1. Introduction
Chronic hepatitis B (CHB) is an infection with the hepatitis B virus (HBV) that lasts for more than six months and causes a long-term liver disease. Currently, 257 million people around the world had a chronic HBV infection and the Western Pacific and Africa were the places where it was most common [
1,
2]. CHB can cause major problems with the liver, such as cirrhosis (when the liver scars), liver failure, and liver cancer. As the illness persists longer and causes more liver inflammation and damage, the likelihood of these issues increases[
3]. In CHB, mitochondrial and endoplasmic reticulum (ER) function changes, which can contribute to liver damage and disease progression [
4,
5]. Patients with CHB had higher levels of mitochondrial DNA (mtDNA) in their serum than healthy, indicating increased mitochondrial dysfunction and damage[
6]. Alterations in mitochondrial morphology, mitochondrial membrane potential, mitochondrial metabolism, and biogenesis have been reported in chronic HBV-infected hepatocytes[
7,
8]. CHB has also been linked to ER stress and its response was found to be activated in liver biopsies from patients with persistent HBV infection, and this was linked to increased liver fibrosis and inflammation[
9,
10]. ER stress and dysfunction of mitochondria are associated to the progression to cirrhosis and end stage Hepatocellular carcinoma (HCC)[
11]. Several factors have been implicated in the development of HBV infection, including the hepatitis B virus x protein (HBx), immune-mediated death of HBV-infected hepatocytes, and liver regeneration[
12]. HBx regulates transcription, signal transmission, proteasome activity, cell cycle progression, and programmed cell deaths[
13].
HBV is a member of the Hepadnaviridae family and has a partially double-stranded DNA genome of 3.2 kb. Its genome contains four open reading frames (ORFs), which encode 3.5, 2.4, 2.1, and 0.7 kb RNAs and are translated into seven different proteins. The 3.5 kb RNA is responsible for encoding the core protein (HBcAg) and the pre-core protein (HBeAg), while the 2.4 and 2.1 kb RNAs encode the small (S), medium (M), and large (L) surface proteins. The regulatory protein HBx, which plays a role in viral replication and pathogenicity, is encoded by the 0.7 kb RNA[
14].Previous research suggests that the regulatory protein HBx may be responsible for various effects attributed to HBV [
13].
HBx induced aberrant aggregation of mitochondrial components near the nucleus periphery triggered by HBx-driven p38 mitogen-activated protein kinase (MAPK), which may eventually lead to cell deaths [
15]. HBx damage mitochondria via interaction with voltage-dependent anion channel (HVDAC3), which is known as mitochondrial porins and forms pores in mitochondrial outer membranes[
16] and interaction alters mitochondrial transmembrane potential, resulting in the formation of reactive oxygen species (ROS) and cellular ATP/ADP ratio [
17]. Furthermore, HBx suppresses the expression of nucleus-encoded genes involved in mitochondrial beta-oxidation of fatty acids, resulting in a low level of cellular ATP due to energy source deficit. HBx-induced Raf-1 kinases pathway or the apoptosis regulator bcl-2-associated X protein (BAX) are translocated to mitochondria by HBx, resulting in hepatic cell proliferation or apoptosis, respectively[
6]. HBV-infected cells synthesize a lot of surface proteins in the endoplasmic reticulum (ER), disrupting ER homeostasis and causing ER stress[
10]. CHB patient’s biopsies reported with enlarge ER due to surface proteins (preS1 and preS2). ER stress or HBx can trigger autophagy [
18]. These findings imply that HBx-induced mitochondrial dysfunction and ER stress could be contributed to hepatocellular carcinoma pathogenesis, including proliferation, metastasis, and other features of carcinogenesis.
Biological networks have been used as a powerful approach for identifying novel dysregulated pathways driving molecular pathology. Weighted protein co-expression network analysis (WPCNA) is a multi-protein study that identifies groupings of proteins that are frequently altered and orchestrates them into modules without relying on a priori specified proteins sets or pathways. WPCNA is a useful technique for determining how networks of proteins and phenotypes are related[
19].
Thiourea and its derivatives are used therapeutically as antioxidants[
20], anti-inflammatory drugs, anti-cancer [
21], fungicides[
22], as well as anti-viral[
23,
24]. Recently, a novel thiourea compound DSA-00 (IR-415) was identified by high-throughput screening of the Maybridge library that suppresses HBV replication like current antivirals[
23].
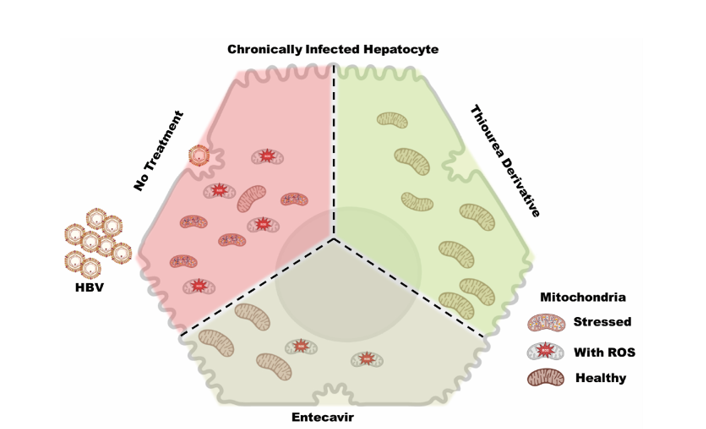
The current study focus on the analysis of proteome alteration in cell (HepG2.2.15) under the influence of currently used anti-virals in comparison to thiourea derivatives in order to identify key molecular changes linked to mitochondrial function and antiviral activity. We validated the proteomic finding of mitochondrial health using flow Cytometery analysis with the ultimate goal of identifying weather repurposed thiourea derivatives could be improved mitochondrial and hepatocyte health.
