Submitted:
19 May 2023
Posted:
22 May 2023
You are already at the latest version
Abstract
Keywords:
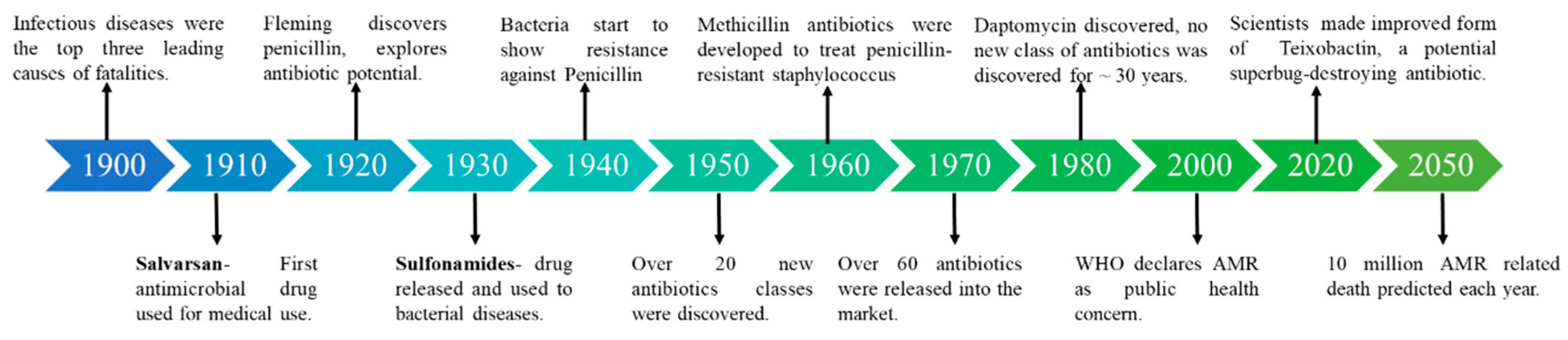
1. Introduction
2. Antibiotics and AMR
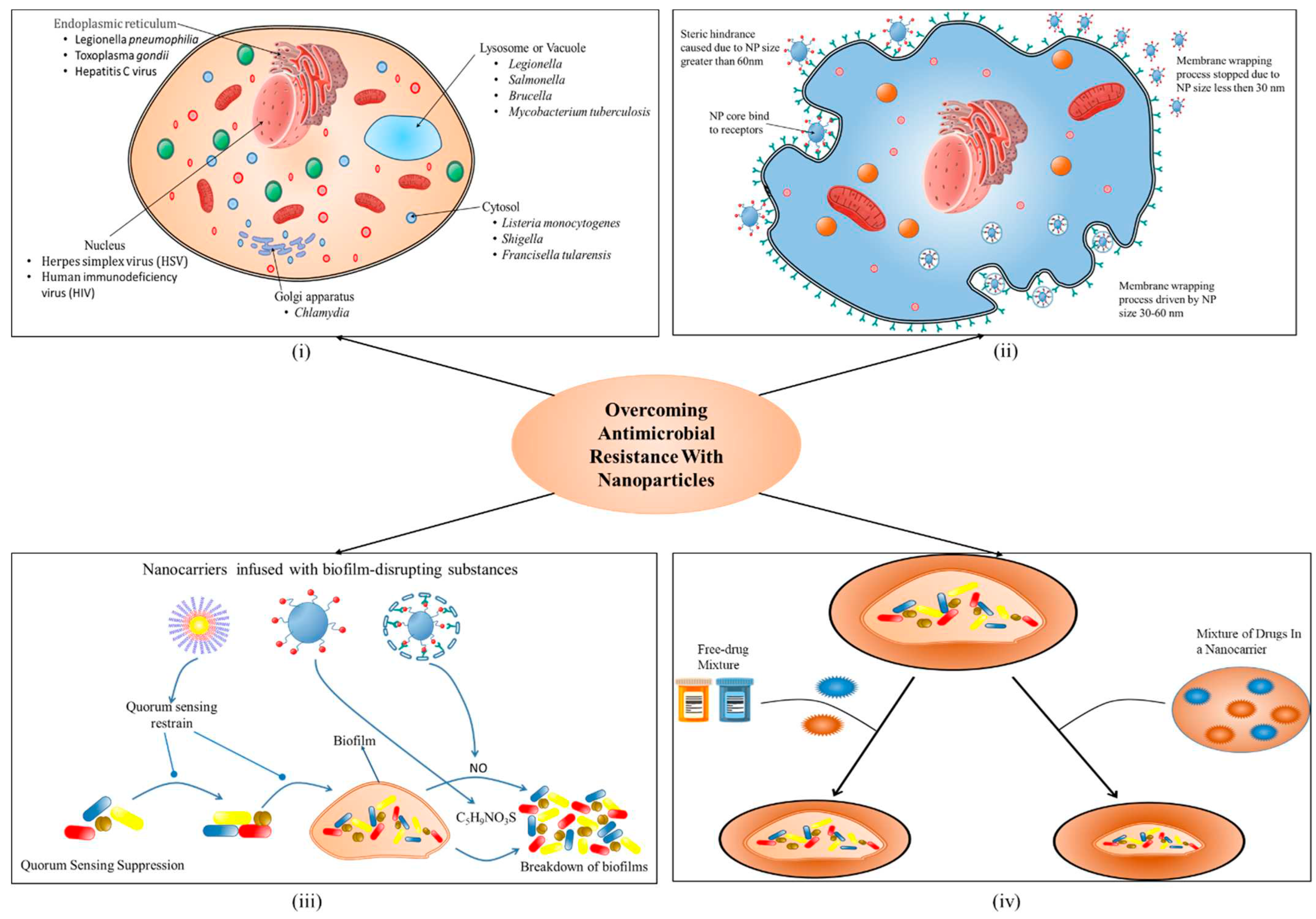
3. Role of nano-biotic in overcoming the challenge of antimicrobial
a. Reduced toxicity and enhanced stability
b. Targeted delivery to sites of infection
c. Stimuli-sensitive drug release
d. Directed towards biofilm microenvironments
e. Combined physical therapy
1. Photo-thermal Therapy (PTT)
| Type of nano-material | Nanoparticle | Particle size (nm) | Shape | Mode of action | Target bacteria | Factors affecting antimicrobial activity | References |
|---|---|---|---|---|---|---|---|
| Organic | Chitosan | 200 nm | Spherical, distinct & regular shape |
|
|
Concentration, pH | [93,94] |
| Quaternary Phosphonium or Sulfonium Groups | 1-100 nm | Polymorphic shape |
|
|
Particle size Shape |
[95,96] | |
| Poly-ε- lysine | 1-100 nm | Star shape |
|
|
Particle size Concentration |
[97,98] | |
| Quaternary ammonium compounds |
1-100 nm | Spherical shape |
|
|
Particle size Concentration |
[98,99] | |
| Inorganic | Silver nanoparticle | 5-100 nm | Sphere, Disk or Triangular shape |
|
|
Morphology | [100,101,102,103] |
| Gold nanoparticle | 1-100 nm | Spherical, star and flower-shaped |
|
|
Particle size | [104,105,106] | |
| Copper nanoparticle | 2- 350 nm | Spherical-mono-dispersed shape |
|
|
Particle size Concentration |
[107,108] | |
| Zinc oxide NP | 1-100 nm | Spherical, rods, needles, and platelets shaped |
|
|
Particle size Concentration Morphology |
[109,110,111] | |
| Magnesium oxide NP | 15-100 nm | Spherical and crystalline structure |
|
|
Particle size Concentration pH |
[112,113] | |
| Titanium oxide NP | 30-45 nm | Spherical |
|
|
Particle size Stability Concentration |
[114,115] | |
| Iron Oxide | 10-20 nm | Rod and Spherical shaped |
|
|
Particle size | [116,117] | |
| Carbon Based | Carbon nano tubes | 1-100 nm | Spiral or Rod shape |
|
|
Particle size Intrinsic properties |
[118,119] |
| Graphene nanoparticles | 12 nm | ellipsoid shape |
|
|
Particle size Particle shape |
[120,121] | |
| Fullerenes | 200 nm | Ball-shape |
|
|
Particle size | [122,123] | |
|
Composite |
Metal matrix | 1-100 nm | Rod, Peanut, Star shaped |
|
|
Particle size | [124,125] |
| Polymer matrix | 5-100 nm | Spherical, Rod shaped |
|
|
Depend on medium content | [126,127] | |
| Ceramic matrix | 1-100 nm | Cylindrical shapes |
|
|
Particle size | [128,129] |
2. Antibacterial Photodynamic Therapy (aPDT)
4. Aspect of One health with nanotechnology and economy
5. Conclusion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chakraborty, N.; Jha, D.; Roy, I.; Kumar, P.; Gaurav, S.S.; Marimuthu, K.; Ng, O.T.; Lakshminarayanan, R.; Verma, N.K.; Gautam, H.K. Nanobiotics against antimicrobial resistance: harnessing the power of nanoscale materials and technologies. J. Nanobiotechnology 2022, 20, 1–25. [Google Scholar] [CrossRef]
- Ipe, D.S.; Kumar, P.T.S.; Love, R.M.; Hamlet, S.M. Silver Nanoparticles at Biocompatible Dosage Synergistically Increases Bacterial Susceptibility to Antibiotics. Front. Microbiol. 2020, 11, 1074. [Google Scholar] [CrossRef] [PubMed]
- Medina, E.; Pieper, D.H. Tackling threats and future problems of multidrug-resistant bacteria. Curr. Top. Microbiol. Immunol. 2016, 398, 3–33. [Google Scholar] [CrossRef] [PubMed]
- Grace, D. Review of evidence on antimicrobial resistance and animal agriculture in developing countries. 2015. [CrossRef]
- Zhu, W.; Wei, Z.; Han, C.; Weng, X. Nanomaterials as Promising Theranostic Tools in Nanomedicine and Their Applications in Clinical Disease Diagnosis and Treatment. Nanomater. 2021, 11, 3346. [Google Scholar] [CrossRef]
- Chakravarty, M.; Vora, A. Nanotechnology-based antiviral therapeutics. Drug Deliv. Transl. Res. 2021, 11, 748–787. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.D.; Yang, S.S.; Wilson, B.K.; McManus, S.A.; Chen, C.V.H.H.; Prud’homme, R.K. Nanoparticle targeting of Gram-positive and Gram-negative bacteria for magnetic-based separations of bacterial pathogens. Appl. Nanosci. 2017, 7, 83–93. [Google Scholar] [CrossRef]
- Chowdhury, N.K.; Deepika; Choudhury, R. ; Sonawane, G.A.; Mavinamar, S.; Lyu, X.; Pandey, R.P.; Chang, C.M. Nanoparticles as an effective drug delivery system in COVID-19. Biomed. Pharmacother. 2021, 143, 112162. [Google Scholar] [CrossRef]
- Beyth, N.; Houri-Haddad, Y.; Domb, A.; Khan, W.; Hazan, R. Alternative antimicrobial approach: Nano-antimicrobial materials. Evidence-based Complement. Altern. Med. 2015, 2015. [Google Scholar] [CrossRef]
- Crofts, T.S.; Gasparrini, A.J.; Dantas, G. Next-generation approaches to understand and combat the antibiotic resistome. Nat. Rev. Microbiol. 2017 157 2017, 15, 422–434. [Google Scholar] [CrossRef]
- Maleki Dizaj, S.; Mennati, A.; Jafari, S.; Khezri, K.; Adibkia, K. Antimicrobial activity of carbon-based nanoparticles. Adv. Pharm. Bull. 2015, 5, 19–23. [Google Scholar] [CrossRef]
