Introduction
Aquaculture is growing rapidly as a food-producing sector globally and flourishing day by day (Hodar et al., 2020; Syed et al., 2022; Habib et al., 2020; Mengo et al., 2023).It has been introduced in various regions of developing nations, including Africa and Asia, to provide local rural communities with the opportunity to raise their standard of living and a means of escaping poverty (Olaganathan & Kar Mun, 2017), generating household income (Moisa et al., 2022: Tran et al., 2023). Fish meal makes up between 50 and 70 percent of the total material in fish feed (Jannathulla et al., 2019); it is highly considered a feed protein source since it has an excellent composition of amino acids and is easy to digest (Olsen & Hasan, 2012). Moreover, the feed cost is 60% to 70% of an aquaculture farm’s total operating expenses out of all input costs. In contrast, the decreasing fish catches (Macusi et al., 2022) and consistent growth in fishmeal consumption create a gloomy future for the aquaculture industry, but not if there is a paradigm shift toward the utilization of non-fish components for fish feed production. Over the next 20 years, aquaculture is projected to expand due to increased demand for fishmeal. Fish oil (FO) and fish meal (FM) could increase pressure on the diminishing stocks of marine fish production (Aladetohun & Sogbesan, 2013; Oliva et al., 2022). In recent years, fishmeal prices have climbed more than twofold globally (FAO, 2013; Lin et al., 2022; Bansemer et al., 2023). In Asia alone, fishmeal consumption for Nile tilapia climbed from 0.8 million tons to 1.7 million tons during the same period, while fish feed output increased from 40% in 2000 to 60% in 2008 (Tacon & Metian, 2008). According to FAO (2001), lowering the amount of fish meal and fish oil in feeds is a huge step toward mitigating the pressure on global marine resource scarcity. Substituting fishmeal at the farm level could reduce expenses associated with production (De Francesco et al., 2007). The essential amino acid compositions of alternative protein sources for fish are not comparable with that of fishmeal; however, mixing several alternative protein sources with various limiting amino acids such as lysine, methionine, threonine, and tryptophan has been strongly suggested (Ogunji & Wirth, 2001). Fish meal (FM) is obviously inadequate to support the huge demand for fish feed with rising aquaculture production, and use of feed has to be declined (Wang et al., 2023; FAO 2022). Therefore, it’s an urgent need to find an alternative protein source to replace FM (Wang et al., 2023; Galkanda et al., 2020). Several sources of plant protein, single-cell protein, and animal protein have partially or entirely replaced the more expensive fishmeal. Animal protein sources have traditionally been regarded as the best alternative to replace fishmeal in the formulation of fish meals due to their higher protein and fat content, superior essential amino acids, and excellent palatability. (Yigit et al., 2006). On the other hand, plant ingredients that contain high protein content, high digestibility of crude protein, and low anti-nutritional components can replace fishmeal as a substitute alternative protein source for fish (Dersjant-Li, 2021). Plant proteins are almost similar to fishmeal in protein content and amino acid digestibility. However, their amino acid profile does not match the amino acid requirement of some fish species as fishmeal does. For example, methionine is the limiting amino acid in soybean meal (SBM), while corn gluten meal is deficient in lysine. Wheat gluten meal is limited in lysine and arginine (Ogello et al., 2014). According to De Francesco et al. (2007), the impact of plant protein as a partial replacement for fishmeal shows contrasting results on the chemical composition of muscles. Soy products, including soybean meal and soy protein concentrate (SPC), have been researched as potential protein substitutes for fishmeal (Trejo-Escamilla et al., 2017) because of their high digestibility, high protein content, well-balanced amino acid, low price, and steady supply, soybean meals are widely used as the most effective substitute for fish meal in feeds for aquaculture (Zhou et al., 2005). In addition, soybean concentrate can replace fishmeal for up to 40% to 100% (Dersjant-Li, 2021). Another plant protein that is used for fishmeal replacement is seaweeds; it contains a good level of minerals, vitamins, carotenoid pigments, bioactive compounds, fatty acids, and amino acids, which are highly required components in fish feed making (Qiu et al., 2018). According to some studies, the partial substitution of fishmeal by seaweeds can positively affect growth, feed utilization, body composition, and resistance to stress and diseases (Guroy et al., 2013). Methionine is an amino acid found in large quantities in some seaweed species, such as Palmaria palmata. However, it is found in much lower amounts in other species of brown seaweed, such as Laminaria digitata (Fleurence, 2004). Cocoa bean shell has also been reported to contain 13.2% to 17.7% crude protein and 13.0% to 16.1% of fiber (Chung et al., 2003). However, theobromine content of 1.3% in cocoa shell limits its usage for feeding, which is a downside as a replacement for fishmeal. Another protein used as a replacement for fishmeal is cacao pod husk (CPH); it is a by-product during cacao production. Using this product as a replacement for fishmeal will eliminate environmental waste since it can be obtained at little to no cost to aquaculture farmers (Ashade, 2010). Navya (2007), studied the growth of Nile tilapia (Oreochromis niloticus) fingerlings fed varied levels of cocoa pod husk diets and discovered that fish weight gains and specific growth rates are lower with above 10% inclusion level than those of the control animals fed fishmeal-based diets. The high fibre content of the cocoa pod husk was the reason for the decrease in growth. Cocoa pod husk also has anti-nutritional factors such as theobromine; however, according to a study by (Ocran (2020), the negative effect of anti-nutritional factors can be eliminated through fermentation. Following the study of Ogello et al. (2014), the main terrestrial by-product meals used as a replacement for fishmeal are blood, insect, feather, and meat and bone. Regardless of its high crude protein content, these alternative proteins commonly lack amino acids, which limits the growth of the aquaculture species. One of the additional animal protein sources that can be utilized to replace fishmeal is poultry by-products. It was thought to be a significant replacement for fishmeal, particularly in rainbow trout since it has a similar composition of amino acids to fishmeal (FM) (Bureau et al., 2000). On the other hand, maggots are usually considered not to have any economic value. However, according to Ajani et al. (2004), it has the potential as a good source of animal protein in fish diets. Adesulu and Mustapha (2000) reported that some essential amino acids, including cystine, histidine, phenylalanine, tryptophan, and tyrosine are present in maggot meal and are higher than that in fishmeal and soybean meal. Utilizing maggot meal as a source of protein for a fish diet is a good way of reducing the cost of waste disposal in the poultry industry, which can help generate additional income for the fish and poultry industry. This review paper was performed to help the aquaculture sector reduce the cost of aquafeeds by identifying sources of substitute protein that can be used in place of fishmeal and to assess the progress in feed development that can be an alternative choice to existing commercial feeds in the aquaculture industry in the Davao region, Philippines.
