1. Introduction
Coronary artery revascularisation has become the most common cardiac surgical procedure performed worldwide. Interestingly, coronary artery bypass grafting as we know it, finds its roots in minimal access approaches, when the first coronary artery bypass grafts were performed through left anterolateral mini-thoracotomies without cardiopulmonary bypass (CPB).
Minimal access cardiac surgery is becoming fashionable, and as more surgeons adopt minimal access techniques there is a need for all cardiac surgeons to be aware of what is becoming available in order that they can offer their patients the best treatment. However, many remain sceptical about minimal access techniques in cardiac surgery and highlight the concerns there are surrounding these approaches and, in many cases, there remains a paucity of evidence demonstrating clear benefit over the traditional median sternotomy, which remains the commonest approach to performing cardiac surgery.
In this review, we aim to focus on the history of minimal access coronary artery revascularisation and move to discuss patient selection and the techniques and evidence supporting the common minimal access approaches to coronary artery revascularisation. These approaches include: minimally invasive direct coronary artery bypass grafting (MIDCAB), totally endoscopic coronary artery bypass grafting (TECAB) and hybrid coronary revascularisation (HCR).
2. Materials and Methods
A search was conducted on the PUBMED online database, using the following search terms “minimally invasive coronary artery revascularisation”, “minimal access coronary artery revascularisation”, “minimally invasive cardiac surgery coronary artery bypass grafting”, “robotic assisted cardiac surgery”, “endoscopic cardiac surgery”, “robotic assisted thoracoscopic surgery coronary artery bypass graft surgery” “minimally invasive direct coronary artery bypass graft”, “total endoscopic coronary artery bypass grafting” and “hybrid coronary revascularisation”. The search was limited to reviews, meta-analyses and randomised controlled trials (RCT) from January 1997 to December 2022.
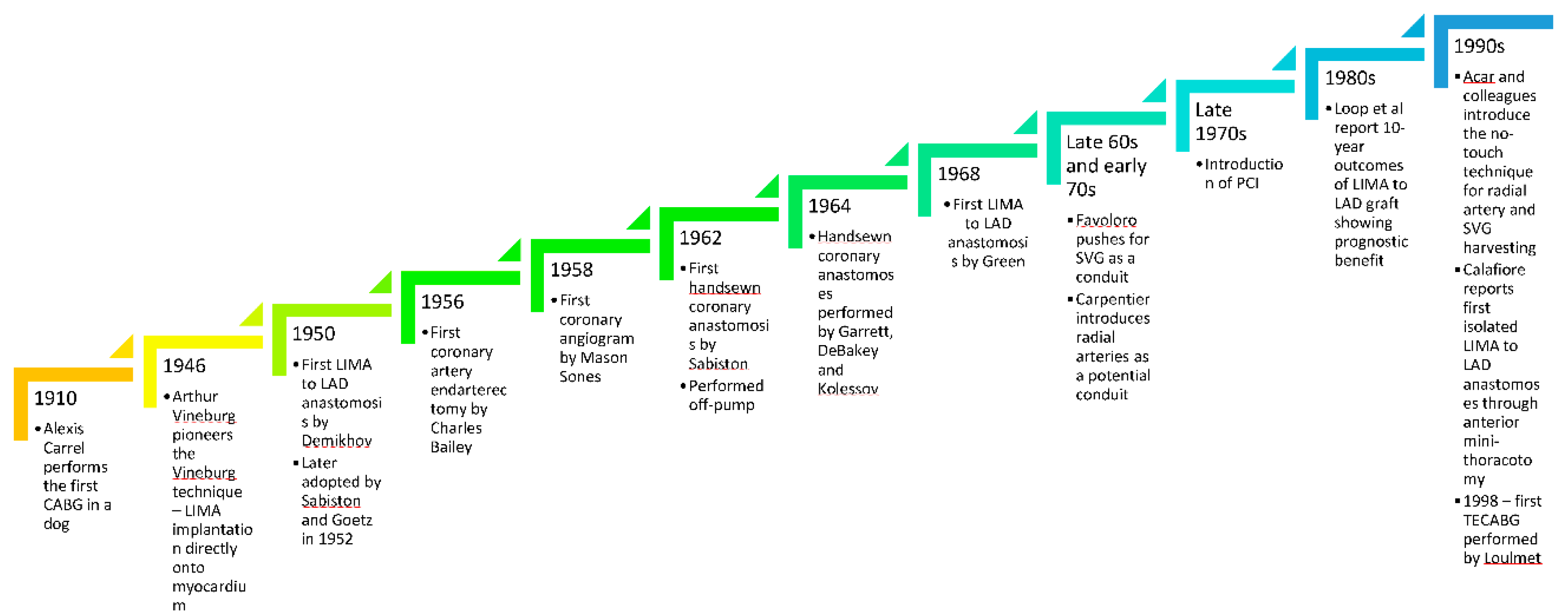
3. History of Coronary Artery Bypass Grafting
The history and evolution of coronary artery bypass grafting (CABG) has been rife with successes and failures. The first CABG was performed by Alexis Carrel [
1,
2] in 1910 in dogs before the advent of coronary angiography or cardiopulmonary bypass (CPB). In the 1930s, John Gibbon invented the CPB machine which revolutionised cardiac surgery [
3]. Later in 1946 Arthur Vineburg [
4,
5] pioneered the Vineburg technique, whereby he implanted the left internal mammary artery (LIMA) directly onto the left ventricular myocardium, which led to symptomatic relief of angina and was shown to still provide good cardiac function 30 years later [
6]. The first LIMA to left anterior descending artery (LAD) anastomosis, using a non-suture technique with tantalum rings, appeared a few years later in 1952, when Demikhov showed graft patency in the LIMA to LAD anastomosis at 2 years, a practice that was also adopted by others in Canada [
7] and the US [
8]. In 1956, Charles Bailey successfully performed coronary artery endarterectomies as a way to treat coronary artery atherosclerosis [
9].
The issue however, remained that the arteries could not be imaged and therefore the uncertainty of which arteries caused the symptoms persisted. This changed in 1958, when Mason Sones [
10] inadvertently performed the first coronary angiogram by accidentally injecting dye in the right coronary artery when attempting to image a patient with rheumatic heart disease. He then went on to further develop coronary angiography – an achievement which changed the history of cardiovascular medicine.
In 1962, Sabiston [
7] performed the first hand-sewn coronary anastomosis, by suturing a saphenous vein graft to the right coronary artery – a procedure performed without CPB, but it was not reported until 1974. Garrett [
11] and DeBakey in Houston also performed hand-sutured coronary anastomoses in 1964, but did not report it until 1973, when the grafts remained patent 7 years later. Kolessov on the other hand, reported his first few CABGs with hand-sutured coronary anastomoses early on in 1967 – all procedures performed without CPB in 1964 [
12]. Despite being heavily involved in pioneering CPB as an artificial circulation for open heart surgery, Kolessov was a great proponent of off pump CABG owing to the large inflammatory response that CPB generated at the time. It was not till 1968 that Green [
13] in New York performed the first hand-sutured LIMA to LAD anastomosis which has since become the cornerstone of coronary artery revascularisation.
