Preprint
Article
Study of the Symptoms’ Severity and Adherence to Therapy of Myelofibrosis Patients Treated With Ruxolitinib
This version is not peer-reviewed.
Submitted:
19 May 2023
Posted:
22 May 2023
You are already at the latest version
A peer-reviewed article of this preprint also exists.
Abstract
We aimed to explore symptoms severity and adherence to therapy for patients with myelofibrosis treated with ruxolitinib in Bulgaria.
It is a prospective, non-interventional study performed at the Specialized hospital for active treatment of hematological diseases in Sofia during 2022 - 2023. Date of diagnosis, demographic characteristics, clinical indicators, ruxolitinib dose, and other data points were collected. Clinical indicators were assessed at baseline, in the middle and at the end of observation. Severity of symptoms was measured with MPN-SAF TSS and adherence to therapy with the Morisky 4 questionnaire 6 times during the observation.
The mean age of diagnosis was 58.5 years, with the average duration of disease of 3 years. Pa-tients’ laboratory results were within physiological ranges, with spleen size experiencing a con-stant decrease. The average value for the severity of the symptoms per MPN-SAF TSS results decreased significantly, indicating better disease control. The average adherence to therapy did not change and remained high at around 9 points, except for one patient.
In conclusion the treatment of myelofibrosis patients with ruxolitinib decreased symptoms se-verity and spleen size. Patients were adherent to the therapy over the observed period but as treatment duration increases the risk of adherence decreasing.
Keywords:
Subject:
Medicine and Pharmacology - Hematology1. Introduction
Rare diseases are an object of intensive studying, especially after the advancement of their therapy with the creation of many new targeted therapies, but little is known about the patients’ adherence to therapy with new molecules. Myelofibrosis (MF) is a rare, hematologic malignancy that is pathologically characterized by bone marrow fibrosis, extramedullary hematopoiesis, and an overactive Janus kinase inhibitors signal transducer and activator of transcription proteins (JAK-STAT) pathway. Clinically, the disease is characterized by splenomegaly, cytopenias, and constitutional symptoms, including fever, night sweats, and weight loss [1]. These constitutional symptoms can be debilitating, compromise quality of life in MF patients, and reduce adherence to therapy [2].
Current pharmacotherapy of myelofibrosis in adults, worldwide, is based on two medicines, authorised for use by the Food and Drug Administration (FDA) and the European Medicines Agency (EMA), and these are Janus kinase inhibitors (JAKi) ruxolitinib and fedratinib [3,4,5,6], with recent studies indicating an increase in the median survival of patients in the last decade from 48 to 63 months [7].
Nonadherence to therapy is recognized as one of the most important and costly problems for global health in the 21st century. According to a European Commission report, non-adherence to therapy is responsible for 194,500 deaths and costs the EU an estimated €125 billion annually. As such, measuring adherence can be extremely useful to better understand patient behavior and outcomes [8]. Adherence to therapy of myelofibrosis patients in real life practice in not studies till now. Most studies measure the adherence in clinical trials but in real life patients might suffer many different obstacles to follow the recommendations. Therefore, studying the adherence to therapy of patients in real life is important to gain information for the probably risk of deterioration in the patient’s status.
MF treatment guidelines recommend assessment of patients’ symptom severity at clinic visits. The Myeloproliferative Neoplasm Symptom Score from the Total Symptom Score (MPN-SAF TSS) is a standardized system that rates 10 specific symptoms associated with MPN on a scale of 1-10. A study that aims to provide up-to-date guidelines, practice recommendations and highlight barriers to adherence in the long-term management of chronic myeloproliferative neoplasms (MPN) examines current non-curative drug therapy for MF, that prolongs survival [9]. Identifying potentially modifiable barriers to treatment adherence allows timely interventions to be implemented and improves overall adherence. Authors consider that the long duration and disease course may contribute to the high risk of nonadherence in this group of patients. Poor adherence to long-term therapies seriously compromises treatment effectiveness.
In this study we aimed to explore symptoms severity and adherence to therapy for patients with myelofibrosis treated with ruxolitinib in an ambulatory department of tertiary care in Bulgaria.
