Submitted:
22 May 2023
Posted:
24 May 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Preparation of GA Loaded In Situ Gel Formulations
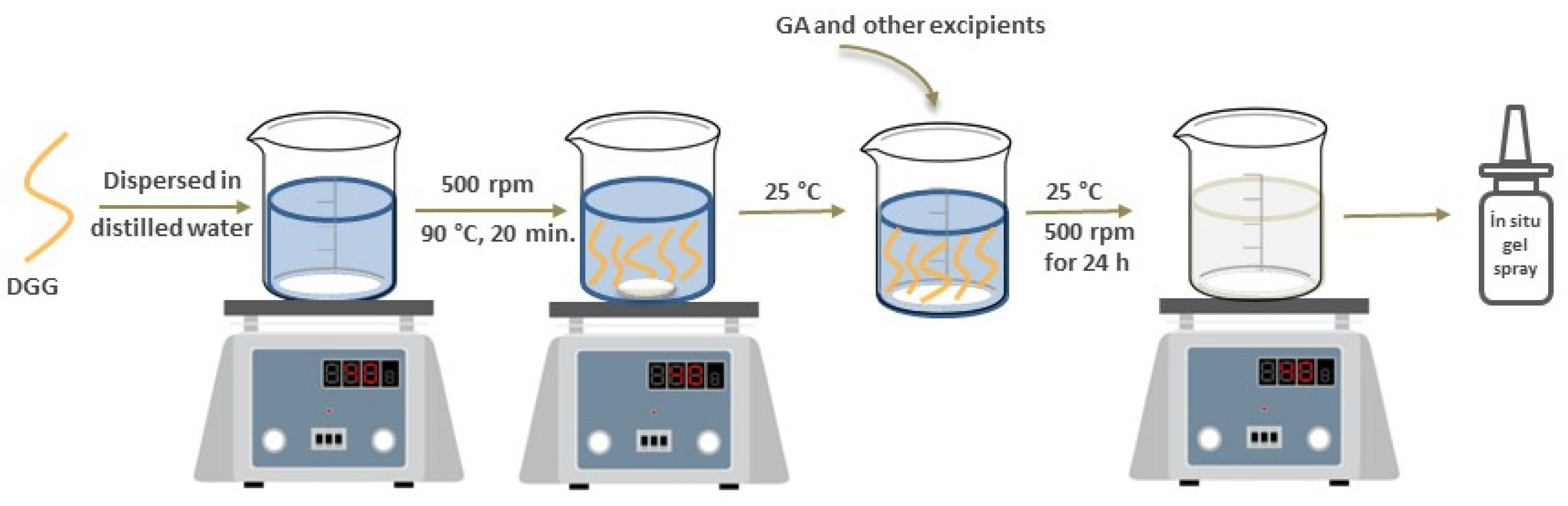
Formulation of In Situ Gelling Systems Using Only Gellan Gum
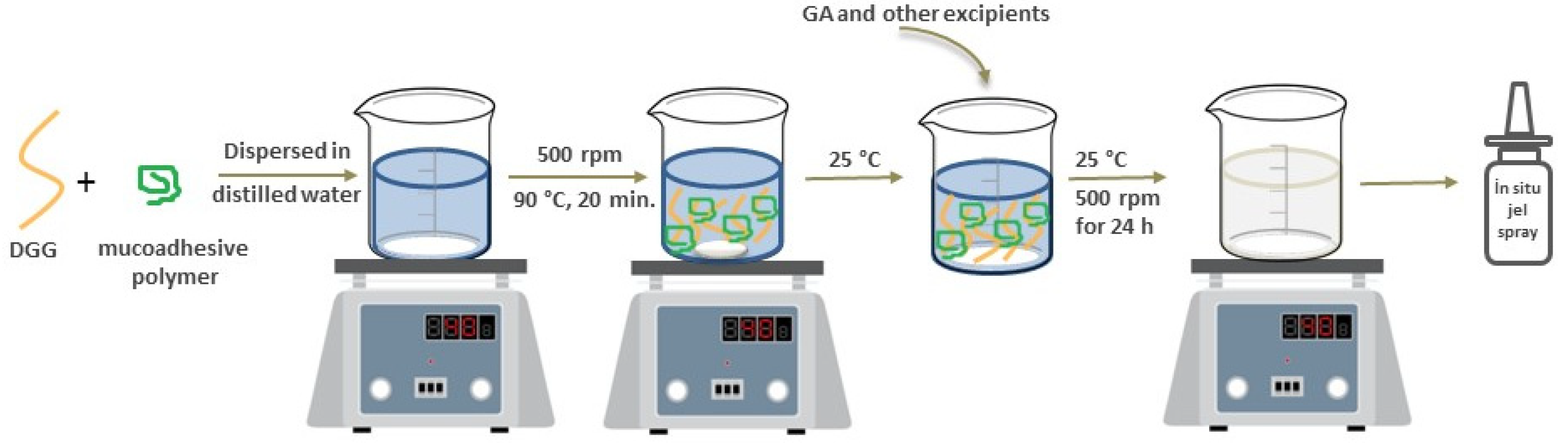
Formulation of In Situ Gelling Systems Using Combined Polymers
2.2.2. Characterization of GA Loaded In Situ Gel Formulations
Gelatin Capacity of In Situ Gel Formulations with/without Mucoadhesive Polymers
Rheological Evaluation
In Situ Gel-Mucin Interaction Study
Mechanical Characteristics of In Situ Gels
Ex Vivo Mucoadhesive Strength Test
Sprayability Analysis
Quantitative Determination of 18β-glycyrrhetinic Acid
Active Substance Content Determination
Fourier Transform Infrared Spectrometry (FTIR) Studies
In Vitro Release Study
Physicochemical Stability
2.2.3. Cell Culture Studies
Cells and Virus
Cytotoxicity Assay
3. Results and Discussion
3.1. Optimization and Characterization of GA Loaded In Situ Gel Formulations
