Submitted:
23 May 2023
Posted:
24 May 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Study design
2.2. Inclusion criteria
2.3. Exclusion criteria
2.4. Laboratory analysis
2.5. Statistical analysis
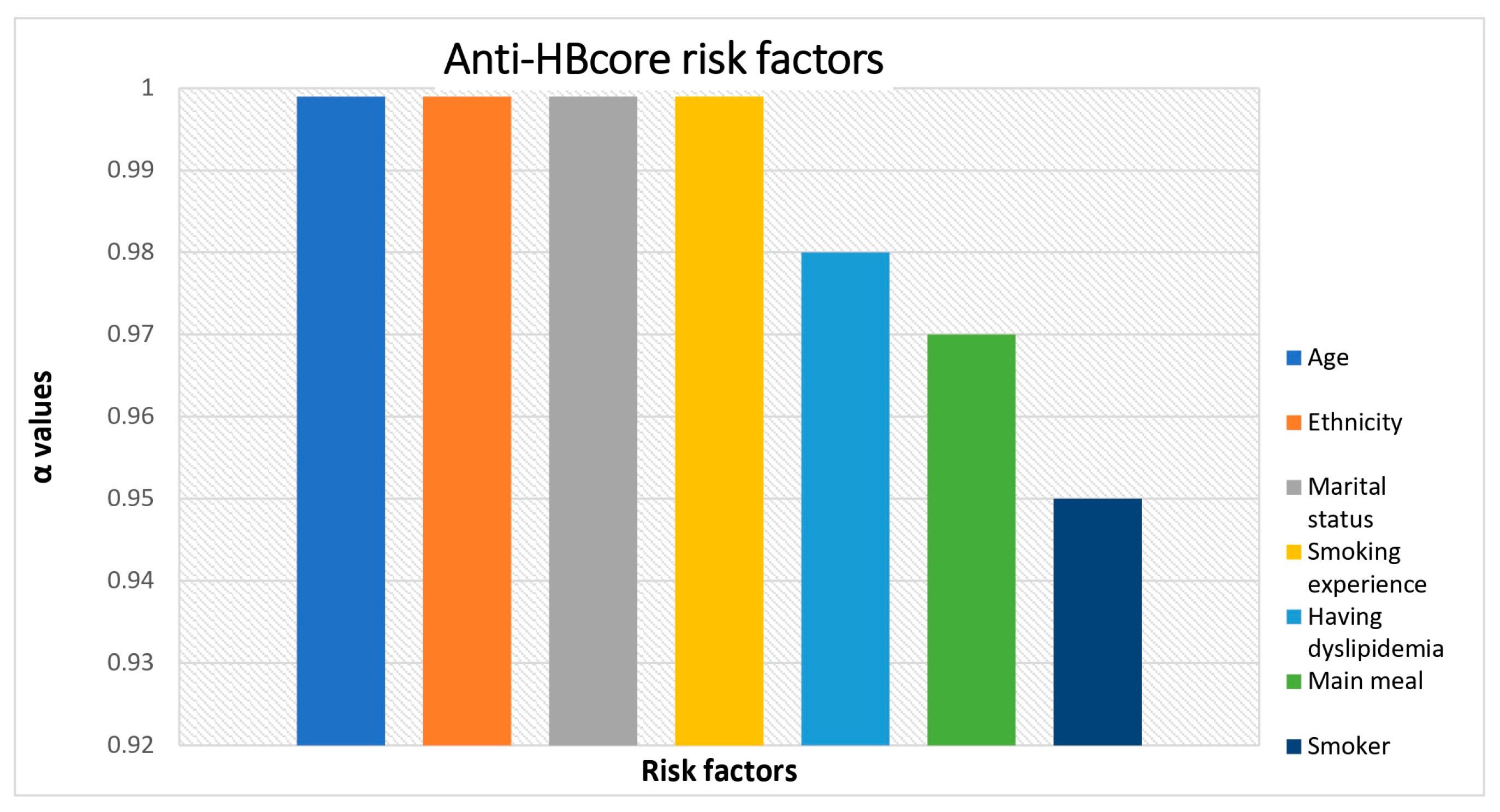
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Declaration of conflicting interests
Abbreviations
| ALT | Alanine aminotransferase |
| Anti-HBs | Antibody to Hepatitis B surface |
| Anti-HBcore | Antibody to hepatitis B core antigen |
| CHB | Chronic hepatitis B infection |
| HBV | Hepatitis B infection |
| HBsAg | Hepatitis B surface antigen |
| HBV DNA | Hepatitis B infection DNA |
| HCV | Hepatitis C infection |
| HIV | Human immunodeficiency virus |
| WHO | The World Health Organization |
References
- Available online: https://www.hepb.org/what-is-hepatitis-b/what-is-hepb/facts-and-figures/.