The study explores the demographic characteristics of the observed patients in its first part, then the changes in the MPN SAF score during the therapy in the second part and at the end the changes in adherence to therapy during the observed period. Statistical correlation between the observed indicators was thoroughly evaluated to answer the question whether there are any statistical dependencies between the observed variables.
2. Materials and Methods
2.1. Design of the study
It is a prospective, non-interventional study performed at the Specialized hospital for active treatment of hematological diseases (SHATHD) in Sofia, Bulgaria during the period January 2022 - February 2023. The SHATHD is the largest center in the country, currently treating more than one third of all patients with MF in Bulgaria.
Out of 67 patients treated with ruxolitinib in the hospital 21 were randomly selected and agreed to participate in the study. The patients were consecutively included in the follow-up by the attending physician, and the data on the demographic characteristics, clinical indicators, severity of symptoms, and adherence to therapy were collected and systematized. Inclusion criteria were MF, regular visits, and treatment in the SHATHD, on ruxolitinib, willing to participate in the observation. Exclusion criteria were non-MF patients, or unwillingness to participate.
The following characteristics were collected in the beginning of observation: demographics, age at diagnosis, age at start of treatment with ruxolitinib, gender, type of myelofibrosis (primary myelofibrosis (PMF), post-polycythemia vera MF (post-PV MF, post-essential thrombocythemia MF (post-ET MF), DIPSS-calculated risk score (Low, intermediate -1, intermediate-2, high risk), JAK2 mutation status (positive homozygous, positive heterozygous, or negative), and other mutations were collected.
From the clinical indicators, ruxolitinib dose, leukocytes, hemoglobin, platelets, lactate dehydrogenase (LDH), spleen size were tracked. Clinical indicators were assessed at baseline, in the middle and at the end of observation. The clinical tests were performed at the hospital after collecting blood samples. Collection of blood samples is a routine practice during the follow up examination of MF patients and is included in the treatment package. Spleen size is measured via Ultrasound and CT scan.
Severity of symptoms was measured with the Myeloproliferative Neoplasm Symptom Assessment Form Total Symptom Score (MPN-SAF TSS). The MPN-SAF TSS questions focus on fatigue, concentration problems, early satiety, inactivity, night sweats, itching, bone pain, abdominal discomfort, weight loss, and fever. The decrease in the values pointing out for the improvement of the symptoms.
Adherence to therapy was measured with the Morisky 4 scale questionnaire, which assesses adherence on a scale of 0-10 points, with 10 points being complete adherence to therapy, and below 8 points non adherence.
Severity of symptoms, and adherence to therapy were monitored 6 times during the observation period, with patient surveys being conducted in the ambulatory department by the attending physician. Patients answer the questions in front of the physician and if any questions appear they were solved on time. The measurement of the severity of symptoms with the Myeloproliferative Neoplasm Symptom Assessment Form Total Symptom Score (MPN-SAF TSS is a routine practice in the clinic but the measurement of adherence to therapy was performed for the first time.
The Ethical committee of the hospital approved the study.
2.2. Statistical analysis
Descriptive statistic, Mann-Freidman test, Pearson correlation analysis were performed with Medcalc ver. 13.
3. Results
3.1. Demographics, Severity of symptoms, and adherence to therapy
Table 1. presents the demographic data for the studied patients after initiation of ruxolitinib therapy.
They were proportionally distributed by gender, with a mean age at diagnosis of 58.5 years, which is typical of adult-onset diseases. The duration of the disease is an average of 3 years, but there is a patient with an 8-year therapy. All observed patients during the follow-up period remained alive.
Table 2 presents the changes in clinical indicators during follow up period. Despite inter-patient variation, the blood results remain within the physiological range of their values. The most important indicator for disease control - spleen size showed a tendency to decrease, indicating the effectiveness of therapy. The observed increases in leukocytes, hemoglobin and platelet levels may be explained both by a change in the disease and by the treatment being carried out.
