Submitted:
22 May 2023
Posted:
23 May 2023
You are already at the latest version
Abstract
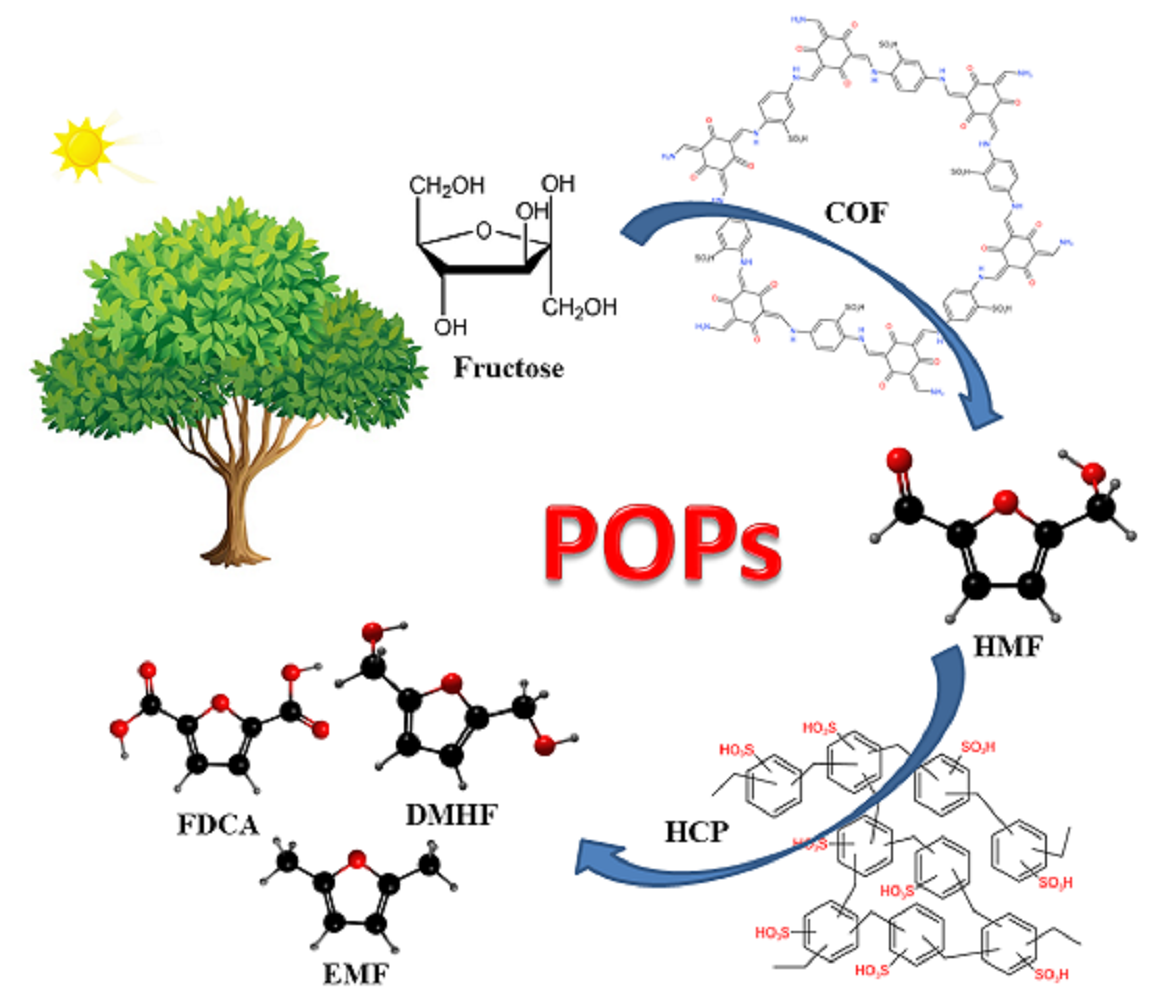
Keywords:
1. Introduction
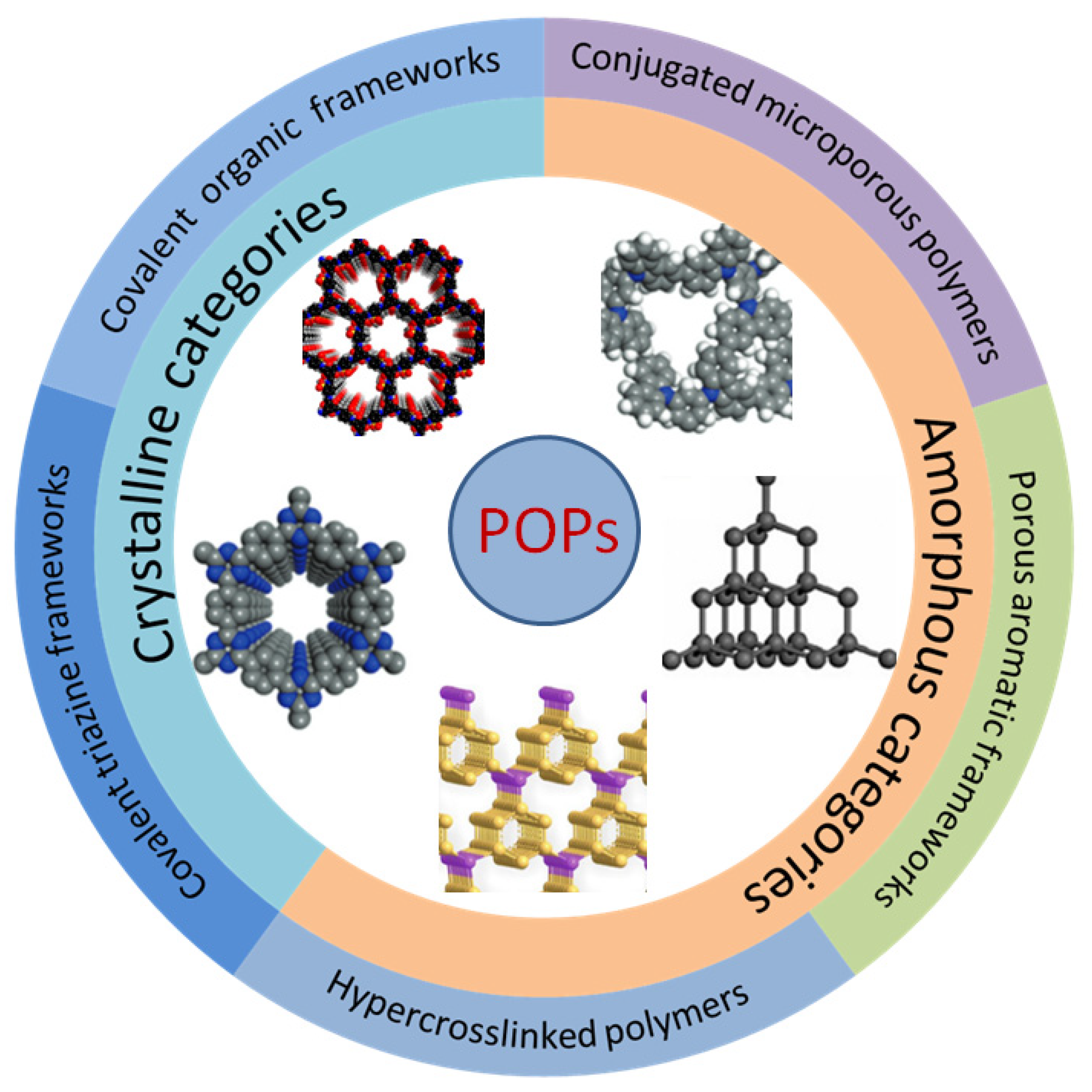
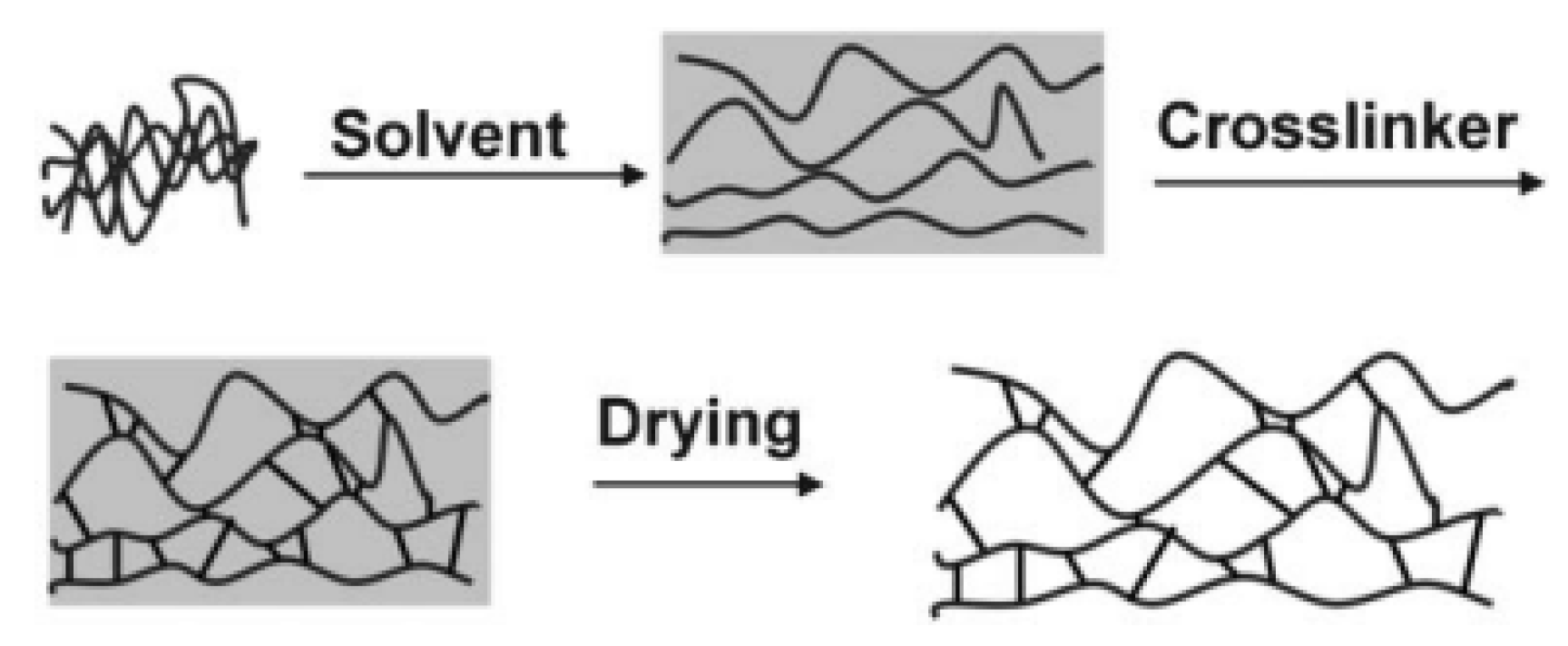
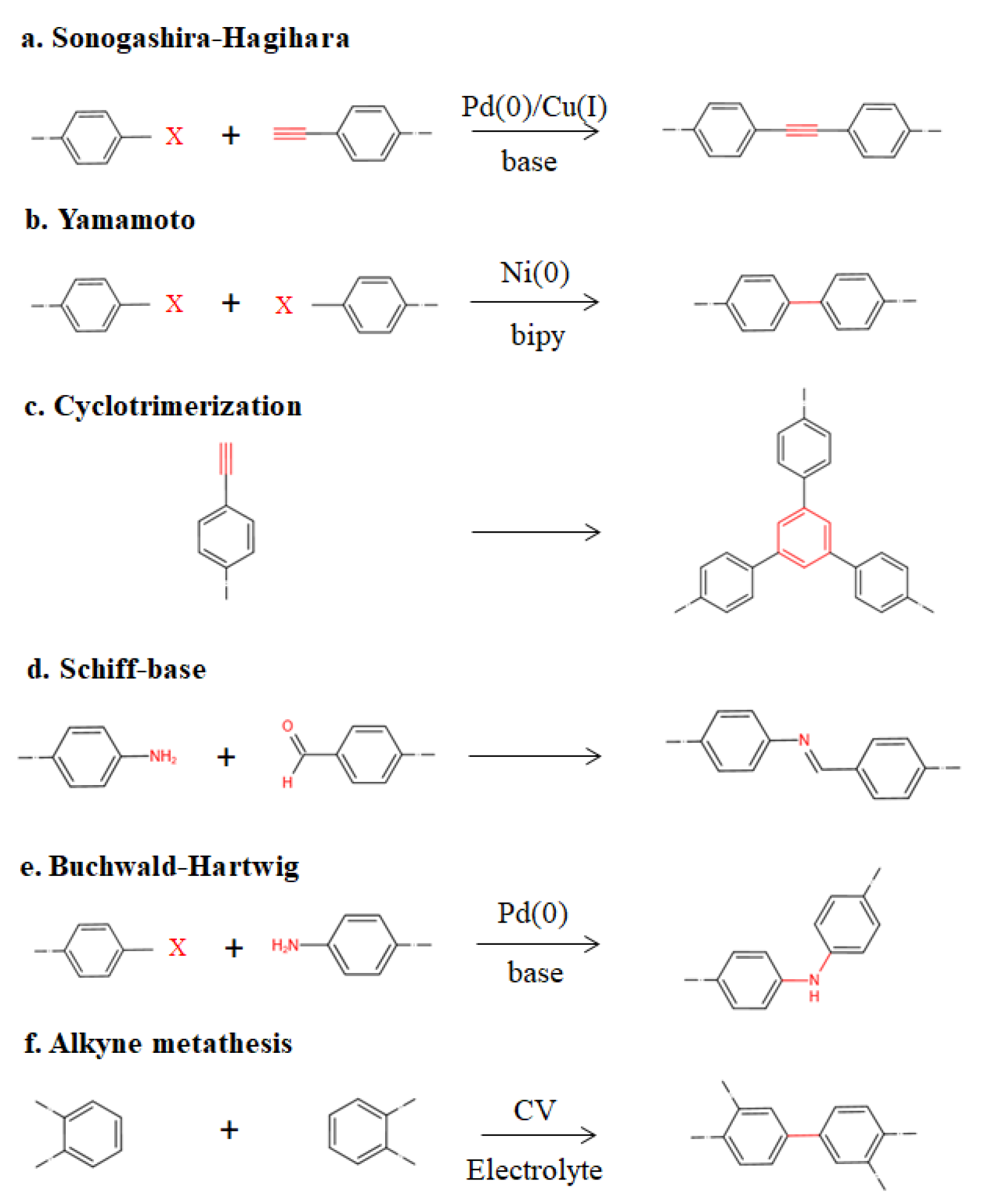
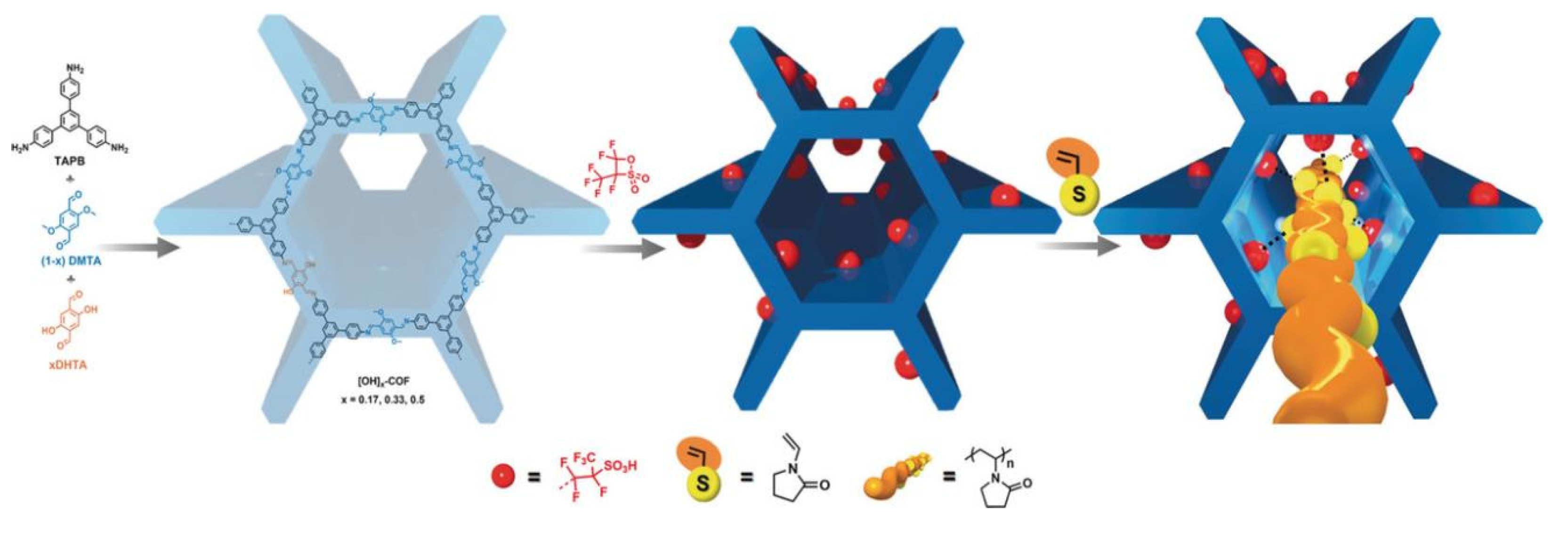
2. Synthesis and Structure of POPs
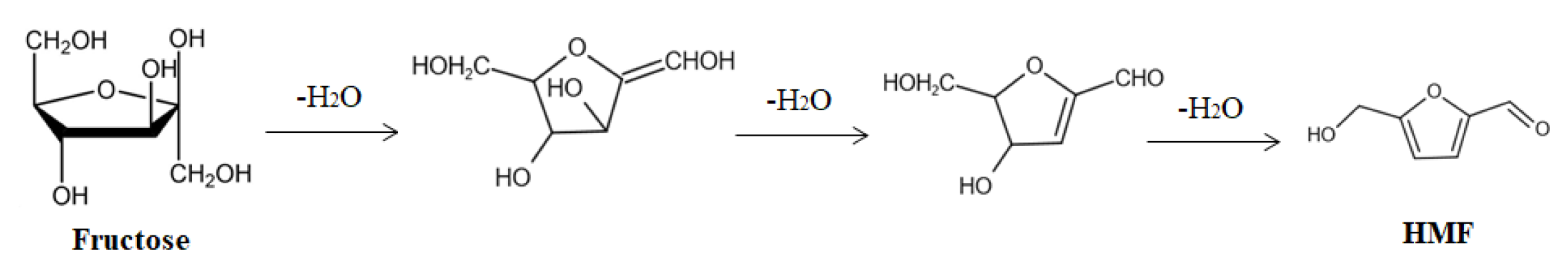
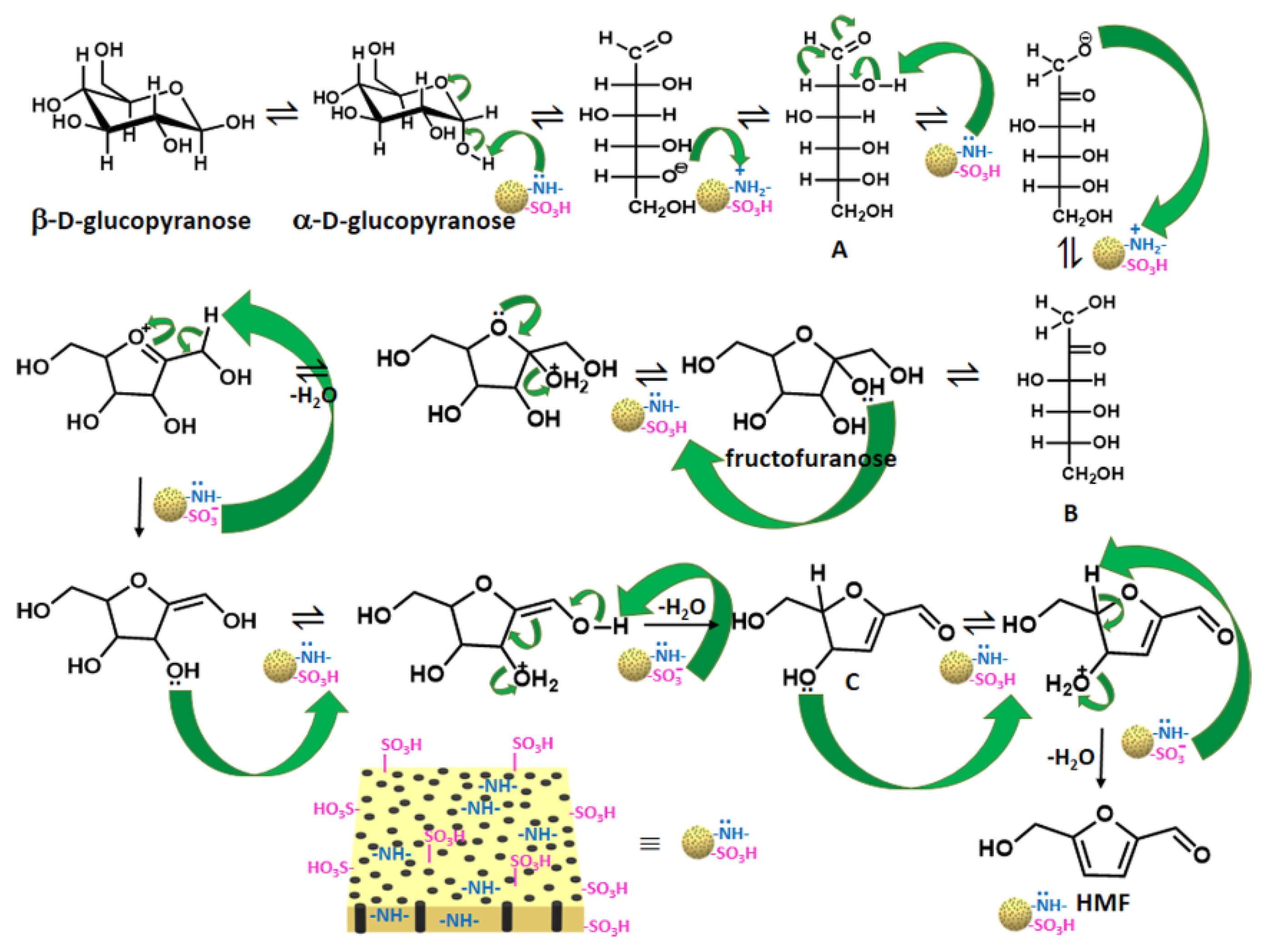
3. Catalytic Conversion of Fructose on POPs
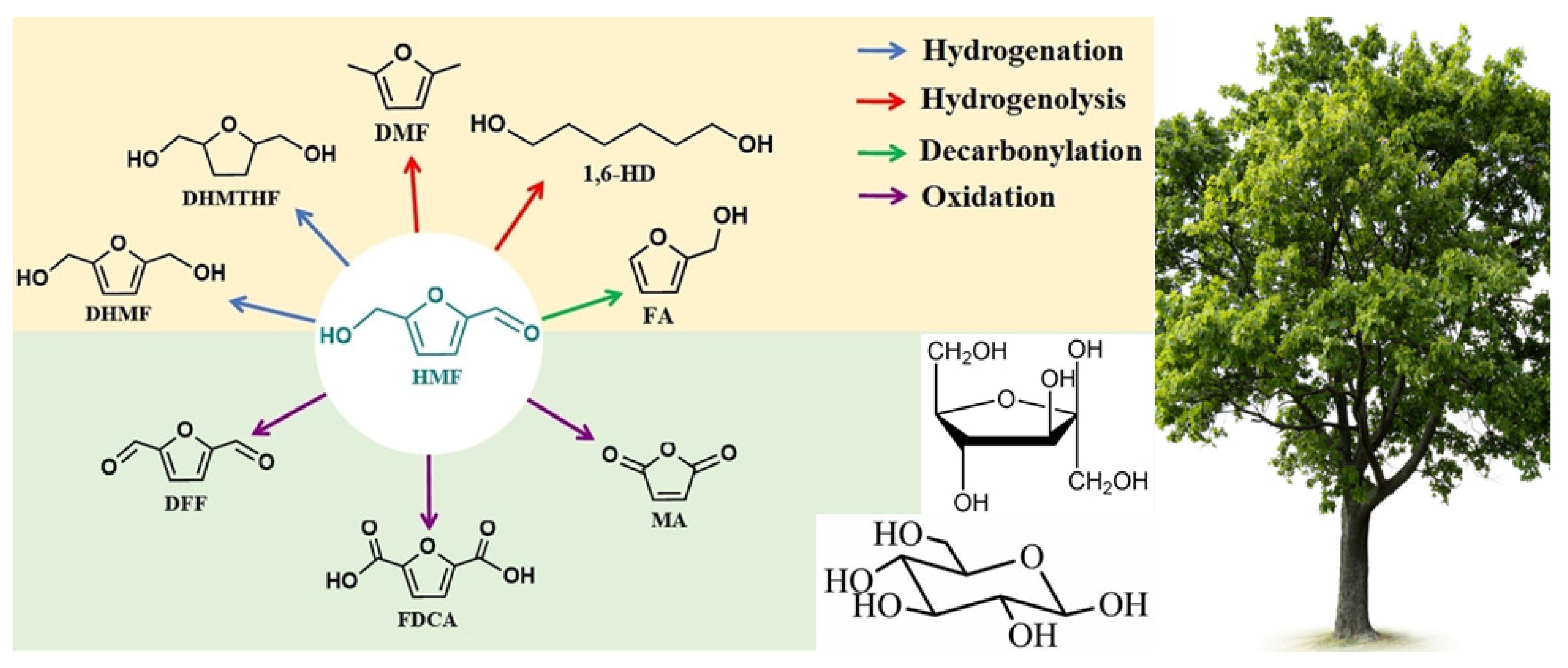
4. Catalytic Conversion of HMF on POPs
5. Conclusion and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Bender, T.A.; Dabrowski, J.A.; Gagné, M.R. Homogeneous catalysis for the production of low-volume, high-value chemicals from biomass. Nature Reviews Chemistry 2018, 2, 35–46. [Google Scholar] [CrossRef]
- Lange, J.-P.; Price, R.; Ayoub, P.M.; Louis, J.; Petrus, L.; Clarke, L.; Gosselink, H. Valeric Biofuels: A Platform of Cellulosic Transportation Fuels. Angewandte Chemie International Edition 2010, 49, 4479–4483. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, W.; Tan, L.; Wang, Y.; He, G. Optimization of metabolic pathways for bioconversion of lignocellulose to ethanol through genetic engineering. Biotechnology Advances 2009, 27, 593–598. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liu, J.; Liu, H.; Xu, G.; Zhang, J.; Liu, J.; Zhou, G.; Li, Q.; Xu, Z.; Fu, Y. Selective Hydrodeoxygenation of 5-Hydroxymethylfurfural to 2,5-Dimethylfuran over Heterogeneous Iron Catalysts. ChemSusChem 2017, 10, 1436–1447. [Google Scholar] [CrossRef] [PubMed]
- Rosatella, A.A.; Simeonov, S.P.; Frade, R.F.M.; Afonso, C.A.M. 5-Hydroxymethylfurfural (HMF) as a building block platform: Biological properties, synthesis and synthetic applications. Green Chemistry 2011, 13, 754. [Google Scholar] [CrossRef]
- Albonetti, S.; Hu, C.; Saravanamurugan, S. Preface to Special Issue on Green Conversion of HMF. ChemSusChem 2022, 15. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, H.; Kong, X.; Zhu, Y. Catalytic Conversion of 5-Hydroxymethylfurfural to High-Value Derivatives by Selective Activation of C−O, C=O, and C=C Bonds. ChemSusChem 2022, 15. [Google Scholar] [CrossRef]
- Ayed, C.; Huang, W.; Kizilsavas, G.; Landfester, K.; Zhang, K.A.I. Photocatalytic Partial Oxidation of 5-Hydroxymethylfurfural (HMF) to 2,5-Diformylfuran (DFF) Over a Covalent Triazine Framework in Water. ChemPhotoChem 2020, 4, 571–576. [Google Scholar] [CrossRef]
- Cai, J.; Li, K.; Wu, S. Recent advances in catalytic conversion of biomass derived 5-hydroxymethylfurfural into 2,5-furandicarboxylic acid. Biomass and Bioenergy 2022, 158, 106358. [Google Scholar] [CrossRef]
- Eerhart AJ, J.E.; Faaij AP, C.; Patel, M.K. Replacing fossil based PET with biobased PEF; process analysis, energy and GHG balance. Energy & Environmental Science 2012, 5, 6407. [Google Scholar]
- Geng, Y.; Wang, Z.; Hu, X.; Li, Y.; Zhang, Q.; Li, Y.; Wang, R.; Zhang, L. Bio-based polyesters based on 2,5-furandicarboxylic acid as 3D-printing materials: Design, preparation and performances. European Polymer Journal 2019, 114, 476–484. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, P.; Liu, Z. Catalytic conversion of carbohydrates to 5-hydroxymethylfurfural from the waste liquid of acid hydrolysis NCC. Carbohydrate Polymers 2016, 142, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Chidambaram, M.; Bell, A.T. A two-step approach for the catalytic conversion of glucose to 2,5-dimethylfuran in ionic liquids. Green Chemistry 2010, 12, 1253. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, Z.; Liu, B.; Xu, Z.; Deng, K. Microwave-assisted rapid conversion of carbohydrates into 5-hydroxymethylfurfural by ScCl3 in ionic liquids. Carbohydrate Research 2013, 375, 68–72. [Google Scholar] [CrossRef]
