1. Introduction
Vitamin D3 (1,25-dihydroxy vitamin D, VD3), the active form of vitamin D, is a compelling immunoregulatory molecule [
1]. Immune cells are sensitive to VD3 after binding cytoplasmic vitamin D receptor (VDR) which allows it to enter the nucleus where they form a new complex with retinoic acid receptor (RXR). In this way, VD3-VDR-RXR bind to VD response elements (VDRE) located in the promoter of genes playing immune stimulatory or inhibitory roles. VDR is expressed constitutively by antigen-presenting cells (APC) such as macrophages and dendritic cells (DC), and in an inducible way by activated T regulatory cells (Treg). Monocytes, macrophages, and T cells can occasionally express enzymes (CYP27A1 and/or CYP27B1) that catalyze the conversion of the inactive precursor into active VD3. Immune cells produced VD3 act locally in an autocrine or paracrine way to regulate the immune response and it exerts pleiotropic effects on both innate and acquired pathways of immunity [
2,
3]. In macrophages and monocytes, VD3 positively regulates its own effect by increasing the expression of VDR and activating enzymes; it also induces monocyte proliferation and expression of pro-inflammatory interleukin-(IL-)1 by macrophages. VD3 hinders the maturation of DC making them switch the cytokine production pattern, that is lower amounts of pro-inflammatory IL-12 and higher levels of inhibitory/regulatory IL-10. An additional notable effect of VD3 on innate cells is the reduction of IL-4 release [
4], a typical cytokine involved in allergic responses. Furthermore, VD3 attenuates the proliferation of effector T cells CD4+ (helper) and the detrimental activity of CD8+ (cytotoxic) influencing the decreased production of the cytokine playing as pan T-cell growth factor, IL-2, and the pro-inflammatory interferon γ (IFN-γ). Furthermore, VD3 blocks B cells proliferation, differentiation, and immunoglobulin production [
4] and indirectly blocks the activity of Th2 cells, effector allergen-specific cells involved in allergic responses. Most importantly, VD3 promotes the development of innate and IL-10-producing inducible Treg playing pivotal roles in allergy development and control [
5]. The induction of durable tolerance towards the allergen is the main scope of allergen immunotherapy (AIT), the only treatment shown to have this capacity. The mechanistic interplay is accepted to rely on the elicitation of several subtypes of central and peripheral allergen-specific Treg that, consequently, increase the production of the inhibitory cytokines IL-10 and Transforming growth factor β
(TGF-β). Lastly, AIT can suppress aberrant Th2 response and induce isotype switch of allergen-specific IgE to IgG production by plasma cells [
6,
7].
The clinical efficacy of AIT has been proven. For illustration, during grass pollen AIT, the number of Th2-type memory cells decreases in conjunction with the increase of Treg and Th1 cells [
8] and Treg results increased in the nasal mucosa of treated patients [
9]. Recently, we showed that 1-year treatment with mite AIT was effective to promote the increase of functional memory Treg, characterized by higher surface inhibitory functional markers [
10].
It is known that, even if AIT determines clear attenuation or disappearance of symptoms, it requires up to five years of treatment and tight adherence and that non-responsive patients occur at 30% frequency [
11].
Hence, it would be important to find an add-on naturally occurring compound able to boost the immunomodulatory activity of AIT and favour its therapeutic effects for a larger number of patients at earlier time. Vitamin D3 might indeed play such a role.
However, there are conflicting findings on its potential role in risk, prevention, and amelioration of allergic diseases likely to reflect the age, the atopy status, the site of manifestation and the geographic area (
Table 1).
Contradictory associations have been described between data referring to the VD3 status of the mother. Higher intake of VD-rich during pregnancy has a preventive effect on allergies in their own atopic children [
12]. But VD3 supplementation appears to inconveniently promote childhood atopic dermatitis [
13]. Another study shows VD3 supplementation reduces the risk of exacerbation of asthma, atopic dermatitis and rhinitis symptoms in children and endogenous levels of VD appear a critical factor in allergy onset, too [
14]. More recently, the findings of a clinical trial do not support the use of vitamin D3 supplementation to prevent severe asthma exacerbations in a group of patients with low vitamin D levels [
15].
Remarkably, in asthmatic children undergoing AIT, the combination treatment with VD3 further enhanced the increase in Tregs, IL-10 and TGF-β [
18,
19] and reduced nasal symptoms and asthma [
16]. Conversely, in non-atopic toddlers, VD3 supplementation was associated with a higher risk of developing asthma [
17], allergic rhinitis and atopic dermatitis.
As evident, the role of endogenous VD in allergy severity and AIT has not been clearly assessed, so far. Previously, our group described an adjuvant role of VD3 supplementation in the murine model of type I mite allergy when administered in combination with Der p 1 immunotherapy [
20]. Starting from these premises, three data sets regarding three cohorts of allergic children (eligible or not for AIT) were retrospectively analyzed to assess whether the endogenous VD or supplemented VD3 might contribute to ameliorating the clinical outcome and/or promote the functionality of regulatory T cells in this unexplored setting.