2. Results
2.1. Alteration of protein profiling during antiviral therapy in chronic HBV infection
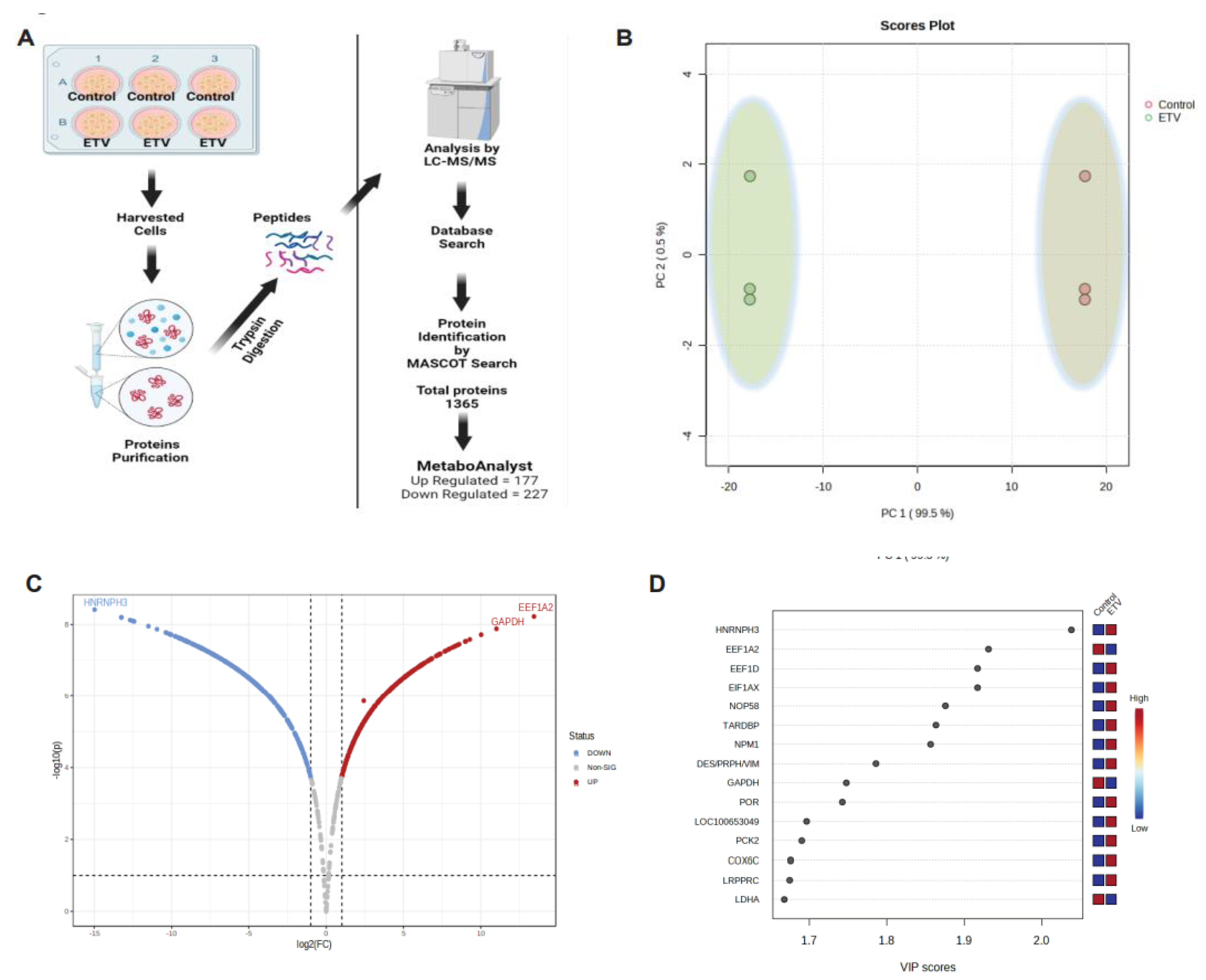
To investigate the alterations in protein profiling during antiviral therapy in chronic HBV infection, HepG2.2.15 cells were treated with Entecavir (ETV) (
Figure 1A), is currently used to treat CHB[
25]. A wide-scale proteome comparison of the ETV-treated and untreated cells showed complete separation of the samples based on their similarity (
Figure 1B). The results showed significant changes in the proteome of the ETV-treated group compared to the untreated group, with 607 proteins (274 UP and 246 Down-regulated) found to be significant (
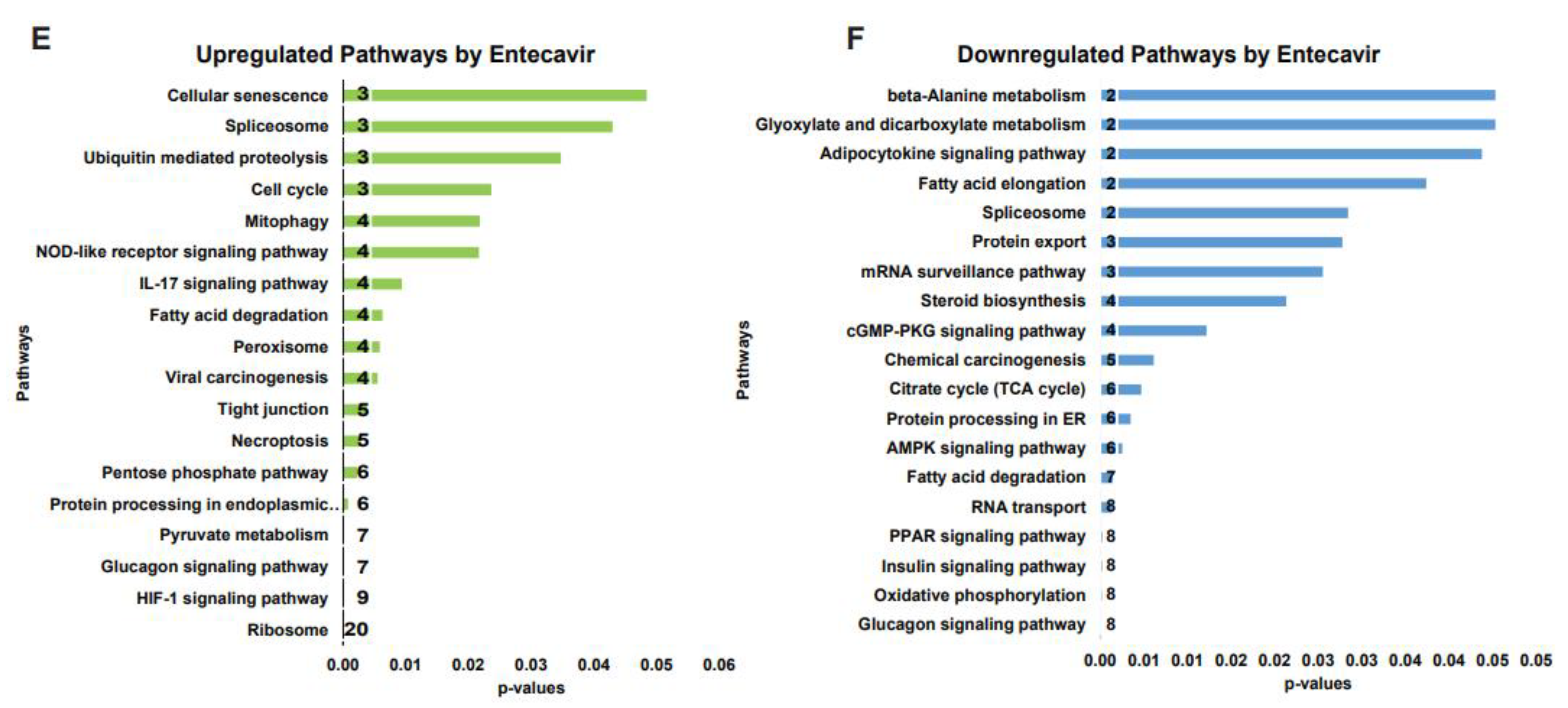
Figure 1C,D.). Further analysis revealed that the upregulated proteins were mostly linked to the ribosome, glycolysis, HIF-1 signaling, pyruvate metabolism, phagosome, pentose phosphate, necroptosis, tight junction, ferroptosis, peroxisome, fatty acid degradation, IL-17 signaling, mitophagy, and pathways related to the cell cycle (p<0.05) (
Figure 1E). On the other hand, the downregulated proteins were mostly linked to mRNA surveillance, TCA cycle, protein processing in the ER, oxidative phosphorylation, insulin signaling, glucagon signaling, and RNA transport (p<0.05) (
Figure 1F), according to the KEGG analysis[
26]. A large proportion of the proteins that were significantly altered were host antiviral proteins, whereas most of the proteins involved in bioenergetics were downregulated. These findings suggest that novel approaches are needed to restore the bioenergetics function and modulate the host RNAi-mediated antiviral defense system.
