Submitted:
22 May 2023
Posted:
24 May 2023
You are already at the latest version
Abstract
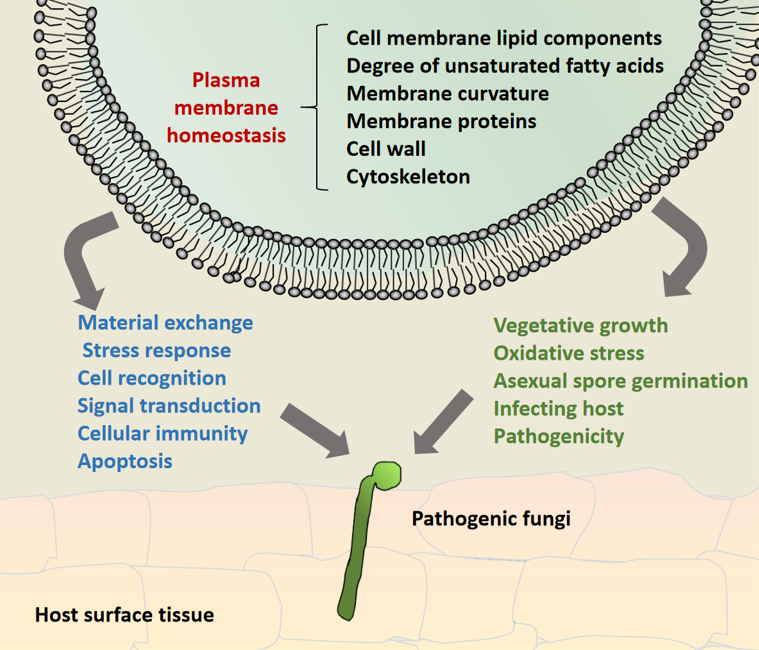
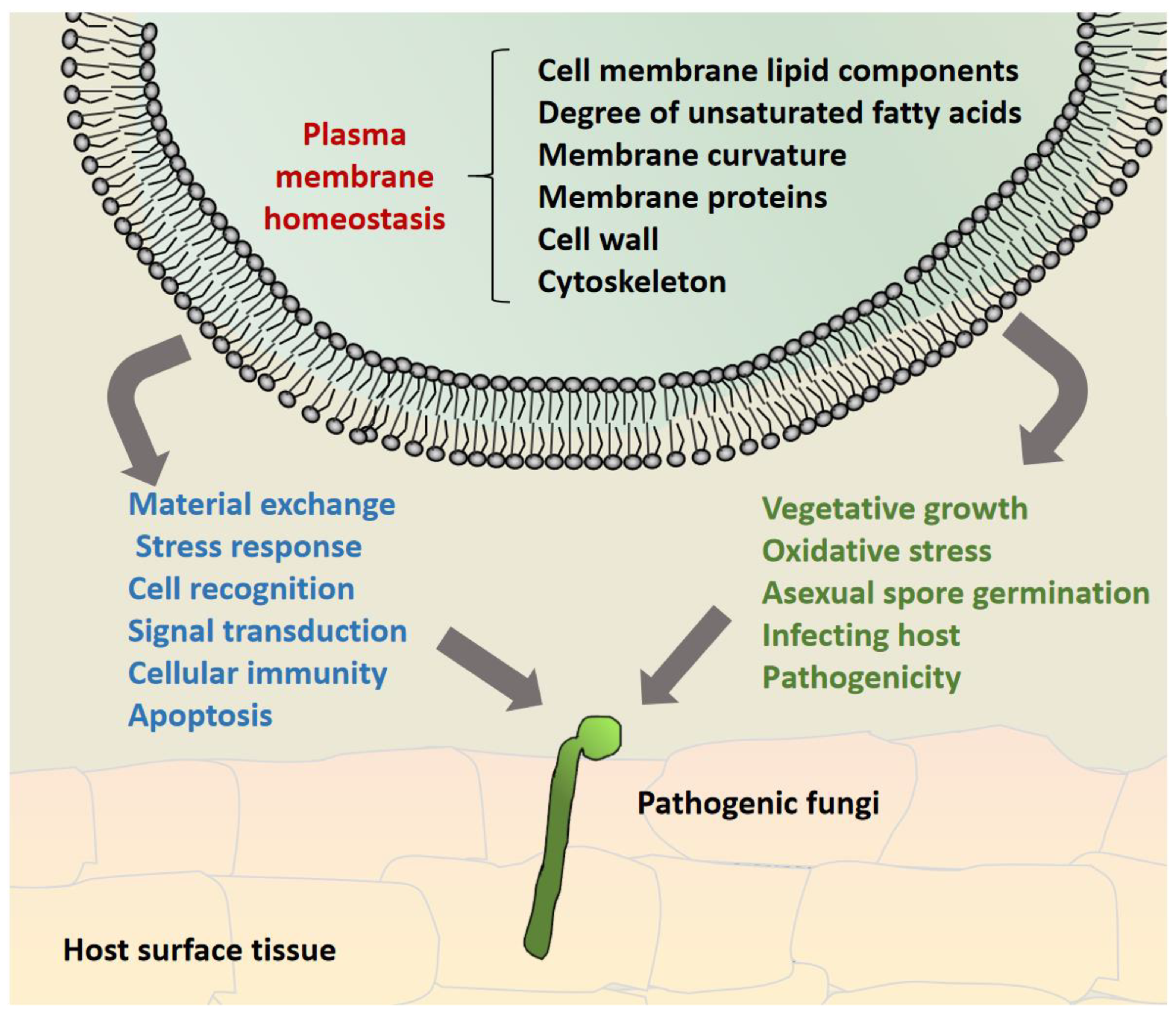
Keywords:
1. Introduction
2. Effect of lipid metabolism on the pathogenicity of fungi
2.1. cytoplasmic membrane lipid components
2.2. Degree of unsaturated fatty acids
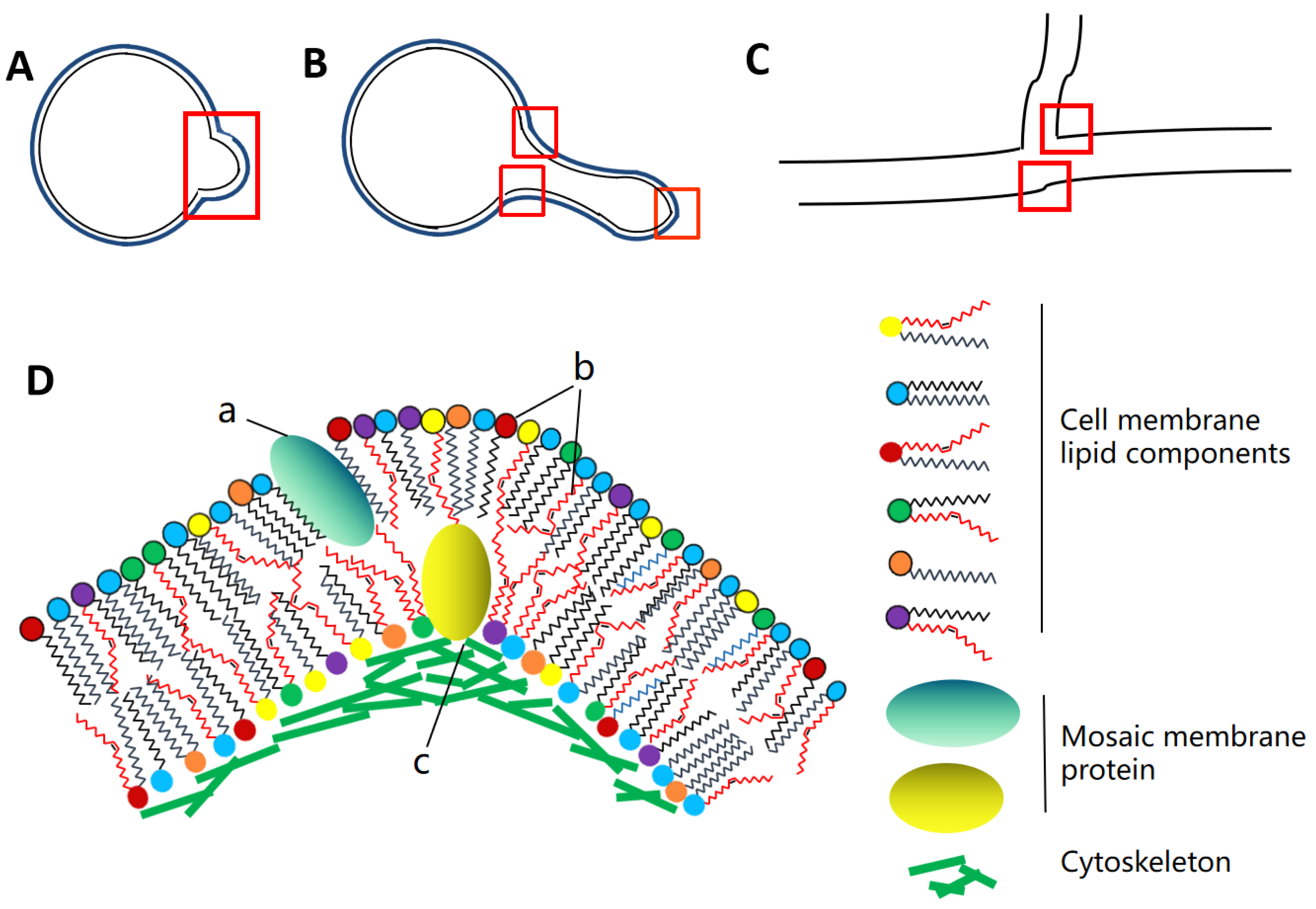
3. Plasma membrane curvature affects membrane homeostasis and the pathogenicity of fungi
4. Effect of membrane proteins on cytoplasmic membrane homeostasis of pathogenic fungi
5. Cell wall affect cytoplasmic membrane homeostasis of the pathogenic fungi
6. Discussion
Acknowledgments
References
- van Meer, G.; Voelker, D.R.; Feigenson, G.W. Membrane lipids: where they are and how they behave. Nat Rev Mol Cell Bio 2008, 9, 112–124. [Google Scholar] [CrossRef] [PubMed]
- Nicolson, G.L. The Fluid-Mosaic Model of Membrane Structure: still relevant to understanding the structure, function and dynamics of biological membranes after more than 40 years. Biochim Biophys Acta 2014, 1838, 1451–1466. [Google Scholar] [CrossRef] [PubMed]
- Sonnino, S.; Prinetti, A. Membrane domains and the "lipid raft" concept. Curr Med Chem 2013, 20, 4–21. [Google Scholar] [PubMed]
- Horn, A.; Jaiswal, J.K. Structural and signaling role of lipids in plasma membrane repair. Curr Top Membr 2019, 84, 67–98. [Google Scholar] [CrossRef] [PubMed]
- Cerioni, L.; Volentini, S.I.; Prado, F.E.; etc. Cellular damage induced by a sequential oxidative treatment on Penicillium digitatum. J Appl Microbiol 2010, 109, 1441–1449. [CrossRef]
- Malinsky, J.; Opekarova, M. New Insight Into the Roles of Membrane Microdomains in Physiological Activities of Fungal Cells. Int Rev Cel Mol Bio 2016, 325, 119–180. [Google Scholar] [CrossRef]
- Ziolkowska, N.E.; Christiano, R.; Walther, T.C. Organized living: formation mechanisms and functions of plasma membrane domains in yeast. Trends Cell Biol 2012, 22, 151–158. [Google Scholar] [CrossRef]
- Harayama, T.; Riezman, H. Understanding the diversity of membrane lipid composition. Nat Rev Mol Cell Bio 2018, 19, 281–296. [Google Scholar] [CrossRef]
