Submitted:
18 May 2023
Posted:
22 May 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Patient Population
2.2. LDR-BT
2.3. Follow-Up
2.4. Outcome Measures
2.5. Statistical Analyses
2.6. Ethical Considerations
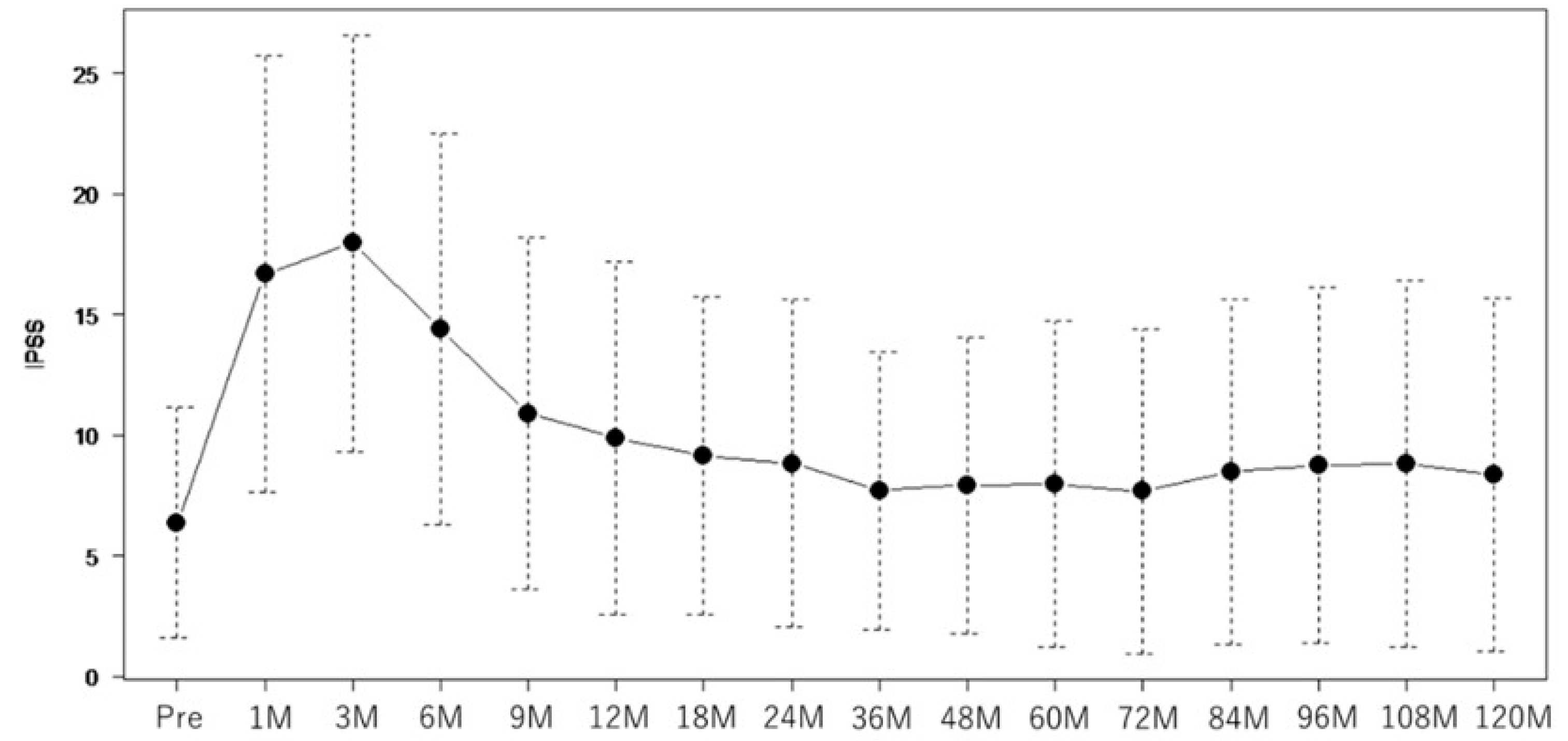
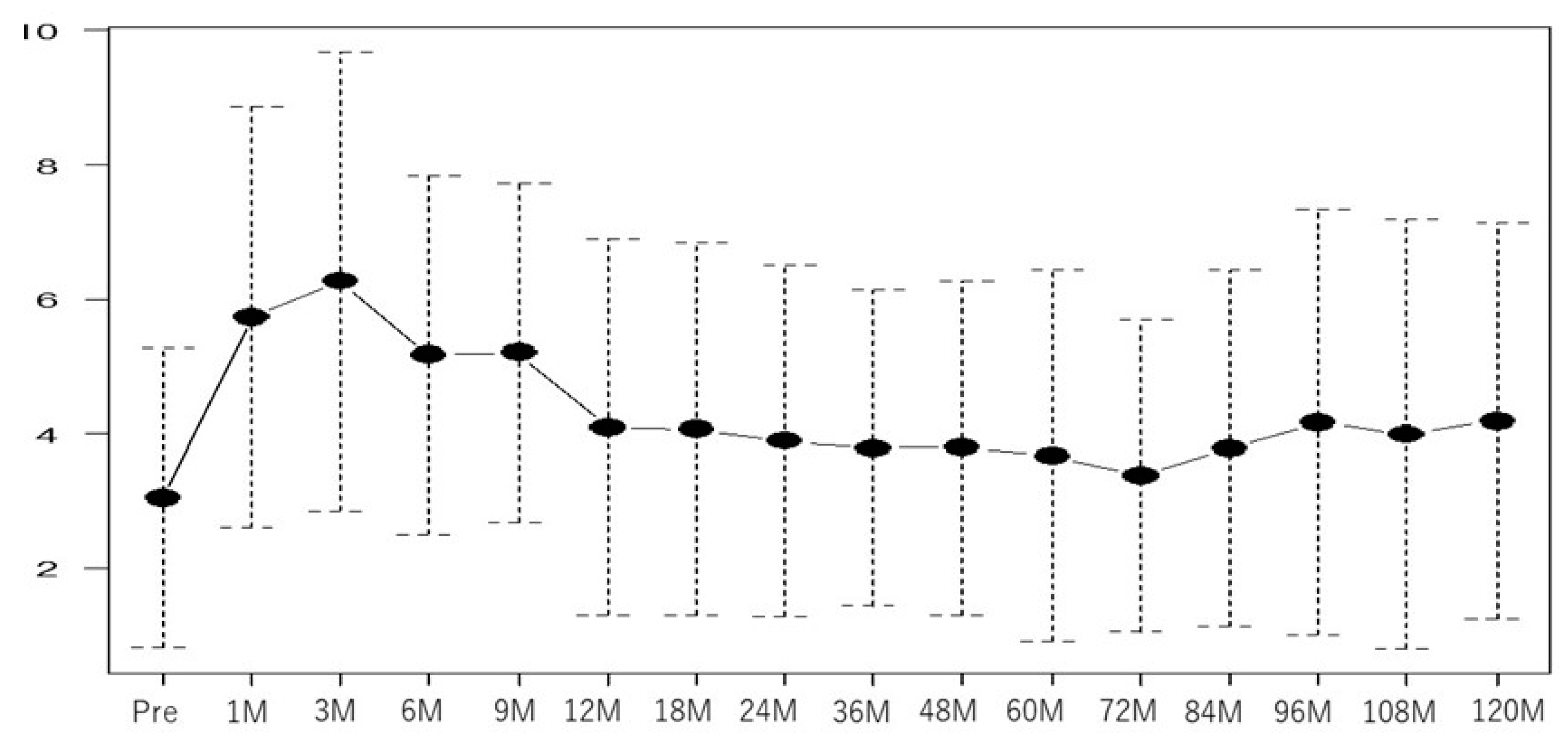
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel R.L.; Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021, 71, 209–249.
- Ganjoho. Projected Cancer Statistics. 2022. Available at https://ganjoho.jp/reg_stat/statistics/stat/short_pred_en.htmlt. Accessed MONTH DAY, YEAR.
- Grimm, P.D., Blasko, J.C., Sylvester, J.E., Meier, R.M., Cavanagh, W. 10-year biochemical (prostate-specific antigen) control of prostate cancer with (125)I brachytherapy. Int J Radiat Oncol Biol Phys. 2001, 51, 31–40. [CrossRef]
- Grimm, P., Sylvester, J. Advances in brachytherapy. Rev Urol. 2004, 6 Suppl 4, S37–S48.
