1. Introduction
Cryptococcal infection is one of the leading causes of death among people with HIV in sub-Saharan Africa (1, 2) despite expansion of antiretroviral therapy (ART) coverage (3). Limited diagnostic and therapeutic options significantly impair the outcome of the disease in this region (3). Point of care tests and an optimized antifungal therapy as recommended by the World Health Organization (WHO) (4, 5) have a major impact on clinical outcome, but these are still lacking in most of African health facilities including in Ethiopia.
However, the first-line recommended antifungals (4), a single, high-dose of liposomal amphotericin B and a 14-day course of flucytosine and fluconazole in the induction phase of cryptococcal treatment are lacking in most health facilities in Africa.
In addition to providing optimal antifungal therapy, performing frequent lumbar puncture (LP) and controlling intracranial pressure, based on clinical needs, have a significant impact on survival in cryptococcal meningitis (6). However, the manometer to measure the intracranial pressure and the LP set are usually missing in this region. Furthermore, patients with previously unknown cryptococcal infection and recent initiation of ART have an increased risk of immune reconstitution inflammatory syndrome (IRIS) and death (7).
In a previous study, we reported that the quick Sequential Organ Failure Assessment (qSOFA) score shows an inadequate performance for the early detection of sepsis in our study centers (8) (9). However, little information is available on the value of the qSOFA to predict mortality or the presence of cryptococcal meningitis in HIV-infected patients.
Due to a lack of diagnostic options and epidemiological data, the timely initiation of adequate sepsis therapy remains challenging. This problem is aggravated in patients with advanced HIV disease because of the large spectrum of potential microbes, including fungi or polymicrobial infections (10). Therefore, irrespective of CD4 count and viral load, we offered blood culture and cryptococcal antigen (CrAg) testing for all HIV-infected patients with suspected sepsis who were presented to adult emergency department or admitted to medical wards. In this study, we assessed the value of the qSOFA score for the early identification of cryptococcal sepsis and associated mortality in HIV-infected patients. In generally, our objective was to investigate the burden of cryptococcal infection and its related mortality among HIV-infected patients with suspected sepsis in a prospective multicenter study in Ethiopia.
2. Materials and Methods
We conducted a prospective observational cohort study in Ethiopia at 1) Asella Referral and Teaching Hospital and 2) Adama Hospital Medical College from September 2019 to November 2020. Eligibility participants had suspected sepsis according to the qSOFA score (score >=1). We collected participants’ socio-demographic and clinical data on admission (baseline) and vital signs at baseline and after 24 hours by trained study personnel. Participants were followed for 28-days either in-person or by phone interview if the participant was discharged or transferred to another hospital.
Blood culture
We collected 40 ml blood samples using blood culture bottles (BacT/ALERT®3D aerobic and anaerobic vials) and incubated them for up to five days (BacT/ALERT®3D, bioMérieux, Marcy-l'Étoile, France). If the instrument detected growth, we immediately performed Gram staining and subcultures on blood, MacConkey, and chocolate agar for bacteria and on Sabouraud dextrose agar for yeasts. Various biochemical tests like catalase, coagulase, urease, oxidase, motility, indole, glucose and lactose fermentation, citrate utilization, gas and H2S production tests were performed to identify the pathogens on site. Then the isolates were preserved at −81°C in Microbank® vials (Pro-Lab Diagnostics Inc., Toronto, Canada) and later exported to Düsseldorf, Germany for confirmation of species identification and antifungal susceptibility testing. The species identification was confirmed by using matrix-assisted laser desorption/ionization-time of flight (MALDI-TOF) mass spectrometry (Vitek® MS, bioMérieux).
Minimum inhibitory concentration (MIC) test
Confirmed samples of Cryptococcus neoformans were cultured at 35°C, and susceptibility testing was performed to determine the susceptibility. We read the antifungal susceptibility testing result at 48 hours of incubation at 30°C following manufacturer instructions of micro dilution according to EUCAST protocol.
Cryptococcal antigen (CrAg) test
In addition to blood culture diagnostics, we collected 5ml of blood for additional point of care tests, including the CrAg lateral flow assay, approved by the U.S. Food and Drug Administration in 2011 (Immy, Norman, Oklahoma, USA) according to the manufacturer’s instructions (11).
