1. Introduction
Functional somatic disorders (FSD) are increasingly common in adolescents with current prevalence estimates of 3-10% [
1,
2,
3,
4]. The adolescents present with persistent and impairing physical symptoms in the health care system, and despite distress and high healthcare use [
5,
6,
7], the young patients and their parents are often left without clear explanations, diagnostic label or treatment advice when seeking medical care [
8,
9].
Several studies regarding youth with FSD have highlighted that the lack of tangible explanations for FSD may increase uncertainty in young patients and parents, which in turn causes distress, mistrust in the diagnostic label of FSD and diminished engagement in available treatment options [
8,
10,
11,
12,
13]. A qualitative study on adolescents with FSD and their parents concluded that there was a need for improved communication with clinicians, with extra focus on discussion of results of medical investigations, especially negative findings with lack of well-defined organic origin of symptoms [
13]. In recent papers, the need for thorough assessment as a first step in management of FSD both in adults and youth has been highlighted [
9,
14,
15,
16]. In adult patients with multi-system FSD the experience and outcome of systematic assessment, psychoeducation and follow-up consultation has been evaluated [
17]. The study showed that such a systematic set-up was associated with clinically relevant improvements on symptom severity, illness worry, illness perceptions and behaviors and positive expectations for treatment and future course [
17]. Positive effects of assessment and psychoeducation have also been reported in a study in children [
18,
19].
A potential barrier for provision of clear and evidence based psychoeducation, is the use of different diagnostic labels for FSD. The heterogeneous symptom presentations of FSD, with different primary symptom (e.g., fatigue, abdominal symptoms or musculoskeletal symptoms) often influence the primary point of contact in the health care system (e.g., different sub-specialties in the pediatric setting or child and adolescent psychiatry). This will often influence the diagnostic categories used (e.g., different functional somatic syndromes, somatic symptom disorder or functional neurological disorder). However, a large overlap has been shown between different diagnostic categories [
20,
21]. Based on this, a unifying classification system of FSD has been proposed depending on number of symptoms and involved symptom clusters (i.e., single-symptom, single-system and multi-system) [
22] recognizing the often more severely affected patients with multi-organ symptomatology [
23,
24].
The present study was part of a randomized controlled trial (RCT) testing a group-based intervention (Acceptance and Commitment Therapy for Health in Adolescents (AHEAD)) for adolescents with multi-system FSD [
25,
26]. The objective of this study was to evaluate: 1) how adolescents presenting with multi-organ symptomatology experienced systematic assessment (e.g., diagnostic certainty and outlook on illness course) and 2) whether systematic assessment and manualized psychoeducation would have a positive impact on self-perceived physical health, symptom severity, illness worry, and potential maladaptive illness perceptions and behaviors prior to further specialized treatment according to randomization in the overall trial.
2. Materials and Methods
2.1. Design
Inclusion for the RCT ran from January 2015 to December 2018. Patients were referred from general practitioners, medical specialists or hospital departments to a tertiary care clinic with special knowledge on assessment and treatment of multi-system FSD. Referrals were screened for eligibility and all potentially eligible adolescents were invited for assessment. After assessment patients were randomized to either AHEAD or enhanced usual care (EUC) with a personalized treatment plan for the general practitioner. The study was conducted in accordance with the Declaration of Helsinki and the RCT was approved by The Danish Data Protection Agency (no. 1-16-02-290-14) and the Committee of Health Research Ethics of Central Denmark Region (no. 1-10-72-181-14). Trial registration before commencement: ClinicalTrials.gov NCT02346071.
2.2. Participants
Eligibility criteria for study participation were: Age 15-19 years, fulfilment of multi-system FSD operationalized as multi-organ Bodily Distress Syndrome (BDS) [
21] of at least one year’s duration and clinician rated moderate to severe impairment based on distress and impairment. Exclusion criteria were acute psychiatric disorder requiring other treatment, a life-time diagnosis of psychosis, serious cognitive deficits, developmental disorders, substance abuse or pregnancy. All adolescents gave oral and written informed consent for inclusion before participation.
2.3. Assessment
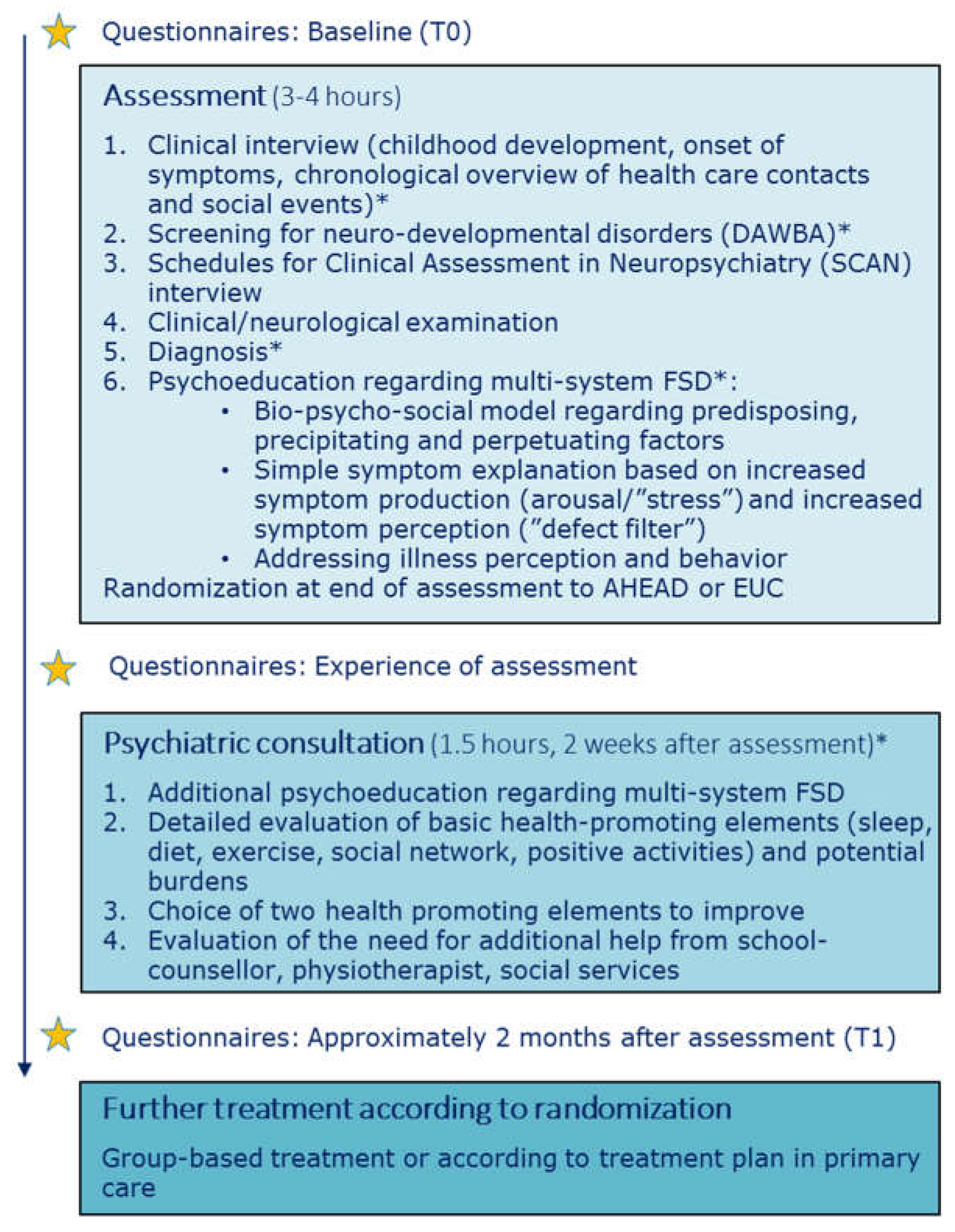
Assessment was regarded as a pivotal part of the intervention in both arms, with five main foci i.e., 1) Systematic assessment of physical symptoms and potential psychiatric comorbidities, 2) Creation of a chronological overview of health care contacts and social events, 3) Clinical/neurological examination, 4) Provision of a clear diagnosis and 5) Psychoeducation regarding FSD. See
Figure 1 for overview of assessment and psychoeducation.