The average value for the severity of the symptoms, measured with MPN-SAF TSS, decreased significantly over the follow-up period, indicating that patients experienced better disease control (Table 3). The average adherence to therapy did not change and remained high throughout, near the maximum value of 10 points. Morisky test response values above 8 are considered to be good adherence, which also corresponds to the behavior of patients with myelofibrosis (Table 3).
At the individual level, values of MPN-SAF-TSS decreased in general (Figure 1). Of all 19 patients for whom all six measurements were available, 12 reported lower indicator values, with the remaining reports still showing a general trend toward decreasing, despite variation.
Inter-patient variability of adherence, as measured by Morisky test is shown on Figure 2. In only one of the patients, a lower adherence was recorded in two of the measurements (third and fifth). We checked the individual patient data for 14th patient and found out that it is a female at the age of 62, with primary MF, homozygotes for JAK2 V617F mutation, and with 8 years length of the disease. She was having a decrease in the spleen size from 18 to 16 mm and clinical indicators within the physiological range. We can assume that the long-lasting therapy is influencing the adherence.
3.2. Statistical analysis
Males in the study are 52.4% and females 47.6% with no statistical difference between their number. Mann-Whitney test shows that there is no statistically significant difference between the average age of both gender groups (p=0,2197), as well as in distribution of patients according to their JAK status (р=0.5174). In contrast was found that there is a difference between the length of the disease in the male and female groups (p=0.0241). The length of disease in male group is 2.7 years longer than female. This could be explained with the fact that the myelofibrosis in general is more common for males, and they probably were diagnosed earlier.
We further explore the changes in the clinical indicators during the observed period. A significant reduction was found in the medians of the leukocytes between the three measurements from 12 to 8 g/l. the reduction between the first and last measurement is statistically significant with p<0.05.
The median values of hemoglobin are also decreasing from 118.5 to 109.5 g/l in a statistically significant manner between the first and last measurements. We also observed the atrong and positive correlation between thе hemoglobin level during second and third measurement (r= 0.952; p=0.003). The correlation allows physicians to predict the changes in the level of hemoglobin for the next period.
Thrombocytes average median level is decreasing from 355 to 281g/l non significantly (p>0.05), bet it is correlated strongly and positively between second and third measurement (r=0.881; p=0.0039) that might have prognostic value for physicians.
Similar are the values of lactate dehydrogenase (LDH) which median values are decreasing from 506.5 to 404 U/l. It was also found a strong and statistically significant correlation between median values changes (r=0,905; p=0,0020).
All clinical indicators remain within their reference intervals.
The median of spleen size statistically significant is decreasing from 23 to 29 cm (p<0.0001). Spleen size is one of the very important prognostic indicators for the progress of the therapy. The fact that it is decreasing is a sign of the therapy success.
The statistical relationships between demographic and clinical characteristics of patients, as well as adherence to therapy and severity of symptoms were further investigated.
Measurement of severity of symptoms with MPN-SAF TSS showed a statistically significant difference in the reduction of mean values /p=0.02216/. Patients reported a steady reduction in values for the whole period of observation. This reduction is clinically relevant and statistically significant, as MPN-SAF TSS decreases indicate an improvement in clinical condition.
There is no statistically significant difference in the mean adherence to therapy measured with Morisky /p=0.7151/. Throughout all 6 inquiries, patient-reported mean values remained steady, with nonsignificant changes. We observed minimally improving adherence throughout the first 5 observations, with the value decreasing minimally as well in the 6th observation of follow-up. A decrease in mean values is indicative of a decrease in adherence and is a negative result. As in other studies, with long-lasting therapy, adherence declines, which we were unfortunately unable to follow longer in our cohort.
Factors that have been studied and that may influence adherence and severity of symptoms are gender, JAK status, disease duration, age.
No statistically significant correlation was found between the Morisky scale, MFN-SAF TSS values and gender (p>0.05). Therefore, gender did not influence adherence to therapy, and severity of symptoms (Table 4). JAK status also did not affect adherence, and severity of symptoms.
Disease duration correlated with severity of symptoms in the third observation (r=0.555), with adherence to therapy and severity of symptoms in the fourth observation (r=-0.397 and r=0.527, respectively) – Table 5. The negative correlation between disease duration and adherence indicates that as treatment progresses, adherence decreases.