Ethical considerations
The institutional ethical review board of Asri University in Asella, Ethiopia, the National Ethical Review Board of the Ethiopian Ministry of Science and Technology in Addis Ababa, Ethiopia, and the Ethics Committee of the Faculty of Medicine, Heinrich-Heine-University, Düsseldorf, Germany approved the study protocol. All patients provided written consent.
3. Results
3.1. Sociodemographic of participants
In total, 82 HIV-infected participants were enrolled in the study. Of the total, 56% (46) were on antiretroviral therapy (ART). The median age was 35 years (IQR: 27-40), and 63% were female. We calculated the qSOFA score of the participants, and 64 (78%) had a qSOFA score of ≥ 2 at baseline, and 53 (65%) had a >2 qSOFA at 24 hours after hospital admission.
Table 1.
Sociodemographic and clinical data of participants.
Table 1.
Sociodemographic and clinical data of participants.
| Variables |
Frequency |
|
Age in years, median (IQR)
|
35 (27-40) |
|
Study site
|
|
| Asella Hospital |
54 (66%) |
| Adama Hospital |
28 (34%) |
|
Sex
|
|
|
Female
|
52 (63%) |
|
Male
|
30 (37%) |
|
HIV Therapy status
|
|
|
ART-naïve
|
36 (44%) |
|
Receiving ART
|
46 (56%0 |
|
qSOFA score
|
|
| ≥2 at baseline |
64 (78%) |
| ≥2 after 24 hours |
53 (65%) |
3.2. Cryptococcal antigenemia
The prevalence of cryptococcal antigenemia was 13% (11/82) and did not differ by ART status. CrAg-positivity was 11% (4/36) in those ART-naïve, and 15% (7/46) among those receiving ART (who knew their HIV serostatus and previously initiated ART with likely poor adherence). CrAg-positivity did not differ by sex (
Table 2). Cryptococcal antigenemia was present in 14% (9/64) with qSOFA score >2 at presentation and 15% (8/53) among participants with qSOFA scores ≥ 2 at 24 hours.
3.3. Bacterial Blood Culture
The overall blood culture positivity rate was 15% (12/82); Cryptococcus neoformans (n=5), Escherichia coli (n=3), Salmonella Typhi (n=1), Staphylococcus aureus (n=1), Streptococcus pneumonia (n=1), and Enterococcus faecalis (n=1). The sensitivity of blood cultures for cryptococcosis was 46% (5/11). All patients who were blood culture positive for yeast of Cryptococcus neoformans are positive for CrAg testing.
Table 3.
List of isolated pathogens from bacterial blood culture.
Table 3.
List of isolated pathogens from bacterial blood culture.
| Name of the microorganisms |
Frequency of isolates |
| Cryptococcus neoformans |
5 |
| Escherichia coli |
3 |
| Salmonella Typhi |
1 |
| Staphylococcus aureus |
1 |
| Streptococcus pneumoniae |
1 |
| Enterococcus faecalis |
1 |
| Total |
12 |
3.4. Minimum inhibitory of concentration (MIC)
Out of the five Cryptococcus yeasts isolated from blood, only one isolate (20%) had elevated fluconazole MIC of 16 mg/L, considered not fully susceptible (
Table 4). None of the isolates were resistant to the most commonly recommended antifungal agents of amphotericin B or flucytosine.