The model for assessment was derived from a model developed and tested in an adult population [
27] and was adapted for adolescents, e.g., with a larger degree of parental involvement and additional focus on assessment of potential underlying neurodevelopmental disorders.
Prior to the assessment, the clinician reviewed all previous medical records, to ensure that the adolescent had been thoroughly medically examined according to symptom presentation, but also to assist the patient and parents in the creation of the chronological overview at the assessment.
2.3.1. Clinical Interview
The adolescent and parents participated in a clinical interview focusing on childhood development from pregnancy/birth till present (see
Figure 1). Both parents were encouraged to participate in the interview.
To facilitate the possibility of linking life stressors (e.g., bullying, accidents, death of close relatives, parental divorce) with the development of physical symptoms a chronological overview of life-time health care contacts and social events was made. The overview was made on a blackboard with previous symptoms, examinations, diagnoses and treatments on the left side of a timeline and important social events (both positive and challenging) on the right side (see Supplementary Material,
Figure S1).
2.3.2. Assessment of Physical Symptoms and Potential Comorbidities
To ensure a systematic assessment of all physical symptoms and potential psychiatric comorbidities, the semi-structured diagnostic interview ‘Schedules for Clinical Assessment in Neuropsychiatry (SCAN)’ was used [
28]. SCAN has a detailed section on functional somatic symptoms and also includes sections for screening and in-depth evaluation of general psychopathology.
In addition, specific sections from the Development and Well-being Assessment (DAWBA) focusing on Attention Deficit Hyperactivity Disorder (ADHD), autism, and conduct disorder were used to screen for specific child psychiatry disorders not covered by the SCAN [
29]. Assessment of potential underlying neurodevelopmental disorders is of importance as these disorders occur with higher prevalence in youth with FSD [
30,
31]. The physicians performing the assessment were trained in child and adolescent psychiatry, psychiatry or community medicine and also certified in conducting the SCAN interview.
2.3.3. Clinical/Neurological Examination
Even though most patients had been extensively examined by their general practitioner or at somatic departments, a clinical/neurological examination was performed. It was prioritized in order to discover and address potential kinesiophobia and positive signs of FSD (e.g., paresthesia not following dermatomes) and to make an evaluation of how the patient handled a physical examination despite having many symptoms. The information from the clinical examination (e.g., potential positive signs of FSD, kinesiophobia) was further used for psychoeducation when addressing illness perception and behavior.
2.3.4. Diagnosis
After finalizing the overall assessment, the clinician evaluated whether the adolescent fulfilled the diagnostic criteria of multisystem FSD conceptualized as multi-organ BDS. If so, the clinician explained in detail about the diagnosis and how this fit with the symptoms experienced by the adolescent. Furthermore, the adolescent and parents were ensured of the negative findings of the physical examination and of previous medical examinations.
2.4. Psychoeducation
Our intention was to provide the adolescents and parents with an evidence-based understanding of FSD and to facilitate a nuanced understanding of their illness. The psychoeducation consisted of 1) A bio-psycho-social model regarding the development and maintenance of bodily distress as the central feature of FSD, 2) A simple bodily focused explanation for symptom production and perception and 3) Perpetuating impact of maladaptive illness perceptions and behaviors.
The generic bio-psycho-social model regarding predisposing, precipitating and perpetuating factors was drawn on a blackboard and specific factors (e.g., important social events) mentioned by the adolescent and/or parents during assessment were incorporated in this model. The adolescent and parents were encouraged to openly express their reflections and understanding in the process in order to facilitate a discussion of their view, potentially shedding light on a purely biomedical understanding of symptom origin or different illness perceptions within the family. This overall discussion allowed for the physician to clarify or address potential misunderstandings e.g., of previous medical results or misconceptions of symptom development and the human body in general.
Next step was a simple symptom explanation based on a model describing the presence of impairing symptoms as a combination of increased symptom production (arousal/’stress’) and increased symptom perception (‘defect filter’). An outline of two persons was drawn on the blackboard, one person with FSD next to a person without FSD and the differences in filter and arousal. Even though the model represented an evident simplification of the complex processes known to cause FSD, it provided a common language in the family but also a clearer understanding of why interventions (e.g., psychological or physiotherapy) may have a positive impact on the physical symptoms by targeting arousal and/or perception.
Lastly, the specific illness related behavior ‘all-or-nothing’ was addressed and the inexpedient strategy of limiting or overdoing things. The adolescents were encouraged to aim for an activity level that was realistic without doing too little or too much. This was explained through an adapted ‘zone of proximal development’ model with three zones i.e., comfort zone, development zone and overload zone. Illness perceptions were indirectly addressed throughout the assessment and psychoeducation e.g., by broadening the perspective on symptom development and giving hope for symptom improvement through treatment.
For a detailed description of the bio-psycho-social model and introduction to common illness related behavior (all-or-nothing) see Additional file 1 in Kallesøe et al. [
32].