Methodology
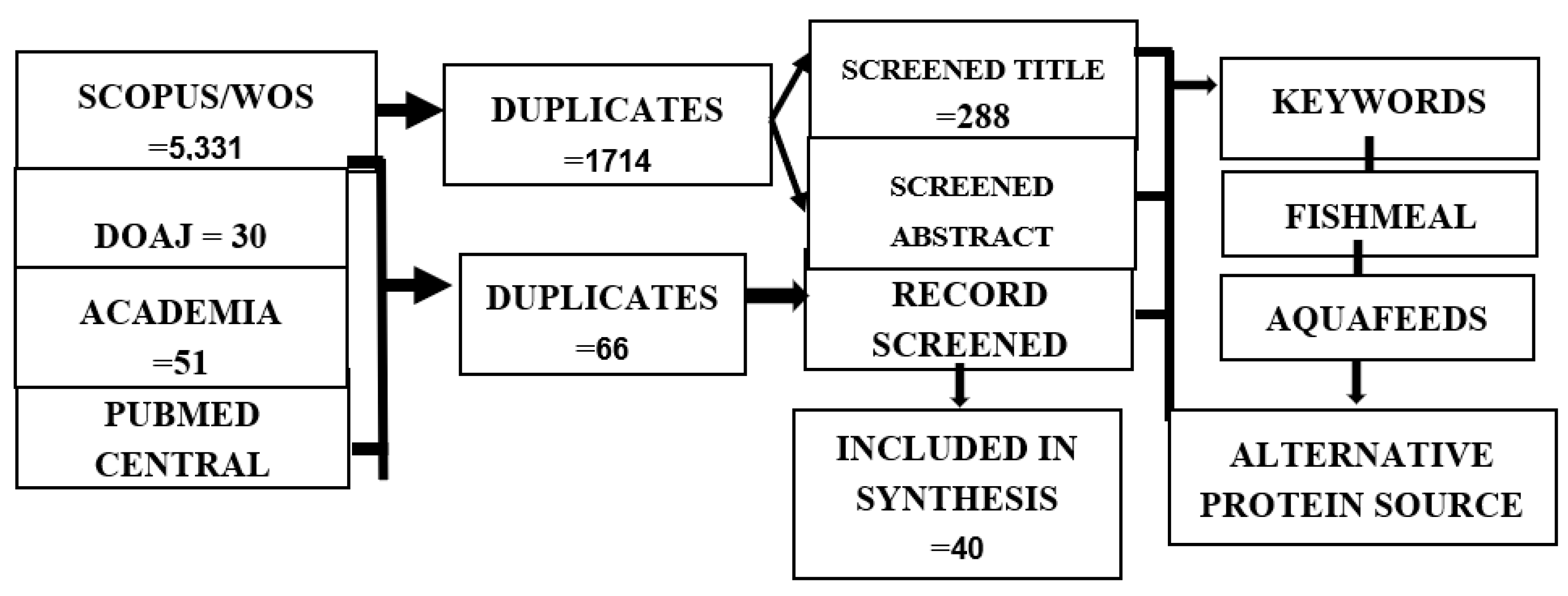
This review paper followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) to address and evaluate the objectives of the study. In order to further understand the possible alternative protein sources for fishmeal and to assess its effect on the growth of cultured species, a comprehensive literature review using the methods used in an earlier paper, e.g., Macusi et al., 2022 and Page et al., 2021, was conducted. The literature searched in this review was limited to the year 2000 and the present on four citation databases: Scopus/WOS, DOAJ (Directory of Open Access Journals), Academia, and PubMed Central. Data searching was first used to locate the records, and then duplicates were eliminated. The articles that did not match the eligibility requirements were then removed throughout the subsequent screening and data extraction process. By looking through t abstracts or contents, the remaining articles were evaluated to check relevance to the topic of interest to meet the qualifying requirements. The literature reviewed was chosen based on inclusion criteria in the final phase based on the publications that passed the eligibility evaluation. The terms “fishmeal,” “alternative protein source,” and “aquafeeds” were used to make sure the search focused only on the literature related to the study.
Exclusion and inclusion criteria were applied to screen the articles used in this review. A total of 5,331 was found in the Scopus database by using the keywords above (see
Figure 1); it was then reduced to 1,714 by eliminating the duplicate articles found in the database. After removing the duplicates, articles were again screened to 288 by removing the articles that did not match the criteria for inclusion based on the title of the articles. Following the