2.2. Biological pathways associated with thiourea derivatives and ETV therapy
ETV is currently used as a treatment for chronic hepatitis B (CHB) due to its ability to suppress HBV infection. However, ETV has minimal impact on cellular energetics[
27]. On the other hand, thiourea derivatives have shown potential in inhibiting HBV replication[
23](Singh et al., 2023; Kumar et al., 2023 in communication). It is important to note that ETV and thiourea derivatives belonged to different classes of antivirals with distinct modes of action for suppressing HBV replication. Based on these finding, we were curious to compare the proteins expression pattern in thiourea derivatives vs. ETV. Again, we used weighted protein network analysis (WPCNA)[
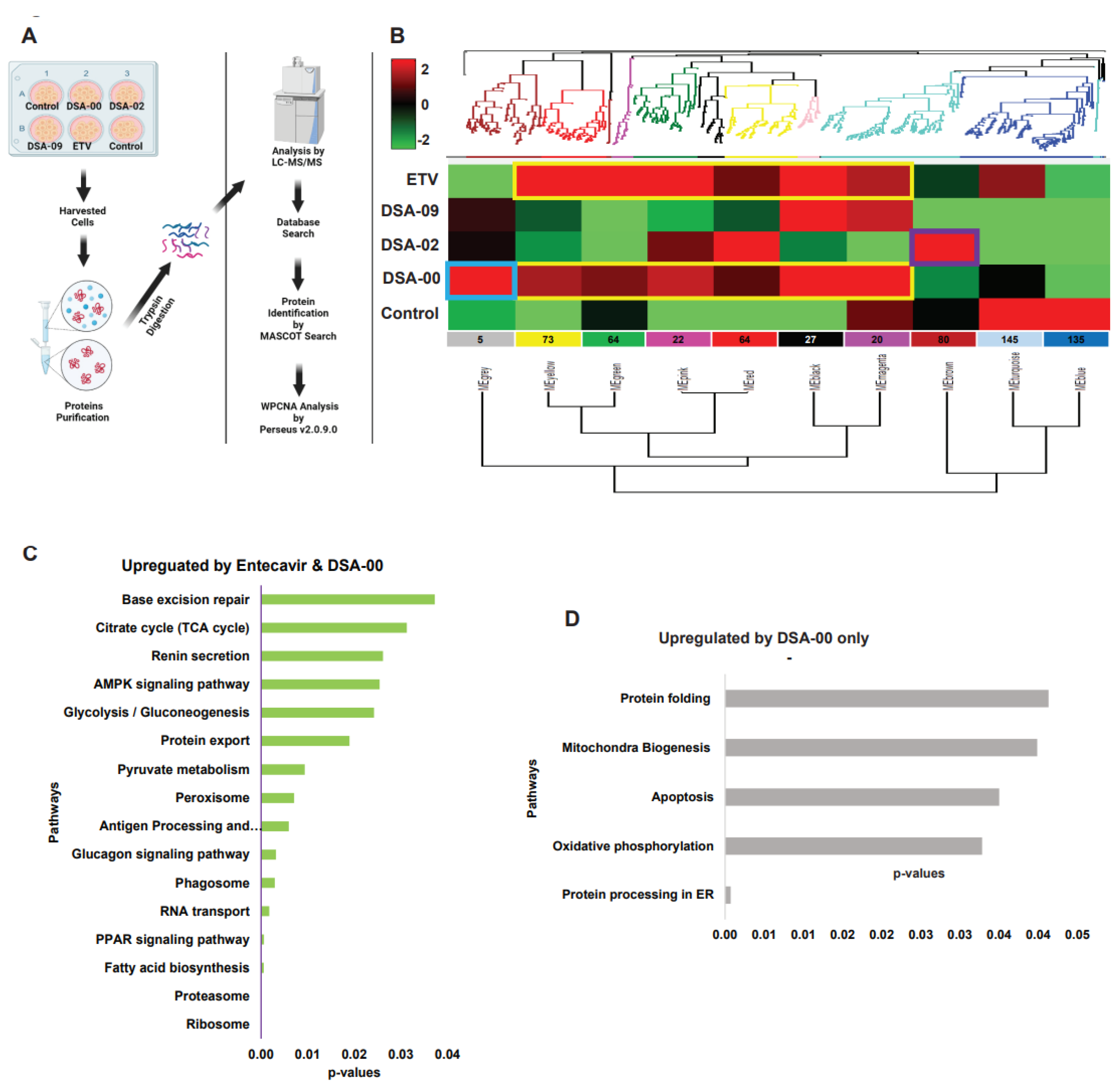
28] approach to identified proteins modules specific to DSA-00, DSA-02, DSA-09, ETV, and untreated cells for correlation patterns among proteins that may all work together in persistence of HBV infection (
Figure 2A). WPCNA revealed 10 clusters (modules), corresponding to a total of 636 strongly linked proteins in DSA-00, DSA-02, DSA-09, ETV, and untreated groups (
Figure 2B). The number of proteins for each module, including the number of proteins with a fold change >2 or <-1.5 untreated compared to the combination of DSA-00, DSA-02, DSA-09, and ETV (p <0.05,
Supplementary Table S1).
