Submitted:
19 May 2023
Posted:
22 May 2023
You are already at the latest version
Abstract
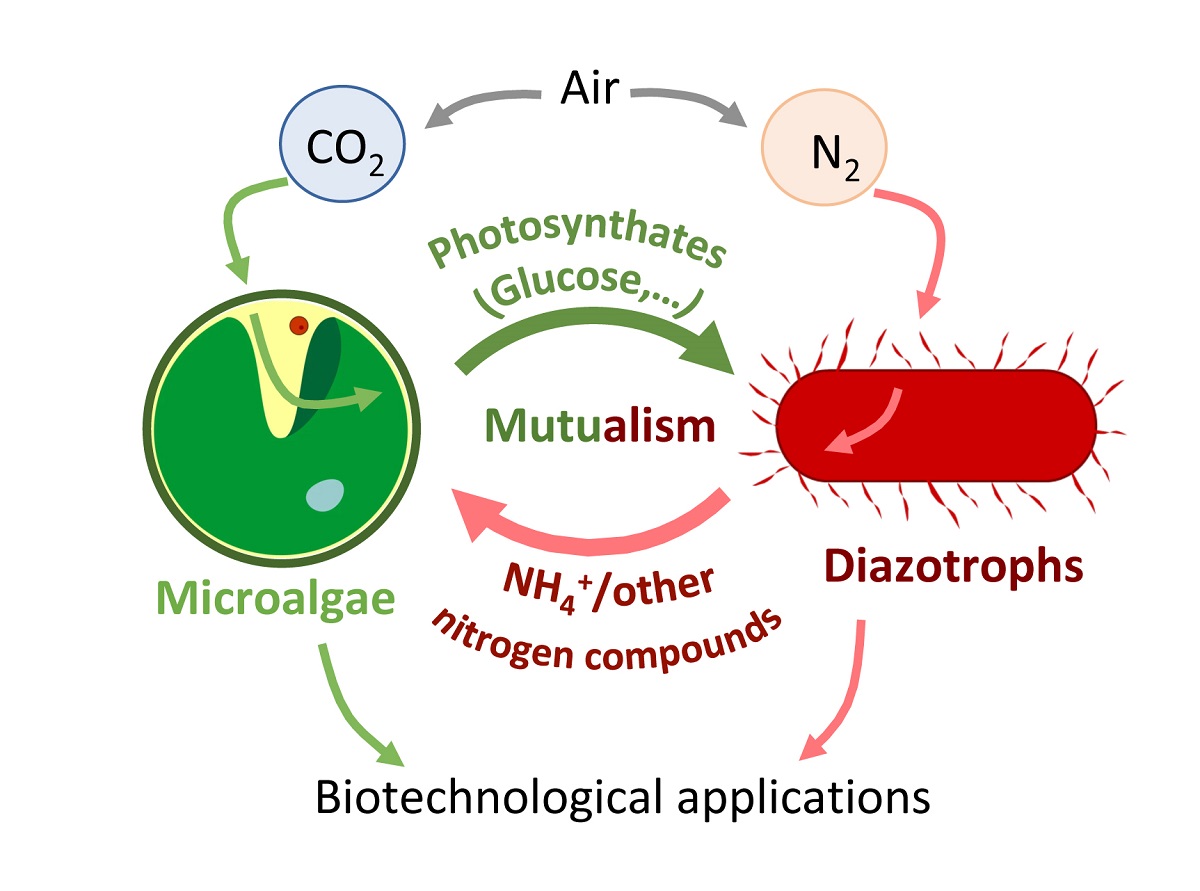
Keywords:
