row, instrument off) and a measurement taken while the acoustic wave generator was operating at 150 Hz for anterior and posterior corneal surfaces. The images demonstrate quantitative modifications in both the anterior and posterior surfaces during the presence of the sound pressure.
3.2. Biomechanical variations and sound frequency
The previous Section showed how the frequency modulation of the sound pressure at a constant signal amplitude can induce slight changes in corneal shape, specifically in central thickness, of the order of a few microns. Those changes, unlike those produced by air-puff tonometry at a macroscopic scale, are microscopic in nature. However, even at microscopic scale, this section presents evidence of how acoustic pressure can induce measurable biomechanical changes in the cornea.
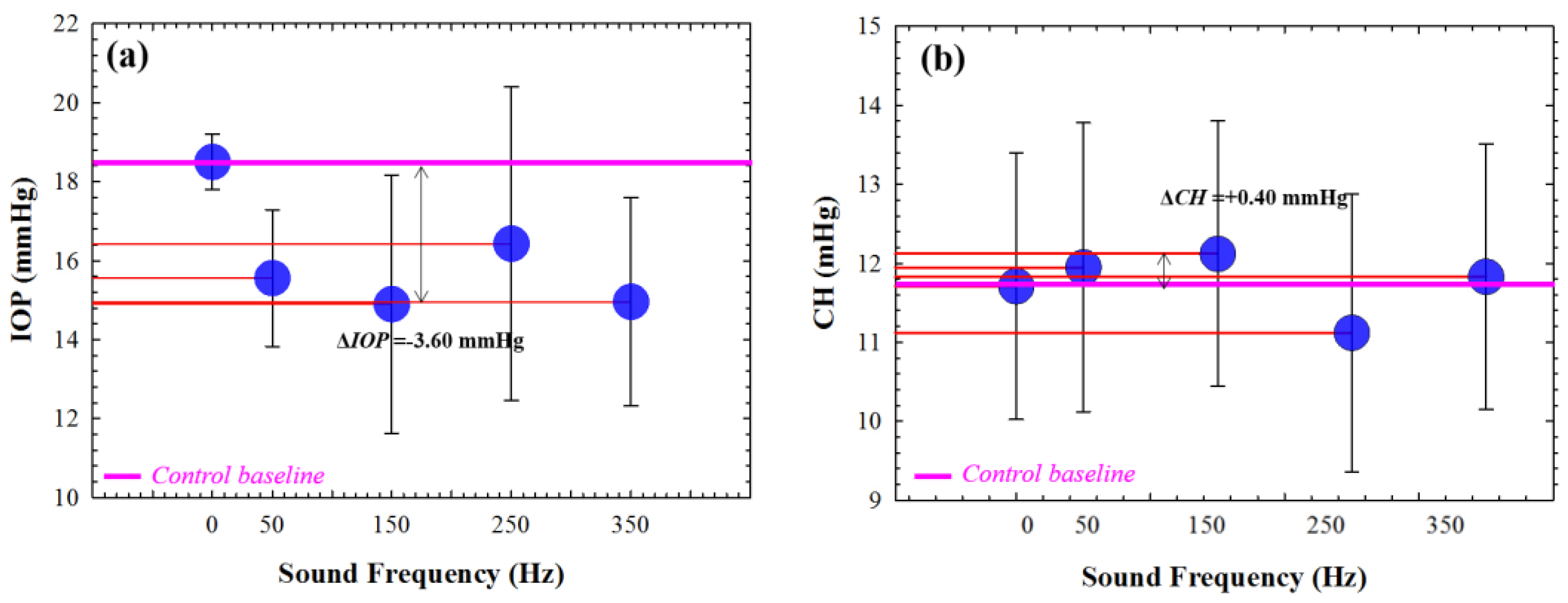
Figure 5 shows the mean IOP values for 6 healthy young adult subjects as a function of the sound frequency. The average control value falls within the normal range stablished between 11 and 21 mmHg [
20]. According to our findings, the IOP is reduced for all the tested frequencies (
Figure 5a), reaching a minimum value at 150 Hz that corresponds to an absolute difference of 3.60 mmHg with respect the control baseline (pink line).