Randomization was done at the end of the assessment day. Specific study information regarding the interventions was given prior to consent and randomization. The whole procedure including information and randomization took approximately 15 minutes.
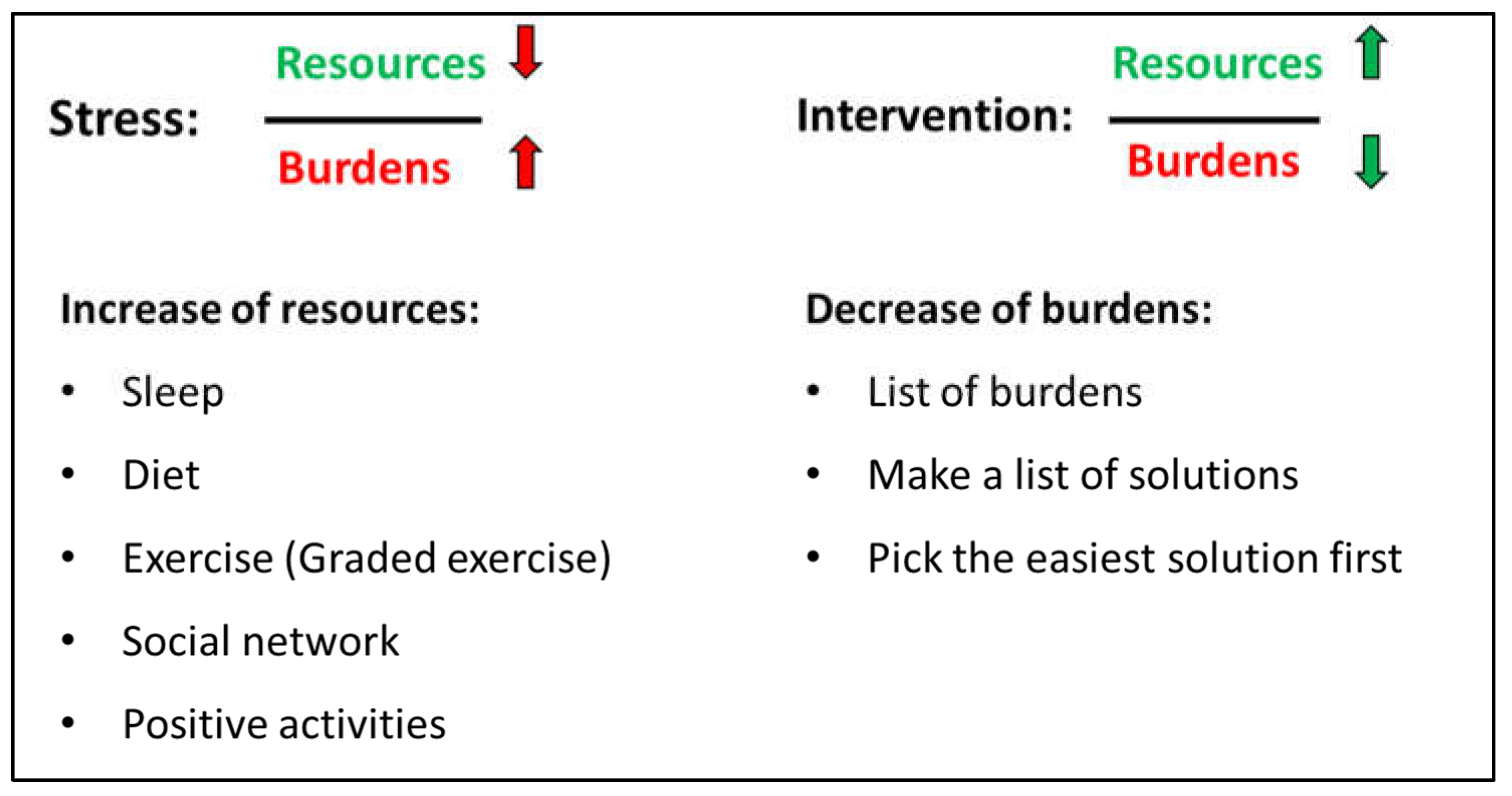
2.5. Psychiatric Consultation
The psychiatric consultation following approx. 2 weeks after the assessment focused on health promoting strategies i.e., sleep, diet, exercise, social network and positive activities. A detailed history of each element was made to identify important elements for improvement. Current burdens were also disclosed. All elements (health promoting strategies and burdens) were integrated elements of the ‘stress-resource fraction’ where stress was described as a low level of resources combined with high level of burdens while intervention for lowering stress was to improve resources and lower burdens (see
Figure 2). After a thorough assessment of resources and burdens, the adolescent had to identify two specific elements to work on e.g., to focus on improvement of sleep and graded exercise.
Parents and/or other close relatives participated in the psychiatric consultation to help uncover resources and burdens but also to support the adolescent in the work-elements they chose.
2.6. Measures
2.6.1. Evaluation of Assessment
Eight questions regarding the adolescents’ impression of the assessment, their certainty regarding their disorder and their expectations for treatment were distributed the day following the assessment via a link in an email. The questions were answered before the follow-up psychiatric consultation.
2.6.2. Other Outcomes
For evaluation of potential impact of systematic assessment, manualized psychoeducation and health promoting strategies the questionnaires chosen for the overall RCT-design were used. The questionnaires were distributed at baseline (before assessment) and approximately two months after assessment prior to engagement in specialized treatment.
Physical health (primary outcome in the RCT) was measured with an aggregate score deriving from the SF-36 subscales PF (physical functioning, 10 items), BP (bodily pain, 2 items) and VT (vitality, 4 items) shown to be sensitive to change in key areas affected in adults with FSD [
27,
33,
34]. Scores range from 15-65 with higher scores indicating better physical health. A change of 4 and above may be regarded as a clinically relevant change and 8 and above as a marked improvement [
35,
36].
Symptom severity was measured with the somatization subscale of the Symptom Checklist Revised (12 items, 5-point scale, score range 0-4) [
37] with higher scores indicating higher symptom severity.
Illness worry was measured by Whiteley-6-R [
38] (6 items, 5-point scale, score range 0-4), a validated modified version of the Whiteley Index. Higher scores indicate more severe symptoms of illness worry.
Illness perception was measured by the Brief Illness Perceptions Questionnaire (BIPQ) [
39] (8 items, 11-point scale, score range 0-80). A higher score reflects a more threatening view of the illness.