1. Introduction
2. Microalgae interaction with diazotrophic bacteria
2.1. Microalgae interaction with non-photosynthetic diazotrophic bacteria
2.1.1. Microalgae interaction with Azotobacter spp.
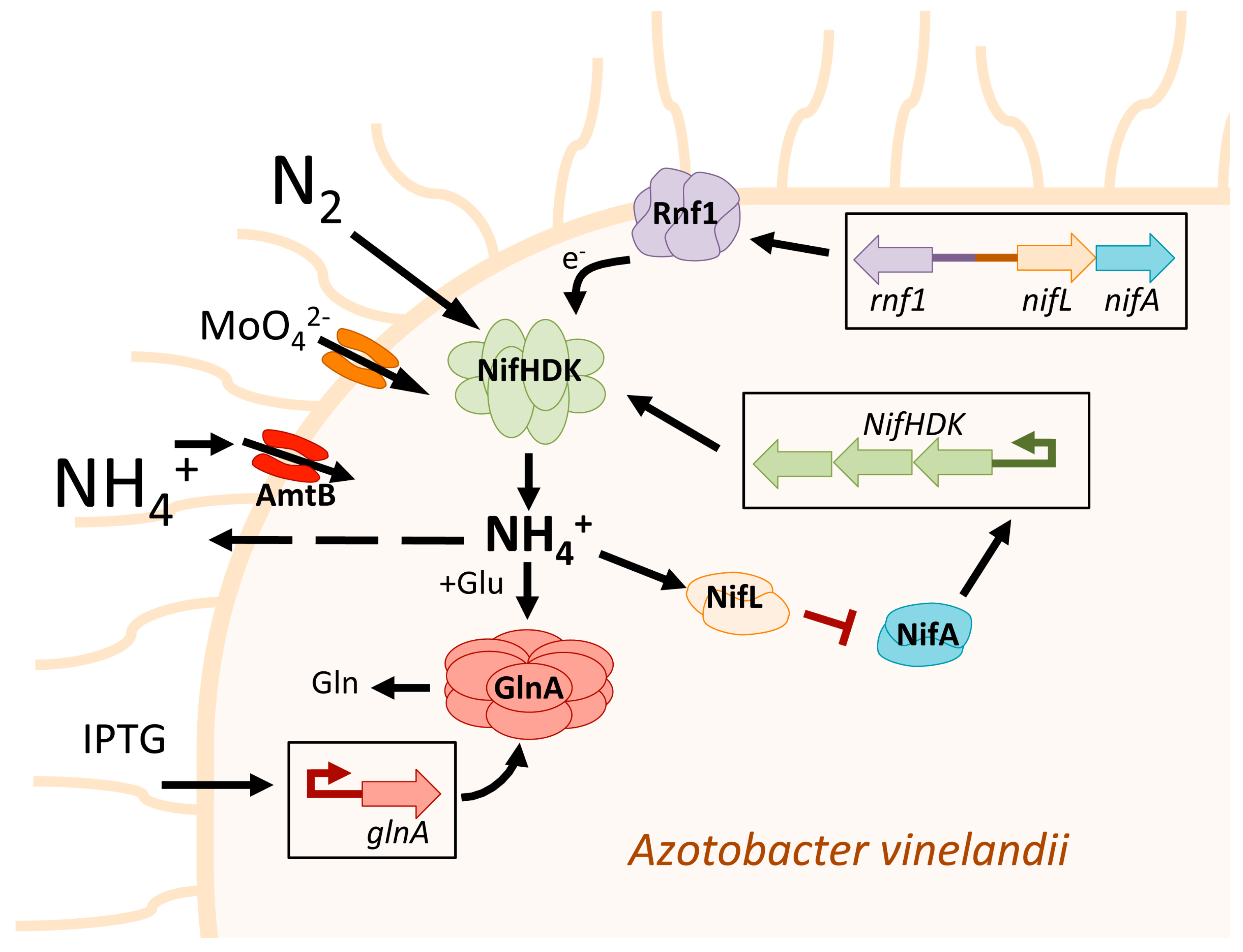
2.1.2. Microalgae interaction with Azospirillum spp.
2.1.3. Microalgae interaction with other diazotrophic bacteria
2.2. Microalgae interaction with photosynthetic diazotrophic bacteria
2.3. Microalgae interaction with diazotrophic bacteria in the corals
2.4. Microalgae interaction with diazotrophic bacteria in the lichens
3. Biotechnological Potential
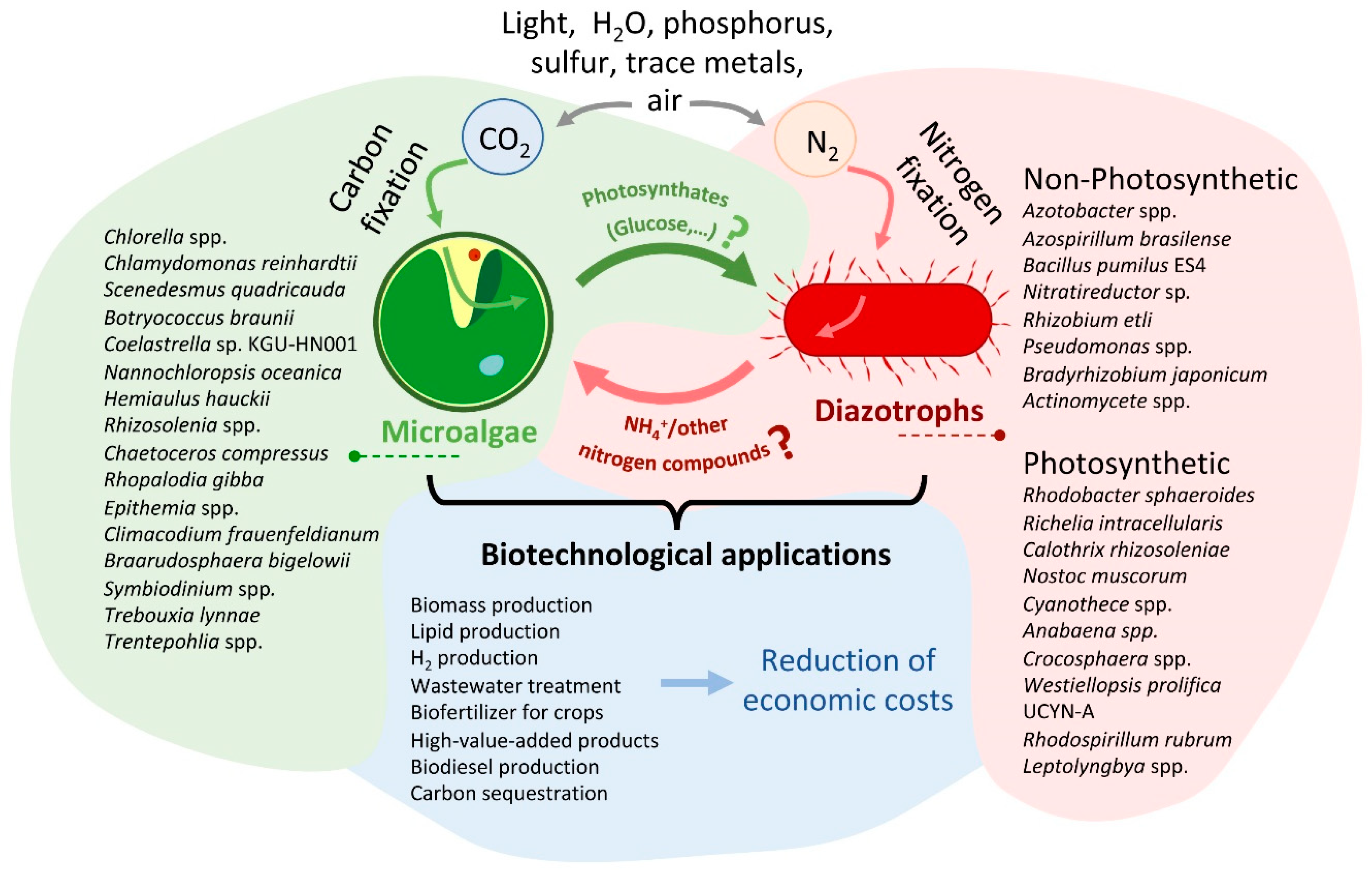
4. Concluding Remarks and Future Outlook
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fabris, M.; Abbriano, R.M.; Pernice, M.; Sutherland, D.L.; Commault, A.S.; Hall, C.C.; Labeeuw, L.; McCauley, J.I.; Kuzhiuparambil, U.; Ray, P.; et al. Emerging Technologies in Algal Biotechnology: Toward the Establishment of a Sustainable, Algae-Based Bioeconomy. Front. Plant Sci. 2020, 11, 279. [Google Scholar] [CrossRef] [PubMed]
- Quintas-Nunes, F.; Brandão, P.R.; Barreto Crespo, M.T.; Glick, B.R.; Nascimento, F.X. Plant Growth Promotion, Phytohormone Production and Genomics of the Rhizosphere-Associated Microalga, Micractinium Rhizosphaerae Sp. Nov. Plants 2023, 12, 651. [Google Scholar] [CrossRef] [PubMed]
- Barreno, E.; Muggia, L.; Chiva, S.; Molins, A.; Bordenave, C.; García-Breijo, F.; Moya, P. Trebouxia Lynnae Sp. Nov. (Former Trebouxia Sp. TR9): Biology and Biogeography of an Epitome Lichen Symbiotic Microalga. Biology (Basel). 2022, 11, 1196. [Google Scholar] [CrossRef] [PubMed]
- Scharfenstein, H.J.; Chan, W.Y.; Buerger, P.; Humphrey, C.; van Oppen, M.J.H. Evidence for de Novo Acquisition of Microalgal Symbionts by Bleached Adult Corals. ISME J. 2022, 16, 1676–1679. [Google Scholar] [CrossRef]
- Winkel, M.; Trivedi, C.B.; Mourot, R.; Bradley, J.A.; Vieth-Hillebrand, A.; Benning, L.G. Seasonality of Glacial Snow and Ice Microbial Communities. Front. Microbiol. 2022, 13, 876848. [Google Scholar] [CrossRef] [PubMed]
- Umen, J.; Herron, M.D. Green Algal Models for Multicellularity. Annu. Rev. Genet. 2021, 55, 603–632. [Google Scholar] [CrossRef] [PubMed]
- Tong, C.Y.; Honda, K.; Derek, C.J.C. A Review on Microalgal-Bacterial Co-Culture: The Multifaceted Role of Beneficial Bacteria towards Enhancement of Microalgal Metabolite Production. Environ. Res. 2023, 228, 115872. [Google Scholar] [CrossRef]
- Ray, A.; Nayak, M.; Ghosh, A. A Review on Co-Culturing of Microalgae: A Greener Strategy towards Sustainable Biofuels Production. Sci. Total Environ. 2022, 802, 149765. [Google Scholar] [CrossRef]
- Calatrava, V.; Tejada-Jimenez, M.; Sanz-Luque, E.; Fernandez, E.; Galvan, A.; Llamas, A. Chlamydomonas Reinhardtii, a Reference Organism to Study Algal–Microbial Interactions: Why Can’t They Be Friends? Plants 2023, 12, 788. [Google Scholar] [CrossRef]
- Bell, W.; Mitchell, R. Chemotactic and Growth Responses of Marine Bacteria to Algal Extracellular Products. Biol. Bull. 1972, 143, 265–277. [Google Scholar] [CrossRef]
- Durán, P.; Flores-Uribe, J.; Wippel, K.; Zhang, P.; Guan, R.; Melkonian, B.; Melkonian, M.; Garrido-Oter, R. Shared Features and Reciprocal Complementation of the Chlamydomonas and Arabidopsis Microbiota. Nat. Commun. 2022, 13, 406. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, N.; Ahmad, F.; Lu, Y. Synergy between Microalgae and Microbiome in Polluted Waters. Trends Microbiol. 2023, 31, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Bunbury, F.; Deery, E.; Sayer, A.P.; Bhardwaj, V.; Harrison, E.L.; Warren, M.J.; Smith, A.G. Exploring the Onset of B 12-Based Mutualisms Using a Recently Evolved Chlamydomonas Auxotroph and B12-Producing Bacteria. Environ. Microbiol. 2022, 24, 3134–3147. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.J.; Francis, M.B. A Designed A. Vinelandii-S. Elongatus Coculture for Chemical Photoproduction from Air, Water, Phosphate, and Trace Metals. ACS Synth. Biol. 2016, 5, 955–961. [Google Scholar] [CrossRef] [PubMed]
- Venkataram, S.; Kuo, H.-Y.; Hom, E.F.Y.; Kryazhimskiy, S. Mutualism-Enhancing Mutations Dominate Early Adaptation in a Two-Species Microbial Community. Nat. Ecol. Evol. 2023, 7, 143–154. [Google Scholar] [CrossRef] [PubMed]
- Threatt, S.D.; Rees, D.C. Biological Nitrogen Fixation in Theory, Practice, and Reality: A Perspective on the Molybdenum Nitrogenase System. FEBS Lett. 2023, 597, 45–58. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Chen, Y.; Huang, K.; Wang, F.; Mei, Z. Molecular Mechanism and Agricultural Application of the NifA–NifL System for Nitrogen Fixation. Int. J. Mol. Sci. 2023, 24, 907. [Google Scholar] [CrossRef]
- Burghardt, L.T.; diCenzo, G.C. The Evolutionary Ecology of Rhizobia: Multiple Facets of Competition before, during, and after Symbiosis with Legumes. Curr. Opin. Microbiol. 2023, 72, 102281. [Google Scholar] [CrossRef]
- Aasfar, A.; Aasfar, A.; Bargaz, A.; Yaakoubi, K.; Hilali, A.; Bennis, I.; Zeroual, Y.; Meftah Kadmiri, I. Nitrogen Fixing Azotobacter Species as Potential Soil Biological Enhancers for Crop Nutrition and Yield Stability. Front. Microbiol. 2021, 12, 1–19. [Google Scholar] [CrossRef]
- Kim, B.H.; Ramanan, R.; Cho, D.H.; Oh, H.M.; Kim, H.S. Role of Rhizobium, a Plant Growth Promoting Bacterium, in Enhancing Algal Biomass through Mutualistic Interaction. Biomass and Bioenergy 2014, 69, 95–105. [Google Scholar] [CrossRef]
- Wirth, R.; Pap, B.; Böjti, T.; Shetty, P.; Lakatos, G.; Bagi, Z.; Kovács, K.L.; Maróti, G. Chlorella Vulgaris and Its Phycosphere in Wastewater: Microalgae-Bacteria Interactions During Nutrient Removal. Front. Bioeng. Biotechnol. 2020, 8, 557572. [Google Scholar] [CrossRef] [PubMed]
