Introduction
Among the vast family of viruses that periodically cause infectious illness in humans, coronaviruses did not initially attract much attention. Isolated in 1965 from a person with acute coryza, they have long been associated with a mild, self-limiting upper respiratory tract infection. Four types of seasonal coronaviruses have been known to be associated with common cold infection in humans: two alpha (229E, OC43) and two beta (NL63, HKU1) [
1,
2]. The situation began to change starting in 2002, when the first highly pathogenic strain of coronavirus emerged, causing a SARS outbreak of more than 8,400 cases. Subsequently, this virus was named SARS-CoV [
3].
Ten years later, another pathogenic
Betacoronavirus representative appeared in the Middle East: the causative agent of Middle East Respiratory Syndrome (MERS). It caused 1,348 infections from 2012-2015, in which 479 people died [
3,
4,
5,
6]. The evolution of pathogenic coronaviruses continued at the end of 2019, on December 31 specifically, when a cluster of SARS patients was detected in a fish market in the Chinese city of Wuhan. It was caused by a new
Betacoronavirus representative, SARS-CoV-2, causing a pandemic of acute respiratory infection pandemic (COVID-19) now in its third year [
7,
8,
9].
According to statistical sources, 630,164,738 people have been infected globally, including 6,577,479 deaths (as of October 14, 2022) [
10,
11]. A characteristic feature of SARS-CoV-2 is relatively rapid viral evolution due to a rather high mutational variability [
12,
13]. Thus, the original Wuhan viral line (2019-nCoV) was replaced by a new variant: B.1.1.7 (Alpha type), first isolated on September 20, 2020 in the UK [
14]. In the same year, a variant, B.1.351 (Beta type) [
15], was identified in S. Africa (May 20). In November, a new variant, B.1.1.28 (P.1), was identified in Brazil, designated as the Gamma viral line [
16]. Around the same time, a virus was isolated in India, designated as B.1.617.2 (Delta variant) [
17]. The latest virus was B.1.1.529, better known as the Omicron variant, which was identified in November 2021 simultaneously in S. Africa and Botswana; it circulated at least until the end of 2022 [
18,
19]. All of the aforementioned coronavirus variants were classified as variants-of-concern (VOC) [
11]. In addition to them, however, there are 7 additional, less pathogenic, strains. These did not show significance in the pandemic process and were quickly forced out of circulation by more pathogenic representatives.
In the Republic of Belarus (RB), the COVID-19 pandemic turned out to be less widespread than in neighboring countries, such as Russia for example [
20]. As of 14/10/2022, 994,037 individuals have been infected in the RB, of which 7,118 (0.7%) have died. In terms of incidence, the Republic ranks 65th among nations globally. At the same time, like other countries globally, it was not bypassed by the well-known SARS-CoV-2 lines that appeared in circulation [
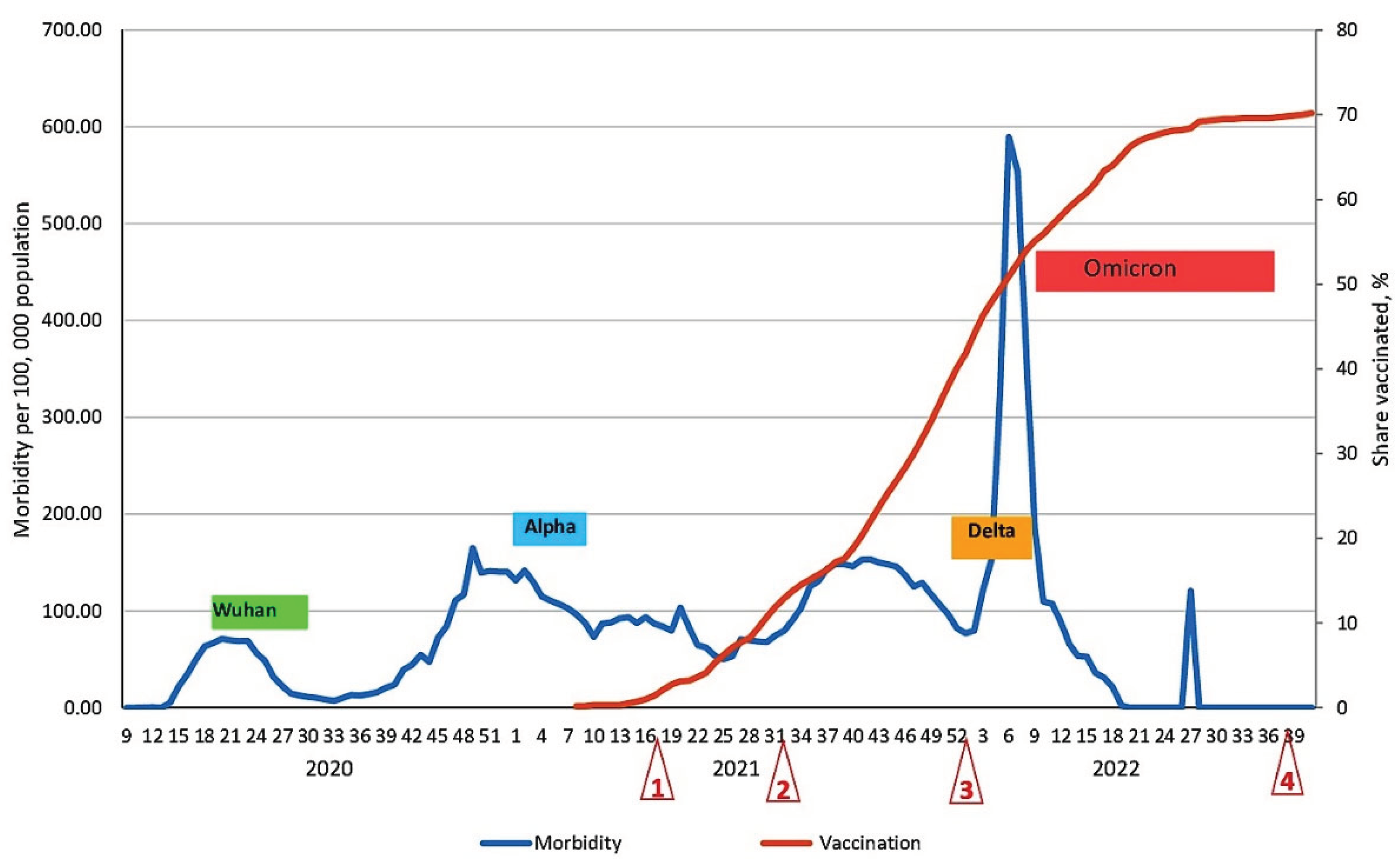
21]. By comparing the ordering of SARS-CoV-2 variants and recorded peaks in morbidity, it was possible, with some degree of probability, to predict the chronological sequence of the listed variants appearing in circulation in the RB (
Figure 1). At the same time, it should be noted that each subsequent viral variant caused a wave of increased incidence in the RB, often 3-4 months later than peaks in many other countries.
The first COVID-19 case was detected only in the 9th week of 2020, that is, at the end of the 2nd month after the first case was detected in Wuhan [
21]. The Alpha SARS-CoV-2 variant was identified in the UK in September 2020, but only in February 2021 did it start circulating in the RB. Similarly, the most transmissible Omicron variant was initially isolated in S. Africa in September 2021, but its rapid, albeit short-lived, spread in RB was not seen until January 2022 (
Figure 1). Thus, the epidemic situation in the RB can be characterized by several features: relatively low population density (45.5 km
-2); later appearance of new SARS-CoV-2 genetic variants in circulation; and relatively low morbidity, not exceeding 600 ‱o (
Figure 1), with mortality varying within 0.7%.
For comparison, in Poland (as of May 5, 2022): pop. density was 121.2 km
-2; morbidity was 15834 ‱o; and COVID-19 mortality was 1.9%. In neighboring Ukraine (pop. density 75.6 km
-2), morbidity was 12152 ‱o, and the COVID-19 mortality rate was 2.17%. In Hungary (pop. density 103.3 km
-2), morbidity was 19803 ‱o, and the mortality rate reached 2.4%. In Lithuania (pop. density 40.6 km
-2), morbidity was unexpected high (39949 ‱o), and the mortality rate was 0.9% [
20,
22].
Another factor likely preventing intense spread of SARS-CoV-2 among the Belarusian population was substantial seroprevalence. According to a cross-sectional study to assess population immunity in Belarus, the average level of seroprevalence for SARS-CoV-2 N protein Abs was 47.1% already by May 2021 (95% CI: 46.3-48.0) [
23]. Vaccination against pathogenic coronavirus was launched in the first half of 2021 and actively carried out in the Republic. This undoubtedly became a decisive prerequisite for increasing the level of population immunity. Indeed, a noticeable decrease in COVID-19 morbidity was observed in the second half of 2022 (
Figure 1).
It should also be noted that booster re-vaccination was launched among the population starting from the 40th week of 2021. In result, vaccine coverage of the population had reached almost 70% of the threshold starting from the 19th week of 2022. A sharp decrease in incidence to only sporadic levels immediately followed. This is fully consistent with existing theories which assert that achievement of 70% seroprevalence represents a threshold leading to interruption of pathogen transmission [
24,
25]. This fact likely explains certain dynamics: the weak collective response to the introduction of the Delta variant into the RB; and the brief peak in morbidity associated with circulation of Omicron B1.1.529 strains (
Figure 1).