- Lehto US, Tenhola H, Taari K, et al. Patients’ perceptions of the negative effects following different prostate cancer treatments and the impact on psychological well-being: A nationwide survey. Br J Cancer 2017;116:864e873.
- Veccia, A., Orazio Caffo. Impact of post-implant dosimetric parameters on the quality of life of patients treated with low-dose rate brachytherapy for localised prostate cancer: results of a single-institution study. Radiat Oncol. 2015, 10, 130. [CrossRef]
- Farris, J.C., Hughes, R.T., Steber, C.R., Craven, T.E., Frizzell, B.A. Patient assessment of lower urinary tract symptoms using the international prostate symptom score following low-dose-rate prostate brachytherapy. Brachytherapy. 2021, 20, 1107–1113.
- Boyce-Fappiano, D., Bathala, T.K., Ye, R., Pasalic, D. Predictors of urinary toxicity with MRI-assisted radiosurgery for low-dose-rate prostate brachytherapy. Brachytherapy. 2020, 19, 574–583.
- Rose, J,N., Araujo, C., Crook, J.M. MRI-defined treatment margins, uriary toxicity, and PSA response in LDR prostate brachytherapy. Brachytherapy. 2022, 21, 216–223. [CrossRef]
- Eriguchi, T., Yorozu, A., Kuroiwa, N., Yagi, Y., Nishiyama, T., Saito, S., Toya, K., Hanada, T., Shiraishi, Y., Ohashi, T., Shigematsu, N. Predictive factors for urinary toxicity after iodine-125 prostate brachytherapy with or without supplemental external beam radiotherapy. Brachytherapy. 2016, 15, 288–295. [CrossRef]
- Cozzarini, C., Rancati, T., Carillo, V., Civardi. F. Multi-variable models predicting specific patient-reported acute urinary symptoms after radiotherapy for prostate cancer: results of a cohort study. Radiother Oncol. 2015, 116, 185–191.
- Miyake,M., Tanaka, N., Asakawa, I., Hori, S., Morizawa, Y. Assessment of lower urinary symptom flare with overactive bladder symptom score and International Prostate Symptom Score in patients treated with iodine-125 implant brachytherapy: long-term follow-up experience at a single institute. BMC Urology. 2017, 17, 62.
- Aaltomaa, S.H., Kataja, V.V., Lahtinen, T., Palmgren, J.E., Forsell, T. Eight years experience of local prostate cancer treatment with permanent I125 seed brachytherapy. Morbidity and outcome results. Radiother Oncol. 2009, 91, 213–216. [CrossRef]
- Wong, C.K., Choi, E.P., Chan, S.W., Tsu, J.H., Fan, C.W., Chu, P.S., Cheung, F.K., Ma, W.K., Mah, I.S.F., Yip, S.K,. et al. Use of the International Prostate Symptom Score (IPSS) in Chinese male patients with benign prostatic hyperplasia. Aging Male. 2017, 20, 241–249. [CrossRef]
- Miller DC, Sanda MG, Dunn RL, et al. Long-term outcomes among localized prostate cancer survivors: Health-related quality-of-life changes after radical prostatectomy, external radiation, and brachy- therapy. J Clin Oncol 2005;23:2772e2780.