- Hou, Q.; Zhen, M.; Li, W.; Liu, L.; Liu, J.; Zhang, S.; Nie, Y.; Bai, C.; Bai, X.; Ju, M. Efficient catalytic conversion of glucose into 5-hydroxymethylfurfural by aluminum oxide in ionic liquid. Applied Catalysis B: Environmental 2019, 253, 1–10. [Google Scholar] [CrossRef]
- Pang, J.; Sun, J.; Zheng, M.; Li, H.; Wang, Y.; Zhang, T. Transition metal carbide catalysts for biomass conversion: A review. Applied Catalysis B: Environmental 2019, 254, 510–522. [Google Scholar] [CrossRef]
- Zhong, R.; Sels, B.F. Sulfonated mesoporous carbon and silica-carbon nanocomposites for biomass conversion. Applied Ca-talysis B: Environmental 2018, 236, 518–545. [Google Scholar] [CrossRef]
- Han, X.; Li, C.; Liu, X.; Xia, Q.; Wang, Y. Selective oxidation of 5-hydroxymethylfurfural to 2,5-furandicarboxylic acid over MnOx–CeO2 composite catalysts. Green Chemistry 2017, 19, 996–1004. [Google Scholar] [CrossRef]
- Nguyen, C.V.; Liao, Y.-T.; Kang, T.-C.; Chen, J.E.; Yoshikawa, T.; Nakasaka, Y.; Masuda, T.; Wu, K.C.-W. A metal-free, high nitrogen-doped nanoporous graphitic carbon catalyst for an effec-tive aerobic HMF-to-FDCA conversion. Green Chemistry 2016, 18, 5957–5961. [Google Scholar] [CrossRef]
- Li, X.; Sun, M.; Rooke, J.C.; Chen, L.; Su, B.-L. Synthesis and applications of hierarchically porous catalysts. Chinese Journal of Catalysis 2013, 34, 22–47. [Google Scholar] [CrossRef]
- Ding, M.; Liu, X.; Ma, P.; Yao, J. Porous materials for capture and catalytic conversion of CO2 at low concentration. Coordination Chemistry Reviews 2022, 465, 214576. [Google Scholar] [CrossRef]
- Sun, M.-H.; Huang, S.-Z.; Chen, L.-H.; Li, Y.; Yang, X.-Y.; Yuan, Z.-Y.; Su, B.-L. Applications of hierarchically structured porous materials from energy storage and conversion, catalysis, photocatalysis, adsorption, separation, and sensing to biomedicine. Chemical Society Reviews 2016, 45, 3479–3563. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.S.; Bao, X.Y.; Guo, W.; Lee, F.Y. Immobilizing catalysts on porous materials. Materials Today 2006, 9, 32–39. [Google Scholar] [CrossRef]
- Gupta, D.; Kumar, R.; Pant, K.K. Hydrotalcite supported bimetallic (Ni-Cu) catalyst: A smart choice for one-pot conversion of biomass-derived platform chemicals to hydrogenated biofuels. Fuel 2020, 277, 118111. [Google Scholar] [CrossRef]
- Xu, J.; Wang, L. Carbon Nanomaterials[M]//Nano-Inspired Biosensors for Protein Assay with Clinical Applications. 2019, 3-38Elsevier, 2019, 3-38.
- Zhao, S.; Wang, D.; Amal, R.; Dai, L. Carbon-Based Metal-Free Catalysts for Key Reactions Involved in Energy Conversion and Storage. Advanced Materials 2019, 31, 1801526. [Google Scholar] [CrossRef] [PubMed]
- Giannakoudakis, D.A.; Zormpa, F.F.; Margellou, A.G.; Qayyum, A.; Len, C.; Colmenares, J.C.; Triantafyllidis, K.S. Carbon-Based Nanocatalysts (CnCs) for Biomass Valorization and Hazardous Organics Remediation. Nanomaterials 2022, 12, 1679. [Google Scholar] [CrossRef] [PubMed]
- Pikunic, J.; Clinard, C.; Cohaut, N.; Gubbins, K.E.; Guet, J.-M.; Pellenq, R.J.-M.; Rannou, I.; Rouzaud, J.-N. Structural Modeling of Porous Carbons: Constrained Reverse Monte Carlo Method. Langmuir 2003, 19, 8565–8582. [Google Scholar] [CrossRef]
- Guo, J.; Jiang, D. Covalent Organic Frameworks for Heterogeneous Catalysis: Principle, Current Status, and Challenges. ACS Central Science 2020, 6, 869–879. [Google Scholar] [CrossRef]
- Ma, D.; Liu, K.; Li, J.; Shi, Z. Bifunctional Metal-Free Porous Organic Framework Heterogeneous Catalyst for Efficient CO2 Conversion under Mild and Cocatalyst-Free Conditions. ACS Sustainable Chemistry & Engineering 2018, 6, 15050–15055. [Google Scholar] [CrossRef]