Regarding the viscoelastic property measured by CH parameter, it is shown a maximum value (increase of 0.40 mmHg with respect to the average control value) for 150 Hz. Those preliminary results suggest that the application of acoustic waves can alter the corneal biomechanics, specifically reducing both IOP and CH at a specific sound frequency of 150 Hz.
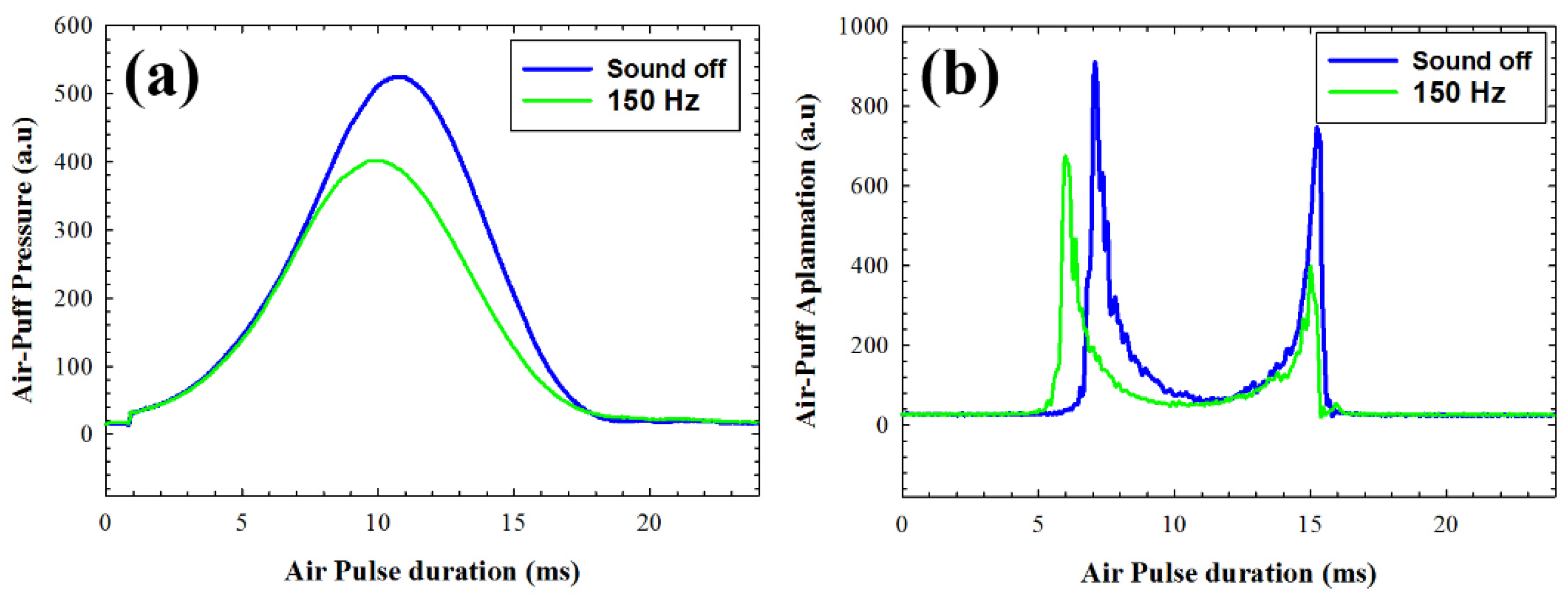
The air-puff system enables the acquisition of stress-strain dynamic measurements during the duration of the air pulse.