The age of the patients at the beginning of the follow-up did not affect the studied indicators, but on the sixth observation a negative and statistically significant correlation appeared between age and adherence to therapy (r=0.605, p<0.05). We can argue that older patients have a worse adherence to therapy.
4. Discussion
In this pilot study we explored the changes in the clinical indicators, severity of symptoms and adherence to therapy in a cohort of 21 patients with a rare disease such as myelofibrosis, treated with ruxolitinib at the ambulatory department of tertiary care. To the best of our knowledge, there is no such study performed in the country. Patients are diagnosed in SHATHD or they are diagnosed in other hospitals and then referred to SHATHD for treatment. Ruxolitinib is the only specific treatment option for patients with MF in Bulgaria. A hematology committee takes the decision if the patient is eligible to be treated with ruxolitinib. When the patient starts treatment, he needs to visit the outpatient department every month. He has regular blood testing and, if necessary, the attending physician can adjust drug dose at his discretion. Every six months the response is evaluated. We use MPN-SAF TSS and spleen size measurement for the evaluation. Ultrasound and CT scan are the methods for spleen size evaluation. The treatment continues until disease progression or intolerance to treatment. If treatment change is indicated, it is discussed with the committee.
Fedratinib is not available on the national market and is not included in the study. The cohort of 21 is representative for all 67 MF patients treated in the hospital. In our study we found that ruxolitinib improves clinical presentation of the patients and decreases spleen size. In addition, the changes in the severity of the disease score, evaluated with MPN-SAF, were statistically significant. We also found that older patients have a worse adherence to therapy, as well as patients on long-standing therapy.
The Morisky Medication Adherence Scale is a widely used questionnaire to indirectly assess patient adherence to medication therapy [10,11,12]. ROMEI is a prospective observational study focused on the treatment of MF patients in real-world settings with ruxolitinib. The study enrolled 215 patients and included a prospective assessment of the medication adherence as a secondary endpoint. Of the 215 patients enrolled, 188 were assessed for medication adherence. It appears that patients with high adherence in week 4 were more likely to maintain the same level over time. No correlation was found between the status of the spleen and the level of adherence demonstrated (data available at the meeting). This analysis shows that a percentage of patients (ranging from 25% to 40%) belong to low-moderate levels of adherence during treatment. Surprisingly, overall, approximately one-third of patients may be exposed to suboptimal treatment over time, and clinicians likely underestimate the number of these patients in clinical practice.
The continuation of the same study suggests that one-third of patients receiving ruxolitinib may be undertreated due to nonadherence, potentially undermining disease control, and indicate a need for better interventions targeting nonadherence to oral therapies [13]. Although no correlation was found between the level of adherence and the response to splenic treatment, probably due to the small number of patients evaluated for both variables, knowledge about the attitude of patients to therapy, especially for long-term and chronic treatment, remains crucial from clinical and pharmacoeconomic studies. Standardized methods for assessing patient adherence, such as the MMAS-8, should be implemented in prospective well-designed clinical trials and could be a valuable tool to guide clinicians in daily practice. Furthermore, real-world studies of adherence complement secondary endpoints used in clinical trials.
Limitations of our study are in the fact that it includes patients from only one clinic, but we have to note that the total number of MF patients in Bulgaria is appr. 280 and out of them nearly 70 (25%) are treated at SHATHD in Sofia. After the introduction of ruxolitinib in 2016 it started being prescribed to all MF patients with manifested symptoms and this is the first national study focusing on the characteristics of those patients, progress of the diseases, and adherence to therapy. Additionally, the number of patients who agreed to participate.
5. Conclusions
The treatment of myelofibrosis patients with ruxolitinib decreases the symptoms severity and is spleen size. In general patients are adherent to the therapy but with the advancement of the length of therapy there is a risk of adherence decrease.
6. Patents
This section is not mandatory but may be added if there are patents resulting from the work reported in this manuscript.
