1. Introduction
Banana is one of the most produced fruits in the world. In 2021, about 125 million tons have been produced [
1]. Bananas are mostly consumed as fresh fruits, but they are also processed into flours, purees, jams, sauces, and snacks (mainly dehydrated bananas, such as banana figs and freeze-dried banana slices). Snacks are usually associated with energy-dense food products high in sodium, sugar and/or fat; however, the consumption of healthier snacks has been a trend, including those based on fruits and vegetables and probiotic-enriched snacks [
2]. Some studies have described the development of probiotic snacks based on fruits, including apples [
3,
4], strawberries [
5], papayas [
6], and bananas [
7]. This is part of the efforts to produce non-dairy probiotic food products, since most probiotic foods in the market are dairy, thus not suitable for consumers with dietary restrictions to milk and derivatives [
8].
However, stress factors during food processing and storage (including thermal, osmotic and oxidative stresses, dehydration, and shear forces) may negatively affect the viability of probiotic bacteria [
9]. That is the main reason why spore-forming probiotic bacteria (mainly from
Bacillus genus), due to their high resistance to environmental stresses, have been the microorganisms of choice in some studies [
5,
10,
11]. The probiotic properties of
B. coagulans have been summarized elsewhere [
12].
Dehydrated fruit slices may be incorporated with probiotics either by direct impregnation with a probiotic suspension [
5,
13,
14], or by having an edible polysaccharide-based coating containing probiotics applied to them [
4,
5,
6], which might help to protect the probiotic, besides potentially helping adhesion of the probiotics to intestinal epithelial cells by hydrogen bonding [
15].
The objective of this study was to obtain probiotic freeze-dried banana slices either by impregnating banana slices with a probiotic suspension or by coating them with a starch-based dispersion containing the probiotics prior to free drying, comparing the products of the two processes.
2. Materials and Methods
2.1. Processing of probiotic banana slices
Two probiotic formulations have been prepared, namely: a probiotic impregnating suspension and a probiotic coating. The impregnation suspension has been prepared by adding 2.5 g of freeze-dried Bacillus coagulans BC4 50 MLD spores (lot C235515A) standardized with maltodextrin and containing about 1011 cfu g−1, as provided by Sacco (Cadorago, Italy), into 500 mL of distilled water (previously autoclaved at 121 °C for 15 min, then cooled back to 25 °C). The probiotic coating was prepared by suspending 10 g of corn starch into 500 mL of distilled water with 3 g of glycerol. The dispersion was kept at 85 °C for 45 min under stirring (150 rpm) for starch gelatinization, then autoclaved at 121 °C for 15 min, cooled back to 25 °C, and added with 2.5 g of the freeze-dried B. coagulans spores.
Over-riped bananas, variety “prata” (Musa sapientum), were purchased from a single supplier in São Carlos, SP, Brazil. They were washed with neutral detergent, rinsed, disinfected with chlorinated water (100 mg L−1) for 5 min, rinsed with water, peeled off, manually cut into 5 mm-thick slices, then blanched in a boiling citric acid 1 wt% solution for 1 min (in order to inactivate enzymes, including polyphenol oxidase, thus avoid enzymatic browning). The slices were separated into four groups, each one containing 60 slices. For each group, the slices received one out of four treatments, namely: COAT-Pro (immersion for 1 min into the starch-based probiotic coating), IMP-Pro (impregnation for 1 min with the probiotic impregnating suspension), COAT (immersion for 1 min into a starch-based coating without probiotics) and C (control—immersion for 1 min into 500 mL of previously autoclaved distilled water). The slices were then pre-frozen in an ultra-freezer at −25 °C for 24 h, then freeze-dried in a Liotop L101 freeze-dryer (Liotop, São Carlos, SP, Brazil) for 6 days.
All equipment and glassware used in the experiment were previously aseptized with ethanol 70 vol% or, when possible, autoclaved at 121 °C for 15 min, in order to avoid interference from contaminants.