- Escriba, P.V.; Busquets, X.; Inokuchi, J.; etc. Membrane lipid therapy: Modulation of the cell membrane composition and structure as a molecular base for drug discovery and new disease treatment. Prog Lipid Res 2015, 59, 38–53. [CrossRef]
- Epand, R.M. Introduction to membrane lipids. Methods Mol Biol 2015, 1232, 1–6. [Google Scholar] [CrossRef]
- Thomas, F.B.; Omnus, D.J.; Bader, J.M.; etc. Tricalbin proteins regulate plasma membrane phospholipid homeostasis. Life Sci Alliance 2022, 5. [CrossRef]
- Akhberdi, O.; Zhang, Q.; Wang, H.; etc. Roles of phospholipid methyltransferases in pycnidia development, stress tolerance and secondary metabolism in the taxol-producing fungus Pestalotiopsis microspore. Microbiol Res 2018, 210, 33–42. [CrossRef] [PubMed]
- Beauvais, A.; Latge, J.P. Membrane and cell wall targets in Aspergillus fumigatus. Drug Resist Update 2001, 4, 38–49. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, C.M.; de Castro, P.A.; Singh, A.; etc. Functional characterization of the Aspergillus nidulans glucosylceramide pathway reveals that LCB Delta8-desaturation and C9-methylation are relevant to filamentous growth, lipid raft localization and Psd1 defensin activity. Mol Microbiol 2016, 102, 488–505. [CrossRef]
- Vernay, A.; Schaub, S.; Guillas, I.; etc. A steep phosphoinositide bis-phosphate gradient forms during fungal filamentous growth. J Cell Biol 2012, 198, 711–730. [CrossRef] [PubMed]
- Li, S.; Du L; Yuen, G.; etc. Distinct ceramide synthases regulate polarized growth in the filamentous fungus Aspergillus nidulans. Mol Biol Cell 2006, 17, 1218–1227. [CrossRef]
- Head, B.P.; Patel, H.H.; Insel, P.A. Interaction of membrane/lipid rafts with the cytoskeleton: impact on signaling and function: membrane/lipid rafts, mediators of cytoskeletal arrangement and cell signaling. Biochim Biophys Acta 2014, 1838, 532–545. [Google Scholar] [CrossRef]
- Markham, P.; Robson, G.D.; Bainbridge, B.W.; etc. Choline: its role in the growth of filamentous fungi and the regulation of mycelial morphology. Fems Microbiol Rev 1993, 10, 287–300. [CrossRef]
- Ghugtyal, V.; Garcia-Rodas, R.; Seminara, A.; etc. Phosphatidylinositol-4-phosphate-dependent membrane traffic is critical for fungal filamentous growth. P Natl Acad Sci Usa 2015, 112, 8644–8649. [CrossRef]
