1. Introduction
Many patients with locally advanced squamous cell carcinoma of the head-and-neck (HNSCC) receive concurrent chemoradiation with cisplatin, either as definitive treatment or, in case of risk factors, following surgery [
1,
2,
3]. However, many patients cannot receive cisplatin, mainly due to pre-treatment decreased renal function. Therefore, alternative systemic agents or combinations have been investigated, including carboplatin alone, carboplatin plus 5-fluorouracil (5-FU), carboplatin plus paclitaxel, mitomycin C plus 5-FU, or cetuximab. Randomized trials demonstrated that chemoradiation with carboplatin plus 5-FU was superior to radiotherapy alone in patients with head-and-neck cancer [
4,
5,
6,
7,
8,
9,
10]. In addition, a randomized trial from Germany found that the addition of mitomycin C plus 5-FU to hyper-fractionated accelerated radiotherapy resulted in significantly better loco-regional control (LRC) and overall survival (OS) [
11,
12].
However, several studies that included combinations with 5-FU found that these regimens were associated with significant acute toxicities [
4,
5,
6,
7,
11,
13,
14]. For example, in the study of Hanemaaijer
et al. that compared concurrent treatment with carboplatin plus 5-FU to cisplatin, more patients in the carboplatin plus 5-FU discontinued their chemotherapy due to chemotherapy-related toxicities [
13]. In another study, discontinuation of chemotherapy showed a trend towards worse OS in patients with oropharynx cancer [
15]. Moreover, chemotherapy-related toxicity may lead to interruption of the radiation treatment, which was shown to impair the patients’ prognoses [
16,
17]. Therefore, concurrent systemic therapies without 5-FU may be preferable for head-and-neck cancer patients unable to receive cisplatin. Such regimens may include cetuximab or carboplatin with or without paclitaxel [
3,
18,
19,
20].
A few studies compared radiotherapy plus concurrent cetuximab to concurrent chemoradiation with carboplatin-based regimens and found that regimens including carboplatin resulted in better LRC and OS [
21,
22,
23,
24,
25]. One of these studies differentiated between carboplatin-based combinations with 5-FU or paclitaxel and carboplatin as a single agent [
23]. Carboplatin alone was not inferior to carboplatin-based combinations with respect to LRC, metastases-free survival (MFS), and OS [
23]. Promising results regarding chemoradiation with carboplatin alone were also reported by other authors [
26,
27,
28,
29]. Several studies compared chemoradiation with concurrent carboplatin alone to concurrent cisplatin [
30,
31,
32,
33,
34,
35,
36,
37,
38,
39,
40,
41]. These studies produced conflicting results regarding treatment outcomes, suggesting either superiority of cisplatin (4 studies), superiority of carboplatin (1 study), or similar efficacy of both agents (6 studies). One study found cisplatin to be superior for stage III but not for stage I or II tumors [
39].
Thus, additional comparative studies are required to better define the potential role of carboplatin alone for chemoradiation of head-and-neck cancer. The present study compared two courses of concurrent carboplatin (AUC 1.0-1.5 on 4-5 days) and two courses of cisplatin (20-25 mg/m
2 on 4-5 days, cumulative dose=200 mg/m
2). Since this cisplatin-regimen was found to be similarly effective but significantly less toxic when compared to three courses of 100 mg/m
2, two courses of fractionated cisplatin have become the standard regimen for SCCHN in our university hospital and selected as reference treatment [
42].
2. Materials and Methods
A total of 176 patients treated with chemoradiation for SCCHN between 2012 and 2022 were included in this retrospective study, which was approved by the Ethics Committee of the University of Lübeck, Germany (file no. 21-034). Of the entire cohort, 131 patients were scheduled for concurrent chemoradiation including two courses of cisplatin, consisting of 20 mg/m2/d1-5 or 25 mg/m2/d1-4. Forty-five patients could not receive cisplatin, mainly due to decreased renal function, and were treated with two courses of concurrent carboplatin (AUC 1.0 on days 1-5 or AUC 1.5 on days 1-4) instead. Planned doses of external beam radiotherapy (EBRT), which was administered as volumetric modulated arc therapy (VMAT), were 60-70 Gy with doses per fraction of 2 Gy, given on five consecutive days per week. Total radiation doses depended on extent of resection and presence of extracapsular spread of lymph node metastases (ECS). Doses were 60 Gy after microscopically complete resection, 66 Gy after microscopically incomplete resection or ECS, and 70 Gy for definitive treatment, respectively. Median doses of EBRT were 66 Gy in the entire cohort and both treatment groups. Ten patients in the cisplatin-group and one patient in the carboplatin-group received a brachytherapy boost of 7.5-16 Gy with 3-5 fractions of 2.5-4 Gy.
Treatment groups were compared with respect to pre-treatment patient and tumor (=baseline) characteristics, outcomes in terms of LRC, MFS and OS, completion of the planned chemotherapy, and for toxicities in terms of oral mucositis, dermatitis, xerostomia, cervical lymph edema, nausea and hematotoxicity. Baseline characteristics included age (≤62 vs. ≥63 years, median age=62 years), gender (female vs. male), Karnofsky performance score (KPS ≤80 vs. 90-100), tumor site (oropharynx/oral cavity vs. hypopharynx/larynx vs. both), primary tumor stage (T1-2 vs. T3 vs. T4), nodal stage (N0-2a vs. N2b-3), histologic grade (G1-2 vs. G3), human papilloma virus (HPV) status (negative vs. positive), upfront surgery (no vs. yes), history of smoking prior to chemoradiation (no vs. yes), smoking during chemoradiation (no vs. yes), and pre-treatment hemoglobin level (<12 vs. ≥12 g/dl). The distributions of these characteristics in both treatment groups are given in
Table 1). In addition to the type of chemotherapy and completion of chemotherapy as planned, these characteristics were evaluated for associations with LRC, MFS, and OS.
Moreover, subgroup analyses comparing carboplatin and cisplatin with respect to LRC, MFS, and OS were performed for patients receiving definitive chemoradiation and for patients treated with adjuvant chemoradiation following surgery.
Statistical analysis regarding the comparison of the treatment groups with respect to distributions of baseline characteristics, completion of chemotherapy, and toxicities were performed with the Chi-square test or, in case of less than 5 patients in one or more cells, with the Fisher’s exact test. LRC, MFS, and OS were referenced from the last day of radiotherapy and corresponding rates were calculated with the Kaplan-Meier method and the log-rank test (univariable analyses). Characteristics that were significantly associated with outcomes (p < 0.05) were included in a multivariable analysis (Cox proportional hazards model). For these analyses, the software BlueSky Statistics 10 GA was used (BlueSky Statistics LLC, Chicago, IL, USA).