- Sakayori, M., Ohashi, T., Momma, T., Kaneda, T., Nishimura, S., Sutani, S., Yamashita, S., Shigematsu, N. Quantitative analysis of genitourinary toxicity after iodine-125 brachytherapy for localized prostate cancer: followup of the International Prostate Symptom Score and Overactive Bladder Symptom Score. Brachytherapy. 2017, 16, 806–814. [CrossRef]
- Gelblum, D.Y., Potters, L., Ashley, R., Waldbaum, R., Wang, X.H., Leibel, S. Urinary morbidity following ultrasound-guided transperineal prostate seed implantation. Int J Radiat Oncol Biol Phys. 1999, 45, 59–67. [CrossRef]
- Onishi, K., Tanaka, N., Miyake, M., Nakai, Y., Anai, S., Torimoto, K., Yamaki, K., Asakawa, I., Hasegawa, M., Fujii, T., et al. Changes in lower urinary tract symptoms after iodine-125 brachytherapy for prostate cancer. Clin Transl Radiat Oncol 2019, 14, 51–58. [CrossRef]
- Ohashi, T., Yorozu, A., Toya, K., Saito, S., Momma, T. Serial changes of international prostate symptom score following I-125 prostate brachytherapy. Int J Clin Oncol. 2006, 11, 320–35. [CrossRef]
- Neill, M., Studer, G., Le, L., et al. The nature and extent of urinary morbidity in relation to prostate brachytherapy urethral dosimetry. Brachytherapy. 2007, 6, 173–179. [CrossRef]
- Thomas, C., Keyes, M., Liu, M., Moravan, V. Segmental urethral dosimetry and urinary toxicity in patients with no urinary symptoms before permanent prostate brachytherapy. Int J Radiat Oncol Biol Phys. 2008, 72, 447–455. [CrossRef]
- Earley, J.J., Abdelbaky, A.M., Cunningham, M.J., Chadwick, E., Langley, S.E.M., Laing, R.W. Correlation between prostate brachytherapy-related ureth,ral stricture and peri-apical urethral dosimetry: a matched case-control study. Radiother Oncol. 2012, 104, 187–191. [CrossRef]
- Steggerda, M.J., Witteveen, T., Van Den Boom, F., Moonen, L.M.F. Is there a relation between the radiation dose to the different sub-segments of the lower urinary tract and urinary morbidity after brachytherapy of the prostate with I-125 seeds? Radiother . 2013, 109, 251–255. [CrossRef]
- Roeloffzen, E.M.A., Monninkhof, E.M., Battermann, J.J., Van Roermund, J.G.H., Moerland, M.A., Van Vulpen, M. Acute urinary retention after I-125 prostate brachytherapy in relation to dose in different regions of the prostate. Int J Radiat Oncol Biol Phys. 2011, 80, 76–84. [CrossRef]
- Cesaretti JA, Stone NN, Stock RG. Urinary symptom flare following I-125 prostate brachytherapy. Int J Radiat Oncol Biol Phys 2003;56:1085–92.
- Keyes, M., Miller, S., Pickles, T., Halperin, R., Kwan, W., Lapointe, V., McKenzie, M., Spadinger, I., Pai. H., Chan, E.K., Morris, W.J. Late urinary side effects 10 years after low-dose-rate prostate brachytherapy: population-based results from a multiphysician practice treating with a standardized protocol and uniform dosimetric goals. Int J Radiat Oncol Biol Phys. 2014, 90, 570–578. [CrossRef]
- Marshall, R.A., Buckstein, M., Stone, N.N., Stock, R. Treatment outcomes and morbidity following definitive brachytherapy with or without external beam radiation for the treatment of localized prostate cancer: 20-year experience at Mount Sinai Medical Center. Urol Oncol. 2014, 32, 38.e1–e7. [CrossRef]
- Stone, N.N., Winoker, J.S., Kaplan, S.A., Stock, R.G. Factors influencing long-term urinary symptoms after prostate brachytherapy. BJU Int. 2018, 122, 831–836. [CrossRef]
- Elizabeth, T.,William, B. Ultra-long-term toxicity of prostate brachytherapy. Brachytherapy. 2021, May- Jun; 20(3): 595–600. [CrossRef]
- Salembier, C., Lavagnini, P., Nickers, P., Mangili, P., Rijnders, A., Polo, A., Venselaar, J., Hoskin, P.R. Tumour and target volumes in permanent prostate brachytherapy: a supplement to the ESTRO/EAU/EORTC recommendations on prostate brachytherapy. Radiother Oncol. 2007, 83, 3–10. [CrossRef]
- Allen ZA, Merrick GS, Butler WM, et al. Detailed urethral dosimetry in the evaluation of prostate brachytherapy-related urinary morbidity. Int J Radiat Oncol Biol Phys 2005;62:981e987.