2.2. Characterization of probiotic banana slices
From each treatment, three 1-g samples were taken before and after freeze-drying for viable cell counting. The samples were homogenized with 9 mL of a previously sterilized saline solution (0.85 wt% NaCl), then subjected to serial dilutions (up to 10−5), which were plated (in triplicate) by the drop plate method on tryptone glucose yeast extract (TGY) agar and incubated at 37 °C for 48 h. The remaining slices were then pre-frozen in an ultra-freezer at −25 °C for 24 h, then freeze-dried in a Liotop L101 freeze-dryer (Liotop, São Carlos, SP, Brazil) for 6 days.
Moreover, the freeze-dried banana slices were subjected to analyses of shear force and surface color.
The shear forces were measured by using a Knife Edge blade and slotted base (HDP/BS, Stable Micro Systems Ltd., Godalming, UK), with a speed of 2 mm s−1, in order to determine the force required to cross-cut each fragment, simulating the action of incisor teeth on a first bite (Paula & Conti-Silva, 2014). The samples were cut into 7 cm × 2 cm samples and the test was done with ten replicates. The result was expressed as the peak force, in Newtons (N).
The surface color was determined by using a Chroma Meter CR 410 (Konica Minolta, USA), with 24 replicates (measured in two points of each face of 6 slices). After measuring the L*, a*, and b* values, chromaticity (C*) and Hue angle (h°) were determined by the following equations:
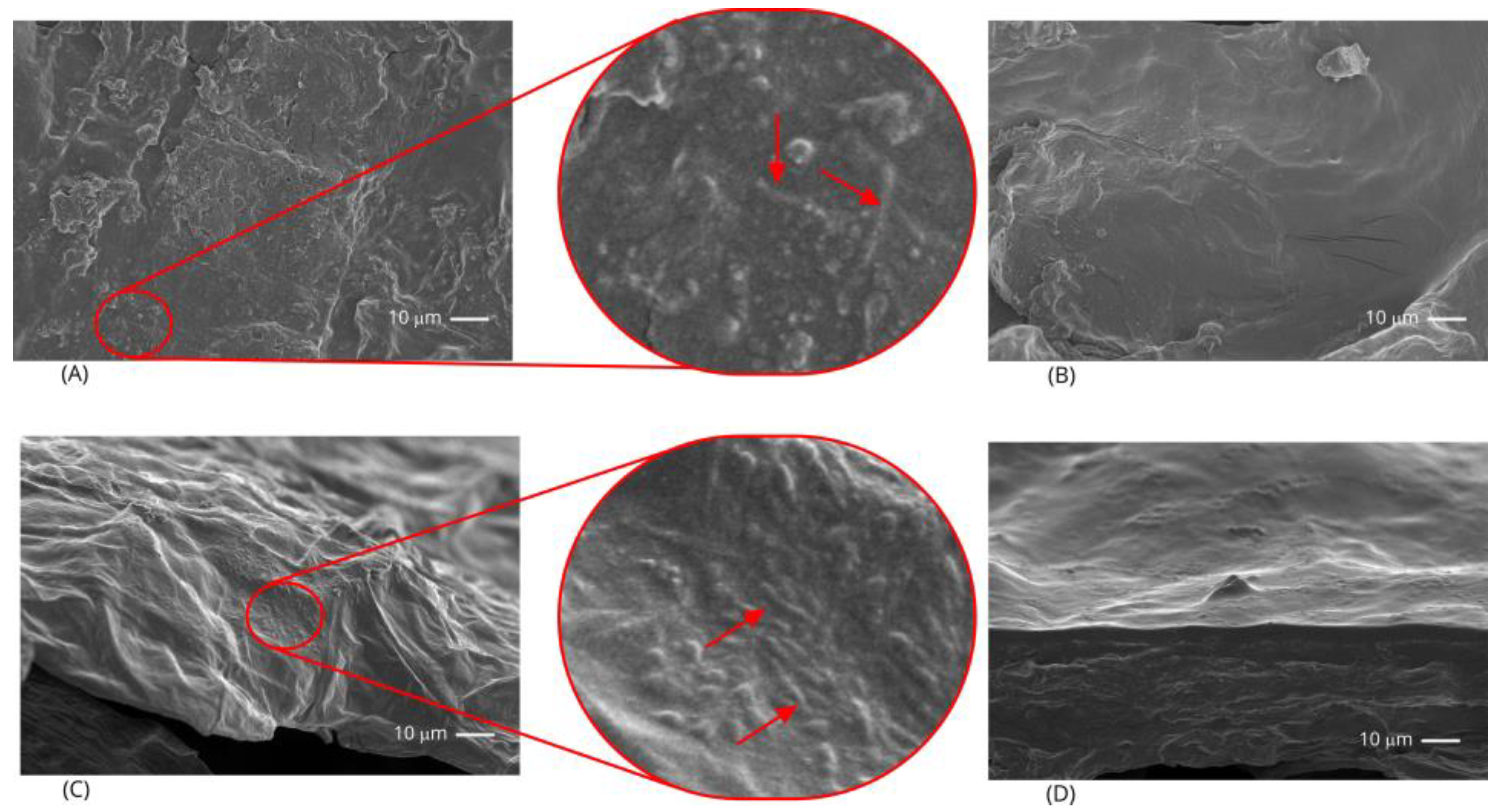