- Zhang, Z.; Jia, J.; Zhi, Y.; Ma, S.; Liu, X. Porous organic polymers for light-driven organic transformations. Chemical Society Reviews 2022, 51, 2444–2490. [Google Scholar] [CrossRef]
- Zhang, T.; Xing, G.; Chen, W.; Chen, L. Porous organic polymers: a promising platform for efficient photocatalysis. Materials Chemistry Frontiers 2020, 4, 332–353. [Google Scholar] [CrossRef]
- Shit, S.C.; Khilari, S.; Mondal, I.; Pradhan, D.; Mondal, J. The Design of a New Cobalt Sulfide Nanoparticle Implanted Porous Organic Polymer Nanohybrid as a Smart and Durable Water-Splitting Photoelectrocatalyst. Chemistry - A European Journal 2017, 23, 14827–14838. [Google Scholar] [CrossRef] [PubMed]
- Kaur, P.; Hupp, J.T.; Nguyen, S.T. Porous Organic Polymers in Catalysis: Opportunities and Challenges. ACS Catalysis 2011, 1, 819–835. [Google Scholar] [CrossRef]
- Hao, Q.; Tao, Y.; Ding, X.; Yang, Y.; Feng, J.; Wang, R.-L.; Chen, X.-M.; Chen, G.-L.; Li, X.; OuYang, H.; Hu, X.; Tian, J.; Han, B.-H.; Zhu, G.; Wang, W.; Zhang, F.; Tan, B.; Li, Z.-T.; Wang, D.; Wan, L.-J. Porous organic polymers: a progress report in China. Science China Chemistry 2023. [Google Scholar] [CrossRef]
- Geng, K.; He, T.; Liu, R.; Dalapati, S.; Tan, K.T.; Li, Z.; Tao, S.; Gong, Y.; Jiang, Q.; Jiang, D. Covalent Organic Frameworks: Design, Synthesis, and Functions. Chemical Reviews 2020, 120, 8814–8933. [Google Scholar] [CrossRef]
- Pei, C.; Ben, T.; Qiu, S. Great Prospects for PAF-1 and its derivatives. Materials Horizons 2015, 2, 11–21. [Google Scholar] [CrossRef]
- Tan, L.; Tan, B. Hypercrosslinked porous polymer materials: design, synthesis, and applications. Chemical Society Reviews 2017, 46, 3322–3356. [Google Scholar] [CrossRef]
- Cooper, A.I. Conjugated Microporous Polymers. Advanced Materials 2009, 21, 1291–1295. [Google Scholar] [CrossRef]
- Wang, S.; Li, H.; Huang, H.; Cao, X.; Chen, X.; Cao, D. Porous organic polymers as a platform for sensing applications. Chemical Society Reviews 2022, 51, 2031–2080. [Google Scholar] [CrossRef]
- Zhang, Y.; Riduan, S.N. Functional porous organic polymers for heterogeneous catalysis. Chem. Soc. Rev. 2012, 41, 2083–2094. [Google Scholar] [CrossRef]
- Kuhn, P.; Antonietti, M.; Thomas, A. Porous, Covalent Triazine-Based Frameworks Prepared by Ionothermal Synthesis. Angewandte Chemie International Edition 2008, 47, 3450–3453. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Mao, N.; Feng, S.; Zhang, C.; Wang, F.; Chen, Y.; Zeng, J.; Jiang, J.-X. Perylene-Containing Conjugated Microporous Polymers for Photocatalytic Hydrogen Evolution. Macromolecular Chemistry and Physics 2017, 218, 1700049. [Google Scholar] [CrossRef]
- Sharma, V.; Sahoo, A.; Sharma, Y.; Mohanty, P. Synthesis of nanoporous hypercrosslinked polyaniline (HCPANI) for gas sorption and electrochemical supercapacitor applications. RSC Advances 2015, 5, 45749–45754. [Google Scholar] [CrossRef]
- Ding, X.; Han, B.-H. Metallophthalocyanine-Based Conjugated Microporous Polymers as Highly Efficient Photosensitizers for Singlet Oxygen Generation. Angewandte Chemie International Edition 2015, 54, 6536–6539. [Google Scholar] [CrossRef] [PubMed]
- Totten, R.K.; Olenick, L.L.; Kim, Y.-S.; Chakraborty, S.; Weston, M.H.; Farha, O.K.; Hupp, J.T.; Nguyen, S.T. A dual approach to tuning the porosity of porous organic polymers: controlling the porogen size and supercritical CO2 processing. Chem. Sci. 2014, 5, 782–787. [Google Scholar] [CrossRef]