- Sarin, S.K.; Kumar, M.; Lau, G.K.; Abbas, Z.; Chan, H.L.; Chen, C.J.; et al. Asian-Pacific clinical practice guidelines on the management of hepatitis B: A 2015 update. Hepatol Int. 2016, 10, 1–98. [Google Scholar] [CrossRef] [PubMed]
- Lok, A.S.; McMahon, B.J. Chronic hepatitis B: Update 2009. Hepatology 2009, 50, 661–662. [Google Scholar] [CrossRef]
- Aliya, K. Epidemiology aspects of the chronic HBV with HDV infection in Kazakhstan. Eurasian Journal of Clinical Sciences 2019, 2, 4–5. [Google Scholar] [CrossRef]
- Jumabayeva, A.; Nersesov, A.; Kulzhanov, M.; Nefedova, M.; Nuraliyeva, G.; Rakhimbekova, G.; Tanabayeva, S.; Fakhradiyev, I. Prevalence of Viral Hepatitis B, C, and D in Kazakhstan. The Scientific World Journal 2022, 2022, 9102565. [Google Scholar] [CrossRef] [PubMed]
- Debra, A.; Kessler, R.N.; Alexandra Jimenez, M.D. In Transfusion Medicine and Hemostasis, 3rd ed.; 2019.
- Miletić, M.; Bingulac-Popović, J.; Stojić Vidović, M.; Hećimović, A.; Berendika, M.; Babić, I.; Đogić, V.; Samardžija, M.; Barišić, K.; Jukić, I.; et al. Anti-HBc prevalence among Croatian blood donors in a 14-year period (2004-2017): Assessment of trends, risks and need for implementing routine testing. Transfus Clin Biol. 2019, 26, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Meffre, C.; Le Strat, Y.; Delarocque-Astagneau, E.; Dubois, F.; Antona, D.; Lemasson, J.M.; Warszawski, J.; Steinmetz, J.; Coste, D.; Meyer, J.F.; et al. Prevalence of hepatitis B and hepatitis C virus infections in France in 2004: Social factors are important predictors after adjusting for known risk factors. J Med Virol. 2010, 82, 546–555. [Google Scholar] [CrossRef] [PubMed]
- Jilg, W.; Hottenträger, B.; Weinberger, K.; Schlottmann, K.; Frick, E.; Holstege, A.; Schölmerich, J.; Palitzsch, K.D. Prevalence of markers of hepatitis B in the adult German population. J Med Virol. 2001, 63, 96–102. [Google Scholar] [CrossRef]
- Liu, X.; Baecker, A.; Wu, M.; Zhou, J.Y.; Yang, J.; Han, R.Q.; Wang, P.H.; Jin, Z.Y.; Liu, A.M.; Gu, X.; et al. Interaction between tobacco smoking and hepatitis B virus infection on the risk of liver cancer in a Chinese population. Int J Cancer 2018, 142, 1560–1567. [Google Scholar] [CrossRef] [PubMed]
- Rutledge, S.M.; Asgharpour, A. Smoking and Liver Disease. Gastroenterol Hepatol (N Y) 2020, 16, 617–625. [Google Scholar] [PubMed]
- Le, T.V.; Vu, T.T.T.; Dang, A.K.; Vu, G.T.; Nguyen, L.H.; Nguyen, B.C.; Tran, T.H.; Tran, B.X.; Latkin, C.A.; Ho, C.S.S.; et al. Understanding Risk Behaviors of Vietnamese Adults with Chronic Hepatitis B in an Urban Setting. Int J Environ Res Public Health 2019, 16, 570. [Google Scholar] [CrossRef]
- Chang, Y.; Liu, Q.Y.; Zhang, Q.; Rong, Y.M.; Lu, C.Z.; Li, H. Role of nutritional status and nutritional support in outcome of hepatitis B virus-associated acute-on-chronic liver failure. World J Gastroenterol. 2020, 26, 4288–4301. [Google Scholar] [CrossRef] [PubMed]
- R.Core, version 4.1.1, USA, 2021.