- Beyth, N.; Houri-Haddad, Y.; Domb, A.; Khan, W.; Hazan, R. Alternative antimicrobial approach: nano-antimicrobial materials. Evid. Based. Complement. Alternat. Med. 2015, 2015. [Google Scholar] [CrossRef] [PubMed]
- Barillo, D.J.; Marx, D.E. Silver in medicine: a brief history BC 335 to present. Burns 2014, 40 (Suppl. 1), S3–S8. [Google Scholar] [CrossRef] [PubMed]
- Hetta, H.F.; Ramadan, Y.N.; Al-Harbi, A.I.; A. Ahmed, E.; Battah, B.; Abd Ellah, N.H.; Zanetti, S.; Donadu, M.G. Nanotechnology as a Promising Approach to Combat Multidrug Resistant Bacteria: A Comprehensive Review and Future Perspectives. Biomed. 2023, 11, 413. [Google Scholar] [CrossRef]
- Ray, P.; Clément, M.; Martini, C.; Abdellah, I.; Beaunier, P.; Rodriguez-Lopez, J.L.; Huc, V.; Remita, H.; Lampre, I. Stabilisation of small mono- and bimetallic gold–silver nanoparticles using calix[8]arene derivatives. New J. Chem. 2018, 42, 14128–14137. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, Y.; Shi, Y.; Song, H.; Yu, C. Antibiotic-Free Antibacterial Strategies Enabled by Nanomaterials: Progress and Perspectives. Adv. Mater. 2020, 32, 1904106. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Chen, L.; Ye, C.; Yu, X. Co-selection of antibiotic resistance via copper shock loading on bacteria from a drinking water bio-filter. Environ. Pollut. 2018, 233, 132–141. [Google Scholar] [CrossRef]
- Li, L.G.; Xia, Y.; Zhang, T. Co-occurrence of antibiotic and metal resistance genes revealed in complete genome collection. ISME J. 2016, 11, 651–662. [Google Scholar] [CrossRef]
- Vats, P.; Kaur, U.J.; Rishi, P. Heavy metal-induced selection and proliferation of antibiotic resistance: A review. J. Appl. Microbiol. 2022, 132, 4058–4076. [Google Scholar] [CrossRef]
- Salam, L.B.; Obayori, S.O.; Nwaokorie, F.O.; Suleiman, A.; Mustapha, R. Metagenomic insights into effects of spent engine oil perturbation on the microbial community composition and function in a tropical agricultural soil. Environ. Sci. Pollut. Res. 2017, 24, 7139–7159. [Google Scholar] [CrossRef]
- McEwen, S.A.; Collignon, P.J. Antimicrobial Resistance: a One Health Perspective. Microbiol. Spectr. 2018, 6. [Google Scholar] [CrossRef]
- Chandy, S. Antimicrobial resistance and inappropriate use of antimicrobials: Can we rise to the challenge? Indian J. Pharmacol. 2015, 47, 347. [Google Scholar] [CrossRef] [PubMed]
- Aslam, B.; Wang, W.; Arshad, M.I.; Khurshid, M.; Muzammil, S.; Rasool, M.H.; Nisar, M.A.; Alvi, R.F.; Aslam, M.A.; Qamar, M.U.; et al. Antibiotic resistance: a rundown of a global crisis. Infect. Drug Resist. 2018, 11, 1645. [Google Scholar] [CrossRef] [PubMed]
- Khanal, S.; Acharya, U.; Trotter, A.B.; Tripathi, P.; Koirala, S.; Pahari, B.; Acharya, S.P. Challenges and opportunities in the implementation of an antimicrobial stewardship program in Nepal. Antimicrob. Steward. Healthc. Epidemiol. 2023, 3, e58. [Google Scholar] [CrossRef]
- Antibiotics and Antimicrobial Resistance Genes. 2020. [CrossRef]
- Manyi-Loh, C.; Mamphweli, S.; Meyer, E.; Okoh, A. Antibiotic Use in Agriculture and Its Consequential Resistance in Environmental Sources: Potential Public Health Implications. Mol. 2018, 23, 795. [Google Scholar] [CrossRef] [PubMed]
- Xiao, K.Q.; Li, B.; Ma, L.; Bao, P.; Zhou, X.; Zhang, T.; Zhu, Y.G. Metagenomic profiles of antibiotic resistance genes in paddy soils from South China. FEMS Microbiol. Ecol. 2016, 92, 23. [Google Scholar] [CrossRef] [PubMed]
- You, Y.; Silbergeld, E.K. Learning from agriculture: Understanding low-dose antimicrobials as drivers of resistome expansion. Front. Microbiol. 2014, 5, 90890. [Google Scholar]
- Lipsitch, M.; Singer, R.S.; Levin, B.R. Antibiotics in agriculture: When is it time to close the barn door? Proc. Natl. Acad. Sci. 2002, 99, 5752–5754. [Google Scholar] [CrossRef]
- Hiller, C.X.; Hübner, U.; Fajnorova, S.; Schwartz, T.; Drewes, J.E. Antibiotic microbial resistance (AMR) removal efficiencies by conventional and advanced wastewater treatment processes: A review. Sci. Total Environ. 2019, 685, 596–608. [Google Scholar] [CrossRef]
- Zhan, Y.; Hu, X.; Li, Y.; Wang, Y.; Chen, H.; Omolo, C.A.; Govender, T.; Li, H.; Huang, F.; Shi, L.; et al. Antimicrobial Hybrid Amphiphile via Dynamic Covalent Bonds Enables Bacterial Biofilm Dispersal and Bacteria Eradication. Adv. Funct. Mater. 2023, 33, 2214299. [Google Scholar] [CrossRef]
- Himanshu; R. Prudencio, C.; da Costa, A.C.; Leal, E.; Chang, C.M.; Pandey, R.P. Systematic Surveillance and Meta-Analysis of Antimicrobial Resistance and Food Sources from China and the USA. Antibiot. 2022, 11, 1471. [Google Scholar] [CrossRef]