- Astafyeva, Y.; Gurschke, M.; Qi, M.; Bergmann, L.; Indenbirken, D.; de Grahl, I.; Katzowitsch, E.; Reumann, S.; Hanelt, D.; Alawi, M.; et al. Microalgae and Bacteria Interaction—Evidence for Division of Diligence in the Alga Microbiota. Microbiol. Spectr. 2022, 10, 633. [Google Scholar] [CrossRef] [PubMed]
- Blifernez-Klassen, O.; Klassen, V.; Wibberg, D.; Cebeci, E.; Henke, C.; Rückert, C.; Chaudhari, S.; Rupp, O.; Blom, J.; Winkler, A.; et al. Phytoplankton Consortia as a Blueprint for Mutually Beneficial Eukaryote-Bacteria Ecosystems Based on the Biocoenosis of Botryococcus Consortia. Sci. Rep. 2021, 11, 1726. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, C.Y.B.; Jacob, A.; Nader, C.; Oliveira, C.D.L.; Matos, Â.P.; Araújo, E.S.; Shabnam, N.; Ashok, B.; Gálvez, A.O. An Overview on Microalgae as Renewable Resources for Meeting Sustainable Development Goals. J. Environ. Manage. 2022, 320, 115897. [Google Scholar] [CrossRef] [PubMed]
- Kazmi, S.S.U.H.; Yapa, N.; Karunarathna, S.C.; Suwannarach, N. Perceived Intensification in Harmful Algal Blooms Is a Wave of Cumulative Threat to the Aquatic Ecosystems. Biology (Basel). 2022, 11, 852. [Google Scholar] [CrossRef] [PubMed]
- Pereira, J.F.; Oliveira, A.L.M.; Sartori, D.; Yamashita, F.; Mali, S. Perspectives on the Use of Biopolymeric Matrices as Carriers for Plant-Growth Promoting Bacteria in Agricultural Systems. Microorganisms 2023, 11, 467. [Google Scholar] [CrossRef]
- Palacios, O.A.; Espinoza-Hicks, J.C.; Camacho-Dávila, A.A.; López, B.R.; de-Bashan, L.E. Differences in Exudates Between Strains of Chlorella Sorokiniana Affect the Interaction with the Microalga Growth-Promoting Bacteria Azospirillum Brasilense. Microb. Ecol. 2022, 22, 02026. [Google Scholar] [CrossRef]
- Sabra, W.; Zeng, A.-P.; Lu¨nsdorf, H.; Lu¨nsdorf, L.; Deckwer, W.-D. Effect of Oxygen on Formation and Structure of Azotobacter Vinelandii Alginate and Its Role in Protecting Nitrogenase. Appl. Environ. Microbiol. 2000, 66, 4037–4044. [Google Scholar] [CrossRef]
- Alleman, A.B.; Mus, F.; Peters, J.W. Metabolic Model of the Nitrogen-Fixing Obligate Aerobe Azotobacter Vinelandii Predicts Its Adaptation to Oxygen Concentration and Metal Availability. MBio 2021, 12, e02593. [Google Scholar] [CrossRef]
- Gyurjan, I.; Turtoczky, I.; Toth, G. .; Paless, G.; Nghia, N.H. Intercellular Symbiosis of Nitrogen-Fixing Bacteria and Green Alga. Acta Bot. Hung 1984, 30, 249–256. [Google Scholar]
- Gyurjan, I.; Nghia, N.H.; Tóth, G.; Turtozky, I.; Stefanovits, P. Photosynthesis, Nitrogen Fixation and Enzyme Activities in Chlamydomonas-Azotobacter Symbioses. Biochem. und Physiol. der Pflanz. 1986, 181, 147–153. [Google Scholar] [CrossRef]
- Preininger, E.; Ponyi, T.; Sarkadi, L.; Nyitrai, P.; Gyurjan, I. Long-Living Azotobacter-Chlamydomonas Association as a Model System for Plant-Microbe Interactions. Symbiosis 2006, 42, 45–50. [Google Scholar]
- Nghia, N.H.; Gyurján, I.; Stefanovits, P.; Turtóczky, I. Establishment of Nitrogen-Fixing Chlamydomonas-Azotobacter Symbioses: Physiological, Biochemical and Morphological Examinations. Endocyt.C.Res 1986, 3, 179–188. [Google Scholar]
- Calatrava, V.; Hom, E.F.Y.; Llamas, A.; Fernández, E.; Galvan, A. Nitrogen Scavenging from Amino Acids and Peptides in the Model Alga Chlamydomonas Reinhardtii. The Role of Extracellular L-Amino Oxidase. Algal Res. 2019, 38, 101395. [Google Scholar] [CrossRef]
- Allen, M.B. Excretion of Organic Compounds by Chlamydomonas. Arch. fiir Mikrobiol. 1956, 24, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Collins, R.P. and K.K. Keto Acids Produced by Chlamydomonas Reinhardtii. Can. J. Microbiol. 1967, 13, 995–999. [Google Scholar] [CrossRef] [PubMed]
- Mus, F.; Dubini, A.; Seibert, M.; Posewitz, M.C.; Grossman, A.R. Anaerobic Acclimation in Chlamydomonas Reinhardtii. J. Biol. Chem. 2007, 282, 25475–25486. [Google Scholar] [CrossRef] [PubMed]
- Vogel, S.L.; Frisch, H.L.; Gotham, I.J. Qualitative Assay of Dissolved Amino Acids and Sugars Excreted by Chlamydomonas Reinhardtii (Chlorophyceae) and Euglena Gracilis (Euglenophyceae). J. Phycol. 1978, 14, 403–406. [Google Scholar] [CrossRef]
- Nghia, N.H.; Gyurján, I.; Stefanovits, P.; Paless, G.; Turtóczky, I. Uptake of Azotobacters by Somatic Fusion of Cell-Wall Mutants of Chlamydomonas Reinhardtii. Biochem. und Physiol. der Pflanz. 1986, 181, 347–357. [Google Scholar] [CrossRef]
- Gyurjan, I.; Koranyi, P.; Paless, G.Y. Ultrastructural Analysis of an Artificial Alga-Bacterium Endosymbiosis After Prolonged Cultivation. Symbiosis 1992, 14, 475–468. [Google Scholar]
- Lorincz, Z.; Preininger, E.; Kósa, A.; Pónyi, T.; Nyitrai, P.; Sarkadi, L.; Kovács, G.M.; Böddi, B.; Gyurján, I. Artificial Tripartite Symbiosis Involving a Green Alga (Chlamydomonas), a Bacterium (Azotobacter) and a Fungus (Alternaria): Morphological and Physiological Characterization. Folia Microbiol. (Praha). 2010, 55, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Marquez, J.C.F.; Nascimento, M. Do; Dublan, M. de los A.; Curatti, L. Association with an Ammonium-Excreting Bacterium Allows Diazotrophic Culture of Oil-Rich Eukaryotic Microalgae. Appl. Environ. Microbiol. 2012, 78, 2345–2352. [Google Scholar] [CrossRef] [PubMed]
- Barney, B.M.; Plunkett, M.H.; Natarajan, V.; Mus, F.; Knutson, C.M.; Peters, J.W. Transcriptional Analysis of an Ammonium-Excreting Strain of Azotobacter Vinelandii Deregulated for Nitrogen Fixation. Appl. Environ. Microbiol. 2017, 83, 01534. [Google Scholar] [CrossRef] [PubMed]
- Plunkett, M.H.; Knutson, C.M.; Barney, B.M. Key Factors Affecting Ammonium Production by an Azotobacter Vinelandii Strain Deregulated for Biological Nitrogen Fixation. Microb. Cell Fact. 2020, 19, 107. [Google Scholar] [CrossRef] [PubMed]