inclusion of criteria based on the abstract, 162 articles were record screened on the Scopus/WOS database. The same method was used on the open-access databases: DOAJ, PubMed, and Academia. One hundred twenty-four (124) articles were found in the three databases; 66 duplicate articles were removed, with fifty-eight (58) articles recorded, screened, and remaining based on the inclusion criteria. A total of forty (40) articles were included in the review on the basis that they passed the eligibility assessment.
RESULTS
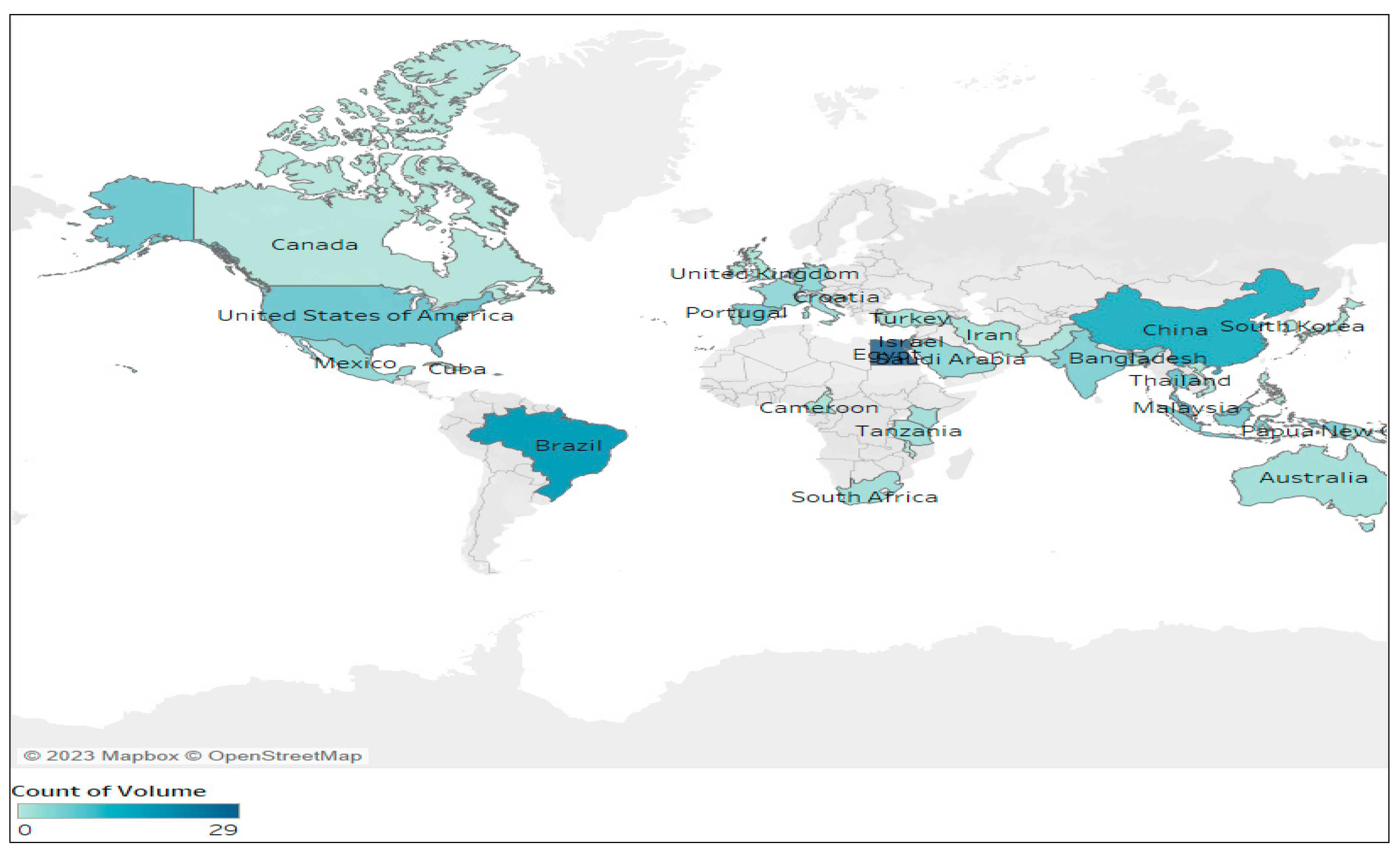
Figure 2 shows the distribution of scientific production of fishmeal for the aquaculture industry; Egypt (18%), Brazil (12%), China (9%), Malaysia (6%), Thailand (5 %), and the USA (5%) top the list. While
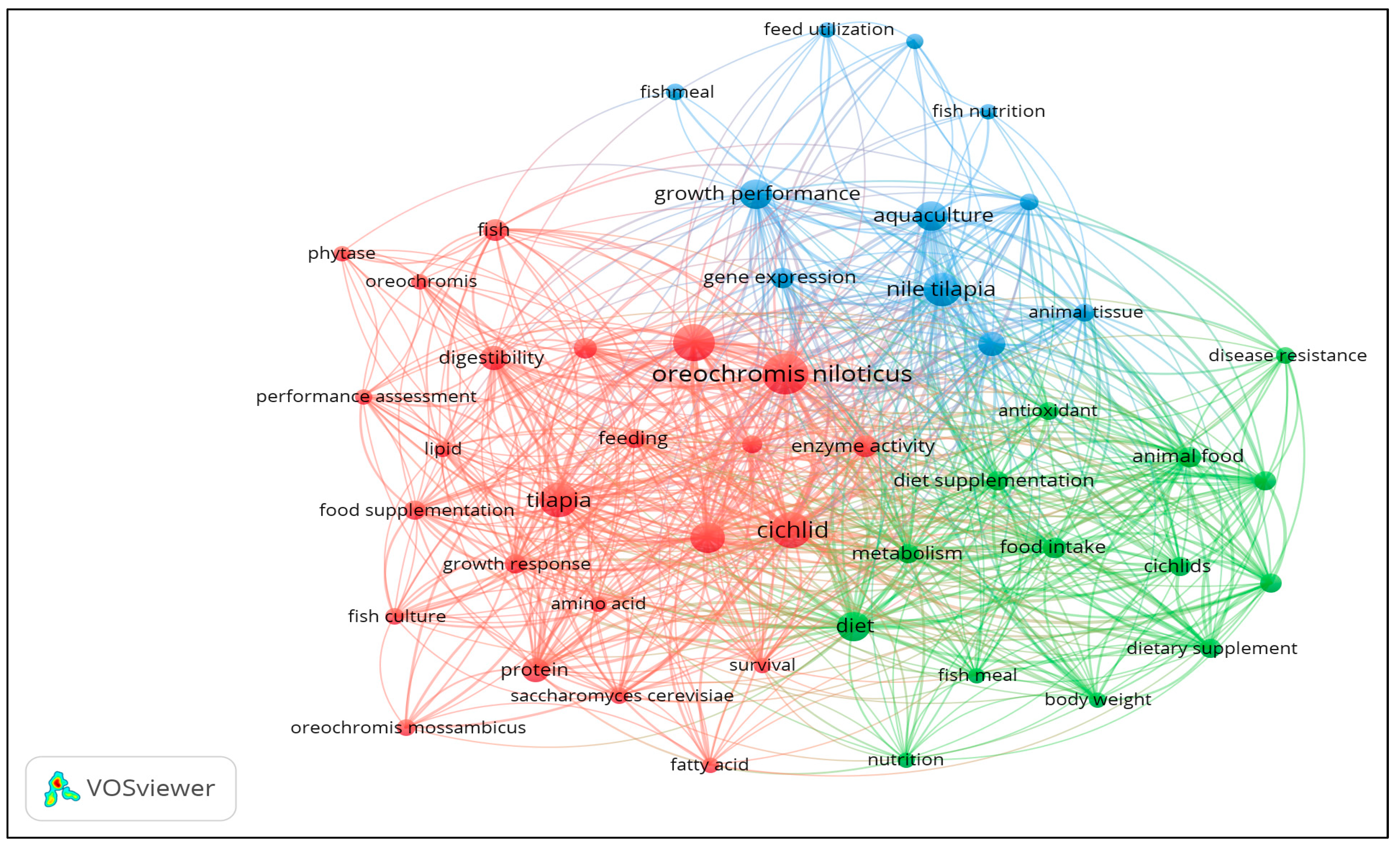
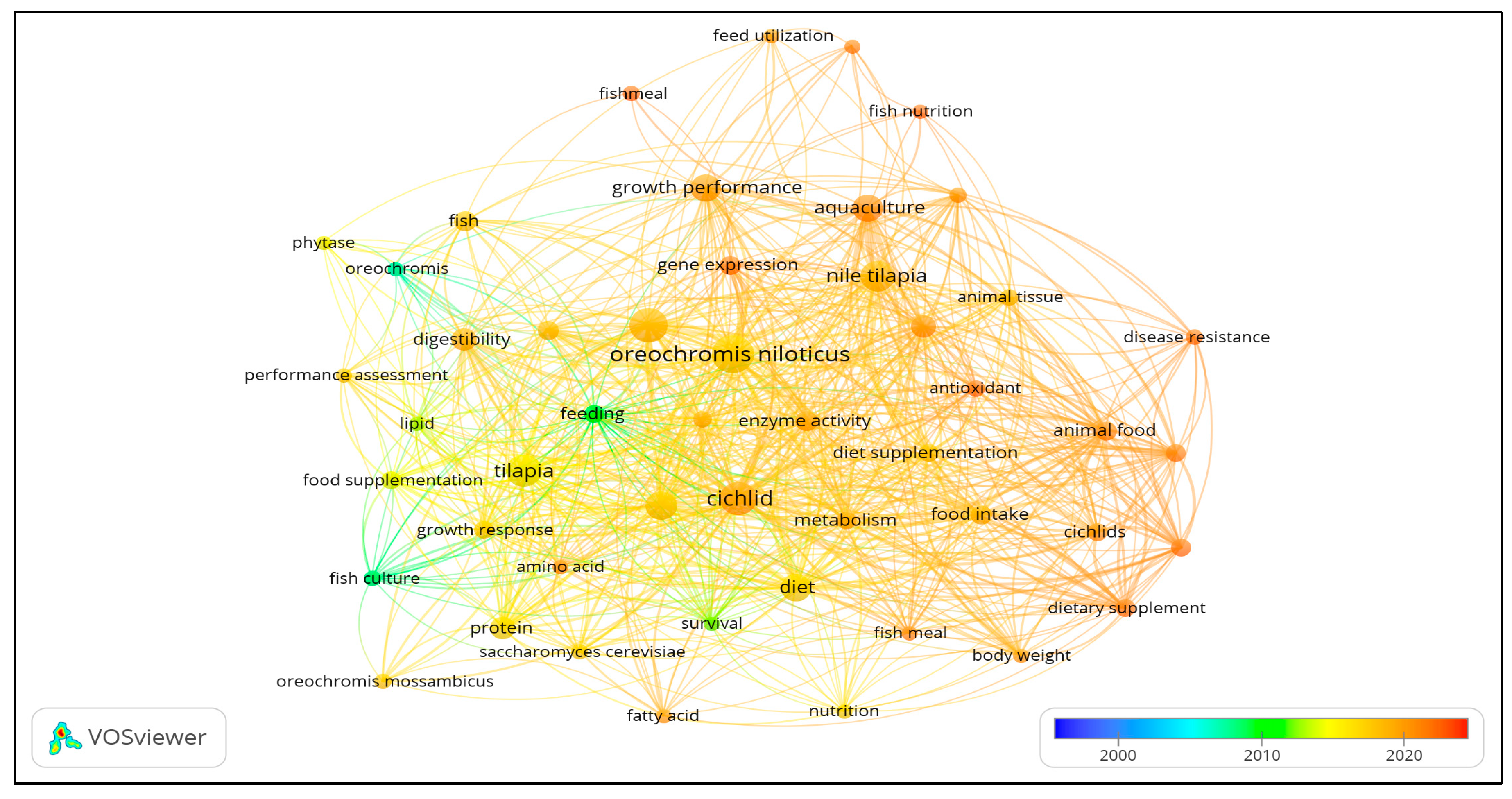
Figure 3 presents the co-occurrence map of authors’ keywords, while
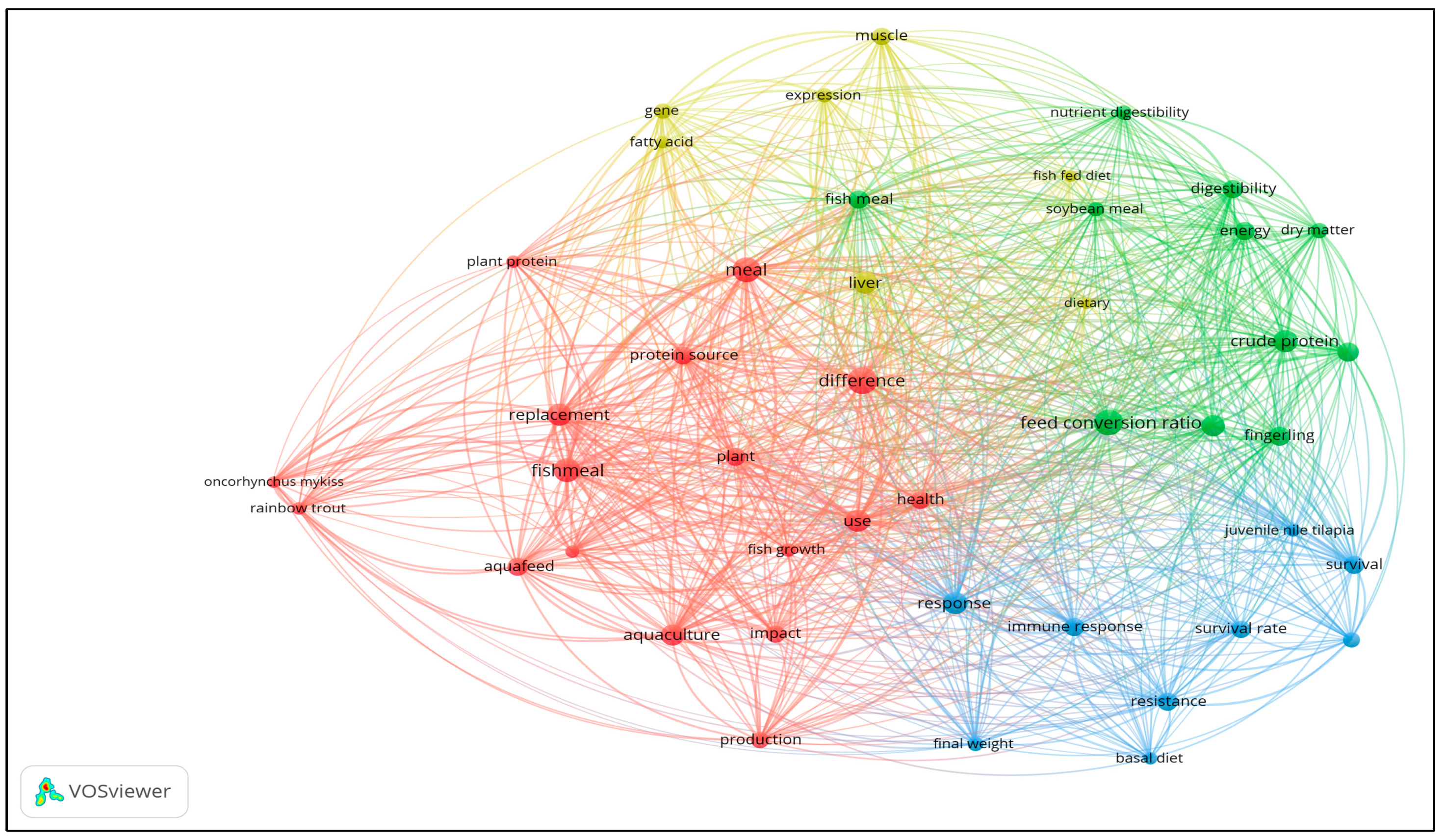
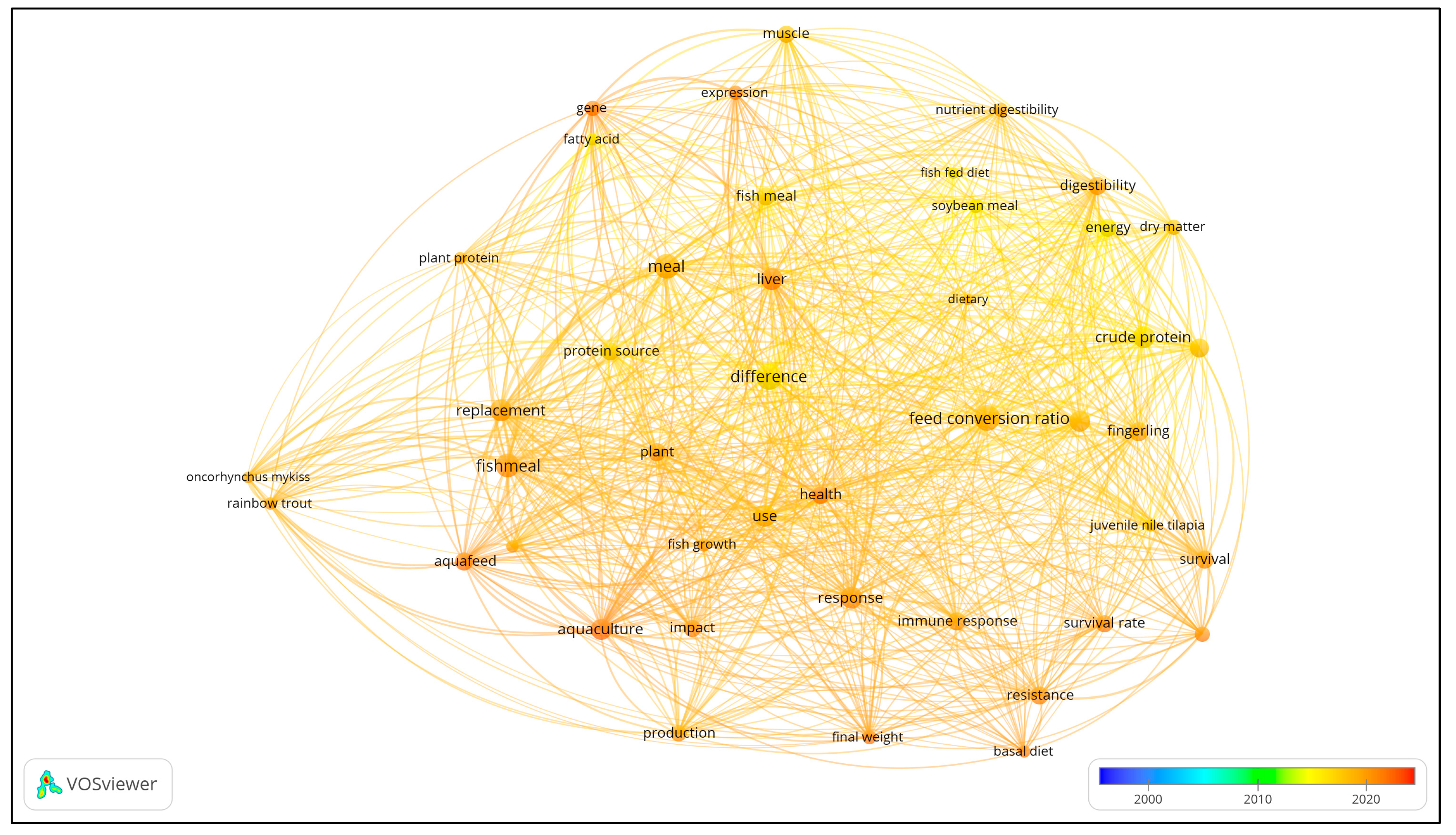
Figure 4 presents the co-occurrence map for titles and abstracts. Forty-nine (49) author and index keywords and 44 text data from the articles’ abstracts and titles were extracted and visualized in the co-occurrence map.
Table 1 presents the co-occurrence classification for the index and author keywords in terms of links, total link strengths, and occurrences. ‘
Oreochromis niloticus,’ ‘growth, ‘Cichlid,’ ‘tilapia,’ and ‘Nile tilapia’ are keywords with the highest occurrences. In contrast ‘Cichlid,’ ‘
Oreochromis niloticus,’ ‘diet,’ ‘growth rate,’ and ‘growth’ are the keywords with the highest total link strengths. The cluster analysis of keywords shows three clusters, as presented in
Table 1. Under cluster 1, Cichlid, growth, growth rate, and
Oreochromis niloticus have the highest link strength referring to the cultured Nile tilapia (