2. Patients, Materials and Methods
2.1. Inclusion criteria
IgE sensitization and symptoms of allergic rhinitis with or without concomitant well-controlled asthma symptoms, who were considered eligible for AIT. All participants were enrolled voluntarily based upon their written informed consent, as well as that of their parents/tutors at Pediatric Allergology and Respiratory Unit, University Hospital of Chieti (Italy)
2.2. Diagnosis
Mite allergy and sensitization to
D. farinae (Der f) and
D. pteronyssinus (Der p) were assessed by positive skin-prick test and serum-specific IgE ≥0.7 kUA/L. Allergy to inhalant and food allergens were measured using the ImmunoCAP system (Phadia, Thermo-Scientific, Milan, Italy), which detects specific IgE in the range between 0 and 100 kUA/L. Diagnosis and severity of rhinitis were posed according to the ARIA classification [
21] applying a 5-point scoring system: 0: no rhinitis; 1: intermittent and mild rhinitis; 2: intermittent and moderate/severe rhinitis; 3: persistent and mild rhinitis; 4: persistent and moderate/severe rhinitis. Diagnosis and severity of asthma were done according to GINA guidelines [
22]: medical history, physical examination findings, and global spirometry, for post-bronchodilator FEV 1≥70% of predicted volume, Asthma Control Test (ACT), and inhaled corticosteroids/long-acting β2 agonist (ICS/LABA) and anti-leukotriene consumption. A visual analogue scale (VAS) was used for last month’s severity rating by using a 10 cm scale where 0 cm means no symptoms, and 10 cm corresponds to the highest level of symptoms. Medication score was assigned by counting the number of patients using antihistamines, and local or systemic steroids.
2.3. Exclusion criteria
reduced lung function (FEV1<70% of the predicted value), with a history of uncontrolled severe asthma (within 3 months before screening), HDM-SIT during the last 5 years, major conditions of the oral cavity, systemic diseases and treated with medications able to interfere with treatment. Systemic side effects after administration of each dose of AIT.
2.4. AIT treatment protocol
Sublingual immunotherapy (SLIT) with the monomeric allergoid LAIS® (Lofarma SpA, Milan, Italy), containing 50% of each Der f and Der p house dust mites. Each day, 1 tablet was kept under the tongue for 1-3 minutes and then swallowed. The patients were asked to refrain from drinking or eating for 15 min. SLIT regimen consisted of an induction phase (500 AU/day) followed by a maintenance phase. Then, all children assumed the maintenance dose of 4000 AU/week, for at least 12 months, as already described [
10].
2.5. VD3 supplementation
VD3 food supplement for the prevention of paediatric musculoskeletal disorders.
2.6. VD serum level
VD levels were determined using an ELISA test (LIAISON® 25-OH Vit Assay Kit; DiaSorin), with a detection range between 7.0 and 150 ng/ml.
2.7. Memory T regulatory cells flow cytometry assessment.
T regulatory cells were analysed to evaluate peripheral effector memory Tregs expressing HLA-DR [
10], as a surrogate endpoint. Briefly, peripheral blood mononuclear cells (PBMC) were stained using a panel of lyophilized reagents (Lyotube #624637) for 30 min, 4°C in the dark. Then, samples underwent an erythrocyte-lyse step with 1X Lysing solution, for 15 min at RT. Then, the samples were centrifuged and washed. For each sample, 1.5 x 10
5 events were acquired by the flow cytometry instrument FACSCanto. The threshold was placed on the Forward Scatter Channel (FSC). To ensure the correct identification of negative and positive populations, cells were plotted using a dot-plot bi-exponential display. Instrument performances, data reproducibility and fluorescence calibrations were sustained and checked by the Cytometer Setup & Tracking Module and further validated by the acquisition of 8-peak Rainbow Beads (Spherotech). Non-specific fluorescence was set out by the fluorescence-minus-one (FMO) control method. Compensation was assessed using CompBeads and single-stained fluorescent samples. Data were analysed using FlowJo v 8.8.6 (TreeStar, USA). Reagents, instruments, and software were purchased by BD Biosciences (Milan, Italy), if not otherwise indicated. HLA-DR surface expression, evaluated in terms of Mean Fluorescence Intensity (MFI), was normalized based on the relative expression of the respective CD4neg lymphocyte compartment. Treg data are expressed as median ± interquartile range (IQR).
