Submitted:
22 May 2023
Posted:
23 May 2023
You are already at the latest version
Abstract
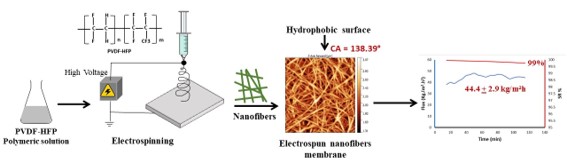
Keywords:
1. Introduction
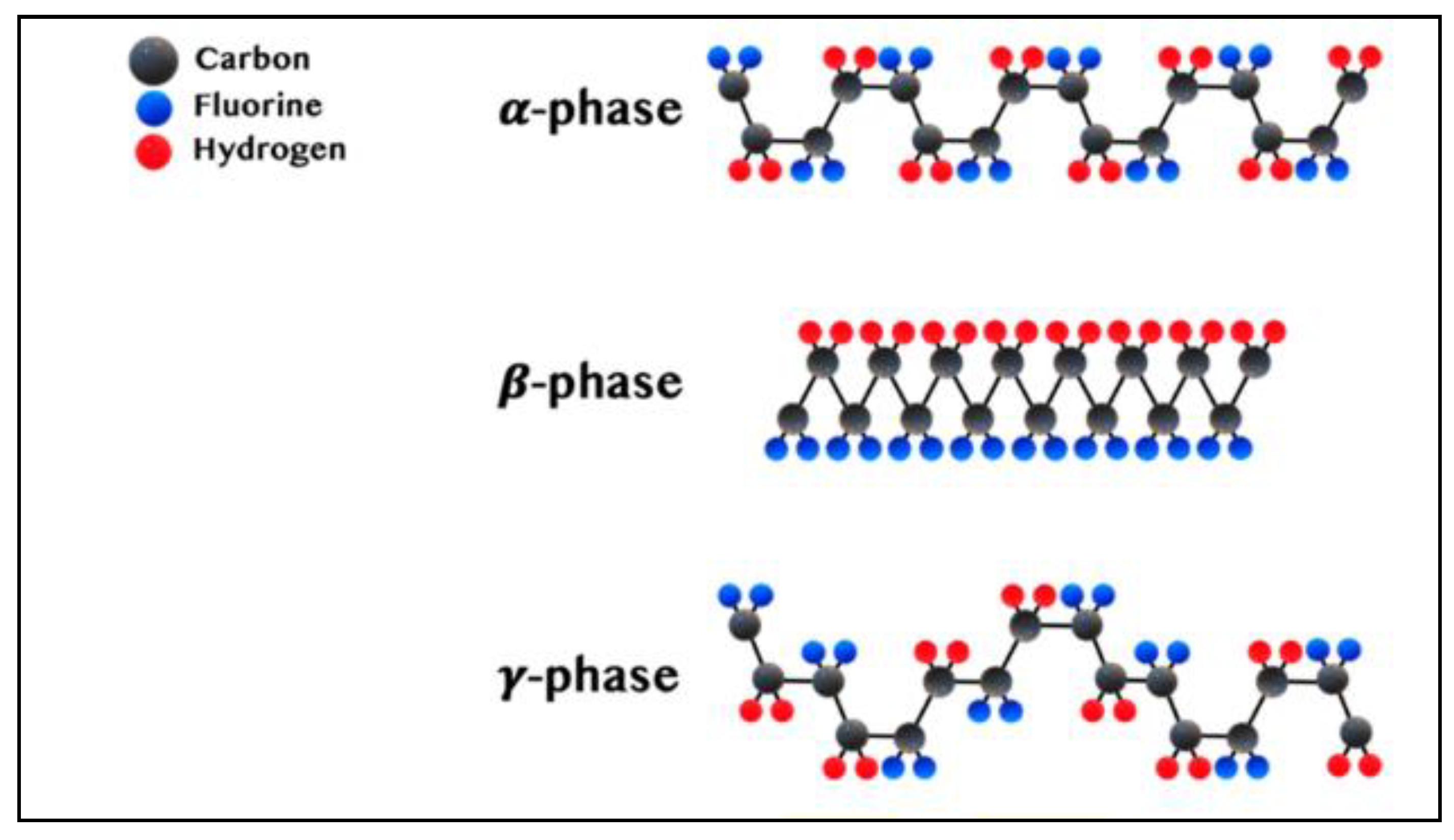
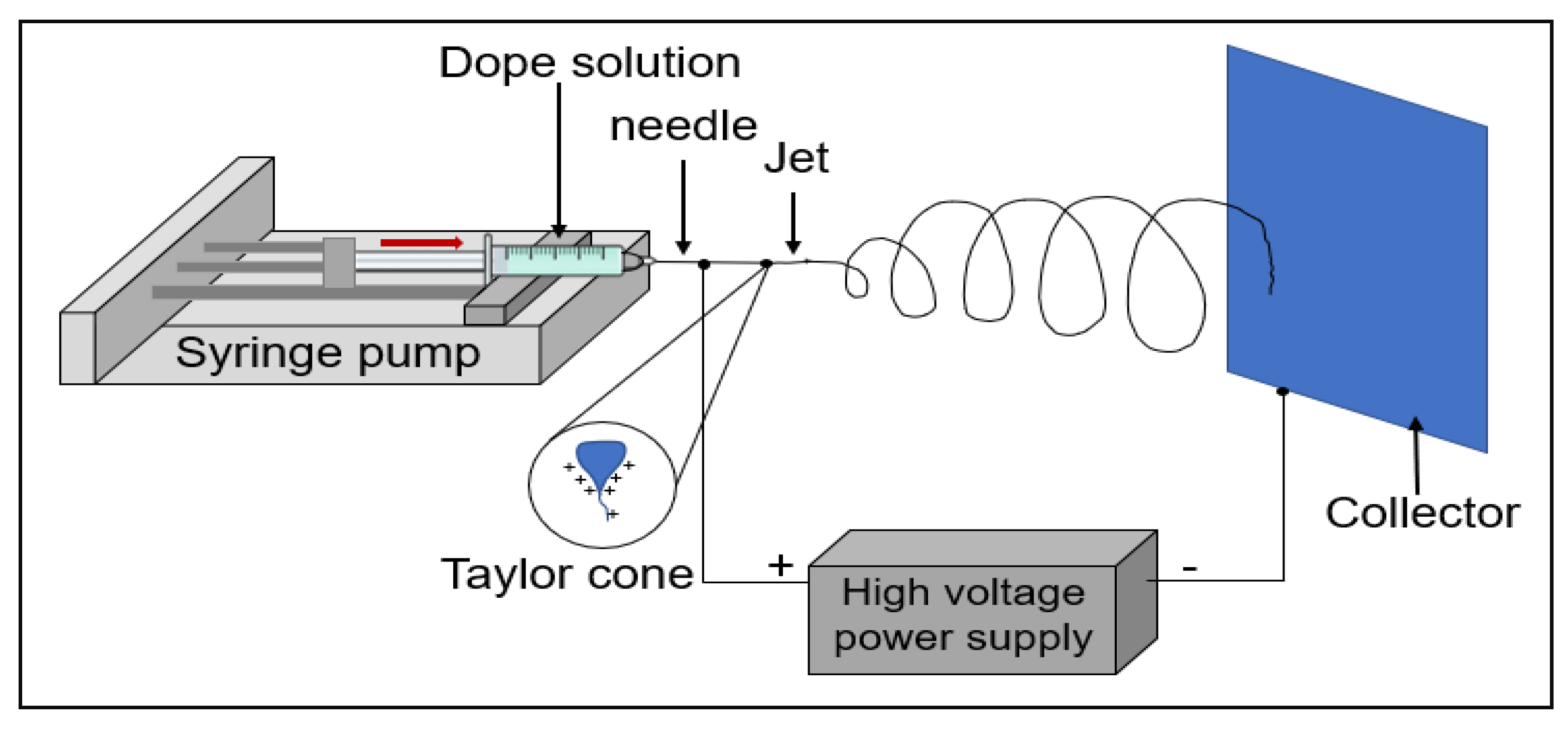
1. Experimental
2.1. Materials
| Dope solution abbreviation | Weight ratio (wt%) of solvents | PVDF-HFP % | LiCl % | |