Figure 6 illustrates a comparison of the air pulse pressure curves and corneal applanation responses in one volunteer under normal operation (acoustic wave generator off) and with the application of acoustic wave pressure at 150 Hz. It can be observed that the the application of external sound pressure implies less air pressure to get the first applanation of the cornea (
Figure 6a). This fact can be associated with reduced corneal stiffness, which aligns with the results presented in
Figure 6b, where the first applanation occurs earlier when acoustic waves are applied. It is noteworthy that in
Figure 6b, an intersection is observed on the left side of the graph between the applanation curves corresponding to normal measurements (blue line) and measurements taken during acoustic pressure (green line). While the cornea still undergoes deformation due to the air pressure (sound off), the effect of acoustic interaction alters the viscoelastic properties over time, causing the cornea to begin relaxation at a point where it would still be deforming.
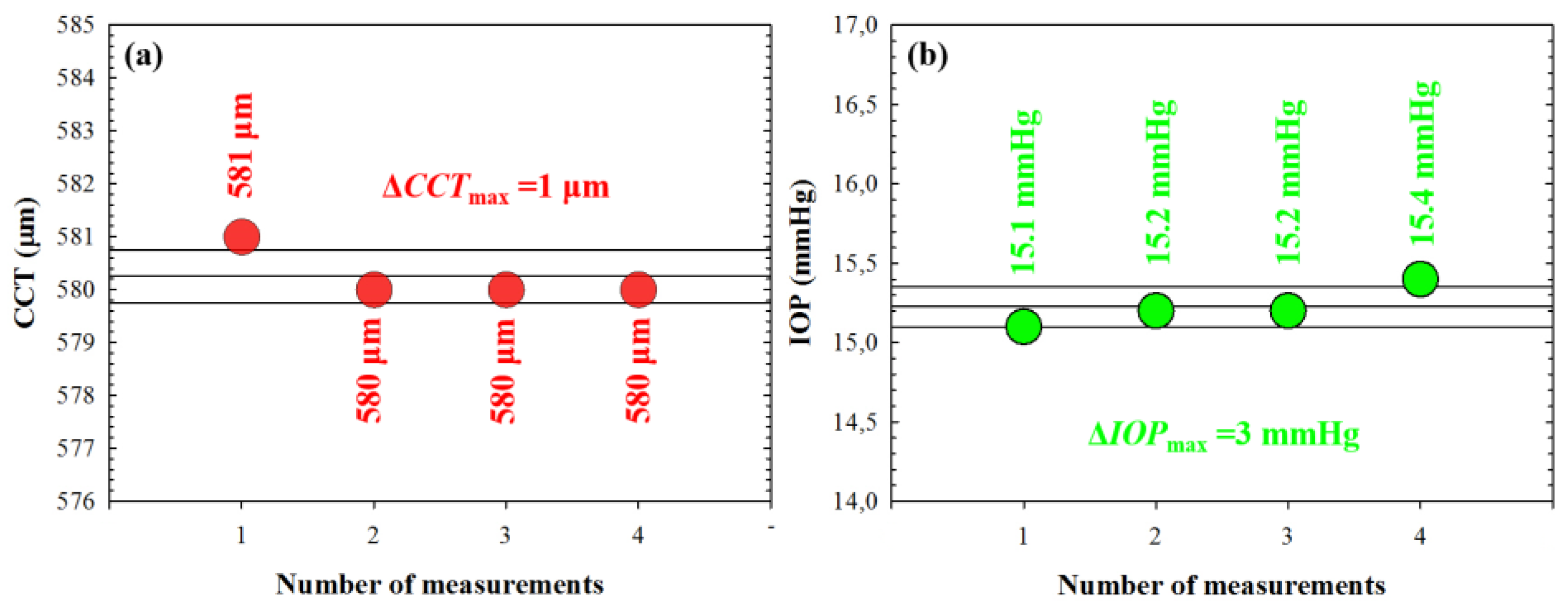
3.3. Structural and biomechanical stability during cornea and acoustic waves interaction
Section 3.1 and 3.2 showed how the application of acoustic pressure by means of our proposed acoustic wave generator can alter the corneal structure at a microscopic scale and modify its biomechanical properties. This raises the question of whether these changes remain stable over time or if they fluctuate randomly. To address this question, we conducted four consecutive measurements on a subject while applying a specific frequency of 150 Hz using both Scheimpflug imaging and the ORA analyzer. The results of these measurements are presented in
Figure 7.