3. Methodology of post hoc analysis
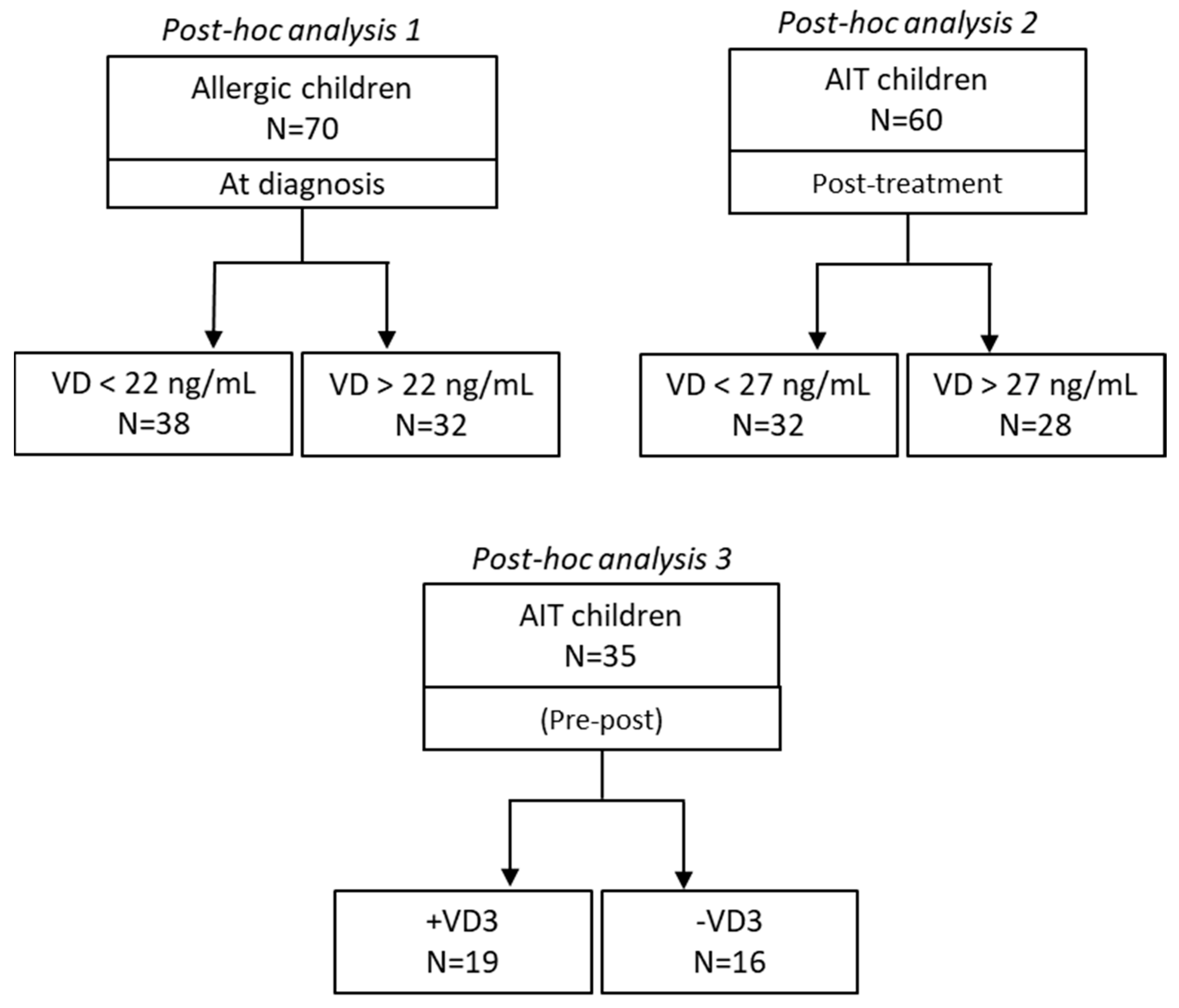
The patient population was selected from the database of the Pediatric Allergology and Respiratory Unit, University of Chieti (Italy) regarding all consecutive subjects with symptoms of allergy who contacted the diagnostic Unit, from September 2019 until June 2021. The specific diagnostic procedures and methods were protocolized as briefly reported in 2. and described in a previous study [
10]. Hundred sixty-five consecutive patients, diagnosed for house dust mite (HDM) allergy, underwent symptomatic therapy or AIT. Three different cohorts were selected posthoc to compare the dichotomous parameter of interest (
Figure 1). The first cohort was on standard symptoms-controlling pharmacological treatment (N=70). The second and the third cohorts (N=60 and N=35, respectively) were treated with AIT, for 12 months. This latter cohort cohort (N=35) was built up to generate two independent sub-cohorts comparable in terms of anthropometric parameters and medical history (
Table 2).
Relevant anthropometric and clinical data as well as laboratory and respiratory data of the patients involved in this analysis are shown in
Table 2 and
Table 4 in the Results section.
Clinical and laboratory data are expressed as means ± standard deviations (SD) unless otherwise indicated. All the measured parameters showed a non-parametric distribution, according to Shapiro-Wilk’s criteria. Statistical comparative analysis between two datasets, below and above the VD cut-off value or ±VD3 food supplement, was determined by Wilcoxon signed-rank test. Statistical analysis was performed using the program Statistical Package for Social Science (SPSS, Chicago, IL, USA). P values <0.05 were considered statistically significant.
4. Results
4.1. Endogenous VD in allergic children – post-hoc analysis 1
Allergic patients of the present study (N=70) were found to be characterized by heterogeneous endogenous levels of serum VD ranging from 17 to 27 ng/ml, hence covering biologically deficient, insufficient, and sufficient values [
23] (
Table 3).
Setting a cut-off value for VD serum concentration equal to the observed mean value (22 ng/ml), the analysis of categorical variables revealed that the group characterized by the lower serum VD (N=38) showed a significantly higher level of IgE (total and specific) and need for ICS-LABA as well as a higher VAS score. Reversely, the group with high VD (N=32) showed sufficient endogenous VD levels, and the lowest IgE levels and medication demand (
Table 4). The two sub-cohorts were otherwise comparable in terms of anthropometric parameters and medical history (
Table 2).
Table 4.
Allergen specificity and ICS-LABA usage of the two sub-cohorts of patients grouped according to the mean serum VD level (22 ng/ml) used as the cut-off value, at the time of diagnosis.
Table 4.
