Submitted:
23 May 2023
Posted:
24 May 2023
You are already at the latest version
Abstract
Keywords:
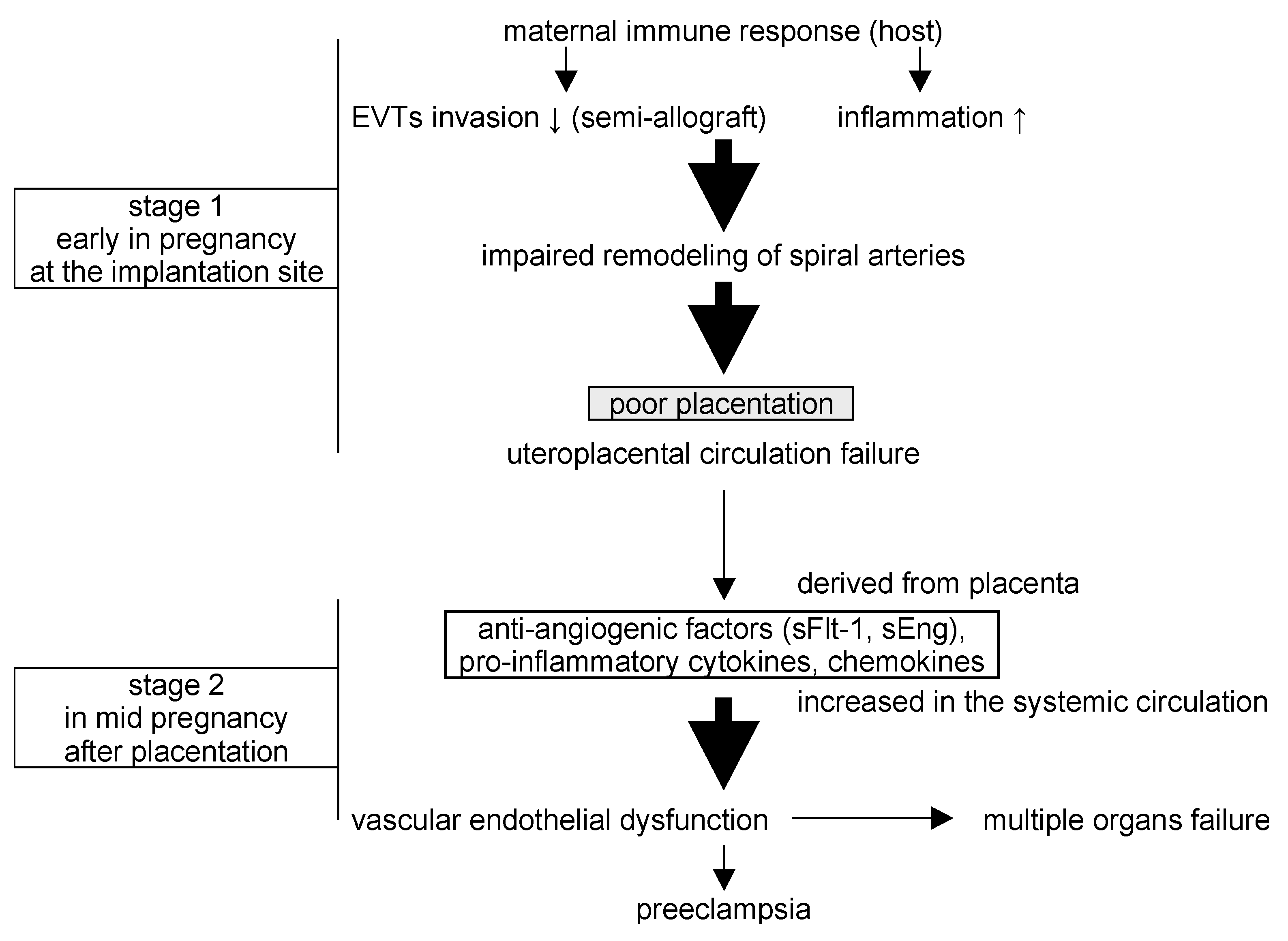
1. Introduction
2. Results
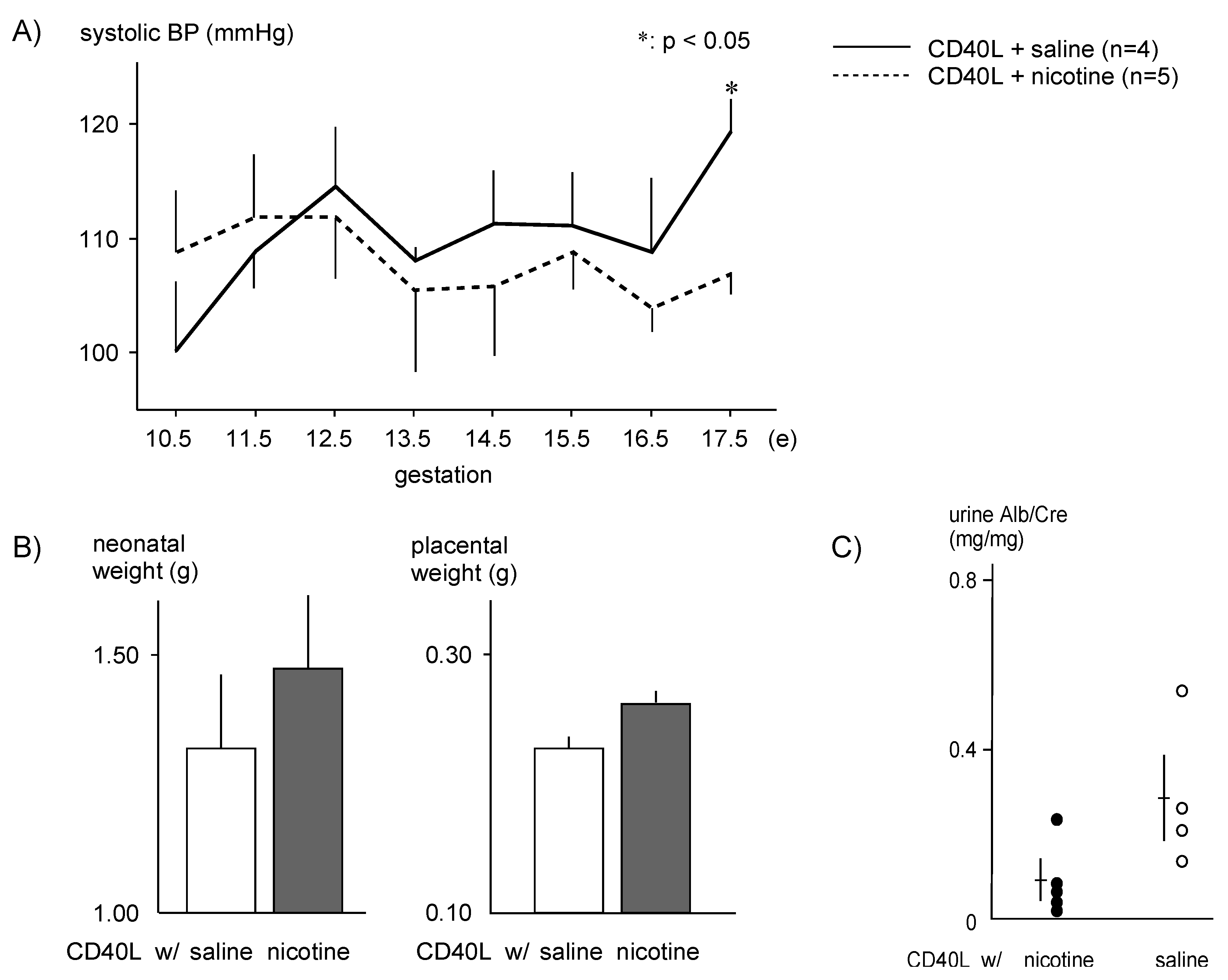
2.1. Physiologic Effects of Nicotine in a PE Mouse Model
2.2. Proteomic Analysis and Bioinformatic Characterization
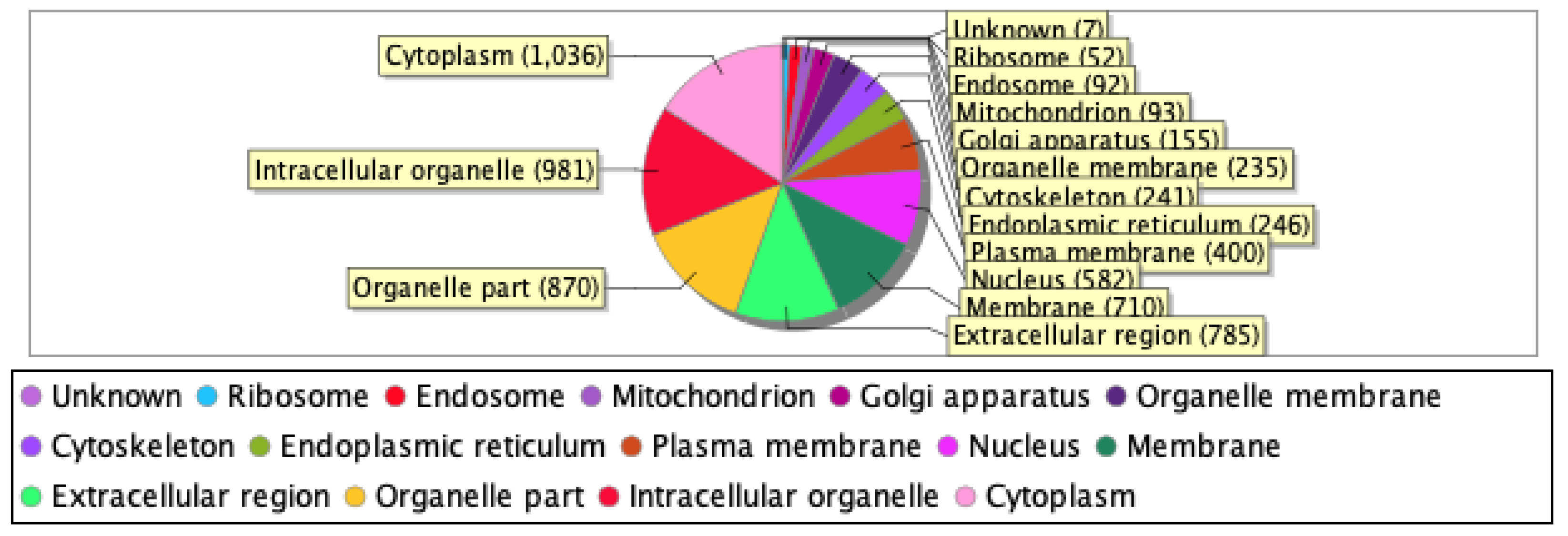
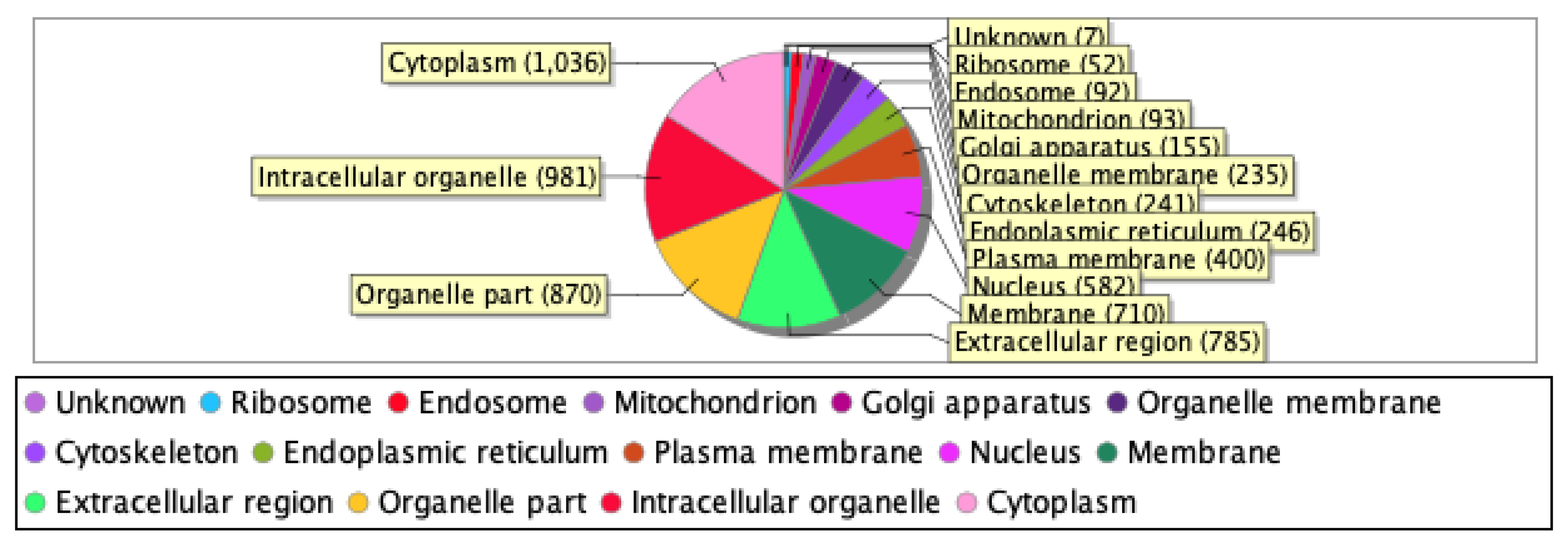
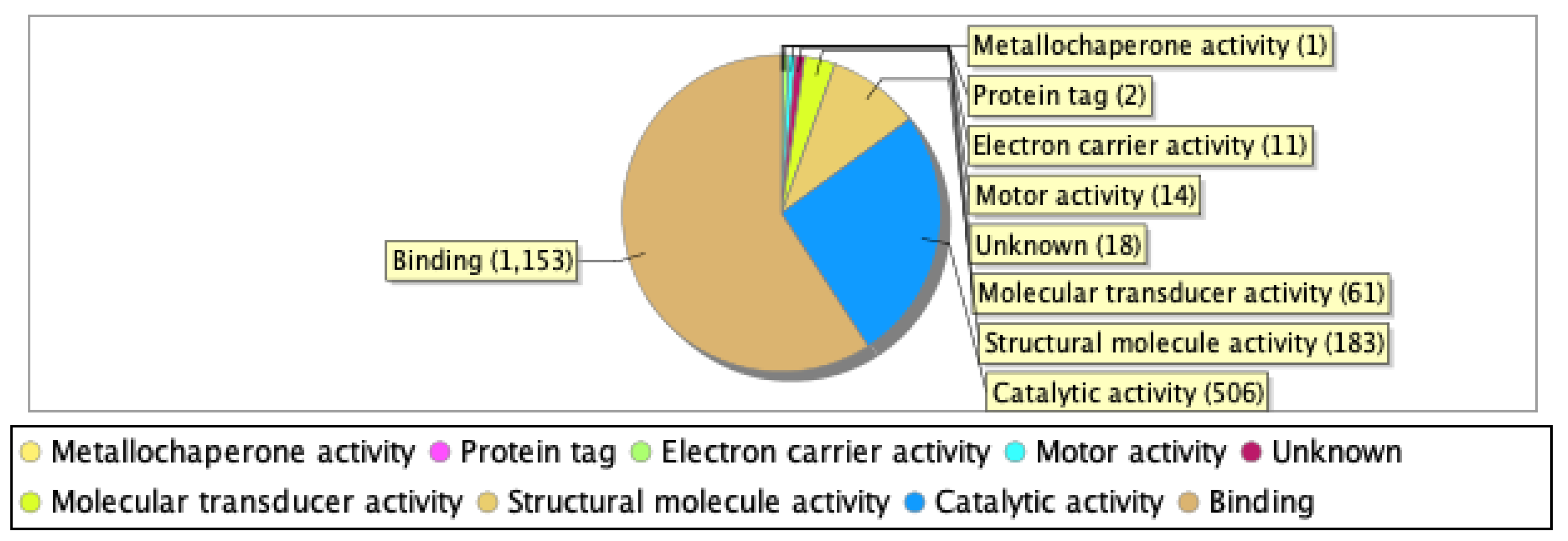
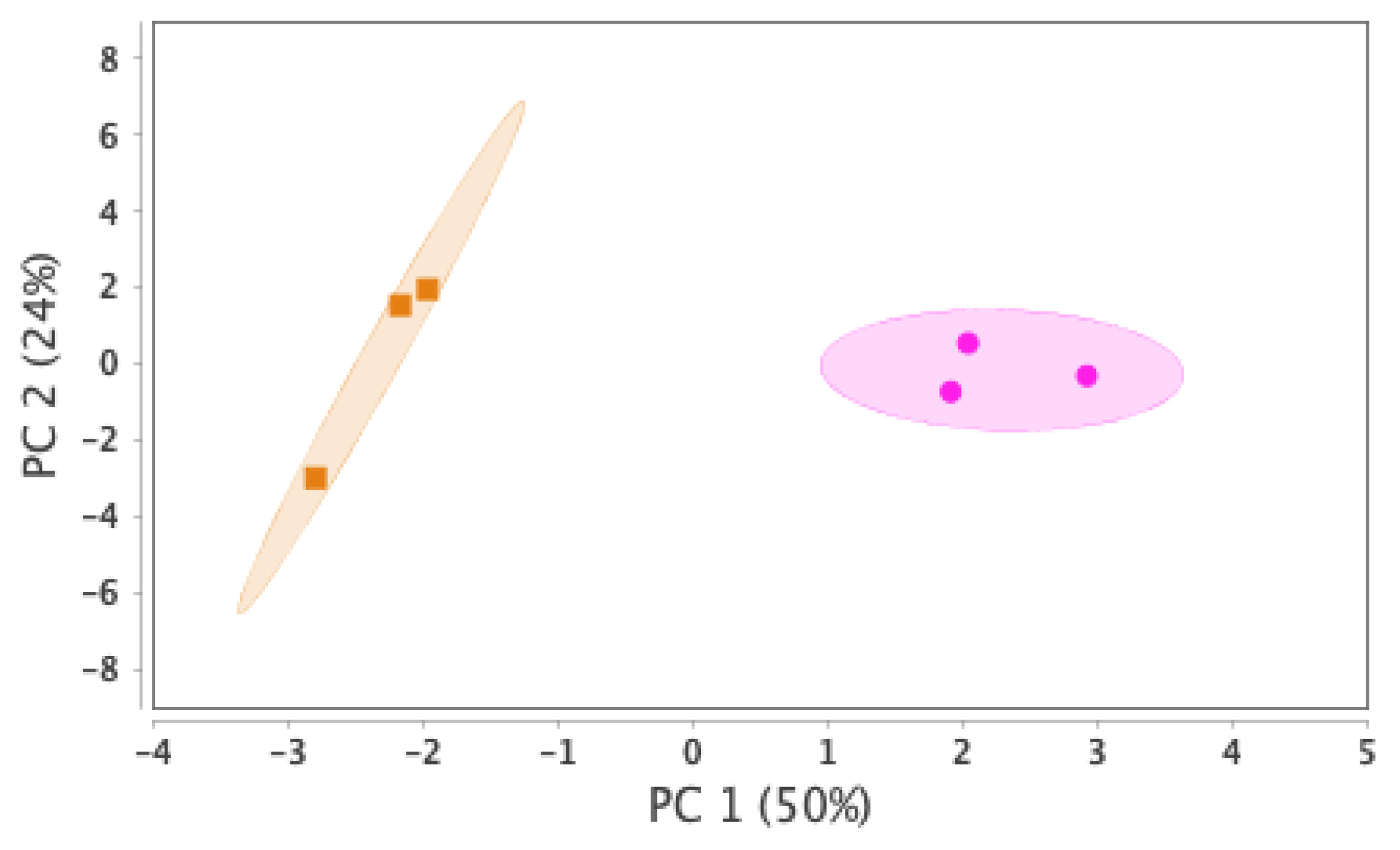
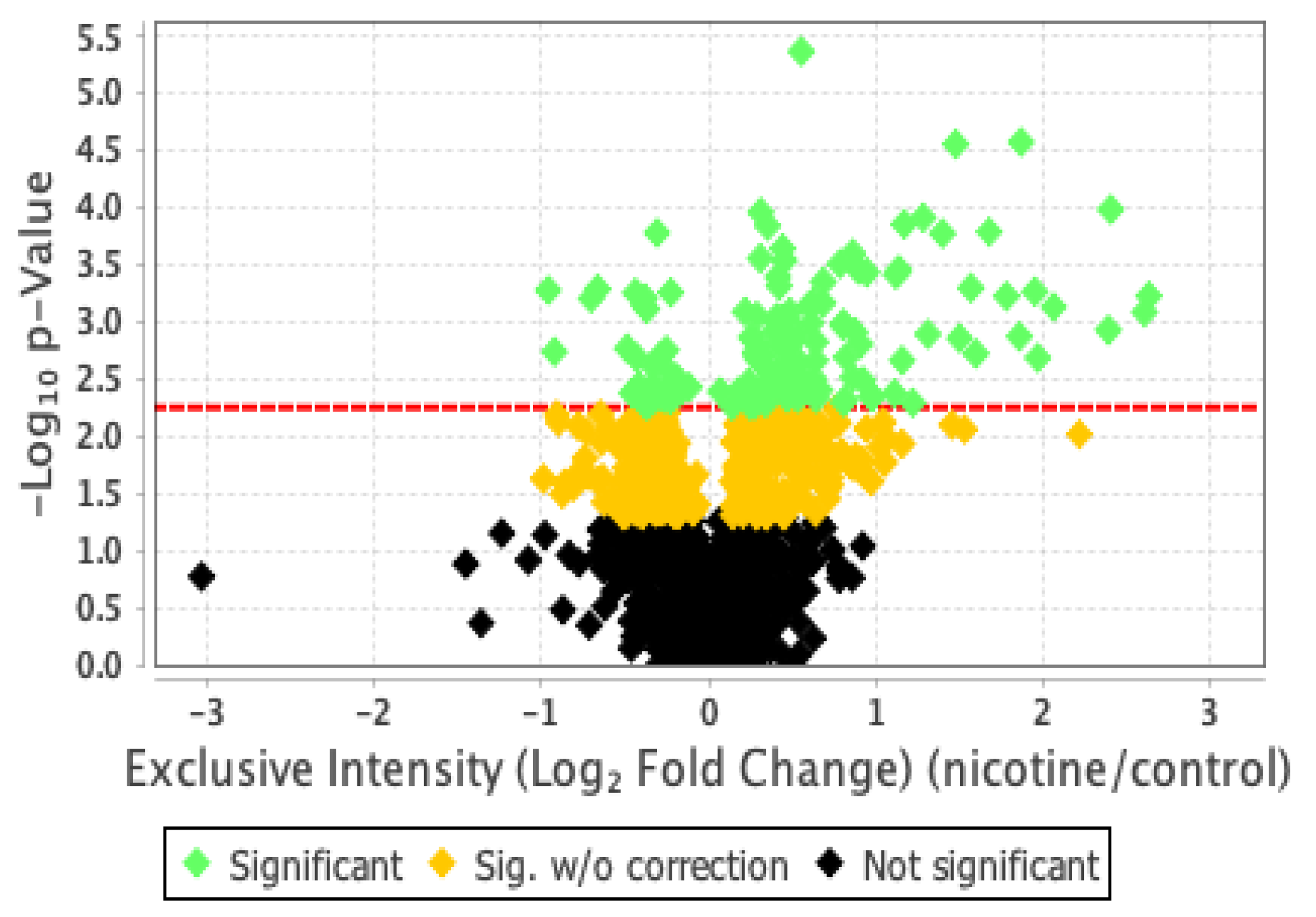
| Upregulated | |||
| Accession number | Protein name | log2FC | p value |
| Q9UGI8 | Testin | 2.67 | p < .05 |
| Q02818 | Nucleobindin-1 | 2.63 | p < .001 |
| P13591 | Neural cell adhesion molecule 1 | 2.60 | p < .001 |
| P02788 | Lactotransferrin | 2.40 | p < .0005 |
| P28827 | Receptor-type tyrosine-protein phosphatase mu | 2.39 | p < .005 |
| P05114 | Non-histone chromosomal protein HMG-14 | 2.31 | p < .01 |
| P24592 | Insulin-like growth factor-binding protein 6 | 2.23 | p < .05 |
| P55285 | Cadherin-6 | 2.21 | p < .01 |
| Q05682 | Caldesmon | 2.06 | p < .001 |
| O00533 | Neural cell adhesion molecule L1-like protein | 2.00 | p < .005 |
| Downregulated | |||
| Accession number | Protein name | log2FC | p value |
| Q6GTS8 | N-fatty-acyl-amino acid synthase/hydrolase | -2.08 | p < .0005 |
| Q7Z7L7 | Protein zer-1 homolog | -2.00 | p < .05 |