Of course, in parallel with changes in coronavirus genetic variants, humoral immunity in the population inevitably evolved as well. When analyzing the COVID-19 morbidity curve alongside vaccination dynamics, we notice a clear relationship. Specifically, the formation of population immunity exerted a dominant, positive influence leading to the suppression, and then the cessation, of the epidemic process among the population (
Figure 1). Analysis of the evolution of SARS-CoV-2 collective immunity in the Belarusian population, in the context of the COVID-19 epidemic (second half of 2020 and the first 10 months of 2022), was the main goal of this randomized longitudinal study.
Materials and Methods
Formation of the volunteer cohort. A study to assess the formation and progression of SARS-CoV-2 collective immunity in the Belarusian population was implemented in 4 stages over 2021-22 (
Figure 1). The 1st stage was carried out (May 14-19, 2021) with 12929 individuals taking part in the survey. In the 2nd stage (Aug. 30 – Sept. 3, 2021), the number of examined individuals decreased by 28%, leaving to 9269 people. By the 3rd stage (Jan. 24 – 28, 2022), the cohort of examined persons decreased by another 11.6%, leaving 8189. In the final 4th stage (Oct. 10 – 14, 2022), the surveyed cohort size was 5755; some in this group had missed the 2nd and/or 3rd stages. To obtain comparable results, only those individuals who participated in all stages of monitoring were selected from the general cohort. These totaled 4661 people, and their results were used for subsequent analysis.
All studies were carried out exclusively on a voluntary basis in which each volunteer (or their legal representative) was familiarized with the study's aims and conditions. The study was conducted in strict accordance with the provisions of the Declaration of Helsinki. Prior to the start of the cross-sectional study, the design was approved by the Bioethics Committee of the RB (protocol No. 2, dated May 13, 2021) and the local ethics committee of the St. Petersburg Pasteur Institute (Proceedings No. 64, dated May 26, 2020).
All volunteers included in the cohort were clinically healthy. The exclusion criterion was: signs of manifest COVID-19 during the survey period. Our methodology for cohort formation and examination has been exhaustively described earlier [
23,
26,
27]. Over the course of four stage examination of individuals in the final cohort, antibodies (Abs) to two main antigens (Ags) were determined in volunteer sera according to previously described methods [
23,
27]. These were Abs to nucleocapsid (Nc) and to S protein receptor-binding domain (RBD). To obtain comparable results, volunteers were randomized during cohort formation by age and region (
Table 1 and
Table 2). Randomization by age yielded a cohort profile similar to the age structure of the Belarusian population [
28].
Upon regional randomization, the greatest volunteer representation was from Minsk (the capital and most populous city) and the Mogilev region. The least was from the Gomel region. In other administrative regions, representation was relatively uniform. Thus, based on the randomization performed, the distribution of volunteers by age and region satisfactorily corresponded to demographic criteria (
Table 2) [
28].
Some of volunteers participated in coronavirus vaccination deployed in the RB. In the 1st stage, 1168 people in the cohort were vaccinated. Of these, 85.3% received the Gam-COVID-Vac vector vaccine (Sputnik V, Gamaleya National Center for Epidemiology and Microbiology, Moscow, Russia), and 14.7% received the inactivated BBIBP-CorV vaccine (Sinopharm Group Co., Ltd., Shanghai, PRC). By the second stage, 1179 volunteers were vaccinated, with 85.3% receiving the Sputnik V vaccine, 14.0% receiving BBIBP-CorV, and about 1% receiving other vaccines. By the 3rd stage, another 1371 volunteers had been vaccinated. At the same time, the share immunized with Sputnik V vaccines was 87.2%, and the share vaccinated with BBIBP-CorV was 12.7%. In the 4th stage, 1196 people were vaccinated, with 85.4% receiving Sputnik V and 14.5% receiving BBIBP-CorV.
Statistical analysis. The data obtained were processed using Excel 2010. Confidence intervals (95% CI) were calculated according to the method of A. Wald and J. Wolfowitz [
29], with the corrections of A. Agresti and B. A. Coull [
30]. Correlation analysis was performed using the Spearman rank correlation method. The statistical significance of differences was calculated by z-test using a corresponding online calculator [
31]. The statistical significance of differences, unless otherwise indicated, was assessed with a probability
p≤0.05.
Results
Characteristics of the COVID-19 epidemic process over the seromonitoring period. The cohort of volunteers who took part in all stages of Ab seroprevalence assessment in the Belarusian population during the COVID-19 pandemic was, as indicated, 4661 individuals. The first stage was conducted from May 14 to 19, 2021 (weeks 19-20). During this period, there was a small peak in morbidity within 88 ‱o. The B.1.1.7 (Alpha) viral variant circulated predominantly in the country, yet without causing a significant increase in morbidity, which did not exceed 100 ‱o.
The second stage of monitoring (30.08-03.09.2021, week 35) featured a more difficult epidemic situation, with the B.1.617.2 (Delta) variant circulating predominantly. Unlike the Alpha variant, it was characterized by higher transmissibility and virulence [
17,
32].
The third stage of monitoring was carried out from January 24-28, 2022 (week 4), two weeks before a sharp increase in morbidity, which reached a maximum of 583.7 ‱o (week 6). Fortunately, this outbreak turned out to be short-lived, and after 2 weeks a rapid decline ensued. This wave of morbidity was caused by B.1.1.529 (Omicron), the circulation of which was still ongoing during the 4th stage of monitoring (10-14.10.2022, week 41).
In summarizing the data, it should be emphasized that fewer SARS-CoV-2 genetic variants were seen in the RB than in the rest of the world. Indeed, a number of variants-of-concern were not detected in the RB (B.1.351, B.1.1.28 (P.1), others), and incidence associated with circulating viral lines was relatively low. A probable reason for this phenomenon could be strong collective immunity to SARS-CoV-2, which had reached 50% (share vaccinated) near the beginning of Omicron circulation (
Figure 1). Considering that, by this time, about 11% of the population had manifested COVID-19 [
23,
33], then the overall level of collective immunity could exceed 60%, even without taking into account asymptomatic infections. Thus, it is logical to assume that a threshold was reached; it was seemingly sufficient to reduce incidence to a sporadic level starting from week 20 (2022). A small one-day outbreak (within 120 ‱o, week 28, 2022) did not change the main trend of near-zero morbidity (
Figure 1).
Evolution of SARS-CoV-2 Collective Immunity among Volunteers Assessed by Serological Dynamics
Distribution of SARS-CoV-2 Seropositivity by Age Group
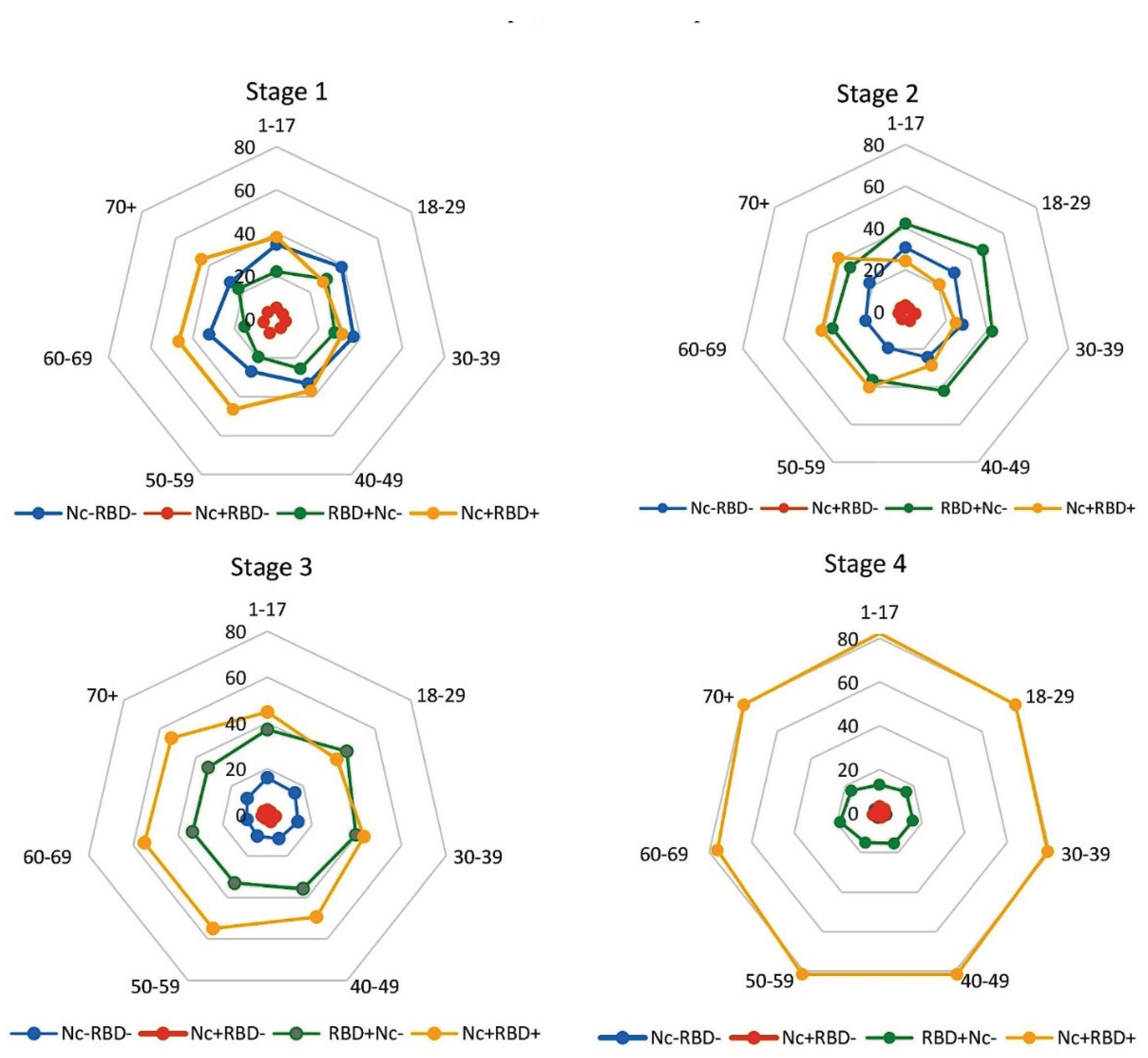
When analyzing the evolution of humoral immunity to SARS-CoV-2, we determined Ab profiles generated in response to the circulation of two main viral antigens in the body, Nc and RBD. For this purpose, four main groups were distinguished in the volunteer cohort. The first group included individuals who did not have specific Abs circulating in their blood (anti-Nc or anti-RBD); it was designated as Nc‒RBD‒. The 2nd group included volunteers in whose blood Nc Abs alone were detected; it was designated as Nc+RBD‒. The 3rd group included volunteers seropositive for RBD Abs alone (RBD+Nc‒). The last group included volunteers with both Ab types in their blood simultaneously (Nc+RBD+). All studies were conducted in a single cohort of 4661 selected volunteers, with all volunteers participating in all monitoring stages.