3. Results
The comparison of both treatment groups with respect to patient and tumor characteristics revealed that significantly more patients in the carboplatin-group were ≥63 years of age (64% vs. 41%,
p = 0.007) and that more patients had poorly differentiated (G3) tumors (61% vs. 40%,
p = 0.017). Otherwise patient and tumor characteristics were balanced between the groups (
Table 1). In the carboplatin-group, non-significantly more patients completed their chemotherapy as planned (78% vs. 66%,
p = 0.15).
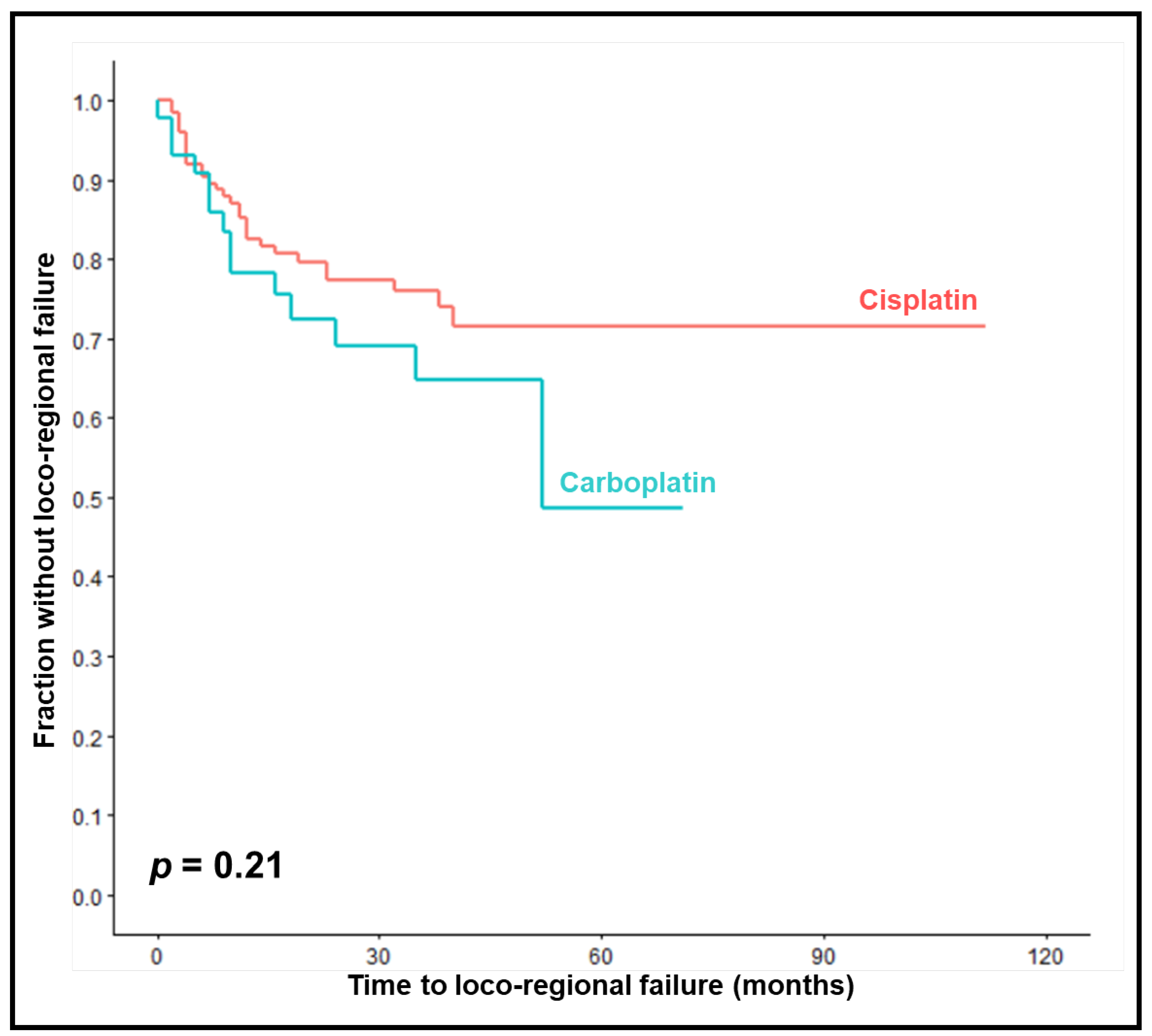
LRC rates at 1, 2 and 3 years were 78%, 69% and 65% in the carboplatin-group compared to 83%, 77% and 76% in the cisplatin-group (
p = 0.21,
Figure 1). On univariable analyses, improved LRC was significantly associated with KPS 90-100 (
p = 0.049) less advanced primary tumor stage (
p < 0.001), less advanced nodal stage (
p = 0.004), upfront surgery (
p < 0.001), and HPV-positivity (
p = 0.001) (
Table 2). A trend was found for completion of chemotherapy (
p = 0.078). In the multivariable analysis of LRC, less advanced primary tumor stage (
p = 0.031) and HPV-positivity (
p = 0.047) were significant (