| Q14139 | Serine/threonine-protein phosphatase 6 catalytic subunit | -1.78 | p < .0005 |
| Q14997 | Proteasome activator complex subunit 4 | -1.66 | p < .05 |
| Q03001 | Dystonin | -1.51 | p < .05 |
| O60341 | Lysine-specific histone demethylase 1A | -1.42 | p < .0001 |
| P68871 | Hemoglobin subunit beta | -1.35 | p < .05 |
| P40306 | Proteasome subunit beta type-10 | -1.34 | p < .05 |
| Q9ULHO | Kinase D-interacting substrate of 220 kDa | -1.26 | p < .001 |
| Q13033 | Striatin-3 | -1.15 | p < .005 |
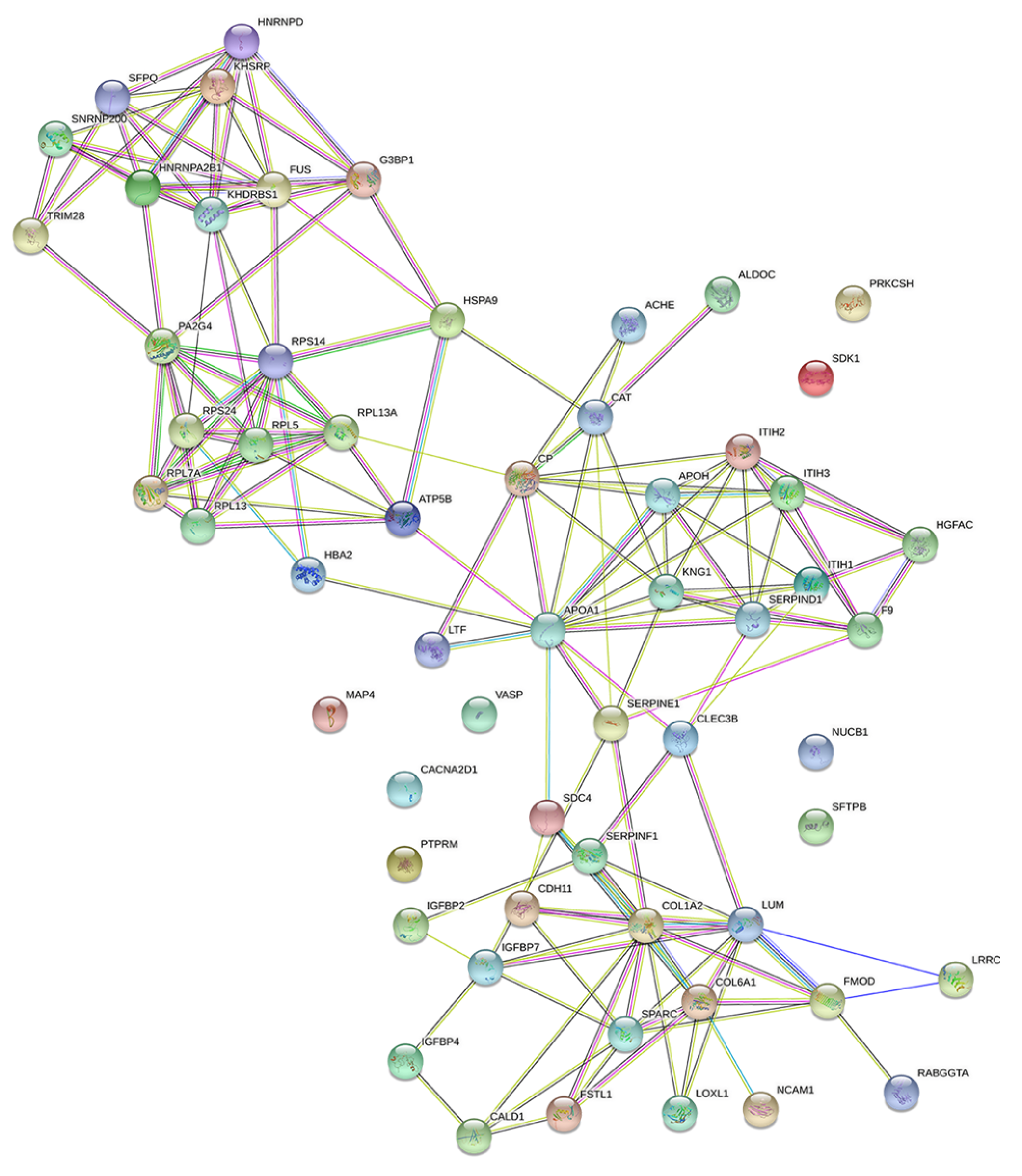
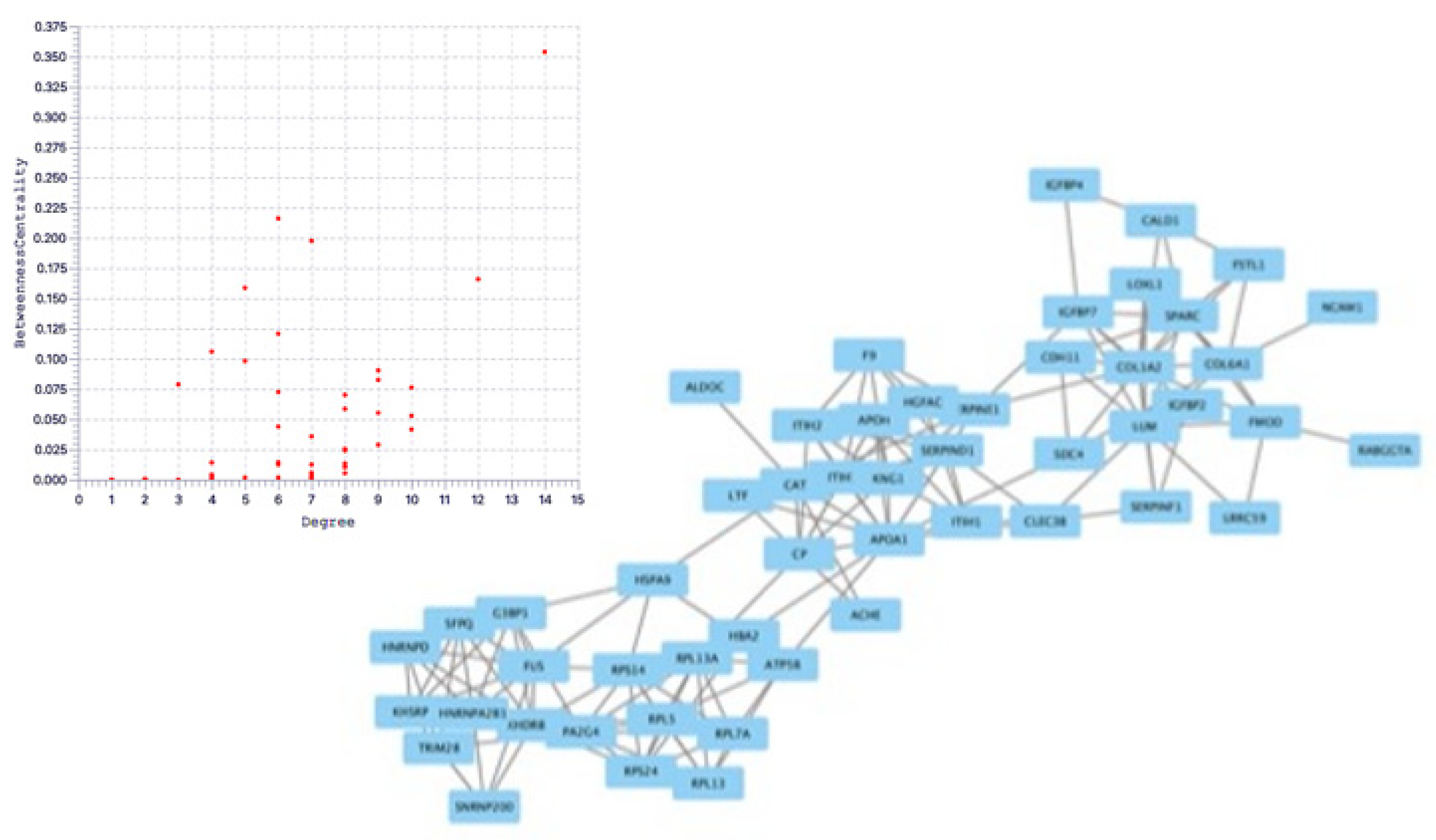
2.3. Protein Network Analysis
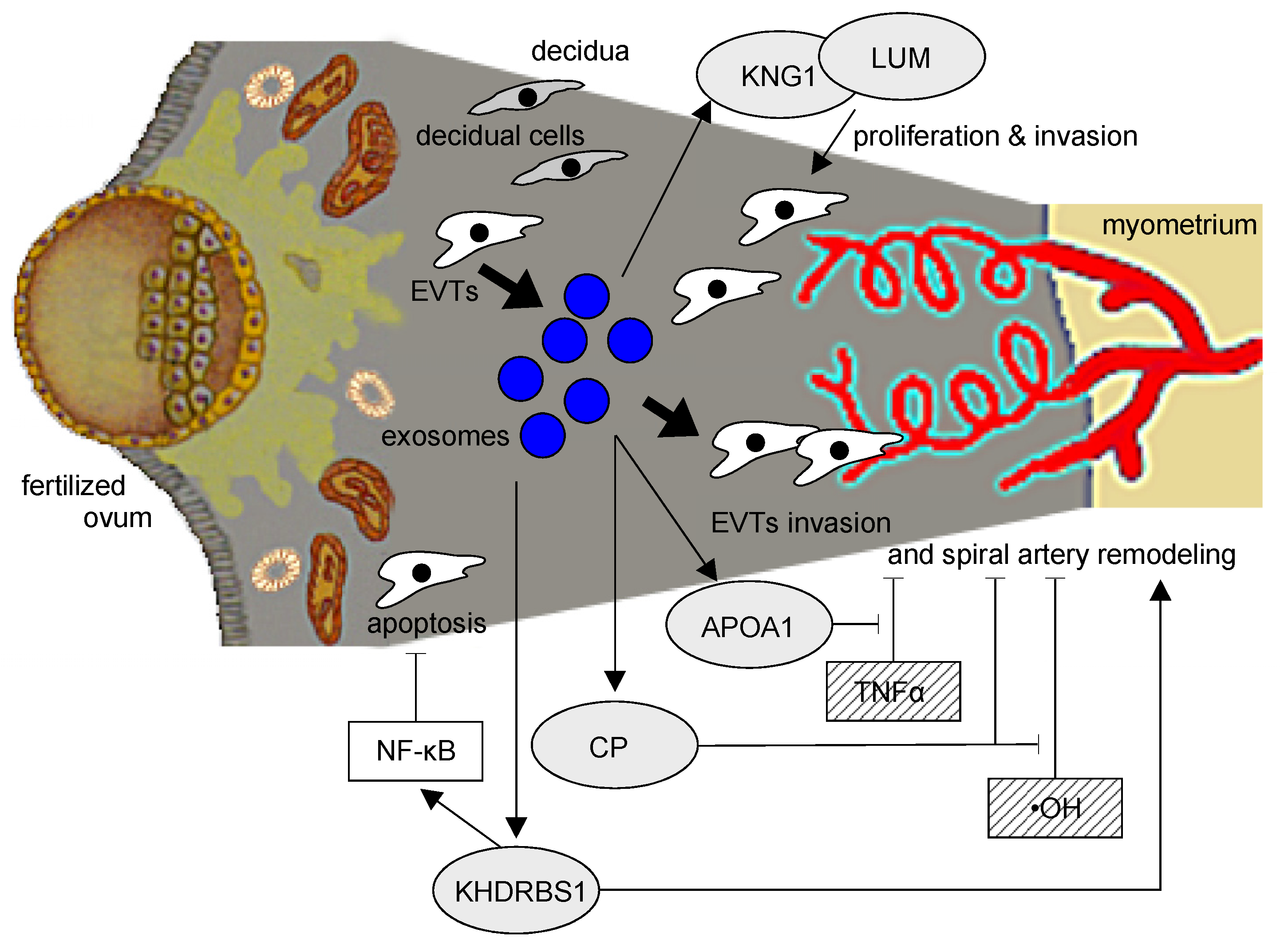
3. Discussion
4. Materials and Methods
4.1. Animal Experiments with a PE Mouse Model
4.2. Cell Culture
4.3. Extraction of Exosomes
4.4. Proteomics Sample Preparation
4.5. Nano LC-MS Analysis
4.6. Statistical Analysis
4.7. DIA (Data Independent Acquisition) Proteomic Analysis
4.8. Bioinformatic Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Granger, J.P.; Spradley, F.T.; Bakrania, B.A. The Endothelin System: A Critical Player in the Pathophysiology of Preeclampsia. Curr. Hypertens. Rep. 2018, 20, 32. [CrossRef]
- Kilbourn, R.G.; Traber, D.L.; Szabó, C. Nitric oxide and shock. Dis. Mon. 1997, 43, 277–348. [CrossRef]