In the 1st stage, a significant predominance of Nc
+RBD
+ over Nc
‒RBD
‒ (
p<0.05) was noted, mainly due to higher seropositivity among older (50-70
+) individuals (
Figure 2). The smallest proportion of seropositive individuals was noted among Nc
+RBD
‒. The share of RBD
+Nc
‒ turned out to be approximately 4.5-fold higher than Nc
+RBD
‒, while being significantly lower than Nc
+RBD
+ and Nc
‒RBD
‒ (
p<0.05), except for the group '18-39 years', where differences with Nc
+RBD
+ were not significant (
Figure 2, Table S1).
By the 2nd stage, the proportion of seronegative (Nc
‒RBD
‒) and specific seropositive groups (Nc
+RBD
‒, Nc
+RBD
+) significantly decreased (
p<0.001). RBD
+Nc
‒, on the contrary, increased significantly (
p<0.0001) (
Figure 2, Table S1). A reason for these changes is likely expansion of vaccination coverage, which by this time amounted to more than 15%. The most widely used preparation during this period was Sputnik V (vector vaccine) whose mechanism leads to production of RBD antigen alone. By the 3rd stage, a change in trend was noted, manifested by a noticeable increase in the share of Nc
+RBD
+, alongside steep decreases in Nc
‒RBD
‒ and Nc
+RBD
‒ (p<0.0001), as well as an insignificant decrease in RBD
+Nc
‒ (
Figure 2, Table S1). By this time, vaccination coverage was 47% (
Figure 1).
The situation that developed by the 4th stage was characterized by an absolute dominance of Nc
+RBD
+ status (
Figure 2, Table S1). Taking into account the short-term outbreak of COVID-19 in the 6th week of 2022, as well as the high level of vaccination (which amounted to 70.2% by the 4th stage), it can be assumed that these factors contributed to the formation of a high level of Nc
+RBD
+ dominant positivity. It averaged 80.0% for the cohort (95% CI: 78.8-91.2), likely becoming one of the reasons for the near-zero COVID-19 morbidity in the Belarusian population starting from week 20 of 2022 (
Figure 1).
Influence of Occupational Factors on the Structure of SARS-CoV-2 Antibody Seroprevalence
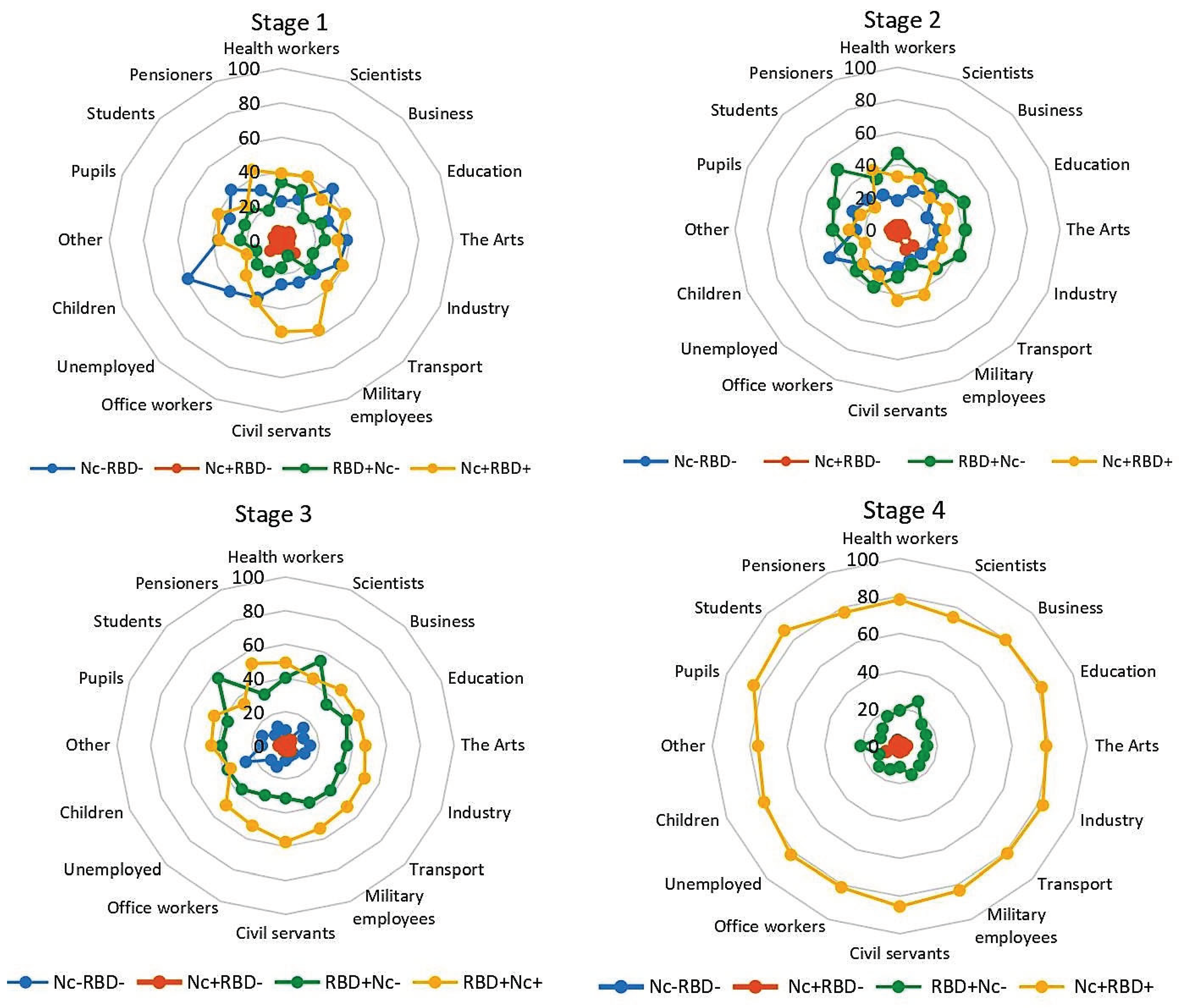
Occupation can have a significant impact on seroprevalence level and structure. There is an extensive list of professions that involve constant wide contact with the surrounding population. Visual, and often tactile, contacts with people are typical for certain professional categories, such as healthcare, education, trade, transport, catering, consumer services (hair salons, massage parlors, spas, etc.), as well as among a number of other people who cannot carry out their work duties remotely or in self-isolation mode. As such, it follows that SARS-CoV-2 Ab seroprevalence among individuals in such professions can have a significant impact on the course and outcome of the COVID-19 epidemic process. Analysis of seropositivity levels, taking into account the professional structure of the surveyed cohort, was the next step in monitoring assessment (
Figure 4, Table S3).
In the 1st stage, a noticeable spread in the shares of Nc
‒RBD
‒, RBD
+Nc
‒ and Nc
+RBD
+ was observed (
Figure 4). When calculating the variance, the largest value was noted in Nc
‒RBD
‒ (81.86); the smallest was in RBD
+Nc
‒ (35.64). The share of Nc
+RBD
‒ was only 5.4% (95% CI: 5.1-6.1); the dispersion was 4.18. The largest proportion of Nc
‒RBD
‒ was observed among children (58.8%; 95% CI: 44.2-72.4). In the Nc
+RBD
+ group, the maximum proportions were found among military personnel (56.7%; 95% CI: 37.4-74.5) and civil servants (53.3%; 95% CI 44.1-62.2) (Table S3).
