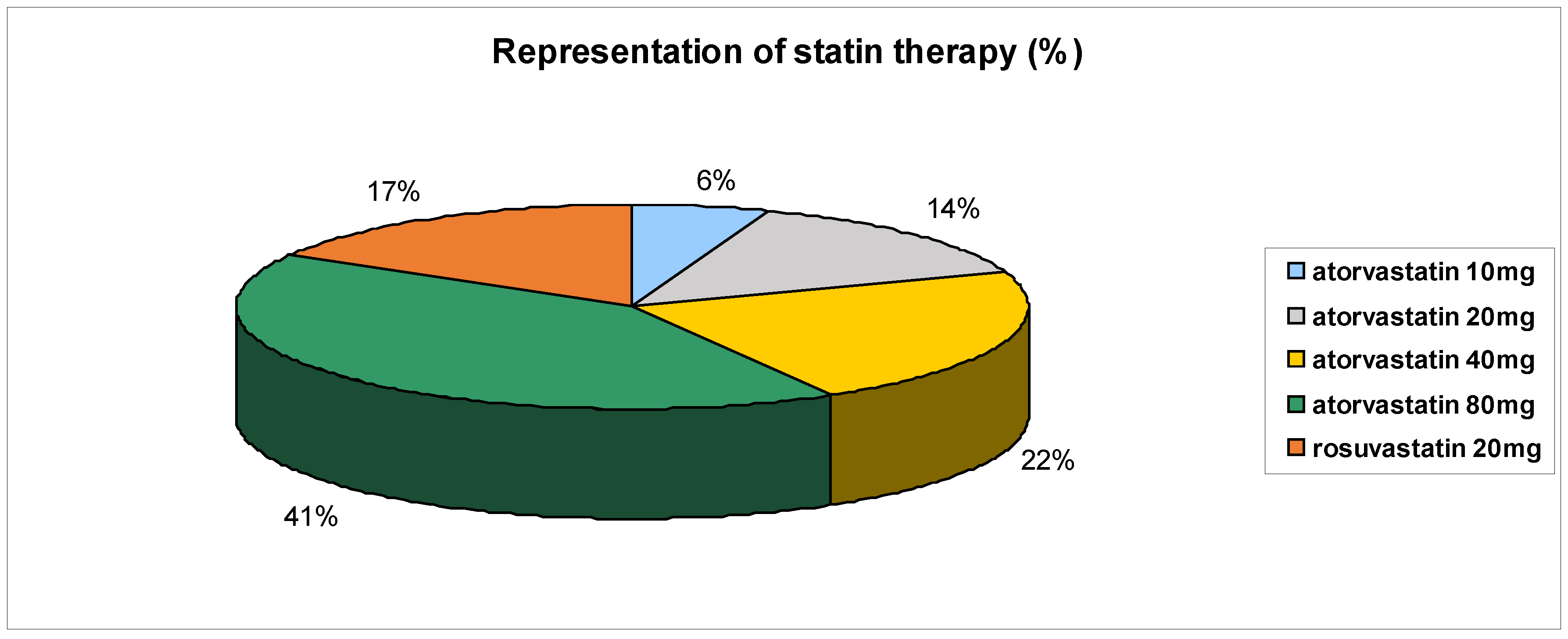Background
Despite advances in preventive cardiology, the cardiovascular (CV) mortality remains high. Metabolic syndrome (MS) is defined as simultaneous presence of lipid and non-lipid related CV and cardio-metabolic risk factors that significantly increase the risk of CV disorders, as well as type 2 diabetes. A common trait of these risk factors is the fact that they cause endothelial dysfunction (ED). ED plays a significant role in the development and clinical manifestation of atherosclerosis.
Omega-3 poly-unsaturated fatty acids (n-3 PUFA) used for primary and secondary prevention of CV disorders are currently associated with a wide range of evidence within the framework of evidence-based medicine. Their anti-inflammatory, anti-thrombotic, anti-arrhythmic and triglyceride-reducing effects (at high doses) are well established, often associated with increase of HDL cholesterol. The objective of the work is to assess the possible effect on n-3 PUFA on endothelial function in MS patients.