Oreochromis niloticus). Under cluster 2, animal feed, animal food, diet, and food intake have the highest total link strength, which refers to the feed diet. For cluster 3, aquaculture, growth performance, immune response, and Nile tilapia have the highest total link strength, which refers to the effect of the diet on the growth of tilapia or cultured species.
In terms of abstract and keywords (see
Figure 4), the most common words based on the cluster analysis revealed four clusters. Words with the highest occurrences include ‘difference,’ ‘feed conversion ratio,’ ‘meal,’ ‘fish meal,’ ‘response,’ and ‘crude protein.’
Table 2 presents the occurrence classification of texts from abstracts and titles in terms of links, total link strength, and occurrences. Under cluster 1, words with the highest total link strength include meal, fishmeal, difference, and replacement which refer to the feed or diet used in the study. Under cluster 2, crude protein, digestibility, feed conversion ratio, and fish meal have the highest total link strength, which has reference to the performance of the diet or feeds used. For cluster 3, immune response, resistance, response, and survival have the highest total link strength, again with reference to the effect of the diet on the growth performance of the fish. Under cluster 4, the liver, gene, muscle, and expression have the highest total link strengths, which refer to the main organ examined in fish to check diet toxicity and immune response.
Figure 4 and
Figure 5 show an overlay visualization of frequently used terms from the the fishmeal studies’ keywords, titles, and abstracts from 2000 to 2022. These present the trend in fishmeal research across the globe during the period. The recent studies are quite varied and include growth performance, gene expression, disease resistance, dietary supplement, replacement, resistance, response, health, and survival.