Author Contributions
Conceptualization, G.P., K.T.; methodology, V.S. and K.T.; software, K.M. and K.T. and N.G.; validation, V.S., G.P. K.M., K.T., formal analysis, V.S. and K.M.; investigation, V.S.; data curation, K.T., K.M.; writing—original draft preparation, V.S. and K.T.; writing—review and editing, G.P., K.T., V.S.; supervision, G.P.; project administration, G.P.; funding acquisition, G.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Bulgarian National Science Fund project 739/25.02.2022, “Evaluation of the compliance and adherence to therapy in case of socially important diseases with high degree of non-adherence” with contract number No. KP-06-KOST/6/14.06.2022.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board (or Ethics Committee) of the SHATHD in Sofia.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author (G.P.), upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Tefferi, A. Primary myelofibrosis: 2019 update on diagnosis, risk-stratification, and management. Am. J. Hematol. 2018, 93, 1551–1560. [Google Scholar] [CrossRef] [PubMed]
- Mesa, R.; Miller, C.B.; Thyne, M.; Mangan, J.; Goldberger, S.; Fazal, S.; Ma, X.; Wilson, W.; Paranagama, D.C.; Dubinski, D.G.; et al. Myeloproliferative neoplasms (MPNs) have a significant impact on patients’ overall health and productivity: The MPN Landmark survey. BMC Cancer 2016, 16, 167. [Google Scholar] [CrossRef] [PubMed]
- United States Food and Drug Administration. JAKAFI (Ruxolitinib) Label; FDA: Silver Spring, MD, USA, 2011.
- EMA. European public assessment report. Available on: https://www.ema.europa.eu/en/medicines/human/EPAR/inrebic (Accessed May 2023).
- EMA. European assessment report. Available on: https://www.ema.europa.eu/en/documents/productinformation/jakavi-epar-product-information_bg.pdf ()Accessed May 2023).
- Wernig, G.; Kharas, M.G.; Okabe, R.; Moore, S.A.; Leeman, D.S.; Cullen, D.E.; Gozo, M.; McDowell, E.P.; Levine, R.L.; Doukas, J.; et al. Efficacy of TG101348, a selective JAK2 inhibitor, in treatment of a murine model of JAK2V617F-induced polycythemia vera. Cancer Cell 2008, 13, 311–320. [Google Scholar] [CrossRef] [PubMed]
- Masarova, L.; Bose, P.; Pemmaraju, N.; et al. Improved survival of patients with myelofibrosis in the last decade: Single-center experience. Cancer. 2022, 128, 1658–1665. [Google Scholar] [CrossRef] [PubMed]
- Guglielmelli, P.; Palandri, F.; Selleri, C.; Cilloni, D.; et al. Adherence to Treatment in Myelofibrosis Patients: Preliminary Results from Italian Romei Observational Study. Blood 2019, 134, 4179. [Google Scholar] [CrossRef]
- Nurgat, Z.; Lawrence, M. Management of Myeloproliferative Neoplasms (MPNs). J Oncol Pharm Pract. 2022, 28, 1400–1410. [Google Scholar] [CrossRef] [PubMed]
- Morisky, D.E.; Ang, A.; Krousel-Wood, M.; Ward, H.J. Predictive validity of a medication adherence measure in an outpatient setting. J Clin Hypertens (Greenwich). 2008, 10, 348–354. [Google Scholar] [CrossRef] [PubMed]
- Krousel-Wood, M.A.; Islam, T.; Webber, L.S.; Re, R.N.; Morisky, D.E.; Muntner, P. New medication adherence scale versus pharmacy fill rates in seniors with hypertension. Am J Manag Care. 2009, 15, 59–66. [Google Scholar] [PubMed]
- Morisky, D.E.; DiMatteo, M.R. Improving the measurement of self-reported medication nonadherence. J Clin Epidemiol. 2011, 64, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Guglielmelli, P.; Palandri, F.; Selleri, C.; Cilloni, D.; Mendicino, F.; et al. ROMEI study group. Adherence to ruxolitinib, an oral JAK1/2 inhibitor, in patients with myelofibrosis: interim analysis from an Italian, prospective cohort study (ROMEI). Leuk Lymphoma. 2022, 63, 189–198. [Google Scholar] [CrossRef] [PubMed]
Figure 1.