- Savchuk, T.; Grinvald, Y.; Ali, M.; Sepetiene, R.; Saussakova, S.; Zhangazieva, K.; Imashpayev, D.; Abdrakhmanova, S. Antibodies to Hepatitis B core antigen prevalence study in Kazakhstan. Immun Inflamm Dis. 2023, 11, e793. [Google Scholar] [CrossRef] [PubMed]
- Eschment, B.; De Cordier, B. Introduction: Ethnic, Civic, or Both? The Ethnicities of Kazakhstan in Search of an Identity and Homeland. Central Asian Affairs 2021, 8, 315–318. [Google Scholar] [CrossRef]
- Gulis, G.; Aringazina, A.; Sangilbayeva, Z.; Zhan, K.; de Leeuw, E.; Allegrante, J.P. Population Health Status of the Republic of Kazakhstan: Trends and Implications for Public Health Policy. Int J Environ Res Public Health 2021, 18, 12235. [Google Scholar] [CrossRef]
- Shaha, M.; Hoque, S.A.; Ahmed, M.F.; Rahman, S.R. Effects of Risk Factors on Anti-HBs Development in Hepatitis B Vaccinated and Nonvaccinated Populations. Viral Immunol. 2015, 28, 217–221. [Google Scholar] [CrossRef] [PubMed]
- Prabina, P.; Jayanthi, S.; Krishna Murthy, C.; Kumar, S.B.; Banu, A.S.; Sakunthala, S.R.; Perumal, J. A Study on Hepatitis B Viral Seromarkers and Associated Risk Factors among the Patients Suffering from Acute and Chronic Hepatitis B Infection. International Journal of Applied and Basic Medical Research 2019, 9, 206–211. [Google Scholar] [CrossRef] [PubMed]
- Ciupe, S.M.; Ribeiro, R.M.; Perelson, A.S. Antibody responses during hepatitis B viral infection. PLoS Comput Biol. 2014, 10, e1003730. [Google Scholar] [CrossRef] [PubMed]
- Murphy, S.E. Biochemistry of nicotine metabolism and its relevance to lung cancer. J Biol Chem. 2021, 296, 100722. [Google Scholar] [CrossRef] [PubMed]
- Ni, Y.H.; Chang, M.H.; Huang, L.M.; et al. Hepatitis B virus infection in children and adolescents in a hyperendemic area: 15 years after mass hepatitis B vaccination. Ann. Intern. Med. 2001, 135, 796–800. [Google Scholar] [CrossRef]
- Elsner, R.A.; Shlomchik, M.J. Germinal center and extrafollicular B cell responses in vaccination, immunity, and autoimmunity. Immunity 2020, 53, 1136–1150. [Google Scholar] [CrossRef]
- Zhang, J.; Ling, N.; Lei, Y.; Peng, M.; Hu, P.; Chen, M. Multifaceted Interaction Between Hepatitis B Virus Infection and Lipid Metabolism in Hepatocytes: A Potential Target of Antiviral Therapy for Chronic Hepatitis B. Front Microbiol. 2021, 12, 636897. [Google Scholar] [CrossRef]
- Li, T.; Yan, H.; Geng, Y.; Shi, H.; Li, H.; Wang, S.; Wang, Y.; Xu, J.; Zhao, G.; Lu, X. Target genes associated with lipid and glucose metabolism in non-alcoholic fatty liver disease. Lipids Health Dis. 2019, 18, 211. [Google Scholar] [CrossRef]