- Cotado-Sampayo, M.; Ramos, P.O.; Perez, R.O.; etc. Specificity of commercial anti-spectrin antibody in the study of fungal and Oomycete spectrin: cross-reaction with proteins other than spectrin. Fungal Genet Biol 2008, 45, 1008–1015. [CrossRef]
- Zhang, L.; Cao, X.; Wang, Z.; etc. Brassinolide alleviated chilling injury of banana fruit by regulating unsaturated fatty acids and phenolic compounds. Sci Hortic-Amsterdam 2022, 297, 110922. [CrossRef]
- Resnick, M.A.; Mortimer, R.K. Unsaturated fatty acid mutants of Saccharomyces cerevisiae. J Bacteriol 1966, 92, 597–600. [Google Scholar] [CrossRef] [PubMed]
- Wisnieski, B.J.; Kiyomoto, R.K. Fatty acid desaturase mutants of yeast: growth requirements and electron spin resonance spin-label distribution. J Bacteriol 1972, 109, 186–195. [Google Scholar] [CrossRef]
- Reich, M.; Gobel, C.; Kohler, A.; etc. Fatty acid metabolism in the ectomycorrhizal fungus Laccaria bicolor. New Phytol 2009, 182, 950–964. [CrossRef] [PubMed]
- Micoogullari, Y.; Basu, S.S.; Ang, J.; etc. Dysregulation of very-long-chain fatty acid metabolism causes membrane saturation and induction of the unfolded protein response. Mol Biol Cell 2020, 31, 7–17. [CrossRef] [PubMed]
- Ballweg, S.; Ernst, R. Control of membrane fluidity: the OLE pathway in focus. Biol Chem 2017, 398, 215–228. [Google Scholar] [CrossRef]
- Zhang, S.; Skalsky, Y.; Garfinkel, D.J. MGA2 or SPT23 is required for transcription of the delta9 fatty acid desaturase gene, OLE1, and nuclear membrane integrity in Saccharomyces cerevisiae. Genetics 1999, 151, 473–483. [Google Scholar] [CrossRef]
- Ballweg, S.; Ernst, R. Control of membrane fluidity: the OLE pathway in focus. Biol Chem 2017, 398, 215–228. [Google Scholar] [CrossRef]
- Ogasawara, Y.; Kira, S.; Mukai, Y.; etc. Ole1, fatty acid desaturase, is required for Atg9 delivery and isolation membrane expansion during autophagy in Saccharomyces cerevisiae. Biol Open 2017, 6, 35–40. [CrossRef]
- Covino, R.; Ballweg, S.; Stordeur, C.; etc. A Eukaryotic Sensor for Membrane Lipid Saturation. Mol Cell 2016, 63, 49–59. [CrossRef]
- Luo, X.; Affeldt, K.J.; Keller, N.P. Characterization of the Far Transcription Factor Family in Aspergillus flavus. G3-Genes Genom Genet 2016, 6, 3269–3281. [Google Scholar] [CrossRef] [PubMed]