| DMF | Acetone | |||
| FA 6-4 P6 | 6 | 4 | 6 | 0.1 |
| FA 6-4 P8 | 6 | 4 | 8 | 0.1 |
| FA 6-4 P10 | 6 | 4 | 10 | 0.1 |
| FA 5-5 P10 | 5 | 5 | 10 | 0.1 |
| FA 4-6 P10 | 4 | 6 | 10 | 0.1 |
2.1. Membrane fabrication
2.1.1. Electrospinning PVDF-HFP dope solutions
2.1.1. The non-solvent induced phase separation (NIPS) method.
2.1. Characterizations of PVDF-HFP nanofibers membranes
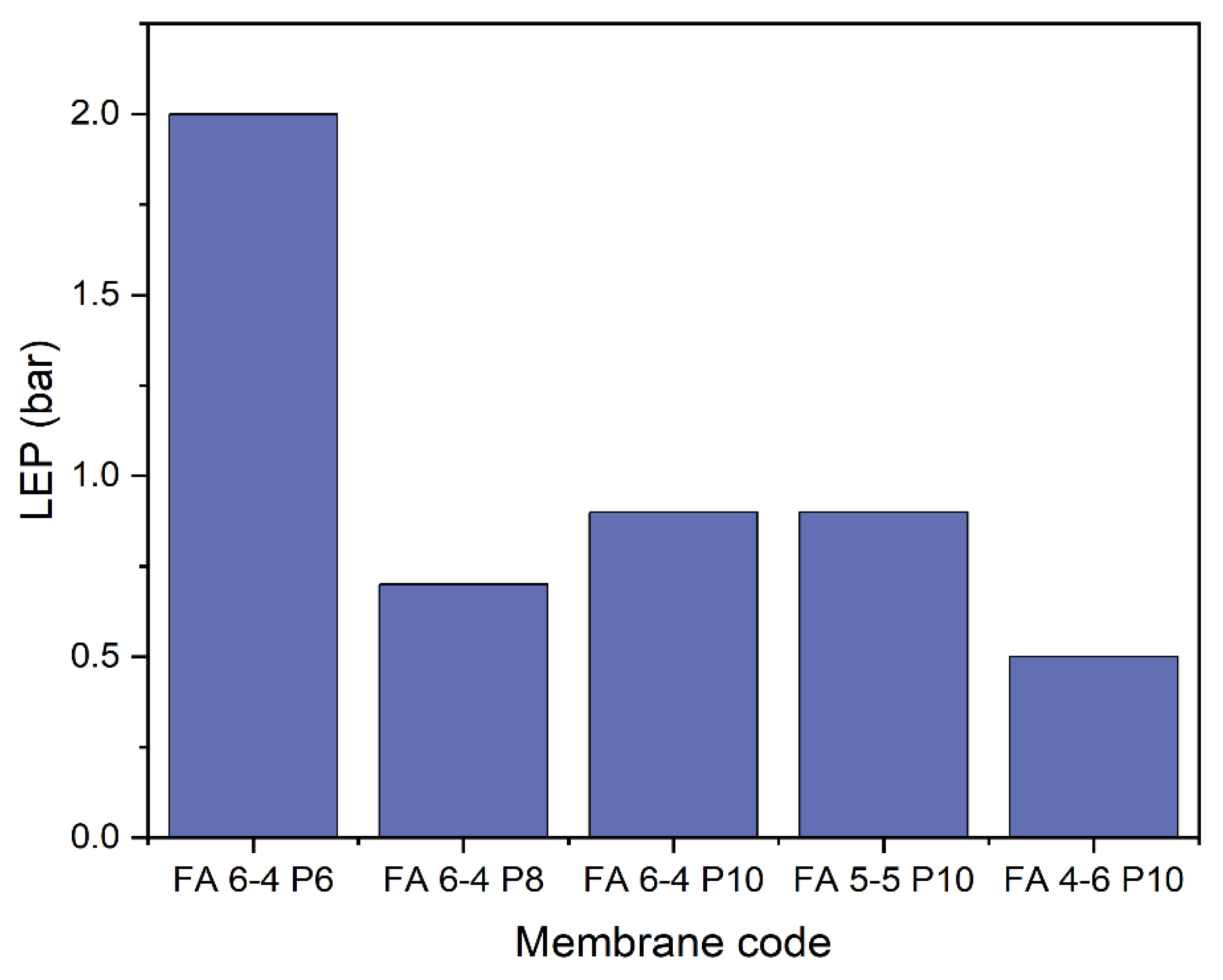
2.1.1. Thickness, mean pore size, and LEP
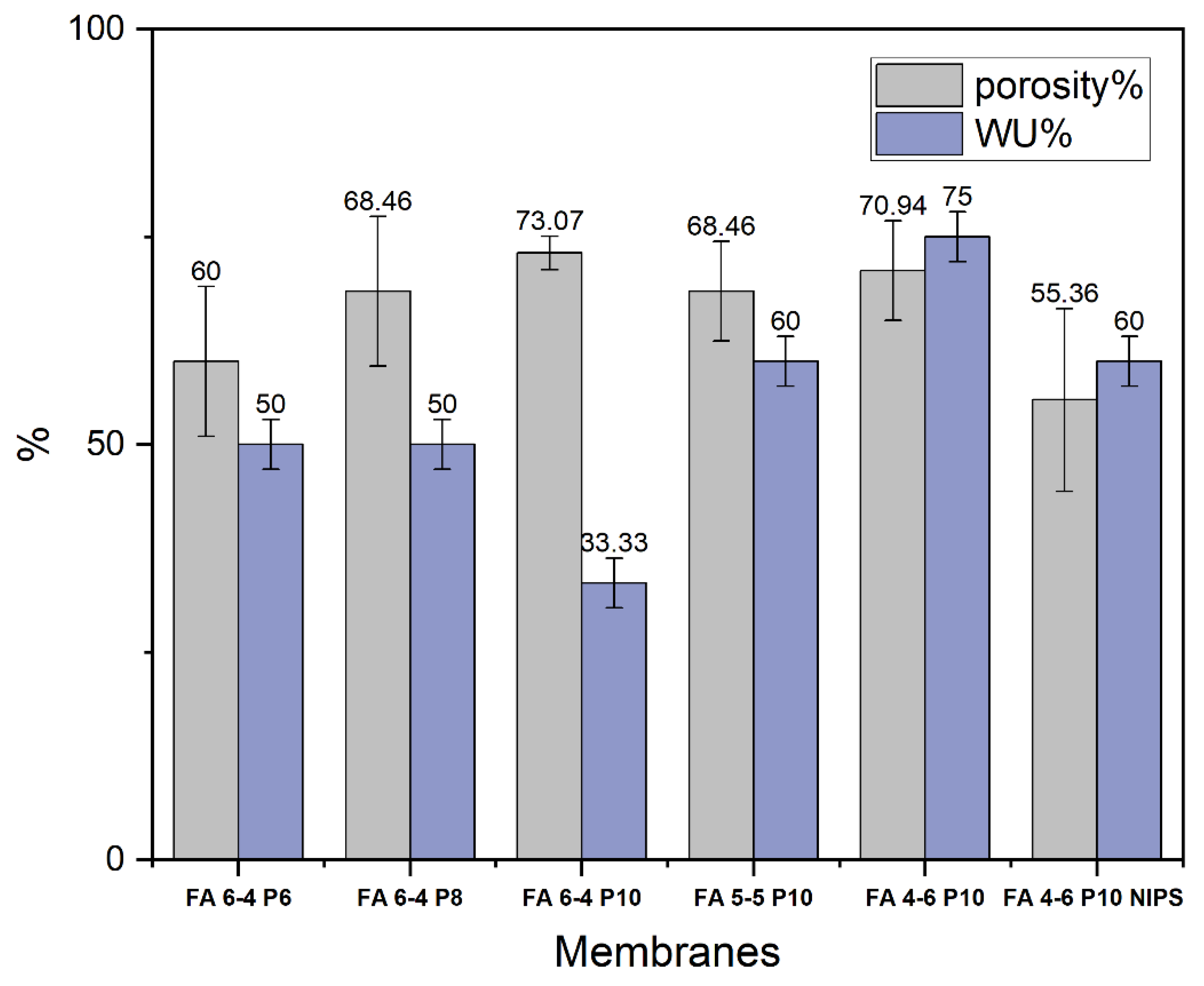
2.1.1. Porosity and water uptake
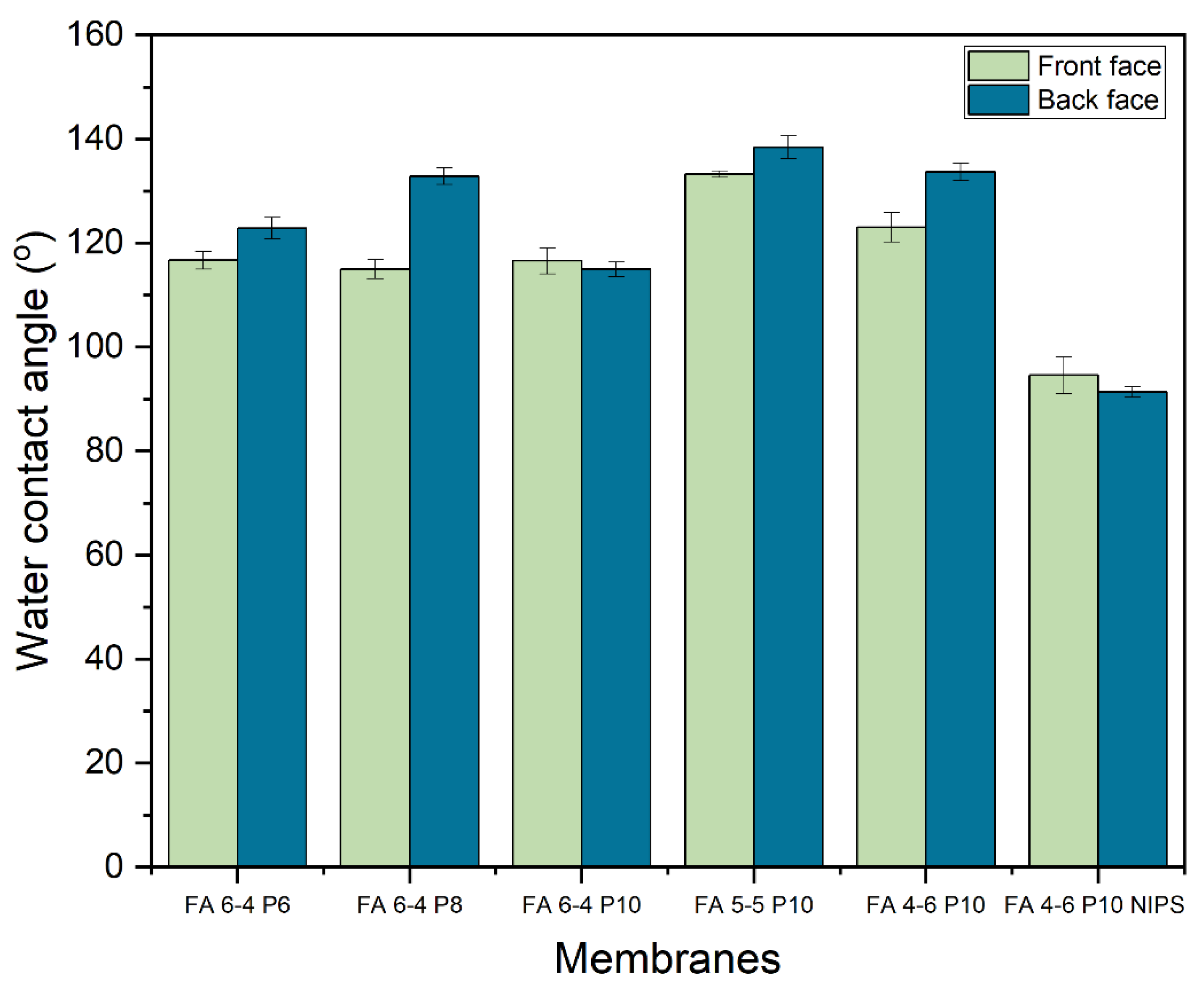
2.1.1. Contact angle (CA)
2.1.1. DSC, FTIR, and XRD
2.1.1. Morphological studies: AFM
2.1.1. Membrane performance: DCMD tests Membrane
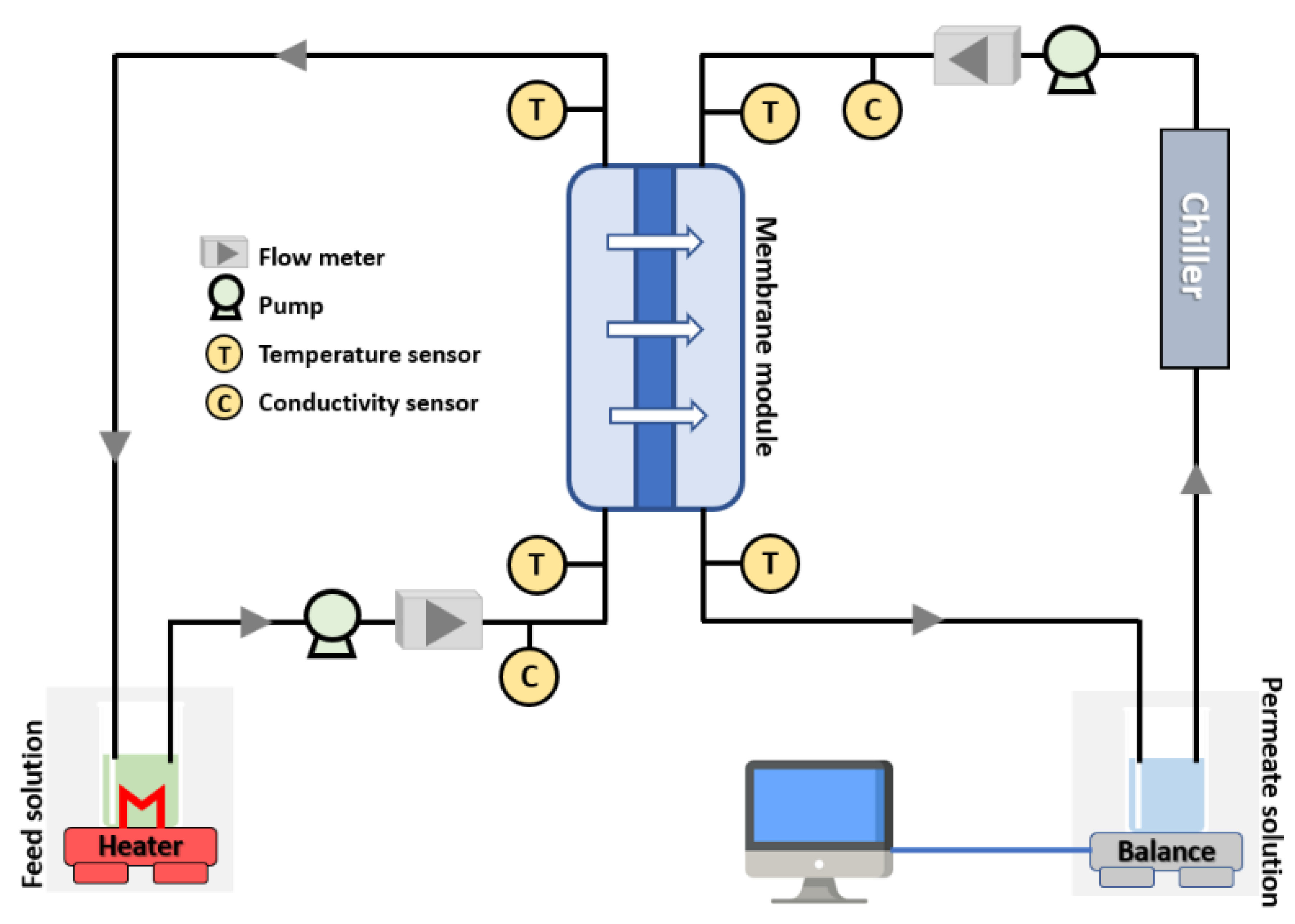
1. Result and discussion
3.1. Dope solution characterizations
| Solvents systems | FA 6-4 | FA 5-5 | FA 4-6 |
| Solubility parameter (MPa)1/2 | 4.34 | 5.69 | 7.11 |
| RED | 0.45 | 0.59 | 0.74 |
| vapor pressure (kPa) | 11.59 | 14.08 | 16.46 |
| Dope solutions | FA 6-4 P6 | FA 6-4 P8 | FA 6-4 P10 | FA 5-5 P10 | FA 4-6 P10 |
| Viscosity (cp) | 70 | 175 | 405 | 375 | 339.5 |
| Surface Tension (mN m-1) |