- Blair, J.M.A.; Webber, M.A.; Baylay, A.J.; Ogbolu, D.O.; Piddock, L.J.V. Molecular mechanisms of antibiotic resistance. Nat. Rev. Microbiol. 2014, 13, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Shi, L.; Su, L.; Van der Mei, H.C.; Jutte, P.C.; Ren, Y.; Busscher, H.J. Nanotechnology-based antimicrobials and delivery systems for biofilm-infection control. Chem. Soc. Rev. 2019, 48, 428–446. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-López, E.; Gomes, D.; Esteruelas, G.; Bonilla, L.; Lopez-Machado, A.L.; Galindo, R.; Cano, A.; Espina, M.; Ettcheto, M.; Camins, A.; et al. Metal-Based Nanoparticles as Antimicrobial Agents: An Overview. Nanomater. 2020, 10, 292. [Google Scholar] [CrossRef]
- Situation Analysis: Antibiotic Use and Resistance in India - One Health Trust.
- Liu, P.Y.; Lee, Y.L.; Lu, M.C.; Shao, P.L.; Lu, P.L.; Chen, Y.H.; Cheng, S.H.; Ko, W.C.; Lin, C.Y.; Wu, T.S.; et al. National surveillance of antimicrobial susceptibility of bacteremic gram-negative bacteria with emphasis on community-acquired resistant isolates: Report from the 2019 surveillance of multicenter antimicrobial resistance in Taiwan (SMART). Antimicrob. Agents Chemother. 2020, 64. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Li, M.; Ren, J.; Qu, X. Metal nanoclusters: novel probes for diagnostic and therapeutic applications. Chem. Soc. Rev. 2015, 44, 8636–8663. [Google Scholar] [CrossRef] [PubMed]
- Sadhu, S.; Rizvi, Z.A.; Pandey, R.P.; Dalal, R.; Rathore, D.K.; Kumar, B.; Pandey, M.; Kumar, Y.; Goel, R.; Maiti, T.K.; et al. Gefitinib Results in Robust Host-Directed Immunity Against Salmonella Infection Through Proteo-Metabolomic Reprogramming. Front. Immunol. 2021, 12, 888. [Google Scholar] [CrossRef]
- Gao, W.; Chen, Y.; Zhang, Y.; Zhang, Q.; Zhang, L. Nanoparticle-based local antimicrobial drug delivery. Adv. Drug Deliv. Rev. 2018, 127, 46–57. [Google Scholar] [CrossRef]
- Murugaiyan, J.; Anand Kumar, P.; Rao, G.S.; Iskandar, K.; Hawser, S.; Hays, J.P.; Mohsen, Y.; Adukkadukkam, S.; Awuah, W.A.; Jose, R.A.M.; et al. Progress in Alternative Strategies to Combat Antimicrobial Resistance: Focus on Antibiotics. Antibiot. 2022, 11, 200. [Google Scholar] [CrossRef]
- Baptista, P. V.; McCusker, M.P.; Carvalho, A.; Ferreira, D.A.; Mohan, N.M.; Martins, M.; Fernandes, A.R. Nano-strategies to fight multidrug resistant bacteria-"A Battle of the Titans". Front. Microbiol. 2018, 9, 1441. [Google Scholar] [CrossRef]
- Wang, L.; Hu, C.; Shao, L. The antimicrobial activity of nanoparticles: present situation and prospects for the future. Int. J. Nanomedicine 2017, 12, 1227–1249. [Google Scholar] [CrossRef]
- Armstead, A.L.; Li, B. Nanomedicine as an emerging approach against intracellular pathogens. Int. J. Nanomedicine 2011, 6, 3281. [Google Scholar] [CrossRef] [PubMed]
- Hoshyar, N.; Gray, S.; Han, H.; Bao, G. The effect of nanoparticle size on in vivo pharmacokinetics and cellular interaction. 2016, 11, 673–692. [Google Scholar] [CrossRef] [PubMed]
- Pal-Bhowmick, I.; Pati Pandey, R.; Jarori, G.K.; Kar, S.; Sahal, D. Structural and functional studies on Ribonuclease S, retro S and retro-inverso S peptides. Biochem. Biophys. Res. Commun. 2007, 364, 608–613. [Google Scholar] [CrossRef] [PubMed]
- Kirtane, A.R.; Verma, M.; Karandikar, P.; Furin, J.; Langer, R.; Traverso, G. Nanotechnology approaches for global infectious diseases. Nat. Nanotechnol. 2021, 16, 369–384. [Google Scholar] [CrossRef] [PubMed]
- Baber, O.; Jang, M.; Barber, D.; Powers, K. Amorphous silica coatings on magnetic nanoparticles enhance stability and reduce toxicity to in vitro BEAS-2B cells. Inhal. Toxicol. 2011, 23, 532–543. [Google Scholar] [CrossRef] [PubMed]
- Insua, I.; Majok, S.; Peacock, A.F.A.; Krachler, A.M.; Fernandez-Trillo, F. Preparation and antimicrobial evaluation of polyion complex (PIC) nanoparticles loaded with polymyxin B. Eur. Polym. J. 2017, 87, 478–486. [Google Scholar] [CrossRef] [PubMed]
- van Gent, M.E.; Ali, M.; Nibbering, P.H.; Kłodzińska, S.N. Current Advances in Lipid and Polymeric Antimicrobial Peptide Delivery Systems and Coatings for the Prevention and Treatment of Bacterial Infections. Pharm. 2021, 13, 1840. [Google Scholar] [CrossRef]
- Hassan, D.; Omolo, C.A.; Fasiku, V.O.; Elrashedy, A.A.; Mocktar, C.; Nkambule, B.; Soliman, M.E.S.; Govender, T. Formulation of pH-Responsive Quatsomes from Quaternary Bicephalic Surfactants and Cholesterol for Enhanced Delivery of Vancomycin against Methicillin Resistant Staphylococcus aureus. Pharm. 2020, 12, 1093. [Google Scholar] [CrossRef]
- Mukherjee, S.; Mishra, A.K.; Peer, G.D.G.; Bagabir, S.A.; Haque, S.; Pandey, R.P.; Raj, V.S.; Jain, N.; Pandey, A.; Kar, S.K. The Interplay of the Unfolded Protein Response in Neurodegenerative Diseases: A Therapeutic Role of Curcumin. Front. Aging Neurosci. 2021, 13, 771. [Google Scholar] [CrossRef]
- Bellotto, O.; Semeraro, S.; Bandiera, A.; Tramer, F.; Pavan, N.; Marchesan, S. Polymer Conjugates of Antimicrobial Peptides (AMPs) with d-Amino Acids (d-aa): State of the Art and Future Opportunities. Pharm. 2022, 14, 446. [Google Scholar] [CrossRef]