- Surma, M.A.; Klose, C.; Peng, D.; etc. A lipid E-MAP identifies Ubx2 as a critical regulator of lipid saturation and lipid bilayer stress. Mol Cell 2013, 51, 519–530. [CrossRef] [PubMed]
- Paege, N.; Warnecke, D.; Zauner, S.; etc. Species-Specific Differences in the Susceptibility of Fungi to the Antifungal Protein AFP Depend on C-3 Saturation of Glycosylceramides. Msphere 2019, 4. [CrossRef]
- Peng, Y.J.; Zhang, H.; Feng, M.G.; etc. SterylAcetyl Hydrolase 1 (BbSay1) Links Lipid Homeostasis to Conidiogenesis and Virulence in the Entomopathogenic Fungus Beauveria bassiana. J Fungi 2022, 8. [CrossRef]
- Turk, M.; Abramovic, Z.; Plemenitas, A.; etc. Salt stress and plasma-membrane fluidity in selected extremophilic yeasts and yeast-like fungi. Fems Yeast Res 2007, 7, 550–557. [CrossRef]
- Sangappillai, V.; Nadarajah, K. Fatty Acid Synthase Beta Dehydratase in the Lipid Biosynthesis Pathway Is Required for Conidiogenesis, Pigmentation and Appressorium Formation in Magnaporthe oryzae S6. Int J Mol Sci 2020, 21. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.Y.; Soanes, D.M.; Kershaw, M.J.; etc. Functional analysis of lipid metabolism in Magnaporthe grisea reveals a requirement for peroxisomal fatty acid beta-oxidation during appressorium-mediated plant infection. Mol Plant Microbe in 2007, 20, 475–491. [CrossRef]
- Goodrich-Tanrikulu, M.; Howe, K.; Stafford, A.; etc. Changes in fatty acid composition of Neurospora crassa accompany sexual development and ascospore germination. Microbiol-Sgm, 1998; 144, Pt 7, 1713–1720. [CrossRef]
- Khunyoshyeng, S.; Cheevadhanarak, S.; Rachdawong, S.; etc. Differential expression of desaturases and changes in fatty acid composition during sporangiospore germination and development in Mucor rouxii. Fungal Genet Biol 2002, 37, 13–21. [CrossRef]
- Thines, E.; Weber, R.W.; Talbot, N.J. MAP kinase and protein kinase A-dependent mobilization of triacylglycerol and glycogen during appressorium turgor generation by Magnaporthe grisea. Plant Cell 2000, 12, 1703–1718. [Google Scholar] [CrossRef]