- Matsubara, K.; Matsubara, Y.; Hyodo, S.; Katayama, T.; Ito, M. Role of nitric oxide and reactive oxygen species in the pathogenesis of preeclampsia. J. Obstet. Gynaecol. Res. 2010, 36, 239–247. [CrossRef]
- Walsh, S.W.; Wang, Y. Deficient glutathione peroxidase activity in preeclampsia is associated with increased placental production of thromboxane and lipid peroxides. Am. J. Obstet. Gynecol. 1993, 169, 1456–1461. [CrossRef]
- Redman, C.W.; Sargent, I.L. Placental stress and pre-eclampsia: a revised view. Placenta. 2009, 30, S38–42. [CrossRef]
- Roberts, J.M.; Hubel, C.A. The two stage model of preeclampsia: variations on the theme. Placenta. 2009, 30, S32–37. [CrossRef]
- Teng, X.; Chen, L.; Chen, W.; Yang, J.; Yang, Z.; Shen, Z. Mesenchymal Stem Cell-Derived Exosomes Improve the Microenvironment of Infarcted Myocardium Contributing to Angiogenesis and Anti-Inflammation. Cell. Physiol. Biochem. 2015, 37, 2415–2424. [CrossRef]
- England, L.; Zhang, J. Smoking and risk of preeclampsia: a systematic review. Front. Biosci. 2007, 12, 2471–2483. [CrossRef]
- Hammoud, A.O.; Bujold, E.; Sorokin, Y.; Schild, C.; Krapp, M.; Baumann, P. Smoking in pregnancy revisited: findings from a large population-based study. Am. J. Obstet. Gynecol. 2005, 192, 1856–1862. [CrossRef]
- West, R. Tobacco smoking: Health impact, prevalence, correlates and interventions. Psychol. Health. 2017, 32, 1018–1036. [CrossRef]