Allergen specificity and ICS-LABA usage of the two sub-cohorts of patients grouped according to the mean serum VD level (22 ng/ml) used as the cut-off value, at the time of diagnosis.
| Serum VD level (ng/ml) |
< 22 |
≥ 22 |
|
| N. of patients |
N=38 |
N=32 |
P |
| Df IgE (kUA/L) |
63.6±30.5 |
34.7±22.8 |
< 0.0001 |
| Dp IgE (kUA/L) |
65.1±32.1 |
42.5±33.0 |
= 0.0051 |
| ICS-LABA Yes/No (%)
|
32/6 (84.0) |
12/20 (37.5) |
< 0.0001 |
4.2. Endogenous VD in AIT children – post-hoc analysis 2
VD serum level, allergy, and respiratory data of the AIT group of patients (N=60) used for diagnosis, AIT prescription, and after 12 months of treatment are shown in
Table 5.
Notably, also in this cohort of patients a wide range of serum VD was detected at baseline and after 12 months post-AIT effective treatment (
Table 5).
Remarkably, the comparison between these two endogenous VD sub-cohorts using the mean value 27 ng/ml as cut-off, showed no significant differences in overall AIT clinical outcome as improvement of respiratory clinical scores (ARIA, ACT), except for oral antihistamine and VAS. Also, allergen IgE serum level was not significantly different by comparing the two sub-cohorts (
Table 6).
4.2. Exogenous supplementation of VD3 in AIT children – post-hoc analysis 3
After 12 months of treatment, the group of AIT patients supplemented with VD3 (+VD3, N=19) showed a significant increase in serum VD levels (baseline: 25.2±2.4 vs. post-treatment 36.1±2.8 ng/ml) (p<0.0001). Instead, no significant changes were found between the same time points in the other sub-cohort of not supplemented AIT children. All relevant clinical parameters (ARIA, antihistamine, nasal corticosteroid, and ICS-LABA consumption) were improved in both groups of patients, regardless VD3, as expected for an effective AIT outcome (
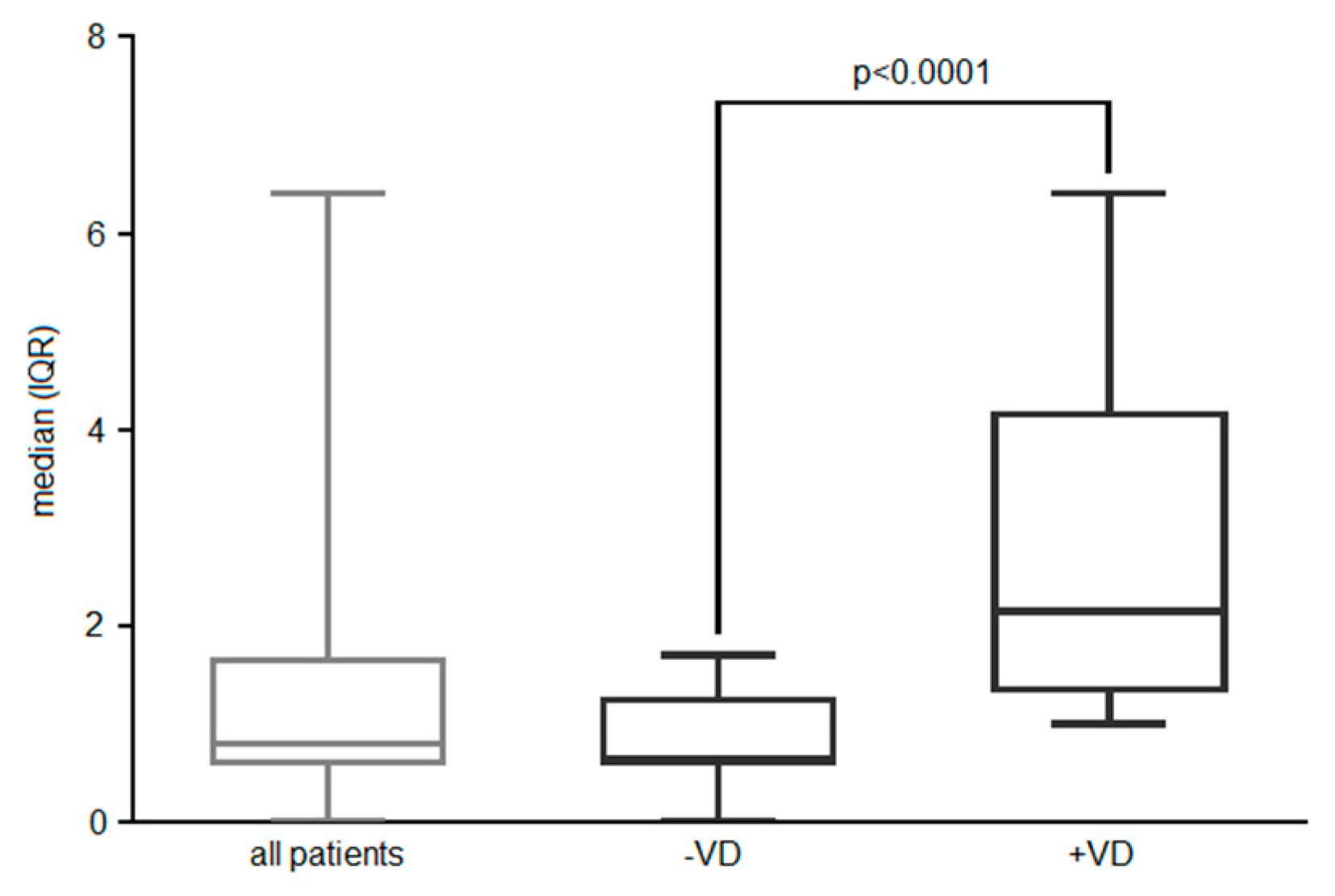
Table 7). Alongside, peripheral effector memory Tregs were also induced in both sub-cohorts of patients (all patients), confirming a previous finding of our group on the tolerogenic role of AIT (
Figure 2).