38.5 | 39.52 | 37.82 | 37.24 | 35 |
3.2. Membrane surface characterizations
| Membrane | Mean Thickness (µm) | Mean pore size (nm) |
| FA 6-4 P6 | 64.8 + 7.8 | 218 + 5 |
| FA 6-4 P8 | 184 + 11.4 | 203 + 5 |
| FA 6-4 P10 | 251 + 15.7 | 217 + 5 |
| FA 5-5 P10 | 103 + 18.9 | 180 + 5 |
| FA 4-6 P10 | 77.5 + 18.7 | 178 + 5 |
| FA 4-6 P10 NIPS | 50.75 + 4.2 | - |
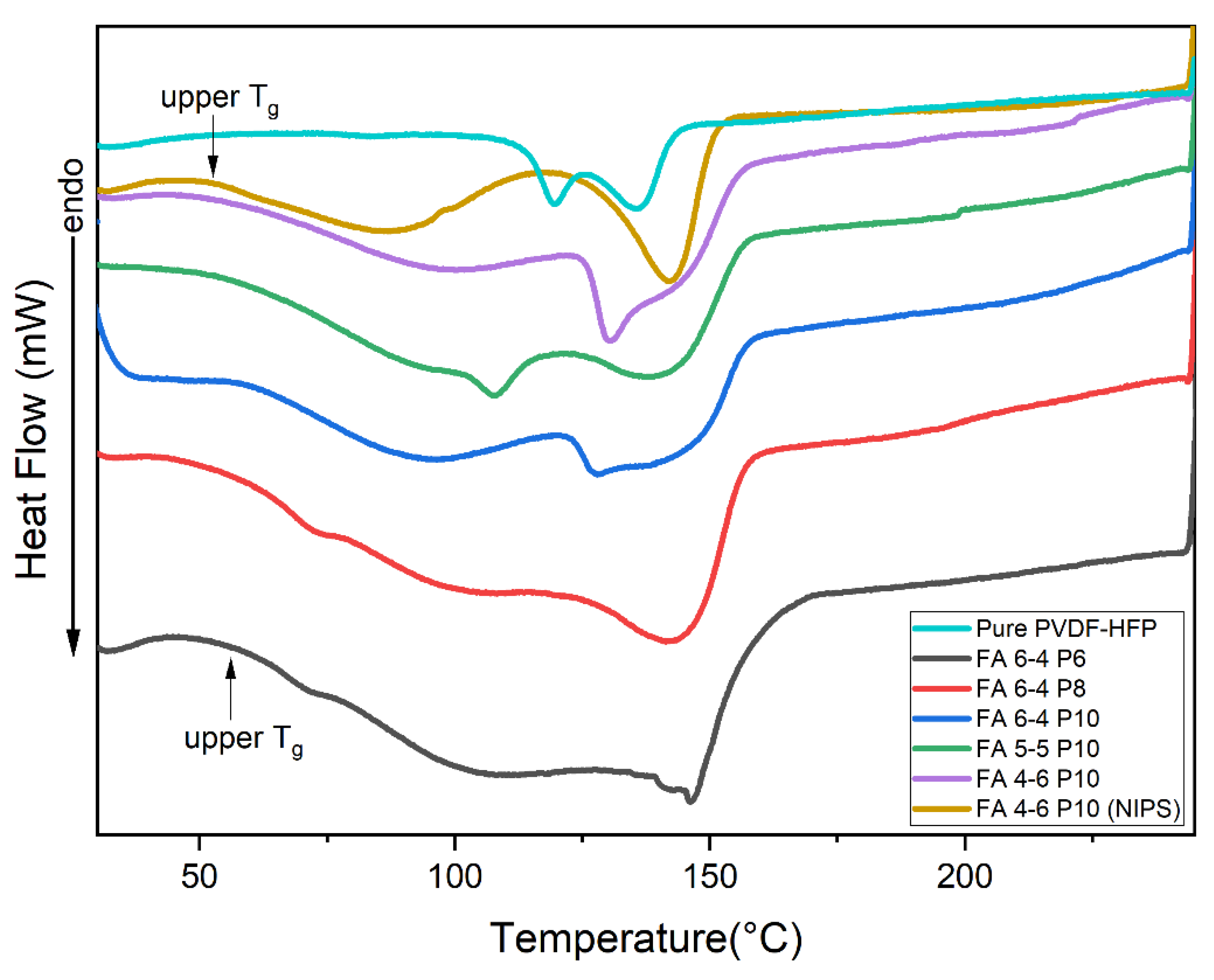
| Membranes | Pure PVDF-HFP | FA 6-4 P6 | FA 6-4 P8 | FA 6-4 P10 | FA 5-5 P10 | FA 4-6 P10 | FA 4-6 P10 (NIPS) |
|---|---|---|---|---|---|---|---|
| Tm (0C) | 119.90 135.37 |
148.0 | 144.0 | 142.5 | 142.1 | 130.6 | 142.32 |
| Upper Tg (0C) | - | 67.36 | 66.98 | 72.07 | 73.5 | 79.23 | 56.61 |
| ΔHm (J g-1) | 12.10 | 3.954 | 9.705 | 8.62 | 12.35 | 11.48 | 10.76 |
| X % | 11.56 | 3.77 | 9.26 | 8.23 | 11.79 | 10.96 + | 10.27 |
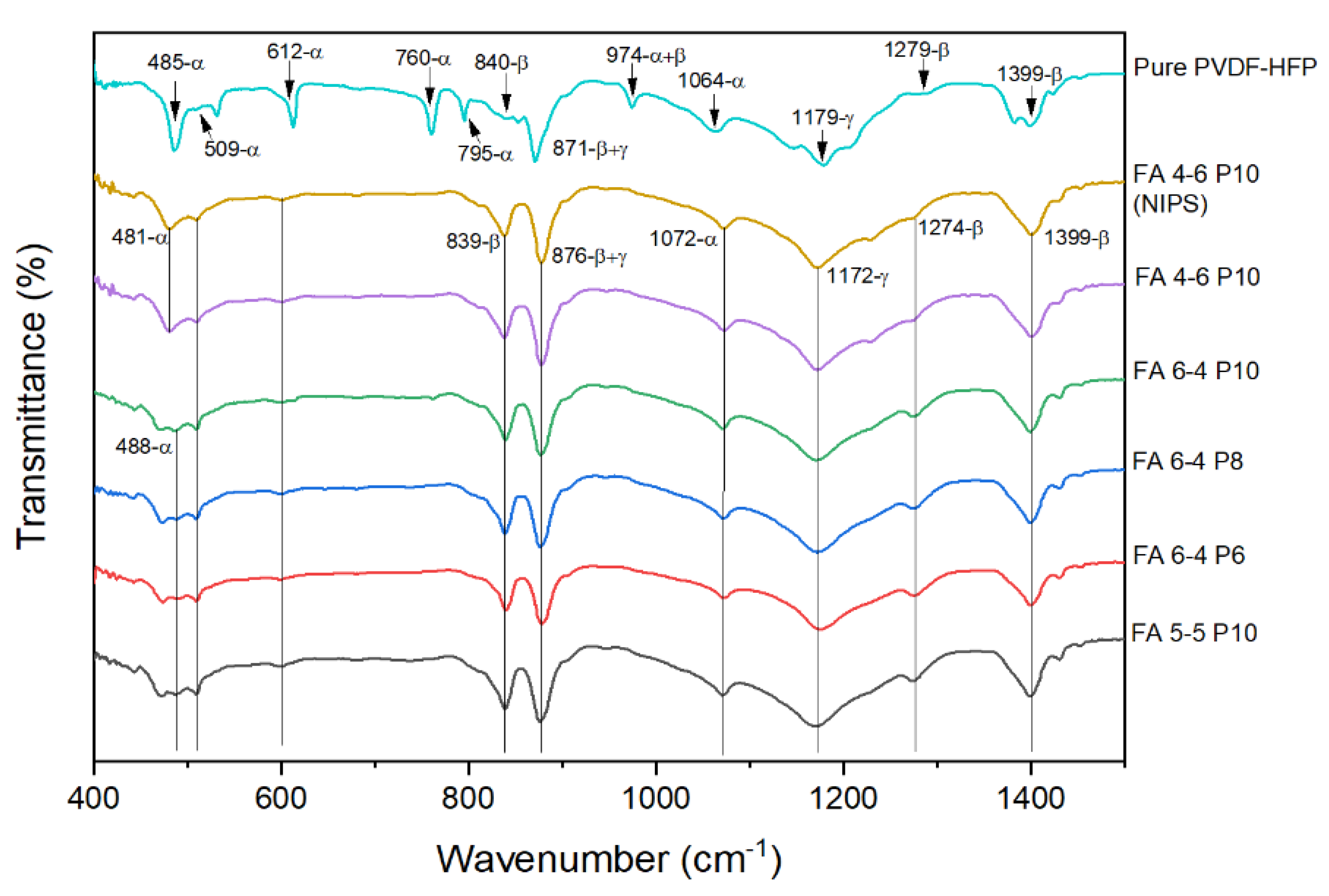
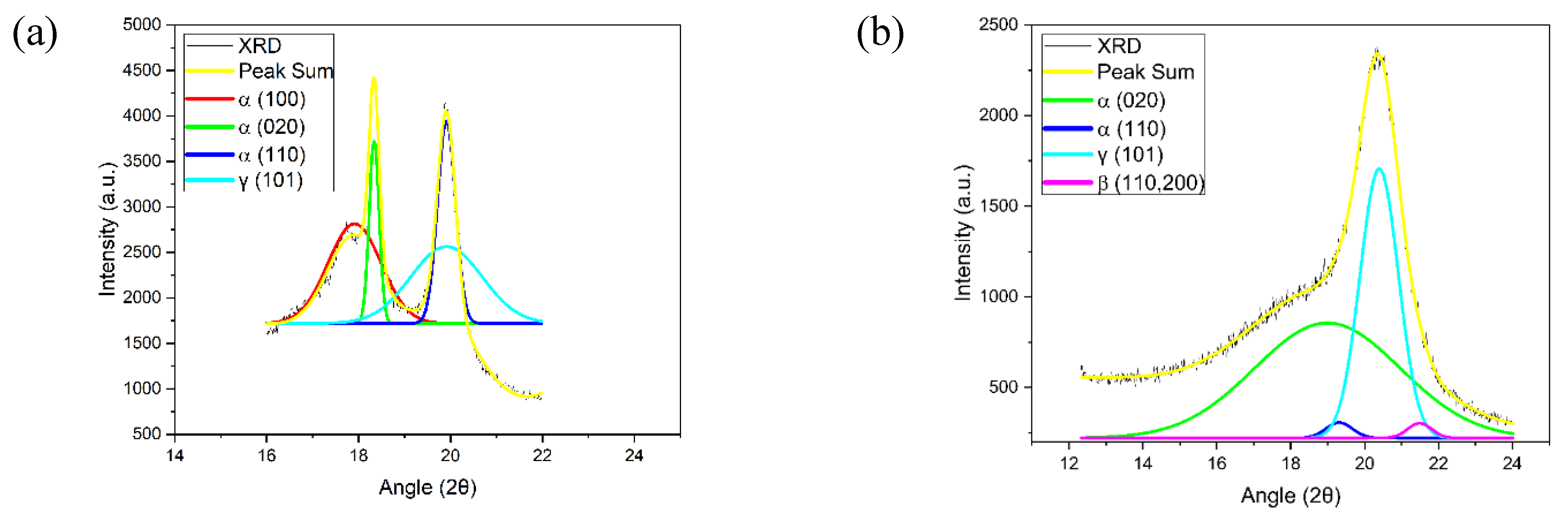
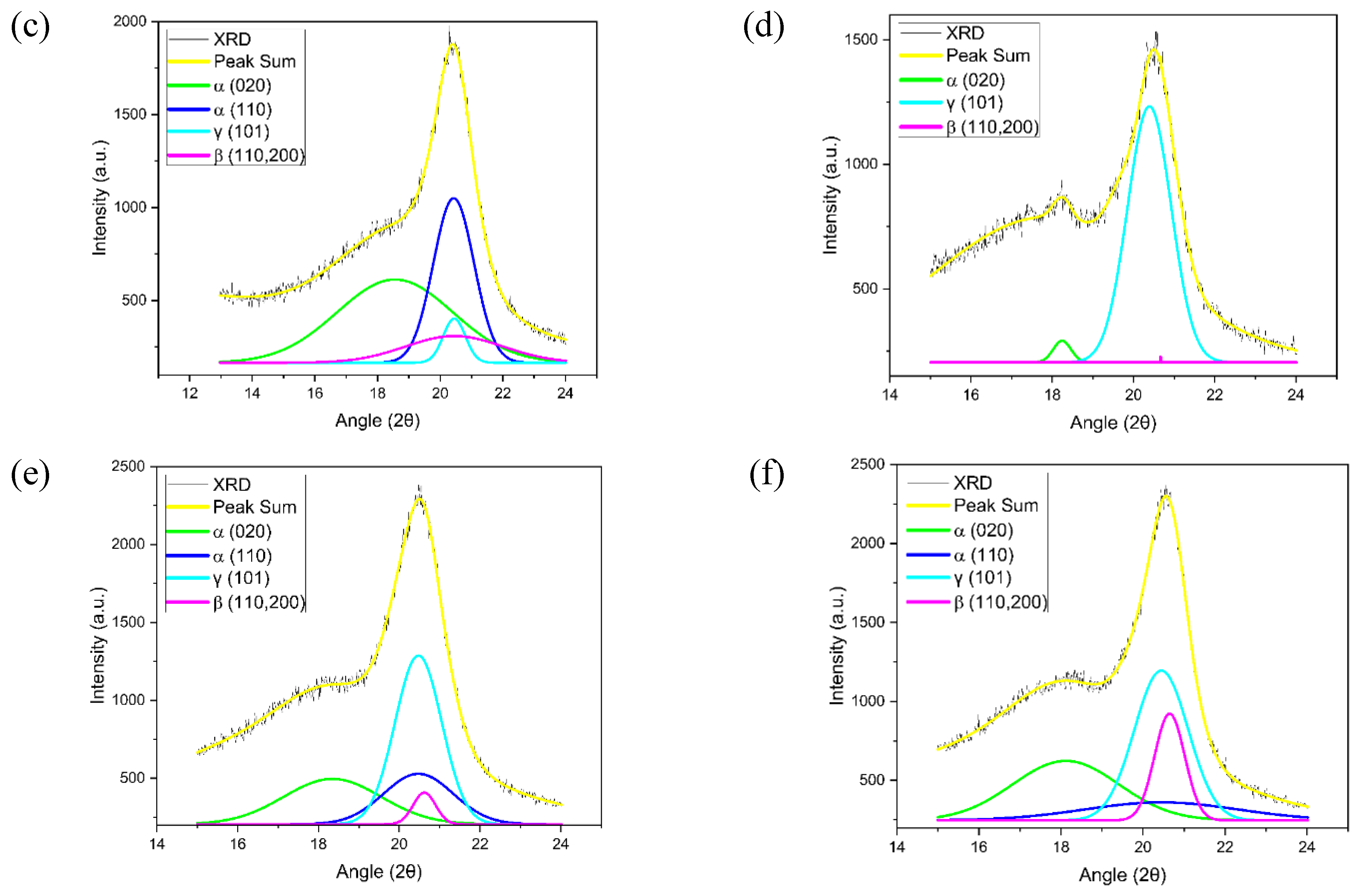
Morphological studies
| Membranes | Ra (nm) | Rq (nm) | AFD (µm) |
|---|---|---|---|
| FA 6-4 P6 | 284.1 | 367.83 | 1.20 + 0.5 |
| FA 6-4 P8 | 373.84 | 477.06 | 1.21 + 0.2 |