Sections (10 mm2, 1-mm thick) were dissected from the banana slice surfaces for scanning electron microscopy (SEM). The specimens were fixed to aluminum stubs using conductive carbon tape and sputter-coated with a 10 nm-thick gold layer by using the ACE600 Sputter Coater (Leica Microsystems, Wetzlar, Germany). Cross-sections were obtained by immersing slices into liquid nitrogen and fracturing them. The samples were fixed to aluminum stubs (with the fractured surface facing upward) using conductive carbon tape and sputter-coated with a 10 nm-thick gold layer. The images were taken with a JSM 6510 (Jeol, Tokyo, Japan) microscope at 5 kV, with a 1,000× magnification.
A sensory evaluation was carried out in individual booths with white light, with 107 panelists with ages ranging from 18 to 60+ years. Each panelist received a two-slice sample of each of three treatments (namely, COAT-Pro, IMP-Pro, and C) in randomized order, each sample being codified with random 3-digit numbers. The panelists were required to fill out a form containing two questions for each sample. The first question was on overall acceptance, in which they were asked about how much they liked each sample, according to a 9-point structured hedonic scale ranging from 1 (“extremely disliked”) to 9 (“extremely liked”). The second question was about the crispiness of the sample, on a 5-point structured ideal scale ranging from −2 (“much less crispy than ideal”) to +2 (“much crispier than ideal”), 0 representing the ideal crispiness. The averages of both the acceptance test and the crispiness test were compared by Tukey tests. The study was approved by the Research Ethics Committee of the Federal University of São Carlos (UFSCar) (CAAE n. 27815920.7.0000.5504).