3.1.1. Gelatin Capacity of In Situ Gel Formulations
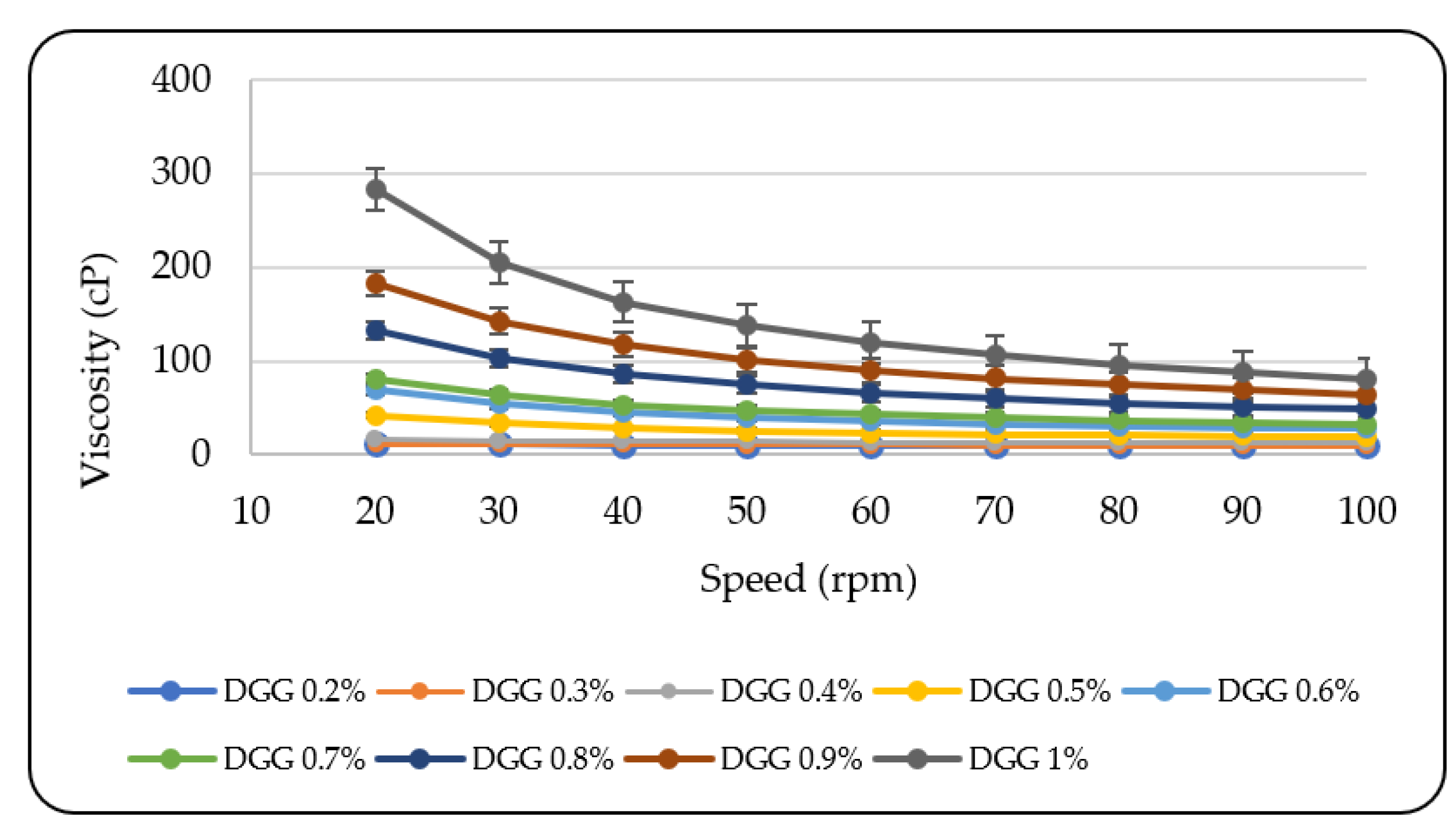
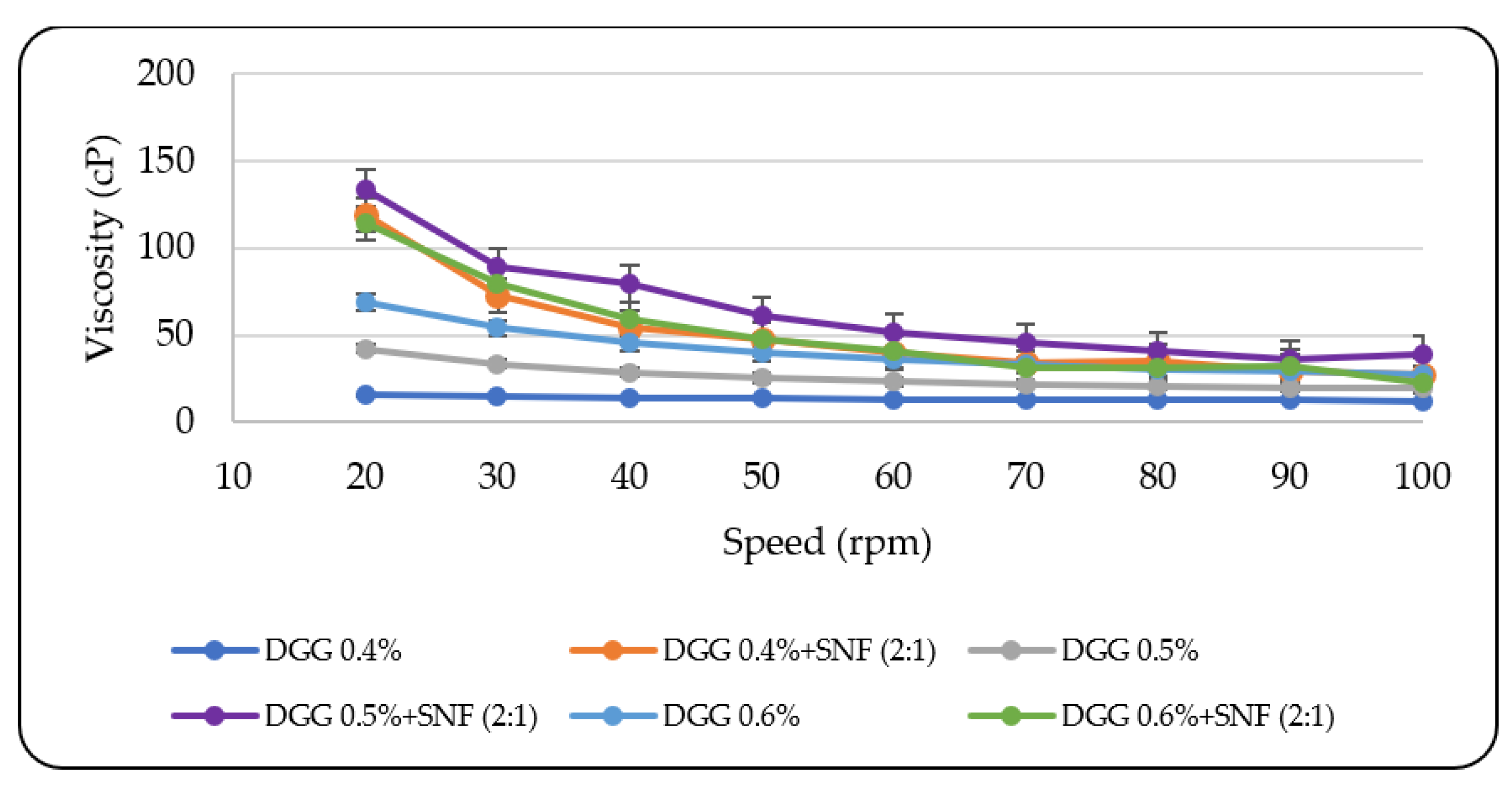
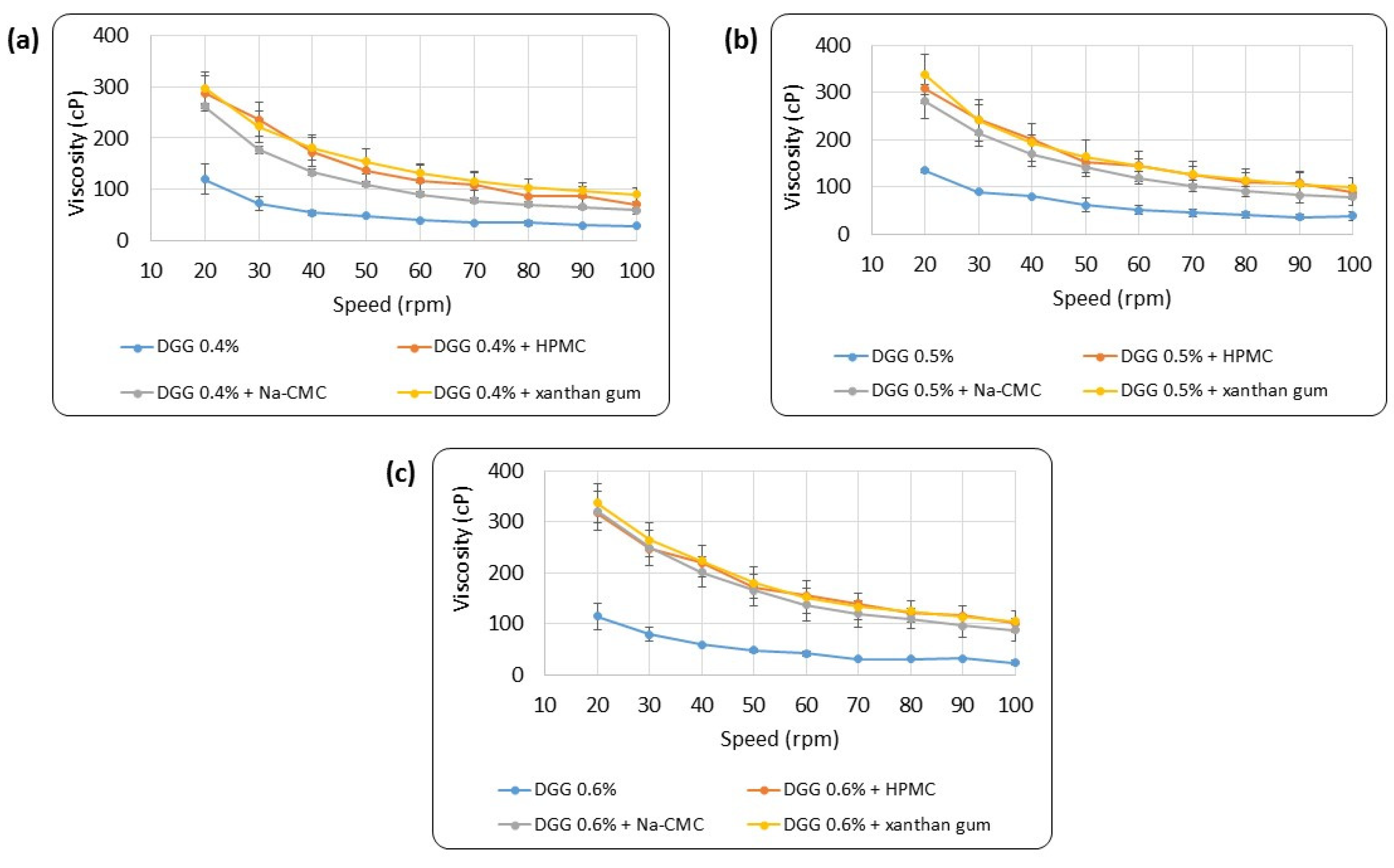
3.1.2. Rheological Evaluation
3.1.3. In Situ Gel-Mucin Interaction Study
3.1.4. Mechanical Characteristics of In Situ Gels
3.1.5. Ex Vivo Mucoadhesive Strength Test
3.1.6. Sprayability
3.1.7. Quantitative Determination of 18β-glycyrrhetinic Acid
3.1.8. Active Substance Content Determination
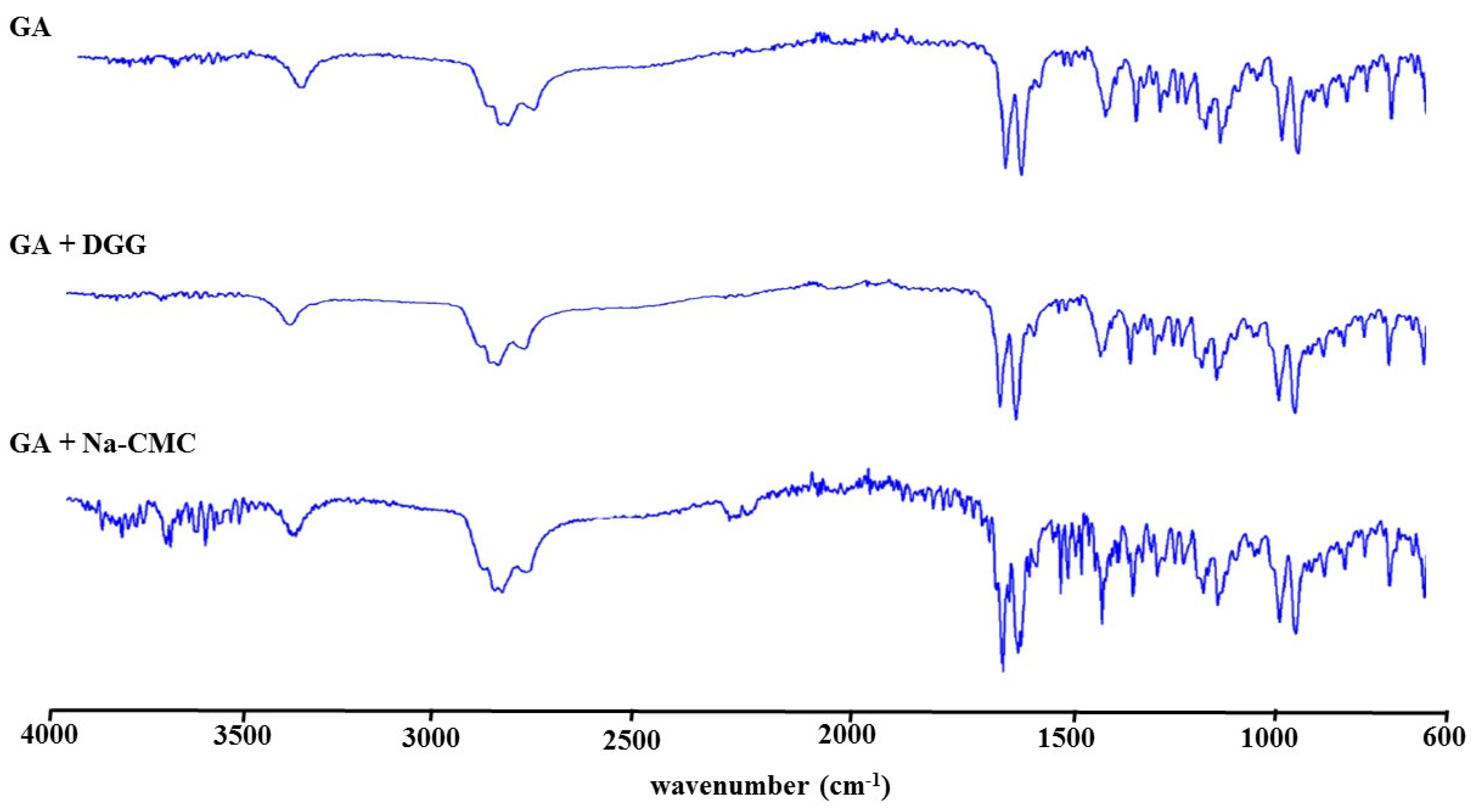
3.1.9. Fourier Transform Infrared Spectrometry (FTIR) Studies
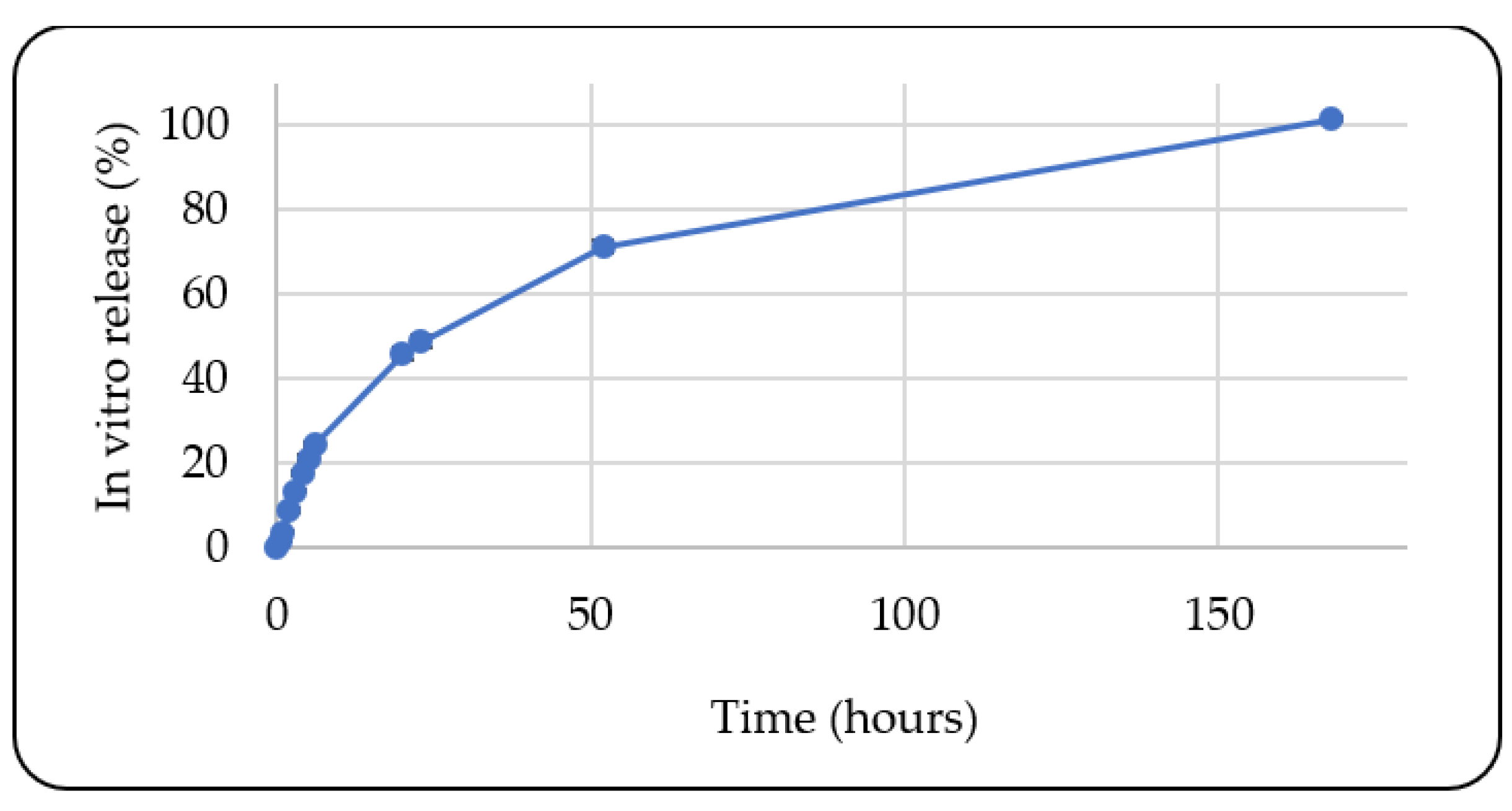
3.1.10. In Vitro Release Study
3.1.11. Physicochemical Stability
3.2. Cell Culture Studies
3.2.1. Cytotoxicity Assay
3.2.2. Virus Titration

3.2.3. Antiviral Activity Assay
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, Q.; et al. Antivirals for respiratory viral infections: Problems and prospects. In Seminars in Respiratory and Critical Care Medicine; Thieme Medical Publishers: New York, NY, USA, 2016. [Google Scholar]