- Lu, S.; Liu, Q.; Han, R.; Guo, M.; Shi, J.; Song, C.; Ji, N.; Lu, X.; Ma, D. Potential applications of porous organic polymers as adsorbent for the adsorption of volatile organic compounds. Journal of Environmental Sciences 2021, 105, 184–203. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Yang, Y. Macrocycle-Based Porous Organic Polymers for Separation, Sensing, and Catalysis. Advanced Materials 2022, 34, 2107401. [Google Scholar] [CrossRef]
- Luo, D.; Li, M.; Ma, Q.; Wen, G.; Dou, H.; Ren, B.; Liu, Y.; Wang, X.; Shui, L.; Chen, Z. Porous organic polymers for Li-chemistry-based batteries: functionalities and characterization studies. Chemical Society Reviews 2022, 51, 2917–2938. [Google Scholar] [CrossRef]
- Liu, X.; Liu, C.; Lai, W.; Lai, W. Porous Organic Polymers as Promising Electrode Materials for Energy Storage Devices. Advanced Materials Technologies 2020, 2000154. [Google Scholar] [CrossRef]
- Tang, Y.; Varyambath, A.; Ding, Y.; Chen, B.; Huang, X.; Zhang, Y.; Yu, D.; Kim, I.; Song, W. Porous organic polymers for drug delivery: hierarchical pore structures, variable morphologies, and biological properties. Biomaterials Science 2022, 10, 5369–5390. [Google Scholar] [CrossRef]
- Côté, A.P.; Benin, A.I.; Ockwig, N.W.; O'keeffe, M.; Yaghi, O.M. Porous, Crystalline, Covalent Organic Frameworks. Science 2005, 310, 1166–1170. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, N.; Ma, L. Recent Advances in Covalent Organic Frameworks for Catalysis. Chemistry – An Asian Journal 2020, 15, 338–351. [Google Scholar] [CrossRef]
- Ben, T.; Ren, H.; Ma, S.; Cao, D.; Lan, J.; Jing, X.; Wang, W.; Xu, J.; Deng, F.; Simmins, J.M.; Qiu, S.; Zhu, G. Targeted Synthesis of a Porous Aromatic Framework with High Stability and Exceptionally High Surface Area. Angewandte Chemie International Edition 2009, 48, 9457–9460. [Google Scholar] [CrossRef]
- Díaz, U.; Corma, A. Ordered covalent organic frameworks, COFs and PAFs. From preparation to application. Coordination Chemistry Reviews 2016, 311, 85–124. [Google Scholar] [CrossRef]
- Yuan, R.; Ren, H.; Yan, Z.; Wang, A.; Zhu, G. Robust tri(4-ethynylphenyl)amine-based porous aromatic frameworks for carbon dioxide capture. Polymer Chemistry 2014, 5, 2266. [Google Scholar] [CrossRef]
- Wood, C.D.; Tan, B.; Trewin, A.; Niu, H.; Bradshaw, D. Hydrogen Storage in Microporous Hypercrosslinked Organic Polymer Networks. Chemistry of Materials 2007, 19, 2034–2048. [Google Scholar] [CrossRef]
- Germain, J.; Fréchet JM, J.; Svec, F. Hypercrosslinked polyanilines with nanoporous structure and high surface area: potential adsorbents for hydrogen storage. Journal of Materials Chemistry 2007, 17, 4989. [Google Scholar] [CrossRef]
- Gao, H.; Ding, L.; Li, W.; Ma, G.; Bai, H.; Li, L. Hyper-Cross-Linked Organic Microporous Polymers Based on Alternating Copolymerization of Bismaleimide. ACS Macro Letters 2016, 5, 377–381. [Google Scholar] [CrossRef]
- Msayib, K.J.; McKeown, N.B. Inexpensive polyphenylene network polymers with enhanced microporosity. Journal of Materials Chemistry A 2016, 4, 10110–10113. [Google Scholar] [CrossRef]
- Li, B.; Gong, R.; Wang, W.; Huang, X.; Zhang, W.; Li, H.; Hu, C.; Tan, B. A New Strategy to Microporous Polymers: Knitting Rigid Aromatic Building Blocks by External Cross-Linker. Macromolecules 2011, 44, 2410–2414. [Google Scholar] [CrossRef]
- Chen, L.; Yang, Y.; Jiang, D. CMPs as Scaffolds for Constructing Porous Catalytic Frameworks: A Built-in Heterogeneous Catalyst with High Activity and Selectivity Based on Nanoporous Metalloporphyrin Polymers. Journal of the American Chemical Society 2010, 132, 9138–9143. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.-B.; Zhan, Z.-P. Conjugated Microporous Polymers for Heterogeneous Catalysis. Chemistry - An Asian Journal 2018, 13, 9–19. [Google Scholar] [CrossRef]