Table 3 below summarizes the alternative proteins that can be used as a fishmeal replacement in aquafeed for culturing fish. Of all these replacements, blood meal can replace the fishmeal 100% with positive growth on the fish. In contrast, poultry-based by-products, feather meal, and bone meal can replace it with 75-100% effectivity, similar to the fishmeal diet. Seaweed can replace about 10% of the fishmeal diet in tests, while soybean meal can replace about 25% of the fishmeal diet, and insect-based diets can replace 50% of the fishmeal diet.
DISCUSSION
Growth effect of alternative protein for fishmeal
From the result of this review, a variety of alternative proteins is used to replace fishmeal in fish diets, including animal and plant-based meals. Growth performance, as determined by final weight and specific growth rate, revealed that extra protein could not be utilized efficiently for growth because growth energy was required to deaminate and extract ingested excess amino acids (Luthada-Raswiswi et al., 2021). In the study conducted by Zlaugotne et al. (2022), feed ingredients impact fish and the environment since it is necessary to evaluate feed materials and whether or not an alternative is possible would be more effective and have less impact on the environment. The alternative fish feed must have high nutritional content and quality and high protein content, adequate amino acids, and digestibility and palatability. In addition, alternative fish feed should have insoluble carbohydrates, fiber, and heavy metals that need to be low because it affects the fish growth process and low feed conversion ratio; feed costs must be economically justified and feed production (Nagappan et al., 2021).