- Akbar, S.; Pinçon, A.; Lanhers, M.C.; Claudepierre, T.; Corbier, C.; Gregory-Pauron, L.; Malaplate-Armand, C.; Visvikis, A.; Oster, T.; Yen, F.T. Expression profile of hepatic genes related to lipid homeostasis in LSR heterozygous mice contributes to their increased response to high-fat diet. Physiol Genomics 2016, 48, 928–935. [Google Scholar] [CrossRef]
- Zeng, Z. Human genes involved in hepatitis B virus infection. World J Gastroenterol. 2014, 20, 7696–7706. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, D.; Sugawa, M.; Kawahara, K. Study on Evaluation of Alanine Aminotransferase (ALT) as Surrogate Marker in Hepatitis Virus Test. J Med Dent Sci. 2016, 63, 45–52. [Google Scholar] [CrossRef]
- Ali, N.; Moiz, B.; Moatter, T. Evaluation of elevated alanine aminotransferase and hepatitis B virus DNA in healthy seronegative blood donors. BMC Res Notes 2012, 5, 272. [Google Scholar] [CrossRef]
- Suk-Fong Lok, A. Hepatitis B Treatment: What We Know Now and What Remains to Be Researched. Hepatol Commun. 2018, 3, 8–19. [Google Scholar] [CrossRef]
| Variables | Total N=5709 |
anti-HBcore | p-value | |
|---|---|---|---|---|
| Negative | Positive | |||
| n=4726 | n=983 | |||
| Age (mean (SD)) | 35.69 (10.57) | 34.42 (10.33) | 41.75 (9.54) | <0.001 |
| Gender, n (%) | 0.46 | |||
| Females | 1466 (31.8) | 1220 (32.1) | 246 (30.7) | |
| Males | 3139 (68.2) | 2583 (67.9) | 556 (69.3) | |
| Ethnicity, n (%) | <0.001 | |||
| Kazakh | 3855 (84.0) | 3146 (83.0) | 709 (88.7) | |
| Russian | 415 (9.0) | 364 (9.6) | 51 (6.4) | |
| Other ethnicities | 320 (7.0) | 281 (7.4) | 39 (4.9) | |
| Education level, n (%) | 0.48 | |||
| Secondary level | 2753 (68.0) | 2269 (67.8) | 484 (69.1) | |
| Vocational level | 549 (13.6) | 464 (13.9) | 85 (12.1) | |
| University level | 745 (18.4) | 614 (18.3) | 131 (18.7) | |
| Married, n (%) | <0.001 | |||
| Yes | 2354 (58.8) | 1845 (55.6) | 509 (74.0) | |
| Not | 1651 (41.2) | 1472 (44.4) | 179 (26.0) | |
| Smoker, n (%) | 0.05 | |||
| Yes | 1041 (25.0) | 836 (24.3) | 205 (27.9) | |
| Not | 3130 (75.0) | 2600 (75.7) | 530 (72.1) | |
| Smoking experience (mean (SD)) | 10.56 (8.79) | 9.83 (8.47) | 13.62 (9.46) | <0.001 |
| Alcohol consumption in the last 48 hours, n (%) | 0.16 | |||
| Yes | 1160 (18.8) | 972 (19.3) | 188 (16.6) | |
| Not | 2866 (71.2) | 2346 (70.7) | 520 (73.4) | |
| Main meal, n (%) | 0.03 | |||
| All | 515 (12.7) | 417 (12.5) | 98 (13.8) | |
| Breakfast | 754 (18.6) | 645 (19.3) | 109 (15.4) | |
| Lunch | 2187 (54.0) | 1805 (54.0) | 382 (53.8) | |
| Dinner | 595 (14.7) | 474 (14.2) | 121 (17.0) | |
| Meals per day, n (%) | 0.10 | |||
| 1-2 times | 386 (9.3) | 330 (9.6) | 56 (7.6) | |
| 3 or more times | 3770 (90.7) | 3091 (90.4) | 679 (92.4) | |
| Eating vegetables and fruits, n (%) | 0.68 | |||
| Several times a day | 581 (14.5) | 487 (14.7) | 94 (13.6) | |