- Tan, Z.; Chen, K.; Liu, P. Possibilities and challenges of China׳s forestry biomass resource utilization. Renewable and Sustainable Energy Reviews 2015, 41, 368–378. [Google Scholar] [CrossRef]
- Peng, W.-H.; Lee, Y.-Y.; Wu, C.; Wu, K.C.-W. Acid–base bi-functionalized, large-pored mesoporous silica nanoparticles for cooperative catalysis of one-pot cellulose-to-HMF conversion. Journal of Materials Chemistry 2012, 22, 23181. [Google Scholar] [CrossRef]
- Binder, J.B.; Cefali, A.V.; Blank, J.J.; Raines, R.T. Mechanistic insights on the conversion of sugars into 5-hydroxymethylfurfural. Energy & Environmental Science 2010, 3, 765. [Google Scholar]
- Moreau, C.; Durand, R.; Razigade, S.; Duhamet, J.; Faugeras, P. Dehydration of fructose to 5-hydroxymethylfurfural over H-mordenites. Applied Catalysis A: General 1996, 145, 211–224. [Google Scholar] [CrossRef]
- Wang, T.; Nolte, M.W.; Shanks, B.H. Catalytic dehydration of C6 carbohydrates for the production of hydroxymethylfurfural (HMF) as a versatile platform chemical. Green Chem. 2014, 16, 548–572. [Google Scholar] [CrossRef]
- Li, C.; Zhang, Z.; Zhao, Z.K. Direct conversion of glucose and cellulose to 5-hydroxymethylfurfural in ionic liquid under microwave irradiation. Tetrahedron Letters 2009, 50, 5403–5405. [Google Scholar] [CrossRef]
- Binder, J.B.; Raines, R.T. Simple Chemical Transformation of Lignocellulosic Biomass into Furans for Fuels and Chemicals. Journal of the American Chemical Society 2009, 131, 1979–1985. [Google Scholar] [CrossRef]
- He, M.; Sun, Y.; Han, B. Green Carbon Science: Efficient Carbon Resource Processing, Utilization, and Recycling towards Carbon Neutrality. Angewandte Chemie International Editio 2022, 61. [Google Scholar] [CrossRef]
- Pan, H.; Liu, X.; Zhang, H.; Yang, K.; Huang, S.; Yang, S. Multi-SO3H functionalized mesoporous polymeric acid catalyst for biodiesel production and fructose-to-biodiesel additive conversion. Renewable Energy 2017, 107, 245–252. [Google Scholar] [CrossRef]
- Herman, M.A.; Birnbaum, M.J. Molecular aspects of fructose metabolism and metabolic disease. Cell Metabolism 2021, 33, 2329–2354. [Google Scholar] [CrossRef]
- Mukherjee, A.; Dumont, M.-J.; Raghavan, V. Review: Sustainable production of hydroxymethylfurfural and levulinic acid: Challenges and opportunities. Biomass and Bioenergy 2015, 72, 143–183. [Google Scholar] [CrossRef]
- Enjamuri, N.; Sarkar, S.; Reddy, B.M.; Mindal, J. Design and Catalytic Application of Functional Porous Organic Polymers: Opportunities and Challenges. The Chemical Record 2019, 19, 1782–1792. [Google Scholar] [CrossRef]
- Haase, F.; Lotsch, B.V. Solving the COF trilemma: towards crystalline, stable and functional covalent organic frameworks. Chemical Society Reviews 2020, 49, 8469–8500. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Hu, Z.; Gao, Y.; Yuan, D.; Kang, Z.; Qian, Y.; Yan, N.; Zhao, D. Synthesis of a Sulfonated Two-Dimensional Covalent Organic Framework as an Efficient Solid Acid Catalyst for Biobased Chemical Conversion. ChemSusChem 2015, 8, 3208–3212. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Tang, Y.; Aguila, B.; Wang, S.; Xiao, F.-S.; Thalloally, P.K.; Al-Enizi, A.M.; Nafady, A.; Ma, S. Reaction Environment Modification in Covalent Organic Frameworks for Catalytic Perfor-mance Enhancement. Angewandte Chemie International Edition 2019, 58, 8670–8675. [Google Scholar] [CrossRef]
- Bhanja, P.; Sharma, S.K.; Chongdar, S.; Paul, B.; Bhaumik, A. Bifunctional crystalline microporous organic polymers: Efficient heterogeneous catalysts for the synthesis of 5-hydroxymethylfurfural. Molecular Catalysis 2021, 515, 111877. [Google Scholar] [CrossRef]
- Chen, D.; Chen, W.; Xing, G.; Zhang, T.; Chen, L. An Upgraded “Two-in-One” Strategy toward Highly Crystalline Covalent Organic Frame-works. Chemistry – A European Journal 2020, 26, 8377–8381. [Google Scholar] [CrossRef]