Moving on to the late 60s and early 70s, Favoloro [
14] in Cleveland Clinic really pushed forward the use of saphenous vein grafts as a conduit during coronary artery revascularisation. However, it was realised early on that owing to intimal [
15,
16] and medial thickening and graft thrombosis secondary to intimal hyperplasia and premature atherosclerosis of the vessel, saphenous vein grafts were prone to stenosis and occlusion. Carpentier [
17] started using radial arteries as a conduit – the early experience of which was not as successful as it is today. The introduction of the no touch technique of vein and radial artery harvesting in the early 1990s by Acar [
18,
19] as well as the use of vasodilators for radial artery grafts significantly improved long-term patency of veins and radial arteries as conduits for coronary artery revascularisation and revived the interest in using radial arteries as a conduit. It was only in the 1980s that the LIMA to LAD anastomosis was proven beyond doubt to have a prognostic benefit when Loop et al in Cleveland clinic reported their 10-year outcomes [
20].
Meanwhile, in the late 1970s, cardiologists had started developing percutaneous catheter-based interventions (PCI), initially with balloon angioplasty [
21] but progressing to stenting and then more recently using drug eluting stents to overcome the complications of in-stent restenosis observed in early versions of bare metal stents. PCI had the overwhelming advantage of being less painful, with a shorter recovery and smaller risk of stroke.
To potentially challenge these advantages of PCI, but still obtain the higher survival rates that surgery conferred, the surgical community began to turn to minimal access coronary surgery. In the mid-90s, Calafiore reported isolated LIMA to LAD anastomoses performed through an anterior thoracotomy [
22]. This has since progressed more recently to coronary revascularisation performed with fully thoracoscopic and robotic methods, with the first TECABG being performed by Loulmet [
23] in 1998. Now, many centres around the world have introduced minimal access coronary surgery with varying permutations: From mini thoracotomy off-pump LIMA to LAD anastomosis in MIDCAB, to fully robotic complete revascularisation.
A timeline summarising major events in coronary artery revascularisation has been summarised in
Figure 1.
4. Minimal Access Coronary Revascularisation – International Guidelines Perspective
The 2018 EACTS/ESC guidelines on myocardial revascularisation do not make any formal recommendation regarding minimal access surgery, but they do mention that it is an attractive alternative to conventional approaches to CABG surgery [
24].
The guidelines do highlight HCR to be an appealing management strategy, whether performed sequentially, i.e., minimal access LIMA to LAD anastomosis followed by PCI to the non-LAD vessels in another setting or performed in a hybrid theatre in one session, quoting the POL-MIDES RCT [
25,
26] where in a small group of 200 patients, conventional surgery and HCR had similar outcomes at 5 years. Of course, it is important to consider if 5-year outcomes are long-term enough to justify non-inferiority of HCR as compared to more traditional approaches though.
Similarly, the 2021 ACC/AHA/SCAI guidelines on coronary artery revascularisation comment that the role of HCR remains unclear and do not make any formal recommendation as to when it can or should be used [
27]. They, however, do not comment on any other method of minimal access of surgical coronary artery revascularisation.
5. Patient Selection and Rationale for Minimal Access Coronary Intervention
The surgical indication for minimal access coronary revascularisation remains unclear in the literature. Some small studies in the early 1990s and 2000s [
28] report the use of minimal access surgical coronary artery revascularisation for patients with isolated coronary artery disease, isolated LAD lesions or proximal right coronary artery disease. However, the conduct of minimal access surgical coronary artery revascularisation, from patient selection, use of CPB or lack thereof, to even conduit selection for different lesion sets, is too varied to make any reasonable conclusion as to where the actual benefit of minimal access CABG lies. The advantage of minimal access coronary artery revascularisation presumably is more apparent in patients with uncontrolled diabetes or multiple co-morbidities, which confer higher risk of sternal wound non-healing, breakdown and infection. In addition, in those performing minimal access CABG off-pump, there are added benefits such as reduced stroke [
29] rate from absence of aortic manipulation and cross-clamping, decreased inflammatory [
30] response from the bypass circuit leading to lower rates of acute kidney injury [
31] and fewer blood transfusions. Moreover, if a mini-thoracotomy is performed, no bone healing is required post-operatively, allowing patients to return to their normal lifestyle more rapidly. With the smaller incisions, patients can be extubated faster and there are fewer complications of respiratory failure. Diegeler et al in a small prospective trial suggested that after post-operative day 4, MIDCABG had lower rates of pain as compared to conventional CABG [
32].
There are some patient features which are favourable to minimal access CABG, including being slim and having a thin, tubular and vertically positioned heart. LAD lesions that tend themselves to minimal access surgery are those with a non-calcified distal segment (approximately 2-4 cm distal to the second diagonal branch), those with an arterial diameter greater than 1.75mm and total occlusion of the LAD with good collateral circulation [
33].
However, it should be kept in mind that while some centers consider multivessel disease a contraindication to minimal access coronary revascularisation, others regularly perform multi vessel grafting using minimal access methods.
6. Contraindications to Minimal Access Coronary Revascularisation
The only absolute contraindications to using a minimal access approach are an occluded left subclavian artery, which prevents the use of the LIMA, particularly in hybrid procedures where the benefit is that of a LIMA to LAD anastomoses, and patients in cardiogenic shock requiring emergent LAD revascularisation, owing to the longer LIMA harvesting time and owing to the longer setup time for certain methods of minimal access surgery [
34].
Relative contraindications depend on the surgeon, institution and their experience. These include extreme obesity which makes access and LIMA harvesting more challenging, deep intramural and calcified LAD grafting sites which are more challenging to identify in a minimal access setting, previous thoracotomy, re-do surgeries and the presence of dense adhesions which all restrict exposure and distort the anatomy, presence of severe pulmonary hypertension with a large left ventricle, making a minimal access approach higher risk and more technically challenging. While some co-morbidities would lend themselves for patients to have better outcomes with minimal access surgery, they can often be prohibitive as well. For example, patients who are unable to tolerate single lung ventilation might not be able to undergo minimal access surgery, despite potentially benefitting greatly from the early extubation and reduced rates of respiratory failure observed with minimal access surgical coronary revascularisations. In addition, the presence of significant peripheral vascular disease may mean that going onto peripheral cardiopulmonary bypass via the femoral vessels may not be an option intra-operatively, if cardiopulmonary bypass were required – such patients should be treated with caution [
33,
34,
35].
7. Techniques of Minimal Access Coronary Artery Revascularisation
In this section we will describe the common approaches to minimal access coronary revascularisation surgery. For each, describing patient positioning as well as some technical considerations. The advantages and disadvantages of the different techniques are summarised in
Table 1.