| FA 6-4 P10 | 382.06 | 455 | 1.78 + 0.4 |
| FA 5-5 P10 | 258.01 | 324.33 | 0.93 + 0.5 |
| FA 4-6 P10 | 593.83 | 697.8 | 1.67 + 0.4 |
| FA 4-6 P10 (NIPS) | 114.39 | 136.46 | - |
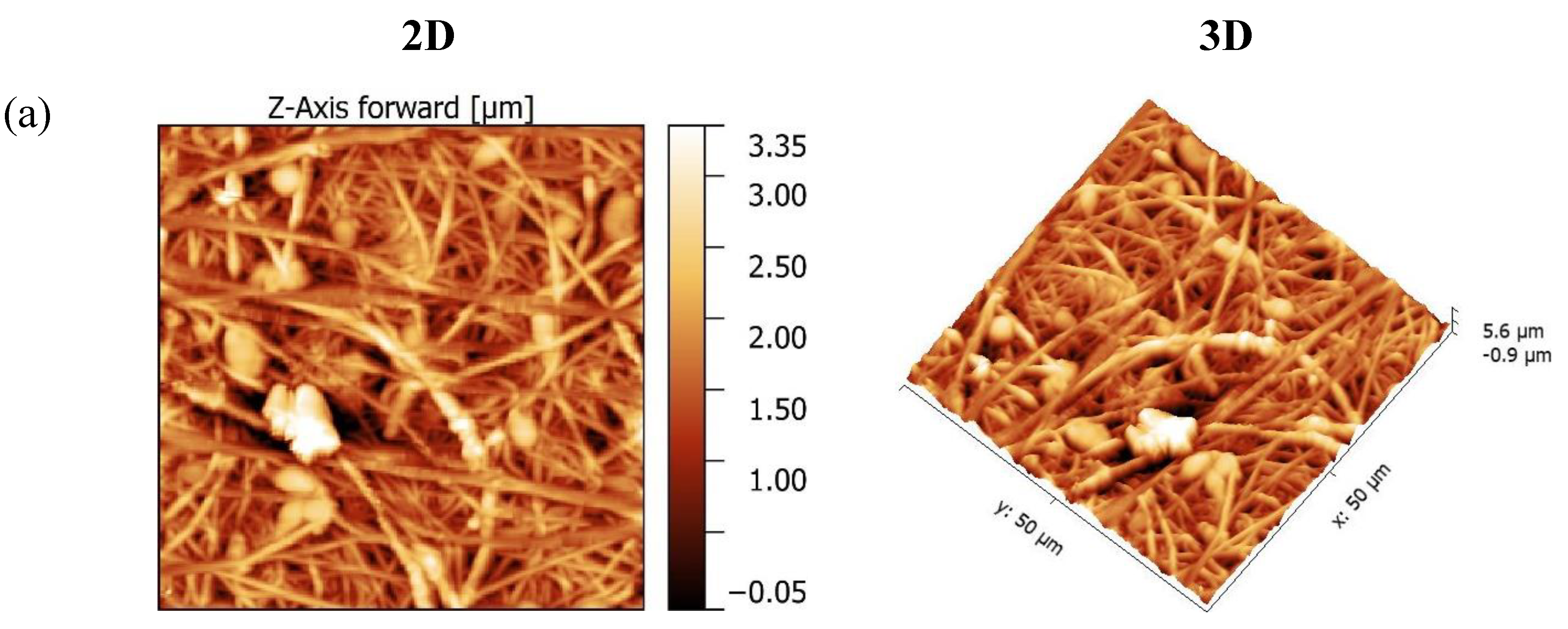
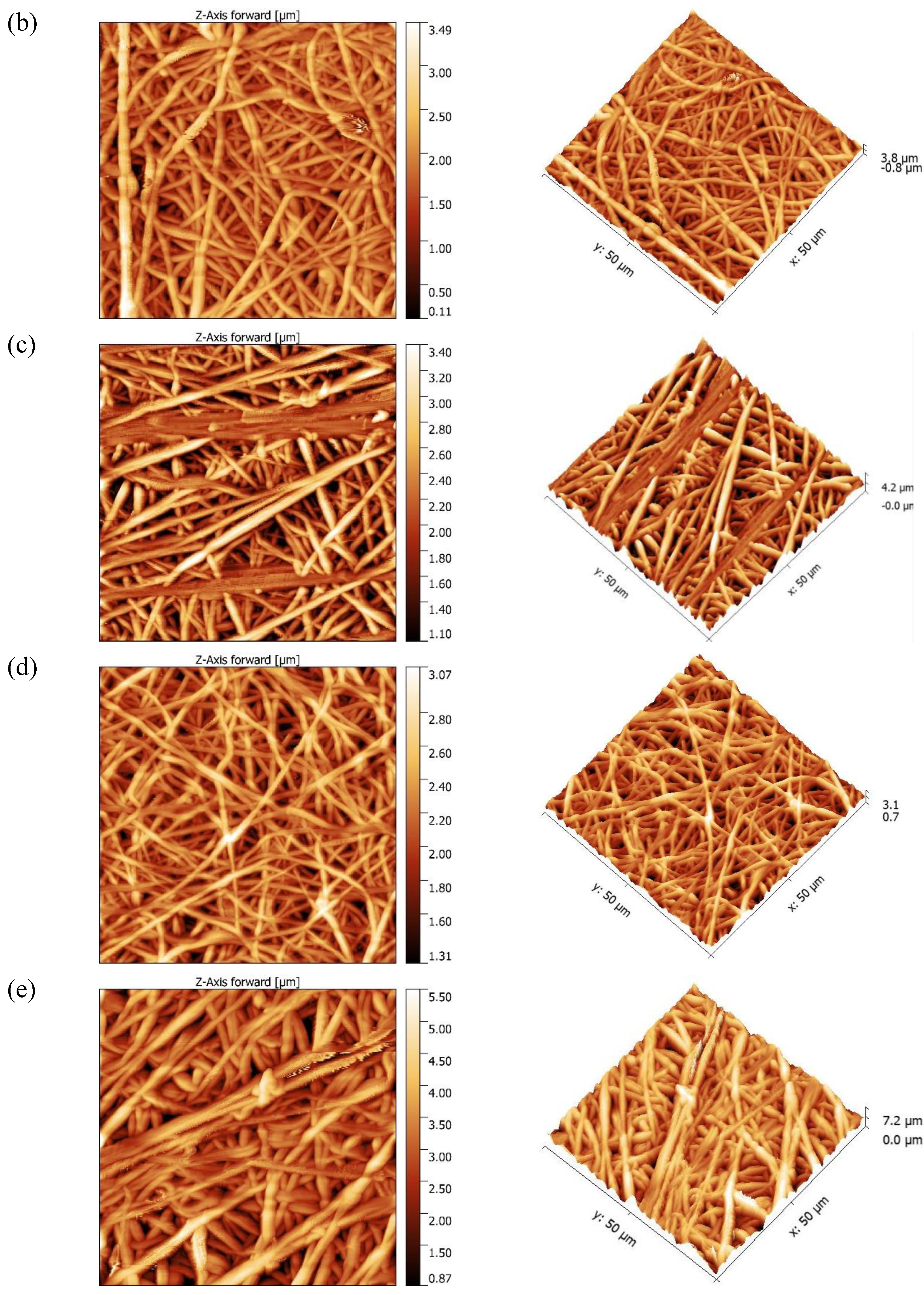
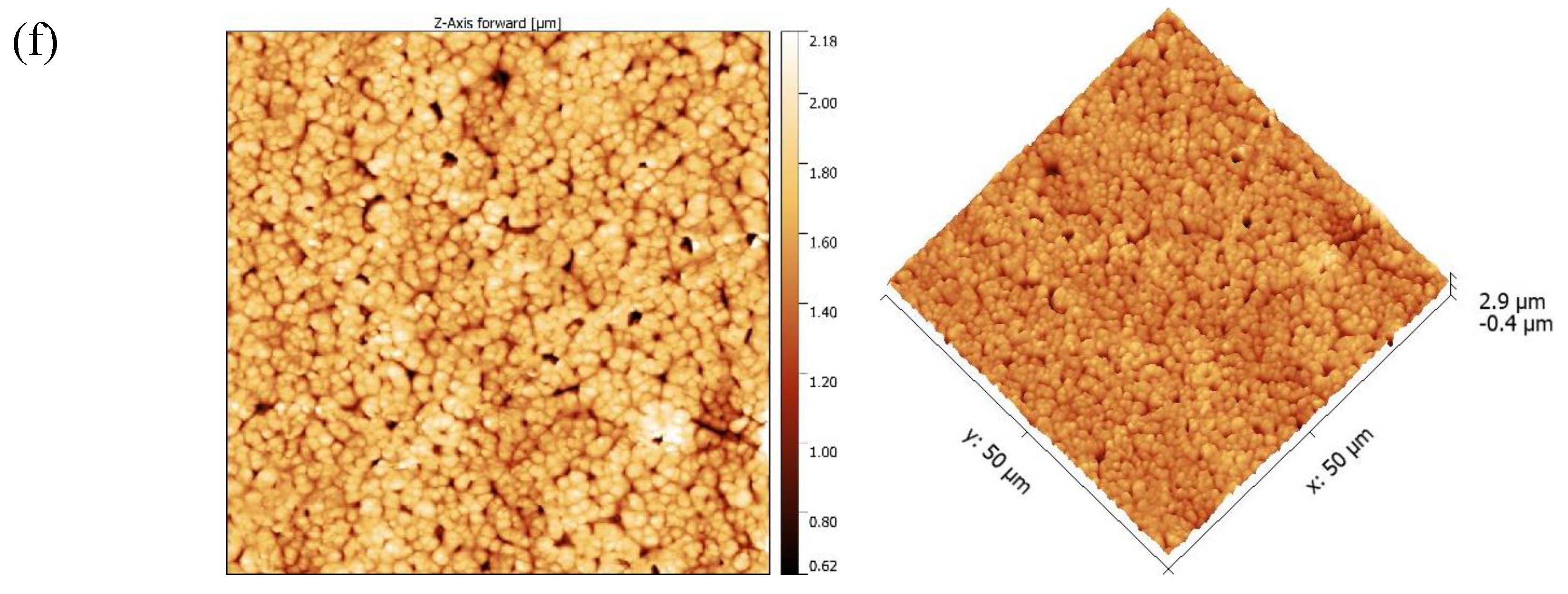
3.3. DCMD application
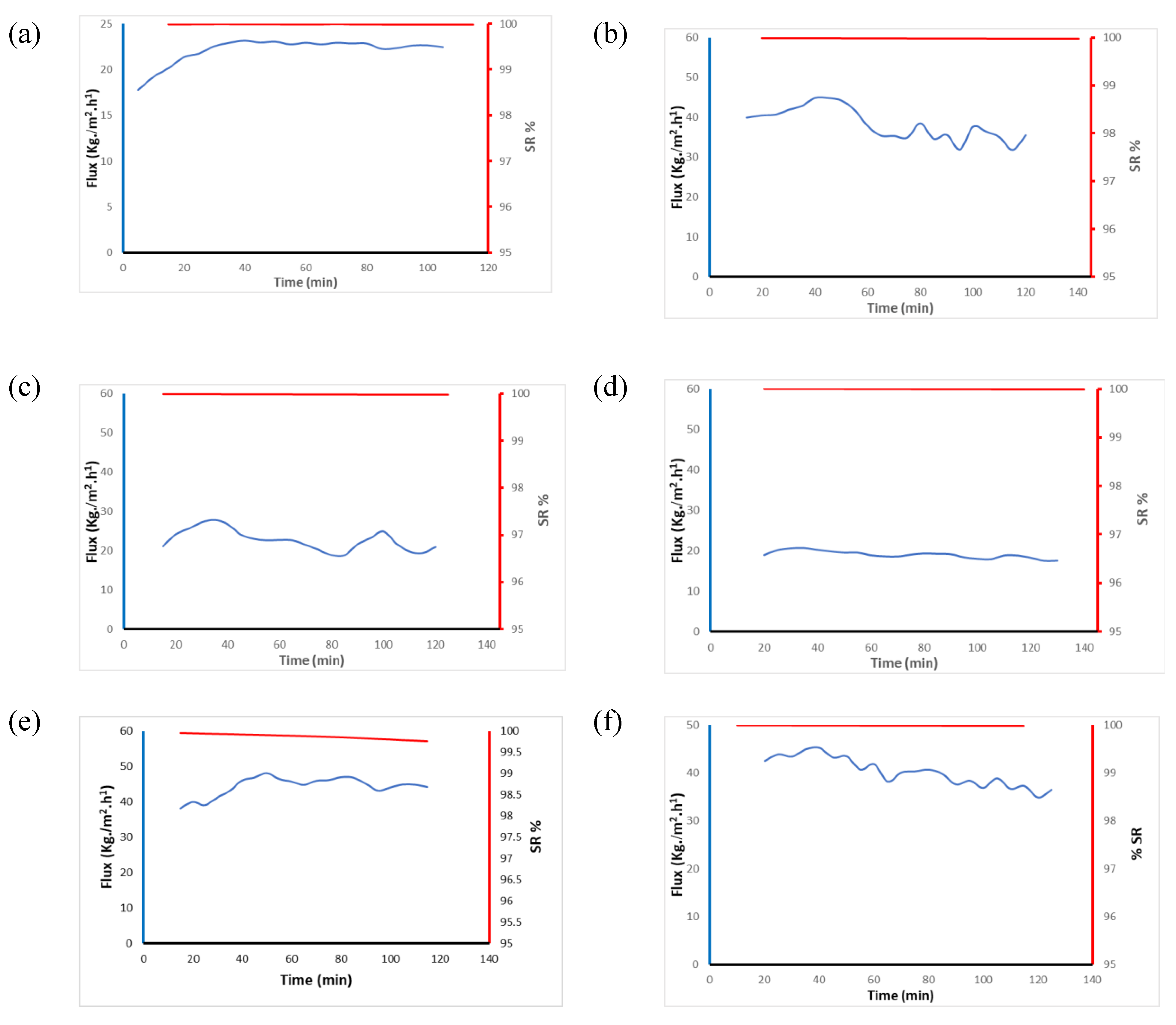
| Membrane code | Average water flux (kg m-2 h-1) | Average salt rejection (%) |
|---|---|---|
| PVDF membrane filter disc | 22.1 + 1.4 | 99.9 |
| FA 6-4 P6 | 38.3 + 4.0 | 91.5 |
| FA 6-4 P8 | 22.7 + 2.6 | 99.9 |
| FA 6-4 P10 | 19.1 + 0.9 | 99.9 |
| FA 5-5 P10 | 44.4 + 2.9 | 99.8 |
| FA 4-6 P10 | 40.3 + 2.9 | 91.7 |
4. Conclusion
Funding
Acknowledgments
Conflicts of Interest
References
- Ravi J, Othman MHD, Matsuura T, Ro’il Bilad M, El-badawy TH, Aziz F, et al. Polymeric membranes for desalination using membrane distillation: A review. Desalination 2020;490. [CrossRef]
- Cui J, Li F, Wang Y, Zhang Q, Ma W, Huang C. Separation and Purification Technology Electrospun nanofiber membranes for wastewater treatment applications. Sep Purif Technol 2020;250:117116.