Table 3).
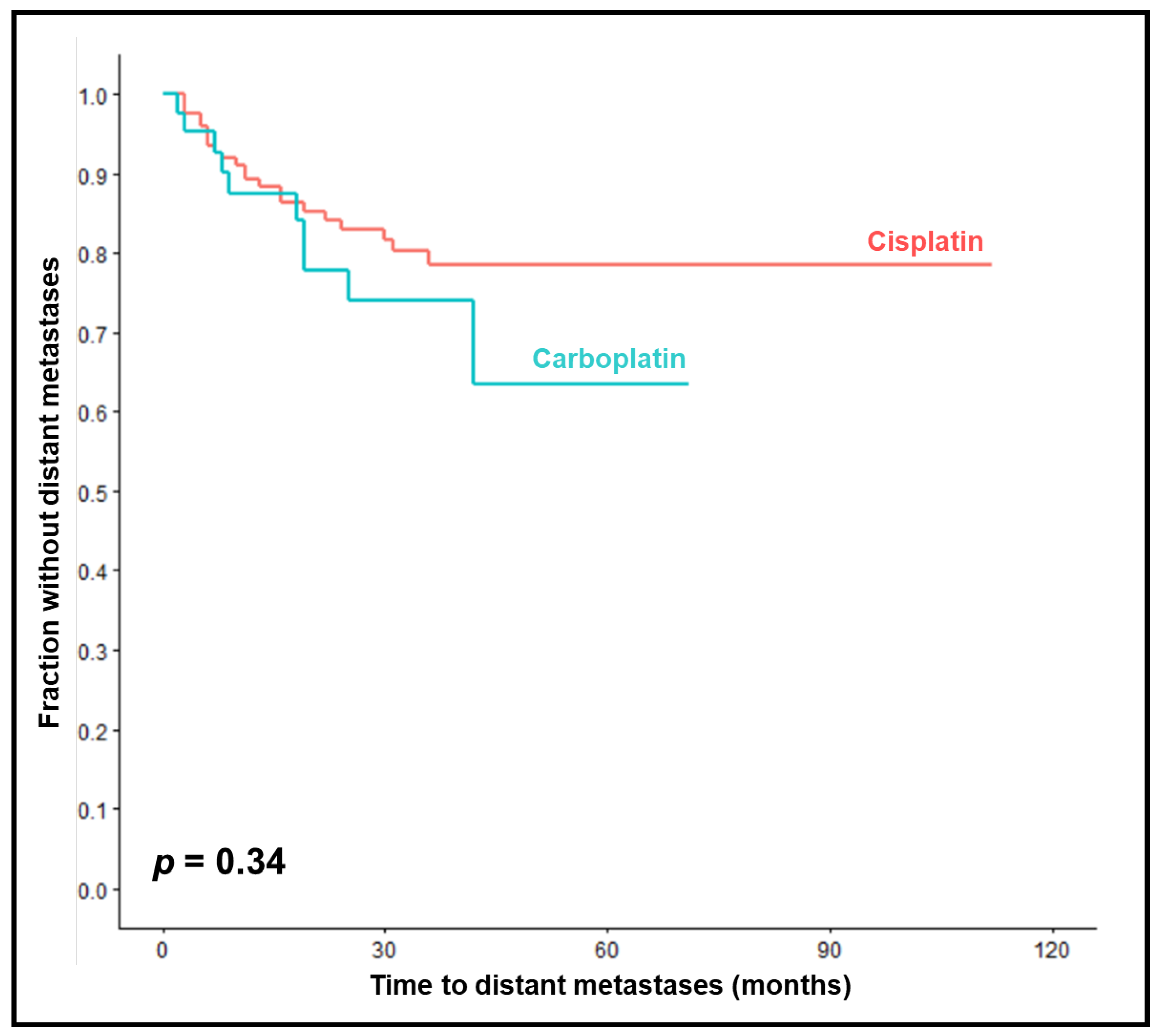
One-year, 2-year and 3-year MFS rates were 87%, 78% and 74% in the carboplatin-group compared to 89%, 83% and 78% in the cisplatin-group (
p = 0.34,
Figure 2). On univariable analyses, improved MFS was significantly associated with KPS 90-100 (
p = 0.031), less advanced nodal stage (
p = 0.013) and HPV-positivity (
p = 0.008) (
Table 4). In the multivariable analysis of MFS, less advanced nodal stage (
p = 0.024) and HPV-positivity (
p = 0.030) were significant (
Table 5).
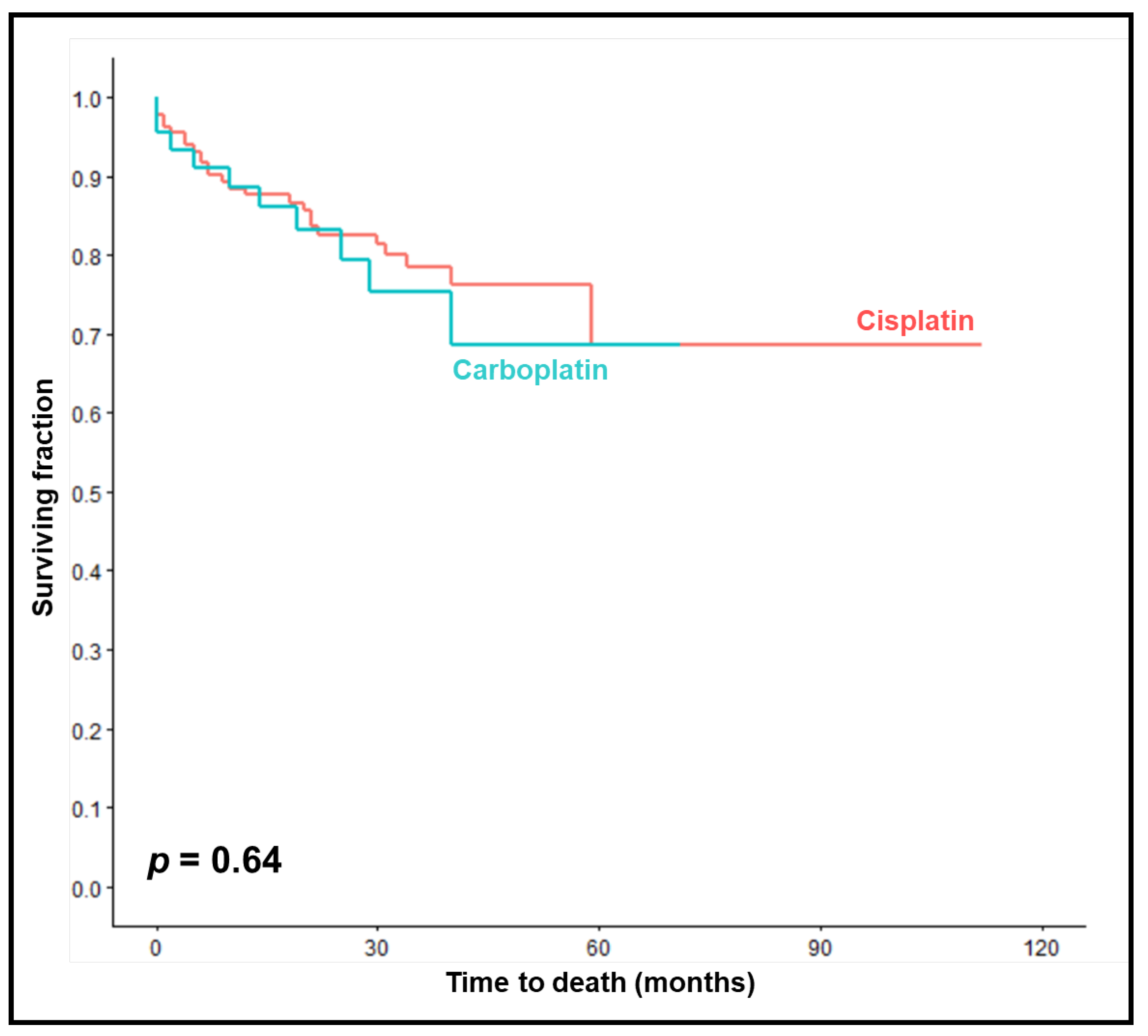
OS rates at 1, 2 and 3 years were 89%, 83% and 75% in the carboplatin-group compared to 88%, 83% and 79% in the cisplatin group (p = 0.64,
Figure 3). On univariable analyses, improved OS was significantly associated with KPS 90-100 (
p = 0.003), HPV-positivity (
p = 0.021), and not smoking during chemoradiation (
p = 0.009) (
Table 6). Trends were found for age ≤62 years (
p = 0.093), pre-treatment hemoglobin levels ≥12 g/dl (
p = 0.093), and completion of chemotherapy (
p = 0.060). In the multivariable analysis of OS, no characteristic achieved significance (
Table 7).