- Kaneko, S.; Maeda, T.; Kume, T.; Kochiyama, H.; Akaike, A.; Shimohama, S.; Kimura, J. Nicotine protects cultured cortical neurons against glutamate-induced cytotoxicity via α7-neuronal receptors and neuronal CNS receptors. Brain. Res. 1997, 765, 135–140. [CrossRef]
- Michalak, E.; Halko-Gąsior, A.; Chomyszyn-Gajewska, M. The impact of tobacco on oral health - based on literature. Przegl. Lek. 2016, 73, 516–519.
- Matsubara, K.; Matsubara, Y.; Mori, M.; Uchikura, Y.; Hamada, K.; Fujioka, T.; Hashimoto, H.; Matsumoto, T. Immune activation during the implantation phase causes preeclampsia-like symptoms via the CD40-CD40 ligand pathway in pregnant mice. Hypertens. Res. 2016, 39, 407–414. [CrossRef]
- Boswijk, E.; Bauwens, M.; Mottaghy, F.M.; Wildberger, J.E.; Bucerius, J. Potential of α7 nicotinic acetylcholine receptor PET imaging in atherosclerosis. Methods. 2017, 130, 90–104. [CrossRef]
- Moccia, F.; Frost, C.; Berra-Romani, R.; Tanzi, F.; Adams, D.J. Expression and function of neuronal nicotinic ACh receptors in rat microvascular endothelial cells. Am. J. Physiol. Heart. Circ. Physiol. 2004, 286, H486-491. [CrossRef]
- Lee, W.O.; Wright, S.M. Production of endothelin by cultured human endothelial cells following exposure to nicotine or caffeine. Metabolism. 1999, 48, 845–848. [CrossRef]