- Mus, F.; Khokhani, D.; Rugoli, E.; Dixon John, R.; Ané, J.-M.; Peters, J. Genetic Determinants of Ammonia Excretion in NifL Mutants of Azotobacter Vinelandii. Appl Env. Microbiol 2022, 88, e01876. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Marquez, J.C.F.; Do Nascimento, M.; Curatti, L. Metabolic Engineering of Ammonium Release for Nitrogen-Fixing Multispecies Microbial Cell-Factories. Metab. Eng. 2014, 23, 154–164. [Google Scholar] [CrossRef] [PubMed]
- Ambrosio, R.; Ortiz-Marquez, J.C.F.; Curatti, L. Metabolic Engineering of a Diazotrophic Bacterium Improves Ammonium Release and Biofertilization of Plants and Microalgae. Metab. Eng. 2017, 40, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Ambrosio, R.; Curatti, L. Deferred Control of Ammonium Cross-Feeding in a N2-Fixing Bacterium-Microalga Artificial Consortium. Appl. Microbiol. Biotechnol. 2021, 105, 2937–2950. [Google Scholar] [CrossRef]
- Barney, B.M.; Eberhart, L.J.; Ohlert, J.M.; Knutson, C.M.; Plunkett, M.H. Gene Deletions Resulting in Increased Nitrogen Release by Azotobacter Vinelandii: Application of a Novel Nitrogen Biosensor. Appl. Environ. Microbiol. 2015, 81, 4316–4328. [Google Scholar] [CrossRef]
- Villa, J.A.; Ray, E.E.; Barney, B.M. Azotobacter Vinelandii Siderophore Can Provide Nitrogen to Support the Culture of the Green Algae Neochloris Oleoabundans and Scenedesmus Sp. BA032. FEMS Microbiol. Lett. 2014, 351, 70–77. [Google Scholar] [CrossRef]
- Barney, B.M. Aerobic Nitrogen-Fixing Bacteria for Hydrogen and Ammonium Production: Current State and Perspectives. Appl. Microbiol. Biotechnol. 2020, 104, 1383–1399. [Google Scholar] [CrossRef] [PubMed]
- Vuolo, F.; Novello, G.; Bona, E.; Gorrasi, S.; Gamalero, E. Impact of Plant-Beneficial Bacterial Inocula on the Resident Bacteriome: Current Knowledge and Future Perspectives. Microorganisms 2022, 10, 2462. [Google Scholar] [CrossRef] [PubMed]
- Elmas, M.; Alexiades, V.; O’neal, L.; Alexandre, G. Modeling Aerotaxis Band Formation in Azospirillum Brasilense. BMC Microbiol. 2019, 19, 101. [Google Scholar] [CrossRef] [PubMed]
- Fukami, J.; Cerezini, P.; Hungria, M. Azospirillum: Benefits That Go Far beyond Biological Nitrogen Fixation. AMB Express 2018, 8, 73. [Google Scholar] [CrossRef]
- Cassan, F.D.; Coniglio, A.; Amavizca, E.; Maroniche, G.; Cascales, E.; Bashan, Y.; de-Bashan, L.E. The Azospirillum Brasilense Type VI Secretion System Promotes Cell Aggregation, Biocontrol Protection against Phytopathogens and Attachment to the Microalgae Chlorella Sorokiniana. Environ. Microbiol. 2021, 23, 6257–6274. [Google Scholar] [CrossRef]
- Cruz-Hernández, M.A.; Mendoza-Herrera, A.; Bocanegra-García, V.; Rivera, G. Azospirillum Spp. from Plant Growth-Promoting Bacteria to Their Use in Bioremediation. Microorganisms 2022, 10, 1057. [Google Scholar] [CrossRef] [PubMed]
- Amavizca, E.; Bashan, Y.; Ryu, C.M.; Farag, M.A.; Bebout, B.M.; De-Bashan, L.E. Enhanced Performance of the Microalga Chlorella Sorokiniana Remotely Induced by the Plant Growth-Promoting Bacteria Azospirillum Brasilense and Bacillus Pumilus. Sci. Rep. 2017, 7, 41310. [Google Scholar] [CrossRef]
- Barbosa-Nuñez, J.A.; Palacios, O.A.; de-Bashan, L.E.; Snell-Castro, R.; Corona-González, R.I.; Choix, F.J. Active Indole-3-Acetic Acid Biosynthesis by the Bacterium Azospirillum Brasilense Cultured under a Biogas Atmosphere Enables Its Beneficial Association with Microalgae. J. Appl. Microbiol. 2022, 132, 3650–3663. [Google Scholar] [CrossRef]
- Peng, H.; de-Bashan, L.E.; Higgins, B.T. Azospirillum Brasilense Reduces Oxidative Stress in the Green Microalgae Chlorella Sorokiniana under Different Stressors. J. Biotechnol. 2021, 325, 179–185. [Google Scholar] [CrossRef]
- de-Bashan, L.E.; Hernandez, J.P.; Morey, T.; Bashan, Y. Microalgae Growth-Promoting Bacteria as “Helpers” for Microalgae: A Novel Approach for Removing Ammonium and Phosphorus from Municipal Wastewater. Water Res. 2004, 38, 466–474. [Google Scholar] [CrossRef]
- Meza, B.; de-Bashan, L.E.; Bashan, Y. Involvement of Indole-3-Acetic Acid Produced by Azospirillum Brasilense in Accumulating Intracellular Ammonium in Chlorella Vulgaris. Res. Microbiol. 2015, 166, 72–83. [Google Scholar] [CrossRef] [PubMed]
- Meza, B.; de-Bashan, L.E.; Hernandez, J.P.; Bashan, Y. Accumulation of Intra-Cellular Polyphosphate in Chlorella Vulgaris Cells Is Related to Indole-3-Acetic Acid Produced by Azospirillum Brasilense. Res. Microbiol. 2015, 166, 399–407. [Google Scholar] [CrossRef] [PubMed]
- Palacios, O.A.; Choix, F.J.; Bashan, Y.; de-Bashan, L.E. Influence of Tryptophan and Indole-3-Acetic Acid on Starch Accumulation in the Synthetic Mutualistic Chlorella Sorokiniana-Azospirillum Brasilense System under Heterotrophic Conditions. Res Microbiol 2016, 167, 367–379. [Google Scholar] [CrossRef] [PubMed]
- Choix, F.J.; Bashan, Y.; Mendoza, A.; De-Bashan, L.E. Enhanced Activity of ADP Glucose Pyrophosphorylase and Formation of Starch Induced by Azospirillum Brasilense in Chlorella Vulgaris. J. Biotechnol. 2014, 177, 22–34. [Google Scholar] [CrossRef] [PubMed]
- Leyva, L.A.; Bashan, Y.; Mendoza, A.; de-Bashan, L.E. Accumulation Fatty Acids of in Chlorella Vulgaris under Heterotrophic Conditions in Relation to Activity of Acetyl-CoA Carboxylase, Temperature, and Co-Immobilization with Azospirillum Brasilense. Naturwissenschaften 2014, 101, 819–830. [Google Scholar] [CrossRef] [PubMed]
- Choix, F.J.; Guadalupe López-Cisneros, C.; Oscar Méndez-Acosta, H. Azospirillum Brasilense Increases CO2 Fixation on Microalgae Scenedesmus Obliquus, Chlorella Vulgaris, and Chlamydomonas Reinhardtii Cultured on High CO2 Concentrations. Microb. Ecol. 2018, 76, 430–442. [Google Scholar] [CrossRef] [PubMed]
- Palacios, O.A.; Gomez-Anduro, G.; Bashan, Y.; de-Bashan, L.E. Tryptophan, Thiamine and Indole-3-Acetic Acid Exchange between Chlorella Sorokiniana and the Plant Growth-Promoting Bacterium Azospirillum Brasilense. FEMS Microbiol Ecol 2016, 92, fiw077. [Google Scholar] [CrossRef] [PubMed]