- Desir, P.; Saha, B.; Vlachos, D.G. Ultrafast flow chemistry for the acid-catalyzed conversion of fructose. Energy & Environ-mental Science 2019, 12, 2463–2475. [Google Scholar] [CrossRef]
- Du, M.; Agrawal, A.M.; Chakraborty, S.; Garibay, S.J.; Limvorapitux, R.; Choi, B.; Madrahimov, S.T.; Nguyen, S.T. Matching the Activity of Homogeneous Sulfonic Acids: The Fructose-to-HMF Conversion Catalyzed by Hierarchically Porous Sulfonic-Acid-Functionalized Porous Organic Polymer (POP) Catalysts. ACS Sustainable Chemistry & Engineering 2019, 7, 8126–8135. [Google Scholar] [CrossRef]
- Das, S.K.; Chatterjee, S.; Mondal, S.; Bhaumik, A. A new triazine-thiophene based porous organic polymer as efficient catalyst for the synthesis of chromenes via multicomponent coupling and catalyst support for facile synthesis of HMF from carbohydrates. Molecular Catalysis 2019, 475, 110483. [Google Scholar] [CrossRef]
- Dong, K.; Zhang, J.; Luo, W.; Su, L.; Huang, Z. Catalytic conversion of carbohydrates into 5-hydroxymethyl furfural over sulfonated hyper-cross-linked polymer in DMSO. Chemical Engineering Journal 2018, 334, 1055–1064. [Google Scholar] [CrossRef]
- Qian, X.; Wang, B.; Zhu, Z.-Q.; Sun, H.-X.; Ren, F.; Mu, P.; Ma, C.; Liang, W.-D.; Li, A. Novel N-rich porous organic polymers with extremely high uptake for capture and reversible storage of volatile iodine. Journal of Hazardous Materials 2017, 338, 224–232. [Google Scholar] [CrossRef] [PubMed]
- Mondal, S.; Mondal, J.; Bhaumik, A. Sulfonated Porous Polymeric Nanofibers as an Efficient Solid Acid Catalyst for the Production of 5-Hydroxymethylfurfural from Biomass. ChemCatChem 2015, 7, 3570–3578. [Google Scholar] [CrossRef]
- Sebati, W.; Ray, S.S.; Moutloali, R. Synthesis of Porous Organic Polymer-Based Solid-Acid Catalysts for 5-Hydroxymethylfurfural Production from Fructose. Catalysts 2019, 9, 656. [Google Scholar] [CrossRef]
- Testa, M.L.; La Parola, V. Sulfonic Acid-Functionalized Inorganic Materials as Efficient Catalysts in Various Applications: A Minireview. Catalysts 2021, 11, 1143. [Google Scholar] [CrossRef]
- Ravi, S.; Choi, Y.; Choe, J.K. Achieving effective fructose-to-5-hydroxymethylfurfural conversion via facile synthesis of large surface phosphate-functionalized porous organic polymers. Applied Catalysis B: Environmental 2020, 271, 118942. [Google Scholar] [CrossRef]
- Vasudevan, V.; Mushrif, S.H. Insights into the solvation of glucose in water, dimethyl sulfoxide (DMSO), tetrahydrofuran (THF) and N,N-dimethylformamide (DMF) and its possible implications on the conversion of glucose to platform chemicals. RSC Advances 2015, 5, 20756–20763. [Google Scholar] [CrossRef]
- Sheldon, R.A. Green and sustainable manufacture of chemicals from biomass: state of the art. Green Chem. 2014, 16, 950–963. [Google Scholar] [CrossRef]
- De Jong, E.; Dam, M.A.; Sipos, L.; Gruter, G.-J.M. Furandicarboxylic Acid (FDCA), A Versatile Building Block for a Very Interesting Class of Polyesters[M]. Smith P B, Gross R A, eds.//ACS Symposium Series. 2012, 1105: 1-13Washington, DC: American Chemical Society, 2012, 1-13. [CrossRef]
- Zhang, W.; Qian, H.; Hou, Q.; Ju, M. The functional and synergetic optimization of the thermal-catalytic system for the selective oxidation of 5-hydroxymethylfurfural to 2,5-diformylfuran: a review. Green Chemistry 2023, 25, 893–914. [Google Scholar] [CrossRef]
- Li, C.; Na, Y. Recent Advances in Photocatalytic Oxidation of 5-Hydroxymethylfurfural. ChemPhotoChem 2021, 5, 502–511. [Google Scholar] [CrossRef]
- Yang, Y.; Mu, T. Electrochemical oxidation of biomass derived 5-hydroxymethylfurfural (HMF): pathway, mechanism, catalysts and coupling reactions. Green Chemistry 2021, 23, 4228–4254. [Google Scholar] [CrossRef]