Interestingly, VD3 supplementation was associated with a significant increase in the endogenous VD level up to fully sufficient values (>30 ng/ml) (
Table 7). In this sub-cohort of patients was observed the uppermost amelioration of allergic symptoms, the lowest corticosteroid requirement and the steeper diminution of total and specific anti-Df IgEs, that is global AIT outcome, after 12 months of treatment. In fact, the clinical improvement according to ARIA, and the reduction in antihistamine consumption, was significantly greater in the sub-cohort group that had taken the VD3 food supplement, compared to the not (p<0.05). A reduced ICS-LABA requirement was registered for the +VD3 sub-cohort, compared to the other one (P<0.0001) (
Table 8).
Furthermore, significant diminution of serum levels of total IgE and specific anti-Df IgE were observed only in the +VD3 cohort, after AIT (
Table 9).
Besides, endogenous VD appeared involved in the extra increase of the functionality of peripheral effector memory Tregs (HLA-DR expression level on CD4
+CD25
+CD127
negCD39
+CD45RA
negHLA-DR
+ peripheral blood lymphocytes) in sub-cohort +VD3 (endogenous level VD>30 ng/ml), compared to those without supplementation (p<0.0001) (
Figure 2).
Figure 2.
HLA expression level expressed as median (IQR) on effector memory Treg in AIT-treated patients, respectively to VD3 supplementation (or endogenous VD>30ng/ml).
Figure 2.
HLA expression level expressed as median (IQR) on effector memory Treg in AIT-treated patients, respectively to VD3 supplementation (or endogenous VD>30ng/ml).
The schematization of the overall results of the three data sets analyzed is shown in
Table 10.
5. Discussion
The rationale of this post-hoc analysis was to address the influence of endogenous vitamin D in children with respiratory mite allergy on allergen immunotherapy (AIT) in children with respiratory mite allergy. Indeed, VD has been described as a pleiotropic immunoregulator. In fact, other investigators have shown that it promotes the maturation of Treg by inducing the surface expression and/or secretion of inhibitory cytokines, which play a pivotal role in allergy control. Furthermore, VD exerts an indirect blocking activity on Th2 effector cells and B cells proliferation, differentiation, and IgE production [
3]. Despite these advancements in the comprehension of immunological mechanisms, the role of VD in human allergic sensitization and symptomatic disease is difficult to disentangle. In fact, variable and incoherent findings are described in clinical settings and trials. Contradictory associations have been described between different VD during pregnancy. Higher intake of VD-rich foods during pregnancy has a preventive effect as reduced incidence of asthmatics, recurrent dyspnea, allergic rhinitis, and food allergy. But high VD3 supplementation appears to inconveniently promote atopic dermatitis in children [
12,
13]. According to the most recent reviews of published data, VD3 supplementation during childhood reduces the risk of exacerbation of asthma, atopic dermatitis, and rhinitis symptoms. Hence, the role of the endogenous level of VD has been envisaged for the first time as a critical factor in allergy [
14]. However, a recent clinical trial (randomized a controlled) does not support the use of VD3 supplementation to prevent exacerbations in a group of children with low VD levels affected by severe asthma [
15]. Conversely, in non-atopic toddlers, VD3 supplementation was associated with a higher risk of developing asthma [
17], allergic rhinitis and atopic dermatitis (
Table 1).
Nevertheless, we described an adjuvant role of VD3 in Der p 2-based experimental MA-AIT in a mouse allergy model [
20]. More recently, an observational study conducted by us showed that one-year AIT, besides the expected amelioration of clinical scores, also increases memory effector Treg [
10]. Furthermore, in asthmatic children undergoing AIT, the combination treatment with VD3 reduced rhinitis and asthma [
16] and further enhanced the increase in Tregs, IL-10 and TGF-β [
18,
19]. Also, a few trials by other investigators have shown inspiring, although not robust, results that foresee the clinical use of VD3 in allergy management [
19].
Hence, we seek out VD potential roles in the severity of the allergy, in the clinical outcomes of effective AIT, and in the underlying immunomodulatory mechanism by analysing post-hoc three different cohorts of mite-allergic children.