We were interested in modules whose expression pattern was similar to ETV and had some unique modules. DSA-00 has a similarity of approximately 70 percent with ETV (Black, Magenta, Red, Pink, Green, and Yellow) and also has unique module (Grey), but DSA-09 has two common modules with ETV and DSA-00 (Black and Magenta). The ETV, DSA-00, and DSA-09 shared modules proteins involved in Ribosome, Proteasome, Fatty acid biosynthesis, PPAR signaling, and RNA transport pathways (
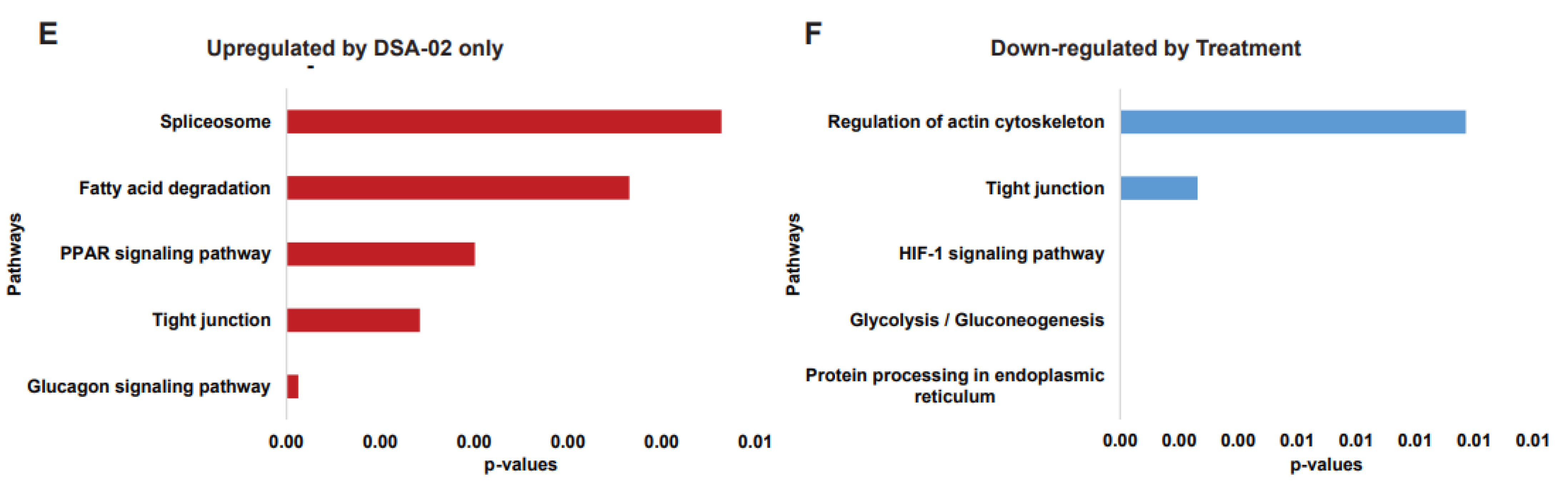
Figure 2C). The DSA-02 has unique Brown module proteins were associated with biological pathways such as Glucagon signaling, tight junction, PPAR signaling, Fatty acid degradation, and Spliceosome (
Figure 2E). DSA-09 induced modules were common with DSA-00 and ETV modules. DSA-00 has a specific grey module other than common modules with ETV, DSA-02 and DSA-09. The grey module proteins involved in Protein processing in ER, Oxidative phosphorylation, Apoptosis, Mitochondria Biogenesis, and Protein folding pathways (
Figure 2F). Additionally, the untreated group has unique modules (Blue and Turquoise), and the proteins in these modules were linked with Regulation of actin cytoskeleton, Tight junction, HIF-1 signaling, Glycolysis/Gluconeogenesis, and protein processing in ER pathways (
Figure 2G). Based on these findings we conclude that, proteomic changes induced by thio-urea derivative are 70% similar to that seen under the treatment with ETV respectively. In addition, our results highlight that thio-urea derivative modulates unique proteins modules majorly linked to mitochondrial and ER activity suggesting that the thio-urea derivative not on provide anti-viral support but also rejuvenates the mitochondria and hepatocyte health in CHB (in –vitro).
2.3. Restoration of bioenergetics in exhausted hepatocytes by thiourea derivatives
It is established that HBx causes mitochondrial dysfunction and inflammation/injury in hepatocytes through disrupting mitochondrial respiration and altering the mitochondrial membrane potential during chronic HBV infections[
29] and thiourea derivatives have been shown to bind to HBx and block the HBV-induced functions (Kumar et al., 2023 in communication) as well as also possess antioxidant activity[
30]. Drawing upon these findings, hypothesized that thiourea derivatives have the potential to restore mitochondrial dysfunction in HBV-related liver diseases. To investigate this hypothesis, we treated HepG2.2.15 cells with DSA-00, DSA-02, DSA-09, and ETV. The proteome profile was subjected to WPCNA which led to the identification of several modules of proteins that were differentially expressed between the groups.
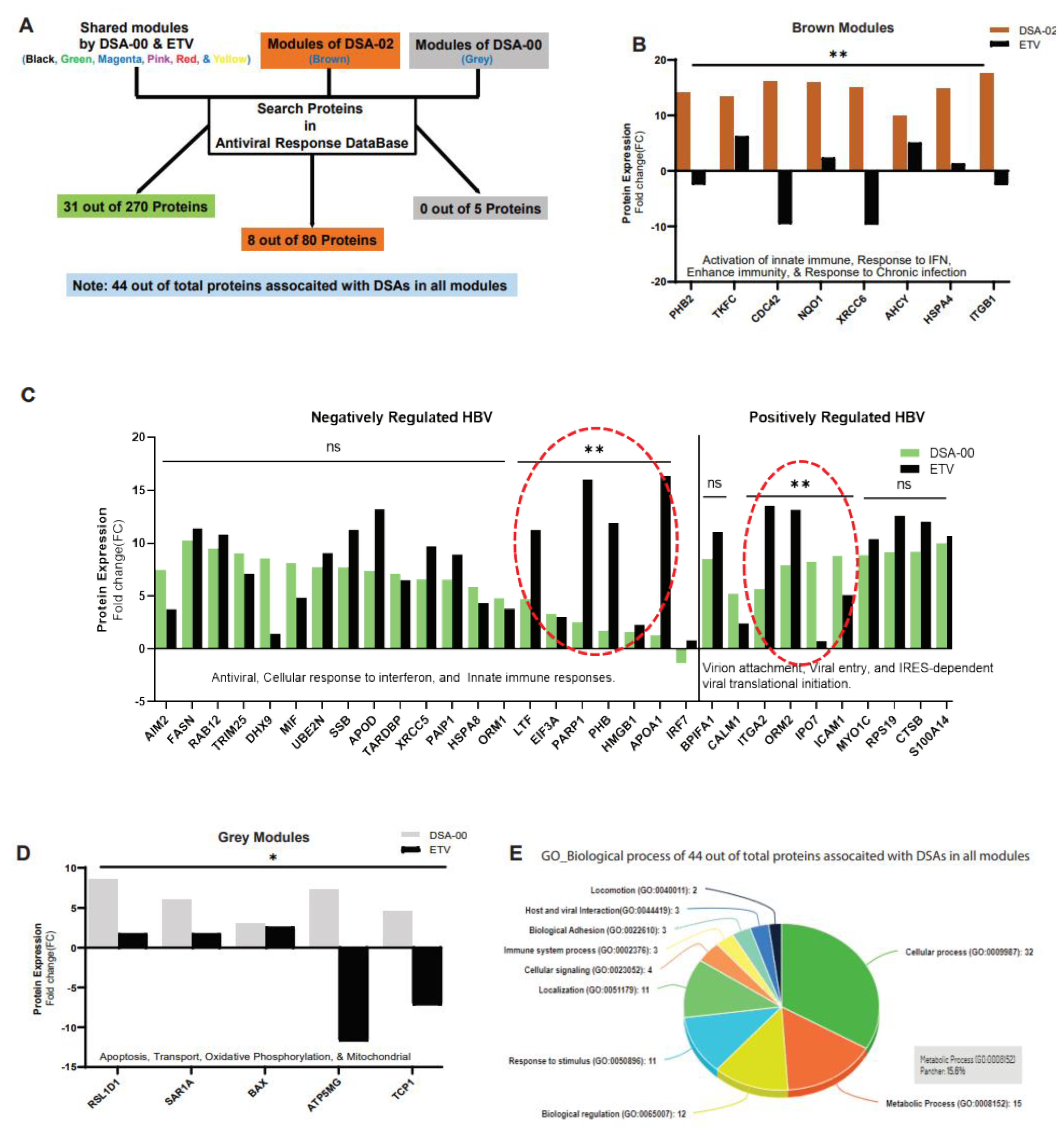
Pathway analysis of these modules using antiviral response proteins database showed that 31 in DSA-00 and 8 proteins DSA-02 were linked with the antiviral response (
Figure 3A). The brown module of DSA-02 contained eight proteins that were associated with enhanced host immunity and responsiveness to chronic infection (
Figure 3B), while DSA-00 had twenty proteins providing negative regulation and ten associated with positive regulation to the HBV infection (
Figure 3C).