Alternative protein sources benefit, implications to food security, and their limits
The most pressing problem facing the aquaculture industry remains the feed cost, and there is considerable pressure on feed companies to develop less expensive formulations that maintain efficient growth at a lower cost per unit gain (Hardy, 2010). To meet this goal, feed companies should lower the fishmeal levels further. Replacing the usual fishmeal with alternative protein sources can significantly benefit the fishing industry since these protein sources are far less expensive than fishmeal. On the other hand, alternative protein allows flexibility in feed formulations when feed ingredients fluctuate, which can also benefit the fishing industry greatly. According to Mulumpwa (2018), a fish product to be adequately available on the market may rely on how alternative protein is incorporated into fish feeds. Using alternative proteins as a replacement for fishmeal has the potential for increasing fish production, hence improving food security. However, challenges to be resolved are food acceptance, food safety issues, and legislation, which can be dealt with by the coordination of government and industry. Moreover, lack of support, mainly, financial from the government could perhaps be one of the limiting factors for the adoption of alternative protein as a replacement for fishmeal.
Adoption viability of alternative protein in the aquaculture industry
The spread of aquaculture production and intensification requires the search for high-quality, new efficient feed ingredients with low cost and sustainable importance (Ashour et al., 2021). Fishmeal, the most expensive component in aquatic diets, is considered one of the most critical challenges in the development of the aquaculture industry. Given the projected increase in production of these species and associated aquafeed demand, substituting fishmeal with alternative protein sources in these diets will considerably reduce the total quantity of fishmeal used (Hua et al., 2019). Significant gains in aquaculture production to supply additional protein, especially for freshwater fish, may also be made by combining alternative proteins or plant-based meals and animal-based meals to meet the needed requirements for fish growth (Holdt & Edwards, 2014). While detailed knowledge is required to balance multiple species, these systems have the added benefits of nutrient bioremediation and positive consumer perception (Park et al., 2018). Given these challenges, there is enormous potential for technological improvements to consistently produce high-quality alternative protein products with enhanced nutritional profiles. Some protein sources, such as fish byproducts and insect meals, are viable and promising alternatives to conventional fishmeal. Feed supplements can also be used to balance the nutrient composition of the feeds and functional ingredients can be used to facilitate the replacement of fishmeal with alternative ingredients. Furthermore, using multiple protein sources allows flexibility in feed formulations when ingredient prices fluctuate, as feed manufacturers often use cost as a determinant in selecting ingredients (Pelletier et al., 2018). Therefore, developing and optimizing alternative protein sources for aquafeeds will ensure a socially and environmentally sustainable future for the aquaculture industry.
Summary and conclusion
This review paper was performed to inform the aquaculture sector to reduce the cost of aquafeeds by identifying sources of substitute protein that can be used in place of fishmeal and to assess the progress in feed development that can be an alternative choice to existing commercial feeds in the aquaculture industry in the Davao region, Philippines.
The literature review followed the Preferred Reporting Items for Systematic Review and Meta-Analyses (PRISMA) using the SCOPUS/WOS (Web of Science), DOAJ (Directory of Open Access Journals), Academia, and Pubmed Central databases with the following search terms: “fishmeal”, “alternative protein source” and “aquafeeds”. This gave a result of 5,331 journal articles from the SCOPUS/WOS databases and 1,714 of which were removed for duplicates. Finally, the titles and abstracts of the articles were screened, giving a total of 162 articles that were used in the study. In comparison, additional 58 articles came from the open DOAJ, Academia and Pubmed databases. Moreover, lack of support, mainly, financial from the government could perhaps be one of the limiting factors for the adoption of alternative protein as a replacement for fishmeal.
Author Contributions
Conceptualization, E.D.M., M.A.C., and A.C.S.; methodology, E.D.M., E.Q.B., M.A.C., A.C.S., A.H., N.F., and M.D.S; software, E.Q.B., M.A.C.; validation, E.D.M, M.A.C., A.C.S., A.H., N.F., M.D.S.; formal analysis, E.D.M., M.A.C., A.C.S., E.Q.B.; investigation, E.D.M, M.A.C., E.Q.B., N.F., A.H., and M.D.S.; resources, E.D.M., A.C.S. ; data curation, E.D.M, and M.A.C. writing—original draft preparation, M.A.C..; writing—review and editing, E.D.M, M.A.C., A.C.S., A.H., N.F., M.D.S.; visualization, E.Q.B., M.A.C.; supervision, E.D.M, A.C.S., N.F., and M.D.S.; project administration, E.D.M.; funding acquisition, E.D.M.. All authors have read and agreed to the publication of this manuscript.
Funding
The first author received funding from the Department of Agriculture, Philippine Rural Development Project (DA-PRDP) and the Department of Science and Technology Region 11 (DOST-XI) with the study entitled: “Enhancing food security, social inclusion, and sustainability in the milkfish aquaculture through the use of indigenous raw materials as feed components”.
Data Availability Statement
The data can be requested from the authors
Conflicts of Interest
The authors declare no conflict of interest
References
- Aladetohun, N.F.; Sogbesan, O.A. Utilization of blood meal as a protein ingredient from animal waste product in the diet of Oreochromis niloticus. Int. J. Fish. Aquac. 2013, 5, 234–237. [Google Scholar]
- Al-asgah, N.A.; Younis, E.S.M.; Abdel-Warith, A.W.A.; Shamlol, F.S. Evaluatiom of red seaweed Gracilaria arcuata as dietary ingredient in African catfish, Clarias gariepinus. Saudi J. Biol. Sci. 2016, 23, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Ashade, O.O.; Osineye, O.M. Effect of replacing maize with cocoa pod husk in the nutrition of Oriochromis niloticus. Fish. Soc. Niger. 2010. [Google Scholar]
- Ashour, M.; Abo-Taleb, H.A.; Hassan, A.K.M.; Abdelzaher, O.F.; Mabrouk, M.M.; Elokaby, M.A.; Alzahrani, O.M.; Mahmoud, S.F.; El-feky, M.M.; Shaban, W.M.; et al. Valorization Use of Amphipod Meal, Gammarus pulex, as a Fishmeal Substitute on Growth Performance, Feed Utilization, Histological and Histometric Indices of the Gut, and Economic Revenue of Grey Mullet. J. Mar. Sci. Eng. 2021, 9, 1336. [Google Scholar] [CrossRef]
- Bansemer, M.S.; Salini, M.J.; Nankervis, L.; Stone, D.A. Reducing dietary wild derived fishmeal inclusion levels in production diets for large yellowtail kingfish (Seriola lalandi). Aquaculture 2023, 572, 739487. [Google Scholar] [CrossRef]
- Bureau, D.P.; Harris, A.M.; Bevan, D.J.; Simmons, L.A.; Azeved, P.A.; Cho, C.Y. Feather meals and meat and bone meals from different origins as protein sources in rainbow trout (Oncorhynchus mykiss) diets. Aquaculture 2000, 181, 281–291. [Google Scholar] [CrossRef]
- Chung, B.Y.; Liyama, K.; Han, K.W. Compositional Characterization of Cacao (Theobroma cacao L.) Hull. Agric. Chem. Biotechnol. 2003, 1, 1216. [Google Scholar]
- Dersjant-Li, Y. The use of soya in aquafeeds. Oilseeds Focus 2021, 7, 29–31. [Google Scholar]
- De Fracesco, M.; Parisi, G.; Perez-Sanchez, J.; Gomez-Requeni, P.; Medale, F.; Kaushik, M.; Mecatti, M.; Poli, B.M. Effect of high-level fish meal replacement by plant proteins in gilthead sea bream (Sparus aurata) on growth and body/fillet quality traits. Aquac. Nutr. 2007, 13, 361–372. [Google Scholar] [CrossRef]
- FAO, Report of the Conference on Aquaculture in the Third Millennium. Bangkok, Thailand, 20-25 February 2000. FAO Fish. Rep. No. 661, 2001, 92.