Individual values of MPN-SAF TTS.
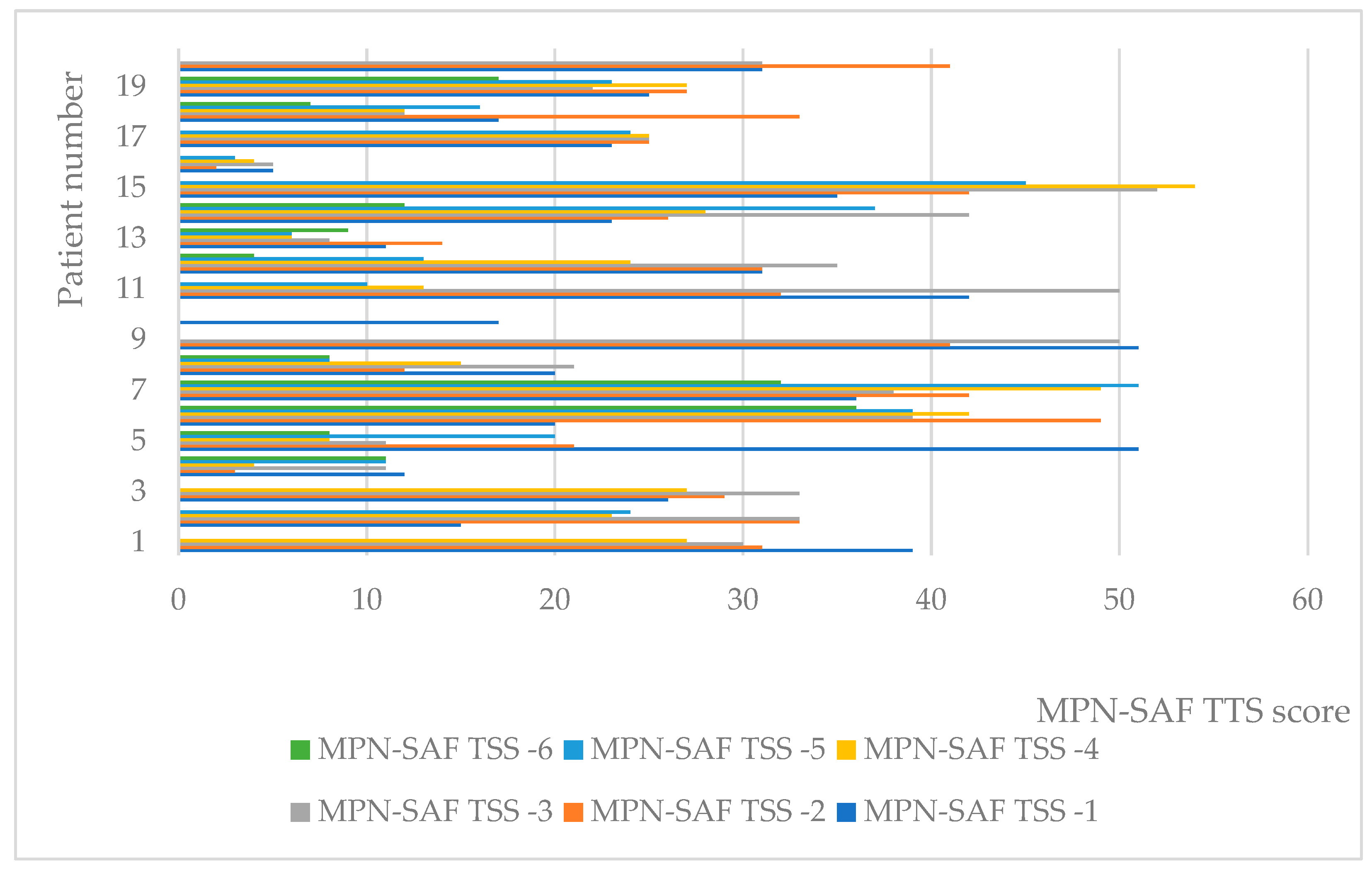
Figure 2.
Adherence to therapy according to Morisky test.