Illness-related behavior was measured by two subscales of the Behavioural Responses to Illness Questionnaire (BRIQ) [
40]: (1) all-or-nothing behavior (6 items, 5-point scale, score range 6-30) and (2) limiting behavior (excessive rest) (7 items, 5-point scale, score range 7-35) with higher scores indicating a higher degree of maladaptive illness-related behavior.
Psychological inflexibility was measured by two questionnaires i.e., 1) the Avoidance and Fusion Questionnaire Youth (AFQ-Y8) (8 items, 5-point scale, score range 7-35 [
41] and 2) the Psychological Inflexibility in Pain (PIPS-12) consisting of two subscales: (a) Avoidance (8 items, 7-point scale, score range 8-56) and (b) Cognitive fusion (4 items, score range 4-28) [
42]. Higher scores indicate a higher degree of psychological inflexibility (i.e., as a single construct or by avoidance and cognitive fusion respectively). The concept of psychological flexibility pertains specifically to Acceptance and Commitment Therapy (ACT), and describes the ability to stay in contact with the present moment regardless of unpleasant thoughts, feelings, and bodily sensations, while choosing one’s behaviors based on the situation and personal values [
43].
2.7. Analysis
Descriptive statistics were used to characterize the sample at baseline. Data were summarized as either mean and standard deviation (SD) or as count and percentage, depending on variables.
Patients’ evaluations of the clinical assessment were presented as percentage.
Clinical outcome data were analyzed using paired t-tests from baseline to two months after assessment. In order to investigate whether the randomization had any influence, mixed models with random intercept, and intervention, time, and their interaction as independent variables were estimated for each of the clinical outcomes. Due to randomization, the main effect of intervention was fixed to zero to reduce bias from potentially different baseline values [
44]. Assumptions of normality and homoscedasticity of residuals and random effects were visually inspected using scatter-, QQ-, and box-plots.
Analyses were performed using Stata version 17.0 for Windows.
3. Results
3.1. Patient Characteristics
Ninety-one adolescents were included in the RCT and attended both assessment and the psychiatric consultation. Ninety percent of the included patients were female and had a long mean symptom duration of 4 years. Forty-four percent had a present psychiatric comorbidity (i.e., anxiety and depressive disorders or attention deficit disorder). Parents reported a lifetime history of FSD (35.2%), psychiatric disorders (44.4%) or substance abuse (12.4%). See
Table 1 and RCT article for further details [
26].
3.2. Evaluation of Assessment
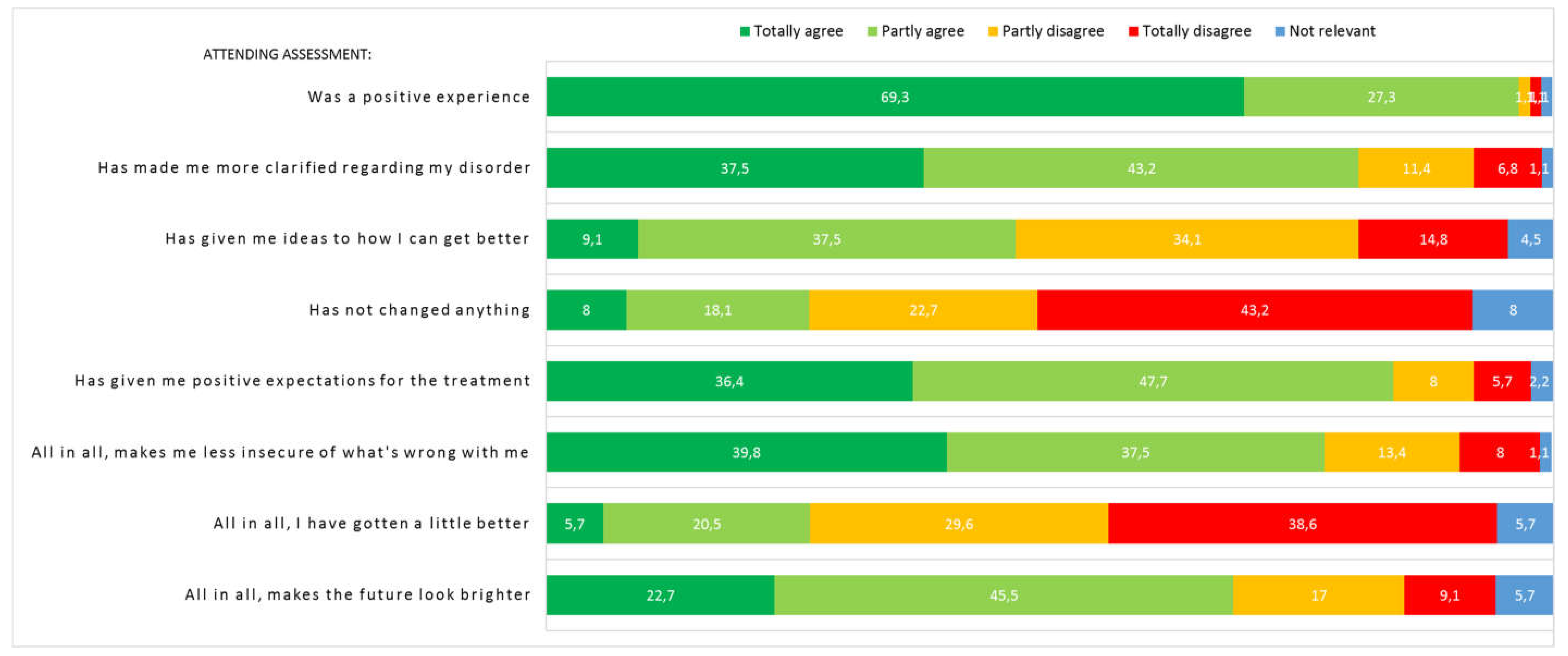
Eighty-eight patients (96.7%) answered the questionnaire regarding experience of assessment. The majority of patients reported that it was a positive experience attending assessment, that they felt less uncertain of what was wrong with them and that it had made them more clarified regarding their disorder (see
Figure 3). One fourth of patients reported that they had improved a bit and almost half of patients that it had given them specific ideas on how to get better. Most patients reported positive expectations for the future treatment and two thirds that it made the future look brighter. Twenty-three patients (26.1%) agreed or partly agreed that assessment had not changed anything, whereof 7 were randomised to AHEAD and 16 to EUC. Experience of assessment divided by randomization groups can be seen in
Supplementary Figure S2.