- Lee, N.Y.; Ko, W.C.; Hsueh, P.R. Nanoparticles in the Treatment of Infections Caused by Multidrug-Resistant Organisms. Front. Pharmacol. 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Pelgrift, R.Y.; Friedman, A.J. Nanotechnology as a therapeutic tool to combat microbial resistance. Adv. Drug Deliv. Rev. 2013, 65, 1803–1815. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, R.; Srivastava, M.; Jain, P.; Pandey, R.P.; Asthana, S.; Kumar, D.; Raj, V.S. Development of potential proteasome inhibitors against Mycobacterium tuberculosis. J. Biomol. Struct. Dyn. 2022, 40, 2189–2203. [Google Scholar] [CrossRef] [PubMed]
- Geersing, A.; de Vries, R.H.; Jansen, G.; Rots, M.G.; Roelfes, G. Folic acid conjugates of a bleomycin mimic for selective targeting of folate receptor positive cancer cells. Bioorg. Med. Chem. Lett. 2019, 29, 1922–1927. [Google Scholar] [CrossRef] [PubMed]
- Layek, B.; Sadhukha, T.; Panyam, J.; Prabha, S. Nano-engineered mesenchymal stem cells increase therapeutic efficacy of anticancer drug through true active tumor targeting. Mol. Cancer Ther. 2018, 17, 1196–1206. [Google Scholar] [CrossRef] [PubMed]
- Simón-Gracia, L.; Hunt, H.; Scodeller, P.; Gaitzsch, J.; Kotamraju, V.R.; Sugahara, K.N.; Tammik, O.; Ruoslahti, E.; Battaglia, G.; Teesalu, T. iRGD peptide conjugation potentiates intraperitoneal tumor delivery of paclitaxel with polymersomes. Biomaterials 2016, 104, 247–257. [Google Scholar] [CrossRef] [PubMed]
- Lehar, S.M.; Pillow, T.; Xu, M.; Staben, L.; Kajihara, K.K.; Vandlen, R.; DePalatis, L.; Raab, H.; Hazenbos, W.L.; Hiroshi Morisaki, J.; et al. Novel antibody–antibiotic conjugate eliminates intracellular S. aureus. Nat. 2015, 527, 323–328. [Google Scholar] [CrossRef]
- Kirtane, A.R.; Verma, M.; Karandikar, P.; Furin, J.; Langer, R.; Traverso, G. Nanotechnology approaches for global infectious diseases. Nat. Nanotechnol. 2021, 16, 369–384. [Google Scholar] [CrossRef]
- Masri, A.; Anwar, A.; Khan, N.A.; Siddiqui, R. The Use of Nanomedicine for Targeted Therapy against Bacterial Infections. Antibiot. 2019, Vol. 8, Page 260 2019, 8, 260. [Google Scholar] [CrossRef]
- Yetisgin, A.A.; Cetinel, S.; Zuvin, M.; Kosar, A.; Kutlu, O. Therapeutic Nanoparticles and Their Targeted Delivery Applications. Mol. 2020, 25, 2193. [Google Scholar] [CrossRef]
- Wang, S.; Gao, Y.; Jin, Q.; Ji, J. Emerging antibacterial nanomedicine for enhanced antibiotic therapy. Biomater. Sci. 2020, 8, 6825–6839. [Google Scholar] [CrossRef]
- Nazir, F.; Tabish, T.A.; Tariq, F.; Iftikhar, S.; Wasim, R.; Shahnaz, G. Stimuli-sensitive drug delivery systems for site-specific antibiotic release. Drug Discov. Today 2022, 27, 1698–1705. [Google Scholar] [CrossRef] [PubMed]
- Visscher, L.E.; Dang, H.P.; Knackstedt, M.A.; Hutmacher, D.W.; Tran, P.A. 3D printed Polycaprolactone scaffolds with dual macro-microporosity for applications in local delivery of antibiotics. Mater. Sci. Eng. C 2018, 87, 78–89. [Google Scholar] [CrossRef] [PubMed]
- Mi, L.; Jiang, S. Synchronizing nonfouling and antimicrobial properties in a zwitterionic hydrogel. Biomaterials 2012, 33, 8928–8933. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, S.; Agrawal, A.; Knabe, C.; Ducheyne, P. Sol–gel silica controlled release thin films for the inhibition of methicillin-resistant Staphylococcus aureus. Biomaterials 2014, 35, 509–517. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yuan, Q.; Feng, W.; Pu, W.; Ding, J.; Zhang, H.; Li, X.; Yang, B.; Dai, Q.; Cheng, L.; et al. Targeted delivery of antibiotics to the infected pulmonary tissues using ROS-responsive nanoparticles. J. Nanobiotechnology 2019, 17, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Thamphiwatana, S.; Gao, W.; Pornpattananangkul, D.; Zhang, Q.; Fu, V.; Li, J.; Li, J.; Obonyo, M.; Zhang, L. Phospholipase A2-responsive antibiotic delivery via nanoparticle-stabilized liposomes for the treatment of bacterial infection. J. Mater. Chem. B 2014, 2, 8201–8207. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, R.; Wang, Y.; Wang, P.; Pu, J.; Xu, X.; Chen, F.; Jiang, L.; Jiang, Q.; Yan, F. Antibiofilm activity of ultra-small gold nanoclusters against Fusobacterium nucleatum in dental plaque biofilms. J. Nanobiotechnology 2022, 20, 1–17. [Google Scholar] [CrossRef]
- Kassinger, S.J.; van Hoek, M.L. Biofilm architecture: An emerging synthetic biology target. Synth. Syst. Biotechnol. 2020, 5, 1–10. [Google Scholar] [CrossRef]
- Flemming, H.C.; Wingender, J. The biofilm matrix. Nat. Rev. Microbiol. 2010, 8, 623–633. [Google Scholar] [CrossRef]
- Flemming, H.C.; Wingender, J.; Szewzyk, U.; Steinberg, P.; Rice, S.A.; Kjelleberg, S. Biofilms: an emergent form of bacterial life. Nat. Rev. Microbiol. 2016, 14, 563–575. [Google Scholar] [CrossRef] [PubMed]
- Ding, M.; Zhao, W.; Song, L.J.; Luan, S.F. Stimuli-responsive nanocarriers for bacterial biofilm treatment. Rare Met. 2022, 41, 482–498. [Google Scholar] [CrossRef] [PubMed]