Stage 2 was characterized by an increase in the proportion of RBD
+Nc
‒ in almost all population groups: from 22.7% (95% CI: 21.6-24.0) in stage 1 to 39.5% (95% CI: 38.1-40.9); differences were significant at
p<0.0001 (
Figure 4, Table S3). The shares of all other categories, on the contrary, decreased: Nc
‒RBD
‒ by 1.3-fold; Nc
+RBD
‒ by 1.5-fold; and Nc
+RBD
+ by 1.2-fold. The differences were significant (
p<0.05).
The general trend of changes in the structure of immunity in later stages was noted in all population groups, regardless of the field of activity. By the 3rd stage, the structure of seropositivity had noticeably changed. The share of Nc+RBD+ increased to 49.5% (95% CI: 48.0-50.4) compared to 32.7% (95% CI: 31.3-34.0) in the 2nd stage; the differences were significant (p<0.001). A pronounced decrease (almost 2-fold) to 12.1% (95% CI: 11.2-13.1) was noted for Nc‒RBD‒. The RBD+Nc‒ value decreased by an average of 3.5% for the cohort to 36.0% (95% CI: 34.6-37.4). The differences with 2nd stage data were significant (p<0.05).
In the 4th stage, the trend towards the formation of stable collective immunity reached its maximum strength, manifested in all population groups as a significant increase in the proportion of Nc
+RBD
+. It ranged from 74.3% (95% CI: 56.7-87.5) in the group 'scientists' to 87.0% (95% CI: 73.7-95.1) in the group 'students'. In result, the proportions of Nc
‒RBD
‒ and Nc
+RBD
- in each population group decreased to 2-3% (
Figure 4, Table S3).
In summarizing the obtained data, we can formulate the main trend regarding collective immunity. Active immunization, carried out across the 4-stage seromonitoring period, was accompanied by the accumulation of SARS-CoV-2 specific Abs, and these likely became the leading factor in reducing pathogen circulation. COVID-19 incidence and spread were nearly stopped, at least in terms of symptomatic infections.
Quantitative Distribution of Nc Abs during Seromonitoring
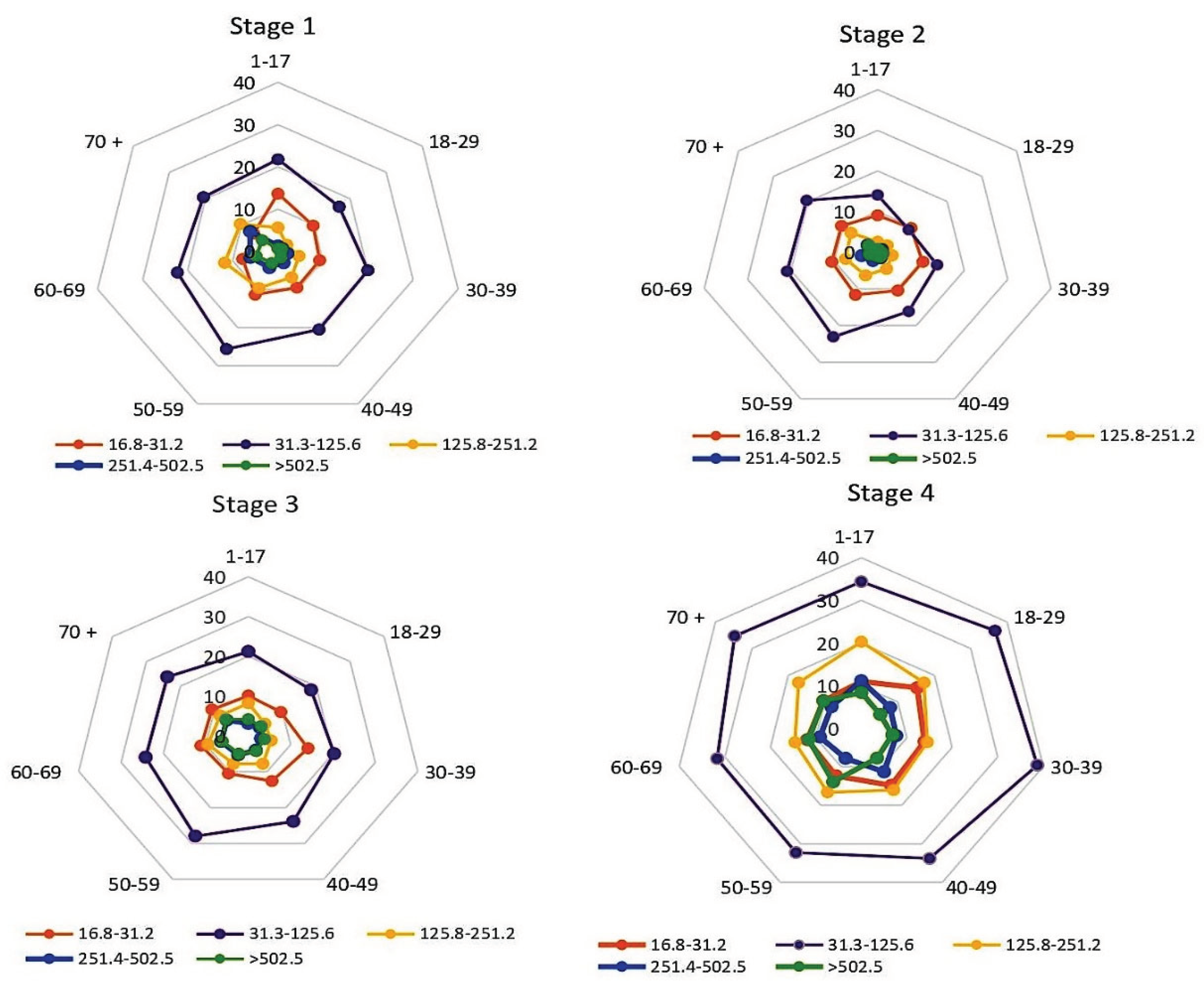
Assessment of general seroprevalence is convenient and informative, but it does not represent fine data on the quantitative distribution of Nc or RBD antibodies. To quantitatively measure the levels of circulating Abs in volunteers at each stage of seromonitoring, an appropriate test system was used, as described in a previous work [
23]. When assessing the distribution of Nc Ab levels in volunteers of all age groups, the predominance of low Ab levels (31.3-125.6 BAU/ml) attracts attention (
Figure 5, Table S4).
The vertical value axis is percentage of all seropositive individuals (normalized to 100%) in the age subgroup with the indicated serological status, %. Sectors: volunteer age intervals, years. Legend: Nc Ab quantitative levels in BAU/ml. The numerical values are given in Supplementary Table S4. Monitoring stage is indicated above the diagrams.
In the 1st stage, the share of such individuals was 21.3% (95% CI: 20.1-22.5), which was 2.2 to 8.4-fold higher than the proportion of volunteers with other Nc Ab levels (
Figure 5, Table S4). The distribution of Nc Ab levels in age groups turned out to be relatively homogeneous, with minor exceptions: a slight predominance of individuals with very low levels (16.8-31.2 BAU/ml) in the children's group; and a somewhat higher share of those with Ab levels of 31.3-125.6 BAU/ml in the group '50-59 years old'. Differences in both groups, relative to the cohort mean, were significant (
p<0.05).
In the 2nd stage, the share of individuals with an Ab level of 31.3-125.6 BAU/ml decreased to an average of 17.5% (95% CI: 16.4-18.6); differences from 1st stage data were significant (
p<0.05). The decrease was due to an almost two-fold decrease in the proportion of such persons among children and adults aged 18–29 years (
Figure 5, Table S4); the differences were significant (
p<0.0001).
In the 3rd stage, antibody distributions almost returned to stage 1 levels. A four-fold increase was noted only among individuals with an Ab level > 502.5 BAU/ml (Table S4).
In the 4th stage, significant increases in the shares of seropositive volunteers were observed at all quantitative levels, with the exception of the interval 16.8-31.2 BAU/ml. In the subgroup 31.3-125.6 BAU/ml, the share of volunteers increased by 1.5-fold (
p<0.0001). In the subgroup 125.8-251.2 BAU/ml, the increase was 2.1-fold (
p<0.0001). In the subgroup 251.4-502.5 BAU/ml, the increase was 2.0-fold (
p<0.001). In the subgroup with maximum Ab levels (>502.5 BAU/ml), the increase was 1.9-fold (
p<0.001). It can be assumed that the noted growth was associated with an increase in the proportion of Nc
+RBD
+ volunteers at this stage, which can be clearly seen in
Figure 2 (Stage 4).