- Liu Q, Chen Z, Pei X, Guo C, Teng K, Hu Y, et al. Review: applications, effects and the prospects for electrospun nanofibrous mats in membrane separation. J Mater Sci 2020;55:893–924. [CrossRef]
- Jain H, Garg MC. Fabrication of polymeric nanocomposite forward osmosis membranes for water desalination—A review. Environ Technol Innov 2021;23. [CrossRef]
- Kugarajah V, Ojha AK, Ranjan S, Dasgupta N, Ganesapillai M, Dharmalingam S, et al. Future applications of electrospun nanofibers in pressure driven water treatment: A brief review and research update. J Environ Chem Eng 2021;9:105107. [CrossRef]
- Francis L, Ahmed FE, Hilal N. Advances in Membrane Distillation Module Configurations. Membranes (Basel) 2022;12. [CrossRef]
- hae SH, Kim JH. Integration of PRO into Desalination Processes. 2017. [CrossRef]
- Liao Y, Loh C, Tian M, Wang R, Fane AG. Progress in Polymer Science Progress in electrospun polymeric nanofibrous membranes for water treatment : Fabrication, modification and applications. Prog Polym Sci J 2018;77:69–94.
- Ismail MS, Mohamed AM, Poggio D, Pourkashanian M. Direct contact membrane distillation: A sensitivity analysis and an outlook on membrane effective thermal conductivity. J Memb Sci 2021;624:119035. [CrossRef]