Our post-hoc analysis unveils that AIT is more effective in children with a fully sufficient serum VD level (>27 ng/ml), naturally occurring or achieved by supplementation, as shown by uppermost amelioration of allergic symptoms of rhinitis and asthma, lowest corticosteroid requirement and diminution of specific IgE (
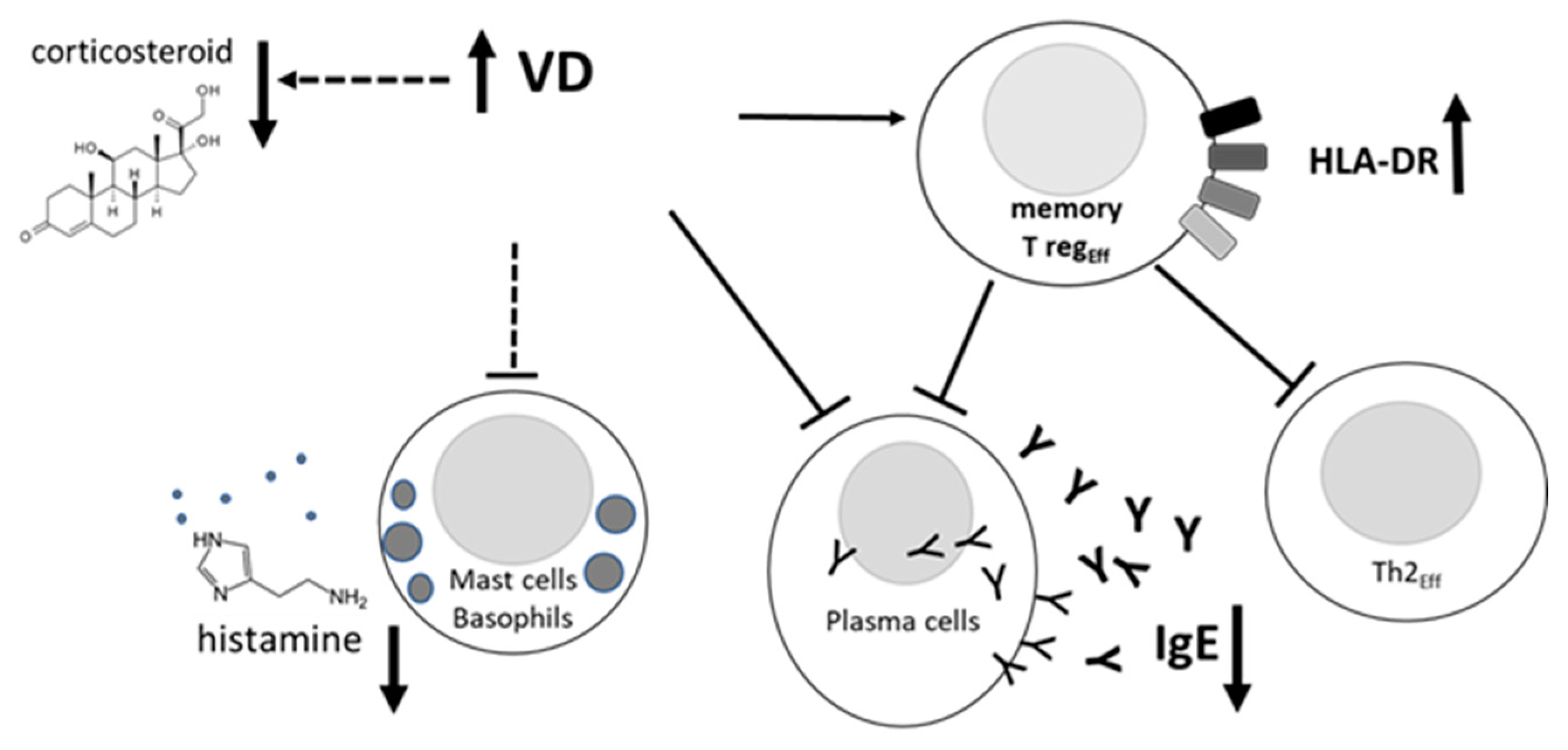
Table 2). Furthermore, the VD might provide empowerment of effector memory Tregs inhibitory function, through the hypothetical mechanism described in
Figure 3. A few takeaway messages can be drawn from our findings: serum endogenous VD levels appear to be inversely associated with allergy severity and medication consumption; furthermore, sufficient endogenous VD level is associated with better improvement of AIT efficacy. Furthermore, VD might play an indirect role in the AIT context, by conditioning the activity of immune cells involved in the underlying immunological mechanism (
Figure 3).
A recent study provides supporting data to our point of view since it shows that better built-up efficacy of allergy immunotherapy is achieved in VD3-supplemented allergic patients [
17]. Clinical studies indicate that VD serum levels are inversely associated with responsiveness to corticosteroids, with the lower level corresponding to the higher drug assumption [
24]. Furthermore, dexamethasone-resistant patients show IL-10-unproductive Tregs [
25]. Also, IL-10 production and sensitivity to glucocorticoids were restored upon co-administration of VD3 in those patients [
25]. Also, VD3 induces Tregs
in vitro and displays an additive effect with glucocorticoids and reversal of glucocorticoid resistance [
26]. A randomized clinical trial has excluded VD3 as an adjuvant supplement for (non-allergic) asthma management, not disproving us since it has been conducted in non-allergic adults with VD insufficiency [
27].
6. Conclusions
We conclude that clinical outcomes of AIT are not affected by endogenous levels of VD and VD3 supplementation. Nevertheless, our findings suggest that it might be useful as an add-on supplement in children with VD deficiency/insufficiency undergoing AIT and characterized by a high need for symptomatic medications. VD role in maintaining antigen-specific long-term Treg-mediated tolerance to the eliciting allergen is worth to be evaluated in the unexplored setting of AIT clinical trials.
Some limitations of our post-hoc analysis are the single-centre geographical area /population and the modest cohort size. Despite that, our investigation deals with a hot and interesting topic and may provide hints for future clinical trials. Further experimental multicentric clinical trials are envisaged to better address the endogenous and exogenous VD role. Serum VD level and peripheral Tregs immunophenotyping could be valuable markers for monitoring AIT during treatment. We highlight also that immunogenic/low-allergenic mite monomeric carbamylated allergoid used as bioactive agent might have contributed to minimising allergic responses and highlight the immunological effects described here.