- Cunha, J.T.; Romaní, A.; Domingues, L. Whole Cell Biocatalysis of 5-Hydroxymethylfurfural for Sustainable Biorefineries. Catalysts 2022, 12, 202. [Google Scholar] [CrossRef]
- Rathod, P.V.; Jadhav, V.H. Efficient Method for Synthesis of 2,5-Furandicarboxylic Acid from 5-Hydroxymethylfurfural and Fructose Using Pd/CC Catalyst under Aqueous Conditions. ACS Sustainable Chemistry & Engineering 2018, 6, 5766–5771. [Google Scholar]
- Li, X.; Yadav, P.; Loh, K.P. Function-oriented synthesis of two-dimensional (2D) covalent organic frameworks – from 3D solids to 2D sheets. Chemical Society Reviews 2020, 49, 4835–4866. [Google Scholar] [CrossRef] [PubMed]
- Cai, M.; Ding, S.; Gibbons, B.; Yang, X.; Kessinger, M.C.; Morris, A.J. Nickel( II )-modified covalent-organic framework film for electrocatalytic oxidation of 5-hydroxymethylfurfural (HMF). Chemical Communications 2020, 56, 14361–14364. [Google Scholar] [CrossRef]
- Guo, L.; Zhang, X.; Gan, L.; Pan, L.; Shi, C.; Huang, Z.-F.; Zhang, X.; Zou, J.-J. Advances in Selective Electrochemical Oxidation of 5-Hydroxymethylfurfural to Produce High-Value Chemicals. Advanced Science 2023, 10, 2205540. [Google Scholar] [CrossRef]
- Pal, P.; Saravanamurugan, S. Recent Advances in the Development of 5-Hydroxymethylfurfural Oxidation with Base (Nonpre-cious)-Metal-Containing Catalysts. ChemSusChem 2019, 12, 145–163. [Google Scholar] [CrossRef]
- Xu, C.; Paone, E.; Rodríguez-Padrón, D.; Luque, R.; Mauriello, F. Recent catalytic routes for the preparation and the upgrading of biomass derived furfural and 5-hydroxymethylfurfural. Chemical Society Reviews 2020, 49, 4273–4306. [Google Scholar] [CrossRef] [PubMed]
- Brandolese, A.; Ragno, D.; Di Carmine, G.; Bernardi, T.; Bortolini, O.; Paolo Giovannini, P.; Ginoble Pandoli, O.; Altomare, A.; Massi, A. Aerobic oxidation of 5-hydroxymethylfurfural to 5-hydroxymethyl-2-furancarboxylic acid and its derivatives by heterogeneous NHC-catalysis. Organic & Biomolecular Chemistry 2018, 16, 8955–8964. [Google Scholar]
- Galkin, K.I.; Ananikov, V.P. When Will 5-Hydroxymethylfurfural, the “Sleeping Giant” of Sustainable Chemistry, Awaken? ChemSusChem 2019, 12, 2976–2982. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Liu, R.; Guo, Y.; Chen, L.; Gao, H. Selective Hydrogenation of Biomass-Based 5-Hydroxymethylfurfural over Catalyst of Palladium Immobilized on Amine-Functionalized Metal–Organic Frameworks. ACS Catalysis 2015, 5, 722–733. [Google Scholar] [CrossRef]
- Yao, Y.; Wu, X.; Gutiérrez, O.Y.; Ji, J.; Wang, S.; Xu, Y.; Zhao, Y.; Wang, S.; Ma, X.; Lercher, J.A. Roles of Cu+ and Cu0 sites in liquid-phase hydrogenation of esters on core-shell CuZnx@C catalysts. Applied Catalysis B: Environmental 2020, 267, 118698. [Google Scholar] [CrossRef]
- Yue, H.; Zhao, Y.; Zhao, S.; Wang, B.; Ma, X.; Gong, J. A copper-phyllosilicate core-sheath nanoreactor for carbon–oxygen hydrogenolysis reactions. Nature Communications 2013, 4, 2339. [Google Scholar] [CrossRef]
- Sarkar, C.; Paul, R.; Chandra Shit, S.; Trinh, Q.T.; Koley, P. Navigating Copper-Atom-Pair Structural Effect inside a Porous Organic Polymer Cavity for Selective Hydrogenation of Biomass-Derived 5-Hydroxymethylfurfural. ACS Sustainable Chemistry & Engineering 2021, 9, 2136–2151. [Google Scholar] [CrossRef]
- Kong, X.; Zhu, Y.; Fang, Z.; Kozinski, J.A.; Butter, L.S.; Xu, L.; Song, H.; Wei, X. Catalytic conversion of 5-hydroxymethylfurfural to some value-added derivatives. Green Chemistry 2018, 20, 3657–3682. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Z.; Jin, S.; Shen, X. Efficient conversion of carbohydrates into 5-hydroxylmethylfurfan and 5-ethoxymethylfurfural over sufonic acid-functionalized mesoporous carbon catalyst. Fuel 2017, 192, 102–107. [Google Scholar] [CrossRef]