First evidence of the benefits of n-3 PUFA originated in 1970-s, with the publication of reports on reduced prevalence of ischemic heart disease and diabetes in Eskimos – Inuits, who live in Greenland, who were found to have high n-3 PUFA levels thanks to excessive intake of fish fat that contains n-3 PUFA [
1]. These reports have been expanded over the recent decades, extended, supplemented and analyzed in additional clinical studies. The results of
GISSI Prevenzione and DART studies present an explanation for several effects of n-3 PUFA [
2,
3]
Clinical data supporting the reduction of the progression of atherosclerosis with n-3 PUFA originate from SCIMO (Study on Prevention of Coronary Atherosclerosis by Intervention with Marine Omega-3 fatty acids), where quantitative analysis of the progression/regression of plaque in coronary arteries has been performed [
4].
Based on extensive evidence-based medicine data, in the primary and secondary prevention of CV diseases, n-3 PUFA have their role in the recommendations by international and global organizations [
5].
The aim of this trial was to evaluate the role of n-3 PUFA supplementation on endothelial function in patients with metabolic syndrome compare to placebo
Methodology
Study Population
80 patients with MS were enrolled in the study (40 patients on n-3 PUFA and 40 patients on corresponding placebo). In n-3 PUFA group there were 56,5% (23) male and 43,5% (17) females with the mean age of 60,5 years, range 31-81 years. MS was classified according to the definition of IDF (2005) (International Diabetes Federation), with discriminating factor being the presence of abdominal obesity [
6].
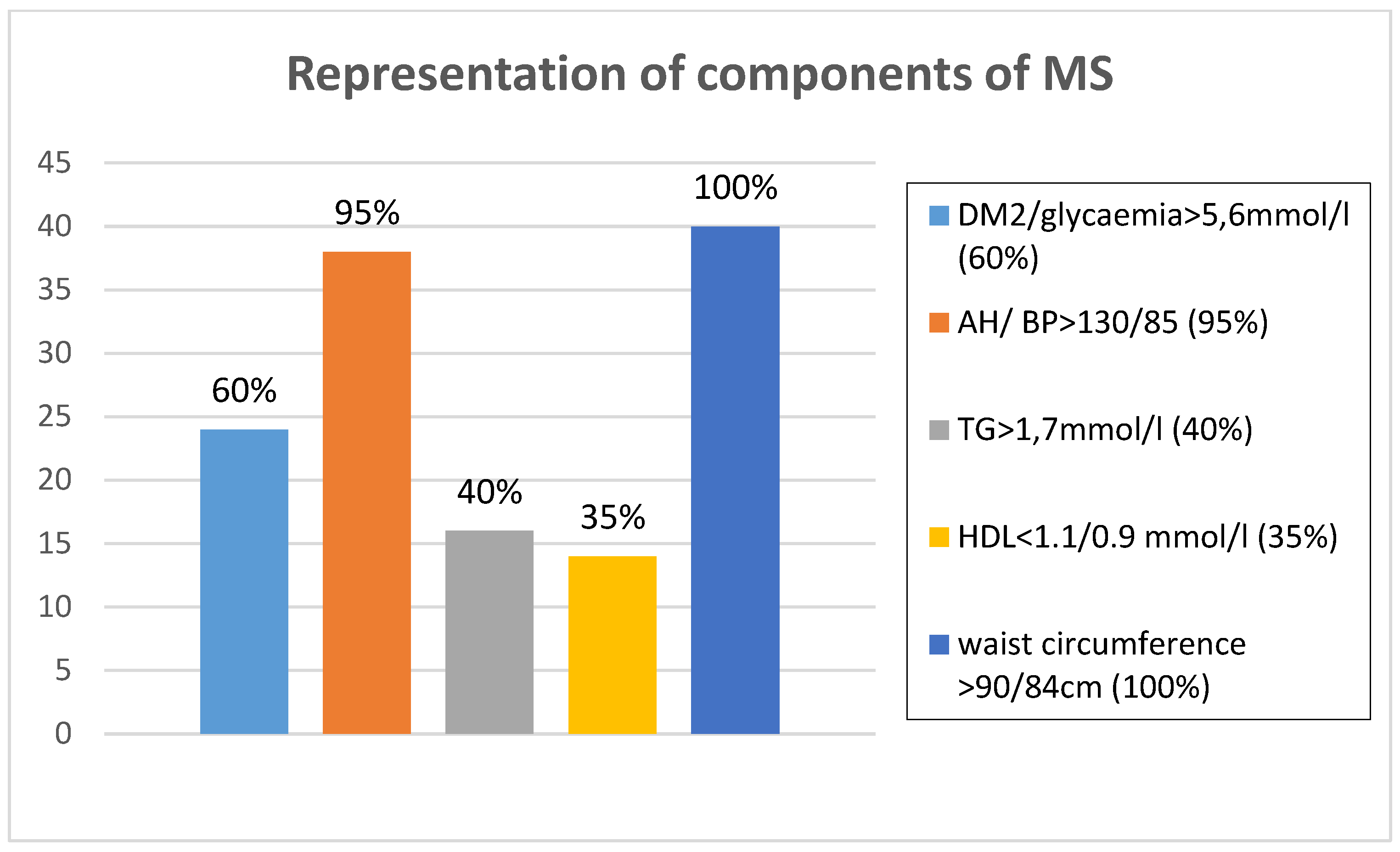
Figure 1 presents the prevalence of the presence of IDF criteria for MS and
Table 1 presents the patient medication at start and at the end of trial in n3 PUFA group of patients. Following
Table 1, statins were most used – representing up to 90% and
Figure 2 presents the use of individual statins at various doses.