3.3. 28-day partcipants follow up outcomes
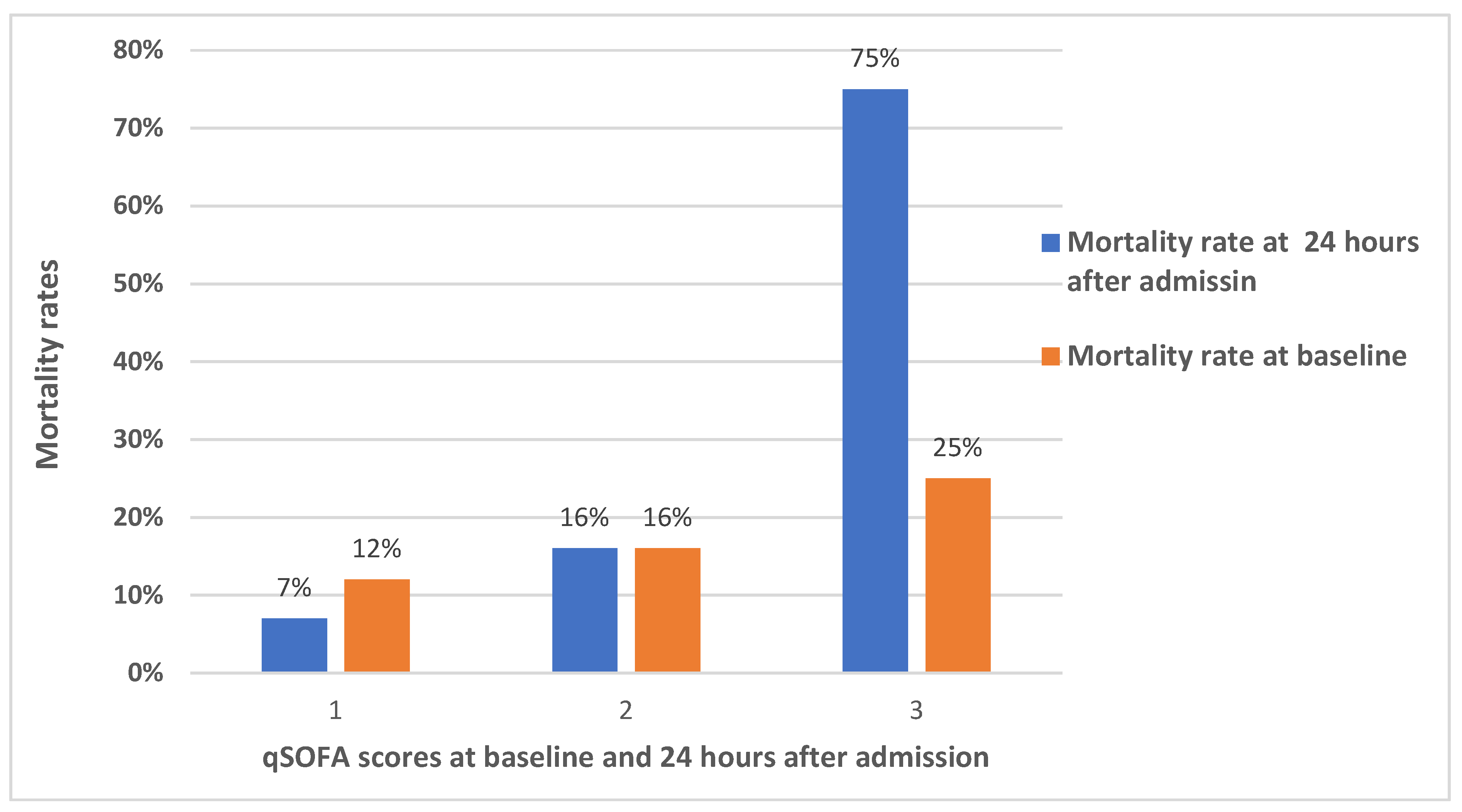
We assessed the 28-day mortality in comparison to the qSOFA score. The mortality rate in HIV-infected patients with baseline qSOFA scores of 1 was 12%, 2 was 16%, and 3 was 25%. Although it was higher in HIV-infected patients with a qSOFA score of 3, the difference at baseline was not statically significant (p=0.567). However, the mortality rate in HIV-infected patients with a qSOFA score of 3 at 24 hours after admission was very high and statistically significant (p=0.011). The mortality rate in participants with a qSOFA score after 24-hours of admission of 1 was 7%, and qSOFA of 2 was 16% (
Figure 1). The 28-day mortality among HIV-infected patients with qSOFA score ≥2 at baseline was 17% (11/65,) and among qSOFA score ≥ 2 at after 24 hours of admission was 21% (11/53).