- Mayhan, W.G.; Sharpe, G.M.; Anding, P. Agonist-induced release of nitric oxide during acute exposure to nicotine. Life Sci. 1999, 65, 1829–1837. [CrossRef]
- Nadler, J.L.; Velasco, J.S.; Horton, R. Cigarette smoking inhibits prostacyclin formation. Lancet. 1983, 1, 1248–1250. [CrossRef]
- Sharma, R.; Lodhi, S.; Sahota, P.; Thakkar, M.M. Nicotine administration in the wake-promoting basal forebrain attenuates sleep-promoting effects of alcohol. J. Neurochem. 2015, 135, 323–331. [CrossRef]
- Klisch, K.; Schraner, E.M. Intraluminal vesicles of binucleate trophoblast cell granules are a possible source of placental exosomes in ruminants. Placenta. 2020, 90, 58–61. [CrossRef]
- Su, Y.; Li, Q.; Zhang, Q.; Li, Z.; Yao, X.; Guo, Y.; Xiao, L.; Wang, X.; Ni, H. Exosomes derived from placental trophoblast cells regulate endometrial epithelial receptivity in dairy cows during pregnancy. J. Reprod. Dev. 2022, 68, 21–29. [CrossRef]
- Cooke, A.L.; Morris, J.; Melchior, J.T.; Street, S.E.; Jerome, W.G.; Huang, R.; Herr, A.B.; Smith, L.E.; Segrest, J.P.; Remaley, A.T.; et al. A thumbwheel mechanism for APOA1 activation of LCAT activity in HDL. J. Lipid. Res. 2018, 59, 1244–1255. [CrossRef]
- Charlton, F.; Bobek, G.; Stait-Gardner, T.; Price, W.S.; Mirabito Colafella, K.M.; Xu, B.; Makris, A.; Rye, K.A.; Hennessy, A. The protective effect of apolipoprotein in models of trophoblast invasion and preeclampsia. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2017, 312, R40–48. [CrossRef]