4. Discussion
We developed a clinically-oriented instrument to deliver acoustic waves to the corneal plane by combining a function/arbitrary waveform generator and a wide-range sub-woofer mounted on vertical orientation. The system was first calibrated by placing a digital sound meter at the position at which the corneal apex should be aligned. Sinusoidal signals at two different peak-to-peak amplitude voltages (1 and 2 Vpp.) were generated within the range frequency of 50-350 Hz (50 Hz step) obtaining a maximum sound pressure around 90 dB (see
Figure 1) at the maximum frequency (350 Hz).
As a proof of concept, we tested the instrument in 6 healthy volunteers in a lab-conducted experiment. In the experimental set up, two commercially available ophthalmic devices were employed to stablish a set of measurements of biomechanics and corneal biometry by using the Ocular Response Analyzer (ORA, Reichter) and a dual Scheimpflug tomography and Placido topography (Ziemer Ophthalmic Systems, AG) systems.
Previously to our work, Akca et al., [
18] reported the observation of three corneal vibrational modes using sound waves within the frequency range of 50-400 Hz in ex-vivo bovine ocular globes. They found a vibration amplitude of ~ 8µm for a fundamental mode at the range of 80-120 Hz and sound pressure level of 100 dB.
In our work, we delivered acoustic sinusoidal acoustic waves at a frequency range of 50-350 Hz (1 Vpp. amplitude) and 90 dB pressure level at the living human cornea using a custom corneal acoustic waveform generator (see description in
Section 2.1). Corneal changes were visualized using a dual Scheimpflug camera and Placido rings analyzer and an Ocular Response Analyzer, to both monitor the corneal structure and biomechanics, respectively.
We found a reduction in the central corneal thickness of 7.33 µm at the sound frequency of 150 Hz together with a flattening of the corneal eccentricity of 0.058 (e
2) (see
Figure 3). Those results were visualized in the elevation maps shown in
Figure 4, then the application of acoustic waves can induce measurable axial deformation of the cornea that is consistent with the results found by Akca et al. [
18] in ex-vivo corneas.
On the one hand, the application acoustic waves of induces a IOP decrease (in average) from 18.5 ± 0.71 to 14.9 ± 3.27 mmHg at a sound frequency of 150 Hz, this induced change is within the reported physiological transient variations of the IOP [
21].
In this sense, corneal hysteresis (CH) is strongly dependent on IOP and is considered as a biomarker of glaucoma disease [
22]. Agarwal et al., [
23] evaluate the relationship between IOP and CH before and after applying prostaglandin analogue therapy in 57 patients with glaucoma, they found correlated reduction in IOP of 18.8 % with increased CH in 5.2 %. Our results showed an IOP reduction of 19.46 % and an increase in CH of 3.5 % during acoustic pressure application with respect to the baseline reference values (see
Figure 5), which are in agreement with those reported by Agarwal et al.
On the other hand, Herndon el al. [
24], compared the CCT values in normal and hypertensive eyes, they found significant greater CCT values of normal eyes compared with ocular hypertension patients. In addition, the positive linear correlation between CCT and IOP was demonstrated by Wei et al. [
25] in a healthy young population, so a decrease in IOP implies a reduction in CCT. In good agreement, our results showed a reduction in CCT of 1.16 % as a consequence of the decrease of 18.8 % in IOP as a consequence of acoustic pressure application at 150 Hz.