7.1. MIDCABG
7.1.1. Description
MIDCABG has been described using multiple methods and approaches in the literature. The first few descriptions of MIDCABG surgery were purely describing a LIMA-LAD anastomosis. The surgical technique has now evolved to include multi-vessel grafting. While most commonly performed via a left anterior mini-thoracotomy in the fourth intercostal space in the infra-mammary fold underneath the nipple with 2/3 of the incision being medial and 1/3 lateral to the nipple [
35], some centres also describe accessing the chest via a upper partial sternotomy or inferior partial sternotomy. MIDCABG started out as being a way of performing open heart coronary artery revascularisation with no sternotomy but has gradually evolved to using endoscopic instruments to facilitate the process.
7.1.2. Positioning and Monitoring
The patient should be placed in an antero-lateral decubitus position with the left chest and left buttock elevated by approximately 20-30 degrees using a bolster, if the approach is to be through a left antero-lateral mini-thoracotomy. If a partial superior or partial inferior sternotomy is to be used, then the patient can be in a supine position. The arms of the patient should be tucked at the sides. Regardless of whether peripheral CPB is used routinely or as a safety measure for emergent situations, the left groin should be prepared and draped. A guidewire is sometimes inserted under ultrasound guidance into the left femoral artery prior to prepping and draping to facilitate emergent institution of CPB if required. External pacing and defibrillator pads, as well as warming blankets, should also be routinely placed and connected. Each institution will have their own monitoring protocols. However, it is advisable to use a pulmonary artery catheter in patients with a left ventricular ejection fraction of <30%, ECG monitoring for ischaemia, urinary bladder catheterisation and temperature probe insertion. Transoesophageal echocardiography is used in patients with poor ventricular function or who are higher risk of becoming haemodynamically unstable.
7.1.3. Operative Steps
The most common approach is through a 5-6cm left anterolateral muscle-sparing mini-thoracotomy in the 4th or 5th intercostal space, 2-3 cm inferior to the nipple [
35]. One-lung ventilation is used to facilitate exposure. A retractor is used for LIMA harvesting, either skeletonised or pedicled, as per surgeon’s preference. In cases where bilateral IMAs will be used, bilateral mini-thoracotomies can be performed. After harvesting the LIMA, beforedividing, the patient is heparinised, the pericardium opened longitudinally, usually 1-2 fingerbreadths lateral to the LIMA pedicle, suspended with traction sutures and the LAD identified. The lateral traction sutures are pulled upward to the upper part of the wound, which rotates the heart, exposing the LAD facilitating anastomosis [
35]. The distal end of the LIMA is then divided and prepared for anastomosis. The edges of the pericardium and selective lung inflation can be used to improve visualisation of the LAD. A suction stabiliser is used to stabilise the LAD for anastomosis. Either a pledgeted tourniquet can be applied around the LAD proximal to the anastomosis, or a soft vascular clamp used to occlude the LAD to allow for a bloodless field. Alternatively, a shunt can also be used. Once the anastomosis is performed, the flow can be verified, following restoration of blood flow through the LAD and removal of the pledgeted tourniquet or vascular clamp, for example using Transit Time Flow Measurement. Haemostasis is performed and heparin reversed. The pericardium is closed around the apex and a chest drain is inserted into the left pleura. The thoracotomy is then closed as per usual [
33,
36].
7.1.4. Evidence
Patel et al published a best evidence topic comparing MIDCABG and PCI for patients with isolated LAD disease in 2014 [
37]. They looked at 13 studies and concluded that both are effective treatments. PCI has higher rates of need for reintervention for symptom recurrence. Despite having a higher upfront cost, MIDCABG is more cost-effective owing to the lower rate of reintervention. There was no significant difference in mortality between both groups.
In 2015, Raja et al [
38], on behalf of the Harefield Cardiac Outcomes Research Group, compared propensity score matched patients undergoing MIDCABG versus full sternotomy revascularisation for isolated LAD disease, with 143 matched sets. In 2018, they compared the short- and long-term outcomes of MIDCABG versus full sternotomy off-pump LIMA to LAD anastomosis for isolated proximal LAD stenosis [
39]. They looked at 668 patients, with 508 patients in the MIDCABG group and 160 patients in the full sternotomy off-pump group. The average operative time was significantly shorter in the full sternotomy group, 141+/-12 min in the median sternotomy group versus 177+/-32 min in the MIDCABG group, p=0.003. There was no significant difference between both groups in terms of the short-term outcomes. The long-term mortality at a median follow-up of 12.95+/-0.45 years was 25% in the full sternotomy off-pump group, as compared to 22.24% in the MIDCABG group, p=0.64.
A study by Repossini et al in 2019 [
40] looked at 1060 patients undergoing MIDCABG, 646 of which had isolated proximal LAD disease and the rest of which had multivessel disease managed either with HCR or MIDCABG and optimal medical therapy. The reported cardiac-related mortality was 92.1% +/- 4.6% at 5 years and 85.3% +/- 6.3% at 15 years, with an overall perioperative mortality of 0.8%.
Manuel et al [
41] recently published their 20-year outcomes of MIDCABG surgery in patients undergoing LIMA to LAD anastomosis. Their cohort consisted of 271 patients – overall survival was 91.9%, 84.7%, 71.3% and 56.5% at 5, 10, 15 and 20 years with patients with isolated LAD disease doing significantly better than patients with multivessel disease (p=0.0035). There were no patients who required reintervention on the LAD post operatively.
Ultimately, there are no robust RCTs comparing MIDCABG and PCI or MIDCABG and conventional CABG via a median sternotomy and this presents a gap in literature.
7.2. TECABG/RACABG
7.2.1. Description
TECABG is currently the least invasive form of surgical coronary artery revascularisation. It is performed via few port sites, occasionally using a remotely controlled robotic system. Robotic-assisted TECABG can be further divided into three surgical techniques: TECABG without CPB, TECABG with CPB and robotic-assisted LIMA harvest followed by off-pump LIMA to LAD manual anastomosis. Other options also include a video-assisted LIMA harvest, followed by manual LIMA to LAD anastomosis via a small anterior mini thoracotomy.
7.2.2. Positioning and Monitoring
The position of the patient depends on the approach. If the procedure is performed without robotic assistance and by using video thoracoscopic assistance, the patient is placed in a left lateral decubitus position, 30-60 degrees from the horizontal line with the arm above their head [
42]. If robotic assisted TECABG is performed, the patient is placed supine with the left side elevated to 30 degrees and the left arm tucked in at the side [
43,
44].
Defibrillation pads are plated on the patient pre-operatively. Monitoring is similar to that for MIDCABG.