- Chen M, Ding W, Zhou M, Zhang H, Ge C, Cui Z, et al. Fouling mechanism of PVDF ultrafiltration membrane for secondary effluent treatment from paper mills. Chem Eng Res Des 2021;167:37–45. [CrossRef]
- Duong HC, Ansari AJ, Hailemariam RH, Woo YC, Pham TM, Ngo LT, et al. Membrane Distillation for Strategic Water Treatment Applications: Opportunities, Challenges, and Current Status. Curr Pollut Reports 2020;6:173–87. [CrossRef]
- Liao Y, Wang R, Tian M, Qiu C, Fane AG. Fabrication of polyvinylidene fluoride (PVDF) nanofibers membranes by electro-spinning for direct contact membrane distillation. J Memb Sci 2013;425–426:30–9. [CrossRef]
- Liang Y, Zhao J, Huang Q, Hu P, Xiao C. PVDF fiber membrane with ordered porous structure via 3D printing near field electrospinning. J Memb Sci 2021;618. [CrossRef]
- Jiang S, Ladewig BP. Green synthesis of polymeric membranes: Recent advances and future prospects. Curr Opin Green Sustain Chem 2020;21:1–8. [CrossRef]
- Huo P, Zhong CT, Xiong XP. Tailoring Morphology of PVDF-HFP Membrane via One-step Reactive Vapor Induced Phase Separation for Efficient Oil-Water Separation. Chinese J Polym Sci (English Ed 2021;39:610–9. [CrossRef]