- McMahon, H.T.; Gallop, J.L. Membrane curvature and mechanisms of dynamic cell membrane remodelling. Nature 2005, 438, 590–596. [Google Scholar] [CrossRef]
- Kunding, A.H.; Mortensen, M.W.; Christensen, S.M.; etc. Intermembrane Docking Reactions Are Regulated by Membrane Curvature. Biophys J 2011, 101, 2693–2703. [CrossRef] [PubMed]
- Roux, A.; Koster, G.; Lenz, M.; etc. Membrane curvature controls dynamin polymerization. Proceedings of the National Academy of Sciences 2010, 107, 4141–4146. [Google Scholar] [CrossRef] [PubMed]
- Mim, C.; Unger, V.M. Membrane curvature and its generation by BAR proteins. Trends Biochem Sci 2012, 37, 526–533. [Google Scholar] [CrossRef]
- Momany, M.; Talbot, N.J. Septins Focus Cellular Growth for Host Infection by Pathogenic Fungi. Front Cell Dev Biol 2017, 5, 33. [Google Scholar] [CrossRef]
- Bridges, A.A.; Jentzsch, M.S.; Oakes, P.W.; etc. Micron-scale plasma membrane curvature is recognized by the septin cytoskeleton. J Cell Biol 2016, 213, 23–32. [CrossRef]
- Cannon, K.S.; Woods, B.L.; Crutchley, J.M.; etc. An amphipathic helix enables septins to sense micrometer-scale membrane curvature. J Cell Biol 2019, 218, 1128–1137. [CrossRef]
- Dagdas, Y.F.; Yoshino, K.; Dagdas, G.; etc. Septin-mediated plant cell invasion by the rice blast fungus, Magnaporthe oryzae. Science 2012, 336, 1590–1595. [CrossRef] [PubMed]
- Nath, S.; Dancourt, J.; Shteyn, V.; etc. Lipidation of the LC3/GABARAP family of autophagy proteins relies on a membrane-curvature-sensing domain in Atg3. Nat Cell Biol 2014, 16, 415–424. [CrossRef]
- Guna, A.; Hegde, R.S. Transmembrane Domain Recognition during Membrane Protein Biogenesis and Quality Control. Curr Biol 2018, 28, R498–R511. [Google Scholar] [CrossRef]
- Douglas, L.M.; Konopka, J.B. Fungal membrane organization: the eisosome concept. Annu Rev Microbiol 2014, 68, 377–393. [Google Scholar] [CrossRef]
- Ota, K.; Butala, M.; Viero, G.; etc. Fungal MACPF-like proteins and aegerolysins: bi-component pore-forming proteins? Subcell Biochem 2014, 80, 271–291. [CrossRef]
- Wang, F.; Wang, Y.; Zhang, X.; etc. Recent progress of cell-penetrating peptides as new carriers for intracellular cargo delivery. J Control Release 2014, 174, 126–136. [CrossRef]