Furthermore, we found that DSA-00 induced a unique grey module that contained only five proteins associated with mitochondrial functions like oxidative phosphorylation, membrane potential, and apoptosis (
Figure 3D). All antiviral response proteins that were up-regulated by DSA-00 (31 proteins), DSA-02 (8 proteins), and DSA-00 (5 proteins) were analyzed for their biological processes using the PANTHER classification system (v.14.0). This analysis revealed that almost 50% of the proteins belonged to metabolic and cellular processes, suggesting that thiourea derivatives may be restore bioenergetics in exhausted hepatocytes by targeting dysfunctional mitochondria (
Figure 3E).
2.4. Restore functional mitochondrial by thiourea derivatives
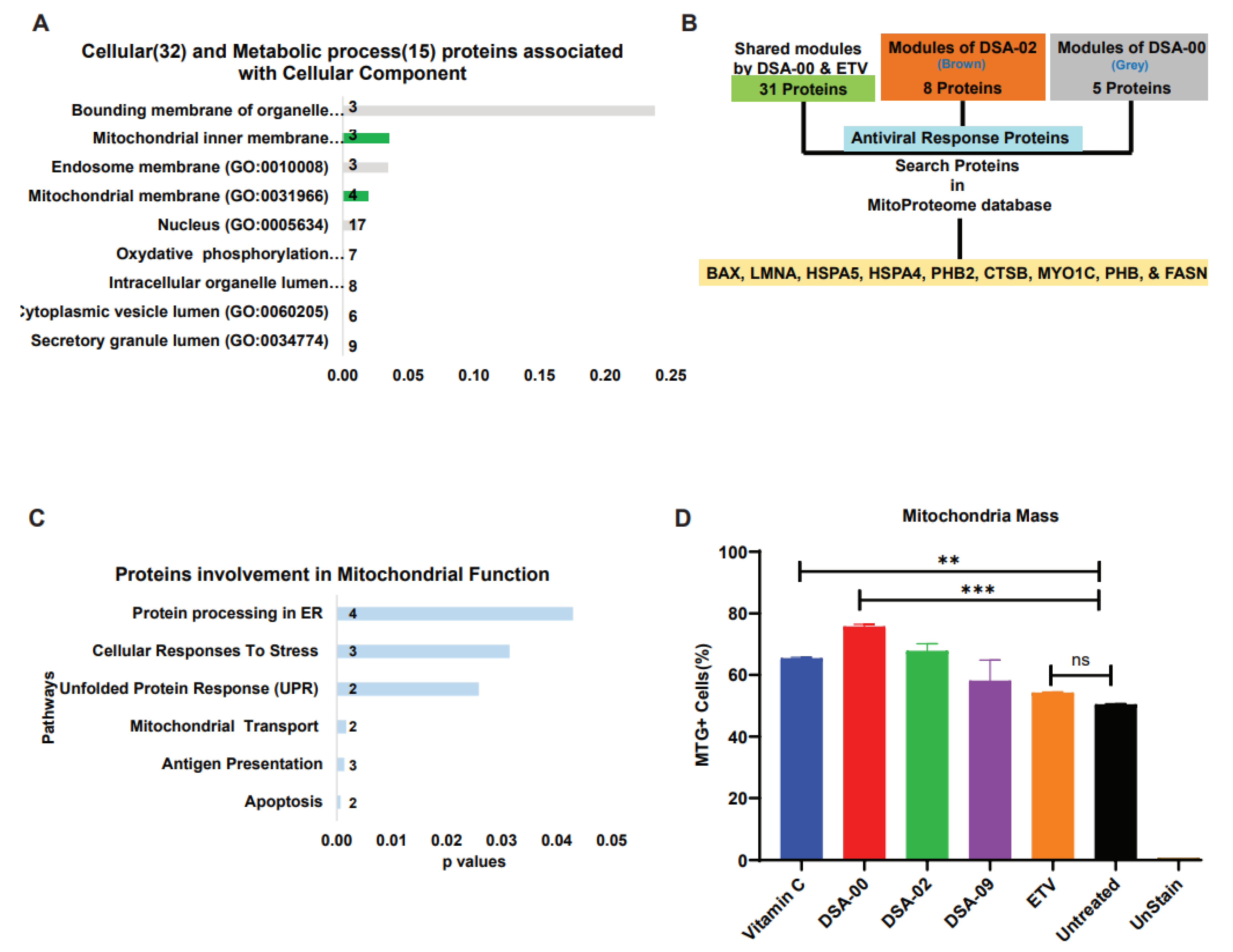
After analyzing the metabolic and cellular process associated proteins, we found that most of the proteins were linked to the mitochondria (
Figure 4A). Based on these results, we hypothesized that thiourea derivatives may have a role in mitochondrial-related functions. To validate these findings, we further blasted the proteins with the MitoProteome database[
31] and found nine proteins (BAX, LMNA, HSPA5, HSPA4, PHB2, CTSB, MYO1C, PHB, and FASN) that were directly linked with mitochondria and its functions (
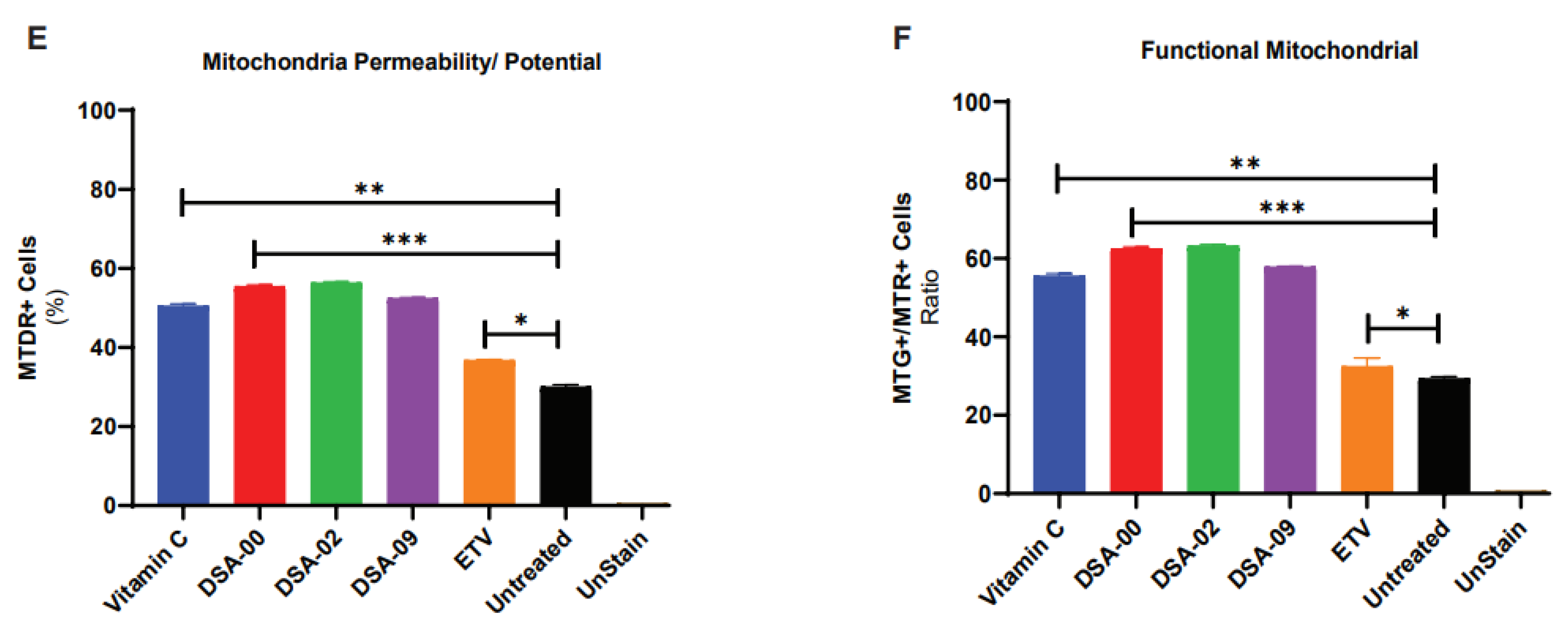
Figure 4B). These results suggested that thiourea derivatives also target the mitochondria, and we hypothesized that they have the potential to restore mitochondrial health and functionality. To validated our hypothesis by treated chronic HBV infected cells with thiourea derivatives, with vitamin C used as the positive control, and ETV used as a positive control for the HBV infection. FACS analysis revealed that thiourea derivatives have the potential to increase mitochondrial mass (
Figure 4C) and restore the membrane potential of mitochondria (
Figure 4D). Therefore, the ratio of mass and membrane potential of mitochondria represents the functionality of the mitochondrial pool. Moreover, these thiourea derivatives were able to restore and maintain the pool of functional mitochondria in chronic HBV infected hepatocytes (
Figure 4E). These observations clearly outline specific role (increase in mitochondrial health) of the thiourea derivative which is unique and was not mentioned ever before. Use of thiourea derivative in CHB care is thus beneficial over or in addition to the currently used antivirals.
3. Discussion
CHB is a major risk to the progression to HCC, the sustained presence of the virus in the liver causes liver inflammation and exhaustion of hepatocytes[
7]. Previous studies have shown that HBV replication and the expression of the non-structural hepatitis B virus X protein (HBx) lead to mitochondrial dysfunction, including alterations in mitochondrial unfolded protein response, biogenesis, mitophagy, and increased production of reactive oxygen species (ROS) such as superoxide and peroxynitrite [
7,
32] HBx has been shown to have diverse roles in regulatory HBV replication, cellular transcription and signal transduction pathways, proteasome activity, and cell cycle progression, and has also been linked to the progression of HBV-associated HCC[
33]. Furthermore, a portion of cytosolic HBx may localize to mitochondria of hepatocytes in in vitro and in vivo systems, where it can modulate mitochondrial membrane potential and alter cytosolic calcium levels via the mitochondrial permeability transition pore (mPTP) [
16,
34]. Based on the crucial role of HBx in the development of chronic HBV infection and progression to HCC. The current study focuses on targeting HBx as a potential therapeutic approach to block HBV replication, restore mitochondrial function, and reduce liver inflammation in CHB patients. One promising candidate for this approach is a thiourea derivative called DSA-00 and its derivatives, which have been shown to efficiently reduce HBx-induce activities, including HBV replication, HBx-induced kinase activity, and HBx-modulated host RNAi machinery [