- Liu Z, Cao R, Wei A, Zhao J, He J. Superflexible/superhydrophilic PVDF-HFP/CuO-nanosheet nanofibrous membrane for efficient microfiltration. Appl Nanosci 2019;9:1991–2000. [CrossRef]
- Das S, Ghosh A. Structure, ion transport, and relaxation dynamics of polyethylene oxide/poly (vinylidene fluoride co-hexafluoropropylene) - Lithium bis(trifluoromethane sulfonyl) imide blend polymer electrolyte embedded with ionic liquid. J Appl Phys 2016;119. [CrossRef]
- Liang Y, Cheng S, Zhao J, Zhang C, Sun S, Zhou N, et al. Heat treatment of electrospun Polyvinylidene fluoride fibrous membrane separators for rechargeable lithium-ion batteries. J Power Sources 2013;240:204–11. [CrossRef]
- Gebreyesus MA, Purushotham Y, Kumar JS. Preparation and characterization of lithium ion conducting polymer electrolytes based on a blend of poly(vinylidene fluoride-co-hexafluoropropylene) and poly(methyl methacrylate). Heliyon 2016;2. [CrossRef]
- Fen W, Zhang H. Recent advances in polymer blend membranes for gas separation and pervaporation. Prog Mater Sci J 2021;116.