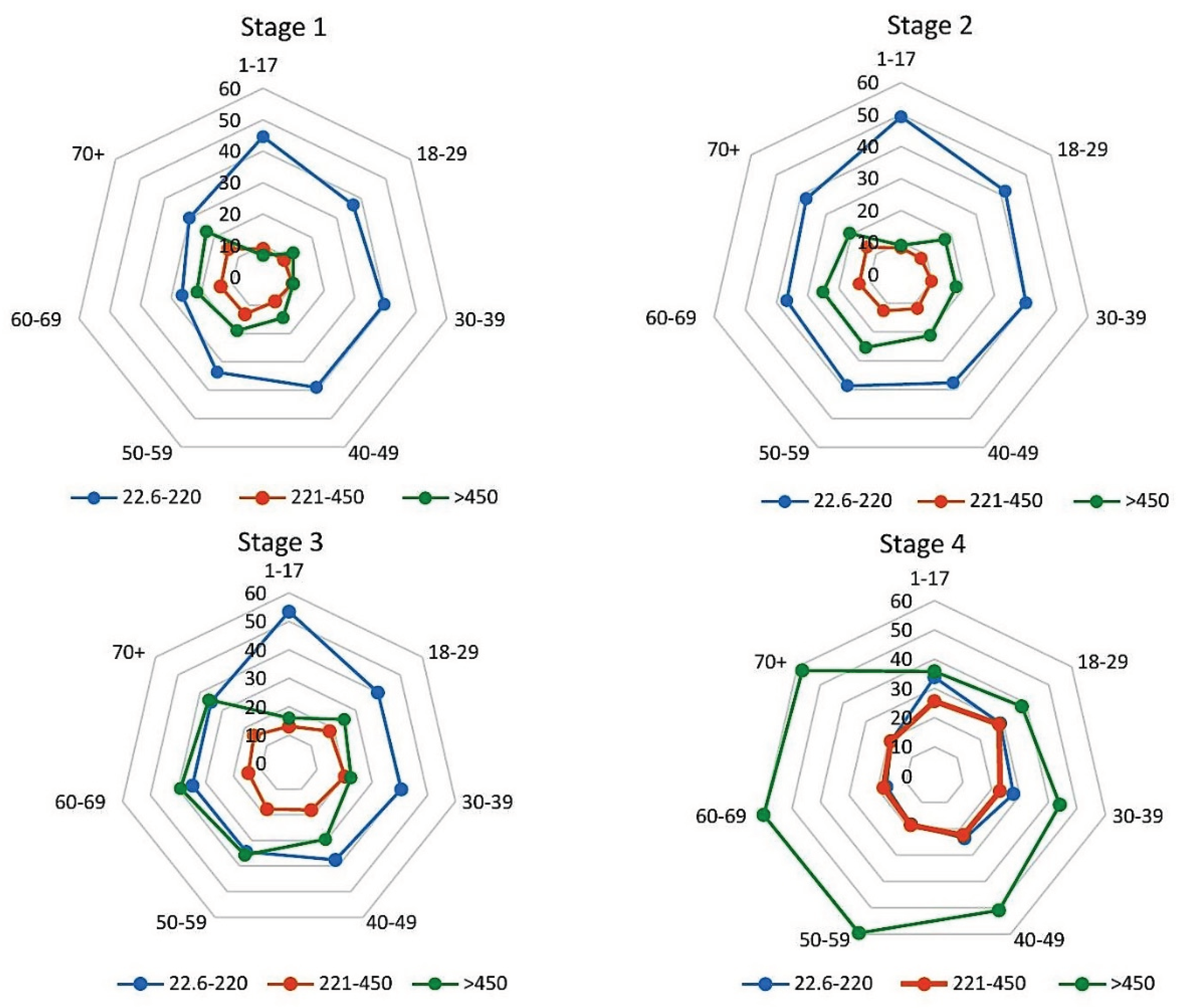
Since it has been shown that neutralizing activity against SARS-CoV-2 is more associated with RBD Abs [
34], these levels were also assessed (
Figure 6, Table S5). In the 1st monitoring stage, the largest proportion of seropositive volunteers carried RBD Abs at the 22.6-220.0 BAU/ml level (
Figure 6). The largest share of such persons was noted in the group aged 1-17 years (44.6%; 95% CI: 40.4-48.8). The smallest was noted in the group aged 60-69 years (26.4%; 95% CI: 23.2-29.8). The differences were significant (
p<0.001).
The vertical value axis is percentage of all seropositive individuals (normalized to 100%) in the age subgroup with the indicated serological status, %. Sectors: volunteer age intervals, years. Legend: RBD Ab quantitative levels in BAU/ml. Quantitative data are given in Supplementary Table S5. Monitoring stage is indicated above the diagrams.
In the 2nd stage, significant changes were not noted. The shares of individuals with minimum (22.6-220 BAU/ml) and maximum (>450 BAU/ml) levels increased by an average of 4.4% (differences from stage 1 were significant,
p<0.05). In the group with Abs in the range 221-450 BAU/ml, there were practically no changes compared with the 1st stage (
Figure 6, Table S5).
By the 3rd stage, an increase in the proportion of volunteers with high RBD Ab levels was noted. The share of volunteers with Ab levels of 221-450 BAU/ml increased by 5.4% to 16.9% (95% CI: 15.8-18.1); differences from 2nd stage data were significant (p<0.001). In the subgroup with Abs >450 BAU/ml, the increase was 10%, reaching 30.0% (95% CI: 28.7-31.4); differences from stage 2 were significant (p<0.0001).
By the 4th stage, the proportion of individuals with an Ab level of 221-450 BAU/ml increased by another 4.7% to 21.6% (95% CI: 20.4-22.8). In the subgroup with Ab levels of >450 BAU/ml, it reached a maximum of 51.0% (95% CI: 49.6-52.4). The differences were significant (p<0.00001).
Thus, the evolution of collective humoral immunity across the stages of seromonitoring was manifested by a significant increase in the proportion of seropositive volunteers with maximum RBD Ab levels and, to a lesser extent, anti-Nc Ab levels. One likely driving factor in this process could be the vaccination pattern in the Belarusian population.
Structure of Volunteer SARS-CoV-2 Vaccination during the Monitoring Period
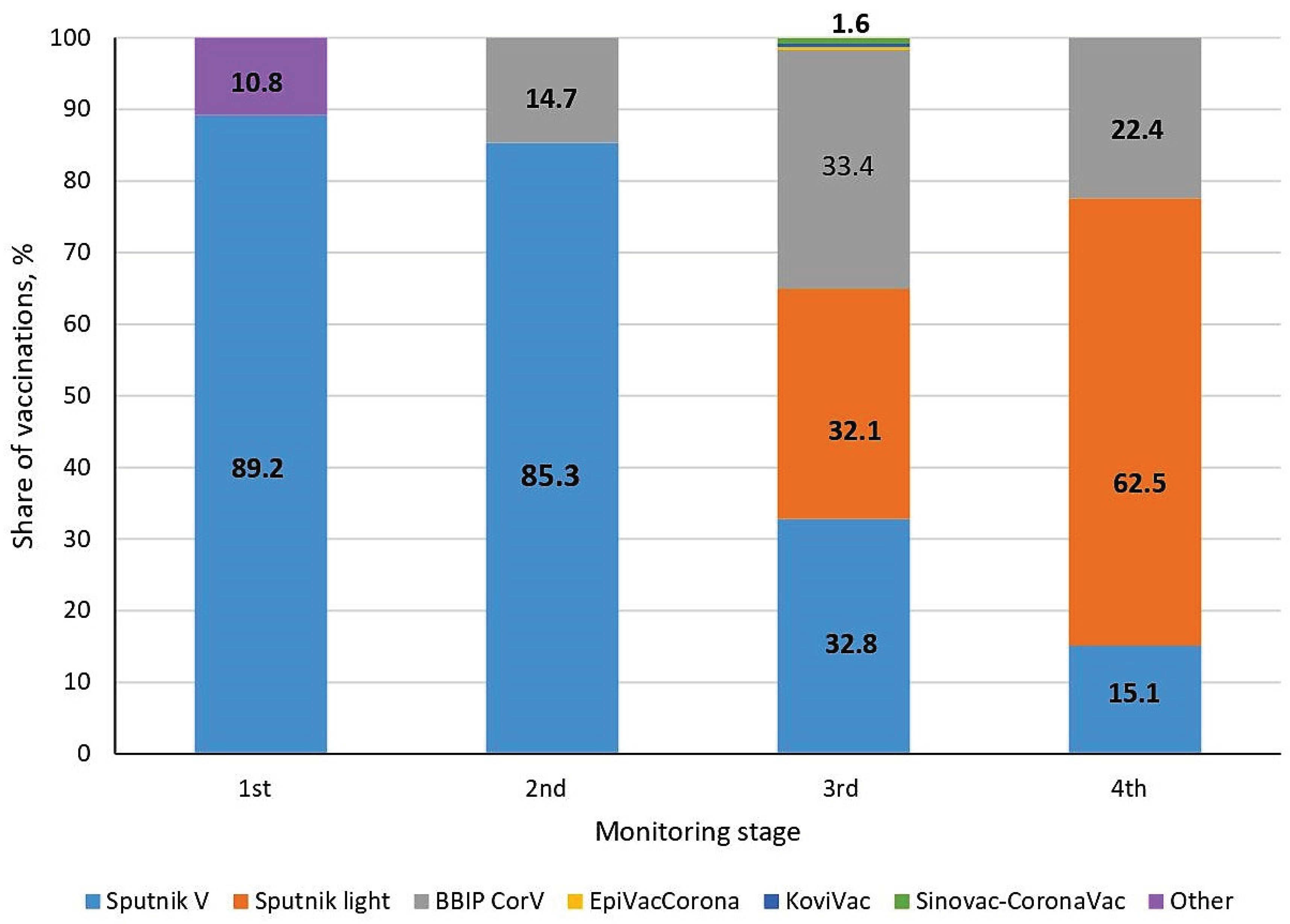
By the 18th week of 2021 (before commencement of this study), the COVID-19 pandemic had already lasted 15 months and passed at least 2 main waves. Ten weeks before the start of seromonitoring, vaccination began in the RB. Coverage by week 18 reached 2.1% of the Belarusian population (
Figure 1). In the initial period, the Sputnik V (Gam-COVID-Vac) vector vaccine was mainly used in the country; its share was 89.2%.