Figure 1.
Representation of components of metabolic syndrome in n3 PUFA group of patients.
Figure 1.
Representation of components of metabolic syndrome in n3 PUFA group of patients.
Figure 2.
Representation of statin therapy (%) in n3 PUFA group of patients.
Figure 2.
Representation of statin therapy (%) in n3 PUFA group of patients.
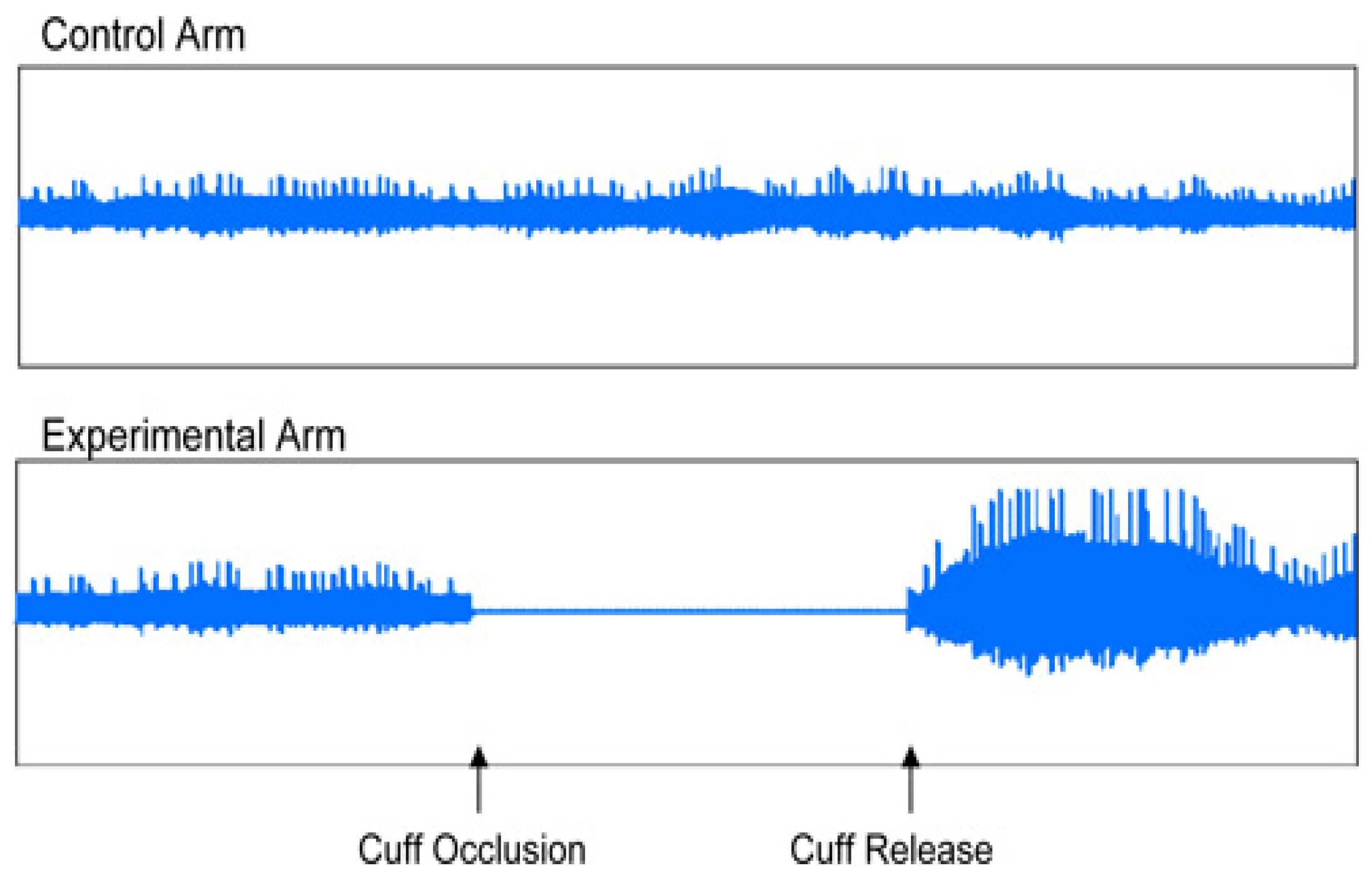
Figure 3.