7.2.3. Operative Steps
TECABG is performed with the help of video-assisted thoracoscopy (VATS) or robot-assisted thoracoscopy (RATS). A controlled pneumothorax is induced using carbon dioxide insufflation. This can help create a visual field without one-lung ventilation. However, sometimes, one-lung ventilation may be required, in which case either a double lumen endotracheal tube or a bronchial blocker can be used. The LIMA and/or RIMA can both be harvested from the left chest using VATS or RATS instruments via ports in the 2nd, 3rd and 4th intercostal spaces, approximately 2cm above and below the anterior axillary line, triangulating towards the mediastinum. However, the port placement can be changed depending on the surgeon, patient body habitus and position of the target vessels. The patient is then heparinised, and the distal ends of the mammary arteries transected. The pericardium is opened longitudinally, anterior to the left phrenic nerve and all target vessels are identified and correlated with angiographic findings. Once the target vessels are identified and located, a 3-4 cm port is created directly above the heart close to the midline in the selected intercostal space. CPB can be instituted peripherally via the femoral vessels. A pledgeted purse-string suture for antegrade cardioplegia is inserted in the ascending aorta. After decompression of the right atrium on CPB, an endoscopic transthoracic clamp is inserted in the 2nd right intercostal space in the anterior axillary line and placed across the ascending aorta. Cardioplegia is then delivered in an antegrade fashion, via an endoscopically placed vent needle in the proximal ascending aorta. It should be noted in cases of robotic assisted TECABG, aortic occlusion in on-pump procedures can also be achieved using an endovascular occluding balloon placed and inflated in the ascending aorta under transoesophageal ultrasound guidance. If the procedure is being carried out off-pump, one of the ports is used to inserted tissue-stabilising devices. In procedures where only the LIMA harvest is performed using the robotic system, once the LIMA is harvested, the robot is undocked and the remainder of the procedure performed as per MIDCABG. Pericardial stay sutures, epicardial stay sutures or gentle traction of the emptied heart through a small subxiphoid incision can help visualise the target vessels to facilitate anastomosis. In cases where the remainder of the procedure is performed using MIDCABG technique, the heart is positioned close to the utility port in the 4th intercostal space close to the midline and the anastomosis is performed manually. If the robotic system is being used for the distal anastomoses as well, the pericardium is opened and the robotic arms used to manipulate the heart and perform the anastomosis, described in detail by Bonatti et al [
43] and Lee et al in 2012 [
45]. Y-grafts to the LIMA are generally used for the non-LAD vessels to avoid aortic manipulation. Alternatively, saphenous vein grafts can be sutured to the axillary artery prior to performing the distal anastomosis – they are endoscopically transferred into the left pleural space through an opening next to the LIMA harvest site. After all anastomoses are completed, haemostasis is performed, and the heparin is reversed with protamine. The pericardium is closed using interrupted sutures apart from channels for the LIMA and/or RIMA. Drains are placed in each intra-thoracic cavity. Ports are closed in a standard fashion, in layers [
46].
7.2.4. Evidence
There have been no RCT comparing the different types of TECABG and comparing TECABG to conventional CABG.
A systematic review by Cao et al [
47] included 44 studies and a total of 8034 patients revealed a pooled perioperative mortality rate of 1.7% and 1.0% after off-pump TECAB and robotic assisted MIDCABG groups, bearing in mind that in the majority of studies, the number of anastomoses was relatively few and patients were relatively young, with a mean age of 60 and good pre-operative left ventricular function, with a mean ejection fraction of more than 55%. Unfortunately, long-term survival was not available owing to limited follow-up rates in the included studies.
Although there have been no RCT’s comparing outcomes of conventional CABG and TECABG, a study by Kofler et al 2017 [
48] compared 134 propensity score matched pairs of conventional CABG and robotic TECABG. The primary endpoints were long-term survival and freedom from major adverse cardiac and cerebral events (MACCE). There was no significant difference in the primary endpoints between both groups at 1, 5 and 10 years. The survival at 1, 5 and 10 years was 99.3%, 96.9% and 81.3% in the robotic group versus 96.3%, 92.2% and 82.6% in the conventional group, p=0.960. Freedom from MACCE in the robotic group at 1, 5 and 10 years was 97.6%, 96.8% and 96.8% versus 100%, 97.7% and 92.8% in the conventional group, p=0.790. Of note, robotic TECABG had significantly longer CPB times (robotic 112+/-100 minutes versus conventional 67+/-48 minutes, p<0.001) and cross -clamp times (robotic 68 +/- 54 minutes versus 38 +/- 27 minutes in the conventional group, p<0.001.)
A meta-analysis by Leonard et al in 2018 looking at the outcomes of TECABG including 17 studies including 3721 patients demonstrated that TECABG has acceptably low operative risk [
49] but there was a severe dearth of data to confidently recommend TECABG. The pooled operative mortality for 3676 patients was 0.8% with 95% CI 0.6-1.2%. Pooled perioperative myocardial infarction event rate for 2556 patients was 2.28% with 95% CI 1.7-3%. The overall pooled graft patency rate was 94.8%. The pooled event rate for perioperative stroke was 1.5% with a 95% CI 1.1-1% with 3353 patients being included.
Gobolos et al [
50] published a systematic review of the clinical outcomes of TECABG over the last 20 years in 2019. The pooled results included 2397 cases and reported a perioperative mortality of 0.8%, with conversion rates of 11.5% and an average surgical time of 291 +/- 57 minutes. Comparing beating heart TECABG (BH-TECABG) and arrested heart TECABG (AH-TECABG) revealed a perioperative mortality of nearly 1% for BH-TECABG and 0.6% for AH-TECABG.
Similarly, a meta-analysis in 2020 by Hammal et al looking specifically at robotic TECABG included 13 studies and reported that although robotic coronary artery surgery was feasible and certainly an appealing alternative to conventional surgery [
51], the level of evidence was too low to make any significant conclusions regarding the benefit of robotic TECABG over conventional CABG in terms of short and long-term outcomes including perioperative mortality, long-term survival, perioperative stroke, perioperative or late MI and rate of revascularisation. The data was too heterogenous to compare pooled event rates between robotic TECABG and conventional CABG.
7.3. HCR
7.3.1. Definition
CABG remains the guideline recommended management option for many patients with multivessel coronary artery disease, with superior long-term survival rates [
52]. It has been posited that the superiority of CABG lies with the LIMA to LAD anastomosis [
53]. For non-LAD lesions, PCI potentially confers similar long-term results as saphenous vein grafts. This principle forms the basis of hybrid minimal access surgery, where the LIMA to LAD anastomosis is performed by minimal access surgery and the other lesions managed percutaneously [
54].
Hybrid coronary artery revascularisation combines the prognostic benefit of the LIMA to LAD anastomosis through minimal access surgery with the advantages of less pain, decreased length of hospital stay and ability to continue dual antiplatelet agents that PCI confers [
55,
56]. While it is difficult to specify a target patient population owing to the lack of RCT evidence, the ideal patient would be a high-risk surgical patient with complex or non-stentable LAD lesions, who would reap the benefits of the LIMA to LAD anastomosis, but with concurrent stentable non-LAD lesions.