Moreover, no significant differences were found between carboplatin and cisplatin regarding acute and late toxicities in terms of oral mucositis grade ≥2 (
p = 0.34) or grade ≥3 (
p = 0.38), dermatitis grade ≥2 (
p = 0.27) or grade ≥3 (p = 1.00), nausea grade ≥2 (
p = 0.50) or grade ≥3 (
p = 0.27), xerostomia grade ≥2 (
p = 0.74) or grade ≥3 (
p = 1.00), lymphedema grade ≥2 (
p = 1.00) or grade ≥3 (
p = 1.00), and hematotoxicity grade ≥2 (
p = 0.74), grade ≥3 (
p = 0.74) or grade 4 (
p = 0.60) (
Table 8).
aunknown in 15 patients; bunknown in 11 patients; cunknown in 6 patients; dunknown in 8 patients; eunknown in 6 patients; funknown in 1 patient; *calculated with the Fisher’s exact test.
In the subgroup analysis of the 79 patients receiving definitive chemoradiation, LRC rates after 1, 2 and 3 years were 71%, 55% and 49% in the carboplatin-group (n = 23) vs. 70%, 61% and 58% in the cisplatin-group (n = 56), respectively (p = 0.53). MFS-rates were 85%, 75% and 69% in the carboplatin-group vs. 94%, 87% and 84% in the cisplatin-group (p = 0.058), and OS-rates were 91%, 81% and 75% vs. 87%, 80% and 74% (p = 0.78), respectively. In the subgroup analysis of the 97 patients receiving adjuvant chemoradiation, LRC rates after 1, 2 and 3 years were 85%, 85% and 85% in the carboplatin-group (n = 22) vs. 92%, 90% and 90% in the cisplatin-group (n = 75), respectively (p = 0.54). MFS-rates were 90%, 82% and 82% in the carboplatin-group vs. 86%, 80% and 75% in the cisplatin-group (p = 0.63), and OS-rates were 86%, 86% and 77% in the carboplatin-group vs. 88%, 84% and 82% in the cisplatin-group (p = 0.84), respectively.
4. Discussion
Standard chemoradiation for SCCHN includes cisplatin, commonly consisting of 100 mg/m
2 on one day, administered every 3 weeks [
1,
2,
3]. Several studies suggested that the minimum cumulative cisplatin-dose during a course of radiotherapy should be 200 mg/m
2 [
43,
44,
45]. Therefore, other cisplatin-regimens including weekly doses of 30-40 mg/m
2 or two courses of fractionated cisplatin, as used in the present study, are also relatively common. However, cisplatin can be associated with significant morbidity, particularly nephrotoxicity. Therefore, many patients are not suitable for this agent. Since the addition of concurrent chemotherapy to radiotherapy improves the outcomes of treatment in patients with SCCHN, replacement of cisplatin by other systemic agents is considered a better option than radiotherapy alone, irrespective of the radiation-regimen [
1,
2,
3,
9,
11,
12]. Several options of systemic therapies without cisplatin exist, for example carboplatin plus/minus 5-FU or paclitaxel, mitomycin C plus 5-FU, or cetuximab. According to previous studies, 5-FU-based regimens were quite toxic, and the anti-epidermal-growth-factor-receptor antibody cetuximab appeared less effective than “classic” chemotherapies [
4,
5,
6,
7,
8,
9,
10,
11,
12,
13,
18,
19,
20,
21,
22,
23,
24,
25,
26,
27,
28,
29]. Thus, carboplatin plus paclitaxel and carboplatin alone remain of the options mentioned above. In the study of Beckham
et al., which compared different platin-based chemotherapy regimens and cetuximab in 316 patients with HPV-negative head-and-neck cancer, carboplatin alone was not inferior to carboplatin plus 5-FU or paclitaxel with respect to LRC (HR: 0.69, 95% CI: 0.23-2.08, p = 0.51), MFS (HR: 1.47, 95% CI: 0.32-6.68, p = 0.62), and OS (HR: 1.30, 95% CI: 0.56-3.04, p = 0.54) [
23]. Whether carboplatin alone was associated with less toxicity than the combined regimen, was not stated but may be suspected. Thus, chemoradiation with carboplatin may be a reasonable alternative for patients not suitable for cisplatin.
Several studies have compared carboplatin alone and cisplatin alone for chemoradiation of head-and-neck cancer [
30,
31,
32,
33,
34,
35,
36,
37,
38,
39,
40,
41]. Some of these studies were additionally included in one or two meta-analyses from 2016 [
46,
47]. However, the results of the studies and meta-analyses were conflicting. Of the studies published prior to 2015, three randomized trials and one matched-pair study found that carboplatin was not inferior to cisplatin [
30,
31,
34,
36]. Moreover, in a randomized phase II trial from 2004 including 119 patients with SCCHN, weekly carboplatin resulted in significantly better 5-year LRC (56.2% vs. 35.5%) and non-significantly better OS (71.4% vs. 66.0%) when compared to daily low-dose cisplatin [
33]. Only two studies published before 2015 suggested that carboplatin was less effective than cisplatin [
32,
35]. One study was retrospective in nature and limited to cancer of the oropharynx or oral cavity [
35]. Of 215 patients screened, only 106 patients (49%) had complete data and were included in the analyses. This may have led to a selection bias. Moreover, non-significantly more patients in the carboplatin-group had cancer of the oral cavity, which generally has a worse prognosis than cancer of the oropharynx. And important prognostic factors including HPV-status, smoking before and during the radiotherapy course, and pre-treatment hemoglobin level were not considered. Therefore, the results of this retrospective study should be interpreted with caution [
35]. The other study had a higher quality being a randomized phase III trial [
32]. It compared radiotherapy alone (41 patients) to chemoradiation with 100 mg/m
2 of cisplatin given on days 2, 22 and 42 (45 patients) and chemoradiation with carboplatin (AUC 7) given on days 2, 22 and 42 (38 patients). In that trial, carboplatin-based chemoradiation was superior to radiotherapy alone but inferior to cisplatin-based treatment with respect to time to progression and median OS [
32]. The two meta-analyses mentioned above were also limited to studies published before 2015 [
46,
47]. Both meta-analyses found that LRC and OS were not significantly different after chemoradiation with carboplatin or cisplatin. However, in a subgroup analysis of non-nasopharynx cancer performed in one meta-analysis, cisplatin resulted in significantly better 3-year OS (HR: 0.66; 95% CI: 0.48-0.91;
p=0.01) [
46].
Since radiotherapy of SCCHN has undergone technical improvements during the last 10 years including increasing use of high-precision radiotherapy with VMAT (like in the present study), it appears reasonable to take a separate look at studies published more recently. No randomized trials but several retrospective studies were identified that were published between 2017 and 2023 [
37,
38,
39,
40,
41]. Three of these studies found that chemoradiation with carboplatin was similarly effective as cisplatin-based treatment [
37,
38,
41]. One study suggested similar efficacy for stage I or II disease but superiority of cisplatin for stage III disease [
39]. In another study that compared chemoradiation with high-dose cisplatin (cumulative dose of ≥200 mg/m
2), chemoradiation with low-dose cisplatin (<200 mg/m
2), chemotherapy with carboplatin, and radiotherapy alone, high-dose cisplatin-based treatment was associated with significantly better OS than the other three regimens [
40]. However, patients in the carboplatin-group and the radiotherapy alone group were significantly older and had a higher comorbidity index than patients of the high-dose cisplatin group, which likely led to a bias in favor of high-dose cisplatin [
40].