- Holguin, G.; Bashan, Y. Nitrogen-Fixation by Azospirillum Brasilense Cd Is Promoted When Co-Cultured with a Mangrove Rhizosphere Bacterium (Staphylococcus Sp.). Soil Bid. Eiochem 1996, 28, 1651–1660. [Google Scholar] [CrossRef]
- de-Bashan, L.E.; Moreno, M.; Hernandez, J.-P.; Bashan, Y. Removal of Ammonium and Phosphorus Ions from Synthetic Wastewater by the Microalgae Chlorella Vulgaris Coimmobilized in Alginate Beads with the Microalgae Growth-Promoting Bacterium Azospirillum Brasilense. Water Res. 2002, 36, 2941–2948. [Google Scholar] [CrossRef]
- Choix, F.J.; de-Bashan, L.E.; Bashan, Y. Enhanced Accumulation of Starch and Total Carbohydrates in Alginate-Immobilized Chlorella Spp. Induced by Azospirillum Brasilense: II. Heterotrophic Conditions. Enzym. Microb Technol 2012, 51, 300–309. [Google Scholar] [CrossRef]
- de-Bashan, L.E.; Schmid, M.; Rothballer, M.; Hartmann, A.; Bashan, Y. Cell-Cell Interaction in the Eukaryote-Prokaryote Model of the Microalgae Chlorella Vulgaris and the Bacterium Azospirillum Brasilense Immobilized in Polymer Beads. J. Phycol. 2011, 47, 1350–1359. [Google Scholar] [CrossRef] [PubMed]
- Covarrubias, S.A.; De-Bashan, L.E.; Moreno, M.; Bashan, Y. Alginate Beads Provide a Beneficial Physical Barrier against Native Microorganisms in Wastewater Treated with Immobilized Bacteria and Microalgae. Appl. Microbiol. Biotechnol. 2012, 93, 2669–2680. [Google Scholar] [CrossRef] [PubMed]
- Pham, T.M.; Bui, X.D.; Trang, L.V.K.; Le, T.M.; Nguyen, M.L.; Trinh, D.M.; Phuong, N.T.D.; Khoo, K.S.; Chew, K.W.; Show, P.L. Isolation of Indole-3-Acetic Acid-Producing Azospirillum Brasilense from Vietnamese Wet Rice: Co-Immobilization of Isolate and Microalgae as a Sustainable Biorefinery. J. Biotechnol. 2022, 349, 12–20. [Google Scholar] [CrossRef]
- Gonzalez, L.E.; Bashan, Y. Increased Growth of the Microalga Chlorella Vulgaris When Coimmobilized and Cocultured in Alginate Beads with the Plant-Growth-Promoting Bacterium Azospirillum Brasilense. Appl. Environ. Microbiol. 2000, 66, 1527–1531. [Google Scholar] [CrossRef] [PubMed]
- O’reilly, A.M.; Scott, J.A. Defined Coimmobilization of Mixed Microorganism Cultures. Enzym. Microb. Technol. 1995, 17, 636–646. [Google Scholar] [CrossRef]
- Hernandez, J.P.; de-Bashan, L.E.; Rodriguez, D.J.; Rodriguez, Y.; Bashan, Y. Growth Promotion of the Freshwater Microalga Chlorella Vulgaris by the Nitrogen-Fixing, Plant Growth-Promoting Bacterium Bacillus Pumilus from Arid Zone Soils. Eur. J. Soil Biol. 2009, 45, 88–93. [Google Scholar] [CrossRef]
- Liu, B.; Eltanahy, E.E.; Liu, H.; Chua, E.T.; Thomas-Hall, S.R.; Wass, T.J.; Pan, K.; Schenk, P.M. Growth-Promoting Bacteria Double Eicosapentaenoic Acid Yield in Microalgae. Bioresour. Technol. 2020, 316, 123916. [Google Scholar] [CrossRef]
- Augimeri, R. V.; Varley, A.J.; Strap, J.L. Establishing a Role for Bacterial Cellulose in Environmental Interactions: Lessons Learned from Diverse Biofilm-Producing Proteobacteria. Front. Microbiol. 2015, 6, 1282. [Google Scholar] [CrossRef]
- Aburai, N.; Tsukagoshi, T.; Sekiguchi, S.; Arakawa, H.; Imamura, Y.; Abe, K. Mutual Supply of Carbon and Nitrogen Sources in the Co-Culture of Aerial Microalgae and Nitrogen-Fixing Bacteria. Algal Res. 2023, 70, 103001. [Google Scholar] [CrossRef]
- Burén, S.; Jiménez-Vicente, E.; Echavarri-Erasun, C.; Rubio, L.M. Biosynthesis of Nitrogenase Cofactors. Chem. Rev. 2020, 120, 4921–4968. [Google Scholar] [CrossRef]
- Zeng, X.; Zhang, C.-C. The Making of a Heterocyst in Cyanobacteria. Annu. Rev. Microbiol. 2022, 76, 597–618. [Google Scholar] [CrossRef] [PubMed]
- Kollmen, J.; Strieth, D. The Beneficial Effects of Cyanobacterial Co-Culture on Plant Growth. Life 2022, 12, 10–3390. [Google Scholar] [CrossRef]
- Gao, M.; Armin, G.; Inomura, K. Low-Ammonium Environment Increases the Nutrient Exchange between Diatom–Diazotroph Association Cells and Facilitates Photosynthesis and N2 Fixation—a Mechanistic Modeling Analysis. Cells 2022, 11, 2911. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, V.; Inomura, K.; Mouw, C.B. Quantitative Analysis of the Trade-Offs of Colony Formation for Trichodesmium. Microbiol. Spectr. 2022, 10, e0202522. [Google Scholar] [CrossRef] [PubMed]
- Inomura, K.; Follett, C.L.; Masuda, T.; Eichner, M.; Prášil, O.; Deutsch, C. Carbon Transfer from the Host Diatom Enables Fast Growth and High Rate of N2 Fixation by Symbiotic Heterocystous Cyanobacteria. Plants 2020, 9, 192. [Google Scholar] [CrossRef] [PubMed]
- Caputo, A.; Nylander, J.A.A.; Foster, R.A. The Genetic Diversity and Evolution of Diatom-Diazotroph Associations Highlights Traits Favoring Symbiont Integration. FEMS Microbiol. Lett. 2019, 366, fny297. [Google Scholar] [CrossRef]
- Anderson, E.E.; Wilson, C.; Knap, A.H.; Villareal, T.A. Summer Diatom Blooms in the Eastern North Pacific Gyre Investigated with a Long-Endurance Autonomous Surface Vehicle. PeerJ 2018, 6, 5387. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, E.J.; Montoya, J.P.; Burns, J.; Mulholland, M.R.; Subramaniam, A.; Capone, D.G. Extensive Bloom of a N2-Fixing Diatom/Cyanobacterial Association in the Tropical Atlantic. Source Mar. Ecol. Prog. Ser. 1999, 185, 273–283. [Google Scholar] [CrossRef]
- Karl, D.M.; Church, M.J.; Dore, J.E.; Letelier, R.M.; Mahaffey, C. Predictable and Efficient Carbon Sequestration in the North Pacific Ocean Supported by Symbiotic Nitrogen Fixation. Proc. Natl. Acad. Sci. U. S. A. 2012, 109, 1842–1849. [Google Scholar] [CrossRef]
- Foster, R.A.; Kuypers, M.M.M.; Vagner, T.; Paerl, R.W.; Musat, N.; Zehr, J.P. Nitrogen Fixation and Transfer in Open Ocean Diatom-Cyanobacterial Symbioses. ISME J. 2011, 5, 1484–1493. [Google Scholar] [CrossRef]
- Foster, R.A.; Tienken, D.; Littmann, S.; Whitehouse, M.J.; Kuypers, M.M.M.; White, A.E. The Rate and Fate of N2 and C Fixation by Marine Diatom-Diazotroph Symbioses. ISME J. 2021, 16, 477–487. [Google Scholar] [CrossRef] [PubMed]