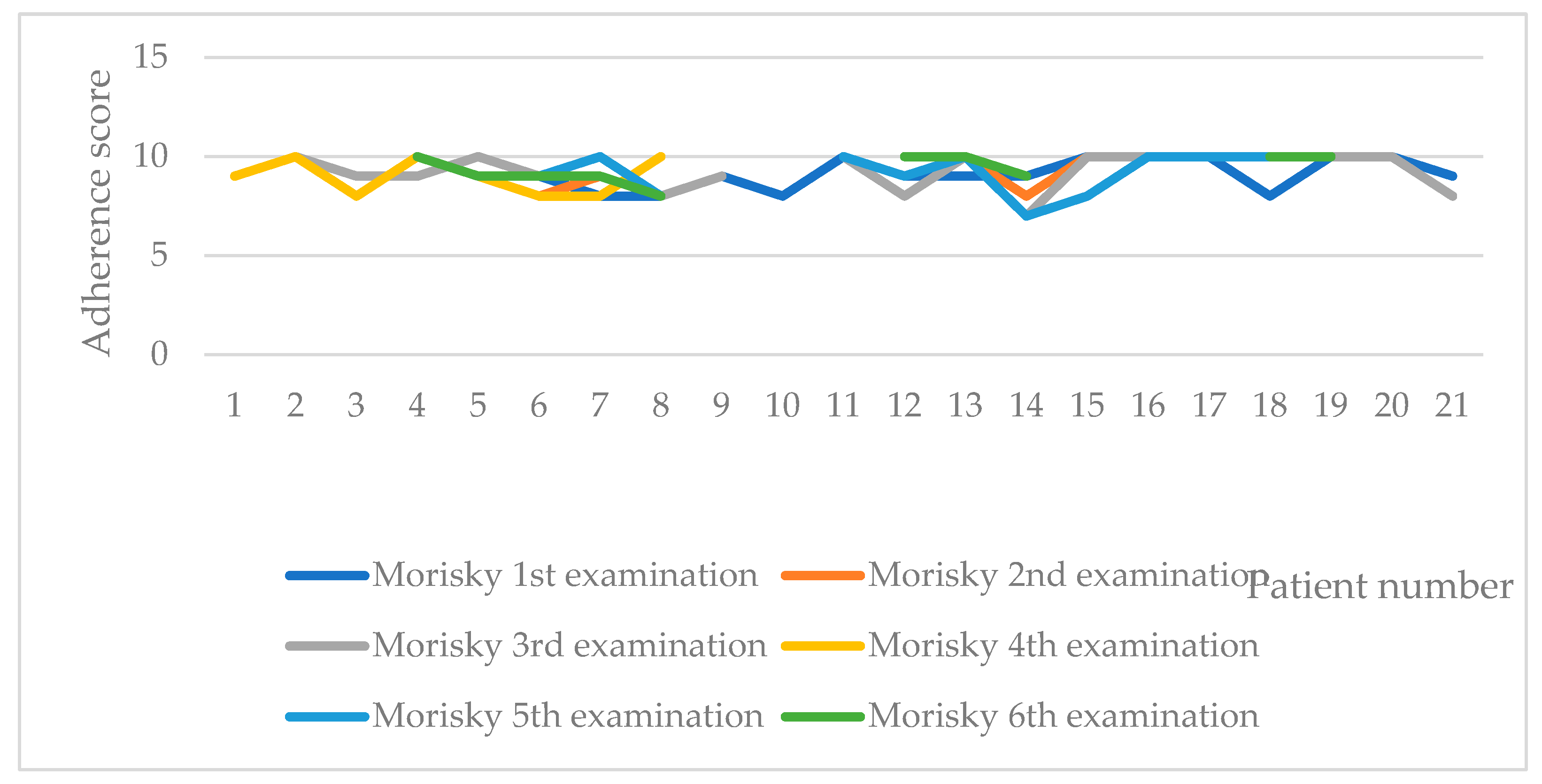
Table 1.