Considering the conflicting results regarding the potential role of chemoradiation with carboplatin alone, it becomes obvious that additional studies are required to properly define its value. Therefore, we performed the present study. According to its results, concurrent chemoradiation with carboplatin was not inferior to concurrent cisplatin with respect to toxicities and treatment outcomes in terms of LRC, MFS, and OS. In contrast to the chemotherapy-regimen, lower primary tumor stage, lower nodal stage, and HPV-positivity were independently associated with improved treatment outcomes on multivariable analyses. A trend was found for an association between higher KPS and better OS. Such associations were already found in previous studies, which demonstrates consistency of the data of our present study [
14,
42,
43,
48,
49].
When interpreting the results of the previous studies, one should be aware that most of those studies comparing carboplatin and cisplatin for chemoradiation of SCCHN were performed in patients receiving definitive treatment. Only two comparative studies did also include patients who received chemoradiation in an adjuvant situation [
33,
36]. One of these studies (phase II trial) found that carboplatin to be superior to cisplatin, and the other study (retrospective matched-pair analysis) suggested that both agents were similarly effective. In addition, a non-comparative study that included also patients in an adjuvant situation found that chemoradiation with carboplatin was well tolerated [
27]. Moreover, a small retrospective study of 47 patients suggested that chemoradiation with carboplatin resulted in non-significantly better median progression-free survival (43 vs. 12 months) and OS (92 vs. 36 months) than radiotherapy combined with cetuximab [
29]. However, the small sample size and the retrospective design must be considered when interpreting these results. In contrast to these studies supporting the use of carboplatin, adjuvant chemoradiation with weekly carboplatin appeared not significantly superior to radiotherapy alone with respect to disease-free survival (DFS) at 2 and 5 years in a phase III trial [
50]. Two-year DFS rates were 71% with and 58% without carboplatin (
p=0.27), and 5-year DFS rates were 53% and 49% (
p=0.72), respectively. Two-year OS rates were 74% vs. 51% (
p=0.04), and 5-year OS rates were 47% vs. 41% (
p=0.61), respectively. However, the trial was prematurely closed due to slow accrual after 76 of planned 200 patients. Moreover, patients were treated in the pre-VMAT era, namely between 1992 and 2002. Therefore, the results of this phase III trial may be of limited validity, which is also stated by the authors. Considering the lack of data in an adjuvant situation, we performed subgroup analyses in patients receiving definitive chemoradiation and patients receiving adjuvant treatment. According to these analyses, a trend was found for improved MFS with cisplatin in patients receiving definitive chemoradiation. However, carboplatin was not significantly inferior to cisplatin in both subgroups with respect to LRC, MFS, or OS. Thus, it may be an option for patients unable to receive cisplatin, irrespective of upfront surgery.
When interpreting the results of our study, its limitations should be regarded including the use of a cisplatin-regimen, which is not the standard in many countries, and the retrospective study design. Retrospective studies always bear the risk of (hidden) selection biases. In our present study, all but two baseline characteristics were balanced between the treatment groups. The distributions of age and histologic grading were more favorable in the cisplatin-group, which supports the findings that carboplatin may be not inferior. Moreover, most patients in the carboplatin-group had a decreased pre-treatment renal function. Despite the results in favor of carboplatin, its real value can only be properly identified in a prospective randomized trial using modern radiotherapy techniques, preferably VMAT.
5. Conclusions
Although patients who received carboplatin had worse pre-treatment renal function, were older and had more aggressive (less differentiated) tumors than patients treated with cisplatin, carboplatin did not result in significantly worse treatment outcomes (LRC, LFS, OS) or toxicities. Thus, concurrent chemoradiation with carboplatin alone appears an option for patients with SCCHN not suitable for cisplatin-based treatment.
Author Contributions
Conceptualization, D.R., I.Z., T.S., C.I., R.P., K.L.B., S.G.H., and N.Y.Y.; methodology, D.R., I.Z., T.S., C.I., R.P., K.L.B., S.G.H., and N.Y.Y.; validation, D.R., I.Z., and N.Y.Y.; formal analysis, D.R. and N.Y.Y.; investigation, D.R. and I.Z.; resources, D.R., T.S., C.I., K.L.B., and S.G.H.; data curation, D.R., I.Z., and N.Y.Y.; writing—original draft preparation, D.R. and N.Y.Y.; writing—review and editing, D.R., I.Z., T.S., C.I., R.P., K.L.B., S.G.H., and N.Y.Y.; visualization, D.R., I.Z., T.S., C.I., R.P., K.L.B., S.G.H., and N.Y.Y. All authors read and agreed to the published version.
Funding
The study has not received external funding.
Institutional Review Board Statement
The trial was approved by the Ethics Committee of the University of Lübeck, Germany (file no. 21-034).
Informed Consent Statement
Since this study is retrospective in nature, written informed consent was not required from patients alive according to the responsible ethics committee.