- FAO, Food and Agriculture Organization. The State of Food Insecurity in the World. Mult. Dimens. Food Secur. 2013, 56.
- FAO. FAO yearbooks of fisheries statistics, food agriculture organization of the United Nations, Rome. In: Retrived from http://www. fao. org/faostat/en/# home. 2022.
- Fleurence, J. Seaweed proteins. In Proteins in Food Processing; Yada, R.Y., Ed.; Woodhead Publishing: Cambridge, UK, 2004; pp. 197–213. [Google Scholar]
- Galkanda-Arachchige, H.S.; Wilson, A.E.; Davis, D.A. Success of fishmeal replacement through poultry by-product meal in aquaculture feed formulations: A meta-analysis. Rev. Aquac. 2020, 12, 1624–1636. [Google Scholar] [CrossRef]
- Guroy, B.; Ergun, S.; Merrifield, D.L. Effect of autoclaved Ulva meal on growth performance, nutrient utilization and fatty acid profile of rainbow trout, Oncorhynchus Mykiss. Aquac. Int. 2013, 21, 605–615. [Google Scholar] [CrossRef]
- Habib, A.; Rahman, M.; Sarker, M.; Musa, N.; Hossain, M.; Shahreza, M.A. Breeding performance of riverine Rohu (Labeo rohita) and growth performance of F1 progenies reared in hapas. J. Sustain Sci. Manag 2020, 15, 24–32. [Google Scholar]
- Hardy, R.W. Utilization of plant proteins in fish diets: Effects of global demand and supplies of fishmeal. Aquac. Res. 2010, 41, 770–776. [Google Scholar] [CrossRef]
- Hodar, A.R.; Vasava, R.J.; Mahayadiya, D.R.; Joshi, N.H. Fish meal and fish oil replacement for aqua feed formulation by using alternative sources: A review. In J. Exp. Zool. India; 2020; Volume 23, pp. 13–21. [Google Scholar]
- Holdt, S.L.; Edwards, M.D. Cost-effective IMTA: A comparison of the production efficiencies of mussels and seaweed. J. Appl. Phycol. 2014, 26, 933–945. [Google Scholar] [CrossRef]
- Hua, K.; Cobcroft, J.M.; Cole, A.; Condon, K.; Jerry, D.R.; Mangott, A.; Praeger, C.; Vucko, M.J.; Zeng, C.; Zenger, K.; et al. The future of aquatic protein: implications for protein sources in aquaculture diets. One Earth 2019, 1, 316–329. [Google Scholar] [CrossRef]
- Lin, Z.; Yoshikawa, S.; Hamasaki, M.; Koyama, T.; Kikuchi, K.; Hosoya, S. Effects of low fishmeal diets on growth performance, blood chemical composition, parasite resistance, and gene expression in the tiger pufferfish, Takifugu rubripes. Aquaculture 2022, 560, 738484. [Google Scholar] [CrossRef]
- Liu, H; Jin, J; Zhu, X; Han, D; Yang, Y; Xie, S. Effect of substitution of dietary fish meal by soybean meal on different sizes of gibel carp (Carassius auratus gibelio): Digestive enzyme gene expressions and activities, and intestinal and hepatic histology. Aquac. Nutr. 2017, 23, 129–147. [Google Scholar] [CrossRef]
- Lu, F.; Haga, Y.; Satoh, S. Effects of replacing fish meal with rendered animal protein and plant protein sources on growth response, biological indices, and amino acid availability for rainbow trout Oncorhynchus mykiss. Fish. Sci. 2015, 81, 95–105. [Google Scholar] [CrossRef]
- Luthada-Raswiswi, R.; Mukaratirwa, S.; O’Brien., G. Animal protein sources as a substitute for fishmeal in aquaculture diets: A systematic review and meta-analysis. Appl. Sci. 2021, 11, 3854. [Google Scholar] [CrossRef]
- Macusi, E.D.; Liguez, C.G.O.; Macusi, E.S.; Liguez, A.K.O.; Digal, L.N. Factors that influence small-scale Fishers’ readiness to exit a declining fishery in Davao Gulf, Philippines. Ocean Coast. Manag. 2022, 230, 106378. [Google Scholar] [CrossRef]
- Macusi, E.D.; Estor, D.E.P.; Borazon, E.Q.; Clapano, M.B.; Santos, M.D. Environmental and Socioeconomic Impacts of Shrimp Farming in the Philippines: A Critical Analysis Using PRISMA. Sustainability 2022, 14, 2977. [Google Scholar] [CrossRef]
- Melenchon, F.; de Mercado, E.; Pula, H.J.; Cardenete, G.; Barroso, F.G.; Fabrikov, D.; Lourenco, H.M.; Pessoa, M.F.; Lagos, L.; Weththasinghe, P.; et al. Fishmeal Dietary Replacement Up to 50%: A Comparative Study of Two Insect Meals for Rainbow Trout (Oncorhynchus mykiss). Animals 2022, 12, 179. [Google Scholar] [CrossRef] [PubMed]
- Mengo, E.; Grilli, G.; Murray, J.M.; Capuzzo, E.; Eisma-Osorio, R.-L.; Eisma-Osorio, R.-L.; Tan, A. Seaweed aquaculture through the lens of gender: Participation, roles, pay and empowerment in Bantayan, Philippines. J. Rural Stud. 2023, 100, 103025. [Google Scholar] [CrossRef]
- Moisa, M.B.; Tufa, C.A.; Gabissa, B.T.; Gurmessa, M.M.; Wedajo, Y.N.; Feyissa, M.E.; Gemeda, D.O. Integration of geospatial technologies with multi-criteria decision analysis for aquaculture land suitability evaluation: The case of Fincha’a River Sub-basin, Western Ethiopia. J. Agric. Food Res. 2022, 10, 100448. [Google Scholar] [CrossRef]
- Mulumpwa, M. The potential of insect meal in improving food security in Malawi: An alternative of soybean and fishmeal in livestock feed. J. Insects Food Feed 2018, 4, 301–312. [Google Scholar] [CrossRef]
- Nagappan, S.; Das, P.; AbdulQuaddir, M.; Thaher, M.; Khan, S.; Mahata, C.; Al-Jabri, H.; Vatland, A.K.; Kumar, G. Potential of microalgae as a sustainable feed ingredient for aquaculture. J. Biotechnol. 2021, 341, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Navya, R. Utilisation of cocoa pod husk as a feed ingredient for labeo rohita (hamilton) fingerlings. 2007. [Google Scholar]