- Eichel, K.; von Zastrow, M. Subcellular Organization of GPCR Signaling. Trends Pharmacol Sci 2018, 39, 200–208. [Google Scholar] [CrossRef] [PubMed]
- Lebreton, S.; Zurzolo, C.; Paladino, S. Organization of GPI-anchored proteins at the cell surface and its physiopathological relevance. Crit Rev Biochem Mol 2018, 53, 403–419. [Google Scholar] [CrossRef] [PubMed]
- Yun, Y.; Guo, P.; Zhang, J.; etc. Flippases play specific but distinct roles in the development, pathogenicity, and secondary metabolism of Fusarium graminearum. Mol Plant Pathol 2020, 21, 1307–1321. [CrossRef] [PubMed]
- Mazheika, I.; Voronko, O.; Kamzolkina, O. Early endocytosis as a key to understanding mechanisms of plasma membrane tension regulation in filamentous fungi. Biol Cell 2020, 112, 409–426. [Google Scholar] [CrossRef]
- Blanchoin, L.; Boujemaa-Paterski, R.; Sykes, C.; etc. Actin dynamics, architecture, and mechanics in cell motility. Physiol Rev 2014, 94, 235–263. [CrossRef]
- Juvvadi, P.R.; Fortwendel, J.R.; Rogg, L.E.; etc. Differential localization patterns of septins during growth of the human fungal pathogen Aspergillus fumigatus reveal novel functions. Biochem Bioph Res Co 2011, 405, 238–243. [CrossRef]
- Vargas-Muniz, J.M.; Renshaw, H.; Richards, A.D.; etc. The Aspergillus fumigatus septins play pleiotropic roles in septation, conidiation, and cell wall stress, but are dispensable for virulence. Fungal Genet Biol 2015, 81, 41–51. [CrossRef]
- Badrane, H.; Nguyen, M.H.; Blankenship, J.R.; etc. Rapid redistribution of phosphatidylinositol-(4,5)-bisphosphate and septins during the Candida albicans response to caspofungin. Antimicrob Agents Ch 2012, 56, 4614–4624. [CrossRef]
- Badrane, H.; Nguyen, M.H.; Clancy, C.J. Highly Dynamic and Specific Phosphatidylinositol 4,5-Bisphosphate, Septin, and Cell Wall Integrity Pathway Responses Correlate with Caspofungin Activity against Candida albicans. Antimicrob Agents Ch 2016, 60, 3591–3600. [Google Scholar] [CrossRef] [PubMed]
- Mela, A.; Momany, M. Septins coordinate cell wall integrity and lipid metabolism in a sphingolipid-dependent process. J Cell Sci 2022, 135. [Google Scholar] [CrossRef] [PubMed]