Data Availability Statement
The data cannot be shared due to data protection regulations. Only evaluation of anonymized data is allowed according to the responsible ethics committee.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lacas, B.; Carmel, A.; Landais, C.; Wong, S.J.; Licitra, L.; Tobias, J.S.; Burtness, B.; Ghi, M.G.; Cohen, E.E.; Grau, C.; et al. Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): An update on 107 randomized trials and 19,805 patients, on behalf of MACH-NC Group. Radiother. Oncol. 2021, 156, 281–293. [Google Scholar] [CrossRef] [PubMed]
- Bernier, J.; Cooper, J.S.; Pajak, T.F.; Van Glabbeke, M.; Bourhis, J.; Forastiere, A.; Ozsahin, E.M.; Jacobs, J.R.; Jassem, J.; Ang, K.-K.; et al. Defining risk levels in locally advanced head and neck cancers: A comparative analysis of concurrent postoperative radiation plus chemotherapy trials of the EORTC (#22931) and RTOG (# 9501). Head Neck 2005, 27, 843–850. [Google Scholar] [CrossRef] [PubMed]
- Budach, W.; Hehr, T.; Budach, V.; Belka, C.; Dietz, K. A meta-analysis of hyperfractionated and accelerated radiotherapy and combined chemotherapy and radiotherapy regimens in unresected locally advanced squamous cell carcinoma of the head and neck. BMC Cancer 2006, 6, 28. [Google Scholar] [CrossRef]
- Calais, G.; Alfonsi, M.; Bardet, E.; Sire, C.; Germain, T.; Bergerot, P.; Rhein, B.; Tortochaux, J.; Oudinot, P.; Bertrand, P. Randomized Trial of Radiation Therapy Versus Concomitant Chemotherapy and Radiation Therapy for Advanced-Stage Oropharynx Carcinoma. JNCI J. Natl. Cancer Inst. 1999, 91, 2081–2086. [Google Scholar] [CrossRef] [PubMed]
- Staar, S.; Rudat, V.; Stuetzer, H.; Dietz, A.; Volling, P.; Schroeder, M.; Flentje, M.; Eckel, H.E.; Mueller, R.-P. Intensified hyperfractionated accelerated radiotherapy limits the additional benefit of simultaneous chemotherapy—results of a multicentric randomized German trial in advanced head-and-neck cancer. Int. J. Radiat. Oncol. Biol. Phys. 2001, 50, 1161–1171. [Google Scholar] [CrossRef] [PubMed]
- Olmi, P.; Crispino, S.; Fallai, C.; Torri, V.; Rossi, F.; Bolner, A.; Amichetti, M.; Signor, M.; Taino, R.; Squadrelli, M.; et al. Locoregionally advanced carcinoma of the oropharynx: conventional radiotherapy vs. accelerated hyperfractionated radiotherapy vs. concomitant radiotherapy and chemotherapy—a multicenter randomized trial. Int. J. Radiat. Oncol. Biol. Phys. 2003, 55, 78–92. [Google Scholar] [CrossRef] [PubMed]
- Denis, F.; Garaud, P.; Bardet, E.; Alfonsi, M.; Sire, C.; Germain, T.; Bergerot, P.; Rhein, B.; Tortochaux, J.; Calais, G. Final Results of the 94–01 French Head and Neck Oncology and Radiotherapy Group Randomized Trial Comparing Radiotherapy Alone With Concomitant Radiochemotherapy in Advanced-Stage Oropharynx Carcinoma. J. Clin. Oncol. 2004, 22, 69–76. [Google Scholar] [CrossRef]
- Denis, F.; Garaud, P.; Bardet, E.; Alfonsi, M.; Sire, C.; Germain, T.; Bergerot, P.; Rhein, B.; Tortochaux, J.; Oudinot, P.; et al. Late toxicity results of the GORTEC 94-01 randomized trial comparing radiotherapy with concomitant radiochemotherapy for advanced-stage oropharynx carcinoma: comparison of LENT/SOMA, RTOG/EORTC, and NCI-CTC scoring systems. Int. J. Radiat. Oncol. Biol. Phys. 2003, 55, 93–98. [Google Scholar] [CrossRef]
- Semrau, R.; Mueller, R.-P.; Stuetzer, H.; Staar, S.; Schroeder, U.; Guntinas-Lichius, O.; Kocher, M.; Eich, H.T.; Dietz, A.; Flentje, M.; et al. Efficacy of intensified hyperfractionated and accelerated radiotherapy and concurrent chemotherapy with carboplatin and 5-fluorouracil: Updated results of a randomized multicentric trial in advanced head-and-neck cancer. Int. J. Radiat. Oncol. Biol. Phys. 2006, 64, 1308–1316. [Google Scholar] [CrossRef]
- Bourhis, J.; Sire, C.; Graff, P.; Grégoire, V.; Maingon, P.; Calais, G.; Gery, B.; Martin, L.; Alfonsi, M.; Desprez, P.; et al. Concomitant chemoradiotherapy versus acceleration of radiotherapy with or without concomitant chemotherapy in locally advanced head and neck carcinoma (GORTEC 99-02): an open-label phase 3 randomised trial. Lancet Oncol. 2012, 13, 145–153. [Google Scholar] [CrossRef]
- Budach, V.; Stuschke, M.; Budach, W.; Baumann, M.; Geismar, D.; Grabenbauer, G.; Lammert, I.; Jahnke, K.; Stueben, G.; Herrmann, T.; et al. Hyperfractionated Accelerated Chemoradiation With Concurrent Fluorouracil-Mitomycin Is More Effective Than Dose-Escalated Hyperfractionated Accelerated Radiation Therapy Alone in Locally Advanced Head and Neck Cancer: Final Results of the Radiotherapy Cooperative Clinical Trials Group of the German Cancer Society 95-06 Prospective Randomized Trial. J. Clin. Oncol. 2005, 23, 1125–1135. [Google Scholar] [CrossRef] [PubMed]
- Budach, V.; Stromberger, C.; Poettgen, C.; Baumann, M.; Budach, W.; Grabenbauer, G.; Marnitz, S.; Olze, H.; Wernecke, K.-D.; Ghadjar, P. Hyperfractionated Accelerated Radiation Therapy (HART) of 70.6 Gy With Concurrent 5-FU/Mitomycin C Is Superior to HART of 77.6 Gy Alone in Locally Advanced Head and Neck Cancer: Long-term Results of the ARO 95-06 Randomized Phase III Trial. Int. J. Radiat. Oncol. Biol. Phys. 2015, 91, 916–924. [Google Scholar] [CrossRef] [PubMed]
- Hanemaaijer, S.H.; Kok, I.C.; Fehrmann, R.S.N.; van der Vegt, B.; Gietema, J.A.; Plaat, B.E.C.; van Vugt, M.A.T.M.; Vergeer, M.R.; Leemans, C.R.; Langendijk, J.A.; et al. Comparison of Carboplatin With 5-Fluorouracil vs. Cisplatin as Concomitant Chemoradiotherapy for Locally Advanced Head and Neck Squamous Cell Carcinoma. Front. Oncol. 2020, 10, 541503. [Google Scholar] [CrossRef]
- Rades, D.; Seidl, D.; Janssen, S.; Bajrovic, A.; Hakim, S.G.; Wollenberg, B.; Schild, S.E. Do we need 5-FU in addition to cisplatin for chemoradiation of locally advanced head-and-neck cancer? Oral Oncol. 2016, 57, 40–45. [Google Scholar] [CrossRef]