- Zhang, J.; Dong, K.; Luo, W.; Guan, H. Catalytic upgrading of carbohydrates into 5-ethoxymethylfurfural using SO3H functionalized hyper-cross-linked polymer based carbonaceous materials. Fuel 2018, 234, 664–673. [Google Scholar] [CrossRef]
- Wang, Z.; Li, H.; Fang, C.; Zhao, W.; Yang, T.; Yang, S. Simply Assembly of Acidic Nanospheres for Efficient Production of 5-Ethoxymethylfurfural from 5-Hydromethylfurfural and Fructose. Energy Technology 2017, 5, 2046–2054. [Google Scholar] [CrossRef]
- Xiang, Y.; Wen, S.; Tian, Y.; Zhao, K.; Guo, D.; Cheng, F.; Xu, Q.; Liu, X.; Yin, D. Efficient synthesis of 5-ethoxymethylfurfural from biomass-derived 5-hydroxymethylfurfural over sulfonated organic polymer catalyst. RSC Advances 2021, 11, 3585–3595. [Google Scholar] [CrossRef] [PubMed]
- Lai, J.; Cheng, F.; Zhou, S.; Wen, S.; Guo, D.; Zhao, W.; Liu, X.; Yin, D. Base-free oxidation of 5-hydroxymethylfurfural to 2, 5-furan dicarboxylic acid over nitro-gen-containing polymers supported Cu-doped MnO2 nanowires. Applied Surface Science 2021, 565, 150479. [Google Scholar] [CrossRef]
- Hayashi, E.; Yamaguchi, Y.; Kamata, K.; Tsunoda, N.; Kumagai, Y.; Oba, F.; Hara, M. Effect of MnO2 Crystal Structure on Aerobic Oxidation of 5-Hydroxymethylfurfural to 2,5-Furandicarboxylic Acid. Journal of the American Chemical Society 2019, 141, 890–900. [Google Scholar] [CrossRef]
- Lee, J.-S.M.; Cooper, A.I. Advances in Conjugated Microporous Polymers. Chemical Reviews 2020, 120, 2171–2214. [Google Scholar] [CrossRef]
- Modak, A.; Nandi, M.; Mondal, J.; Bhaumik, A. Porphyrin based porous organic polymers: novel synthetic strategy and exceptionally high CO2 adsorption capacity. Chem. Commun. 2012, 48, 248–250. [Google Scholar] [CrossRef]
- Saha, B.; Gupta, D.; Abu-Omar, M.M.; Modak, A.; Bhaumik, A. Porphyrin-based porous organic polymer-supported iron(III) catalyst for efficient aerobic oxidation of 5-hydroxymethyl-furfural into 2,5-furandicarboxylic acid. Journal of Catalysis 2013, 299, 316–320. [Google Scholar] [CrossRef]
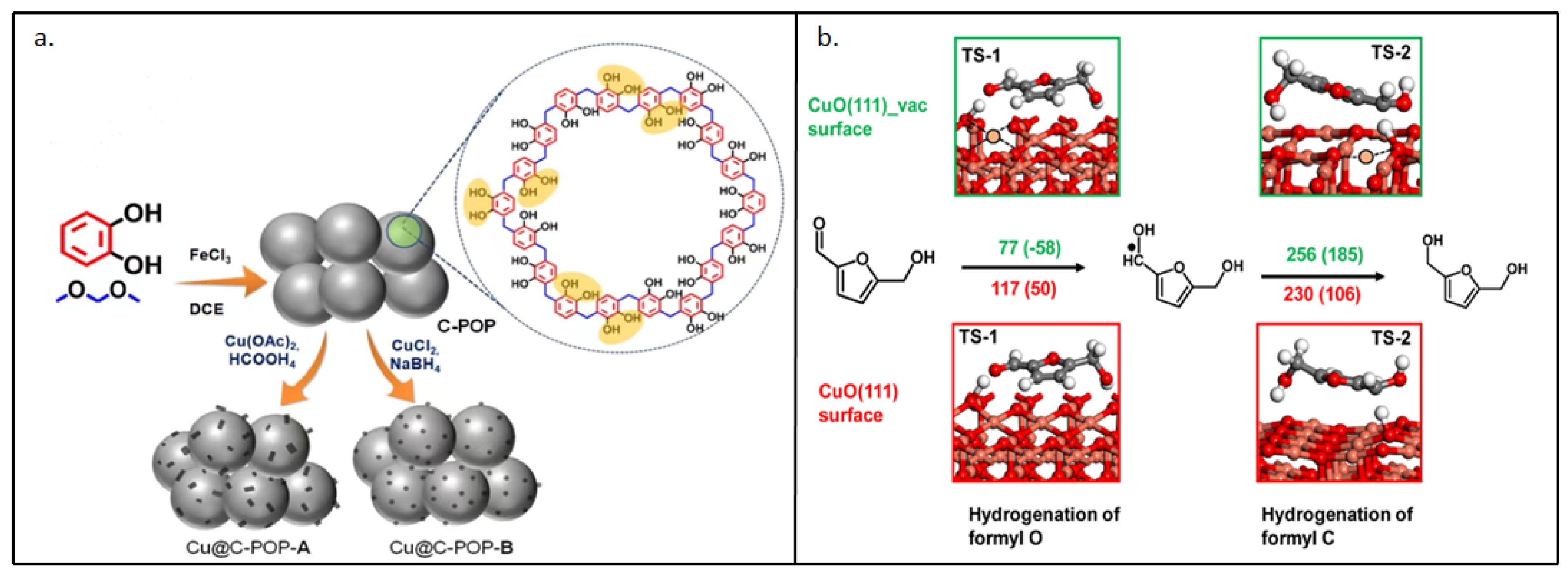
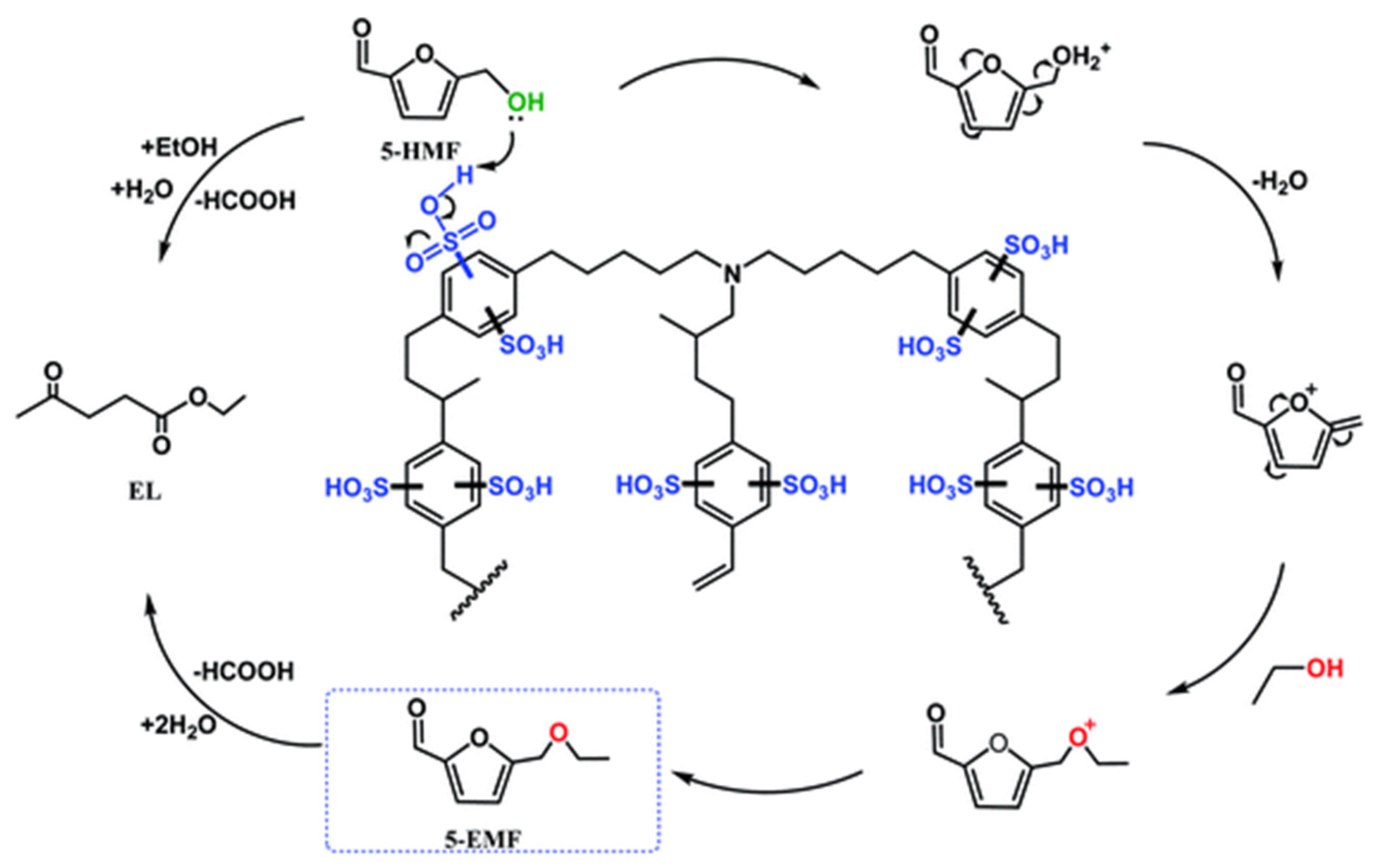
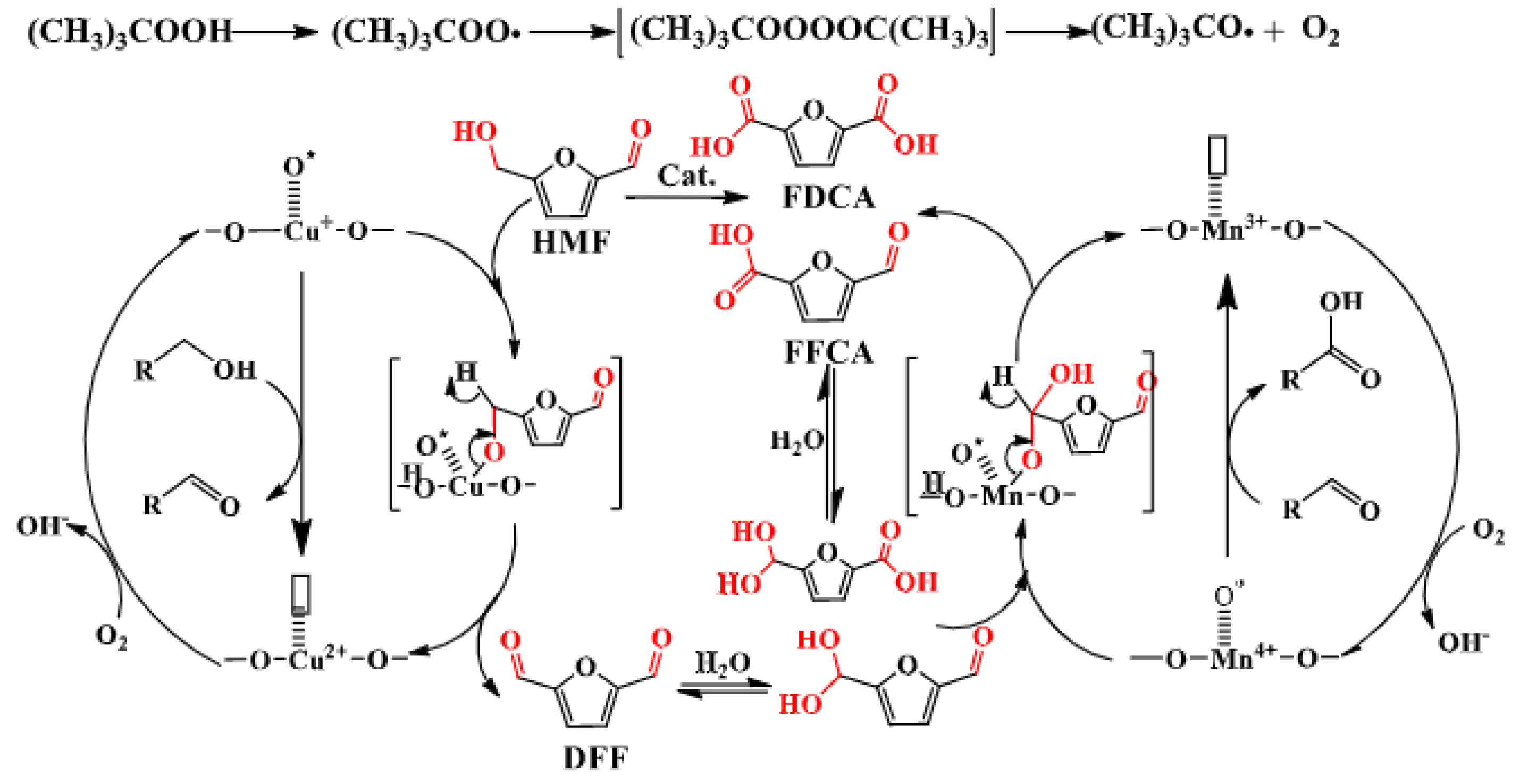
| Catalyst | Substrate | Solvent system | Time (min) |
Temperature (℃) |
Xsubstrate (%) |
HMF Yield (%) |
Reference |
|---|---|---|---|---|---|---|---|
| TFP-DABA | Fructose | DMSO | 60 | 100 | >99 | 97 | [77] |
| PVP@[SO3H]0.17-COF | Fructose | Tetrahydrofuran | 30 | 100 | >99.5 | 97.6 | [78] |
| POPDS | Fructose | DMSO | 20 | 140 | 98 | 86 | [79] |
| POPSDS | Fructose | DMSO | 20 | 140 | 98 | 89 | [79] |
| HO3S-POP | Fructose | Dioxane aqueous | 15 | 140 | 100 | >70 | [82] |
| STrzDBTH | D-Fructose | DMSO | 20 | 140 | 100 | 96.2 | [83] |
| HCP-x | Fructose | DMSO | 30 | 139.85 | >99 | 96.7 | [84] |
| SPPTPA-1 | Fructose | DMSO | 20 | 140 | 100 | 94.6 | [86] |
| FeSPPTPA | Fructose | DMSO | 20 | 100 | 95 | 96.6 | [87] |
| B-POP | Fructose | DMSO/ Dioxane |
30 | 130 | 100 | 85 | [89] |
| Catalyst | Substrate | Solvent system | Oxidant | Pressure (Mpa) |
Time (min) |
Temperature (℃) |
Xsubstrate (%) |
Yield (%) |
Reference |
|---|---|---|---|---|---|---|---|---|---|
| HCP-x | Fructose |
Ethanol/ DMSO |
Air | 0.1 | 480 | 104.85 | 99.8 | 78.9(EMF); 15.4(HMF) |
[111] |
| PDVTA-SO3H | HMF | Ethanol | Air | 0.1 | 30 | 110 | 99.8 | 87.5(EMF) | [113] |
| Cu-MnO2 @PDVTA |
HMF | Tert-butyl alcohol | TBHP /Air |
0.1 | 720 | 80 | 95 | 96.8(FDCA) | [114] |
| FeⅢ-POP-1 | Fructose | Water | Air | 1 | 600 | 100 | 100 | 79(FDCA) | [118] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