Demographic characteristics of included patients on ruxolitinib therapy.
| Characteristic | N | Average(SD) |
| Total number | 21 | |
| Female | 10 | 48% |
| Male | 11 | 52% |
| Age at the moment of observation | from 39 to 76 years | 61.2 (8.4) |
| Age at diagnosis | from 34 to 70 years | 58.49 (9.13) |
| Time from diagnosis of the disease | from 0.7 to 8 years | 3.16 (1.88) |
| Type of myelofibrosis | ||
| PMF | 10 | 48% |
| Post-PV MF | 9 | 42% |
| Post-ET MF | 2 | 10% |
| Risk group | ||
| Intermediate-1 risk | 5 | 24% |
| Intermediate-2 risk | 5 | 24% |
| Unknown | 2 | 10% |
| High risk | 3 | 14% |
| Low risk | 6 | 28% |
| JAK2 V617F mutation status | ||
| Homozygote mutation | 6 | 28% |
| Heterozygote mutation | 8 | 38% |
| Negative for mutation | 4 | 20% |
| No information | 3 | 14% |
| Ruxolitinib dose | ||
| 5 mg 2 x daily | 3 | 14% |
| 10 mg 2 x daily | 4 | 20% |
| 15 mg 2 x daily | 8 | 38% |
| 20 mg 2 x daily | 3 | 14% |
| No information | 2 | 10% |
Table 2.
Changes in the clinical indicators of the included patients.
| Indicator | First measurement – average (SD) | Second measurement – average (SD) | Third measurement – average (SD) |
|---|---|---|---|
| Leukocytes (G/l) | 14.38 (7.9) | 20.02 (16.16) | 18.57 (3.26) |
| Hemoglobin (g/l) | 106.92 (24.2) | 117.82 (23.44) | 110.38 (17.13) |
| Thrombocytes (G/l) | 328.3 (150.1) | 354.27 (165.26) | 260.75 (93.25) |
| LDH (U/l) | 688.38 (337.67) | 751.9 (547.34) | 626.25 (414.63) |
| Spleen size (cm) | 21.8 (2.88) | 19.5 (3) | 19.33 (2.2) |
Table 3.
Results from the evaluation of the severity symptoms and adherence to therapy.
| Instrument | 1st inquiry Average (SD) |
2nd inquiry Average (SD) |
3rd inquiry Average (SD) |
4th inquiry Average (SD) |
5th inquiry Average (SD) |
6th inquiry Average (SD) |
|---|---|---|---|---|---|---|
| MРN-SAF TSS | 26.7 (11.06) |
28.8 (12.2) |
26.09 (13.9) |
20.4 (12.8) |
18.3 (12.9) |
14.4 (8.36) |
| Morisky | 9.1 (0.7) | 8.8 (1.3) | 9.5 (0.9) | 9.1 (0.8) | 9.3 (0.8) | 9.3 (0.7) |
Table 4.
Pearson Correlation between Morisky, MPN-SAF TSS scale and gender,.
| Variable | GENDER=1 | GENDER=2 | |||
|---|---|---|---|---|---|
| Median | Average Rank | Median | Average Rank | P a | |
| MOR | 9,0000 | 12,0500 | 9,0000 | 10,0455 | 0,4300 |
| MPN_SAF_TSS | 25,5000 | 11,3000 | 18,5000 | 9,7000 | 0,5446 |
Table 5.
Correlation between Morisky, MPN-SAF TSS scale and disease duration.
| Disease duration (DD) | Morisky 3rd observation | MPN_SAF_TSS_3rd observation | |
| DD | Correlation coefficient Significance Level P n |
-0,153 0,5208 20 |
0,555 0,0137 19 |
| Disease duration | Morisky 4th observation | MPN_SAF_TSS_4th observation | |
| DD | Correlation coefficient Significance Level P n |
-0,397 0,1024 18 |
0,527 0,0298 17 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions, or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Copyright: This open access article is published under a Creative Commons CC BY 4.0 license, which permit the free download, distribution, and reuse, provided that the author and preprint are cited in any reuse.
Alerts
MDPI Initiatives
Important Links
© 2025 MDPI (Basel, Switzerland) unless otherwise stated