To conclude, we present a new and versatile system to generate acoustic waves for corneal applications. As a proof of concept, the system was tested in 6 volunteers delivering a maximum sound pressure of 90 dB of sinusoidal acoustic waves at the range of 50-350 Hz (50 Hz step size). At the given frequency of 150 Hz, we found corneal deformation and changes in both IOP and CH that are consistent with the reported literature. Therefore, the application of acoustic waves allows to modify the corneal biomechanics and the structure of the cornea the physiological transient physiological variations. Future work will focus on a clinical study of a large population of healthy subjects and patients with hypertensive and glaucomatous eyes.
Author Contributions
Conceptualization, F.A.; methodology, F.A. M.C.M. and L.R.; formal analysis, F.A..; investigation, M.C.M and L.R.; writing—original draft preparation, F.A.; writing—review and editing, F.A., M.C.M., L.R.; project administration, F.A.; funding acquisition, F.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by FUNDACIÓN BANCARIA IBERCAJA, grant number, 223221: JIUZ-2021-CIE-01.
Informed Consent Statement
Patient consent was waived due to the participants are members of the same research group.
Data Availability Statement
All data generated in this study are shown in the manuscript.
Acknowledgments
Authors thank prof. Julio Amaré and Juanjo Lanuza from the “Departamento de Fisica Aplicada” of the University of Zaragoza for their technical support in the electronic design and mechanical assembly of the corneal waveform generator.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Piñero, D.P.; Alcón, N. Corneal biomechanics: a review. Clin Exp Optom 2015, 98(2), 107-16. [CrossRef]
- Kling, S.; Hafezi, F. Corneal biomechanics – a review. Ophtalmic Physiol Opt 2017, 37(3), 240-252.
- Wilson, A.; Marshall, J. A review of corneal biomechanics: Mechanisms for measurement and the implications for refractive surgery. Indian J Ophthalmol 2020, 68(12), 2679-2690. [CrossRef]
- Meek K.M. Corneal collagen-its role in maintaining corneal shape and transparency. Biophys Rev 2009, 1(2), 83-93.
- Fernández,J.; Rodríguez-Vallejo, M.; Martínez, J.; Tauste, A.; Piñero, D.P. Corneal biomechanics after laser refractive surgery: Unmasking differences between techniques. J Cataract Refract Surg 2018, 44(3), 390-398. [CrossRef]
- Cao, K.; Liu, L.; Yu, T.; Chen, F.; Bai, J., Liu, T. Changes in corneal biomechanics during small-incision lenticule extraction (SMILE) and femtosecond-assisted laser in situ keratomileusis (FS-LASIK). Lasers Med Sci 2020, 35(3):599-609. [CrossRef]
- Wallace, H.B.; McKelvie, J.; Green, C.R.; Misra, S.L. Corneal Curvature: the Influence of Corneal Accommodation and Biomechanics on Corneal Shape. Transl Vis Sci Technol 2019, 8;8(4):5. [CrossRef]
- Wu, D.; Liu, C.; Li, B.; Wang, D.; Fang, X. Influence of Cap Thickness on Corneal Curvature and Corneal Biomechanics After SMILE: A Prospective, Contralateral Eye Study. J Refract Surg 2020, 36(2):82-88. [CrossRef]
- Kaushik, S.; Pandav, S.S. Ocular Response Analyzer. J Curr Glaucoma Pract. 2012, 6(1):17-19.
- Baptista P.M.; Ambrosio, R.; Oliveira, L.; Meneres, P.; Beirao, J.M. Corneal Biomechanical Assessment with Ultra-High-Speed Scheimpflug Imaging During Non-Contact Tonometry: A Prospective Review. Clin Ophthalmol 2021, 6;15:1409-1423. [CrossRef]
- Karmiris, E.; Tsiripidis, K.; Gartaganis, P.S.; Totou, S.; Vasilopoulou, M.G.; Patelis, A.; Giannakis, I.; Chalkiadaki, E. Comparison of intraocular pressure obtained by Goldmann applanation tonometer, Corvis ST and an airpuff tonometer in healthy adults. Eur J Ophthalmol 2021, 27:11206721211069227. [CrossRef]
- Eltony, A.M.; Shaom P.; Yun, S.H. Measuring mechanical anisotropy of the cornea with Brillouin microscopy. Nat Commun 2022, 15,13(1):1354.