- Borchardt, R.A.; Rolston, K.V. Respiratory tract infections: Emerging viral pathogens. JAAPA 2012, 25, 19–20. [Google Scholar] [CrossRef]
- Kim, Y.I.; et al. The antiviral effects of RSV fusion inhibitor, MDT-637, on clinical isolates, vs its achievable concentrations in the human respiratory tract and comparison to ribavirin. Influenza Other Respir. Viruses 2017, 11, 525–530. [Google Scholar] [CrossRef]
- Ferkol, T.; Schraufnagel, D. The global burden of respiratory disease. Ann. Am. Thorac. Soc. 2014, 11, 404–406. [Google Scholar] [CrossRef]
- Yeolekar, L.R.; et al. Respiratory viruses in acute respiratory tract infections in Western India. Indian J. Pediatr. 2008, 75, 341–345. [Google Scholar] [CrossRef]
- Corti, M.; Palmero, D.; Eiguchi, K. Respiratory infections in immunocompromised patients. Curr. Opin. Pulm. Med. 2009, 15, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Englund, J.; Feuchtinger, T. Viral infections in immunocompromised patients. Biol. Blood Marrow Transplant. 2011, 17, S2. [Google Scholar] [CrossRef] [PubMed]
- Gainer, S.M.; et al. Increased mortality of solid organ transplant recipients with H1N1 infection: A single center experience. Clin. Transplant. 2012, 26, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Shahani, L.; Ariza-Heredia, E.J.; Chemaly, R.F. Antiviral therapy for respiratory viral infections in immunocompromised patients. Expert Rev. Anti-Infect. Ther. 2017, 15, 401–415. [Google Scholar] [CrossRef]
- Behzadi, M.A.; Leyva-Grado, V.H. Overview of current therapeutics and novel candidates against influenza, respiratory syncytial virus, and Middle East respiratory syndrome coronavirus infections. Front. Microbiol. 2019, 10, 1327. [Google Scholar] [CrossRef]
- Collins, P.L.; Fearns, R.; Graham, B.S. Respiratory syncytial virus: Virology, reverse genetics, and pathogenesis of disease. Challenges and opportunities for respiratory syncytial virus vaccines.