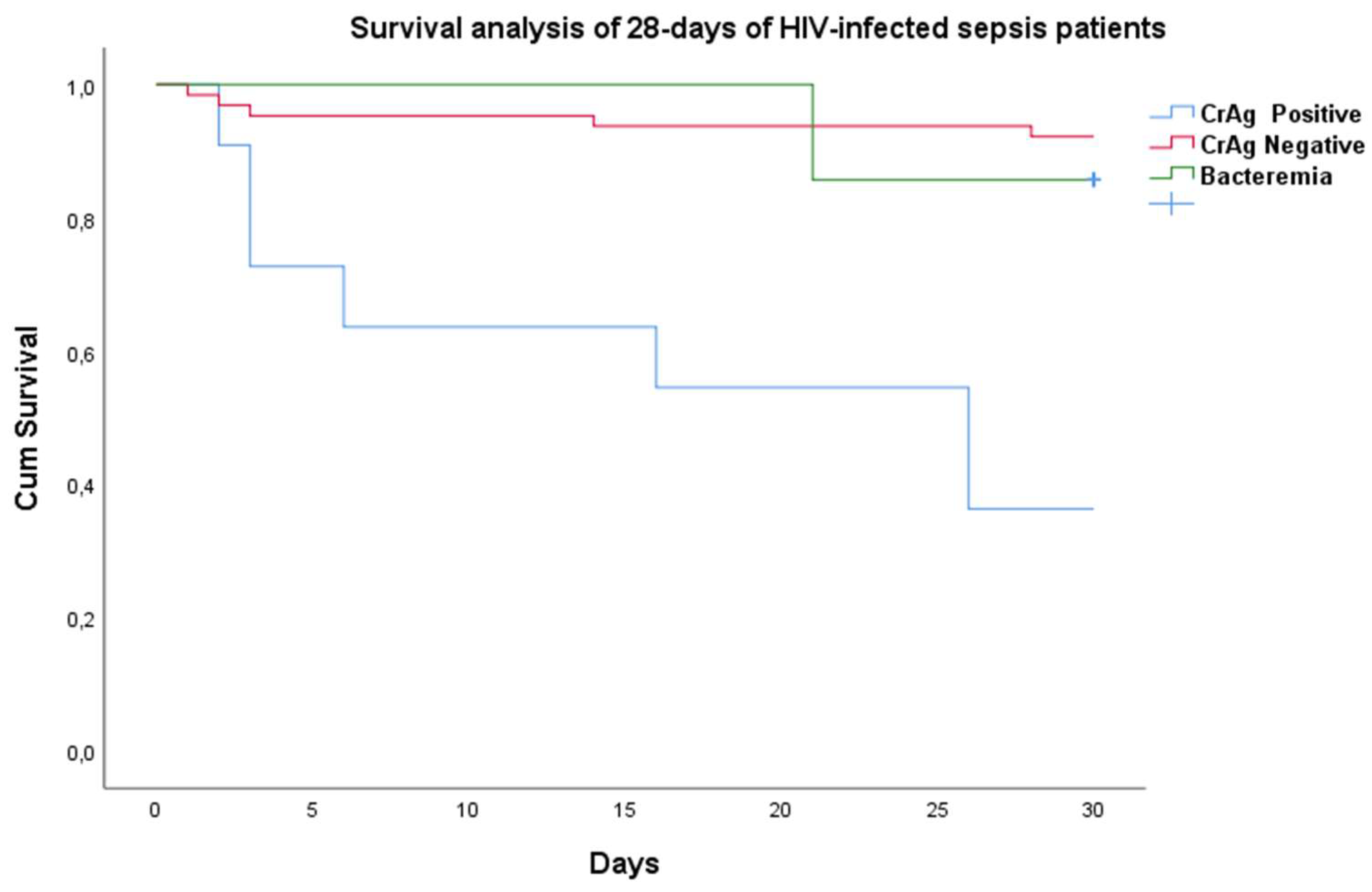
The overall 28-day mortality rate was 16% (13/82). Although all CrAg-positive patients received high-dose fluconazole monotherapy (1200 mg/d), the mortality rate was 64% (7/11). This mortality rate was significantly higher in CrAg-positive patients (64%) than in CrAg-negative HIV-infected suspected sepsis patients (9% (6/71); p<0.001). As shown in
Figure 2, the majority of deaths among CrAg-positive patients occurred in the initial 15 days.
4. Discussion
Cryptococcus neoformans has recently been ranked among the very critical pathogenic fungi (12), and cryptococcosis is the neglected killer among HIV-infected individuals in low and middle-income countries. In this study, we found that in addition to CrAg screening in HIV-infected persons with low CD4 counts per WHO recommendations and preemptive fluconazole therapy, CrAg screening in HIV-infected patients attending emergency outpatient or internal medicine department irrespective of CD4 count can prevent the under diagnosis of cryptococcosis and associated mortality in resource-limited settings.