- Baine, M.; Dorius, T.; Bennion, N.; Alam, M.; Smith, L.; Zhen, W.; Ganti, A. Chemoradiotherapy for locally advanced squamous cell carcinoma of the oropharynx: Does completion of systemic therapy affect outcomes? Oral Oncol. 2017, 73, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Rades, D.; Stoehr, M.; Kazic, N.; Hakim, S.G.; Walz, A.; Schild, S.E.; Dunst, J. Locally Advanced Stage IV Squamous Cell Carcinoma of the Head and Neck: Impact of Pre-Radiotherapy Hemoglobin Level and Interruptions During Radiotherapy. Endocrine 2008, 70, 1108–1114. [Google Scholar] [CrossRef] [PubMed]
- Xiang, M.; Gensheimer, M.F.; Pollom, E.L.; Holsinger, F.C.; Colevas, A.D.; Le, Q.-T.; Beadle, B.M. Prolongation of definitive head and neck cancer radiotherapy: Survival impact and predisposing factors. Radiother. Oncol. 2020, 156, 201–208. [Google Scholar] [CrossRef]
- Bonner, J.A.; Harari, P.M.; Giralt, J.; Cohen, R.B.; Jones, C.U.; Sur, R.K.; Raben, D.; Baselga, J.; Spencer, S.A.; Zhu, J.; et al. Radiotherapy plus cetuximab for locoregionally advanced head and neck cancer: 5-year survival data from a phase 3 randomised trial, and relation between cetuximab-induced rash and survival. Lancet Oncol. 2010, 11, 21–28. [Google Scholar] [CrossRef]
- Nassif, S.; Wichmann, J.; Strube, D.; Vassis, S.; Christiansen, H.; Steinmann, D. Cisplatin Versus Carboplatin and Paclitaxel in Radiochemotherapy for Patients With Locally Advanced Head and Neck Squamous Cell Carcinoma. Vivo 2022, 36, 821–832. [Google Scholar] [CrossRef]
- Maring, S.; Elsayad, K.; Stenner, M.; Rudack, C.; Haverkamp, U.; Rehkämper, J.; Wardelmann, E.; Eich, H.T. Efficacy of Carboplatin/Paclitaxel-Based Radiochemotherapy in Locally Advanced Squamous Cell Carcinoma of Head and Neck. Oncol. Res. Treat. 2018, 41, 736–743. [Google Scholar] [CrossRef]
- Zumsteg, Z.S.; Lok, B.H.; Ho, A.S.; Drill, E.; Zhang, Z.; Riaz, N.; Shiao, S.L.; Ma, J.; McBride, S.M.; Tsai, C.J.; et al. The toxicity and efficacy of concomitant chemoradiotherapy in patients aged 70 years and older with oropharyngeal carcinoma in the intensity-modulated radiotherapy era. Cancer 2016, 123, 1345–1353. [Google Scholar] [CrossRef]
- Xiang, M.; Colevas, A.D.; Holsinger, F.C.; Le, Q.-T.X.; Beadle, B.M. Survival After Definitive Chemoradiotherapy With Concurrent Cisplatin or Carboplatin for Head and Neck Cancer. J. Natl. Compr. Cancer Netw. 2019, 17, 1065–1073. [Google Scholar] [CrossRef]
- Beckham, T.H.; Barney, C.; Healy, E.; Wolfe, A.R.; Branstetter, A.; Yaney, A.; Riaz, N.; McBride, S.M.; Tsai, C.J.; Kang, J.; et al. Platinum-based regimens versus cetuximab in definitive chemoradiation for human papillomavirus-unrelated head and neck cancer. Int. J. Cancer 2020, 147, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Thanikachalam, K.; Krishnan, J.; Siddiqui, F.; Ali, H.Y.; Sheqwara, J. Carboplatin versus cetuximab chemoradiation in cisplatin ineligible locally advanced head and neck squamous cell carcinoma. J. Clin. Oncol. 2020, 38, e18555–e18555. [Google Scholar] [CrossRef]
- Sun, L.; Candelieri-Surette, D.; Anglin-Foote, T.; Lynch, J.A.; Maxwell, K.N.; D’avella, C.; Singh, A.; Aakhus, E.; Cohen, R.B.; Brody, R.M. Cetuximab-Based vs Carboplatin-Based Chemoradiotherapy for Patients With Head and Neck Cancer. JAMA Otolaryngol. Neck Surg. 2022, 148, 1022–1028. [Google Scholar] [CrossRef] [PubMed]
- Hamauchi, S.; Yokota, T.; Onozawa, Y.; Ogawa, H.; Onoe, T.; Kamijo, T.; Iida, Y.; Nishimura, T.; Onitsuka, T.; Yasui, H. Safety and efficacy of concurrent carboplatin plus radiotherapy for locally advanced head and neck cancer patients ineligible for treatment with cisplatin. Jpn. J. Clin. Oncol. 2015, 45, 1116–1121. [Google Scholar] [CrossRef] [PubMed]
- Prabhash, K.; Noronha, V.; Sharma, V.; Joshi, A.; Patil, V.; Laskar, S. Carboplatin-based concurrent chemoradiation therapy in locally advanced head and neck cancer patients who are unfit for cisplatin therapy. Indian J. Cancer 2017, 54, 453–457. [Google Scholar] [CrossRef]
- Lu, S.M.; Iganej, S.; Abdalla, I.A.; Buchschacher, G.L. Concurrent Radiotherapy and Triweekly Carboplatin for the Definitive Treatment of Locally Advanced Laryngeal Carcinoma. Am. J. Clin. Oncol. 2018, 41, 595–600. [Google Scholar] [CrossRef]
- Hamauchi, S.; Yokota, T.; Mizumachi, T.; Onozawa, Y.; Ogawa, H.; Onoe, T.; Kamijo, T.; Iida, Y.; Nishimura, T.; Onitsuka, T.; et al. Safety and efficacy of concurrent carboplatin or cetuximab plus radiotherapy for locally advanced head and neck cancer patients ineligible for treatment with cisplatin. Int. J. Clin. Oncol. 2019, 24, 468–475. [Google Scholar] [CrossRef]
- Gasparini, G.; Testolin, A.; Maluta, S.; Cristoferi, V.; Pozza, F. Treatment of locally advanced squamous-cell carcinoma of the head and neck with concurrent radiochemotherapy - randomized comparison of cisplatin versus carboplatin. Int. J. Oncol. 1993, 2, 185–190. [Google Scholar] [CrossRef]
- Jeremic, B.; Shibamoto, Y.; Stanisavljevic, B.; Milojevic, L.; Milicic, B.; Nikolic, N. Radiation therapy alone or with concurrent low-dose daily either cisplatin or carboplatin in locally advanced unresectable squamous cell carcinoma of the head and neck: a prospective randomized trial. Radiother. Oncol. 1997, 43, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Fountzilas, G.; Ciuleanu, E.; Dafni, U.; Plataniotis, G.; Kalogera-Fountzila, A.; Samantas, E.; Athanassiou, E.; Tzitzikas, J.; Ciuleanu, T.; Nikolaou, A.; et al. Concomitant Radiochemotherapy vs Radiotherapy Alone in Patients with Head and Neck Cancer: A Hellenic Cooperative Oncology Group Phase III Study. Med Oncol. 2004, 21, 095–108. [Google Scholar] [CrossRef]