There are three options for HCR: simultaneous revascularisation in a hybrid theatre, surgery followed by PCI or PCI followed by surgery [
57,
58]. The latter option could follow such an example, where the culprit artery causing an infarct is a non-LAD artery which can be stented, perhaps acutely, with concurrent LAD lesions requiring surgery performed soon thereafter. Whether the surgery is performed via MIDCABG or TECABG is up to the Heart team and the institution’s experience.
7.3.2. Evidence
The POL-MIDES (HYBRID) trial in 2014 published by Gasior
et al randomized 200 patients with multivessel disease to undergo either HCR (n=98) or CABG (n=100). The primary endpoint was evaluating the feasibility of HCR, which was defined as the percentage of patients who had a completely hybrid approach with LIMA to LAD followed by PCI with Drug-eluting stents. 93.9% of patients randomized to HCR group had a complete hybrid procedure with 6.1% converting to a standard CABG. The secondary endpoints were post-procedure and angiographic measurements of the graft patency and restenosis rates at 12 months, among others. The mortality from CABG was 2.9% as compared to 2% in the HCR group, p=0.1. HCR had a higher HYBRID patency score (free of stenosis/occlusions grafted or ratio of stented arteries to total number of grafted and stented arteries) at 90% as compared to 81% in the CABG group, p=0.01 [
25].
In 2019, Ganyukov et al, in the Hybrid coronary REvascularisation Versus Stenting or Surgery (HREVS) prospective randomised safety and efficacy study compared conventional CABG (n=50), HCR (n=52) or multi-vessel PCI (n=53), with residual ischaemia as their primary endpoint. They concluded that the percentage of ischaemic myocardium in CABG, HCR and PCI were 6.7% (95% CI 4.6%-8.8%), 6.4% (95% CI 4.3%-8.5%) and 7.9% (95% CI 5.9%-9.8%), p=0.45. The rates of MACCE, one of their secondary endpoints, in CABG, HCR and PCI were 12%, 13.4% and 13.2% respectively, p=0.83. The main limitation quoted was that the study was severely underpowered and therefore not conclusive [
59].
In 2020, Esteves et al published their results of a pilot RCT, the Myocardial hybrid revascularization versus coronary artery bypass GraftING (MERGING study) for complex triple-vessel disease comparing HCR to conventional CABG, with 40 patients in the hybrid arm and 20 patients in the conventional CABG arm. They concluded that HCR, while feasible, was associated with higher rates of MACCE defined as all-case death, stroke, MI and unplanned revascularisation during the first 2 years as compared to conventional surgery, with 19.3% MACCE rate at 2 years in the HCR group versus 5.9% MACCE rate in the conventional group [
60].
Guan et al in 2019 [
28] published a meta-analysis comparing other modalities of minimal access CABG with HCR which summarised 8 observational studies which concluded that HCR was non-inferior to other modalities of minimal access CABG, in terms of in-hospital mortality, rates of MACCE, shock, perioperative MI, long-term survival, cost and surgical complications. On the other hand, Nagraj et al 2022 concluded in their meta-analysis including 12 observational studies and 2 RCTs comparing HCR to conventional CABG via a median sternotomy in multi-vessel coronary artery disease that although feasible, HCR did not have any clear benefits over conventional surgery [
61].
8. Nomenclatures
The large variation in the nomenclature used to describe minimal access surgical techniques for coronary artery revascularization renders interpretation of literature challenging and makes comparison of the different techniques challenging. Just to name a few, the terms MIDCABG, MICS CABG, TECABG, AH-TECABG [
62], PA CABG [
63] and RACABG have all been used to describe various minimal access cardiac surgery. Some of these terms are used interchangeably by some authors but considered distinct by others. For example, some papers claim that MIDCABG and MICS CABG are completely different modalities, while others use the terms interchangeable. Similarly, some papers consider TECABG and RACABG to be distinct modalities, while some authors describe in detail how they use either VATS or RATS to perform TECABG. We would posit that standardization of terms is an imperative step to allow robust comparison of minimal access techniques, be it as compared to each other or to conventional CABG.
9. Future Perspectives
Minimal access techniques are gaining popularity in all areas of surgery. The number of cardiac surgical centres with access to minimal access techniques and surgical robots is continuously increasing. Mitral valve surgery is a particularly hot area for minimal access surgery – and publication of the results of the UK Mini Mitral Trial are eagerly awaited. For coronary revascularization, it is important that these new techniques are cautiously adopted and experience accumulated. For this, large RCTs are required, to develop the evidence base to support use of these techniques and demonstrate conclusively that they are beneficial to patient outcomes. Demonstrating this through trials will be essential to gaining wider adoption of these techniques, and for some the ability to justify the expense of the technology to hospital management.
Thankfully, there are trials ongoing. For example, the Minimally Invasive Coronary Surgery Compared to STernotomy Coronary Artery Bypass Grafting RCT (MIST trial) is an upcoming prospective RCT, comparing the outcomes of minimal access coronary revascularization to those of conventional CABG [
64]. The primary outcome is the quality of life using the physical function score of Short Form Health Survey (SF-36) four weeks after surgery. Secondary outcomes include MACCE and Target Vessel Revascularisation at 1 year after surgery, the number of bypass grafts, the percentage of arterial graft use, use of transfusion intra-operatively and post-operatively, rates of re-exploration for bleeding, post-operative pain, duration of intubation, length of stay on intensive care unit, length of hospital stay, rates of post-operative atrial fibrillation and wound infection, post-operative angina and quality of life in terms of their mental health. It is currently still in the enrolment phase, projected to be completed primarily in March 2024.
10. Conclusion
Minimal access surgery appears to be the future. It is increasingly demanded by referring cardiologists, but also patients who both perceive the surgery to be superior. Minimal access coronary artery revascularization represents a very appealing management approach to coronary artery disease. It incorporates the benefits of surgical revascularization with some of the advantages of off-pump surgery and PCI, with less pain, shorter hospital stays, earlier mobilization and earlier return to work for patients. However, the challenge is to ensure that that the benefits of surgical revascularization with complete revascularization and patency of grafts remain uncompromised by using a minimal access approach. Given the paucity of RCTs regarding methods of minimal access coronary artery revascularization, it is challenging to make any robust recommendations. Part of this comes from the large variation in nomenclature of methods of minimal access coronary artery revascularization and the very slow uptake of minimal access methods across different surgical units.
Author Contributions
Conceptualization, J.A.; methodology, R.P.; writing—original draft preparation, R.P.; writing—review and editing, R.P. and J.A.; supervision, J.A. All authors have read and agreed to the published version of the manuscript.