- Shafique, M.; et al. Hurdles in vaccine development against respiratory syncytial virus. In The Burden of Respiratory Syncytial Virus Infection in the Young; IntechOpen: London, UK, 2019. [Google Scholar]
- Wat, D. The common cold: A review of the literature. Eur. J. Intern. Med. 2004, 15, 79–88. [Google Scholar] [CrossRef]
- Hall, C.B.; Long, C.E.; Schnabel, K.C. Respiratory syncytial virus infections in previously healthy working adults. Clin. Infect. Dis. 2001, 33, 792–796. [Google Scholar] [CrossRef]
- Collins, P.L.; Graham, B.S. Viral and host factors in human respiratory syncytial virus pathogenesis. J. Virol. 2008, 82, 2040–2055. [Google Scholar] [CrossRef]
- Morris, J.; Blount, R., Jr.; Savage, R. Recovery of cytopathogenic agent from chimpanzees with goryza. Proc. Soc. Exp. Biol. Med. 1956, 92, 544–549. [Google Scholar] [CrossRef]
- Chanock, R.; Finberg, L. Recovery from Infants with Respiratory Illness of a Virus related to Chimpanzee Coryza Agent (CCA). II. Epidemiologie Aspects of Infection in Infants and Young Children. Am. J. Hyg. 1957, 66, 291–300. [Google Scholar]
- Glezen, W.P.; et al. Risk of primary infection and reinfection with respiratory syncytial virus. Am. J. Dis. Child. 1986, 140, 543–546. [Google Scholar] [CrossRef]
- Murphy, B.R.; et al. Current approaches to the development of vaccines effective against parainfluenza and respiratory syncytial viruses. Virus Res. 1988, 11, 1–15. [Google Scholar] [CrossRef]
- Britto, C.J.; et al. Respiratory viral infections in chronic lung diseases. Clin. Chest Med. 2017, 38, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Falsey, A.R.; et al. Respiratory syncytial virus infection in elderly and high-risk adults. N. Engl. J. Med. 2005, 352, 1749–1759. [Google Scholar] [CrossRef] [PubMed]
- Long, S.S., C. G. Prober, and M. Fischer, Principles and practice of pediatric infectious diseases E-Book. 2022, Elsevier Health Sciences.
- Walsh, E.; et al. RSV-associated hospitalization in adults in the USA: A retrospective chart review investigating burden, management strategies, and outcomes. Health Sci. Rep. 2022, 5, e556. [Google Scholar] [CrossRef]
- Peebles, R.S., Jr.; Graham, B.S. Pathogenesis of respiratory syncytial virus infection in the murine model. Proc. Am. Thorac. Soc. 2005, 2, 110–115. [Google Scholar] [CrossRef]
- Kapikian, A.Z.; et al. An epidemiologic study of altered clinical reactivity to respiratory syncytial (RS) virus infection in children previously vaccinated with an inactivated RS virus vaccine. Am. J. Epidemiol. 1969, 89, 405–421. [Google Scholar] [CrossRef]
- Kim, H.W.; et al. Respiratory syncytial virus disease in infants despite prior administration of antigenic inactivated vaccine. Am. J. Epidemiol. 1969, 89, 422–434. [Google Scholar] [CrossRef] [PubMed]
- Alwan, W.; Kozlowska, W. Distinct types of lung disease caused by functional subsets of antiviral T cells. J. Exp. Med. 1994, 179, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, C.; Drews, S.J.; Marchant, D.J. Respiratory syncytial virus: Infection, detection, and new options for prevention and treatment. Clin. Microbiol. Rev. 2017, 30, 277–319. [Google Scholar] [CrossRef] [PubMed]
- Antonini, T.M.; et al. Sofosbuvir-based regimens in HIV/HCV coinfected patients after liver transplantation: Results from the ANRS CO23 CUPILT study. Transplantation 2018, 102, 119–126. [Google Scholar] [CrossRef]
- Kim, J.-A.; et al. Favipiravir and ribavirin inhibit replication of Asian and African strains of Zika virus in different cell models. Viruses 2018, 10, 72. [Google Scholar] [CrossRef]
- Zhurilo, N.I.; et al. Isosteric ribavirin analogues: Synthesis and antiviral activities. Bioorganic Med. Chem. Lett. 2018, 28, 11–14. [Google Scholar] [CrossRef]
- Bonavia, A.; et al. Identification of broad-spectrum antiviral compounds and assessment of the druggability of their target for efficacy against respiratory syncytial virus (RSV). Proc. Natl. Acad. Sci. 2011, 108, 6739–6744. [Google Scholar] [CrossRef]
- Devincenzo, J.P. Therapy of respiratory syncytial virus infection. Pediatr. Infect. Dis. J. 2000, 19, 786–790. [Google Scholar] [CrossRef]
- Sun, Z.; et al. Respiratory syncytial virus entry inhibitors targeting the F protein. Viruses 2013, 5, 211–225. [Google Scholar] [CrossRef]
- Chemaly, R.; et al. Aerosolized ribavirin: The most expensive drug for pneumonia. Transpl. Infect. Dis. 2016, 18, 634–636. [Google Scholar] [CrossRef] [PubMed]
- Trang, T.P.; et al. Comparative effectiveness of aerosolized versus oral ribavirin for the treatment of respiratory syncytial virus infections: A single-center retrospective cohort study and review of the literature. Transpl. Infect. Dis. 2018, 20, e12844. [Google Scholar] [CrossRef] [PubMed]
- Simoes, E.A.; et al. Past, present and future approaches to the prevention and treatment of respiratory syncytial virus infection in children. Infect. Dis. Ther. 2018, 7, 87–120. [Google Scholar] [CrossRef] [PubMed]
- Domachowske, J.B.; Anderson, E.J.; Goldstein, M. The future of respiratory syncytial virus disease prevention and treatment. Infect. Dis. Ther. 2021, 10, 47–60. [Google Scholar] [CrossRef] [PubMed]
- Geskey, J.M.; Thomas, N.J.; Brummel, G.L. Palivizumab: A review of its use in the protection of high risk infants against respiratory syncytial virus (RSV). Biol. Targets Ther. 2007, 1, 33–43. [Google Scholar]
- Anderson, E.J.; et al. Effectiveness of palivizumab in high-risk infants and children: A propensity score weighted regression analysis. Pediatr. Infect. Dis. J. 2017, 36, 699. [Google Scholar] [CrossRef] [PubMed]
- Blanken, M.O.; et al. Respiratory syncytial virus and recurrent wheeze in healthy preterm infants. N. Engl. J. Med. 2013, 368, 1791–1799. [Google Scholar] [CrossRef] [PubMed]
- Resch, B. Product review on the monoclonal antibody palivizumab for prevention of respiratory syncytial virus infection. Hum. Vaccines Immunother. 2017, 13, 2138–2149. [Google Scholar] [CrossRef]
- Yamaguchi, H.; et al. Novel effects of glycyrrhetinic acid on the central nervous system tumorigenic progenitor cells: Induction of actin disruption and tumor cell-selective toxicity. Eur. J. Med. Chem. 2010, 45, 2943–2948. [Google Scholar] [CrossRef]