- Fu, K.; Sun, X.; Wier, E.M.; Hodgson, A.; Liu, Y.; Sears, C.L.; Wan, F. Sam68/KHDRBS1 is critical for colon tumorigenesis by regulating genotoxic stress-induced NF-κB activation. Elife. 2016, 5, 10. [CrossRef]
- Erices, R.; Corthorn, J.; Lisboa, F.; Valdés, G. Bradykinin promotes migration and invasion of human immortalized trophoblasts. Reprod. Biol. Endocrinol. 2011, 9, 97. [CrossRef]
- Maurer, M.; Bader, M.; Bas, M.; Bossi, F.; Cicardi, M.; Cugno, M.; Howarth, P.; Kaplan, A.; Kojda, G.; Leeb-Lundberg, F.; et al. New topics in bradykinin research. Allergy. 2011, 66, 1397–1406. [CrossRef]
- Appunni, S.; Rubens, M.; Ramamoorthy, V.; Anand, V.; Khandelwal, M.; Saxena, A.; McGranaghan, P.; Odia, Y.; Kotecha, R.; Sharma, A. Lumican, pro-tumorigenic or anti-tumorigenic: A conundrum. Clin. Chim. Acta. 2021, 514, 1–7. [CrossRef]
- Giatagana, E.M.; Berdiaki, A.; Tsatsakis, A.; Tzanakakis, G.N.; Nikitovic, D. Lumican in Carcinogenesis-Revisited. Biomolecules. 2021, 11. [CrossRef]
- Chang, W.M.; Li, L.J.; Chiu, I.A.; Lai, T.C.; Chang, Y.C.; Tsai, H.F.; Yang, C.J.; Huang, M.S.; Su, C.Y.; Lai, T.L.; et al. The aberrant cancer metabolic gene carbohydrate sulfotransferase 11 promotes non-small cell lung cancer cell metastasis via dysregulation of ceruloplasmin and intracellular iron balance. Transl. Oncol. 2022, 25, 101508. [CrossRef]