| Every day | 2737 (68.4) | 2254 (68.1) | 483 (69.7) | |
| Sometimes | 683 (17.1) | 567 (17.1) | 116 (16.7) | |
| The percentage of food with meat and meat products, n (%) | 0.37 | |||
| ≤ 25% | 1113 (27.3) | 935 (27.8) | 178 (25.1) | |
| 26%-50% | 1953 (48.0) | 1606 (47.8) | 347 (48.9) | |
| 51%-75% | 726 (17.8) | 597 (17.8) | 129 (18.2) | |
| > 76% | 279 (6.9) | 223 (6.6) | 56 (7.9) | |
| Having dyslipidemia, n (%) | 0.02 | |||
| Yes | 150 (4.3) | 113 (3.9) | 37 (6.2) | |
| Not | 3359 (95.7) | 2797 (96.1) | 562 (93.8) | |
| Have family members had hepatitis in the last 6 months? n (%) | 0.41 | |||
| Yes | 68 (1.6) | 59 (1.7) | 9 (1.2) | |
| Not | 4096 (98.4) | 3367 (98.3) | 729 (98.8) | |
| Having transfusion of donated blood or its components in the last 12 months, n (%) | 0.57 | |||
| Yes | 56 (1.2) | 44 (1.1) | 12 (1.4) | |
| Not | 4653 (98.8) | 3835 (98.9) | 818 (98.6) | |
| Having intravenous or intramuscular injections, acupuncture, tattoos or piercings in the last 4 months | 0.71 | |||
| Yes | 247 (5.2) | 201 (5.2) | 46 (5.6) | |
| Not | 4458 (94.8) | 3678 (94.8) | 780 (94.4) | |
| Having surgical interventions (including cosmetic surgery or organ removal), n (%) | 0.18 | |||
| Yes | 722 (15.3) | 608 (15.7) | 114 (13.7) | |
| Not | 3989 (84.7) | 3273 (84.3) | 716 (86.3) | |
| Variables | anti-HBs | p-value | |
|---|---|---|---|
| Negative | Positive | ||
| n=211 (19.22%) | n=887 (80,78%) | ||
| Age (mean (SD)) | 40.02 (10.43) | 41.27 (9.50) | 0.117 |
| Gender, n (%) | 0.795 | ||
| Male | 62 (22.5) | 223 (21.1) | |
| Female | 129 (67.5) | 493 (68.9) | |
| Ethnicity, n (%) | 0.094 | ||
| Kazakh | 161 (84.3) | 629 (88.2) | |
| Russian | 12 (6.3) | 47 (6.6) | |
| Other ethnicities | 18 (9.4) | 37 (5.2) | |
| Education level, n (%) | 0.031 | ||
| Secondary level | 92 (59.4) | 439 (70.1) | |
| Vocational level | 27 (17.4) | 73 (11.7) | |
| University level | 36 (23.2) | 114 (18.2) | |
| Married, n (%) | 0.627 | ||
| Yes | 105 (70.9) | 454 (73.3) | |
| Not | 43 (29.1) | 165 (26.7) | |
| Smoker, n (%) | 0.366 | ||
| Yes | 40 (24.2) | 184 (28.1) | |
| Not | 125 (75.8) | 470 (71.9) | |
| Smoking experience (mean (SD)) | 15.18 (8.86) | 13.15 (9.57) | 0.306 |
| Alcohol consumption in the last 48 hours, n (%) | 1.00 | ||
| Yes | 43 (26.4) | 168 (26.7) | |
| Not | 120 (73.6) | 461 (73.3) | |
| Main meal, n (%) | 0.382 | ||
| All | 22 (13.9) | 87 (13.7) | |
| Breakfast | 31 (19.6) | 100 (15.8) | |
| Lunch | 85 (53.8) | 335 (52.8) | |
| Dinner | 20 (12.7) | 112 (17.7) | |
| Meals per day, n (%) | 0.062 | ||
| 1-2 times | 6 (3.6) | 54 (8.3) | |
| 3 or more times | 159 (96.4) | 600 (91.7) | |
| Eating vegetables and fruits, n (%) | 0.081 | ||
| Several times a day | 26 (17.1) | 81 (13.0) | |