- Saxena P, Shukla P. A comprehensive review on fundamental properties and applications of poly ( vinylidene fluoride ) ( PVDF ). Adv Compos Hybrid Mater 2021;4:8–26. [CrossRef]
- Kalimuldina G, Turdakyn N, Abay I, Medeubayev A, Nurpeissova A, Adair D, et al. A Review of Piezoelectric PVDF Film by Electrospinning and Its Applications. Sensors 2020;20. [CrossRef]
- Oumghar K, Chakhchaoui N, Farhane R, Eddiai A, Meddad M, Cherkaoui O, et al. Enhanced piezoelectric properties of PVdF-HFP/PZT nanocomposite for energy harvesting application. IOP Conf Ser Mater Sci Eng 2020;827:6–11. [CrossRef]
- Polat, K. Energy harvesting from a thin polymeric film based on PVDF-HFP and PMMA blend. Appl Phys A Mater Sci Process 2020;126:1–8. [CrossRef]
- Martina P, Gayathri R, Pugalenthi MR, Cao G, Liu C, Prabhu MR. Nanosulfonated silica incorporated SPEEK/SPVdF-HFP polymer blend membrane for PEM fuel cell application. Ionics (Kiel) 2020;26:3447–58. [CrossRef]
- Russo F, Ursino C, Sayinli B, Koyuncu I, Galiano F, Figoli A. Advancements in Sustainable PVDF Copolymer Membrane Preparation Using Rhodiasolv® PolarClean As an Alternative Eco-Friendly Solvent. Clean Technol 2021;3:761–86. [CrossRef]
- Sarkar S, Chakraborty S. Nanocomposite polymeric membrane a new trend of water and wastewater treatment: A short review. Ground Sustain Dev 2021;12. [CrossRef]
- Saleem H, Trabzon L, Kilic A, Zaidi SJ. Recent advances in nanofibrous membranes: Production and applications in water treatment and desalination. Desalination 2020;478. [CrossRef]
- Tabe, S. A review of electrospun nanofiber membranes. J Membr Sci Res 2017;3:228–39. [CrossRef]
- Su CI, Shih JH, Huang MS, Wang CM, Shih WC, Liu Y sheng. A study of hydrophobic electrospun membrane applied in seawater desalination by membrane distillation. Fibers Polym 2012;13:698–702. [CrossRef]
- Liao Y, Wang R, Fane AG. Engineering superhydrophobic surface on poly(vinylidene fluoride) nanofibers membranes for direct contact membrane distillation. J Memb Sci 2013;440:77–87. [CrossRef]
- Wu X, Wu X, Wang T, Zhao L, Bach Y. Omniphobic surface modification of electrospun nanofibers membrane via vapor deposition for enhanced anti-wetting property in membrane distillation. J Membr Sci J 2020;606.
- Beyraghi F, Mirfarsi SH, Rowshanzamir S, Karimi A, Parnian MJ. Optimal thermal treatment conditions for durability improvement of highly sulfonated poly(ether ether ketone) membrane for polymer electrolyte fuel cell applications. Int J Hydrogen Energy 2020;45:13441–58. [CrossRef]
- Samadianfard R, Seifzadeh D, Habibi-Yangjeh A. Sol-gel coating filled with SDS-stabilized fullerene nanoparticles for active corrosion protection of the magnesium alloy. Surf Coatings Technol 2021;419:127292. [CrossRef]
- Rahimpour A, Jahanshahi M, Mollahosseini A, Rajaeian B. Structural and performance properties of UV-assisted TiO 2 deposited nano-composite PVDF/SPES membranes. Desalination 2012;285:31–8. [CrossRef]
- Nthunya LN, Gutierrez L, Lapeire L, Verbeken K, Zaouri N, Nxumalo EN, et al. Fouling-resistant PVDF nanofibre membranes for the desalination of brackish water in membrane distillation. Sep Purif Technol 2019;228. [CrossRef]
- Rezaei M, Samhaber W. Wetting behaviour of superhydrophobic membranes coated with nanoparticles in membrane distillation. Chem Eng Trans 2016;47:373–8. [CrossRef]
- Russo F, Ursino C, Avruscio E, Desiderio G, Perrone A, Santoro S, et al. Innovative poly (Vinylidene fluoride) (PVDF) electrospun nanofibers membrane preparation using DMSO as a low toxicity solvent. Membranes (Basel) 2020;10:1–17. [CrossRef]
- Nuamcharoen P, Kobayashi T, Potiyaraj P. Influence of volatile solvents and mixing ratios of binary solvent systems on morphology and performance of electrospun poly(vinylidene fluoride) nanofibers. Polym Int 2021;70:1465–77. [CrossRef]
- Bahrami M, Karimi-Sabet J, Hatamnejad A, Dastbaz A, Moosavian MA. Optimization and modification of PVDF dual-layer hollow fiber membrane for direct contact membrane distillation; application of response surface methodology and morphology study. Korean J Chem Eng 2018;35:2241–55. [CrossRef]
- Serhan M, Sprowls M, Jackemeyer D, Long M, Perez ID, Maret W, et al. Total iron measurement in human serum with a smartphone. AIChE Annu Meet Conf Proc 2019;2019-Novem. [CrossRef]
- Hu X, Chen X, Giagnorio M, Wu C, Luo Y, Hélix-Nielsen C, et al. Beaded electrospun polyvinylidene fluoride (PVDF) membranes for membrane distillation (MD). J Memb Sci 2022;661. [CrossRef]
- Zheng R, Chen Y, Wang J, Song J, Li X-M, He T. Preparation of omniphobic PVDF membrane with hierarchical structure for treating saline oily wastewater using direct contact membrane distillation. J Memb Sci 2018;555:197–205. [CrossRef]
- Shalu, Singh VK, Singh RK. Development of ion conducting polymer gel electrolyte membranes based on polymer PVdF-HFP, BMIMTFSI ionic liquid and the Li-salt with improved electrical, thermal and structural properties. J Mater Chem C 2015;3:7305–18. [CrossRef]
- Gradišar Centa U, Mihelčič M, Bobnar V, Remškar M, Slemenik Perše L. The Effect of PVP on Thermal, Mechanical, and Dielectric Properties in PVDF-HFP/PVP Thin Film. Coatings 2022;12. [CrossRef]
- Yadav PS, Kumar V, Singh UP, Bhat HR, Mazumder B. Physicochemical characterization and in vitro dissolution studies of solid dispersions of ketoprofen with PVP K30 and d-mannitol. Saudi Pharm J 2013;21:77–84. [CrossRef]
- Atanassov A, Kostov G, Kiryakova D, Borisova-Koleva L. Properties of clay nanocomposites based on poly(vinylidene fluoride-co- Hexafluoropropylene). J Thermoplast Compos Mater 2014;27:126–41. [CrossRef]
- Dillon DR, Tenneti KK, Li CY, Ko FK, Sics I, Hsiao BS. On the structure and morphology of polyvinylidene fluoride-nanoclay nanocomposites. Polymer (Guildf) 2006;47:1678–88. [CrossRef]
- Malmonge LF, Malmonge JA, Sakamoto WK. Study of pyroelectric activity of PZT/PVDF-HFP composite. Mater Res 2003;6:469–73. [CrossRef]
- Merlini C, Barra GMO, Medeiros Araujo T, Pegoretti A. Electrically pressure sensitive poly(vinylidene fluoride)/polypyrrole electrospun mats. RSC Adv 2014;4:15749–58. [CrossRef]
- Chakhchaoui N, Farhan R, Eddiai A, Meddad M, Cherkaoui O, Mazroui M, et al. Improvement of the electroactive b-phase nucleation and piezoelectric properties of PVDF-HFP thin films influenced by TiO2 nanoparticles. Mater Today Proc 2019;39:1148–52. [CrossRef]
- Yadav A, Singh K, Panda AB, Labhasetwar PK, Shahi VK. Membrane distillation crystallization for simultaneous recovery of water and salt from tannery industry wastewater using TiO2 modified poly(vinylidene fluoride-co-hexafluoropropylene) nanocomposite membranes. J Water Process Eng 2021;44. [CrossRef]
- Shanthi PM, Hanumantha PJ, Albuquerque T, Gattu B, Kumta PN. Novel Composite Polymer Electrolytes of PVdF-HFP Derived by Electrospinning with Enhanced Li-Ion Conductivities for Rechargeable Lithium-Sulfur Batteries. ACS Appl Energy Mater 2018;1:483–94. [CrossRef]
- He Z, Rault F, Lewandowski M, Mohsenzadeh E, Salaün F. Electrospun PVDF nanofibers for piezoelectric applications: A review of the influence of electrospinning parameters on the β phase and crystallinity enhancement. Polymers (Basel) 2021;13:1–23. [CrossRef]
- Szewczyk PK, Gradys A, Kim SK, Persano L, Marzec M, Kryshtal A, et al. Enhanced Piezoelectricity of Electrospun Polyvinylidene Fluoride Fibers for Energy Harvesting. ACS Appl Mater Interfaces 2020;12:13575–83. [CrossRef]
- Abbas D, Mu’min MS, Bonanno M, Thiele S, Böhm T. Active solution heating and cooling in electrospinning enabling spinnability from various solvents. J Appl Polym Sci 2022;139:1–11. [CrossRef]
- Ndlwana, L.; Sikhwivhilu, K.; Moutloali, R.; Ngila, J.C. Heterogeneous Functionalization of Polyethersulfone: A New Approach for pH-Responsive Microfiltration Membranes with Enhanced Antifouling Properties. J. Membr. Sci. Res. 2020, 6, 178–187. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