Some volunteers could not name the type of vaccine received. The data for these individuals were grouped separately and designated as 'Other' (
Figure 7). There were 10.8% of such individuals in the 1st stage of the study. Such uncertainties were no longer observed in subsequent monitoring stages.
In the 2nd monitoring stage, the structure of vaccine usage changed somewhat: the share of Sputnik V decreased by 3.9%; but at the same time, the BBIBP-CorV whole-virion vaccine was added, the share of which was 14.7%.
In stage 3, the range of vaccines expanded as much as possible. At the same time, the share of Sputnik V decreased to 32.8%, and the share of BBIBP-CorV increased to 33.4%. In addition, the Sputnik Light vector vaccine (32.1%), and four preparations in minor quantities (EpiVacCorona, CoviVac and Sinovac-CoronaVac) were added. The total share of the latter group was only 1.6%.
By the last stage, the range of the main vaccines used was preserved; only their proportions changed. The share of Sputnik V decreased to 15.1%, the share of Sputnik Light increased, and BBIBP-CorV decreased by 11% to 22.4%.
Changes in the list of vaccines used in the 3rd and 4th stages can be explained to some extent by the booster re-vaccination campaign, in which Sputnik Light was most often used. The campaign started on weeks 39-40 of 2021. By the 4th stage, re-vaccination coverage amounted to almost 40% of the total number of people who had fully completed immunization. In this regard, it can be reasonably argued that the most important consequence of the implementation of the primary immunization and booster revaccination programs was a sharp decrease in the number of illnesses to nearly zero starting from the 19th week of 2022. The short-term surge in infections noted on the 27th week (2022) was an isolated event that did not affect the general trend of the epidemic process.
Discussion
The COVID-19 epidemic in the Republic of Belarus was milder than in neighboring countries. According to official data for the entire pandemic period, almost 990,000 people fell ill in the country. According to this indicator, Belarus ranks 75th in the world [
33]. Such low morbidity is probably due not only to relatively low population density (45.5 km
-2), but also to active vaccination, which by mid-October 2022 amounted to 70.2% of the population. This was accompanied by a set of other measures to prevent the spread of COVID-19 in the Republic [
35].
As in other countries, SARS-CoV-2 during the epidemic period in the RB has undergone specific evolution associated with antigenic variability of the virus (
Figure 1). The beginning of the epidemic, like the rest of the world, was due to circulation of the ancestral (Wuhan) viral strain, which became the source of almost instantaneous infection of people globally. In the RB, the first cases of infection were detected in the 15th week of 2020. The strain disappeared from circulation by about week 30 of 2020 and was replaced by the first mutated strain, B.1.1.7 (Alpha). In weeks 24-25 of 2021, the B.1.612.2 (Delta) variant forced out Alpha from circulation, and from the 1st week of 2022 it was replaced by line B.1.1.629 (Omicron). In fairness, it should be noted that successive viral variants did not cause massive morbidity. For the most part, COVID-19 morbidity did not exceed 100-170 cases per 100,000 population.
One of the reasons for this situation could be collective humoral immunity formed after COVID-19 illness. The first stage of the study (assessing the state of collective humoral immunity) was carried out from May 14 - 19, 2021. By this time, the COVID-19 pandemic had already lasted for 17 months, and about 380,000 people had experienced symptomatic illness (about 4.7% of the total population). To this should be added about 45% of the population who had experienced asymptomatic infection [
23]. In total, at least 50% could have had an immune response to either Nc or RBD antigen, or both.
The results of serological testing of the population for the presence of SARS-CoV-2 Abs in the 1st monitoring stage generally confirmed this hypothesis. When assessing total seroprevalence in the entire age-stratified cohort, the proportion of individuals who had any specific Abs (to Nc, RBD, or both) was 67.8% (95% CI: 66.5-69.2). The majority of volunteers were seropositive for both Ags (Nc
+RBD
+). In terms of age, the highest seropositivity was seen among those aged 50-70
+; the lowest was seen in those aged 18-29 years (Table S1). It can be assumed that the higher level of seropositivity in older volunteers (about 70.0%) is due to the presence of pre-existing cross-immunity elicited as a result of anamnestic contact with endemic strains of coronaviruses [
36]. As for the low seropositivity in the age group of 18-29 years, 61.3% (95% CI: 56.4-66.1), a certain proportion of them were students, among whom up to 40%, according to some data, are skeptical about the idea of vaccination against SARS-CoV-2 [
37,
38,
39]. This was probably an additional reason why more than a third of volunteers of this age did not have specific Abs (Table S1).
As the COVID-19 vaccination campaign expanded, the pattern of circulating Abs changed markedly. The proportion of seronegative volunteers (Nc-RBD-) decreased from 32.2% (95% CI: 30.8-33.5) to 24.2% (95% CI: 23.0-25.4). The shares of other subpopulations varied in different directions: Nc+RBD- decreased by 1.5-fold to 3.7% (95% CI: 3.2-4.2); Nc+RBD+ decreased by 1.2-fold to 32.7 (95% CI: 31.3-34.0); while RBD+Nc‒, on the contrary, increased 1.7-fold and amounted to 39.5% (95% CI: 38.1-40.9). Distribution by age interval was relatively uniform. Significant increases were noted in the RBD+Nc‒ subgroup among people aged 18-29, as well as in the Nc+RBD+ subgroup among older volunteers aged 50 to 70+ (p<0.05 in both cases).
As vaccination coverage increased, there was a trend towards an increase in RBD positivity in the Belarusian population. By stage 3, in particular, the share of Nc+RBD+ individuals increased by 1.5-fold to 49.5% (95% CI: 48.0-50.9). The growth in fully seropositive individuals was accompanied by a slight decrease in the share of RBD+Nc‒ by 3.5% and a decrease in Nc‒RBD‒ by 2-fold.
The outlined trend reached its greatest expression by the 4th stage. The share Nc
+RBD
+ increased to 80.0% (95% CI: 78.8-91.2), while RBD
+Nc
‒ and Nc
‒RBD
‒ decreased by 2.3 and 5.8-fold, respectively (Table S1). The described processes were noted during stratification by age, region, and occupational group (
Figure 2,
Figure 3 and
Figure 4, Tables S1–S3). Some minor group differences were seen leading to some heterogeneity, yet the overall evolution of collective immunity was not affected in any substantial way by subgroup differences.
Assessment of collective immunity would not be complete without a quantitative analysis of peripheral blood Ab content. As part of this study, the quantitative content of circulating anti-Nc and anti-RBD Abs was assessed (
Figure 5 and
Figure 6; Tables S4 and S5). The 1st stage survey showed a predominance of individuals with Nc Ab content within the range 31.5-125.5 BAU/ml in the cohort. The share of such volunteers in the whole group was 21.3% (95% CI: 20.1-22.5); differences between cohort age groups were not significant. In the 2nd stage, a significant decrease in the proportion of individuals with such Abs (31.5-125.5 BAU/ml) down to 17.5% (95% CI: 16.4-18.6) was revealed (
p<0.0001). In the remaining groups, a weak growth in the number of individuals was noted for all Nc Ab levels (
Figure 5, Table S4). In the 3rd and 4th stages, due to the general increase in Nc Ab seroprevalence in the population, the number of volunteers with Nc Abs increased evenly in all serological intervals.
The distribution patterns of seropositive volunteers by RBD Ab level generally mirrored Nc Ab distributions, with the exception of certain features (
Figure 6, Table S5). In the first two stages of seromonitoring (carried out an interval of 4 months), individuals with RBD Ab levels of 22.6-220 BAU/ml prevailed in all age groups. This was especially pronounced among children, where their proportions were 44.6% (95% CI: 40.4-48.8) and 49.3% (95% CI: 45.0-53.7), respectively. Starting from stage 3, the proportion of people with maximum RBD Ab levels (>450 BAU/ml) increased significantly. By stage 4, it reached an average of 51.0% (95% CI: 49.6-52.4). In those older than 40 years, it ranged from 51.0 to 59.9%. In all cases, the differences were significant (
p<0.0001).
Overall, the described seropositivity dynamics clearly indicate the evolution of collective humoral immunity towards the formation of a full response following not only to infection (cumulative incidence), but also the use of specific SARS-CoV-2 vaccines. In result, these processes led to the simultaneous circulation of Nc and RBD Abs, with a predominance of anti-RBD Abs. A likely prerequisite for this could be the predominant use of vector vaccines, in particular Sputnik V, during primary vaccination (stages 1, 2) and booster vaccination (stages 3, 4). At the same time, the total share of both Sputnik vaccines in the overall structure varied from 89.2% in the 1st stage to 77.6% in the 4th (
Figure 7).