- Flores, E.; Romanovicz, D.K.; Nieves-Morión, M.; Foster, R.A.; Villareal, T.A. Adaptation to an Intracellular Lifestyle by a Nitrogen-Fixing, Heterocyst-Forming Cyanobacterial Endosymbiont of a Diatom. Front. Microbiol. 2022, 13, 799362. [Google Scholar] [CrossRef] [PubMed]
- Hilton, J.A.; Foster, R.A.; James Tripp, H.; Carter, B.J.; Zehr, J.P.; Villareal, T.A. Genomic Deletions Disrupt Nitrogen Metabolism Pathways of a Cyanobacterial Diatom Symbiont. Nat. Commun. 2013, 4, 1767. [Google Scholar] [CrossRef] [PubMed]
- Nieves-Morión, M.; Flores, E.; Foster, R.A. Predicting Substrate Exchange in Marine Diatom-Heterocystous Cyanobacteria Symbioses. Environ. Microbiol. 2020, 22, 2027–2052. [Google Scholar] [CrossRef]
- Gautam, K.; Tripathi, J.K.; Pareek, A.; Sharma, D.K. Growth and Secretome Analysis of Possible Synergistic Interaction between Green Algae and Cyanobacteria. J. Biosci. Bioeng. 2019, 127, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Meenakshi, V.K.; Gomathy, S.; Senthamarai, S.; Paripooranaselvi, M.; Chamundeswari, K.P. GC-MS Determination of the Bioactive Components of Microcosmus Exasperatus Heller, 1878. J. Curr. Chem. Pharm. Sc. 2012, 2, 271–276. [Google Scholar]
- Zhai, X.; Lenon, G.B.; Xue, C.C.L.; Li, C.G. Euonymus Alatus: A Review on Its Phytochemistry and Antidiabetic Activity. Evid Based Complement Altern. Med 2016, 2016, 9425714. [Google Scholar] [CrossRef]
- Moulin, S.L.Y.; Frail, S.; Doenier, J.; Braukmann, T.; Yeh, E. The Endosymbiont of Epithemia Clementina Is Specialized for Nitrogen Fixation within a Photosynthetic Eukaryote. bioRxiv Prepr. 2023. [Google Scholar] [CrossRef]
- Kneip, C.; Voß, C.; Lockhart, P.J.; Maier, U.G. The Cyanobacterial Endosymbiont of the Unicellular Algae Rhopalodia Gibba Shows Reductive Genome Evolution. BMC Evol. Biol. 2008, 8, 30. [Google Scholar] [CrossRef]
- Carpenter, E.J.; Janson, S. Intracellular Cyanobacterial Symbionts in the Marine Diatom Climacodium Frauenfeldianum (Bacillariophyceae). J. Phycol. 2000, 36, 540–544. [Google Scholar] [CrossRef]
- Hagino, K.; Onuma, R.; Kawachi, M.; Horiguchi, T. Discovery of an Endosymbiotic Nitrogen-Fixing Cyanobacterium UCYN-A in Braarudosphaera Bigelowii (Prymnesiophyceae). PLoS One 2013, 8, e81749. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, T.; Ikegami, Y.; Nakayama, T.; Ishida, K. ichiro; Inagaki, Y.; Inouye, I. Spheroid Bodies in Rhopalodiacean Diatoms Were Derived from a Single Endosymbiotic Cyanobacterium. J. Plant Res. 2011, 124, 93–97. [Google Scholar] [CrossRef] [PubMed]
- Turk-Kubo, K.A.; Mills, M.M.; Arrigo, K.R.; van Dijken, G.; Henke, B.A.; Stewart, B.; Wilson, S.T.; Zehr, J.P. UCYN-A/Haptophyte Symbioses Dominate N2 Fixation in the Southern California Current System. ISME Commun. 2021, 1, 42. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Marín, M. del C.; Magasin, J.D.; Zehr, J.P. Open Ocean and Coastal Strains of the N2-Fixing Cyanobacterium UCYN-A Have Distinct Transcriptomes. PLoS One 2023, 18, e0272674. [Google Scholar] [CrossRef]
- Thompson, A.W.; Foster, R.A.; Krupke, A.; Carter, B.J.; Musat, N.; Vaulot, D.; Kuypers, M.M.M.; Zehr, J.P. Unicellular Cyanobacterium Symbiotic with a Single-Celled Eukaryotic Alga. Science (80-. ). 2012, 337, 1546–1554. [Google Scholar] [CrossRef] [PubMed]
- Schvarcz, C.R.; Wilson, S.T.; Caffin, M.; Stancheva, R.; Li, Q.; Turk-Kubo, K.A.; White, A.E.; Karl, D.M.; Zehr, J.P.; Steward, G.F. Overlooked and Widespread Pennate Diatom-Diazotroph Symbioses in the Sea. Nat. Commun. 2022, 13, 799. [Google Scholar] [CrossRef] [PubMed]
- Bove, C.B.; Valadez-Ingersoll, M.; Davies, S.W. Help Me, Symbionts, You’re My Only Hope: Approaches to Accelerate Our Understanding of Coral Holobiont Interactions. Integr. Comp. Biol. 2022, 62, 1756–1769. [Google Scholar] [CrossRef] [PubMed]
- Thirukanthan, C.S.; Azra, M.N.; Lananan, F.; Sara’, G.; Grinfelde, I.; Rudovica, V.; Vincevica-Gaile, Z.; Burlakovs, J. The Evolution of Coral Reef under Changing Climate: A Scientometric Review. Animals 2023, 13, 949. [Google Scholar] [CrossRef]
- Benavides, M.; Bednarz, V.N.; Ferrier-Pagès, C. Diazotrophs: Overlooked Key Players within the Coral Symbiosis and Tropical Reef Ecosystems? Front. Mar. Sci. 2017, 4, 10. [Google Scholar] [CrossRef]
- Mague, T.H.; Holm-Hansen, O. Nitrogen Fixation on a Coral Reef. Phycologia 1975, 14, 87–92. [Google Scholar] [CrossRef]
- Shashar, N.; Cohen, Y.; Loya, Y.; Sar, N. Nitrogen Fixation (Acetylene Reduction) in Stony Corals: Evidence for Coral-Bacteria Interactions. Mar. Ecol. Prog. Ser. 1994, 111, 259–264. [Google Scholar] [CrossRef]
- Morrow, K.M.; Pankey, M.S.; Lesser, M.P. Community Structure of Coral Microbiomes Is Dependent on Host Morphology. Microbiome 2022, 10. [Google Scholar] [CrossRef] [PubMed]
- Lema, K.A.; Willis, B.L.; Bourneb, D.G. Corals Form Characteristic Associations with Symbiotic Nitrogen-Fixing Bacteria. Appl. Environ. Microbiol. 2012, 78, 3136–3144. [Google Scholar] [CrossRef] [PubMed]
- Benavides, M.; Houlbreque, F.; Camps, M.; Lorrain, A.; Grosso, O.; Bonnet, S. Diazotrophs: A Non-Negligible Source of Nitrogen for the Tropical Coral Stylophora Pistillata. J. Exp. Biol. 2016, 219, 2608–2612. [Google Scholar] [CrossRef] [PubMed]
- Meunier, V.; Geissler, L.; Bonnet, S.; Rädecker, N.; Perna, G.; Grosso, O.; Lambert, C.; Rodolfo-Metalpa, R.; Voolstra, C.R.; Houlbrèque, F. Microbes Support Enhanced Nitrogen Requirements of Coral Holobionts in a High CO2 Environment. Mol. Ecol. 2021, 30, 5888–5899. [Google Scholar] [CrossRef]
- Camps, M.; Benavides, M.; Lema, K.A.; Bourne, D.G.; Grosso, O.; Bonnet, S. Released Coral Mucus Does Not Enhance Planktonic N2 Fixation Rates. Aquat. Microb. Ecol. 2016, 77, 51–63. [Google Scholar] [CrossRef]