| Factor | Total 203 |
|
|---|---|---|
| Age, years Gleason score |
66 (50–78) | |
| 6 | 143 (50.7) | |
| 7 | 59 (49.3) | |
| PSA cTstage |
6 (1.1–14.7) | |
| T1c | 165 | |
|
NADT |
T2a | 37 |
| T2b | 2 | |
| Yes | 159 (78.3) | |
| No | 44 (21.7) | |
| IPSS OABSS |
5 (0–25) | |
| 2 (0–15) | ||
| OAB | Yes | 39 (23.4) |
| No | 128 (76.6) | |
| UFM (mL) | 9 (9–49) | |
| Intra_PV (mL) | 25.4(8.77–46.88) |
| Factor | Median | Range |
|---|---|---|
| Needle | 22 | 13–35 |
| Seed | 75 | 35–108 |
| PD90 (Gy) | 172 | 105–241 |
| PV100 (mL) | 96 | 77–100 |
| UD5 (Gy) | 235 | 171–400 |
| UD10 (Gy) | 222 | 168–332 |
| UD30 (Gy) | 218 | 159–319 |
| UD90 (Gy) | 141 | 63–241 |
| 2 years | 5 years | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Moderate (n=98) | Severe (n=15) | Moderate (n=75) | Severe (n=12) | |||||||||
| Factor | UVA | MVA | UVA | MVA | UVA | MVA | UVA | MVA | ||||
| p | OR (95% CI) | P | p | OR (95% CI) | p | p | OR (95% CI) | p | P | OR (95% CI) | p | |
| Age | 0.61 | 0.72 | 0.19 | 0.99 (0.91–1.07) | 0.83 | 0.35 | ||||||
| GS | 0.34 | 0.56 | 0.53 | 1.00 | ||||||||
| PSA | 0.76 | 0.42 | 0.59 | 0.71 | ||||||||
| NADT | 0.22 | 0.09 | 0.65 (0.11–3.94) | 0.64 | 0.86 | 0.14 | 0.55 (0.21–1.46) | 0.23 | ||||
| Needle | 0.41 | 0.71 | 0.73 | 0.72 | ||||||||
| Seed | 0.11 | 1.02 (1.00–1.04) | 0.02 | 0.20 | 1.02 (0.96–1.07) | 0.56 | 0.59 | 0.94 | ||||
| Intra_PV | 0.53 | 0.37 | 0.24 | 0.61 | ||||||||
| PD90 | 0.82 | 0.37 | 0.63 | 0.21 | ||||||||
| PV100 | 0.41 | 0.75 | 0.53 | 0.33 | ||||||||
| UD5 | 0.49 | 0.25 | 0.78 | 0.62 | ||||||||
| UD10 | 0.16 | 1.00 (0.99–1.01) | 0.97 | 0.10 | 0.97 (0.94–1.01) | 0.18 | 0.20 | 1.00 (0.98–1.02) | 0.90 | 0.11 | 0.99 (0.98–1.04) | 0.91 |
| UD30 | 0.56 | 0.38 | 0.71 | 0.76 | ||||||||
| UD90 | 0.83 | 0.47 | 0.84 | 0.20 | ||||||||
| Baseline IPSS | <0.01 | 1.3 (1.18–1.42) | <0.01 | 0.08 | 1.12 (0.95–1.31) | 0.17 | <0.01 | 1.19 (1.10–1.29) | <0.01 | 0.02 | 1.18 (1.06–1.31) | <0.01 |
| Baseline UFM | 0.53 | 0.72 | 0.60 | 0.09 | 1.01 (0.94–1.08) | 0.75 | ||||||
| OAB at 2 years (n=60) | OAB at 5 years (n=40) | |||||
|---|---|---|---|---|---|---|
| UVA | MVA | UVA | MVA | |||
| P | OR (95% CI) | p | p | OR (95% CI) | p | |
| Age | 0.52 | 0.45 | ||||
| GS | 0.12 | 0.97 | ||||
| PSA | 0.71 | 0.27 | ||||
| NADT | 0.56 | 0.19 | ||||
| Needle | 0.84 | 0.86 | ||||
| Seed | 0.16 | 0.22 | ||||
| Intra_PV | 0.24 | 0.47 | ||||
| PD90 | 0.42 | 0.72 | ||||
| PV100 | 0.99 | 0.87 | ||||
| UD5 | 0.23 | 0.75 | ||||
| UD10 | 0.19 | 0.99 (0.98–1.01) | 0.78 | 0.40 | ||
| UD30 | 0.40 | 0.75 | ||||
| UD90 | 0.92 | 0.61 | ||||
| Baseline OABSS | <0.01 | 1.77 (1.35–2.31) | <0.01 | 0.03 | 2.5 (1.10–5.80) | 0.02 |
| Baseline UFM | 0.02 | 1.11 (1.03–1.20) | <0.01 | 0.61 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