23](Kumar et al., 2023 communicated).
Using a proteomic approach, we wanted to identify the proteomic perturbation associated with the decrease in HBV viral replication and improvement of mitochondrial functions and exhaustion of hepatocytes during CHB infection. To achieve this, proteome profile of an in vitro model of chronically infected hepatocytes was compared to chronically infected hepatocytes under the treatment of commonly used anti-viral ETV and different thiourea derivatives respectively. Instead of performing conventional differential expression analysis based on predetermined protein sets or pathways, we conducted a modular analysis that used an unsupervised hierarchical clustering method to examine the link between groups of co-expressed proteins. We used the WPCNA method, which enables the identification of protein co-expression and the determination of how well a particular protein is connected to other proteins in order to determine the pathways jointly involved in a biological process. The protein co-expression networks of intricate biological processes are segmented into modules by WPCNA. Findings with WPCNA analysis identified 10 different modules (
Figure 2), with Black, Magenta, Red, Pink, Green, and Yellow modules having the most shared modules between DSA-00 and ETV treatment. These modules were mostly correlated with ribosome, proteasome, fatty acid biosynthesis, PPAR signaling, RNA transport pathways, according to enrichment analysis. Brown module was unique for the DSA-02 treatment, with proteins significantly associated with biological processes such as Glucagon signaling, tight junction, PPAR signaling, fatty acid degradation, spliceosome, etc. The Grey modules had only five proteins, which were upregulated by the treatment of DSA-00 (
Figure 2E). KEGG analysis of these proteins revealed that they were significantly involved in mitochondrial biogenesis, oxidative phosphorylation, and unfolded protein response.
Furthermore, we looked at proteins involved in the antiviral response by blasting with the antiviral response database, and we found 44 proteins in Black, Magenta, Red, Pink, Green, Yellow, Brown, and Grey modules (
Figure 3D). We measured the correlation between the changed biological functions in DSA-00, DSA-02, and ETV groups and found that antiviral response proteins were associated with positively regulated and negatively regulated HBV in DSA-00+ETV groups. DSA-02 induced proteins that hit the antiviral response. Additionally, DSA-00 had a unique Grey module, which had five proteins associated with mitochondrial functions.
The aim of our study was to repurpose drugs that have the potential to reduce HBV infection and restore the exhausted hepatocytes that are notably exhausted during CHB infection. Restoration of dysfunctional mitochondria has been correlated with the improvement of cell health in the past, although not being previously linked to thiourea derivatives efficacy. Moreover, the blast hits with mitoproteome database [
31] revealed that thiourea derivatives are suitable candidates that have the efficacy to restore dysfunctional mitochondria as well as have the potential to reduce HBV infection.
Limitations of the investigation include the fact that it is a pilot study and that just one type of in-vitro CHB model (HepG2.2.15) was employed. Instead of measuring mitochondrial respiration, oxygen consumption rate (OCR), and ATP production in the validation research, just test the mitochondrial health using flow cytometry.