- Dutt, Y.; Dhiman, R.; Singh, T.; Vibhuti, A.; Gupta, A.; Pandey, R.P.; Raj, V.S.; Chang, C.M.; Priyadarshini, A. The Association between Biofilm Formation and Antimicrobial Resistance with Possible Ingenious Bio-Remedial Approaches. Antibiotics 2022, 11. [Google Scholar] [CrossRef] [PubMed]
- Folliero, V.; Franci, G.; Dell’annunziata, F.; Giugliano, R.; Foglia, F.; Sperlongano, R.; De Filippis, A.; Finamore, E.; Galdiero, M. Evaluation of Antibiotic Resistance and Biofilm Production among Clinical Strain Isolated from Medical Devices. Int. J. Microbiol. 2021, 2021. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Romero, M.; Travanut, A.; Monteiro, P.F.; Jordana-Lluch, E.; Hardie, K.R.; Williams, P.; Alexander, M.R.; Alexander, C. Dual bioresponsive antibiotic and quorum sensing inhibitor combination nanoparticles for treatment of Pseudomonas aeruginosa biofilms in vitro and ex vivo. Biomater. Sci. 2019, 7, 4099–4111. [Google Scholar] [CrossRef]
- He, J.; Zhu, X.; Qi, Z.; Wang, C.; Mao, X.; Zhu, C.; He, Z.; Li, M.; Tang, Z. Killing dental pathogens using antibacterial graphene oxide. ACS Appl. Mater. Interfaces 2015, 7, 5605–5611. [Google Scholar] [CrossRef]
- Lee, J.H.; El-Fiqi, A.; Mandakhbayar, N.; Lee, H.H.; Kim, H.W. Drug/ion co-delivery multi-functional nanocarrier to regenerate infected tissue defect. Biomaterials 2017, 142, 62–76. [Google Scholar] [CrossRef]
- Imlay, J.A. The molecular mechanisms and physiological consequences of oxidative stress: lessons from a model bacterium. Nat. Rev. Microbiol. 2013, 11, 443–454. [Google Scholar] [CrossRef]
- Gao, L.; Liu, Y.; Kim, D.; Li, Y.; Hwang, G.; Naha, P.C.; Cormode, D.P.; Koo, H. Nanocatalysts promote Streptococcus mutans biofilm matrix degradation and enhance bacterial killing to suppress dental caries in vivo. Biomaterials 2016, 101, 272–284. [Google Scholar] [CrossRef]
- Tian, X.; Jiang, X.; Welch, C.; Croley, T.R.; Wong, T.Y.; Chen, C.; Fan, S.; Chong, Y.; Li, R.; Ge, C.; et al. Bactericidal Effects of Silver Nanoparticles on Lactobacilli and the Underlying Mechanism. ACS Appl. Mater. Interfaces 2018, 10, 8443–8450. [Google Scholar] [CrossRef]
- Zhi, D.; Yang, T.; O’Hagan, J.; Zhang, S.; Donnelly, R.F. Photothermal therapy. J. Control. Release 2020, 325, 52–71. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Aw, J.; Xing, B. Nanostructures for NIR light-controlled therapies. Nanoscale 2017, 9, 3698–3718. [Google Scholar] [CrossRef] [PubMed]
- Teodorescu, F.; Oz, Y.; Quéniat, G.; Abderrahmani, A.; Foulon, C.; Lecoeur, M.; Sanyal, R.; Sanyal, A.; Boukherroub, R.; Szunerits, S. Photothermally triggered on-demand insulin release from reduced graphene oxide modified hydrogels. J. Control. Release 2017, 246, 164–173. [Google Scholar] [CrossRef] [PubMed]
- Turcheniuk, K.; Boukherroub, R.; Szunerits, S. Gold–graphene nanocomposites for sensing and biomedical applications. J. Mater. Chem. B 2015, 3, 4301–4324. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Jiang, G.; Zhang, Y.; Liu, D.; Xu, B.; Zhou, J. Near-infrared light triggered and separable microneedles for transdermal delivery of metformin in diabetic rats. J. Mater. Chem. B 2017, 5, 9507–9513. [Google Scholar] [CrossRef]
- Venditti, I. Engineered Gold-Based Nanomaterials: Morphologies and Functionalities in Biomedical Applications. A Mini Review. Bioeng. 2019, 6, 53. [Google Scholar] [CrossRef]
- Chen, Q.; Wang, C.; Zhan, Z.; He, W.; Cheng, Z.; Li, Y.; Liu, Z. Near-infrared dye bound albumin with separated imaging and therapy wavelength channels for imaging-guided photothermal therapy. Biomaterials 2014, 35, 8206–8214. [Google Scholar] [CrossRef]
- Justin, R.; Chen, B. Strong and conductive chitosan–reduced graphene oxide nanocomposites for transdermal drug delivery. J. Mater. Chem. B 2014, 2, 3759–3770. [Google Scholar] [CrossRef]
- Yu, Y.; Li, P.; Zhu, C.; Ning, N.; Zhang, S.; Vancso, G.J. Multifunctional and Recyclable Photothermally Responsive Cryogels as Efficient Platforms for Wound Healing. Adv. Funct. Mater. 2019, 29, 1904402. [Google Scholar] [CrossRef]
- Yan, D.; Li, Y.; Liu, Y.; Li, N.; Zhang, X.; Yan, C. Antimicrobial Properties of Chitosan and Chitosan Derivatives in the Treatment of Enteric Infections. Molecules 2021, 26. [Google Scholar] [CrossRef]
- Jha, R.; Mayanovic, R.A. A Review of the Preparation, Characterization, and Applications of Chitosan Nanoparticles in Nanomedicine. Nanomater. 2023, 13, 1302. [Google Scholar] [CrossRef] [PubMed]
- Alkhalifa, S.; Jennings, M.C.; Granata, D.; Klein, M.; Wuest, W.M.; Minbiole, K.P.C.; Carnevale, V. Analysis of the Destabilization of Bacterial Membranes by Quaternary Ammonium Compounds: A Combined Experimental and Computational Study. ChemBioChem 2020, 21, 1510–1516. [Google Scholar] [CrossRef] [PubMed]
- Eygeris, Y.; Patel, S.; Jozic, A.; Sahay, G.; Sahay, G. Deconvoluting Lipid Nanoparticle Structure for Messenger RNA Delivery. Nano Lett. 2020, 20, 4543–4549. [Google Scholar] [CrossRef] [PubMed]