We reported a 15% blood culture positivity rate in HIV-infected patients among suspected sepsis and this figure is higher than our previous finding of a 5.4% bacterial blood culture positivity rate in febrile patients in which most participants were from the same hospital (13). The pathogens profile detected by blood cultures is somewhat different from that of non-HIV-infected patients with suspected sepsis. In current study, we isolated C. neoformans (n=5), E. coli (n=3), Salmonella Typhi (n=1), S. aureus (n=1), S. pneumoniae (n=1), and E. faecalis (n=1). With the exception of E. coli and S. aureus, the detected pathogens have not commonly been isolated in our previous study (8). However, all pathogens are known to be typical causes of bloodstream infections in HIV-infected individuals in resource-limited settings including Mycobacterium tuberculosis, which was not targeted in this study (14, 15).
Another important point is that all patients in whom blood culture were positive for Cryptococcus neoformans are also positive for CrAg testing, which is not the case vice versa. This clearly showed us that the sensitivity of the CrAg testing for the diagnosis of cryptococcosis is better than that of blood culture.
We reported a 28-day mortality of 21% among HIV-infected patients with a qSOFA score ≥2 at 24 hours upon admission and 7% among patients with a qSOFA score <2, respectively. This mortality rate is lower than our previously reported figures from the same hospital among adult sepsis patients with a qSOFA score ≥2 (8). However, the mortality was very high among CrAg-positive patients treated with high-dose fluconazole monotherapy (1200 mg/d) when compared with CrAg-negative HIV-infected suspected sepsis patients (64% vs. 9%); p<0.001) (
Figure 2). This finding is largely consistent with our earlier report that 68% of cryptococcal patients died within the first three months of fluconazole therapy at 1200 mg/d (16) and the recent report from Sierra Leone that 63% of CrAg-positive participants died within a month (17). There is considerable evidence that high-dose fluconazole monotherapy is inadequate for cryptococcal meningitis treatment, but that fluconazole is effective as preemptive therapy in asymptomatic antigenemia patients or when no brain infection is involved (18).
Our results showed that a qSOFA score of HIV-infected patients ≥ 2 had no statically significant effect on the proportion of cryptococcal antigenemia both at baseline and after 24 hours. Thus, a higher qSOFA value at 24 hours predicted mortality in HIV-infected patients (
Figure 1), but a higher qSOFA value at baseline did not predict mortality. Therefore, new modeling approaches should be developed to easily predict cryptococcal infection and associated mortality with considering other factors such as CD4 count and intracranial pressure (19), or CrAg serum titers (20).
In resource limited settings, applying the existing diagnostic and therapeutic options without delay is crucial in order to minimize the cryptococcal-associated mortality among HIV-infected individuals. Several approaches exist to reduce cryptococcal mortality. One approach is to expand ART services and improve patient adherence and thus immune status to reduce susceptibility to cryptococcal infections. Even though we reported higher prevalence of among on ART in this study, this could be due to their poor adherence for ART. Another approach is implementation of the current WHO recommendations of which CrAg screening among patients with advanced HIV disease and preemptive fluconazole treatment in asymptomatic CrAg-positive individuals without delay. There is strong evidence that CrAg-positive HIV-infected patients with CrAg-negative CSF show no difference in mortality compared to CrAg-negative subjects when treated with fluconazole alone (18). The other option we have pointed out in this study is to offer CrAg testing to all HIV-infected patients with suspected sepsis or newly HIV-diagnosed, taking into account other common opportunistic infections. Then, an adequate antifungal treatment should be administered to CrAg-positive patients, and the patients should be closely monitored. For instance, a multicounty study has shown that a single dose of liposomal amphotericin B at 10 mg/kg followed by a combination of 5-flucytosine and fluconazole is cost-effective and safe (21).
A few data are available on the resistance profile of
C. neoformans yeasts in Africa. In this study, we reported that 20% of isolates were resistant to fluconazole (see
Table 4), although the sample size was too small. There were no isolates resistant to the first line recommended antifungal agents amphotericin B and 5-flucytosine. However, in most African countries, fluconazole is the only antifungal available in many health facilities for cryptococcal meningitis treatment. The emerging fluconazole resistance of
C. neoformans and the high mortality rate of cryptococcal patients receiving fluconazole monotherapy (21) require timely action to make other recommended antifungals available.