4. Material and Methods
4.1. Cell culture, reagents and treatments
The cell lines used in this study were HepG2.2.15 cells, which were grown in Dulbecco’s modified Eagle’s medium (DMEM, Invitrogen, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (Gibco of Invitrogen), GlutaMax, 10 mM HEPES, 100 unit/ml penicillin, 100 µg/ml streptomycin, and 10% FBS at 37°C in a humidified atmosphere containing 5% CO2.
DSA-00, DSA-02, DSA-09, Entecavir (ETV), and Ascorbic Acid (Vitamin C, Sigma-Aldrich). The compounds were dissolved in dimethyl sulfoxide (DMSO) and preserved at -20°C or -80°C until used. Cells were treated with 10 µM concentration of treatment for 24 h, further cells were trypsinized and used for proteomics analysis.
4.2. Untargeted proteomics analysis using UHPLC coupled with High Resolution-MS/MS
Untargeted proteomic analysis of cell culture samples was performed using ultra-high performance liquid chromatography (UHPLC) coupled with high resolution tandem mass spectrometry (HRMS/MS)[
35]. The trypsinized and collected by centrifugation at 5000 RPM. The cells pellet was then resuspended in RIPA lysis buffer for the isolation of total proteins from cultured cells. Detailed methods for the untargeted proteomics analysis are provided in the supporting information (SI).
4.3. MTR and MTG HepG2.2.15 cell staining
MTR CM-H2XROS (Molecular Probes) and MTG (Molecular Probes) staining were done according to the manufacturer’s instructions (Molecular Probes). Detailed methods are provided in the supporting information (SI).
4.4. Statistical and correlation analysis
The results are presented as mean and S.D. Statistical analysis was performed using Graph Pad Prism, version 8 (GraphPad Software, La Jolla, CA, USA;
www.graphpad.com. A difference was considered to be statistically significant at *P< 0.05 and **P< 0.01, and not significant (NS). Detailed methods for statistical and correlation analysis are provided in the supporting information (SI).
5. Conclusions
Our research concentrated on CHB, which causes mitochondrial dysfunction, HBV reactivation, and HBV replication. Thiourea derivatives known for the antiviral activity and restoration of host RNA interference mediated defense machinery. Here, we repurposed thiourea derivatives, they have the potential to restoration of the dysfunctional mitochondria. Mitochondrion-targeted antioxidants considerably enhanced mitochondrial and antiviral hepatocytes functions, revealing a crucial role for reactive oxygen species (ROS) in cell exhaustion. our study provides valuable insights into the potential of thiourea derivatives for the treatment of CHB and restoration of exhausted hepatocytes. Further experimental validation of our findings is necessary to confirm their potential clinical application.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org.
Author Contributions
Conceptualization, J.K.,PT, and J.S.M.; methodology, J.K., and J.S.M.; software, J.K.; validation, J.K. and P.T.; formal analysis, J.K.; investigation, J.K..; resources, J.K., and AKS; data curation, J.K.; writing-original draft preparation, J.K.,; writing-review and editing, J.S.M., and V.K.; visualization, J.K.; supervision, V.K.; project administration, DS; funding acquisition, V.K. All authors have read and agreed to the published version of the manuscript.
Acknowledgements
This work was supported by grants to VK from the Department of Biotechnology (DBT), Government of India (Grant No. BT/PR30082/Med/29/1341/2018) and J.C. Bose National Fellowship (Grant No. SR/S2/JCB-80)/2012) of the Department of Science and Technology, Government of India, New Delhi. JK, PT, and AKS received Senior Research Fellowships respectively from the Council of Scientific and Industrial Research, New Delhi and University Grants Commission, New Delhi for the period of this study.
References
- Jeng, W.J., G.V. Papatheodoridis, and A.S.F. Lok, Hepatitis B. Lancet, 2023. 401(10381): p. 1039-1052.
- WHO, World Health Statistics 2022, Retrieved from:https://www.who.int/news/item/20-05-2022-world-health-statistics-2022. 2022.
- Yeh, M.-L.; Hung, C.-H.; Tseng, K.-C.; Lai, H.-C.; Chen, C.-Y.; Kuo, H.-T.; Wang, J.-H.; Chen, J.-J.; Lee, P.-L.; Chien, R.-N.; et al. Long-term outcome of liver complications in patients with chronic HBV/HCV co-infection after antiviral therapy: a real-world nationwide study on Taiwanese Chronic Hepatitis C Cohort (T-COACH). Hepatol. Int. 2021, 15, 1109–1121. [Google Scholar] [CrossRef] [PubMed]
- Li, D., et al., A potent human neutralizing antibody Fc-dependently reduces established HBV infections. Elife, 2017. 6.
- Han, C.Y.; Rho, H.S.; Kim, A.; Kim, T.H.; Jang, K.; Jun, D.W.; Kim, J.W.; Kim, B.; Kim, S.G. FXR Inhibits Endoplasmic Reticulum Stress-Induced NLRP3 Inflammasome in Hepatocytes and Ameliorates Liver Injury. Cell Rep. 2018, 24, 2985–2999. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Hann, H.-W.; Wan, S.; Hann, R.S.; Wang, C.; Lai, Y.; Ye, X.; Evans, A.; Myers, R.E.; Ye, Z.; et al. Cell-free circulating mitochondrial DNA content and risk of hepatocellular carcinoma in patients with chronic HBV infection. Sci. Rep. 2016, 6, 23992–23992. [Google Scholar] [CrossRef] [PubMed]
- Loureiro, D.; Tout, I.; Narguet, S.; Bed, C.M.; Roinard, M.; Sleiman, A.; Boyer, N.; Pons-Kerjean, N.; Castelnau, C.; Giuly, N.; et al. Mitochondrial stress in advanced fibrosis and cirrhosis associated with chronic hepatitis B, chronic hepatitis C, or nonalcoholic steatohepatitis. Hepatology 2022, 77, 1348–1365. [Google Scholar] [CrossRef]
- Lin, C.; Ou, Q. Emerging role of mitochondria in response to HBV infection. J. Clin. Lab. Anal. 2022, 36, e24704. [Google Scholar] [CrossRef]
- Kim, J.Y.; Garcia-Carbonell, R.; Yamachika, S.; Zhao, P.; Dhar, D.; Loomba, R.; Kaufman, R.J.; Saltiel, A.R.; Karin, M. ER Stress Drives Lipogenesis and Steatohepatitis via Caspase-2 Activation of S1P. Cell 2018, 175, 133–145. [Google Scholar] [CrossRef]
- Li, Y.; Xia, Y.; Cheng, X.; Kleiner, D.E.; Hewitt, S.M.; Sproch, J.; Li, T.; Zhuang, H.; Liang, T.J. Hepatitis B Surface Antigen Activates Unfolded Protein Response in Forming Ground Glass Hepatocytes of Chronic Hepatitis B. Viruses 2019, 11, 386. [Google Scholar] [CrossRef]
- Ghany, M.G.; Feld, J.J.; Chang, K.-M.; Chan, H.L.Y.; Lok, A.S.F.; Visvanathan, K.; A Janssen, H.L. Serum alanine aminotransferase flares in chronic hepatitis B infection: the good and the bad. Lancet Gastroenterol. Hepatol. 2020, 5, 406–417. [Google Scholar] [CrossRef] [PubMed]
- Morikawa, K.; Shimazaki, T.; Takeda, R.; Izumi, T.; Umumura, M.; Sakamoto, N. Hepatitis B: progress in understanding chronicity, the innate immune response, and cccDNA protection. Ann. Transl. Med. 2016, 4, 337–337. [Google Scholar] [CrossRef] [PubMed]
- Slagle, B.L.; Bouchard, M.J. Role of HBx in hepatitis B virus persistence and its therapeutic implications. Curr. Opin. Virol. 2018, 30, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Yuen, M.F., et al., Hepatitis B virus infection. Nat Rev Dis Primers, 2018. 4: p. 18035.