- Olson, N.D.; Ainsworth, T.D.; Gates, R.D.; Takabayashi, M. Diazotrophic Bacteria Associated with Hawaiian Montipora Corals: Diversity and Abundance in Correlation with Symbiotic Dinoflagellates. J. Exp. Mar. Bio. Ecol. 2009, 371, 140–146. [Google Scholar] [CrossRef]
- Bednarz, V.N.; Grover, R.; Maguer, J.F.; Fine, M.; Ferrier-Pagès, C. The Assimilation of Diazotroph-Derived Nitrogen by Scleractinian Corals Depends on Their Metabolic Status. MBio 2017, 8, e02058. [Google Scholar] [CrossRef]
- Ceh, J.; Kilburn, M.R.; Cliff, J.B.; Raina, J.B.; Van Keulen, M.; Bourne, D.G. Nutrient Cycling in Early Coral Life Stages: Pocillopora Damicornis Larvae Provide Their Algal Symbiont (Symbiodinium) with Nitrogen Acquired from Bacterial Associates. Ecol. Evol. 2013, 3, 2393–2400. [Google Scholar] [CrossRef]
- Pogoreutz, C.; Rädecker, N.; Cárdenas, A.; Gärdes, A.; Voolstra, C.R.; Wild, C. Sugar Enrichment Provides Evidence for a Role of Nitrogen Fixation in Coral Bleaching. Glob. Chang. Biol. 2017, 23, 3838–3848. [Google Scholar] [CrossRef]
- Xiang, N.; Meyer, A.; Pogoreutz, C.; Rädecker, N.; Voolstra, C.R.; Wild, C.; Gärdes, A. Excess Labile Carbon Promotes Diazotroph Abundance in Heat-Stressed Octocorals. R. Soc. Open Sci. 2023, 10, 221268. [Google Scholar] [CrossRef] [PubMed]
- Bednarz, V.N.; van de Water, J.A.J.M.; Rabouille, S.; Maguer, J.F.; Grover, R.; Ferrier-Pagès, C. Diazotrophic Community and Associated Dinitrogen Fixation within the Temperate Coral Oculina Patagonica. Environ. Microbiol. 2019, 21, 480–495. [Google Scholar] [CrossRef] [PubMed]
- Cardini, U.; van Hoytema, N.; Bednarz, V.N.; Rix, L.; Foster, R.A.; Al-Rshaidat, M.M.D.; Wild, C. Microbial Dinitrogen Fixation in Coral Holobionts Exposed to Thermal Stress and Bleaching. Environ. Microbiol. 2016, 18, 2620–2633. [Google Scholar] [CrossRef]
- Grimm, M.; Grube, M.; Schiefelbein, U.; Zühlke, D.; Bernhardt, J.; Riedel, K. The Lichens’ Microbiota, Still a Mystery? Front. Microbiol. 2021, 12, 623839. [Google Scholar] [CrossRef] [PubMed]
- Almer, J.; Resl, P.; Gudmundsson, H.; Warshan, D.; Andrésson, Ó.S.; Werth, S. Symbiont-Specific Responses to Environmental Cues in a Threesome Lichen Symbiosis. Mol. Ecol. 2023, 32, 1045–1061. [Google Scholar] [CrossRef] [PubMed]
- Jung, P.; Brust, K.; Schultz, M.; Büdel, B.; Donner, A.; Lakatos, M. Opening the Gap: Rare Lichens With Rare Cyanobionts – Unexpected Cyanobiont Diversity in Cyanobacterial Lichens of the Order Lichinales. Front. Microbiol. 2021, 12, 728378. [Google Scholar] [CrossRef] [PubMed]
- Lindgren, H.; Moncada, B.; Lücking, R.; Magain, N.; Simon, A.; Goffinet, B.; Sérusiaux, E.; Nelsen, M.P.; Mercado-Díaz, J.A.; Widhelm, T.J.; et al. Cophylogenetic Patterns in Algal Symbionts Correlate with Repeated Symbiont Switches during Diversification and Geographic Expansion of Lichen-Forming Fungi in the Genus Sticta (Ascomycota, Peltigeraceae). Mol. Phylogenet. Evol. 2020, 150, 106860. [Google Scholar] [CrossRef]
- Sigurbjörnsdóttir, M.A.; Andrésson, Ó.S.; Vilhelmsson, O. Nutrient Scavenging Activity and Antagonistic Factors of Non-Photobiont Lichen-Associated Bacteria: A Review. World J. Microbiol. Biotechnol. 2016, 32, 68. [Google Scholar] [CrossRef]
- Muggia, L.; Fernández-Brime, S.; Grube, M.; Wedin, M. Schizoxylon as an Experimental Model for Studying Interkingdom Symbiosis. FEMS Microbiol. Ecol. 2016, 92, fiw165. [Google Scholar] [CrossRef]
- Friedl, T.; Büdel, B. Photobionts. In Lichen biology; Nash, T.H., Ed.; Cambridge University Press, Cambridge, UK, 2008; pp. 9–26.
- Lawrey, J.D. Biological Role of Lichen Substances. Source Bryol., Summer 1986, 89, 111–122. [Google Scholar] [CrossRef]
- Millbank, J.W.; Kershaw, K.A. Nitrogen Metabolism in Lichens: I. Nitrogen Fixation in the Cephalodia of Peltigera Aphthosa. New Phytol. 1969, 68, 721–729. [Google Scholar] [CrossRef]
- Hyvärinen, M.; Härdling, R.; Tuomi, J. Cyanobacterial Lichen Symbiosis: The Fungal Partner as an Optimal Harvester. Oikos 2002, 98, 498–504. [Google Scholar] [CrossRef]
- Cornejo, C.; Scheidegger, C. New Morphological Aspects of Cephalodium Formation in the Lichen Lobaria Pulmonaria (Lecanorales, Ascomycota). Lichenologist 2013, 45, 77–87. [Google Scholar] [CrossRef]
- Finger-Higgens, R.; Duniway, M.C.; Fick, S.; Geiger, E.L.; Hoover, D.L.; Pfennigwerth, A.A.; Van Scoyoc, M.W.; Belnap, J. Decline in Biological Soil Crust N-Fixing Lichens Linked to Increasing Summertime Temperatures. PNAS 2022, 119, e2120975119. [Google Scholar] [CrossRef] [PubMed]
- Raggio, J.; Green, T.G.A.; Crittenden, P.D.; Pintado, A.; Vivas, M.; Pérez-Ortega, S.; De Los Ríos, A.; Sancho, L.G. Comparative Ecophysiology of Three Placopsis Species, Pioneer Lichens in Recently Exposed Chilean Glacial Forelands. Symbiosis 2012, 56, 55–66. [Google Scholar] [CrossRef]
- De los Ríos, A.; Raggio, J.; Pérez-Ortega, S.; Vivas, M.; Pintado, A.; Green, T.G.A.; Ascaso, C.; Sancho, L.G. Anatomical, Morphological and Ecophysiological Strategies in Placopsis Pycnotheca (Lichenized Fungi, Ascomycota) Allowing Rapid Colonization of Recently Deglaciated Soils. Flora 2011, 206, 857–864. [Google Scholar] [CrossRef]
- Rai, A.N.; Bergman, B. Cyanolichens. Proc. R. Irish Acad. 2002, 102, 19–22. [Google Scholar] [CrossRef]
- Palmqvist, K.; Dahlman, L.; Valladares, F.; Tehler, A.; Sancho, L.G.; Mattsson, J.E. CO2 Exchange and Thallus Nitrogen across 75 Contrasting Lichen Associations from Different Climate Zones. Oecologia 2002, 133, 295–306. [Google Scholar] [CrossRef]
- Schneider, K.; Resl, P.; Spribille, T. Escape from the Cryptic Species Trap: Lichen Evolution on Both Sides of a Cyanobacterial Acquisition Event. Mol. Ecol. 2016, 25, 3453–3468. [Google Scholar] [CrossRef]
- Grube, M.; Berg, G. Microbial Consortia of Bacteria and Fungi with Focus on the Lichen Symbiosis. Fungal Biol. Rev. 2009, 23, 72–85. [Google Scholar] [CrossRef]