- Tan, Z.; Shi, Y.; Xing, B.; Hou, Y.; Cui, J.; Jia, S. The antimicrobial effects and mechanism of ε-poly-lysine against Staphylococcus aureus. Bioresour. Bioprocess. 2019, 6, 1–10. [Google Scholar] [CrossRef]
- Zheng, M.; Pan, M.; Zhang, W.; Lin, H.; Wu, S.; Lu, C.; Tang, S.; Liu, D.; Cai, J. Poly(α-l-lysine)-based nanomaterials for versatile biomedical applications: Current advances and perspectives. Bioact. Mater. 2021, 6, 1878–1909. [Google Scholar] [CrossRef]
- Gerba, C.P. Quaternary ammonium biocides: Efficacy in application. Appl. Environ. Microbiol. 2015, 81, 464–469. [Google Scholar] [CrossRef]
- Li, W.R.; Xie, X.B.; Shi, Q.S.; Zeng, H.Y.; Ou-Yang, Y.S.; Chen, Y. Ben Antibacterial activity and mechanism of silver nanoparticles on Escherichia coli. Appl. Microbiol. Biotechnol. 2010, 85, 1115–1122. [Google Scholar] [CrossRef]
- Mikhailova, E.O. Silver Nanoparticles: Mechanism of Action and Probable Bio-Application. J. Funct. Biomater. 2020, 11, 84. [Google Scholar] [CrossRef]
- Stabryla, L.M.; Johnston, K.A.; Millstone, J.E.; Gilbertson, L.M. Emerging investigator series: it’s not all about the ion: support for particle-specific contributions to silver nanoparticle antimicrobial activity. Environ. Sci. Nano 2018, 5, 2047–2068. [Google Scholar] [CrossRef]
- Cheon, J.Y.; Kim, S.J.; Rhee, Y.H.; Kwon, O.H.; Park, W.H. Shape-dependent antimicrobial activities of silver nanoparticles. Int. J. Nanomedicine 2019, 14, 2773–2780. [Google Scholar] [CrossRef]
- Rudramurthy, G.R.; Swamy, M.K.; Sinniah, U.R.; Ghasemzadeh, A. Nanoparticles: Alternatives Against Drug-Resistant Pathogenic Microbes. Mol. 2016, 21, 836. [Google Scholar] [CrossRef] [PubMed]
- Beyth, N.; Yudovin-Farber, I.; Perez-Davidi, M.; Domb, A.J.; Weiss, E.I. Polyethyleneimine nanoparticles incorporated into resin composite cause cell death and trigger biofilm stress in vivo. Proc. Natl. Acad. Sci. U. S. A. 2010, 107, 22038–22043. [Google Scholar] [CrossRef] [PubMed]
- Su, C.; Huang, K.; Li, H.H.; Lu, Y.G.; Zheng, D.L. Antibacterial Properties of Functionalized Gold Nanoparticles and Their Application in Oral Biology. J. Nanomater. 2020, 2020. [Google Scholar] [CrossRef]
- Sharma, P.; Goyal, D.; Chudasama, B. Antibacterial activity of colloidal copper nanoparticles against Gram-negative (Escherichia coli and Proteus vulgaris) bacteria. Lett. Appl. Microbiol. 2022, 74, 695–706. [Google Scholar] [CrossRef] [PubMed]
- Hassan, S.E.-D.; Salem, S.S.; Fouda, A.; Awad, M.A.; El-Gamal, M.S.; Abdo, A.M. New approach for antimicrobial activity and bio-control of various pathogens by biosynthesized copper nanoparticles using endophytic actinomycetes. J. Radiat. Res. Appl. Sci. 2018, 11, 262–270. [Google Scholar] [CrossRef]
- Sirelkhatim, A.; Mahmud, S.; Seeni, A.; Kaus, N.H.M.; Ann, L.C.; Bakhori, S.K.M.; Hasan, H.; Mohamad, D. Review on Zinc Oxide Nanoparticles: Antibacterial Activity and Toxicity Mechanism. Nano-Micro Lett. 2015, 7, 219. [Google Scholar] [CrossRef] [PubMed]
- Chandra, H.; Patel, D.; Kumari, P.; Jangwan, J.S.; Yadav, S. Phyto-mediated synthesis of zinc oxide nanoparticles of Berberis aristata: Characterization, antioxidant activity and antibacterial activity with special reference to urinary tract pathogens. Mater. Sci. Eng. C 2019, 102, 212–220. [Google Scholar] [CrossRef]
- Jin, S.E.; Jin, H.E. Antimicrobial Activity of Zinc Oxide Nano/Microparticles and Their Combinations against Pathogenic Microorganisms for Biomedical Applications: From Physicochemical Characteristics to Pharmacological Aspects. Nanomater. 2021, 11, 263. [Google Scholar] [CrossRef]
- Leung, Y.H.; Ng, A.M.C.; Xu, X.; Shen, Z.; Gethings, L.A.; Wong, M.T.; Chan, C.M.N.; Guo, M.Y.; Ng, Y.H.; Djurišic̈, A.B.; et al. Mechanisms of Antibacterial Activity of MgO: Non-ROS Mediated Toxicity of MgO Nanoparticles Towards Escherichia coli. Small 2014, 10, 1171–1183. [Google Scholar] [CrossRef]
- Fouda, A.; Eid, A.M.; Abdel-Rahman, M.A.; EL-Belely, E.F.; Awad, M.A.; Hassan, S.E.D.; AL-Faifi, Z.E.; Hamza, M.F. Enhanced Antimicrobial, Cytotoxicity, Larvicidal, and Repellence Activities of Brown Algae, Cystoseira crinita-Mediated Green Synthesis of Magnesium Oxide Nanoparticles. Front. Bioeng. Biotechnol. 2022, 10, 849921. [Google Scholar] [CrossRef]
- Khashan, K.S.; Sulaiman, G.M.; Abdulameer, F.A.; Albukhaty, S.; Ibrahem, M.A.; Al-Muhimeed, T.; Alobaid, A.A. Antibacterial Activity of TiO2 Nanoparticles Prepared by One-Step Laser Ablation in Liquid. Appl. Sci. 2021, 11, 4623. [Google Scholar] [CrossRef]
- Bekele, E.T.; Gonfa, B.A.; Zelekew, O.A.; Belay, H.H.; Sabir, F.K. Synthesis of Titanium Oxide Nanoparticles Using Root Extract of Kniphofia foliosa as a Template, Characterization, and Its Application on Drug Resistance Bacteria. J. Nanomater. 2020, 2020. [Google Scholar] [CrossRef]