- Ocran, J.N. Feed resources and policy options on feed for aquaculture production in Africa: A review. 2020, 8, 19–23. [Google Scholar]
- Ogello, E.O.; Munguti, J.M.; Sakakura, Y.; Hagiwara, A. Complete replacement of fish meal in the ddiet of Nile tilapia tilapia (Oreochromis niloticus L.) grow-out with alternative protein sources. A review. Int. J. Adv. Res. 2014, 2, 962–978. [Google Scholar]
- Ogunji, J.O.; Wirth, M. Alternative protein sources as substitutes for fishmeal in the diet of young tilapia Oreochromis niloticus (Linn.). The Israeli Journal of Aquaculture–Bamidgeh 2001, 53, 34–43. [Google Scholar] [CrossRef]
- Olaganathan, R.; Kar Mun, A.T. Impact of aquaculture on the livelihoods and food security of rural communities. Int. J. Fish. Aquat. Stud. 2017, 5, 278. [Google Scholar]
- Oliva-Teles, A.; Enes, P.; Couto, A.; Peres, H. Replacing fish meal and fish oil in industrial fish feeds. Feed Feed. Pract. Aquac. 2022, 231–268. [Google Scholar]
- Olmos, J.; Lopez, L.M.; Gorriño, A.; Galavis, M.A.; Mercado, V. Bacillus subtilis Effects on Growth Performance and Health Status of Totoaba macdonaldi Fed with High Levels of Soy Protein Concentrate. Animals 2022, 12, 3422. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffman, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Int. J. Surg. 2021, 88, 105906. [Google Scholar] [CrossRef] [PubMed]
- Park, M.; Shin, S.K.; Do, Y.H.; Yarish, C.; Kim, J.K. Application of open water integrated multi-trophic aquaculture to intensive monoculture: A review of the current status and challenges in Korea. Aquaculture 2018, 497, 174–183. [Google Scholar] [CrossRef]
- Pelletier, N.; Klinger, D.H.; Sims, N.A.; Yoshioka, J.R.; Kittinger, J.N. Nutritional attributes, substitutability, scalability, and environmental intensity of an illustrative subset of current and future protein sources for aquaculture feeds: Joint consideration of potential synergies and trade-offs. Environ. Sci. Technol. 2018, 52, 5532–5544. [Google Scholar] [CrossRef] [PubMed]
- Qiu, X.; Neori, A.; Kim, J.K.; Yarish, C.; Shpigel, M.; Guttman, L.; Ben Ezra, D.; Odintsov, V.; Davis, D.A. Evaluation of green seaweed Ulva sp. as a replacement of fish meal in plant-based practical diets for Pacific white shrimp, Litopenaeus vannamei. J. Appl. Phycol. 2018, 30, 1305–1305. [Google Scholar] [CrossRef]
- Silva, D.M.; Valente, L.M.P.; Sousa-Pinto, I.; Pereira, R.; Pires, M.A.; Seixas, F.; Rema, P. Evaluation of IMTA-produced seaweeds (Gracilaria, Porphyra, and Ulva) as dietary ingredients in Nile tilapia, Oreochromis niloticus L., juveniles. Effects on growth performance and gut histology. J. Appl. Phycol. 2015, 27, 1671–1680. [Google Scholar] [CrossRef]
- Syed, R.; Masood, Z.; Hassan, H.U.; Khan, W.; Mushtaq, S.; Ali, A.; Shah, M.I.A. Growth performance, haematological assessment and chemical composition of Nile tilapia, Oreochromis niloticus (Linnaeus, 1758) fed different levels of Aloe vera extract as feed additives in a closed aquaculture system. Saudi J. Biol. Sci. 2022, 29, 296–303. [Google Scholar] [CrossRef]
- Tran, T.Q.; Van Vu, H.; Nguyen, T.V. Aquaculture, household income and inequality in Vietnam’s coastal region. Mar. Policy 2023, 153, 105634. [Google Scholar] [CrossRef]
- Trejo-Escamilla, I.; Galaviz, M.A.; Flores-Ibarra, M.; Alvarez Gonzalez, C.A.; Lopez, L.M. Replacement of fishmeal by soya protein concentrate in the diets of Totoaba macdonaldi (Gilbert, 1890) juveniles: Effect on the growth performance, in vitro digestibility, digestive enzymes and the haematological and biochemistry parameters. Aquac. Res. 2017, 48, 4038–4057. [Google Scholar] [CrossRef]
- Waite, R.; Beveridge, M.; Brummett, R.; Castine, S.; Chaiyawannakam, N.; Kaushik, S.; Mungkung, R.; Nawapakpilai, S.U.; Phillips, M.I. Improving Productivity and Environmental Performance of Aquaculture; WorldFish: Penang, Malaysia, 2014. [Google Scholar]
- Wang, X.; Luo, H.; Zheng, Y.; Wang, D.; Wang, Y.; Zhang, W.; Shao, J. Effects of poultry by-product meal replacing fish meal on growth performance, feed utilization, intestinal morphology and microbiota communities in juvenile large yellow croaker (Larimichthys crocea). Aquac. Rep. 2023, 30, 101547. [Google Scholar] [CrossRef]
- Yigit, M.; Erdem, M.; Koshio, S.; Ergun, S.; Turker, A.; Karaali, B. Substituting fish meal with poultry by-product meal in diets for black Sea turbot Psetta maeotica. Aquac. Nutr. 2006, 12, 340–347. [Google Scholar] [CrossRef]
- Zhou, Q.C.; Mai, K.S.; Tan, B.P.; Liu, Y.J. Partial replacement of fishmeal by soybean meal in diets for juvenile cobia (Rachycentron canadum). Aquac. Nutr. 2005, 11, 175–182. [Google Scholar] [CrossRef]
- Zlaugotne, B.; Pubule, J.; Blumberga, D. Advantages and disadvantages of using more sustainable ingredients in fish feed. Heliyon 2022, 8, e10527. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).