| Every day | 110 (72.4) | 433 (69.7) | |
| Sometimes | 16 (10.5) | 107 (17.2) | |
| The percentage of food with meat and meat products, n (%) | 0.507 | ||
| ≤ 25% | 39 (24.8) | 160 (25.2) | |
| 26%-50% | 80 (51.0) | 311 (48.9) | |
| 51%-75% | 31 (19.7) | 115 (18.1) | |
| > 76% | 7 (4.5) | 50 (7.9) | |
| Having cholesterol disorder, n (%) | 0.838 | ||
| Yes | 7 (5.2) | 33 (6.2) | |
| Not | 127 (94.8) | 503 (93.8) | |
| Have family members had hepatitis in the last 6 month? n (%) | 1.00 | ||
| Yes | 2 (1.2) | 8 (1.2) | |
| Not | 163 (98.8) | 648 (98.8) | |
| Having transfusion of donated blood or its components in the last 12 months, n (%) | 0.126 | ||
| Yes | 5 (3.0) | 8 (1.1) | |
| Not | 162 (97.0) | 736 (98.9) | |
| Having intravenous or intramuscular injections, acupuncture, tattoos or piercings in the last 4 months | 0.856 | ||
| Yes | 8 (4.8) | 41 (5.5) | |
| Not | 158 (95.2) | 699 (94.5) | |
| Having surgical interventions (including cosmetic surgery or organ removal), n (%) | 0.166 | ||
| Yes | 31 (18.6) | 104 (14.0) | |
| Not | 136 (81.4) | 640 (86.0) | |
| ALT level, n (%) | 0.479 | ||
| Normal | 196 (94.2) | 819 (95.7) | |
| Elevated | 12 (5.8) | 37 (4.3) | |
| Variables | anti-HBcore | anti-HBs | ||
|---|---|---|---|---|
| Adjusted OR (95%CI) |
p-value | Adjusted OR (95%CI) |
p-value | |
| Age | 1.06 (1.05; 1.07) | <0.001 | 1.02 (1.00; 1.04) | 0.09 |
| Gender | 0.32 | 0.64 | ||
| Females | Ref. | Ref. | ||
| Males | 1.11 (0.90; 1.38) | 1.11 (0.71; 1.72) | ||
| Ethnicity | ||||
| Kazakh | Ref. | Ref. | ||
| Russian | 0.65 (0.46; 0.93) | 0.03 | 0.80 (0.39; 1.63) | 1.00 |
| Other ethnicities | 0.56 (0.37; 0.85) | 0.01 | 0.36 (0.18; 0.72) | <0.01 |
| Education level | ||||
| Secondary level | Ref. | Ref. | ||
| Vocational level | 0.77 (0.57; 1.04) | 0.18 | 0.50 (0.28; 0.89) | 0.04 |
| University level | 1.02 (0.79; 1.31) | 1.00 | 0.78 (0.47; 1.30) | 0.70 |
| Married | <0.01 | 0.74 | ||
| Yes | Ref. | Ref. | ||
| No | 0.71 (0.57; 0.89) | 0.92 (0.58; 1.46) | ||
| Have family members had hepatitis in the last 6 months? | 0.61 | 0.74 | ||
| Yes | Ref. | Ref. | ||
| No | 1.23 (0.55; 2.71) | 1.31 (0.26; 6.53) | ||
| Having transfusion of donated blood or its components in the last 12 months | 0.92 | 0.07 | ||
| Yes | Ref. | Ref. | ||
| No | 0.96 (0.44; 2.10) | 3.31 (0.89; 12.3) | ||
| Having intravenous or intramuscular injections, acupuncture, tattoos or piercings in the last 4 months | 0.21 | 0.89 | ||
| Yes | Ref. | Ref. | ||
| No | 0.79 (0.55; 1.15) | 0.89 (0.40; 1.99) | ||
| Having surgical interventions (including cosmetic surgery or organ removal) | 0.07 | 0.19 | ||
| Yes | Ref. | Ref. | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