- Almendras, K.; García, J.; Carú, M.; Orlando, J. Nitrogen-Fixing Bacteria Associated with Peltigera Cyanolichens and Cladonia Chlorolichens. Molecules 2018, 23, 3077. [Google Scholar] [CrossRef] [PubMed]
- Swamy, C.T.; Gayathri, D.; Devaraja, T.N.; Bandekar, M.; D’Souza, S.E.; Meena, R.M.; Ramaiah, N. Plant Growth Promoting Potential and Phylogenetic Characteristics of a Lichenized Nitrogen Fixing Bacterium, Enterobacter Cloacae. J. Basic Microbiol. 2016, 56, 1369–1379. [Google Scholar] [CrossRef] [PubMed]
- Eymann, C.; Lassek, C.; Wegner, U.; Bernhardt, J.; Fritsch, O.A.; Fuchs, S.; Otto, A.; Albrecht, D.; Schiefelbein, U.; Cernava, T.; et al. Symbiotic Interplay of Fungi, Algae, and Bacteria within the Lung Lichen Lobaria Pulmonaria L. Hoffm. as Assessed by State-of-the-Art Metaproteomics. J. Proteome Res. 2017, 16, 2160–2173. [Google Scholar] [CrossRef] [PubMed]
- Shukla, V.; Kumari, R.; Patel, D.K.; Upreti, D.K. Characterization of the Diversity of Mycosporine-like Amino Acids in Lichens from High Altitude Region of Himalaya. Amino Acids 2016, 48, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Sivaramakrishnan, R.; Suresh, S.; Kanwal, S.; Ramadoss, G.; Ramprakash, B.; Incharoensakdi, A. Microalgal Biorefinery Concepts’ Developments for Biofuel and Bioproducts: Current Perspective and Bottlenecks. Int. J. Mol. Sci. 2022, 23, 2623. [Google Scholar] [CrossRef] [PubMed]
- Amaro, H.M.; Salgado, E.M.; Nunes, O.C.; Pires, J.C.M.; Esteves, A.F. Microalgae Systems - Environmental Agents for Wastewater Treatment and Further Potential Biomass Valorisation. J. Environ. Manage. 2023, 337, 117678. [Google Scholar] [CrossRef] [PubMed]
- Vuppaladadiyam, A.K.; Yao, J.G.; Florin, N.; George, A.; Wang, X.; Labeeuw, L.; Jiang, Y.; Davis, R.W.; Abbas, A.; Ralph, P.; et al. Impact of Flue Gas Compounds on Microalgae and Mechanisms for Carbon Assimilation and Utilization. ChemSusChem 2018, 11, 334–355. [Google Scholar] [CrossRef]
- Xu, P.; Li, J.; Qian, J.; Wang, B.; Liu, J.; Xu, R.; Chen, P.; Zhou, W. Recent Advances in CO2 Fixation by Microalgae and Its Potential Contribution to Carbon Neutrality. Chemosphere 2023, 319, 137987. [Google Scholar] [CrossRef]
- Zhang, B.; Li, W.; Guo, Y.; Zhang, Z.; Shi, W.; Cui, F.; Lens, P.N.L.; Tay, J.H. Microalgal-Bacterial Consortia: From Interspecies Interactions to Biotechnological Applications. Renew. Sustain. Energy Rev. 2020, 118, 109563. [Google Scholar] [CrossRef]
- Ramanan, R.; Kim, B.-H.; Cho, D.-H.; Oh, H.-M.; Kim, H.-S. Algae-Bacteria Interactions: Evolution, Ecology and Emerging Applications. Biotechnol. Adv. 2016, 34, 14–29. [Google Scholar] [CrossRef]
- Zhou, Z.; Li, Q.; Song, K.; Wang, R.; Wen, S.; Zhang, D.; Cong, W. Exploration of Applying Growth-Promotion Bacteria of Chlorella Sorokiniana to Open Cultivation Systems. Bioprocess Biosyst. Eng. 2021, 44, 1567–1576. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z.; Xiao, J.; Ye, H.; Fu, G.; Li, G.; Wu, W.; Li, F. Determination of Nitrogen Sources and Losses in Surface Runoff from Different Lands at a Watershed Scale. Environ. Sci. Pollut. Res. 2023, 23, 26459. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Cheng, X.; Wang, Q. Enhanced Lipid Production in Chlamydomonas Reinhardtii by Co-Culturing With Azotobacter Chroococcum. Front. Plant Sci. 2018, 9, 741. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.J.; Xiao, L.I.; Wang, H.N.; Yin, Y.H.; Jun, X.L.; Sheng, G.B. Enhanced Biomass Production and Lipid Accumulation by Co-Cul-Tivation of Chlorella Vulgaris with Azotobacter Mesorhizobium Sp. Chin Biotech 2019, 39, 56–64. [Google Scholar]
- Silaban, A.; Bai, R.; Gutierrez-Wing, M.T.; Negulescu, I.I.; Rusch, K.A. Effect of Organic Carbon, C: N Ratio and Light on the Growth and Lipid Productivity of Microalgae/Cyanobacteria Coculture. Eng. Life Sci. 2014, 14, 47–56. [Google Scholar] [CrossRef]
- Kim, M.S.; Baek, J.S.; Yun, Y.S.; Jun Sim, S.; Park, S.; Kim, S.C. Hydrogen Production from Chlamydomonas Reinhardtii Biomass Using a Two-Step Conversion Process: Anaerobic Conversion and Photosynthetic Fermentation. Int. J. Hydrogen Energy 2006, 31, 812–816. [Google Scholar] [CrossRef]
- Melis, A.; Melnicki, M.R. Integrated Biological Hydrogen Production. Int. J. Hydrogen Energy 2006, 31, 1563–1573. [Google Scholar] [CrossRef]
- Fakhimi, N.; Gonzalez-Ballester, D.; Fernández, E.; Galván, A.; Dubini, A. Algae-Bacteria Consortia as a Strategy to Enhance H2 Production. Cells 2020, 9, 1353. [Google Scholar] [CrossRef]
- Oruganti, R.K.; Katam, K.; Show, P.L.; Gadhamshetty, V.; Upadhyayula, V.K.K.; Bhattacharyya, D. A Comprehensive Review on the Use of Algal-Bacterial Systems for Wastewater Treatment with Emphasis on Nutrient and Micropollutant Removal. Bioengineered 2022, 13, 10412–10453. [Google Scholar] [CrossRef]
- Dong, H.; Liu, W.; Zhang, H.; Wang, Z.; Feng, F.; Zhou, L.; Duan, H.; Xu, T.; Li, X.; Ma, J. Enhanced Biomass Production and Wastewater Treatment in Attached Co-Culture of Chlorella Pyrenoidosa with Nitrogen-Fixing Bacteria Azotobacter Beijerinckii. Bioprocess Biosyst. Eng. 2023, 46, 707–716. [Google Scholar] [CrossRef]
- Osorio-Reyes, J.G.; Valenzuela-Amaro, H.M.; Pizaña-Aranda, J.J.P.; Ramírez-Gamboa, D.; Meléndez-Sánchez, E.R.; López-Arellanes, M.E.; Castañeda-Antonio, M.D.; Coronado-Apodaca, K.G.; Gomes Araújo, R.; Sosa-Hernández, J.E.; et al. Microalgae-Based Biotechnology as Alternative Biofertilizers for Soil Enhancement and Carbon Footprint Reduction: Advantages and Implications. Mar. Drugs 2023, 21, 93. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Hu, B.; Zheng, C.; Deng, Z.; Duan, J.; Qin, K.; Cui, X.; Li, S. Application of Anabaena Azotica- And Chlorella Pyrenoidosa-Based Algal Biotechnology in Green Production of Algae-Rich Crataegi Fructus. J. Healthc. Eng. 2022, 2022, 424890. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).