Taking into account the previously noted effectiveness of vector vaccines [
40], the widespread use of the Sputnik family of vaccines may have become a significant factor behind decreasing COVID-19 incidence in the population. Another pattern confirming such a process is the inverse relationship between population humoral immunity and morbidity: an increase in post-vaccination resistance is inevitably accompanied by a decrease in morbidity [
23,
41,
42,
43,
44,
45,
46].
The combined increase in the content of the two main antibody types indicates the formation of hybrid immunity [
47], usually featuring maximum protection against the “aggression” of a pathogenic agent [
40]. The use of a range of specific vaccines made it possible to create the required level of COVID-19 resistance in the population (
Figure 7).
In the initial period, the Gam-COVID-Vac vector vaccine (Sputnik V) was mainly used, which made it possible to form a stable pool of RBD Abs, which laid the foundation for the formation of hybrid immunity. In the 2nd stage, usage of vector vaccines remained almost at the initial level. In the 3rd stage, usage ratios were: almost 2/3 vector vaccines and 1/3 inactivated vaccine (BBIBP-CorV). In the 4th stage, the spectrum of vaccines was preserved, but the ratio changed due to expanded usage of the Sputnik Light vaccine.
Conclusion
Consistent use of vector vaccines that do not contain Nc antigens was sufficient to form a maximal level of post-vaccination immunity. The additional introduction of the whole-virion inactivated BBIBP-CorV vaccine into the practice of vaccination served as a prerequisite for maximal growth of hybrid immunity and, ultimately, the near-complete elimination of new COVID-19 cases. Based on the data obtained, we can formulate a key requirement in the formation of robust coronavirus immunity: maximum vaccination coverage using a wide range of vaccines, with both vector and inactivated whole-virion platforms present. This approach, combined with anamnestic morbidity, makes it possible to form the most durable adaptive immunity.
Author Contributions
Author contributions: AYP, AAT — conceptualization; AMD; AMM; AAT - data curation; VAI, SAE, VSS - formal analysis; IVD, ING, AVG, VGD, OBZ. OAP, APR – investigation; EVZ – methodology TAA, AMD - project administration; ALS; LVR; EOS – resources; AMM, SAE, TVA - supervision; IAK,NPS – validation; VSS, ESR - writing - original draft; VSS; TAA, SAE - writing - review & editing
Funding
This research received no external funding.
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose. The authors have no competing interests to declare relevant to the content of this article.
Ethics approval
The study was organized in accordance with the provisions of the Declaration of Helsinki and approved by the ethics committees of the Republic of Belarus (protocol No 2, dated 05.13.2021) and the St. Petersburg Pasteur Institute (protocol No. 64, dated 26.05.2020).
Consent to Publication
Not applicable.
References
- Smirnov, V.S.; Zarubaev, V.V.; Petlenko, S.V. Biology of the pathogen and control of influenza and SARS. St. Petersburg; Hippocrates Publishing House. 2020. ISBN 978-5-8232-0643-3.
- Shchelkanov, M.Yu.; Popova, A.Yu.; Dedkov, V.G.; Akimkin, V.G.; Maleev, V.V. ; History of study and modern classification of coronaviruses (Nidovirales: Coronaviridae) // Russian Journal of Infection and Immunity = Infektsiya i immunitet. 2020,10, 2, 221–246. [CrossRef]
- Shahrajabian, M.H.; Sun, W.; Chenga, Q. Product of natural evolution (SARS, MERS, and SARS-CoV-2); deadly diseases, from SARS to SARS-CoV-2 Hum Vaccin Immunother. 2021, 17, 1, 62–83. [CrossRef]
- Zaki, A. M.; van Boheemen, S.; Bestebroer, T.M.; Osterhaus, A.D.; Fouchier, R.A. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N. Engl. J. Med. 2012, 367, 1814–20. [Google Scholar] [CrossRef] [PubMed]
- Adegboye, O.; Saffary, T.; Adegboye, M.; Elfaki, F. Individual and network characteristics associated with hospital-acquired Middle East Respiratory Syndrome coronavirus. J. Infect. Public Health. 2019, 12, 343–49. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Yin, J.; Wu, X.; Wang, D.; Li, C. Environment and COVID-19 incidence: A critical review. Review J. Environ. Sci (China) 2023, 124, 933–951. [Google Scholar] [CrossRef]
- Xu, X.; Chen, P.; Wang, J.; Feng, J.; Zhou, H.; Li, X.; Zhong, W.; Hao, P. Evolution of the novel coronavirus from the ongoing Wuhan outbreak and modeling of its spike protein for risk of human transmission. Sci. China Life Sci. 2020, 63. [Google Scholar] [CrossRef]
- van Dorp, L.; Houldcroft, C.J.; Richard, D. ; Balloux. F. COVID-19, the first pandemic in the post-genomic era. Curr. Opin. Virol. 2021, 50, 40–48. [Google Scholar] [CrossRef]
- Rochman, N.D.; Wolf, Y.I.; Faure, G.; Mutz, P.; Zhang, F.; Koonin, E.V. Ongoing global and regional adaptive evolution of SARS-CoV-2. Proc. Natl. Acad. Sci U S A. 2021, 118, 29,e2104241118. [Google Scholar] [CrossRef]
- COVID-19 Data Repository by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University. URL: https://github.com/CSSEGISandData/COVID-19. Accessed 23/12/2022.
- Worldometers. URL: https://www.worldometers.info/coronavirus/ Accessed 23/12/2022.
- Singh, D.; Yi, S.V. On the origin and evolution of SARS-CoV-2. Review Exp. Mol. Med. 2021, 53, 4, 537–547. [Google Scholar] [CrossRef]
- Dubey, A.; Choudhary, S; Kumar, P.; Tomar, S. Emerging SARS-CoV-2 Variants: Genetic Variability and Clinical Implications. Curr. Microbiol. 2021, 79, 1, 20. [Google Scholar] [CrossRef] [PubMed]
- Lubinski, B.; Fernandes, M.H.V.; Frazier, L.; Tang, T.; Daniel, S.; Diel, D.G.; Jaimes, J.A.; Whittaker, G.R. Functional evaluation of the P681H mutation on the proteolytic activation of the SARS-CoV-2 variant B.1.1.7 (Alpha) spike. iScience. 2022, 25, 1, 103589. [Google Scholar] [CrossRef]
- Gómez, C. E.; Perdiguero, B.; Esteban, M. Emerging SARS-CoV-2 Variants and Impact in Global Vaccination Programs against SARS-CoV-2/COVID-19. Vaccines (Basel) 2021, 9, 3, 243. [Google Scholar] [CrossRef]
- Buss, L.F.; Sabino, E.C. Intense SARS-CoV-2 transmission among affluent Manaus residents preceded the second wave of the epidemic in Brazil. Lancet Glob. Health. 2021, 9, 11, e1475–e1476. [Google Scholar] [CrossRef] [PubMed]
- Mlcochova, P.; Kemp, S.A.; Dhar, M.S.; Papa, G.; Meng, B.; Ferreira, I. A. T. M. , et al. SARS-CoV-2 B.1.617.2 Delta variant replication and immune evasion. Nature. 2021; 599(7883): 114–119. [CrossRef]
- Khandia, R.; Singhal, S.; Alqahtani, T.; Kamal, M.A.; El-Shall, N.A.; Nainu, F.; Desingu, P.A.; Dhama, K. Emergence of SARS-CoV-2 Omicron (B.1.1.529) variant, salient features, high global health concerns and strategies to counter it amid ongoing COVID-19 pandemic Environ. Res. 2022,112816. [CrossRef]
- Farahat, R.A.; Abdelaal, A.; Umar, T.P.; El-Sakka, A.A.; Benmelouka, A.Y.; Albakri, K.; Ali, I.; Al-Ahdal., T.; Abdelazeem., B.; Sah., R.; Rodriguez-Morales, A.J. The emergence of SARS-CoV-2 Omicron subvariants: current situation and future trends. Infez. Med. 2022, 30, 4, 480–494. [Google Scholar] [CrossRef]
- Coronavirus-monitor URL: https://coronavirus-monitor.info/ Accessed 25/12/2022.
- Chen, N.; Zhou, M.; Dong, X.; Qu, J.; Gong, F.; Han, Y.; Qiu, Y.; Wang, J.; Liu, Y.; Wei, Y.; Xia, J.; Yu, T.; Zhang, X.; Zhang, L. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020, 395, 10223, 507–513. [Google Scholar] [CrossRef]
- Countrymeters. URL: https://countrymeters.info/ru Accessed 12/12/2022.