Time between assessment and evaluation at T1 prior to start of specialized treatment was a median of 62 days, (IQR 28-103). Assessment and psychiatric consultation did not in itself cause a clinically relevant improvement of physical health (see
Table 2). With regard to impact on secondary outcomes and treatment targets, respectively, there was a decline in symptom severity and illness worry as well as in negative illness perception, limiting illness behaviour and psychological inflexibility on one scale (both sub-scales of PIPS-12). At T1, there was no observed differences in improvements between AHEAD and EUC on all outcomes (see interaction effects
Table 2). Mean and SD at T0 and T1 for both randomization groups can be seen in
Table S1.
Table 2 displays the unadjusted mean differences and their 95% confidence intervals from baseline (T0) to right before start of specialized treatment (T1), approx. two months apart, for all patients included in the RCT. A negative value means a decrease in the respective scores. For physical health and mental health, a positive value means an increase in self-reported physical and mental health. Interaction effect and their 95% confidence intervals from the mixed analysis are also displayed, to evaluate whether randomization influenced change.
AFQ-Y8: Avoidance and Fusion Questionnaire Youth; B-IPQ: Illness Perception Questionnaire; BRIQ: Behavioural Response to Illness Questionnaire; CI: Confidence interval; MCS: Mental component summary; PIPS: Psychological Inflexibility in Pain questionnaire; SF-36: The Short Form (36) Health Survey; SCL-som: Symptom Checklist Revised somatization subscale
4. Discussion
The present study embedded in the AHEAD trial has shown that a thorough clinical assessment and psychoeducation was associated with improved diagnostic certainty, positive outlook on future treatment and a general hope for a better future for the young patients. Furthermore, the combination of assessment, psychoeducation and health promoting strategies was associated with an improvement of symptom severity, illness worry, illness perception, limiting behaviour and psychological flexibility two months after assessment.
A higher diagnostic certainty was obtained through systematic assessment, provision of a clear diagnosis (i.e., multi-system FSD) and manualized psychoeducation with inclusion of personal elements from the systematic assessment. The negative consequences of not providing a clear diagnosis has been highlighted in previous pediatric studies. A qualitative study in adolescents with physical symptoms without organic pathology addressed that a lack of diagnosis and medical explanation for the symptoms is difficult to accept for the patients and parents [
13]. Moulin et al. further observed that diagnostic uncertainty fuelled uncertainty of how to handle the symptoms in everyday life but also had emotional consequences where parents sometimes wished for more serious pathology just to have a diagnosis that could alleviate the disbelief from their social circles [
13]. A paper presenting clinical vignettes illustrated that lack of diagnosis and dismissive and misguided manners by health care professionals leave the young patients and parents with an experience of not being taken seriously [
9]. Experiences from adults also underline that medical reassurance is not sufficient when targeting the diagnostic uncertainty [
45]. When young patients and parents are not met with individualised bio-psycho-social explanations taking all life-aspects into account including infections, social life-stressors and psychological aspects, the young patients and parents may find their own justification for the symptoms often focusing on biomedical/physical factors for symptom explanation [
13,
46]. The tendency to seek a biomedical explanation, may stand in the way of relevant treatment options including psychological interventions. Hence, using a bio-psycho-social framework in the assessment of the young patient with FSD in a mindful way to explore potential complex interactions is important [
9,
47].
The vast majority of adolescents in this study reported that attending assessment was a positive experience supporting that the bio-psycho-social model can be successfully implemented balancing all elements of the model including the sharing of psychological factors (e.g., personal sensitive information) without making the adolescents feel dismissed by the physician. This is an important experience, as research has shown a risk of feeling dismissed when the explanation is not approached respectfully [
8,
9,
48]. Furthermore, more than 4 out of 5 adolescents reported that attending assessment gave them positive expectations for future treatment and many also agreed that it made the future look brighter in general. In the adult literature it has been shown that positive treatment expectations are associated with better overall outcome of treatment [
49,
50,
51]. The impact of treatment expectations have not been thoroughly examined in youth but a study in children with chronic pain did show that a higher degree of readiness to self-manage pain was associated with greater improvements from pre- to post-treatment and the use of more adaptive coping strategies [
52]. As the systematic assessment and psychoeducation in the present study succeeded in creating positive expectations and general hope, this may be an important step towards better outcome as suggested by adult literature.
Two months following assessment the overall intervention including psychoeducation and personalized health promoting strategies was associated with an early reduction in symptom severity, illness worry, negative illness perceptions, limiting illness behaviour and psychological inflexibility. In an adult FSD study, an early treatment response was predictive of better treatment outcome, especially regarding illness worry [
53]. Early predictors of positive treatment response have not been thoroughly investigated in youth. However, specific treatment elements that may be important to address in treatment have been identified in mediation studies across different single-system FSD in children and adolescents [
54]. These include specific behaviours and perceptions e.g., improvement of avoidance/limiting behaviour [
55,
56] and catastrophic cognitions [
57] in gastrointestinal FSD and pain impairment beliefs in children with chronic pain [
58]. It could be suggested to consider these elements early on in psychoeducation, as done in the present study. The early improvements seen in the present study, may serve as a first step towards a broader and more adaptive understanding and behaviour towards the disorder. The positive impact of psychoeducation in itself has been shown in a pediatric RCT study on the effect of cognitive behavioural therapy (CBT) for chronic fatigue syndrome by Chalder et al. [
19], although CBT displayed important advantages at long term follow up [
18]. This supports that psychoeducation may serve as a relevant minimal first step intervention, though more intensive treatment will be necessary for patients with more severe clinical presentations.
The present study has several limitations. First, this is a study embedded in the AHEAD trial and therefore not specifically designed to evaluate preliminary effects of assessment, psychoeducation and health promoting strategies. The observed changes from baseline to approximately two months after assessment may therefore be due to spontaneous improvements. However, as the adolescents had been ill for a mean of 3.9 years with multi-system involvement, spontaneous improvements are expected to be low. Second, randomization was done right after assessment prior to evaluation of the effect of assessment and psychoeducation, potentially influencing outcome with different incentives to work with the health promoting strategies. However, interaction effects did not display that randomization group influenced change. Third, the time period between T0 and T1 differed in length due to start of group-treatment defining the T1 measurement with some patients having a short time between the two measurement point, which may have influenced the potential for improvement. Fourth, the design does not leave room for evaluation of the impact of assessment and psychoeducation as a standalone intervention, and it is therefore not possible to evaluate long-term effects on relevant outcomes such as symptom severity and health-care use. Last, despite the importance of parental factors, we did not evaluate the parents’ experience of assessment which may differ from the experience of the adolescents potentially influencing the course of symptoms.