Author Contributions
Conceptualization, methodology C.P. and D.V.; writing and original draft preparation, C.P.; review and editing, C.P. and D.V.; funding acquisition C.P. and D.V. All authors have read and agreed to the published version of the manuscript.
Funding
C.P. was funded by the Italian Ministry of University and Research, MIUR (FAR 2021). D.V. was funded by the Department of Medicine and Science Aging, University of Chieti-Pescara, Italy, grant number CUP D55F21004250005 and CUP D53C22002740005. The APC was funded by the Department of Medicine and Science Aging, University of Chieti-Pescara, Italy.
Institutional Review Board Statement
The present study was approved by the local Ethical Committee of the University “G. d’Annunzio”, Chieti-Pescara (Protocol No. 22/2019).
Informed Consent Statement
Patient consent was waived since participating patients cannot be identified (including by the patients themselves).
Acknowledgements
Pediatric Allergology and Respiratory Unit, University of Chieti (Italy).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Nagpal, S.; Na, S.; Rathnachalam, R. Noncalcemic Actions of Vitamin D Receptor Ligands. Endocr Rev 2005, 26, 662–687. [Google Scholar] [CrossRef]
- Edfeldt, K.; Liu, P.T.; Chun, R.; Fabri, M.; Schenk, M.; Wheelwright, M.; Keegan, C.; Krutzik, S.R.; Adams, J.S.; Hewison, M.; et al. T-Cell Cytokines Differentially Control Human Monocyte Antimicrobial Responses by Regulating Vitamin D Metabolism. Proc Natl Acad Sci U S A 2010, 107, 22593–22598. [Google Scholar] [CrossRef] [PubMed]
- Smolders, J.; Thewissen, M.; Damoiseaux, J. Control of T Cell Activation by Vitamin D. Nat Immunol 2011, 12, 3. [Google Scholar] [CrossRef] [PubMed]
- Mora, J.R.; Iwata, M.; Von Andrian, U.H. Vitamin Effects on the Immune System: Vitamins A and D Take Centre Stage. Nat Rev Immunol 2008, 8, 685–698. [Google Scholar] [CrossRef]
- Akdis, M.; Blaser, K.; Akdis, C.A. T Regulatory Cells in Allergy. Chem Immunol Allergy 2006, 91, 159–173. [Google Scholar] [CrossRef] [PubMed]
- Akdis, C.A.; Akdis, M. Mechanisms of Immune Tolerance to Allergens: Role of IL-10 and Tregs. J Clin Invest 2014, 124, 4678–4680. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Wang, Z.C.; Wang, N.; Zhou, P.C.; Chen, C.L.; Song, J.; Pan, L.; Liao, B.; Zhang, X.H.; Yang, Y.S.; et al. Allergen Immunotherapy Improves Defective Follicular Regulatory T Cells in Patients with Allergic Rhinitis. Journal of Allergy and Clinical Immunology 2019, 144, 118–128. [Google Scholar] [CrossRef]
- Suárez-Fueyo, A.; Ramos, T.; Galán, A.; Jimeno, L.; Wurtzen, P.A.; Marin, A.; de Frutos, C.; Blanco, C.; Carrera, A.C.; Barber, D.; et al. Grass Tablet Sublingual Immunotherapy Downregulates the TH2 Cytokine Response Followed by Regulatory T-Cell Generation. Journal of Allergy and Clinical Immunology 2014, 133, 130–138.e2. [Google Scholar] [CrossRef]
- Radulovic, S.; Jacobson, M.R.; Durham, S.R.; Nouri-Aria, K.T. Grass Pollen Immunotherapy Induces Foxp3-Expressing CD4+CD25+ Cells in the Nasal Mucosa. Journal of Allergy and Clinical Immunology 2008, 121, 1467–1472.e1. [Google Scholar] [CrossRef]
- Petrarca, C.; Lanuti, P.; Petrosino, M.I.; di Pillo, S.; Mistrello, G.; Compalati, E.; Otzuki, T.; Marchisio, M.; Pierdomenico, L.; Paganelli, R.; et al. Peripheral Effector Memory Regulatory T Cells Are Incremented and Functionally Enhanced in Successful Mite Monomeric Allergoid Sublingual Immunotherapy. Allergy 2021, 76, 2208–2211. [Google Scholar] [CrossRef]
- Ferrando, M.; Racca, F.; Madeira, L.N.G.; Heffler, E.; Passalacqua, G.; Puggioni, F.; Stomeo, N.; Canonica, G.W. A Critical Appraisal on AIT in Childhood Asthma. Clinical and Molecular Allergy 2018, 16, 6. [Google Scholar] [CrossRef] [PubMed]
- Miyake, Y.; Tanaka, K.; Okubo, H.; Sasaki, S.; Arakawa, M. Maternal Consumption of Dairy Products, Calcium, and Vitamin D during Pregnancy and Infantile Allergic Disorders. Annals of Allergy, Asthma and Immunology 2014, 113, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.; Sun, Y.; Zeng, Z.; Liu, Y.; Peng, S.; Wei, P. Vitamin D Supplementation in Pregnant Women or Infants for Preventing Allergic Diseases: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Chin Med J (Engl) 2022, 135, 276–284. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Zhou, Q.; Zhang, G.; Tian, X.; Li, Y.; Wang, Z.; Zhao, Y.; Chen, Y.; Luo, Z. Vitamin D Supplementation and Allergic Diseases during Childhood: A Systematic Review and Meta-Analysis. Nutrients 2022, 14. [Google Scholar] [CrossRef]