- Arakha, M.; Pal, S.; Samantarrai, D.; Panigrahi, T.K.; Mallick, B.C.; Pramanik, K.; Mallick, B.; Jha, S. Antimicrobial activity of iron oxide nanoparticle upon modulation of nanoparticle-bacteria interface. Sci. Reports 2015, 5, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Arias, L.S.; Pessan, J.P.; Vieira, A.P.M.; De Lima, T.M.T.; Delbem, A.C.B.; Monteiro, D.R. Iron Oxide Nanoparticles for Biomedical Applications: A Perspective on Synthesis, Drugs, Antimicrobial Activity, and Toxicity. Antibiot. 2018, 7, 46. [Google Scholar] [CrossRef] [PubMed]
- Dizaj, S.M.; Mennati, A.; Jafari, S.; Khezri, K.; Adibkia, K. Antimicrobial Activity of Carbon-Based Nanoparticles. Adv. Pharm. Bull. 2015, 5, 19. [Google Scholar] [CrossRef]
- Weise, K.; Winter, L.; Fischer, E.; Kneis, D.; de la Cruz Barron, M.; Kunze, S.; Berendonk, T.U.; Jungmann, D.; Klümper, U. Multiwalled Carbon Nanotubes Promote Bacterial Conjugative Plasmid Transfer. Microbiol. Spectr. 2022, 10. [Google Scholar] [CrossRef] [PubMed]
- Pulingam, T.; Thong, K.L.; Ali, M.E.; Appaturi, J.N.; Dinshaw, I.J.; Ong, Z.Y.; Leo, B.F. Graphene oxide exhibits differential mechanistic action towards Gram-positive and Gram-negative bacteria. Colloids Surfaces B Biointerfaces 2019, 181, 6–15. [Google Scholar] [CrossRef]
- Amaro-Gahete, J.; Benítez, A.; Otero, R.; Esquivel, D.; Jiménez-Sanchidrián, C.; Morales, J.; Caballero, Á.; Romero-Salguero, F.J. A Comparative Study of Particle Size Distribution of Graphene Nanosheets Synthesized by an Ultrasound-Assisted Method. Nanomater. 2019, 9, 152. [Google Scholar] [CrossRef]
- Palomino, L.; Chipoco Haro, D.A.; Gakiya-Teruya, M.; Zhou, F.; La Rosa-Toro, A.; Krishna, V.; Rodriguez-Reyes, J.C.F. Polyhydroxy Fullerenes Enhance Antibacterial and Electrocatalytic Activity of Silver Nanoparticles. Nanomaterials 2022, 12, 3321. [Google Scholar] [CrossRef]
- Heredia, D.A.; Durantini, A.M.; Durantini, J.E.; Durantini, E.N. Fullerene C60 derivatives as antimicrobial photodynamic agents. J. Photochem. Photobiol. C Photochem. Rev. 2022, 51, 100471. [Google Scholar] [CrossRef]
- Jiao, J.; Zhang, S.; Qu, X.; Yue, B. Recent Advances in Research on Antibacterial Metals and Alloys as Implant Materials. Front. Cell. Infect. Microbiol. 2021, 11. [Google Scholar] [CrossRef] [PubMed]
- Skłodowski, K.; Chmielewska-Deptuła, S.J.; Piktel, E.; Wolak, P.; Wollny, T.; Bucki, R. Metallic Nanosystems in the Development of Antimicrobial Strategies with High Antimicrobial Activity and High Biocompatibility. Int. J. Mol. Sci. 2023, Vol. 24, Page 2104 2023, 24, 2104. [Google Scholar] [CrossRef] [PubMed]
- Díez-Pascual, A.M.; Luceño-Sánchez, J.A. Antibacterial Activity of Polymer Nanocomposites Incorporating Graphene and Its Derivatives: A State of Art. Polymers 2021, 13. [Google Scholar] [CrossRef] [PubMed]
- Selvarajan, V.; Obuobi, S.; Ee, P.L.R. Silica Nanoparticles—A Versatile Tool for the Treatment of Bacterial Infections. Front. Chem. 2020, 8, 526915. [Google Scholar] [CrossRef] [PubMed]
- de Amadio, T.M.; Hotza, D.; Rodrigues Neto, J.B. BACTERICIDAL EFFECTIVENESS OF FREEZE-CAST CERAMIC FILTERS IMPREGNATED WITH SILVER NANOPARTICLES. Brazilian J. Chem. Eng. 2018, 35, 1267–1274. [Google Scholar] [CrossRef]
- Chan, K.F.; Zaid, M.H.M.; Mamat, M.S.; Liza, S.; Tanemura, M.; Yaakob, Y. Recent Developments in Carbon Nanotubes-Reinforced Ceramic Matrix Composites: A Review on Dispersion and Densification Techniques. Cryst. 2021, 11, 457. [Google Scholar] [CrossRef]
- Panáček, A.; Kvítek, L.; Smékalová, M.; Večeřová, R.; Kolář, M.; Röderová, M.; Dyčka, F.; Šebela, M.; Prucek, R.; Tomanec, O.; et al. Bacterial resistance to silver nanoparticles and how to overcome it. Nat. Nanotechnol. 2017, 13, 65–71. [Google Scholar] [CrossRef]
- Mesquita, M.Q.; Dias, C.J.; Neves, M.G.P.M.S.; Almeida, A.; Faustino, M.A.F. Revisiting Current Photoactive Materials for Antimicrobial Photodynamic Therapy. Mol. 2018, 23, 2424. [Google Scholar] [CrossRef]
- Brancaleon, L.; Moseley, H. Laser and non-laser light sources for photodynamic therapy. Lasers Med. Sci. 2002, 17, 173–186. [Google Scholar] [CrossRef]
- Darabpour, E.; Kashef, N.; Mashayekhan, S. Chitosan nanoparticles enhance the efficiency of methylene blue-mediated antimicrobial photodynamic inactivation of bacterial biofilms: An in vitro study. Photodiagnosis Photodyn. Ther. 2016, 14, 211–217. [Google Scholar] [CrossRef]
- Xie, L.; Sun, G.; Su, F.; Guo, X.; Kong, Q.; Li, X.; Huang, X.; Wan, L.; Song, W.; Li, K.; et al. Hierarchical porous carbon microtubes derived from willow catkins for supercapacitor applications. J. Mater. Chem. A 2016, 4, 1637–1646. [Google Scholar] [CrossRef]
- Afkhami, F.; Akbari, S.; Chiniforush, N. Entrococcus faecalis Elimination in Root Canals Using Silver Nanoparticles, Photodynamic Therapy, Diode Laser, or Laser-activated Nanoparticles: An In Vitro Study. J. Endod. 2017, 43, 279–282. [Google Scholar] [CrossRef] [PubMed]
- Antimicrobial resistance and infection prevention and control. Available online: https://openwho.org/courses/IPC-AMR-EN.