5. Conclusions
The present study embedded in the AHEAD trial showed that systematic assessment and psychoeducation were associated with improved diagnostic certainty, positive treatment expectations and a brighter outlook on the future for the young patient. Furthermore, improvements were observed on important clinical outcomes including symptom severity, illness worry, illness perception, limiting behavior and psychological flexibility prior to specialized treatment. The results underpin the importance and potential positive implications of systematic assessment and psychoeducation. Hence, these elements may be in their own right in specialized treatment of adolescents with severe FSD.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org, Figure S1: Chronological overview of health care contacts and social events, Figure S2 Experience of assessment divided by randomization group; Table S1: Mean and SD at T0 and T1.
Author Contributions
Conceptualization, KHK and CUR; methodology, KHK, KBW, EØ and CUR; validation, KBW and EØ; formal analysis, KHK, KBW and EØ.; investigation, KHK and CUR; data curation, KHK, KBW and EØ; writing—original draft preparation, KHK; writing—review and editing, KHK, KBW, EØ and CUR.; visualization, KHK; supervision, CUR; project administration, KHK and CUR; funding acquisition, KHK and CUR. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by TrygFonden, grant number: 100408 and the Danish Medical Association, grand number: 2013-5480/912523-63;.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Committee of Health Research Ethics of Central Denmark Region (protocol code 1-10-72-181-14, date of approval July 3rd 2014). The trial was also approved by The Danish Data Protection Agency (protocol code 1-16-02-290-14, date of approval May 8th 2014).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The individual participant data that underlie the results reported in this article will be available for researchers who provide a methodologically sound proposal to achieve their aims. Proposals should be directed to the first author karkal@rm.dk; to gain access. Data requestors will need to sign a data access agreement. The data are available immediately following publication and no specific end date has been set.
Acknowledgments
The authors want to thank the participating young patients and their families. They wish to thank the main funders of the study, that is, TrygFonden (grant number 100408) and co-funders, that is, The Danish Medical Association (grant number 2013-5480/912523-63) for making the research project possible. The treatment manual can be obtained from the first author.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Janssens, K.A.; Klis, S.; Kingma, E.M.; Oldehinkel, A.J.; Rosmalen, J.G. Predictors for Persistence of Functional Somatic Symptoms in Adolescents. J. Pediatr. 2014, 164, 900–905. [Google Scholar] [CrossRef] [PubMed]
- Lieb, R.; Pfister, H.; Mastaler, M.; Wittchen, H.U. Somatoform syndromes and disorders in a representative population sample of adolescents and young adults: prevalence, comorbidity and impairments. Acta Psychiatr Scand. 2000, 101, 194–208. [Google Scholar] [CrossRef] [PubMed]
- Hagquist, C.; Due, P.; Torsheim, T.; Välimaa, R. Cross-country comparisons of trends in adolescent psychosomatic symptoms – a Rasch analysis of HBSC data from four Nordic countries. Heal. Qual. Life Outcomes 2019, 17, 27. [Google Scholar] [CrossRef] [PubMed]
- Vesterling, C.; Schütz-Wilke, J.; Bäker, N.; Bolz, T.; Eilts, J.; Koglin, U.; Rademacher, A.; Goagoses, N. Epidemiology of Somatoform Symptoms and Disorders in Childhood and Adolescence: A Systematic Review and Meta-Analysis. Heal. Soc. Care Community 2023, 2023, 6242678. [Google Scholar] [CrossRef]
- Hoftun, G.B.; Romundstad, P.R.; Zwart, J.-A.; Rygg, M. Chronic idiopathic pain in adolescence – high prevalence and disability: The young HUNT study 2008. (1872-6623 (Electronic)). [CrossRef] [PubMed]
- Saunders, N.R.; Gandhi, S.; Chen, S.; Vigod, S.; Fung, K.; De Souza, C.; Saab, H.; Kurdyak, P. Health Care Use and Costs of Children, Adolescents, and Young Adults With Somatic Symptom and Related Disorders. JAMA Netw. Open 2020, 3, e2011295–e2011295. [Google Scholar] [CrossRef] [PubMed]
- Vila, M.; Kramer, T.; Obiols, J.E.; Garralda, M.E. Abdominal pain in British young people: Associations, impairment and health care use. J. Psychosom. Res. 2012, 73, 437–442. [Google Scholar] [CrossRef]
- Hinton, D.; Kirk, S. Families' and healthcare professionals' perceptions of healthcare services for children and young people with medically unexplained symptoms: a narrative review of the literature. Heal. Soc. Care Community 2015, 24, 12–26. [Google Scholar] [CrossRef]
- Kozlowska, K.; Sawchuk, T.; Waugh, J.L.; Helgeland, H.; Baker, J.; Scher, S.; Fobian, A.D. Changing the culture of care for children and adolescents with functional neurological disorder. Epilepsy Behav. Rep. 2021, 16, 100486. [Google Scholar] [CrossRef]
- Pincus, T.; Noel, M.; Jordan, A.; Serbic, D. Perceived diagnostic uncertainty in pediatric chronic pain. Pain 2018, 159, 1198–1201. [Google Scholar] [CrossRef]
- Neville, A.; Jordan, A.; Beveridge, J.K.; Pincus, T.; Noel, M. Diagnostic Uncertainty in Youth With Chronic Pain and Their Parents. J. Pain 2019, 20, 1080–1090. [Google Scholar] [CrossRef] [PubMed]
- Meldrum, M.L.; Tsao, J.C.; Zeltzer, L.K. , “I can’t be what I want to be”: children’s narratives of chronic pain experiences and treatment outcomes. Pain medicine (Malden, Mass.) 2009, 10, 1018–1034. [Google Scholar] [CrossRef] [PubMed]
- Moulin, V.; Akre, C.; Rodondi, P.; Ambresin, A.; Suris, J. A qualitative study of adolescents with medically unexplained symptoms and their parents. Part 2: How is healthcare perceived? J. Adolesc. 2015, 45, 317–326. [Google Scholar] [CrossRef] [PubMed]