Funding
This review received no external funding
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| AH -TECABG |
Totally Endoscopic Arrested Heart Coronary Artery Bypass Grafting |
| CABG |
Coronary Artery Bypass Grafting |
| CPB |
Cardiopulmonary bypass |
| HCR |
Hybrid Coronary Revascularisation |
| LAD |
Left Anterior Descending Artery |
| LIMA |
Left Internal Mammary Artery |
| MICS CABG |
Minimally Invasive Coronary Artery Bypass Grafting |
| MIDCABG |
Minimally invasive Coronary Artery Bypass Grafting |
| PA CABG |
Port Access Coronary Artery Bypass Grafting |
| RACABG |
Robotic Assisted Coronary Artery Bypass Grafting |
| RATS |
Robot Assisted Thoracoscopic Surgery |
| RCT |
Randomised Control Trial |
| RIMA |
Right Internal Mammary Artery |
| TECABG |
Totally Endoscopic Coronary Artery Bypass Grafting |
| VATS |
Video Assisted Thoracoscopic Surgery |
References
- Carrel, A. On the Experimental Surgery of the Thoracic Aorta and Heart. Ann. Surg. 1910, 52, 83–95. [Google Scholar] [CrossRef] [PubMed]
- Melly, L.; Torregrossa, G.; Lee, T.; Jansens, J.-L.; Puskas, J.D. Fifty years of coronary artery bypass grafting. J. Thorac. Dis. 2018, 10, 1960–1967. [Google Scholar] [CrossRef] [PubMed]
- Diodato, M.; Chedrawy, E.G. Coronary artery bypass graft surgery: The past, present, and future of myocardial revascularisation. Surg. Res. Pract. 2014, 2014, 1–6. [Google Scholar] [CrossRef]
- Vineberg, A.; Miller, G. Internal mammary coronary anastomosis in the surgical treatment of coronary artery insufficiency. Can. Med. Assoc. J. 1951, 64, 204–210. [Google Scholar]
- Thomas, J.L. The Vineberg legacy: Internal mammary artery implantation from inception to obsolescence. Tex. Heart Inst. J. 1999, 26, 107–113. [Google Scholar]
- Rozsival, V. Outcome of Vineberg's operation after 31 years. Heart 2006, 92, 1070. [Google Scholar] [CrossRef]
- Sabiston, D.C., Jr.; William, F.; Rienhoff, L., Jr. ; The coronary circulation. Johns. Hopkins Med. J. 1974, 134, 314–329. [Google Scholar]
- Goetz, R.H.; Rohman, M.; Haller, J.D.; et al. Internal mammary-coronary artery anastomosis. A nonsuture method employing tantalum rings. J. Thorac. Cardiovasc. Surg. 1961, 41, 378–386. [Google Scholar] [CrossRef]
- Bailey, C.P.; May, A.; Lemmon, W.M. Survival after coronary endarterectomy in man. J. Am. Med. Assoc. 1957, 164, 641–646. [Google Scholar] [CrossRef] [PubMed]
- Sones, F.M., Jr.; Shirey, E.K. Cine coronary arteriography. Mod. Concepts Cardiovasc. Dis. 1962, 31, 735–738. [Google Scholar] [CrossRef]
- Garrett, H.E.; Dennis, E.W.; DeBakey, M.E. Aortocoronary bypass with saphenous vein graft. Seven-year follow-up. JAMA 1973, 223, 792–794. [Google Scholar] [CrossRef] [PubMed]
- Kolessov, VI. Mammary artery-coronary artery anastomosis as method of treatment for angina pectoris. J. Thorac. Cardiovasc. Surg. 1967, 54, 535–544. [Google Scholar] [CrossRef]
- Green, G.E.; Stertzer, S.H.; Reppert, E.H. Coronary arterial bypass grafts. Ann. Thorac. Surg. 1968, 5, 443–450. [Google Scholar] [CrossRef] [PubMed]
- Favaloro, R.G. Saphenous vein autograft replacement of severe segmental coronary artery occlusion: Operative technique. Ann. Thorac. Surg. 1968, 5, 334–339. [Google Scholar] [CrossRef] [PubMed]
- Marti, M.C.; Bouchardy, B.; Cox, J.N. Aorto-coronary by-pass with autogenous saphenous vein grafts: Histopathological aspects. Virchows Arch. A Pathol. Pathol. Anat. 1971, 352, 255–266. [Google Scholar] [CrossRef] [PubMed]
- Cuminetti, G.; Gelsomino, S.; Curello, S.; et al. Contemporary use of arterial and venous conduits in coronary artery bypass grafting: Anatomical, functional and clinical aspects. Neth. Heart J. 2017, 25, 4–13. [Google Scholar] [CrossRef]
- Carpentier, A.; Guermonprez, J.L.; Deloche, A.; et al. The aorta-to-coronary radial artery bypass graft. A technique avoiding pathological changes in grafts. Ann. Thorac. Surg. 1973, 16, 111–121. [Google Scholar] [CrossRef]
- Acar, C.; Jebara, V.A.; Portoghese, M.; et al. Revival of the radial artery for coronary artery bypass grafting. Ann. Thorac. Surg. 1992, 54, 652–659. [Google Scholar] [CrossRef]
- Acar, C.; Ramsheyi, A.; Pagny, J.Y.; et al. The radial artery for coronary artery bypass grafting: Clinical and angiographic results at five years. J. Thorac. Cardiovasc. Surg. 1998, 116, 981–989. [Google Scholar] [CrossRef]
- Loop, F.D.; Lytle, B.W.; Cosgrove, D.M.; et al. Influence of the internal-mammary-artery graft on 10-year survival and other cardiac events. N. Engl. J. Med. 1986, 314, 1–6. [Google Scholar] [CrossRef]
- Gruntzig, A. Transluminal dilatation of coronary-artery stenosis. Lancet 1978, 1, 263. [Google Scholar] [CrossRef]
- Calafiore, A.M.; Giammarco, G.D.; Teodori, G.; et al. Left anterior descending coronary artery grafting via left anterior small thoracotomy without cardiopulmonary bypass. Ann. Thorac. Surg. 1996, 61, 1658–1663. [Google Scholar] [CrossRef] [PubMed]
- Loulmet, D.; Carpentier, A.; d'Attellis, N.; et al. Endoscopic coronary artery bypass grafting with the aid of robotic assisted instruments. J. Thorac. Cardiovasc. Surg. 1999, 118, 4–10. [Google Scholar] [CrossRef]
- Neumann, F.-J.; Sousa-Uva, M.; Ahlsson, A.; Alfonso, F.; Banning, A.P.; Benedetto, U.; et al. ESC/EACTS Guidelines on myocardial revascularization. European Heart J. 2018, 40, 87–165. [Google Scholar] [CrossRef] [PubMed]
- Gasior, M.; Zembala, M.O.; Tajstra, M.; Filipiak, K.; Gierlotka, M.; Hrapkowicz, T.; Hawranek, M.; Polonski, L.; Zembala, M. POL-MIDES (HYBRID) Investigators. Hybrid revascularization for multivessel coronary artery disease. JACC Cardiovasc. Interv. 2014, 7, 1277–1283. [Google Scholar] [CrossRef] [PubMed]