- Sun, M.G.; Son, T.; Crutison, J.; Guaiquil, V.; Lin, S.; Nammari, L.; Klatt, D.; Yao, X.; Rosenblatt, M.I.; Royston, T.J. Optical coherence elastography for assessing the influence of intraocular pressure on elastic wave dispersion in the cornea. J Mech Behav Biomed Mater 2022, 128:105100. [CrossRef]
- Qin, X.; Tian, L.; Zhang, H.; Zhang, D.; Jie, Y.; Zhang, H.X.; Li, L. Determine Corneal Biomechanical Parameters by Finite Element Simulation and Parametric Analysis Based on ORA Measurements. Front Bioeng Biotechnol 2022, 13;10:862947. [CrossRef]
- Wardosanidze, Z.V. About the possible acoustic functions of the eye. Am J Biom Sci Res 2021, 15(1):79-81. [CrossRef]
- Coquart, L.; Depeursinge, C.; Gurnier, A.; Ohayon, R. A fluid-structure interaction problem in biomechanics: Prestressed vibrations of the eye by the finite element method. J Biomech 1992, 25(10), 1105-1118. [CrossRef]
- Shih, P.J.; Guo, Y.R. Resonance frequency of fluid-filled and prestressed spherical shell - A model of the human eyeball. The Journal of the Acoustical Society of America, 2016, 139(4), 1784. [CrossRef]
- Akca, B.I.; Chang, E.W.; Kling, S.; Ramier, A.; Scarcelli, G.; Marcos, S.; Yun, S.H. Observation of sound-induced corneal vibrational modes by optical coherence tomography. Biomed Opt Express. 2015, 11, 6(9):3313-9. [CrossRef]
- Ramier, A.; Tavakol, B.; Yun, S.H. Effect of intraocular pressure on the vibrational resonance of the cornea measured by optical coherence tomography. Invest Ophthalmol Vis Sci 2017, 58(8), 4326.
- Martin, X.D. Normal intraocular pressure in man. Ophthalmologica. 1992, 205(2),57-63. [CrossRef]
- Bakke, E.F.; Hisdal, J.; Semb, S.O. Intraocular Pressure Increases in Parallel with Systemic Blood Pressure during Isometric Exercise. Invest Ophthalmol Vis Sci 2009, 50(2),760-764. [CrossRef]
- Zimprich, L.; Diedrich, J.; Bleeker, A.; Schweitzer, J.A. Corneal Hysteresis as a Biomarker of Glaucoma: Current Insights. Clin Ophthalmol 2020, 14, 2255-2264. [CrossRef]
- Agarwal, D.; Ehrlich, J.; Shimmyo, M.; Radcliffe, N. The relationship between corneal hysteresis and the magnitude of intraocular pressure reduction with topical prostaglandin therapy. The British journal of ophthalmology 2011, 96, 254-7. [CrossRef]
- Herndon, L.W.; Choudhri, S.A.: Cox. T.; Damji, K.F.;Shields, M.B.; Allingham, R.R. Central corneal thickness in normal, glaucomatous, and ocular hypertensive eyes. Arch Ophthalmol 1997, 115(9):1137-41. [CrossRef]
- Wei, W.; Fan, Z.; Wang, L.; Li, Z. Jiao, W.; et al. Correlation Analysis between Central Corneal Thickness and Intraocular Pressure in Juveniles in Northern China: The Jinan City Eye Study. PLOS ONE 2011, 9(8): e104842. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).