- Popova, A.Y.; Tarasenko, A.A.; Smolenskiy, V.Y.; Egorova, S.A. ; Smirnov. V.S.; Dashkevich, A.M.; Svetogor, T.N.; Glinskaya, I.N.; Skuranovich, A.L.; Milichkina, A.M.; Dronina, A.M.; Samoilovich, E.O.; Khamitova, I.V.; Semeiko, G.V.; Amvrosyeva, T.V.; Shmeleva, N.P.; Rubanik, L.V.; Esmanchik, O.P.; Karaban, I.A.; Drobyshevskaya, V.G.; Sadovnikova, G.V.; Shilovich, M.V.; Podushkina, E.A.; Kireichuk, V.V.; Petrova, O.A.; Bondarenko, S.V.; Salazhkova, I.F.; Tkach, L.M.; Shepelevich, L.P.; Avtukhova, N.L.; Ivanov, V.M.; Babilo, A.S.; Navyshnaya, M.V.; Belyaev, N.N.; Zueva, E.V.; Volosar, L.A.; Verbov, V.N.; Likhachev, I.V.; Zagorskaya, T.O.; Morozova, N.F.; Korobova, Z.R.; Gubanova, A.V.; Totolian, A.A. Herd immunity to SARS-CoV-2 among the population of the Republic of Belarus amid the COVID-19 pandemic // Russian Journal of Infection and Immunity = Infektsiya i immunitet, 2021, vol. 11, no. 5, pp. 887–904. [CrossRef]
- Randolph, H.E.; Barreiro, L. B Herd Immunity: Understanding COVID-19. Immunity. 2020, 52, 5, 737–741. [Google Scholar] [CrossRef]
- Rostami, A.; Sepidarkish, M.; Leeflang, M.M.G.; Riahi, S.M.; Shiadeh, M.N.; Esfandyari, S.; Mokdad, A.H.; Hotez, P.J.; Gasser, R.B. SARS-CoV-2 seroprevalence worldwide: a systematic review and meta-analysis. Clin. Microbiol Infect. 2021, 27, 3, 331–340. [Google Scholar] [CrossRef]
- Popova, A.Y.; Totolian, A.A, . Methodology for assessing herd immunity to the SARS‐CoV‐2 virus in the
context of the COVID‐19 pandemic. Russian Journal of Infection and Immunity = Infektsiya i immunitet,
2021, 11, 4, 609–616. [CrossRef]
- Popova, A.Y.; Kasymov, O.T.; Smolenski, V.Y.; Smirnov, V.S.; Egorova, S.A.; Nurmatov, Z.S.; Milichkina, A. M.; Suranbaeva, G.S.; Khamitova, I.V.; Zueva, E.V.; Ivanov, V.A.; Nuridinova, Z.N.; Derkenbaeva, A.А.; Drobyshevskaya, V.G.; Sattarova, G.Z.; Gubanova, A.V.; Zhimbaeva, O.B.; Razumovskaya, A.Р.; Verbov, V.N.; Likhachev, I.V.; Totolian A., A. SARS-CoV-2 Herd Immunity of the Kyrgyz Population in 2021. Med. Microbiol. Immunol. 2022, 211, 195–210. [Google Scholar] [CrossRef] [PubMed]
- Total population by age and sex, marital status, level of education, nationalities, language, sources of income existence in the Republic of Belarus. Statistical bulletin. Minsk 2020. URL: https://www.belstat.gov.by/ofitsialnaya-statistika/solialnaya-sfera/naselenie-i-migratsiya/naselenie/statisticheskie-izdaniya/index_17854/ Accessed 23/01/2023.
- Wald, A.; Wolfowitz J. Confidence Limits for Continuous Distribution Functions.” The Annals of Mathematical Statistics.1939, 10, 2, 105–118. Available: www.jstor.org/stable/2235689. Acessed: 10.07.2021.
- Agresti, A.; Coull, B.A. Approximate Is Better than "Exact" for Interval Estimation of Binomial Proportions. The American Statistician. 1998; 52, 2, 119-126. [CrossRef]
- Significant Difference Calculator (z-test). RADAR Research Company. URL: https://radar-research.ru/software/z-test_calculator. Accessed 07.04.2022.
- Butt, A.A.; Dargham, S.R.; Chemaitelly, H.; Al Khal, A.; Tang, P.; Hasan, M.R.; Coyle, P.V.; Thomas, A.G.; Borham, A.M.; Concepcion, E.G.; Kaleeckal, A.H.; Latif, A.N.; Bertollini, R.; Abou-Samra, A.B.; Abu-Raddad, L.J. Severity of Illness in Persons Infected With the SARS-CoV-2 Delta Variant vs Beta Variant in Qatar. JAMA Intern. Med. 2022, 182, 2,197–205. [Google Scholar] [CrossRef]
- Coronavirus statistics in the world. URL: https://gogov.ru/covid-19/world. Accessed 25/12/2022.
- Zhang, H.; Liu, X.; Liu, Q.; Mei, H.; Wang, Y.; Cui, G.; Zhao, S. Serological reactivity of inactivated SARS-CoV-2 vaccine based on an S-RBD neutralizing antibody assay Clinical Trial Int. J. Infect. Dis. 2022, 117, 169–173. [Google Scholar] [CrossRef]
- Dashkevich, A.M.; Kolomiets, N.D.; Glinskaya, I.N.; Skuranovich, A.L.; Tarasenko, A.A.; Karaban, I.A. COVID-19 pandemic. Measures to prevent the spread in the Republic of Belarus. Issues of organization and informatization of healthcare. 2022, 2, 4-11. URL: https://belcmt.by/docs/Journal_2022/N_2/1-1.
- Crowley, A.R.; Natarajan, H.; Hederman, A.P.; Bobak, C.A.; Weiner, J.A.; Wieland-Alter, W.; Lee, J.; Bloch, E.M.; Tobian, A.A.R.; Redd, A.D.; Blankson, J.N.; Wolf, D.; Goetghebuer, T.; Marchant, A.; Connor, R.I.; Wright., P.F.; Ackerman, M.E. Boosting of cross-reactive antibodies to endemic coronaviruses by SARS-CoV-2 infection but not vaccination with stabilized spike. Elife. 2022, 11, e75228. [Google Scholar] [CrossRef]
- Getachew, D.; Yosef, T.; Solomon, N.; Tesfaye, M.; Bekele, E. Predictors of unwillingness to receive COVID -19 vaccines among Ethiopian Medical students. PLoS One. 2022, 17, 11, e0276857. [Google Scholar] [CrossRef] [PubMed]
- Sadaqat, W.; Habib, S.; Tauseef, A.; Akhtar, S.; Hayat, M.; Shujaat, S.A.; Mahmood, A. Determination of COVID-19 Vaccine Hesitancy Among University Students. Cureus. 2021, 13, 8, e17283. [Google Scholar] [CrossRef]
- Bolatov, A.K.; Seisembekov, T.Z.; Askarova, A.Zh.; Pavalkis, D. Barriers to COVID-19 vaccination among medical students in Kazakhstan: development, validation, and use of a new COVID-19 Vaccine Hesitancy Scale. Hum. Vaccin. Immunother. 2021, 17, 12, 4982–4992. [Google Scholar] [CrossRef]
- Totolian, A.A.; Smirnov, V.S.; Krasnov, A.A.; Ramsay, E.S.; Dedkov, V.G.; Popova, A.Y. ; COVID-19 Case Numbers as a Function of Regional Testing Strategy, Vaccination Coverage, and Vaccine Type. Research Square. Preprint. [CrossRef]
- Madhi, S.A. COVID-19 herd immunity v. learning to live with the virus. S. Afr. Med. J. 2021, 111, 9, 852–856. [Google Scholar] [CrossRef]
- Saban, M.; Kaim, A.; Myers, V.; Wilf-Miron, R. COVID-19 Vaccination, Morbidity, and Mortality During a 12-Month Period in Israel: Can We Maintain a "Herd Immunity" State? Popul. Health Manag. 2022, 25, 5, 684–691. [Google Scholar] [CrossRef]
- Chen, YT. The Effect of Vaccination Rates on the Infection of COVID-19 under the Vaccination Rate below the Herd Immunity Threshold. Int. J. Environ. Res. Public Health. 2021, 18, 14, 7491. [Google Scholar] [CrossRef]
- Datta S, Roy A. Herd Immunity Against Coronavirus: A Review. Recent Pat Biotechnol. 2022;16(3):256-265. [CrossRef]
- Sanz-Leon, P.; Hamilton, L.H.W.; Raison, S.J.; Pan, A.J.X.; Stevenson, N.J.; Stuart, R.M.; Abeysuriya, R.G.; Kerr, C.C.; Lambert, S.B; Roberts, J.A. Modelling herd immunity requirements in Queensland: impact of vaccination effectiveness, hesitancy and variants of SARS-CoV-2. Philos. Trans. A, Math. Phys. Eng.Sci. 2022, 380, 2233, 20210311. [Google Scholar] [CrossRef]
- McBryde, E.S.; Meehan, M.T.; Caldwell, J.M.; Adekunle, A.I.; Ogunlade, S.T.; Kuddus, M.A.; Ragonnet, R.; Jayasundara, P.; Trauer, J.M.; Cope, R.C. Modelling direct and herd protection effects of vaccination against the SARS-CoV-2 Delta variant in Australia. Med. J. Aust. 2021, 215, 9, 427–432. [Google Scholar] [CrossRef]
- Crotty, S. Hybrid immunity: COVID-19 vaccine responses provide insights into how the immune system perceives threats. Science 2021, 372, 6549, 1392–1393. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).