Unlike bacteria, multidrug resistance in fungi is rare and not yet widespread, although the number of fungi resistant to antifungals is increasing from time to time (22). Therefore, it is timely recommended to introduce fungal diagnostics and susceptibility testing for isolates to combat the spread of fungal resistance and prevent the emergence of multidrug resistance like Candida aureus.
In this study, we found no correlation of resistance between fluconazole and amphoteric B or 5-flucytosine in the five isolated yeasts, and this result is consistent with previous reports (23). Therefore, for effective treatment and prevention of resistant yeasts expansion, the use of antifungal combination therapy is strongly recommended for cryptococcal treatment.
Our study has some limitations. The sample size was too small to generalize the data, and we did not consider tuberculosis and cytomegalovirus which are also common causes of sepsis and/or contributors to mortality among HIV-infected patients.
5. Conclusions
Cryptococcal infection is the leading cause of mortality among HIV-infected suspected sepsis patients at the study sites. With the limited therapeutic options, and fluconazole monotherapy as only treatment currently available, the 64% 28-day mortality rate was alarmingly high. In this study, we found that a higher qSOFA score 24 hours after admission was not related to a positive CrAg test but predicted high 28-day mortality in HIV-infected patients. In addition to scaling up ART coverage in the country and improvement of adherence to HIV therapy, capacity for HIV testing should be strengthened to reduce the number of HIV-infected individuals who are diagnosed lately. The CrAg screening in newly HIV infection diagnosed and HIV positive who are attending hospital irrespective of CD4 count and viral load can minimize the cryptococcal infections missing cases in resource limited settings. Expanding the fungal laboratory capacities in the country and performing susceptibility testing for pathogenic fungal isolates are very important to minimize resistance to antifungals and to improve patient care. These findings warrant the need of a bundle approach for diagnostics and management of HIV-infected patients presenting with sepsis in Africa, including CrAg testing and mycobacterial testing.
Author Contributions
Conceptualization, TBT, DRB and TF; Methodology and performing tests TBT, CRM and TW; writing—original draft preparation TBT, and TF; writing—review and editing DRB, TF, TL, CRM, HMO, and BOJ. All authors have read and agreed to the published version of the manuscript.
Funding
the Heinz Ansmann Foundation for HIV/AIDS research supports the first author of this study, TBT, and the Hirsch Institute for Tropical Medicine, with stipends. The U.S. National Institute of Allergy and Infectious Diseases support DRB for CrAg screening (U01AI125003). There was no influence of the funding organization on analysis or interpretation of the described data.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by Institutional Review Board of Arsi University(Ref.No. AU/HSC/120/6443/08) Ministry of Sciences and Higher Educations National Ethical Review Committee (Ref. No. MosHE/RD/141/2769/19 and 04/08/2019 date of approval).” for studies involving human.
Informed Consent Statement
Written informed consent has been obtained from the patients.
Data Availability Statement
Acknowledgments
The authors thank the study participants for their willing to be a part of this study. The authors do have a great appreciation of laboratory team of the Hirsch Institute of Tropical Medicine in Asella, Asella referral and teaching hospital, Adama Hospital Medical College, Ethiopia and the staff of the Institute of Medical Microbiology and Hospital Hygiene, Heinrich Heine University, Düsseldorf, Germany for supporting this work.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results”.
References
- Chaka, W.; Berger, C.; Huo, S.; Robertson, V.; Tachiona, C.; Magwenzi, M.; Magombei, T.; Mpamhanga, C.; Katzenstein, D.; Metcalfe, J. Presentation and outcome of suspected sepsis in a high-HIV burden, high antiretroviral coverage setting. Int. J. Infect. Dis. 2020, 96, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Rajasingham, R.; Smith, R.M.; Park, B.J.; Jarvis, J.N.; Govender, N.P.; Chiller, T.M.; Denning, D.W.; Loyse, A.; Boulware, D.R. Global burden of disease of HIV-associated cryptococcal meningitis: an updated analysis. Lancet Infect. Dis. 2017, 17, 873–881. [Google Scholar] [CrossRef] [PubMed]
- Muzazu, S.G.Y.; Assefa, D.G.; Phiri, C.; Getinet, T.; Solomon, S.; Yismaw, G.; Manyazewal, T. Prevalence of cryptococcal meningitis among people living with human immuno-deficiency virus and predictors of mortality in adults on induction therapy in Africa: A systematic review and meta-analysis. Front. Med. 2022, 9, 989265. [Google Scholar] [CrossRef] [PubMed]
-
Guidelines for Diagnosing Pamcdaa, Adolescents and Children Living with HIV; World Health Organization: Geneva, 2022.