Normal endothelial function (vasodilatation).
Figure 3.
Normal endothelial function (vasodilatation).
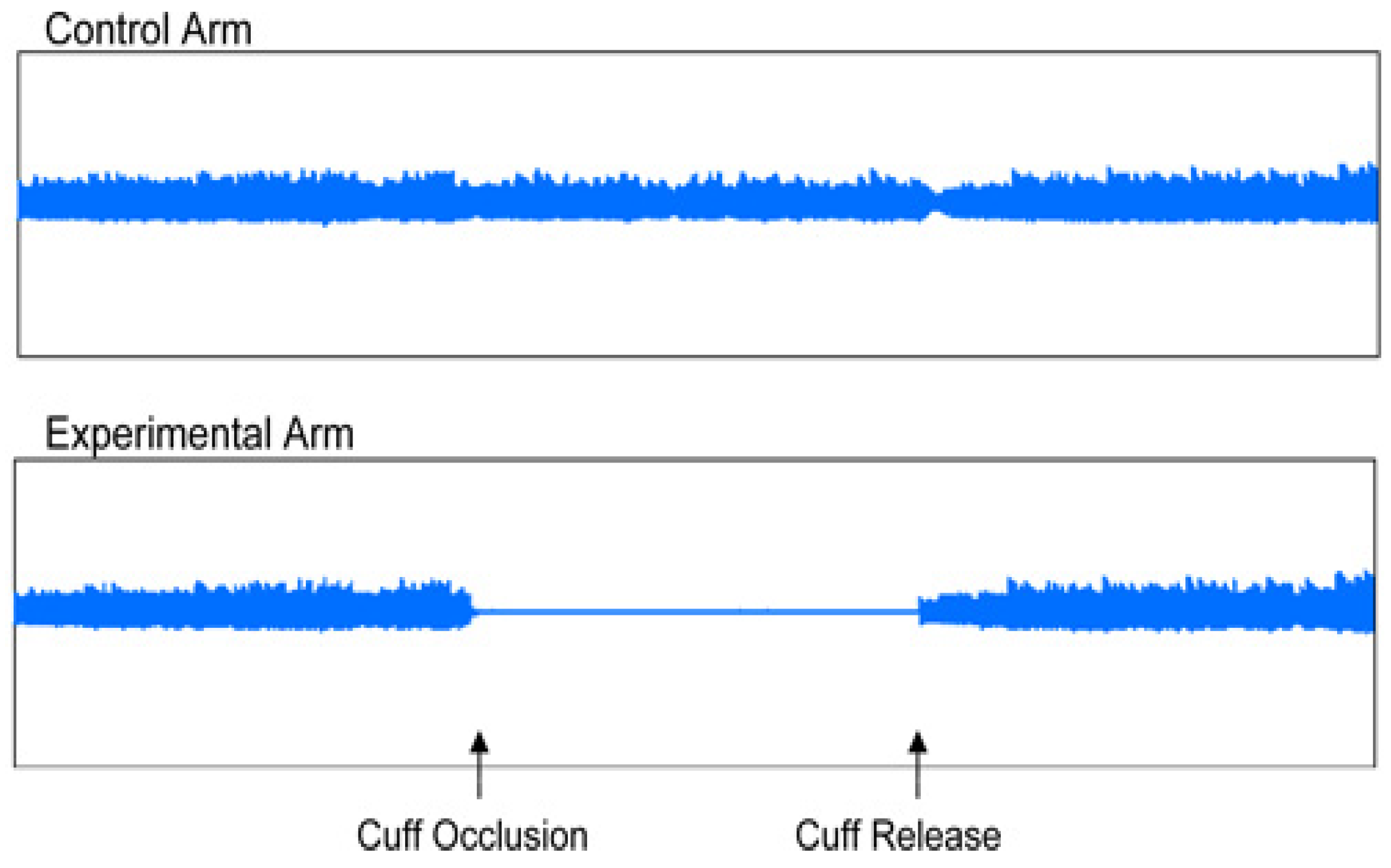
Figure 4.