- Homma, A.; Shirato, H.; Furuta, Y.; Nishioka, T.; Oridate, N.; Tsuchiya, K.; Nagahashi, T.; Aoyama, H.; Inuyama, Y.; Fukuda, S. Randomized Phase II Trial of Concomitant Chemoradiotherapy Using Weekly Carboplatin or Daily Low-Dose Cisplatin for Squamous Cell Carcinoma of the Head and Neck. Cancer J. 2004, 10, 326–332. [Google Scholar] [CrossRef] [PubMed]
- Chitapanarux, I.; Lorvidhaya, V.; Kamnerdsupaphon, P.; Sumitsawan, Y.; Tharavichitkul, E.; Sukthomya, V.; Ford, J. Chemoradiation comparing cisplatin versus carboplatin in locally advanced nasopharyngeal cancer: Randomised, non-inferiority, open trial. Eur. J. Cancer 2007, 43, 1399–1406. [Google Scholar] [CrossRef] [PubMed]
- Rades, D.; Ulbricht, T.; Hakim, S.; Schild, S. Cisplatin superior to carboplatin in adjuvant radiochemotherapy for locally advanced cancers of the oropharynx and oral cavity. Strahlenther. und Onkol. 2011, 188, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Wilkins, A.; Rosenfelder, N.; Schick, U.; Gupta, S.; Thway, K.; Nutting, C.; Harrington, K.; Newbold, K.; Bhide, S. Equivalence of cisplatin and carboplatin-based chemoradiation for locally advanced squamous cell carcinoma of the head and neck: A matched-pair analysis. Oral Oncol. 2013, 49, 615–619. [Google Scholar] [CrossRef]
- Nagasaka, M.; Zaki, M.; Issa, M.; Kim, H.; Abrams, J.; Sukari, A. Definitive chemoradiotherapy with carboplatin for squamous cell carcinoma of the head and neck. Laryngoscope 2017, 127, 2260–2264. [Google Scholar] [CrossRef]
- Amini, A.; Eguchi, M.; Jones, B.L.; Stokes, W.A.; Gupta, A.; McDermott, J.D.; Massarelli, E.; Bradley, C.J.; Karam, S.D. Comparing outcomes of concurrent chemotherapy regimens in patients 65 years old or older with locally advanced oropharyngeal carcinoma. Cancer 2018, 124, 4322–4331. [Google Scholar] [CrossRef]
- Iganej, S.; Beard, B.W.; Chen, J.; Buchschacher, G.L Jr.; Abdalla, I.A.; Thompson, L.D.R.; Bhattasali, O. Triweekly carboplatin as a potential de-intensification agent in concurrent chemoradiation for early-stage HPV-associated oropharyngeal cancer. Oral Oncol. 2019, 97, 18-22.
- McCusker, M.G.; Mehra, R.; Amr, S.; Taylor, R.J.; Cullen, K.J.; Goloubeva, O.G. Comparison of efficacy and toxicity of chemoradiation regimens for head and neck squamous cell carcinoma primary treatment. Head Neck 2021, 44, 749–759. [Google Scholar] [CrossRef]
- Dechaphunkul, A.; Danchaivijitr, P.; Jiratrachu, R.; Dechaphunkul, T.; Sookthon, C.; Jiarpinitnun, C.; Paoin, C.; Setakornnukul, J.; Suktitipat, B.; Pattaranutaporn, P.; et al. Real-world evidence of cisplatin versus carboplatin in patients with locally advanced nasopharyngeal carcinoma receiving concurrent chemoradiotherapy: A multicenter analysis. Asia-Pacific J. Clin. Oncol. 2022, 19, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Rades, D.; Seidl, D.; Janssen, S.; Bajrovic, A.; Hakim, SG.; Wollenberg, B.; Karner, K.; Strojan, P.; Schild, S.E. Chemoradiation of locally advanced squamous cell carcinoma of the head-and-neck (LASCCHN): Is 20mg/m(2) cisplatin on five days every four weeks an alternative to 100mg/m(2) cisplatin every three weeks? Oral Oncol. 2016, 59, 67–72. [Google Scholar] [CrossRef]
- Strojan, P.; Vermorken, J.B.; Beitler, J.J.; Saba, N.F.; Haigentz, M., Jr.; Bossi, P.; Worden, F.P.; Langendijk, J.A.; Eisbruch, A.; Mendenhall, W.M.; et al. Cumulative cisplatin dose in concurrent chemoradiotherapy for head and neck cancer: A systematic review. Head Neck 2015, 38, E2151–E2158. [Google Scholar] [CrossRef] [PubMed]
- Oliva, M.; Huang, S.H.; Xu, W.; Su, J.; Hansen, A.R.; Bratman, S.V.; Ringash, J.; Jang, R.; Cho, J.; Bayley, A.; et al. Impact of cisplatin dose and smoking pack-years in human papillomavirus–positive oropharyngeal squamous cell carcinoma treated with chemoradiotherapy. Eur. J. Cancer 2019, 118, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Babar, A.; Woody, N.M.; Ghanem, A.I.; Tsai, J.; Dunlap, N.E.; Schymick, M.; Liu, H.Y.; Burkey, B.B.; Lamarre, E.D.; Ku, J.A.; et al. Outcomes of Post-Operative Treatment with Concurrent Chemoradiotherapy (CRT) in High-Risk Resected Oral Cavity Squamous Cell Carcinoma (OCSCC): A Multi-Institutional Collaboration. Curr. Oncol. 2021, 28, 2409–2419. [Google Scholar] [CrossRef]
- Guan, J.; Li, Q.; Zhang, Y.; Xiao, N.; Chen, M.; Zhang, Y.; Li, L.; Chen, L. A meta-analysis comparing cisplatin-based to carboplatin-based chemotherapy in moderate to advanced squamous cell carcinoma of head and neck (SCCHN). Oncotarget 2016, 7, 7110–7119. [Google Scholar] [CrossRef] [PubMed]
- Aguiar, P.N. Jr.; Tadokoro, H.; da Silva, G.F.; Landgraf, M.M.; Noia Barreto, C.M.; Filardi, B.A.; Lopes, G.L. Jr.; Oliveira, P.; de Mello, R.A. Definitive chemoradiotherapy for squamous head and neck cancer: cisplatin versus carboplatin? A meta-analysis. Future Oncol. 2016, 12, 2755–2764. [Google Scholar] [CrossRef] [PubMed]
- Seidl, D.; Janssen, S.; Strojan, P.; Bajrovic, A.; E Schild, S.; Rades, D. Prognostic Factors After Definitive Radio(Chemo) Therapy of Locally Advanced Head and Neck Cancer. 2016, 36, 2523–2526.
- Ang, K.K.; Harris, J.; Wheeler, R.; Weber, R.; Rosenthal, D.I.; Nguyen-Tân, P.F.; Westra, W.H.; Chung, C.H.; Jordan, R.C.; Lu, C.; et al. Human Papillomavirus and Survival of Patients with Oropharyngeal Cancer. N. Engl. J. Med. 2010, 363, 24–35. [Google Scholar] [CrossRef]
- Argiris, A.; Karamouzis, M.V.; Johnson, J.T.; Heron, D.E.; Myers, E.; Eibling, D.; Cano, E.; Urba, S.; Gluckman, J.; Grandis, J.R.; et al. Long-Term Results of a Phase III Randomized Trial of Postoperative Radiotherapy With or Without Carboplatin in Patients With High-Risk Head and Neck Cancer. Laryngoscope 2008, 118, 444–449. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).