- Medina, N.; Rodriguez-Tudela, J.L.; Pérez, J.C.; Mercado, D.; Bonilla, O.; Arathoon, E.; Alastruey-Izquierdo, A. Epidemiology and Mortality of Cryptococcal Disease in Guatemala: Two-Year Results of a Cryptococcal Antigen Screening Program. Microorganisms 2022, 10, 1388. [Google Scholar] [CrossRef] [PubMed]
- Meda, J.; Kalluvya, S.; Downs, J.A.; Chofle, A.A.; Seni, J.; Kidenya, B.; Fitzgerald, D.W.; Peck, R.N. Cryptococcal meningitis management in Tanzania with strict schedule of serial lumber punctures using intravenous tubing sets: an operational research study. JAIDS J. Acquir. Immune Defic. Syndr. 2014, 66, e31–e36. [Google Scholar] [CrossRef]
- Rhein, J.; Hullsiek, K.H.; E Evans, E.; Tugume, L.; Nuwagira, E.; Ssebambulidde, K.; Kiggundu, R.; Mpoza, E.; Musubire, A.K.; Bangdiwala, A.S.; et al. Detrimental Outcomes of Unmasking Cryptococcal Meningitis With Recent ART Initiation. Open Forum Infect. Dis. 2018, 5, ofy122. [Google Scholar] [CrossRef]
- Fuchs, A.; Tufa, T.B.; Hörner, J.; Hurissa, Z.; Nordmann, T.; Bosselmann, M.; Abdissa, S.; Sorsa, A.; Orth, H.M.; Jensen, B.-E.O.; et al. Clinical and microbiological characterization of sepsis and evaluation of sepsis scores. PLOS ONE 2021, 16, e0247646. [Google Scholar] [CrossRef]
- Seymour, C.W.; Liu, V.X.; Iwashyna, T.J.; Brunkhorst, F.M.; Rea, T.D.; Scherag, A.; Rubenfeld, G.; Kahn, J.M.; Shankar-Hari, M.; Singer, M.; Deutschman, C.S. Assessment of clinical criteria for sepsis: for the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). Jama 2016, 315, 762–774. [Google Scholar] [CrossRef]
- Letang, E.; Rakislova, N.; Martinez, M.J.; Hurtado, J.C.; Carrilho, C.; Bene, R.; Mandomando, I.; Quintó, L.; Nhampossa, T.; Chicamba, V.; et al. Minimally Invasive Tissue Sampling: A Tool to Guide Efforts to Reduce AIDS-Related Mortality in Resource-Limited Settings. Clin. Infect. Dis. 2021, 73, S343–S350. [Google Scholar] [CrossRef]
- Boulware, D.R.; Rolfes, M.A.; Rajasingham, R.; von Hohenberg, M.; Qin, Z.; Taseera, K.; Schutz, C.; Kwizera, R.; Butler, E.K.; Meintjes, G.; et al. Multisite Validation of Cryptococcal Antigen Lateral Flow Assay and Quantification by Laser Thermal Contrast. Emerg. Infect. Dis. 2014, 20, 45–53. [Google Scholar] [CrossRef]
- 12. WHO fungal priority pathogens list to guide research daphaG, 2: Organization, 2022.