- Henningsen, P.; Zipfel, S.; Sattel, H.; Creed, F. Management of Functional Somatic Syndromes and Bodily Distress. Psychother. Psychosom. 2018, 87, 12–31. [Google Scholar] [CrossRef] [PubMed]
- Kangas, M.; Kallesoe, K.H.; Rask, C.U. Functional Somatic Syndromes (FSS) in Children and Adolescents. Zeitschrift für Psychologie 2020, 228, 81–92. [Google Scholar] [CrossRef]
- Ibeziako, P.; Brahmbhatt, K.; Chapman, A.; De Souza, C.; Giles, L.; Gooden, S.; Latif, F.; Malas, N.; Namerow, L.; Russell, R.; et al. Developing a Clinical Pathway for Somatic Symptom and Related Disorders in Pediatric Hospital Settings. Hosp. Pediatr. 2019, 9, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, H.F.; Holsting, A.; Frostholm, L.; Rask, C.; Jensen, J.; Høeg, M.D.; Schröder, A. “Understand your illness and your needs”: Assessment-informed patient education for people with multiple functional somatic syndromes. Patient Educ. Couns. 2019, 102, 1662–1671. [Google Scholar] [CrossRef]
- Lloyd, S.; Chalder, T.; Rimes, K.A. Family-focused cognitive behaviour therapy versus psycho-education for adolescents with chronic fatigue syndrome: Long-term follow-up of an RCT. Behav. Res. Ther. 2012, 50, 719–725. [Google Scholar] [CrossRef]
- Chalder, T.; Deary, V.; Husain, K.; Walwyn, R. Family-focused cognitive behaviour therapy versus psycho-education for chronic fatigue syndrome in 11- to 18-year-olds: a randomized controlled treatment trial. Psychol. Med. 2009, 40, 1269–1279. [Google Scholar] [CrossRef]
- Petersen, M.W.; Schröder, A.; Jørgensen, T.; Ørnbøl, E.; Dantoft, T.M.; Eliasen, M.; Benros, M.E.; Fink, P. Irritable bowel, chronic widespread pain, chronic fatigue and related syndromes are prevalent and highly overlapping in the general population: DanFunD. Sci. Rep. 2020, 10, 1–10. [Google Scholar] [CrossRef]
- Fink, P.; Schröder, A. One single diagnosis, bodily distress syndrome, succeeded to capture 10 diagnostic categories of functional somatic syndromes and somatoform disorders. J. Psychosom. Res. 2010, 68, 415–426. [Google Scholar] [CrossRef] [PubMed]
- Burton, C.; on behalf of the EURONET-SOMA Group; Fink, P. ; Henningsen, P.; Löwe, B.; Rief, W. Functional somatic disorders: discussion paper for a new common classification for research and clinical use. BMC Med. 2020, 18, 34. [Google Scholar] [CrossRef] [PubMed]
- Tomenson, B.; Essau, C.; Jacobi, F.; Ladwig, K.H.; Leiknes, K.A.; Lieb, R.; Meinlschmidt, G.; McBeth, J.; Rosmalen, J.; Rief, W.; et al. Total somatic symptom score as a predictor of health outcome in somatic symptom disorders. 203. [CrossRef]
- Petersen, M.W.; Rosendal, M.; Ørnbøl, E.; Fink, P.; Jørgensen, T.; Dantoft, T.M.; Schröder, A. The BDS checklist as measure of illness severity: a cross-sectional cohort study in the Danish general population, primary care and specialised setting. BMJ Open 2020, 10, e042880. [Google Scholar] [CrossRef] [PubMed]
- Kallesøe, K.H.; Schröder, A.; Wicksell, R.K.; Fink, P.; Ørnbøl, E.; Rask, C.U. Comparing group-based acceptance and commitment therapy (ACT) with enhanced usual care for adolescents with functional somatic syndromes: a study protocol for a randomised trial. BMJ Open 2016, 6, e012743. [Google Scholar] [CrossRef] [PubMed]
- Kallesøe, K.H.; et al. , Group-based Acceptance and Commitment Therapy (AHEAD) for adolescents with multiple functional somatic syndromes: A randomised trial. JCPP Advances 2021, e12047. [Google Scholar] [CrossRef]
- Schröder, A.; Rehfeld, E.; Ørnbøl, E.; Sharpe, M.; Licht, R.W.; Fink, P. Cognitive–behavioural group treatment for a range of functional somatic syndromes: Randomised trial. Br. J. Psychiatry 2012, 200, 499–507. [Google Scholar] [CrossRef]
- Wing, J.K.; et al., SCAN.; et al. Schedules for Clinical Assessment in Neuropsychiatry. Archives of General Psychiatry 1990, 47, 589–593. [Google Scholar] [CrossRef]
- Goodman, R.; et al. , The Development and Well-Being Assessment: description and initial validation of an integrated assessment of child and adolescent psychopathology. Journal of child psychology and psychiatry, and allied disciplines 2000, 41, 645–655. [Google Scholar] [CrossRef]
- McWilliams, A.; Reilly, C.; Gupta, J.; Hadji-Michael, M.; Srinivasan, R.; Heyman, I. Autism spectrum disorder in children and young people with non-epileptic seizures. Seizure 2019, 73, 51–55. [Google Scholar] [CrossRef]
- Lipsker, C.W.; Bölte, S.; Hirvikoski, T.; Lekander, M.; Holmström, L.; Wicksell, R.K. Prevalence of autism traits and attention-deficit hyperactivity disorder symptoms in a clinical sample of children and adolescents with chronic pain. J. Pain Res. 2018, ume 11, 2827–2836. [Google Scholar] [CrossRef]
- Kallesøe, K.H.; Schröder, A.; Wicksell, R.K.; Preuss, T.; Jensen, J.S.; Rask, C.U. Feasibility of group-based acceptance and commitment therapy for adolescents (AHEAD) with multiple functional somatic syndromes: a pilot study. BMC Psychiatry 2020, 20, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Bjorner, J.B.; Damsgaard, M.T.; Watt, T.; Groenvold, M. Tests of Data Quality, Scaling Assumptions, and Reliability of the Danish SF-36. J. Clin. Epidemiology 1998, 51, 1001–1011. [Google Scholar] [CrossRef]
- Rief, W.; Burton, C.; Frostholm, L.; Henningsen, P.; Kleinstäuber, M.; Kop, W.J.; Löwe, B.; Martin, A.; Malt, U.; Rosmalen, J.; et al. Core Outcome Domains for Clinical Trials on Somatic Symptom Disorder, Bodily Distress Disorder, and Functional Somatic Syndromes: European Network on Somatic Symptom Disorders Recommendations. Psychosom. Med. 2017, 79, 1008–1015. [Google Scholar] [CrossRef]
- Ware, J.E. and M. Kosinski, SF-36 Physical and Mental Health Summary Scales: A Manual for Users of Version 1. Quality Metric., 2001. Second Edition.