3. Results and Discussion
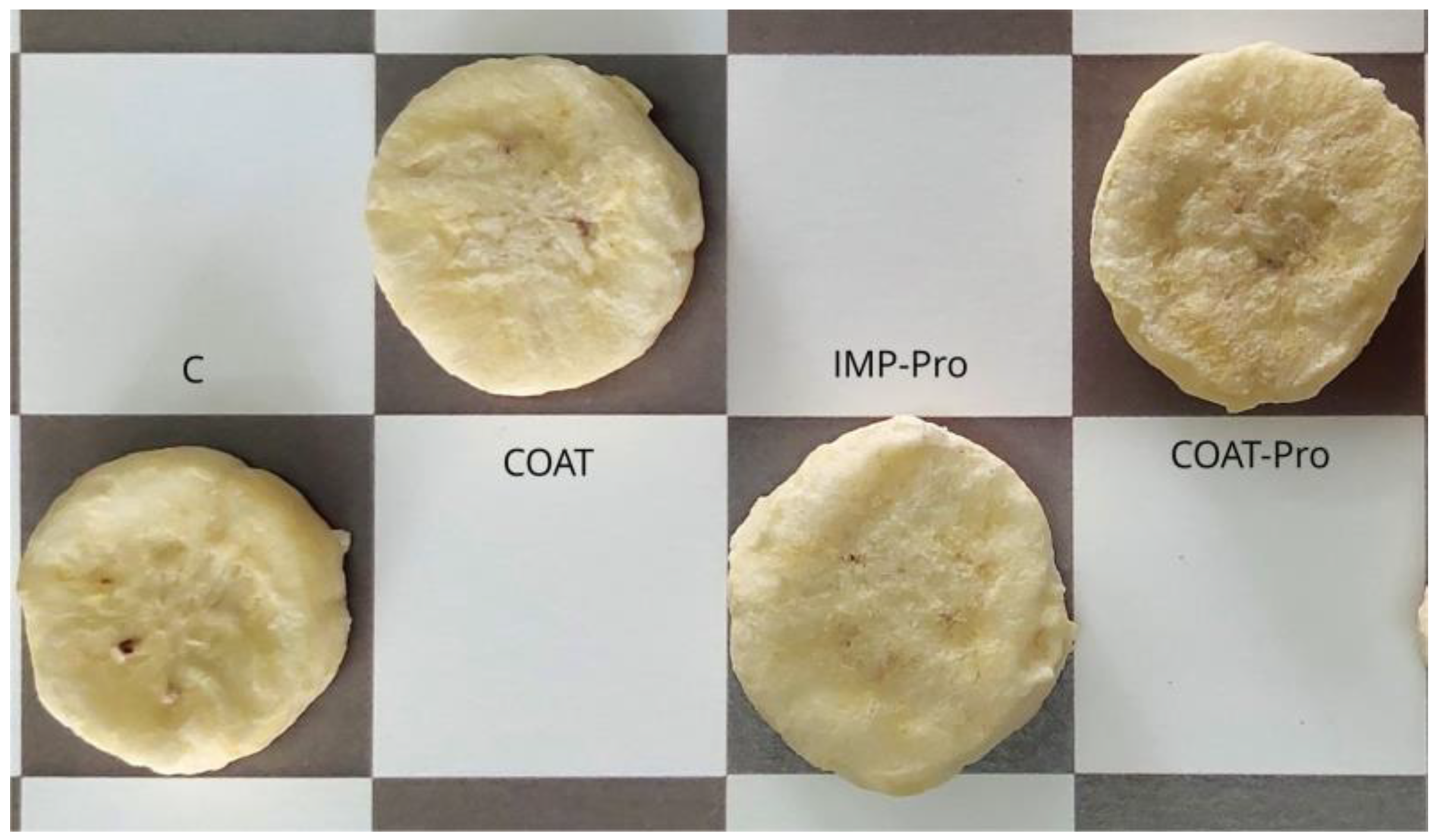
The freeze-dried banana slices presented light color, very similar to the one of fresh slices (
Figure 1), as expected from previously blanched freeze-dried slices. They were also very similar to each other, irrespective of the treatments. The SEM micrographs of the probiotic-containing slices (
Figure 2) showed irregular rough structures (mainly on the surface of impregnated slices). Also, the impregnated slices revealed the presence of bacteria on their surfaces, while the coated slices did not, probably because the bacteria were embedded into the starch matrix.
The viable cell counts of the slices before and after freeze drying were compared. Those of the slices impregnated with probiotics were reduced after freeze drying, while those of the starch-probiotic-coated slices were not significantly reduced (
Table 1), indicating that the starch coating provided some protection to the bacteria, corroborating studies showing protective effects of polysaccharides on probiotic microorganisms [
16,
17].