- Tajstra, M.; Hrapkowicz, T.; Hawranek, M.; Filipiak, K.; Gierlotka, M.; Zembala, M.; et al. Hybrid coronary revascularization in selected patients with Multivessel disease. JACC: Cardiovasc. Interv. 2018, 11, 847–852. [Google Scholar] [CrossRef]
- Lawton, J.S.; Tamis-Holland, J.E.; Bangalore, S.; Bates, E.R.; Beckie, T.M.; Bischoff, J.M.; et al. ACC/AHA/SCAI guideline for Coronary artery revascularization: A report of the american college of cardiology/american heart association joint committee on clinical practice guidelines. Circulation 2022, 145. [Google Scholar]
- Guan, Z.; Zhang, Z.; Gu, K.; Wang, H.; Lin, J.; Zhou, W.; et al. Minimally invasive CABG or hybrid coronary revascularization for Multivessel Coronary Diseases: Which is best? A systematic review and metaanalysis. Heart Surgery Forum. 2019, 22. [Google Scholar] [CrossRef]
- Misfeld, M.; Brereton, R.J.; Sweetman, E.A.; Doig, G.S. Neurologic complications after off-pump coronary artery bypass grafting with and without aortic manipulation: Meta-analysis of 11,398 cases from 8 studies. J. Thorac. Cardiovasc. Surg. 2011, 142, e11–e17. [Google Scholar] [CrossRef]
- Czerny, M.; Baumer, H.; Kilo, J.; et al. Inflammatory response and myocardial injury following coronary artery bypass grafting with or without cardiopulmonary bypass. Eur. J. Cardiothorac. Surg. 2000, 17, 737–742. [Google Scholar] [CrossRef]
- Shroyer, A.L.; Hattler, B.; Wagner, T.H.; et al. Five-year outcomes after on-pump and off-pump coronary-artery bypass. N. Engl. J. Med. 2017, 377, 623–632. [Google Scholar] [CrossRef]
- Diegeler, A.; Walther, T.; Metz, S.; et al. Comparison of MIDCAB versus conventional CABG surgery regarding pain and quality of life. Heart Surg. Forum 1999, 2, 290–295. [Google Scholar] [PubMed]
- Garg, S.; Raja, S.G. Minimally invasive direct coronary artery bypass (MIDCAB) grafting. AME Med. J. 2020, 5, 19. [Google Scholar] [CrossRef]
- McGinn, J.T.; Usman, S.; Lapierre, H.; Pothula, V.R.; Mesana, T.G.; Ruel, M. Minimally invasive coronary artery bypass grafting. Circulation. 2009, 120, S78–S84. [Google Scholar] [CrossRef]
- Subramanian, V.A. Midcab approach for Single Vessel Coronary Artery Bypass Graft. Oper. Tech. Card. and Thorac. Surg. 1998, 3, 2–15. [Google Scholar] [CrossRef]
- Greenspu, H.G.; Adourian, U.A.; Fonger, J.D.; Fan, J.S. Minimally invasive direct coronary artery bypass (MIDCAB): Surgical techniques and anesthetic considerations. J. Cardiothoracic Vasc. Anes. 1996, 10, 507–509. [Google Scholar] [CrossRef]
- Patel, A.J.; Yates, M.T.; Soppa, G.K. What is the optimal revascularization technique for isolated disease of the left anterior descending artery: Minimally invasive direct coronary artery bypass or percutaneous coronary intervention? Interact. Cardiovasc. Thorac. Surg. 2014, 19, 144–148. [Google Scholar] [CrossRef] [PubMed]
- Raja, S.G.; Benedetto, U.; Alkizwini, E.; Gupta, S.; Amrani, M. Harefield Cardiac Outcomes Research Group. Propensity Score Adjusted Comparison of MIDCAB Versus Full Sternotomy Left Anterior Descending Artery Revascularization. Innovations (Phila). 2015, 10, 174–178. [Google Scholar] [CrossRef]
- Raja, S.G.; Garg, S.; Rochon, M.; Daley, S.; De Robertis, F.; Bahrami, T. Short-term clinical outcomes and long-term survival of minimally invasive direct coronary artery bypass grafting. Ann. Cardiothorac. Surg. 2018, 7, 621–627. [Google Scholar] [CrossRef] [PubMed]
- Repossini, A.; Di Bacco, L.; Nicoli, F.; et al. Minimally invasive coronary artery bypass: Twenty-year experience. J. Thorac. Cardiovasc. Surg. 2019, 158, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Manuel, L.; Fong, L.S.; Betts, K.; Bassin, L.; Wolfenden, H. LIMA to LAD grafting returns patient survival to age-matched population: 20-year outcomes of MIDCAB surgery. Interact. CardioVasc Thorac. Surg. 2022. [CrossRef] [PubMed]
- Benetti, F.J.; Ballester, C.; Sani, G.; Doonstra, P.; Grandjean, J. Video assisted coronary bypass surgery. J. Card. Surg. 1995, 10, 620–625. [Google Scholar] [CrossRef]
- Bonatti, J.; Wehman, B.; de Biasi, A.R.; et al. Totally endoscopic quadruple coronary artery bypass grafting is feasible using robotic technology. Ann. Thorac. Surg. 2012, 93, e111–e112. [Google Scholar] [CrossRef] [PubMed]
- Balkhy, H.H. Robotic totally endoscopic coronary artery bypass grafting: It's now or never! JTCVS Tech. 2021, 10, 153–157. [Google Scholar] [CrossRef]
- Lee, J.D.; Srivastava, M.; Bonatti, J. History and current status of robotic totally endoscopic coronary artery bypass. Circ J. 2012, 76, 2058–2065. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, A.; Robic, B.; Starinieri, P.; Polus, F.; Stinkens, R.; Stessel, B. A new viewpoint on endoscopic CABG: Technique description and clinical experience. J. Cardio. 2020, 75, 614–620. [Google Scholar] [CrossRef] [PubMed]
- Cao, C.; Indraratna, P.; Doyle, M.; Tian, D.H.; Liou, K.; Munkholm-Larsen, S.; Uys, C.; Virk, S. A systematic review on robotic coronary artery bypass graft surgery. Ann. Cardiothorac. Surg. 2016, 5, 530–543. [Google Scholar] [CrossRef] [PubMed]
- Kofler, M.; Stastny, L.; Reinstadler, S.; et al. Robotic Versus Conventional Coronary Artery Bypass Grafting. Direct Comparison of Long-Term Clinical Outcome. Innovations 2017, 12, 239–246. [Google Scholar] [PubMed]
- Leonard, J.R.; Rahouma, M.; Abouarab, A.A.; Schwann, A.N.; Scuderi, G.; Lau, C.; et al. Totally endoscopic coronary artery bypass surgery: A meta-analysis of the current evidence. Intern. J. Cardio. 2018, 261, 42–46. [Google Scholar] [CrossRef]