- Roelants, F.M.; Su, B.M.; von Wulffen, J.; etc. Protein kinase Gin4 negatively regulates flippase function and controls plasma membrane asymmetry. J Cell Biol 2015, 208, 299–311. [CrossRef] [PubMed]
- Takeshita, N. Control of Actin and Calcium for Chitin Synthase Delivery to the Hyphal Tip of Aspergillus. Curr Top Microbiol 2020, 425, 113–129. [Google Scholar] [CrossRef]
- Seidl-Seiboth, V.; Zach, S.; Frischmann, A.; etc. Spore germination of Trichoderma atroviride is inhibited by its LysM protein TAL6. Febs J 2013, 280, 1226–1236. [CrossRef]
- Zhang, L.B.; Tang, L.; Guan, Y.; etc. Subcellular localization of Sur7 and its pleiotropic effect on cell wall integrity, multiple stress responses, and virulence of Beauveria bassiana. Appl Microbiol Biot 2020, 104, 6669–6678. [CrossRef]
- Rojas, E.R.; Huang, K.C.; Theriot, J.A. Homeostatic Cell Growth Is Accomplished Mechanically through Membrane Tension Inhibition of Cell-Wall Synthesis. Cell Syst 2017, 5, 578–590. [Google Scholar] [CrossRef]
- Radeck, J.; Lautenschlager, N.; Mascher, T. The Essential UPP Phosphatase Pair BcrC and UppP Connects Cell Wall Homeostasis during Growth and Sporulation with Cell Envelope Stress Response in Bacillus subtilis. Front Microbiol 2017, 8, 2403. [Google Scholar] [CrossRef]
- Athanasopoulos, A.; Andre, B.; Sophianopoulou, V.; etc. Fungal plasma membrane domains. Fems Microbiol Rev 2019, 43, 642–673. [CrossRef]
- Kubicek, C.P.; Starr, T.L.; Glass, N.L. Plant cell wall-degrading enzymes and their secretion in plant-pathogenic fungi. Annu Rev Phytopathol 2014, 52, 427–451. [Google Scholar] [CrossRef]
- Commer, B.; Shaw, B.D. Current views on endocytosis in filamentous fungi. Mycology 2020, 12, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.J.; Ding, J.L.; Lin, H.Y.; etc. A virulence-related lectin traffics into eisosome and contributes to functionality of cytomembrane and cell-wall in the insect-pathogenic fungus Beauveria bassiana. Fungal Biol-Uk 2021, 125, 914–922. [CrossRef]
- Zhu, X.; Li, L.; Wang, J.; etc. acuolar Protein-Sorting Receptor MoVps13 Regulates Conidiation and Pathogenicity in Rice Blast Fungus Magnaporthe oryzae. J Fungi 2021, 7. [CrossRef]
- Vangalis, V.; Papaioannou, I.A.; Markakis, E.A.; etc. Hex1, the Major Component of Woronin Bodies, Is Required for Normal Development, Pathogenicity, and Stress Response in the Plant Pathogenic Fungus Verticillium dahliae. J Fungi 2020, 6. [CrossRef]