- Forno, E.; Bacharier, L.B.; Phipatanakul, W.; Guilbert, T.W.; Cabana, M.D.; Ross, K.; Covar, R.; Gern, J.E.; Rosser, F.J.; Blatter, J.; et al. Effect of Vitamin D3 Supplementation on Severe Asthma Exacerbations in Children With Asthma and Low Vitamin D Levels: The VDKA Randomized Clinical Trial. JAMA 2020, 324, 752–760. [Google Scholar] [CrossRef] [PubMed]
- Jerzynska, J.; Stelmach, W.; Rychlik, B.; Lechańska, J.; Podlecka, D.; Stelmach, I. The Clinical Effect of Vitamin D Supplementation Combined with Grass-Specific Sublingual Immunotherapy in Children with Allergic Rhinitis. Allergy and asthma proceedings : the official journal of regional and state allergy societies 2016, 37, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Nwaru, B.I.; Hadkhale, K.; Hämäläinen, N.; Takkinen, H.M.; Ahonen, S.; Ilonen, J.; Toppari, J.; Niemelä, O.; Haapala, A.M.; Veijola, R.; et al. Vitamin D Intake during the First 4 Years and Onset of Asthma by Age 5: A Nested Case-Control Study. Pediatr Allergy Immunol 2017, 28, 641–648. [Google Scholar] [CrossRef]
- Taher, Y.A.; van Esch, B.C.A.M.; Hofman, G.A.; Henricks, P.A.J.; van Oosterhout, A.J.M. 1alpha,25-Dihydroxyvitamin D3 Potentiates the Beneficial Effects of Allergen Immunotherapy in a Mouse Model of Allergic Asthma: Role for IL-10 and TGF-Beta. J Immunol 2008, 180, 5211–5221. [Google Scholar] [CrossRef]
- Baris, S.; Kiykim, A.; Ozen, A.; Tulunay, A.; Karakoc-Aydiner, E.; Barlan, I.B. Vitamin D as an Adjunct to Subcutaneous Allergen Immunotherapy in Asthmatic Children Sensitized to House Dust Mite. Allergy 2014, 69, 246–253. [Google Scholar] [CrossRef]
- Petrarca, C.; Clemente, E.; Amato, V.; Gatta, A.; Cortese, S.; Lamolinara, A.; Rossi, C.; Zanotta, S.; Mistrello, G.; Paganelli, R.; et al. Vitamin D3 Improves the Effects of Low Dose Der p 2 Allergoid Treatment in Der p 2 Sensitized BALB/c Mice. Clin Mol Allergy 2016, 14, 7. [Google Scholar] [CrossRef]
- Bousquet, J.; Bedbrook, A.; Czarlewski, W.; Onorato, G.L.; Arnavielhe, S.; Laune, D.; Mathieu-Dupas, E.; Fonseca, J.; Costa, E.; Lourenço, O.; et al. Guidance to 2018 Good Practice: ARIA Digitally-Enabled, Integrated, Person-Centred Care for Rhinitis and Asthma. Clin Transl Allergy 2019, 9, 1–19. [Google Scholar] [CrossRef]
- GLOBAL STRATEGY FOR ASTHMA MANAGEMENT AND PREVENTION.
- Wei, F.; Wang, Z.; Wang, J.; Xu, H.; Zhou, H. Serum Vitamin D Levels among Children Aged 0-12 Years in the First Affiliated Hospital of Harbin Medical University, China. Journal of Public Health (United Kingdom) 2018, 40. [Google Scholar] [CrossRef] [PubMed]
- Searing, D.A.; Zhang, Y.; Murphy, J.R.; Hauk, P.J.; Goleva, E.; Leung, D.Y.M. Decreased Serum Vitamin D Levels in Children with Asthma Are Associated with Increased Corticosteroid Use. Journal of Allergy and Clinical Immunology 2010, 125, 995–1000. [Google Scholar] [CrossRef] [PubMed]
- Xystrakis, E.; Kusumakar, S.; Boswell, S.; Peek, E.; Urry, Z.; Richards, D.F.; Adikibi, T.; Pridgeon, C.; Dallman, M.; Loke, T.K.; et al. Reversing the Defective Induction of IL-10-Secreting Regulatory T Cells in Glucocorticoid-Resistant Asthma Patients. Journal of Clinical Investigation 2006, 116, 146–155. [Google Scholar] [CrossRef]
- Urry, Z.; Chambers, E.S.; Xystrakis, E.; Dimeloe, S.; Richards, D.F.; Gabryšová, L.; Christensen, J.; Gupta, A.; Saglani, S.; Bush, A.; et al. The Role of 1α,25-Dihydroxyvitamin D3 and Cytokines in the Promotion of Distinct Foxp3+ and IL-10+ CD4+ T Cells. Eur J Immunol 2012, 42, 2697–2708. [Google Scholar] [CrossRef]
- Castro, M.; King, T.S.; Kunselman, S.J.; Cabana, M.D.; Denlinger, L.; Holguin, F.; Kazani, S.D.; Moore, W.C.; Moy, J.; Sorkness, C.A.; et al. Effect of Vitamin D3 on Asthma Treatment Failures in Adults with Symptomatic Asthma and Lower Vitamin D Levels: The VIDA Randomized Clinical Trial. JAMA 2014, 311, 2083–2091. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).