- Göbölös, L.; Ramahi, J.; Obeso, A.; et al. Robotic Totally Endoscopic Coronary Artery Bypass Grafting: Systematic Review of Clinical Outcomes from the Past two Decades. Innovations 2019, 14, 5–16. [Google Scholar] [CrossRef] [PubMed]
- Hammal, F.; Nagase, F.; Menon, D.; Ali, I.; Nagendran, J.; Stafinski, T. Robot-assisted coronary artery bypass surgery: A systematic review and meta-analysis of comparative studies. Canadian J. Surg. 2020, 63. [Google Scholar] [CrossRef] [PubMed]
- Holm, N.R.; Mäkikallio, T.; Lindsay, M.M.; Spence, M.S.; Erglis, A.; Menown, I.B.; et al. Percutaneous coronary angioplasty versus coronary artery bypass grafting in the treatment of unprotected left main stenosis: Updated 5-year outcomes from the randomised, non-inferiority Noble trial. Lancet. 2020, 395, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Kappetein, A.P.; Head, S.J. CABG, stents, or hybrid procedures for left main disease? EuroIntervention 2015, 11, V111–4. [Google Scholar] [CrossRef] [PubMed]
- Katz, M.R.; Van Praet, F.; de Canniere, D.; et al. Integrated coronary revascularization: Percutaneous coronary intervention plus robotic totally endoscopic coronary artery bypass. Circulation 2006, 114, I473–I476. [Google Scholar] [CrossRef]
- Deppe, A.-C.; Liakopoulos, O.J.; Kuhn, E.W.; Slottosch, I.; Scherner, M.; Choi, Y.-H.; et al. Minimally invasive direct coronary bypass grafting versus percutaneous coronary intervention for single-vessel disease: A meta-analysis of 2885 patients†. Eur Jour CardioThorac Surg. 2014, 47, 397–406. [Google Scholar] [CrossRef] [PubMed]
- Kiaii, B.; Teefy, P. Hybrid coronary artery revascularization: A review and current evidence. Innov. Technol. Tech. Cardiothorac. Vasc. Surg. 2019, 14, 394–404. [Google Scholar] [CrossRef] [PubMed]
- Saha, T.; Naqvi, S.Y.; Goldberg, S. Hybrid revascularization: A Review. Cardiology 2018, 140, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Harskamp, R.E.; Zheng, Z.; Alexander, J.H.; Williams, J.B.; Xian, Y.; Halkos, M.E.; et al. Status quo of hybrid coronary revascularization for multi-vessel coronary artery disease. Ann. Thorac. Surg. 2013, 96, 2268–2277. [Google Scholar] [CrossRef]
- Ganyukov, V.; Kochergin, N.; Shilov, A.; Tarasov, R.; Skupien, J.; Szot, W.; et al. Randomized clinical trial of surgical vs. Percutaneous vs. hybrid revascularization in multivessel coronary artery disease: Residual myocardial ischemia and clinical outcomes at one year—Hybrid coronary revascularization versus stenting or surgery (HREVS). Jour Interv. Card. 2020, 2020, 1–11. [Google Scholar] [CrossRef]
- Esteves, V.; Oliveira, M.A.; Feitosa, F.S.; Mariani, J.; Campos, C.M.; Hajjar, L.A.; et al. Late clinical outcomes of myocardial hybrid revascularization versus coronary artery bypass grafting for complex triple-vessel disease: Long-term follow-up of the randomized merging clinical trial. Cathe Cardiovasc. Interv. 2020, 97, 259–264. [Google Scholar] [CrossRef]
- Nagraj, S.; Tzoumas, A.; Kakargias, F.; Giannopoulos, S.; Ntoumaziou, A.; Kokkinidis, D.G.; et al. Hybrid coronary revascularization (HCR) versus coronary artery bypass grafting (CABG) in multivessel coronary artery disease (MVCAD): A meta-analysis of 14 studies comprising 4226 patients. Cathe Cardiovasc Interv. 2022, 100, 1182–1194. [Google Scholar] [CrossRef]
- Bonatti, J.; Schachner, T.; Bonaros, N.; Öhlinger, A.; Danzmayr, M.; Jonetzko, P.; et al. Technical challenges in totally endoscopic robotic coronary artery bypass grafting. J. Thorac. Cardiovasc. Surg. 2006, 131, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Groh, M.A.; Sutherland, S.E.; Burton, H.G.; Johnson, A.M.; Ely, S.W. Port-access coronary artery bypass grafting: Technique and comparative results. Ann. Thorac. Surg. 1999, 68, 1506–1508. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.H.; Wells, G.A.; Glineur, D.; Fortier, J.; Davierwala, P.M.; Kikuchi, K.; et al. Minimally invasive coronary surgery compared to sternotomy coronary artery bypass grafting: The mist trial. Contemp. Clin. Trials. 2019, 78, 140–145. [Google Scholar] [CrossRef]
- Ruel, M. Nonsternotomy multivessel coronary artery bypass grafting: A key development in cardiac surgery. J. Thorac. Cardiovasc. Surg. Techniques. 2021, 10, 162–167. [Google Scholar] [CrossRef]
- Bonatti, J.; Schachner, T.; Bonaros, N.; Oehlinger, A.; Ruetzler, E.; Friedrich, G.; et al. How to improve performance of robotic totally endoscopic coronary artery bypass grafting. Amer J. Surg. 2008, 195, 711–716. [Google Scholar] [CrossRef] [PubMed]
- Ruel, M.; Shariff, M.A.; Lapierre, H.; Goyal, N.; Dennie, C.; Sadel, S.M.; et al. Results of the minimally invasive coronary artery bypass grafting angiographic patency study. J. Thorac. Cardiovasc. Surg. 2014, 147, 203–209. [Google Scholar] [CrossRef]
- Bonatti, J.; Schachner, T.; Bernecker, O.; Chevtchik, O.; Bonaros, N.; Ott, H.; et al. Robotic totally endoscopic coronary artery bypass: Program development and learning curve issues. J. Thorac. Cardiovasc. Surg. 2004, 127, 504–510. [Google Scholar] [CrossRef]
- Van Praet, K.M.; Kofler, M.; Nazari Shafti, T.Z.; El Al, A.A.; van Kampen, A.; Amabile, A.; et al. Minimally invasive coronary revascularisation surgery: A focused review of the available literature. ICR 2021, 16. [Google Scholar] [CrossRef]
- Ruel, M. Commentary: Sternotomy for every cardiac surgery patient ain't the future, so let's get going. J. Thorac. Cardiovasc. Surg. 2023, 165, 129–131. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).