- Kong, S.-Z.; et al. The protective effect of 18β-Glycyrrhetinic acid against UV irradiation induced photoaging in mice. Exp. Gerontol. 2015, 61, 147–155. [Google Scholar] [CrossRef]
- Pugalendi, K. Protective effect of 18β-glycyrrhetinic acid on lipid peroxidation and antioxidant enzymes in experimental diabetes. J. Pharm. Res. 2011, 4, 107–111. [Google Scholar]
- Zhao, C.-H.; et al. Inhibition of human enterovirus 71 replication by pentacyclic triterpenes and their novel synthetic derivatives. Chem. Pharm. Bull. 2014, 62, 764–771. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.-R.; et al. 18β-Glycyrrhetinic acid derivatives possessing a trihydroxylated A ring are potent gram-positive antibacterial agents. J. Nat. Prod. 2016, 79, 721–731. [Google Scholar] [CrossRef] [PubMed]
- Aly, A.M.; Al-Alousi, L.; Salem, H.A. Licorice: A possible anti-inflammatory and anti-ulcer drug. Aaps Pharmscitech 2005, 6, E74–E82. [Google Scholar] [CrossRef] [PubMed]
- Pugalendi, K.V. Antihyperglycemic effect of 18β-glycyrrhetinic acid, aglycone of glycyrrhizin, on streptozotocin-diabetic rats. Eur. J. Pharmacol. 2009, 606, 269–273. [Google Scholar]
- Jeong, H.G.; et al. Hepatoprotective effects of 18β-glycyrrhetinic acid on carbon tetrachloride-induced liver injury: Inhibition of cytochrome P450 2E1 expression. Pharmacol. Res. 2002, 46, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Pastorino, G.; et al. Liquorice (Glycyrrhiza glabra): A phytochemical and pharmacological review. Phytother. Res. 2018, 32, 2323–2339. [Google Scholar] [CrossRef] [PubMed]
- Hardy, M.E.; et al. 18 β-glycyrrhetinic acid inhibits rotavirus replication in culture. Virol. J. 2012, 9, 1–7. [Google Scholar] [CrossRef]
- Yeh, C.F.; et al. Water extract of licorice had anti-viral activity against human respiratory syncytial virus in human respiratory tract cell lines. J. Ethnopharmacol. 2013, 148, 466–473. [Google Scholar]
- Higgins, T.S.; et al. Intranasal antiviral drug delivery and coronavirus disease 2019 (COVID-19): A state of the art review. Otolaryngol. Head Neck Surg. 2020, 163, 682–694. [Google Scholar] [CrossRef]
- Cao, S.-l.; et al. In situ gel based on gellan gum as new carrier for nasal administration of mometasone furoate. Int. J. Pharm. 2009, 365, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Andrews, G.P.; Laverty, T.; Jones, D.S. Mucoadhesive polymeric platforms for controlled drug delivery. Eur. J. Pharm. Biopharm. 2009, 71, 505–518. [Google Scholar] [CrossRef] [PubMed]
- Phillipson, M.; et al. The gastric mucus layers: Constituents and regulation of accumulation. Am. J. Physiol. -Gastrointest. Liver Physiol. 2008, 295, G806–G812. [Google Scholar] [CrossRef]
- Salamat-Miller, N.; Chittchang, M.; Johnston, T. The use of mucoadhesive polymers in buccal drug delivery. Adv. Drug Deliv. Rev. 2005, 57, 1666–1691. [Google Scholar] [CrossRef]
- Hagesaether, E.; Hiorth, M.; Sande, S.A. Mucoadhesion and drug permeability of free mixed films of pectin and chitosan: An in vitro and ex vivo study. Eur. J. Pharm. Biopharm. 2009, 71, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Musumeci, T.; Bonaccorso, A.; Puglisi, G. Epilepsy disease and nose-to-brain delivery of polymeric nanoparticles: An overview. Pharmaceutics 2019, 11, 118. [Google Scholar] [CrossRef]
- Sosnik, A.; Neves, J.D.; Sarmento, B. Mucoadhesive polymers in the design of nano-drug delivery systems for administration by non-parenteral routes: A review. Prog. Polym. Sci. 2014, 39, 2030–2075. [Google Scholar] [CrossRef]
- Altuntaş, E.; Yener, G. Formulation and evaluation of thermoreversible in situ nasal gels containing mometasone furoate for allergic rhinitis. AAPS PharmSciTech 2017, 18, 2673–2682. [Google Scholar] [CrossRef]
- Shinde, J.V.; et al. In situ mucoadhesive nasal gels of metoclopramide hydrochloride: Preformulation and formulation studies. J Pharm Res 2008, 1, 88–96. [Google Scholar]
- Salunke, S.R.; Patil, S.B. Ion activated in situ gel of gellan gum containing salbutamol sulphate for nasal administration. Int. J. Biol. Macromol. 2016, 87, 41–47. [Google Scholar] [CrossRef]
- Jansson, B.; et al. The influence of gellan gum on the transfer of fluorescein dextran across rat nasal epithelium in vivo. Eur. J. Pharm. Biopharm. 2005, 59, 557–564. [Google Scholar] [CrossRef] [PubMed]
- Morsi, N.; et al. Nanoemulsion-based electrolyte triggered in situ gel for ocular delivery of acetazolamide. Eur. J. Pharm. Sci. 2017, 104, 302–314. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; et al. Formulation and evaluation of in situ gelling systems for intranasal administration of gastrodin. Aaps Pharmscitech 2011, 12, 1102–1109. [Google Scholar] [CrossRef]
- Hao, J.; et al. Fabrication of an ionic-sensitive in situ gel loaded with resveratrol nanosuspensions intended for direct nose-to-brain delivery. Colloids Surf. B Biointerfaces 2016, 147, 376–386. [Google Scholar] [CrossRef] [PubMed]
- Hassan, E.E.; Gallo, J.M. A simple rheological method for the in vitro assessment of mucin-polymer bioadhesive bond strength. Pharm. Res. 1990, 7, 491–495. [Google Scholar] [CrossRef]
- Mayol, L.; et al. A novel poloxamers/hyaluronic acid in situ forming hydrogel for drug delivery: Rheological, mucoadhesive and in vitro release properties. Eur. J. Pharm. Biopharm. 2008, 70, 199–206. [Google Scholar] [CrossRef]
- Callens, C.; et al. Rheological study on mucoadhesivity of some nasal powder formulations. Eur. J. Pharm. Biopharm. 2003, 55, 323–328. [Google Scholar] [CrossRef]
- Gadhave, D.; et al. Nose-to-brain delivery of amisulpride-loaded lipid-based poloxamer-gellan gum nanoemulgel: In vitro and in vivo pharmacological studies. Int. J. Pharm. 2021, 607, 121050. [Google Scholar] [CrossRef]
- Gadhave, D.; et al. Intranasal teriflunomide microemulsion: An improved chemotherapeutic approach in glioblastoma. J. Drug Deliv. Sci. Technol. 2019, 51, 276–289. [Google Scholar] [CrossRef]
- Spray, N.; Solution, I. Suspension, and Spray Drug Products; Chemistry, Manufacturing and Controls Documentation. US Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER).