- Yang, H.; Bao, Y.; Jin, F.; Jiang, C.; Wei, Z.; Liu, Z.; Xu, Y. Ceruloplasmin inhibits the proliferation, migration and invasion of nasopharyngeal carcinoma cells and is negatively regulated by miR-543. Nucleotides. Nucleic. Acids. 2022, 41, 474–488. [CrossRef]
- Chen, J.; Qiu, M.; Huang, Z.; Chen, J.; Zhou, C.; Han, F.; Qu, Y.; Wang, S.; Zhuang, J.; Li, X. Nicotine suppresses the invasiveness of human trophoblasts by downregulation of CXCL12 expression through the α-7 subunit of the nicotinic acetylcholine receptor. Reprod. Sci. 2020, 27, 916–924. [CrossRef]
- Zong, Y.; Zhang, S.T.; Zhu, S.T. Nicotine enhances migration and invasion of human esophageal squamous carcinoma cells which is inhibited by nimesulide. World. J. Gastroenterol. 2009, 15, 2500–2505. [CrossRef]
- Zhang, J.; Klebanoff, M.A.; Levine, R.J.; Puri, M.; Moyer, P. The puzzling association between smoking and hypertension during pregnancy. Am. J. Obstet. Gynecol. 1999, 181, 1407–1413. [CrossRef]
- Han, X.; Li, W.; Li, P.; Zheng, Z.; Lin, B.; Zhou, B.; Guo, K.; He, P.; Yang, J. Stimulation of α7 nicotinic acetylcholine receptor by nicotine suppresses decidual M1 macrophage polarization against inflammation in lipopolysaccharide-induced preeclampsia-like mouse model. Front. Immunol. 2021, 12, 642071. [CrossRef]