Endothelial dysfunction.
Figure 4.
Endothelial dysfunction.
Table 1.
Medication in n-3 PUFA group of evaluated patients.
Table 1.
Medication in n-3 PUFA group of evaluated patients.
| Medication |
N. of Patients at Start |
N. of Patients at End |
p |
% |
| ACEi |
25 |
22 |
NS |
62.50 |
| Beta-blocker |
32 |
32 |
NS |
80.00 |
| Calcium channel blocker |
14 |
14 |
NS |
35.00 |
| Sartans-AT1 Blockers |
12 |
12 |
NS |
30.00 |
| Alfa-blocker |
12 |
12 |
NS |
30.00 |
| Diuretics |
30 |
28 |
NS |
75.00 |
| Acetylsalicylic acid |
15 |
15 |
NS |
37.00 |
| Trimetazidin |
18 |
18 |
NS |
45.00 |
| Insulin |
6 |
6 |
NS |
15.00 |
| Oral Antidiabetics |
12 |
12 |
NS |
30.00 |
| Nitrates |
10 |
10 |
NS |
25.00 |
| Clopidogrel |
7 |
7 |
NS |
17.00 |
| Allopurinol |
8 |
8 |
NS |
20.00 |
| Proton pump inhibitors |
2 |
2 |
NS |
5.00 |
| Warfarin |
3 |
3 |
NS |
7.50 |
| Dabigatran |
1 |
1 |
NS |
2.50 |
| Rivaroxaban |
2 |
2 |
NS |
5.00 |
| Ivabradine |
4 |
4 |
NS |
10.00 |
| Propaphenone |
1 |
1 |
NS |
2.50 |
| Amiodarone |
1 |
1 |
NS |
2.50 |
| Digoxin |
2 |
2 |
NS |
5.00 |
| L-thyroxine |
3 |
3 |
NS |
7.50 |
| Prasugrel |
1 |
1 |
NS |
2.50 |
| Statin |
36 |
34 |
NS |
90.00 |
| Fibrat |
8 |
8 |
NS |
20.00 |
| Ezetimibe |
6 |
6 |
NS |
15.00 |
Patient population was based on patients examined at the out-patient cardiology office (academic based) Kosice as well as the patients referred by cooperating cardiologists. Patients who took any nutrition supplements containing n-3 PUFA in the course of the last 6 months were not enrolled. When enrolling the patients, we focused especially on clinically stable patients who were not expected to have their pharmacotherapy or lifestyle changed in the course of the observed study period.
Upon signing the informed consent form, all patients had physical checkup, including blood pressure measurement, waist and hip circumference measurements, body height and body weight. Laboratory tests were performed, and the patient was evaluated using EndoPAT device. At the beginning of the study, the patients initially received n-3 PUFA (ZenixX Vital) at the dose of 2.4 grams per day, divided in three daily doses of 800 mg. All tests were performed before starting the study therapy and at the end of the subject period after three months (± 1 week). The effect of treatment with n-3 PUFA on ED was assessed on the following basis:
1. selected laboratory markers and ED risk factors: glutathione-peroxidase (GPX), homocysteine (Hcy), lipoprotein (a) (Lp(a)), apo-lipoprotein B (ApoB)
2. markers of arterial stiffness – augmentation index (AI) and index of reactive hyperemia (RHI) – endothelium function parameter – examined using EndoPAT 2000 device
Laboratory Markers Evaluation
Of the assessed parameters, upon sample collection the Lp(a), ApoB and Hcy were processed by local laboratory, specific ED markers such as GPX were sent in vials with heparine to the Department of Experimental Medicine, Pavol Jozef Safarik University, Kosice, Slovakia where centrifuged plasma was frozen (-80°C) until individual tests were to be performed. Upon thawing, 50 ul of heparinized blood was added to 2 ml of dilution solution. Upon measurement of Hb concentration in the resulting hemolysate, the GPX activity was determined using RANSEL kit (RANDOX, Great Britain) using automatic analyzer Daytona. GPX activity in control samples was determined with each measurement series [
7].