- Tufa, T.B.; Mackenzie, C.R.; Orth, H.M.; Wienemann, T.; Nordmann, T.; Abdissa, S.; Hurissa, Z.; Schönfeld, A.; Bosselmann, M.; Häussinger, D.; et al. Prevalence and characterization of antimicrobial resistance among gram-negative bacteria isolated from febrile hospitalized patients in central Ethiopia. Antimicrob. Resist. Infect. Control. 2022, 11, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Taramasso, L.; Tatarelli, P.; Di Biagio, A. Bloodstream infections in HIV-infected patients. Virulence 2016, 7, 320–328. [Google Scholar] [CrossRef] [PubMed]
- Kiertiburanakul, S.; Watcharatipagorn, S.; Chongtrakool, P.; Santanirand, P. Epidemiology of Bloodstream Infections and Predictive Factors of Mortality among HIV-Infected Adult Patients in Thailand in the Era of Highly Active Antiretroviral Therapy. Jpn. J. Infect. Dis. 2012, 65, 28–32. [Google Scholar] [CrossRef]
- Beyene, T.; Zewde, A.G.; Balcha, A.; Hirpo, B.; Yitbarik, T.; Gebissa, T.; Rajasingham, R.; Boulware, D.R. Inadequacy of High-Dose Fluconazole Monotherapy Among Cerebrospinal Fluid Cryptococcal Antigen (CrAg)–Positive Human Immunodeficiency Virus-Infected Persons in an Ethiopian CrAg Screening Program. Clin. Infect. Dis. 2017, 65, 2126–2129. [Google Scholar] [CrossRef] [PubMed]
- Lakoh, S.; Rickman, H.; Sesay, M.; Kenneh, S.; Burke, R.; Baldeh, M.; Jiba, D.F.; Tejan, Y.S.; Boyle, S.; Koroma, C.; et al. Prevalence and mortality of cryptococcal disease in adults with advanced HIV in an urban tertiary hospital in Sierra Leone: a prospective study. BMC Infect. Dis. 2020, 20, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Faini, D.; Kalinjuma, A.V.; Katende, A.; Mbwaji, G.; Mnzava, D.; Nyuri, A.; Glass, T.R.; Furrer, H.; Hatz, C.; Boulware, D.R.; et al. Laboratory-Reflex Cryptococcal Antigen Screening Is Associated With a Survival Benefit in Tanzania. Am. J. Ther. 2019, 80, 205–213. [Google Scholar] [CrossRef]
- Zhao, T.; Xu, X.-L.; Nie, J.-M.; Chen, X.-H.; Jiang, Z.-S.; Liu, S.-Q.; Yang, T.-T.; Yang, X.; Sun, F.; Lu, Y.-Q.; et al. Establishment of a novel scoring model for mortality risk prediction in HIV-infected patients with cryptococcal meningitis. BMC Infect. Dis. 2021, 21, 1–10. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, R.; Shen, Y.; Liu, L.; Qi, T.; Wang, Z.; Mehraj, V.; Routy, J.; Lu, H. Serum cryptococcal antigen titre as a diagnostic tool and a predictor of mortality in HIV-infected patients with cryptococcal meningitis. HIV Med. 2018, 20, 69–73. [Google Scholar] [CrossRef]
- Lawrence, D.S.; Muthoga, C.; Meya, D.B.; Tugume, L.; Williams, D.; Rajasingham, R.; Boulware, D.R.; Mwandumba, H.C.; Moyo, M.; Dziwani, E.N.; et al. Cost-effectiveness of single, high-dose, liposomal amphotericin regimen for HIV-associated cryptococcal meningitis in five countries in sub-Saharan Africa: an economic analysis of the AMBITION-cm trial. Lancet Glob. Heal. 2022, 10, e1845–e1854. [Google Scholar] [CrossRef] [PubMed]
- Fisher, M.C.; Alastruey-Izquierdo, A.; Berman, J.; Bicanic, T.; Bignell, E.M.; Bowyer, P.; Bromley, M.; Brüggemann, R.; Garber, G.; Cornely, O.A.; et al. Tackling the emerging threat of antifungal resistance to human health. Nat. Rev. Microbiol. 2022, 20, 557–571. [Google Scholar] [CrossRef]
- Gerlach, E.S.; Altamirano, S.; Yoder, J.M.; Luggya, T.S.; Akampurira, A.; Meya, D.B.; Boulware, D.R.; Rhein, J.; Nielsen, K. ATI-2307 exhibits equivalent antifungal activity in Cryptococcus neoformans clinical isolates with High and low Fluconazole IC50. Front. Cell. Infect. Microbiol. 2021, 11, 695240. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).