- Liu, Y.; Yang, J.; Bao, J.; Li, X.; Ye, A.; Zhang, G.; Liu, H. Activation of the cholinergic anti-inflammatory pathway by nicotine ameliorates lipopolysaccharide-induced preeclampsia-like symptoms in pregnant rats. Placenta. 2017, 49, 23–32. [CrossRef]
- Morisaki, N.; Obara, T.; Piedvache, A.; Kobayashi, S.; Miyashita, C.; Nishimura, T.; Ishikuro, M.; Sata, F.; Horikawa, R.; Mori, C.; et al. Association between smoking and hypertension in pregnancy among Japanese women: a meta-analysis of birth cohort studies in the Japan Birth Cohort Consortium (JBiCC) and JECS. J. Epidemiol. 2022, 10.2188. [CrossRef]
- Kawashima, A.; Koide, K.; Hasegawa, J.; Arakaki, T.; Takenaka, S.; Maruyama, D.; Matsuoka, R.; Sekizawa, A. Maternal smoking history enhances the expression of placental growth factor in invasive trophoblasts at early gestation despite cessation of smoking. PLoS. One. 2015, 10, e0134181. [CrossRef]
- Pillay, P.; Moodley, K.; Moodley, J.; Mackraj, I. Placenta-derived exosomes: potential biomarkers of preeclampsia. Int. J. Nanomedicine. 2017, 12, 8009–8023. [CrossRef]
- Matsubara, K.; Abe, E.; Ochi, H.; Kusanagi, Y.; Ito, M. Changes in serum concentrations of tumor necrosis factor alpha and adhesion molecules in normal pregnant women and those with pregnancy-induced hypertension. J. Obstet. Gynaecol. Res. 2003, 29, 422–426. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