- Ware, J.E., Jr.; et al. , Differences in 4-year health outcomes for elderly and poor, chronically ill patients treated in HMO and fee-for-service systems. Results from the Medical Outcomes Study. Jama 1996, 276, 1039–1047. [Google Scholar] [PubMed]
- Derogatis, L.R.; Cleary, P.A. Confirmation of the dimensional structure of the scl-90: A study in construct validation. Journal of clinical psychology 1977, 33, 981–989. [Google Scholar] [CrossRef]
- Carstensen, T.B.W.; Ørnbøl, E.; Fink, P.; Pedersen, M.M.; Jørgensen, T.; Dantoft, T.M.; Benros, M.E.; Frostholm, L. Detection of illness worry in the general population: A specific item on illness rumination improves the Whiteley Index. J. Psychosom. Res. 2020, 138, 110245. [Google Scholar] [CrossRef] [PubMed]
- Broadbent, E.; Wilkes, C.; Koschwanez, H.; Weinman, J.; Norton, S.; Petrie, K.J. A systematic review and meta-analysis of the Brief Illness Perception Questionnaire. Psychol. Heal. 2015, 30, 1361–1385. [Google Scholar] [CrossRef] [PubMed]
- Spence, M.; Moss-Morris, R.; Chalder, T. The Behavioural Responses to Illness Questionnaire (BRIQ): a new predictive measure of medically unexplained symptoms following acute infection. Psychol. Med. 2004, 35, 583–593. [Google Scholar] [CrossRef]
- Greco, L.A.; Lambert, W.; Baer, R.A. Psychological inflexibility in childhood and adolescence: Development and evaluation of the Avoidance and Fusion Questionnaire for Youth. Psychol. Assess. 2008, 20, 93–102. [Google Scholar] [CrossRef]
- Wicksell, R.K.; Lekander, M.; Sorjonen, K.; Olsson, G.L. The Psychological Inflexibility in Pain Scale (PIPS) – Statistical properties and model fit of an instrument to assess change processes in pain related disability. Eur. J. Pain 2010, 14, 771–e1. [Google Scholar] [CrossRef]
- Hayes, S.C.; Strosahl, K.D.; Wilson, K.G. Acceptance and commitment therapy: The process and practice of mindful change, 2nd ed.; Guilford Press: New York, NY, USA, 2012; xiv, 402-xiv, 402. [Google Scholar]
- Wan, F. Statistical analysis of two arm randomized pre-post designs with one post-treatment measurement. BMC Med Res. Methodol. 2021, 21, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Rief, W.; Heitmüller, A.M.; Reisberg, K.; Rüddel, H. Why Reassurance Fails in Patients with Unexplained Symptoms—An Experimental Investigation of Remembered Probabilities. PLOS Med. 2006, 3, e269. [Google Scholar] [CrossRef] [PubMed]
- Hulgaard, D.R.; Rask, C.U.; Risor, M.B.; Dehlholm, G. Illness perceptions of youths with functional disorders and their parents: An interpretative phenomenological analysis study. Clin. Child Psychol. Psychiatry 2019, 25, 45–61. [Google Scholar] [CrossRef] [PubMed]
- Helgeland, H.; Gjone, I.H.; Diseth, T.H. The biopsychosocial board—A conversation tool for broad diagnostic assessment and identification of effective treatment of children with functional somatic disorders. Hum. Syst. Ther. Cult. Attach. 2022, 2, 144–157. [Google Scholar] [CrossRef]
- Hulgaard, D.R.; Rask, C.U.; Risør, M.B.; Dehlholm, G. ‘I can hardly breathe’: Exploring the parental experience of having a child with a functional disorder. J. Child Heal. Care 2019. p. 1367493519864745. [Google Scholar] [CrossRef] [PubMed]
- Stewart, J.A.; Egloff, N.; von Känel, R.; Grolimund, J.; Studer, M.; Holtforth, M.G. Motivation for Psychological Treatment Predicts Favorable Outcomes in Multimodal Interdisciplinary Treatment for Chronic Somatoform Pain. Psychother. Psychosom. 2016, 86, 60–61. [Google Scholar] [CrossRef] [PubMed]
- Cormier, S.; Lavigne, G.L.; Choinière, M.; Rainville, P. Expectations predict chronic pain treatment outcomes. Pain 2016, 157, 329–338. [Google Scholar] [CrossRef]
- Vos-Vromans, D.; Huijnen, I.; Rijnders, L.; Winkens, B.; Knottnerus, J.; Smeets, R. Treatment expectations influence the outcome of multidisciplinary rehabilitation treatment in patients with CFS. J. Psychosom. Res. 2016, 83, 40–45. [Google Scholar] [CrossRef]
- Logan, D.E.; Conroy, C.; Sieberg, C.B.; Simons, L.E. Changes in willingness to self-manage pain among children and adolescents and their parents enrolled in an intensive interdisciplinary pediatric pain treatment program. Pain 2012, 153, 1863–1870. [Google Scholar] [CrossRef]
- Kleinstäuber, M.; Lambert, M.J.; Hiller, W. Early response in cognitive-behavior therapy for syndromes of medically unexplained symptoms. BMC Psychiatry 2017, 17, 1–15. [Google Scholar] [CrossRef]
- Leake, H.B.; Moseley, G.L.; Stanton, T.R.; Heathcote, L.C.; Pate, J.W.; Wewege, M.A.; Lee, H. Using Mediation Analysis to Understand How Treatments for Paediatric Pain Work: A Systematic Review and Recommendations for Future Research. Children 2021, 8, 147. [Google Scholar] [CrossRef] [PubMed]
- Bonnert, M.; Olén, O.; Bjureberg, J.; Lalouni, M.; Hedman-Lagerlöf, E.; Serlachius, E.; Ljótsson, B. The role of avoidance behavior in the treatment of adolescents with irritable bowel syndrome: A mediation analysis. Behav. Res. Ther. 2018, 105, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Lalouni, M.; Hesser, H.; Bonnert, M.; Hedman-Lagerlöf, E.; Serlachius, E.; Olén, O.; Ljótsson, B. Breaking the vicious circle of fear and avoidance in children with abdominal pain: A mediation analysis. J. Psychosom. Res. 2020, 140, 110287. [Google Scholar] [CrossRef] [PubMed]
- Levy, R.L.M.; Langer, S.L.; Romano, J.M.; Labus, J.; Walker, L.S.; Murphy, T.B.; van Tilburg, M.A.; Feld, L.D.B.; Christie, D.L.; Whitehead, W.E. Cognitive Mediators of Treatment Outcomes in Pediatric Functional Abdominal Pain. Clin. J. Pain 2014, 30, 1033–1043. [Google Scholar] [CrossRef]
- Wicksell, R.K.; Olsson, G.L.; Hayes, S.C. Mediators of change in Acceptance and Commitment Therapy for pediatric chronic pain. Pain 2011, 152, 2792–2801. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).