Assessment of Endothelial Dysfunction Using EndoPAT 2000
EndoPAT 2000 is a simple method for non-invasive measurement of ED. The method involves the measurement of the changes of vascular tone in peripheral vascular bed (PAT – peripheral arterial tone), using plethysmography, i.e. the measurement is independent of the investigator. EndoPAT assessment involves vasodilation – hyperemia following previous application of tourniquette on the extremity. EndoPAT measures the signal from the vascular bed of the entire finger, including microcirculation. The test enables differentiation between systemic and endothel-induced vaso-reactivity, the measurement is performed simultaneously on both upper extremities and the resulting curves are compared. The assessment needs to be performed in a separate, quiet and warm room. Patient preparation before the assessment is required – the patient should be fasting for at least 4 hours, avoiding caffeine, nicotine, vitamins or any other medication for 8 hours, as these may affect the vascular tone [
8]. During the test, usually taking about 20 minutes, the patient is in supine or semi-sitting position, most commonly with closed eyes, no talking and no movement of upper extremities to avoid interference with the electrode signals. The sensing electrodes were placed on both index fingers, subsequently they were inflated, and signal check was carried out. The signal was recorded by the computer and observed by investigator in the form of 2 curves throughout the test. During the first 5 minutes the upper extremity was left without occlusion, subsequently the cuff was inflated using pressure gauge to the pressure of at least 200 mmHg for at least 5 minutes, after that the cuff was deflated and the signal after occlusion was observed for at least additional five minutes. The outcome of the test was automatically assessed using the system software, using two parameters: reactive hyperemia index (RHI) and augmentation index (AI) [
8].
Statistical Analysis
To describe the population, basic description statistics was used for continuous variable – number, mean value, standard deviation, minimum and maximum values and 95% confidence interval for mean value. For categorical values, absolute and relative frequency was used. To compare the mean values of continuous parameters for two and more groups, scatter analysis was used. Chi-square test was used to compare to categorical variables. Pair t-test was used to determine the effect of treatment, comparing the parameter values at the beginning and at the end of the therapy. When testing hypotheses, we considered significance value of 0.05.
Discussion
Based not only from evidence based medicine data but also from our pilot trial we could conclude that n-3 PUFA represent a benefit for MS patients due to the lipid-lowering effect – through positive effect on atherogenic dyslipidemia. We have also demonstrated that the supplementation with n-3 PUFA leads to the improvement of endothelial dysfunction in the MS patients, therefore we can assume the effect on atherogenic process at this level, as well as the potential use of n-3 PUFA in comprehensive treatment of MS, since ED represent the basic common denominator in the pathogenesis of all components of MS.
ED occurs in the early stages of atherosclerosis, where disorders in the reactivity of blood vessels precede the structural changes of the vascular wall, resulting from the joint action of all atherogenic and athero-protective factors. All known risk factors of atherosclerosis, such as dyslipidemy, hypertension, diabetes, smoking, age, menopause are associated with ED. In the recent 10 years, attention has been focused on additional important risk factors of atherosclerosis, i.e. also ED, including: ApoB, ApoA, triglycerides, triglyceride-rich lipoproteins, small dense LDL particles, oxidized LDL, oxidized LDL antibodies, Lp(a), Hcy, C-reactive protein measured using high-sensitivity method (hsCRP) [
9]. Further below we shall discuss the risk factors and markers of ED that we observed.
Measurement of ApoB in MS patients is very important. These patients sometimes have normal levels, but in reality their LDL consist of the population of so-called “small dense LDL particles”, with very high atherogenic potential. These small dense LDLD-particles contain greater percentage of Apob and the measurement of ApoB helps us to better estimate the proportion of this high-risk population of LDL. The AMORIS study (Apolipoprotein MOrtality RISk study), as well as the large international study INTERHEART have shown that apoprotein B levels as well as the apoB/apoA-I ratio significantly improve the estimate of cardiovascular risk [
10]. In our work we have shown, that supplementation of n-3 PUFA in patients with MS has lead to significant decrease of ApoB. Similar results have been shown also by multicenter randomized study MARINE [
11]. Even though it has to be admitted that only EPA (eicosapentaenoic acid) was supplemented in this study – as compared to our study where a combination of EPA+DHA (deoxyhexaenic acid) was used), (EPA at the dose of at least 155g, DHA at least 520g). However, the observation period was 12 weeks as well.