- Grobuschek, N.; et al. Mass uniformity of nasal sprays. Sci. Pharm. 2003, 71, 151–164. [Google Scholar] [CrossRef]
- Zhou, N.; et al. A simple method for evaluation pharmacokinetics of glycyrrhetinic acid and potential drug-drug interaction between herbal ingredients. Sci. Rep. 2019, 9, 11308. [Google Scholar] [CrossRef] [PubMed]
- Nodilo, L.N.; et al. In situ gelling nanosuspension as an advanced platform for fluticasone propionate nasal delivery. Eur. J. Pharm. Biopharm. 2022, 175, 27–42. [Google Scholar] [CrossRef] [PubMed]
- Francis, T. Diagnostic procedures for virus and rickettsial diseases. 1956, American Public Health Association.
- Andrighetti-Fröhner, C.; et al. Cytotoxicity and potential antiviral evaluation of violacein produced by Chromobacterium violaceum. Memórias Do Inst. Oswaldo Cruz 2003, 98, 843–848. [Google Scholar] [CrossRef] [PubMed]
- Ho, W.S.; et al. Antiviral activity of daphnoretin isolated from Wikstroemia indica. Phytother. Res. Int. J. Devoted Pharmacol. Toxicol. Eval. Nat. Prod. Deriv. 2010, 24, 657–661. [Google Scholar] [CrossRef]
- Doğan, H.H.; Duman, R. The Anti Hrsv Activity of Ferula Halophila Peşmen Aqueous and Methanol Extract by Mtt Assay. Trak. Univ. J. Nat. Sci. 2021. [Google Scholar] [CrossRef]
- Abdulla, N.A.; et al. Intranasal delivery of Clozapine using nanoemulsion-based in-situ gels: An approach for bioavailability enhancement. Saudi Pharm. J. 2021, 29, 1466–1485. [Google Scholar] [CrossRef]
- Qian, S.; Wong, Y.C.; Zuo, Z. Development, characterization and application of in situ gel systems for intranasal delivery of tacrine. Int. J. Pharm. 2014, 468, 272–282. [Google Scholar] [CrossRef]
- Galgatte, U.C.; Kumbhar, A.B.; Chaudhari, P.D. Development of in situ gel for nasal delivery: Design, optimization, in vitro and in vivo evaluation. Drug Deliv. 2014, 21, 62–73. [Google Scholar] [CrossRef]
- Zahir-Jouzdani, F.; et al. In situ gelling and mucoadhesive polymers: Why do they need each other? Expert Opin. Drug Deliv. 2018, 15, 1007–1019. [Google Scholar] [CrossRef]
- Jelkmann, M.; et al. A gellan gum derivative as in-situ gelling cationic polymer for nasal drug delivery. Int. J. Biol. Macromol. 2020, 158, 1037–1046. [Google Scholar] [CrossRef]
- Wu, Y.; et al. Research progress of in-situ gelling ophthalmic drug delivery system. Asian J. Pharm. Sci. 2019, 14, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Pal, K.; Paulson, A.T.; Rousseau, D. Biopolymers in controlled-release delivery systems. In Modern biopolymer science; Elsevier: Amsterdam, The Netherlands, 2009; pp. 519–557. [Google Scholar]
- Khouryieh, H.; et al. Intrinsic viscosity and viscoelastic properties of xanthan/guar mixtures in dilute solutions: Effect of salt concentration on the polymer interactions. Food Res. Int. 2007, 40, 883–893. [Google Scholar] [CrossRef]
- Lin, H.-R.; Sung, K. Carbopol/pluronic phase change solutions for ophthalmic drug delivery. J. Control. Release 2000, 69, 379–388. [Google Scholar] [CrossRef] [PubMed]
- Almeida, H.; et al. In situ gelling systems: A strategy to improve the bioavailability of ophthalmic pharmaceutical formulations. Drug Discov. Today 2014, 19, 400–412. [Google Scholar] [CrossRef] [PubMed]
- Fini, A.; Bergamante, V.; Ceschel, G.C. Mucoadhesive gels designed for the controlled release of chlorhexidine in the oral cavity. Pharmaceutics 2011, 3, 665–679. [Google Scholar] [CrossRef]
- Madhav, N. Oral mucoadhesive drug delivery systems: A review. JBI 2011, 2229, 7499. [Google Scholar]
- Jones, D.S.; Woolfson, A.D.; Brown, A.F. Textural, viscoelastic and mucoadhesive properties of pharmaceutical gels composed of cellulose polymers. Int. J. Pharm. 1997, 151, 223–233. [Google Scholar] [CrossRef]
- Peppas, N.A.; Buri, P.A. Surface, interfacial and molecular aspects of polymer bioadhesion on soft tissues. J. Control. Release 1985, 2, 257–275. [Google Scholar] [CrossRef]
- Draget, K.; et al. Gel strength of Ca-limited alginate gels made in situ. In in Fourteenth International Seaweed Symposium: In Proceedings of the Fourteenth International Seaweed Symposium, Brest, France, 16–21 August 1992. [Google Scholar]
- Senderak, E.T. Content uniformity acceptance limit for a validation batch—Suppositories, transdermal systems, and inhalations. Drug Dev. Ind. Pharm. 2009, 35, 735–737. [Google Scholar] [CrossRef]
- Sharma, M. Mucoadhesive nanoformulations and their potential for combating COVID-19. Nanomedicine 2021, 16, 2497–2501. [Google Scholar]
- Vigani, B.; et al. Recent advances in the development of in situ gelling drug delivery systems for non-parenteral administration routes. Pharmaceutics 2020, 12, 859. [Google Scholar] [CrossRef] [PubMed]
- Karavasili, C.; Fatouros, D.G. Smart materials: In situ gel-forming systems for nasal delivery. Drug Discov. Today 2016, 21, 157–166. [Google Scholar] [CrossRef]
- Food, U.; Administration, D. Guidance for industry: Drug stability guidelines; USFDA: Theodore Roosevelt, WA, USA, 2008. [Google Scholar]
- Pires, P.C.; et al. Strategies to improve drug strength in nasal preparations for brain delivery of low aqueous solubility drugs. Pharmaceutics 2022, 14, 588. [Google Scholar] [CrossRef] [PubMed]
- Chattopadhyay, D.; et al. Recent advancements for the evaluation of anti-viral activities of natural products. New Biotechnol. 2009, 25, 347–368. [Google Scholar] [CrossRef]
- Faber, T.E. Immunity to respiratory syncytial virus: A clinical perspective; Utrecht University: Utrecht, The Netherlands, 2017. [Google Scholar]
- Proença-Módena, J.L.; et al. Respiratory Viral Infections. Tropical Infectious Diseases: Principles, Pathogens and Practice 2011, 378.