- Palma-Guerrero, J.; Huang, I.C.; Jansson, H.B.; etc. Chitosan permeabilizes the plasma membrane and kills cells of Neurospora crassa in an energy dependent manner. Fungal Genet Biol 2009, 46, 585–594. [CrossRef] [PubMed]
- Wen, Z.; Tian, H.; Xia, Y.; etc. MaPmt1, a protein O-mannosyltransferase, contributes to virulence through governing the appressorium turgor pressure in Metarhizium acridum. Fungal Genet Biol 2020, 145, 103480. [CrossRef]
- Klein, D.A.; Paschke, M.W. Filamentous fungi: the indeterminate lifestyle and microbial ecology. Microb Ecol 2004, 47, 224–235. [Google Scholar] [CrossRef]
- Phillips, R.; Ursell, T.; Wiggins, P.; etc. Emerging roles for lipids in shaping membrane-protein function. Nature 2009, 459, 379–385. [CrossRef]
- Fabri, J.; Rocha, M.C.; Malavazi, I. Overview of the Interplay Between Cell Wall Integrity Signaling Pathways and Membrane Lipid Biosynthesis in Fungi: Perspectives for Aspergillus fumigatus. Curr Protein Pept Sc 2020, 21, 265–283. [Google Scholar] [CrossRef]
- Pedersen, R.; Drubin, D.G. Type I myosins anchor actin assembly to the plasma membrane during clathrin-mediated endocytosis. J Cell Biol 2019, 218, 1138–1147. [Google Scholar] [CrossRef]
- Christianson, J.C.; Carvalho, P. Order through destruction: how ER-associated protein degradation contributes to organelle homeostasis. Embo J 2022, 41, e109845. [Google Scholar] [CrossRef] [PubMed]
- Christianson, J.C.; Ye, Y. Cleaning up in the endoplasmic reticulum: ubiquitin in charge. Nat Struct Mol Biol 2014, 21, 325–335. [Google Scholar] [CrossRef] [PubMed]
- Galluzzi, L.; Kepp, O.; Trojel-Hansen, C.; etc. Mitochondrial control of cellular life, stress, and death. Circ Res 2012, 111, 1198–1207. [CrossRef] [PubMed]
- Nakatogawa, H. Mechanisms governing autophagosome biogenesis. Nat Rev Mol Cell Bio 2020, 21, 439–458. [Google Scholar] [CrossRef]
- Larsen, J.B.; Jensen, M.B.; Bhatia, V.K.; etc. Membrane curvature enables N-Ras lipid anchor sorting to liquid-ordered membrane phases. Nat Chem Biol 2015, 11, 192–194. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).