- Kim, S., et al., Hepatitis B virus x protein induces perinuclear mitochondrial clustering in microtubule- and Dynein-dependent manners. J Virol, 2007. 81(4): p. 1714-26.
- Rahmani, Z.; Huh, K.-W.; Lasher, R.; Siddiqui, A. Hepatitis B Virus X Protein Colocalizes to Mitochondria with a Human Voltage-Dependent Anion Channel, HVDAC3, and Alters Its Transmembrane Potential. J. Virol. 2000, 74, 2840–2846. [Google Scholar] [CrossRef]
- Foo, J.; Bellot, G.; Pervaiz, S.; Alonso, S. Mitochondria-mediated oxidative stress during viral infection. Trends Microbiol. 2022, 30, 679–692. [Google Scholar] [CrossRef]
- Wang, D.; Wang, Q.; Shan, F.; Liu, B.; Lu, C. Identification of the risk for liver fibrosis on CHB patients using an artificial neural network based on routine and serum markers. BMC Infect. Dis. 2010, 10, 251–251. [Google Scholar] [CrossRef]
- Stuart, J.M.; Segal, E.; Koller, D.; Kim, S.K. A Gene-Coexpression Network for Global Discovery of Conserved Genetic Modules. Science 2003, 302, 249–255. [Google Scholar] [CrossRef]
- Naz, S.; Zahoor, M.; Umar, M.N.; Alghamdi, S.; Sahibzada, M.U.K.; UlBari, W. Synthesis, characterization, and pharmacological evaluation of thiourea derivatives. Open Chem. 2020, 18, 764–777. [Google Scholar] [CrossRef]
- Ghorab, M.M.; El-Gaby, M.S.; Alsaid, M.S.; Elshaier, Y.A.; Soliman, A.M.; El-Senduny, F.F.; Badria, F.A.; Sherif, A.Y. Novel Thiourea Derivatives Bearing Sulfonamide Moiety as Anticancer Agents Through COX-2 Inhibition. Anti-Cancer Agents Med. Chem. 2017, 17, 1411–1425. [Google Scholar] [CrossRef]
- Antypenko, L.; Meyer, F.; Kholodniak, O.; Sadykova, Z.; Jirásková, T.; Troianova, A.; Buhaiova, V.; Cao, S.; Kovalenko, S.; Garbe, L.; et al. Novel acyl thiourea derivatives: Synthesis, antifungal activity, gene toxicity, drug-like and molecular docking screening. Arch. der Pharm. 2018, 352, e1800275. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Kaushik, A.; Khurana, S.; Varshney, A.; Singh, A.K.; Dahiya, P.; Thakur, J.K.; Sarin, S.K.; Gupta, D.; Malhotra, P.; et al. An RNAi-based high-throughput screening assay to identify small molecule inhibitors of hepatitis B virus replication. J. Biol. Chem. 2017, 292, 12577–12588. [Google Scholar] [CrossRef] [PubMed]
- Küçükgüzel, I.; Tatar, E.; Küçükgüzel, .G.; Rollas, S.; De Clercq, E. Synthesis of some novel thiourea derivatives obtained from 5-[(4-aminophenoxy)methyl]-4-alkyl/aryl-2,4-dihydro-3H-1,2,4-triazole-3-thiones and evaluation as antiviral/anti-HIV and anti-tuberculosis agents. Eur. J. Med. Chem. 2008, 43, 381–392. [CrossRef]
- Zhang, Z.; Zhou, Y.; Yang, J.; Hu, K.; Huang, Y. The effectiveness of TDF versus ETV on incidence of HCC in CHB patients: a meta analysis. BMC Cancer 2019, 19, 1–9. [Google Scholar] [CrossRef]
- Kanehisa, M.; Furumichi, M.; Sato, Y.; Kawashima, M.; Ishiguro-Watanabe, M. KEGG for taxonomy-based analysis of pathways and genomes. Nucleic Acids Res. 2022, 51, D587–D592. [Google Scholar] [CrossRef] [PubMed]
- Santantonio, T.A. and M. Fasano, Chronic hepatitis B: Advances in treatment. World J Hepatol, 2014. 6(5): p. 284-92.
- Seyfried, N.T.; Dammer, E.B.; Swarup, V.; Nandakumar, D.; Duong, D.M.; Yin, L.; Deng, Q.; Nguyen, T.; Hales, C.M.; Wingo, T.; et al. A Multi-network Approach Identifies Protein-Specific Co-expression in Asymptomatic and Symptomatic Alzheimer’s Disease. Cell Syst. 2016, 4, 60–72. [Google Scholar] [CrossRef]
- Ling, L.; Zheng, D.; Zhang, Z.; Xie, W.; Huang, Y.; Chen, Z.; Wang, X.; Li, D. Effect of HBx on inflammation and mitochondrial oxidative stress in mouse hepatocytes. Oncol. Lett. 2020, 19, 2861–2869. [Google Scholar] [CrossRef]
- Arbuthnot, P. and M. Kew, Hepatitis B virus and hepatocellular carcinoma. Int J Exp Pathol, 2001. 82(2): p. 77-100.
- Calvo, S.E.; Clauser, K.R.; Mootha, V.K. MitoCarta2.0: an updated inventory of mammalian mitochondrial proteins. Nucleic Acids Res. 2015, 44, D1251–D1257. [Google Scholar] [CrossRef] [PubMed]
- Brenner, C.; Ventura-Clapier, R.; Jacotot, E. Mitochondria and Cytoprotection. Biochem. Res. Int. 2012, 2012, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Slagle, B.L.; Bouchard, M.J. Hepatitis B Virus X and Regulation of Viral Gene Expression. Cold Spring Harb. Perspect. Med. 2016, 6, a021402. [Google Scholar] [CrossRef]
- Sivasudhan, E.; Blake, N.; Lu, Z.; Meng, J.; Rong, R. Hepatitis B Viral Protein HBx and the Molecular Mechanisms Modulating the Hallmarks of Hepatocellular Carcinoma: A Comprehensive Review. Cells 2022, 11, 741. [Google Scholar] [CrossRef]
- Tripathi, G.; Sharma, N.; Bindal, V.; Yadav, M.; Mathew, B.; Sharma, S.; Gupta, E.; Maras, J.S.; Sarin, S.K. Protocol for global proteome, virome, and metaproteome profiling of respiratory specimen (VTM) in COVID-19 patient by LC-MS/MS-based analysis. STAR Protoc. 2021, 3, 101045. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).