- Hinchliffe, S. More than one world, more than one health: Re-configuring interspecies health. Soc. Sci. Med. 2015, 129, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Dadgostar, P. <p>Antimicrobial Resistance: Implications and Costs</p>. Infect. Drug Resist. 2019, 12, 3903–3910. [Google Scholar] [CrossRef] [PubMed]
- Babo Martins, S.; Rushton, J.; Stärk, K.D.C. Economic Assessment of Zoonoses Surveillance in a ‘One Health’ Context: A Conceptual Framework. Zoonoses Public Health 2016, 63, 386–395. [Google Scholar] [CrossRef] [PubMed]
- Malik, S.; Muhammad, K.; Waheed, Y. Nanotechnology: A Revolution in Modern Industry. Molecules 2023, 28. [Google Scholar] [CrossRef]
- Pandey RP, Mukherjee R, Priyadarshini A, Gupta A, Vibhuti A, Leal E, Sengupta U, Katoch VM, Sharma P, Moore CE, Raj VS, Lyu X. Potential of nanoparticles encapsulated drugs for possible inhibition of the antimicrobial resistance development. Biomed Pharmacother. 2021, 141, 111943. [Google Scholar]
- Global Nanotechnology Market Outlook & Growth Forecast Report. Available online: https://www.bccresearch.com/market-research/nanotechnology/global-nanotechnology-market.html.
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; Rodriguez-Torres, M.D.P.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnology 2018, 16, 1–33. [Google Scholar] [CrossRef]
- Antimicrobial resistance : tackling a crisis for the health and wealth of nations / the Review on Antimicrobial Resistance chaired by Jim O’Neill. | Wellcome Collection.
- One Health for One Planet.
- Rushton, J.; Häsler, B.; de Haan, N.; Rushton, R. Economic benefits or drivers of a ‘One Health’ approach: Why should anyone invest? Onderstepoort J. Vet. Res. 2012, 79, 5. [Google Scholar] [CrossRef]
| S.no. | Country | Organizations | Role |
|---|---|---|---|
| 1. | India | National action Plan (NAP) https://iica.nic.in/sob_nap.aspx |
Implementation of a comprehensive and multi-sectorial NAP with a coordinated body, an operational strategy, and the creation of a monitoring system. |
| 2. | National centre for diseases control (NCDC) https://ncdc.gov.in |
In collaboration with the State Governments, disease surveillance facilitates the prevention and control of communicable diseases. | |
| 3. | Antimicrobial Resistance Surveillance and Research Network (AMRSN) http://www.carss.cn |
Collect evidence and identify trends and patterns of drug-resistant diseases. | |
| 4. | Food safety and standard authority of India (FSSAI) |
Collect and compile data on food consumption, contaminants in food and food product, identification of developing risks, and the implementation of a quick warning system. | |
| 5. | Taiwan | Taiwan centers for diseases control https://www.cdc.gov.tw/En |
Strengthening surveillance system, conduct hospital accreditation, run stewardship program. |
| 6. | Infection control society of Taiwan https://nics.org.tw/ |
Formulating recommendations, engage in infection control activities in response to the government health policies. | |
| 7. | The infectious disease society of Taiwan (IDST) http://www.idsroc.org.tw/ |
Formation of rules and contributions in the prevention and control of antimicrobial resistance. | |
| 8. | Surveillance of Multicenter Antimicrobial Resistance in Taiwan (SMART) |
Monitor the in vitro resistance of clinically important bacteria obtained from Taiwanese hospitals. | |
| 9. | U.S.A. | Food and Drug Administration (FDA) https://www.fda.gov/ | Ensures public health through safeguarding the safety, effectiveness, and security of human and veterinary drugs, biological products, and medical devices. |
| 10. | National Antimicrobial Resistance Monitoring System (NARMS) https://www.cdc.gov/narms/index.html |
Tracks changes in antimicrobial susceptibility of select foodborne enteric bacteria found in ill people, retail meats, and food animals | |
| 11. | Pan American Health Organization (PAHO) https://www.paho.org/en/together-fight-antimicrobial-resistance | Fostering strategic collaborations among Member States and other partners to promote health equity, fight against diseases, and enhance the quality of life and lifespan | |
| 12. | Centers for Disease Control and Prevention https://www.cdc.gov/ |
As the nation’s health protection agency, CDC saves lives and protects people from health threats | |
| 13. | Europe | European Antimicrobial Resistance Surveillance Network (EARS-Net) https://www.ecdc. europa.eu/en/ |
Play a crucial role in increasing awareness among political leaders, public health officials, the scientific community, and the general public. |
| 14. | Healthcare-associated Infections Surveillance Network (HAI-Net) https://www.ecdc.europa.eu/en/ |
Provide baseline endemic infection rates by conducting surveillance. | |
| 15. | Organisation for Economic Co-operation and Development (OECD) https://www.oecd.org/els/health-systems/antimicrobial-resistance.htm | Promote policies that cultivate prosperity, equality, opportunity, and well-being for everyone. | |
| 16. | Joint Programming Initiative on Antimicrobial Resistance (JPIAMR) https://www.jpiamr.eu/ | Establish connections between research networks, research institutes/centers, and collaborate on implementing research focused on Antimicrobial Resistance (AMR) using a "One Health" approach. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