Another risk factor of ED is hyperhomocysteinemia (HHcy), even though its causal relation to CV risk is subject to controversial discussion, as even large randomized studies such as NORVIT, HOPE-2, SEARCH failed to show that the reduction of Hcy would lead to decrease of CV risk [
12]. Although the multicenter, randomized, placebo-controlled, double blind study SU.FOL.OM3 (Supplémentation en Folates et Omega-3) has shown significant decrease of Hcy following the treatment with n-3 PUFA, surprisingly, this has not resulted in the reduced CV morbidity [
13]. In our study, following the supplementation with n-3 PUFA, we observed significant decrease of Hcy levels, but to demonstrate the effect of CV morbidity, longer observation is needed as well as larger patient population would be required, possible also higher dosage of n-3 PUFA than that used in SU.FOL.OM3, this could therefore be the objective of future clinical studies. However, an interesting finding involves the fact that Hcy, probably through increased vascular oxidative stress and atherothrombosis – partially suppresses the expression go GPX-1 gene, located at the 3p21.3 chromosome. Among many factors that contribute to the risk of atherosclerosis in plasma, great emphasis is placed on GPX-3, basic extracellular peroxidase, that plays a significant role in the modulation of oxidative stress. Lack of GPX-3 is associated with reduced biological availability of the nitric oxide and increased activation of platelets [
14,
15]. In our study, following the treatment with n-3 PUFA, we have shown significant increase of GPX, thus confirming the parallel antioxidant effect of n-3 PUFA, that significantly affects the ED and the process of atherogenesis.
Another independent risk factors of development of CV disorders is the increased level of Lp(a), as shown by currently available meta-analyses of epidemiological studies. Lp(a) increases the risk of stroke and death associated with vascular events in elderly males – independently of LDL-cholesterol levels. Reduction of Lp(a) is the secondary priority, following the reduction of LDL cholesterol and total cholesterol [
16]. In our population n-3 PUFA did not significantly decreased the Lp(a), but enrolling plasma levels in our observed patients did not reach significant values at all.
Endothelial dysfunction plays important role in Long Covid syndrome as well reported by recent papers, where COVID-19 could impair ED directly as viral effect or via cytokines inflammatory response (17) on endothelial cells reducing nitric oxid bioavailability. More common in patients with non-respiratory symptoms, Long Covid-19 symptoms could be persistent due to ED, that’s why better care for the patients could be done by n-3 PUFFA supplementation. These conclusions need to be confirmed by another pilot, placebo controlled trial.
In addition to laboratory assessment of AD, non-invasive measurement of ED using EndoPAT 2000 is currently coming to the center of attention. This method involves the measurement of the changes of vascular tone in the peripheral vascular bed (PAT – peripheral arterial tone) using plethysmography. The common principle for EndoPAT 2000 and flow mediated dilation (FMD) is the vasodilation-hyperemia, following the previous occlusion of the extremity. A significant advantage compared to FMD is the fact that EndoPAT 2000 is also a measure for arterial stiffness. Arterial stiffness is increased in various pathological conditions, such as ischemic heart disease, MS, chronic kidney disease and others [
1]. In our work, we assessed the endothelial function using EndoPAT 2000, based on the values of two parameters – RHI and AI. There are no official reference values available for RHI, but in general, RHI values under 1,67 are classified as ED, while the higher values of RHI are considered normal or represent the improvement of the endothelial function. Normal range of AI is between -30% and -10%, the limit value of AI is between -10% and 10% and abnormal values of AI are above 10% [
19,
20]. This method was used to demonstrate the improvement of the parameters of ED and arterial stiffness in our population of MS patients after 3 months of n-3 PUFA supplementation vs placebo group of patients.
Limitations of the Study
The study was limited by the low number of subjects, affecting the results of the effect on ED. Another limitation is the duration of observation period – a 3-month observation is not necessarily sufficient to assess the change of endothelial function. Additionally, it has to be said, that our study did not involve a homogenous population of subjects (the subjects took statins at various doses, in monotherapy as well as in combination with other agents, and for different length of time before the start of the combination therapy), possibly affecting the results due to their known pleiotropic effects. Another source of possible errors involved the assessment using the highly sensitive device – EndoPAT 2000, despite our effort to ensure strict observation of the requirements and procedures.