Table 2 presents the values of shear force and color (chromaticity and Hue angle) of the banana slices from different treatments. There were no significant differences within treatments regarding the color attributes, i.e., neither the presence of coatings nor of probiotics changed the instrumental color, corroborating the appearance similarities shown at
Figure 1. The shear force of the coated slices was significantly higher than those of the uncoated ones, indicating that the presence of a coating increased the force required to fracture the slices, that is to say, coated slices became less crispy. On the other hand, the sensory panel has not perceived significant differences in crispiness within treatments (
Table 3), indicating that the presence of the starch coating has not impaired the sensory crispiness. Also, although the COAT-Pro and IMP-Pro treatments have not received significantly different acceptances, the COAT-Pro samples were more accepted than the control, which was unexpected. The starch coating may have changed the texture in some way as to enhance their sensory acceptability but, since a detailed texture analysis has not been carried out, this is just speculation.
4. Conclusions
Probiotic banana slices have been successfully obtained either by impregnating them with Bacillus coagulans suspension or by coating them with a starch dispersion containing the bacteria, previously to freeze-drying. The presence of the starch coating has avoided a significant loss of cell viability upon freeze-drying, but both processes resulted in viable cell counts above 7 log ufc.g−1. The instrumental texture (shear force) test indicated that the coated slices were less crispy than the impregnated ones but, according to the sensory test, the panelists have not perceived differences in texture (in terms of ideal crispiness) between impregnated and coated slices. The coated slices were as well accepted as the impregnated ones, but more accepted than the non-probiotic control slices. Both methods (impregnation and coating) are simple, and have presented satisfactory results, both in terms of probiotic viability and sensory acceptability.
Author Contributions
Conceptualization, H.M.C.A.; methodology, H.M.C.A., C.M.N., G.M.N.M. and V.F.S.; formal analysis, H.M.C.A.; investigation, C.M.N., G.M.N.M. and L.R.P.; writing—original draft preparation, review and editing, H.M.C.A.; supervision, H.M.C.A.; funding acquisition, H.M.C.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Brazilian National Council for Scientific and Technological Development (CNPq), grant number 408940/2018-2.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to them containing information that could compromise research participant privacy.
Acknowledgments
Author G.M.N.M. thanks the Coordination for the Improvement of Higher Education Personnel (CAPES, grant number 88887.600295/2021-00), and authors C.M.N. and L.R.P. thank CNPq (grant numbers 164843/2021-3 and 154026/2021-2) for their scholarships. Author H.M.C.A. acknowledges CNPq for her Research Productivity Fellowships (308777/2021-2).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Shahbandeh, M. World Production of Bananas in 2021, by Region. Available online: https://www.statista.com/statistics/264003/production-of-bananas-worldwide-by-region/.
- Azeredo, H.M.C.; Matos, M.C.; Niro, C.M. Something to Chew on: Technological Aspects for Novel Snacks. Journal of the Science of Food and Agriculture 2021, 102, 2191–2198. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, M.V.; Bambace, M.F.; Quintana, G.; Gomez-Zavaglia, A.; del Rosario Moreira, M. Prebiotic-Alginate Edible Coating on Fresh-Cut Apple as a New Carrier for Probiotic Lactobacilli and Bifidobacteria. LWT 2021, 137, 110483. [Google Scholar] [CrossRef]