- Dhakar, R.C.; et al. A review on factors affecting the design of nasal drug delivery system. Int. J. Drug Deliv. 2011, 3, 194. [Google Scholar]
- Gizurarson, S. Anatomical and histological factors affecting intranasal drug and vaccine delivery. Curr. Drug Deliv. 2012, 9, 566–582. [Google Scholar] [CrossRef]
- Robinson, T.E.; Moakes, R.J.; Grover, L.M. Low acyl gellan as an excipient to improve the sprayability and mucoadhesion of iota carrageenan in a nasal spray to prevent infection with SARS-CoV-2. Front. Med. Technol. 2021, 3, 687681. [Google Scholar] [CrossRef] [PubMed]
- Takeda, Y.; et al. Acidic electrolyzed water potently inactivates SARS-CoV-2 depending on the amount of free available chlorine contacting with the virus. Biochem. Biophys. Res. Commun. 2020, 530, 1–3. [Google Scholar] [CrossRef]
- de Vries, R.D.; et al. Intranasal fusion inhibitory lipopeptide prevents direct-contact SARS-CoV-2 transmission in ferrets. Science 2021, 371, 1379–1382. [Google Scholar] [CrossRef]
- Mann, B.J.; et al. TaffiX nasal powder forms an effective barrier against SARS-CoV-2. Biomed J Sci Tech Res 2021, 33, 25483–25485. [Google Scholar] [CrossRef]
- Moakes, R.J.; et al. Formulation of a Composite Nasal Spray Enabling Enhanced Surface Coverage and Prophylaxis of SARS-COV-2. Adv. Mater. 2021, 33, 2008304. [Google Scholar] [CrossRef] [PubMed]

| Ingredients | Composition (%w/w) | ||
|---|---|---|---|
| DGG | 0.40 | 0.50 | 0.60 |
| GA | 0.10 | 0.10 | 0.10 |
| Xanthan gum, HPMC, Na-CMC or Carbopol® 974P NF |
0.50 | 0.50 | 0.50 |
| Dexpanthenol | 0.20 | 0.20 | 0.20 |
| Glycerin | 1.00 | 1.00 | 1.00 |
| Benzalkonium chloride | 0.02 | 0.02 | 0.02 |
| Distilled water | 97.88 | 97.78 | 97.68 |
| Formulation | Ƞp | Ƞm | Ƞt | Ƞb | Fb (Pa) |
|---|---|---|---|---|---|
| DGG 0.4% - HPMC | 125.68 | 13.95 | 238.13 | 98.50 | 3.94 |
| DGG 0.5% - HPMC | 168.68 | 13.95 | 294.35 | 111.73 | 4.47 |
| DGG 0.6% - HPMC | 251.36 | 13.95 | 343.96 | 78.65 | 3.15 |
| DGG 0.4% - Na-CMC | 55.59 | 13.95 | 191.82 | 122.28 | 4.89 |
| DGG 0.5% - Na-CMC | 72.48 | 13.95 | 321.08 | 234.65 | 9.39 |
| DGG 0.6% - Na-CMC | 74.01 | 13.95 | 327.43 | 239.46 | 9.58 |
| DGG 0.4% - xanthan gum | 99.22 | 13.95 | 317.51 | 204.34 | 8.17 |
| DGG 0.5% - xanthan gum | 148.83 | 13.95 | 317.04 | 154.26 | 6.17 |
| DGG 0.6% - xanthan gum | 188.52 | 13.95 | 317.51 | 115.04 | 4.60 |
| Formulation | Ƞp | Ƞm | Ƞt | Ƞb | Fb(Pa) |
|---|---|---|---|---|---|
| DGG 0.5% - Na-CMC 0.1% | 20.28 | 16.51 | 298.84 | 262.06 | 8.91 |
| DGG 0.5% - Na-CMC 0.3% | 54.28 | 16.51 | 315.03 | 244.23 | 8.30 |
| DGG 0.5% - Na-CMC 0.5% | 74.23 | 16.51 | 371.08 | 280.34 | 9.53 |
| DGG 0.5% - Na-CMC 0.7% | 116.41 | 16.51 | 371.37 | 238.44 | 8.11 |
| Formulation | Adhesion strength (g) | |
|---|---|---|
| DGG 0.5% - Na-CMC 0.1% | 14.33 ± 8.40 | |
| DGG 0.5% - Na-CMC 0.3% | 15.67 ± 5.20 | |
| DGG 0.5% - Na-CMC 0.5% | 19.67 ± 5.90 | |
| DGG 0.5% - Na-CMC 0.7% | 27.67 ± 6.80 |
| Formulation | T1 | T7 | T14 | ||
|---|---|---|---|---|---|
| Mean ± SD |
Mean ± SD |
Weight deviation (%) |
Mean ± SD | Weight deviation (%) |
|
| DGG 0.5% - Na-CMC 0.1% |
0.323 ± 0.003 | 0.319 ± 0.002 | 1.24 | 0.320 ± 0.003 | 0.93 |
| DGG 0.5% - Na-CMC 0.3% |
0.319 ± 0.002 | 0.326 ± 0.001 | 2.19 | 0.323 ± 0.002 | 1.25 |
| DGG 0.5% - Na-CMC 0.5% |
0.306 ± 0.001 | 0.299 ± 0.002 | 2.29 | 0.302 ± 0.001 | 1.30 |
| DGG 0.5% - Na-CMC 0.7% |
0.299 ± 0.003 | 0.287 ± 0.001 | 4.01 | 0.294 ± 0.001 | 1.67 |
| Cytotoxicity | ||
|---|---|---|
| Sample Type | MNTC (µg/mL) |
CC50 (µg/mL) |
| GA | 8.33 | 47.59 |
| GA in situ gel | 4.16 | 15.29 |
| Placebo in situ gel | 4.16 | 14.84 |
| Ribavirin | 0.98 | 117.00 |
| Sample Type | Simultaneous | Pre-infection | ||
|---|---|---|---|---|
| EC50 (µg/mL) |
SI | EC50 (µg/mL) |
SI | |
| GA | 0.435 | 109.65 | 0.115 | 415.00 |
| GA in situ gel | 0.050 | 306.00 | 0.154 | 100.00 |
| Placebo in situ gel | 0.790 | 18.83 | 2.005 | 7.40 |
| Ribavirin | 4.189 | 28.00 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