- Galvão, A.M.M.T.; Rodrigues, S.; Fernandes, F.A.N. Probiotic Dried Apple Snacks: Development of Probiotic Coating and Shelf-Life Studies. Journal of Food Processing and Preservation 2020, 44, e14974. [Google Scholar] [CrossRef]
- Oliveira, A.S.; Niro, C.M.; Bresolin, J.D.; Soares, V.F.; Ferreira, M.D.; Sivieri, K.; Azeredo, H.M.C. Dehydrated Strawberries for Probiotic Delivery: Influence of Dehydration and Probiotic Incorporation Methods. LWT 2021, 144, 111105. [Google Scholar] [CrossRef]
- Monteiro, S.S.; Silva, W.P.; Monteiro, S.S.; Gomes, J.P.; Pereira, E.M.; Ferreira, J.P. Probiotic Coating Applied to Papaya Slices for High Quality Snack Production by Convective Drying. Journal of Food Processing and Preservation 2022, 46, e16183. [Google Scholar] [CrossRef]
- Niro, C.M.; de Medeiros, J.A.; Bresolin, J.D.; Dionísio, A.P.; Salgaço, M.K.; Sivieri, K.; Azeredo, H.M. Banana Leathers as Influenced by Polysaccharide Matrix and Probiotic Bacteria. Food Hydrocolloids for Health 2022, 2, 100081. [Google Scholar] [CrossRef]
- Neffe-Skocińska, K.; Rzepkowska, A.; Szydłowska, A.; Kołozyn-Krajewska, D. Trends and Possibilities of the Use of Probiotics in Food Production. Alternative and Replacement Foods 2018, 17, 65–94. [Google Scholar] [CrossRef]
- Kiepś, J.; Dembczyński, R. Current Trends in the Production of Probiotic Formulations. Foods 2022, 11, 2330. [Google Scholar] [CrossRef] [PubMed]
- Elshaghabee, F.M.F.; Rokana, N.; Gulhane, R.D.; Sharma, C.; Panwar, H. Bacillus As Potential Probiotics: Status, Concerns, and Future Perspectives. Frontiers in Microbiology 2017, 1490–1490. [Google Scholar] [CrossRef] [PubMed]
- Soares, M.B.; Martinez, R.C.R.; Pereira, E.P.R.; Balthazar, C.F.; Cruz, A.G.; Ranadheera, C.S.; Sant’Ana, A.S. The Resistance of Bacillus, Bifidobacterium, and Lactobacillus Strains with Claimed Probiotic Properties in Different Food Matrices Exposed to Simulated Gastrointestinal Tract Conditions. Food Research International 2019, 125, 108542. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Yu, Z.; Liu, W.; Zhao, J.; Zhang, H.; Zhai, Q.; Chen, W. Probiotic Characteristics of Bacillus coagulans and Associated Implications for Human Health and Diseases. Journal of Functional Foods 2020, 64, 103643. [Google Scholar] [CrossRef]
- Akman, P.K.; Uysal, E.; Ozkaya, G.U.; Tornuk, F.; Durak, M.Z. Development of Probiotic Carrier Dried Apples for Consumption as Snack Food with the Impregnation of Lactobacillus paracasei. LWT 2019, 103, 60–68. [Google Scholar] [CrossRef]
- Zura-Bravo, L.; Rodriguez, A.; Stucken, K.; Vega-Gálvez, A. Drying Kinetics of Probiotic-Impregnated Murta (Ugni molinae T.) Berries. J Food Sci Technol 2019, 56, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Guillén, M.C.; Montero, M.P. Enhancement of Oral Bioavailability of Natural Compounds and Probiotics by Mucoadhesive Tailored Biopolymer-Based Nanoparticles: A Review. Food Hydrocolloids 2021, 118, 106772–106772. [Google Scholar] [CrossRef]
- Alfaro-Galarza, O.; López-Villegas, E.O.; Rivero-Perez, N.; Tapia-Maruri, D.; Jiménez-Aparicio, A.R.; Palma-Rodríguez, H.M.; Vargas-Torres, A. Protective Effects of the Use of Taro and Rice Starch as Wall Material on the Viability of Encapsulated Lactobacillus paracasei subsp. paracasei. LWT 2020, 117, 108686. [Google Scholar] [CrossRef]
- Pech-Canul, A.D.L.C.; Ortega, D.; García-Triana, A.; González-Silva, N.; Solis-Oviedo, R.L. A Brief Review of Edible Coating Materials for the Microencapsulation of Probiotics. Coatings 2020, 10, 197. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).