INTRODUCTION
Bronchopulmonary dysplasia (BPD) is one of the most severe consequences of very preterm birth. Advances in many non-nutrition-related aspects of care, particularly avoiding invasive ventilation1 and fluid overload,2 using caffeine,3 and late systemic corticosteroids4 in those infants who cannot be weaned from mechanical ventilation, are evidence-based strategies to lower the risk of developing BPD. However, contrary to documented decrease in most neonatal morbidity, the incidence of BPD remains unchanged5 or is even increasing.6-8
On the other hand, it has been reported that for every ten percent increase in human milk feeding in preterm neonates, the risk of BPD is lowered by 9.5% 9 and that a daily intake of 50 mL/kg/day of human milk during the first month of life can minimize the incidence of BPD.10 Aiming to summarize the results of previous research on this topic, a meta-analysis found that feeding exclusively mother’s milk might reduce the incidence of BPD.11 Another meta-analysis found a significant lower incidence of BPD in donor milk (DM) fed preterm infants compared to their formula-fed counterparts.12
BPD pathophysiology is still not completely understood, but there is an agreement to define it as a multifactorial disease in which a combination of oxidative stress and inflammation cause damage in the developing lung of preterm infants that are particularly vulnerable to injury. 13Among all antioxidant therapies and strategies, human milk is one of the most straightforward and accessible. Human milk is rich in antioxidants, which play a major role in maintaining the equilibrium between oxidative stress and antioxidant systems.14,15
Therefore, promoting mother’s own milk (OMM) is one of the priorities of modern neonatal care. An estimated 75% of high-risk Spanish neonates have access to DM. The option of giving DM, when MOM is not available or sufficient to cover baby's needs, is almost always present in Spanish NICUs.16
In this context, we undertook an observational cohort study to further explore relationship between human milk feeding and BPD rates over the last years in Spain.
MATERIALS AND METHODS
A retrospective descriptive study was designed, based on data obtained prospectively from the Spanish neonatal network SEN1500, a database created in 2002. It collects the perinatal data on the morbidity and mortality of premature infants born with a birth weight less than 1500 g, either born at the 64 collaborating centers or admitted to them in the first 28 days of life.17 SEN1500 includes all premature infants born alive from 22.0 weeks. The population represents approximately two-thirds of the extremely premature babies born in Spain.
Extremely preterm infants from 22.0 to 26.6 weeks born between 2004 and 2019 were included in this study. Those who died before discharge or with major congenital malformations were excluded. Our aim was to explore the clinical factors related to the development of moderate-severe BPD, focusing mainly on the presence or absence of human milk at discharge. BPD was defined as the need for supplementary oxygen or positive pressure at 36 weeks of postmenstrual age (PMA)18,
Other independent variables potentially related to BDP included were: gestational age (GA) estimated in weeks based on the last menstrual date and ultrasound calculation, sex, birthweight, multiple pregnancy, in vitro fertilisation (IVF), place of birth (inborn or outborn), antenatal steroids (complete or partial course), caesarean section, maternal chorioamnionitis, maternal hypertensive disorder, Apgar score at 1' and 5', Clinical Index Risk for Babies (CRIB) score,19 use and duration of invasive mechanical ventilation (iMV), high frequency oscillatory ventilation (HFOV), non- invasive mechanical ventilation including nasal continuous airway pressure (nCPAP) and nasal intermittent positive pressure (nIPPV), inhaled nitric oxide (iNO), surfactant administration and number of doses, prophylactic indomethacin, postnatal systemic steroids, early onset sepsis, late onset sepsis (LOS), need for inotropic therapy, necrotizing enterocolitis (NEC) requiring surgery, patent ductus arteriosus (PDA) managed with medical or surgical treatment, major cerebral lesion defined as intraventricular haemorrhage (IVH) grade 3 or more according to Papile criteria,20 and/or periventricular leukomalacia (PVL), defined as the presence of changes in periventricular white matter detected by ultrasound 3 weeks after birth; retinopathy of prematurity (ROP) grade 3 or more according to the ROP International Classification,21 or ROP surgically treated.
The Ethics and Research Committee of the Hospital Universitari Germans Trias i Pujol approved the study. The SEN1500 data registry is anonymous and was utilized under data protection laws and regulations approved by the ethics and research committees of the collaborating centers.
Statistical analysis:
Data were expressed as means (SD) for continuous variables or as numbers/percentages for categorical variables. The normality of distribution and equality of variances were evaluated through the Kolmogorov-Smirnov and Levene’s tests, respectively. The Student T or U Mann-Whitney tests compared independent variables according to their size and characteristics between two groups. The ANOVA or Kruskal-Wallis non-parametric test was used to compare differences among more than two groups. A Pearson chi-square test was used to compare categorical variables between groups. The odds ratio and 95% confidence intervals were calculated. Multivariate analysis was performed by logistic regression to model the probability of dichotomous events (moderate-severe BPD, yes/no). The Statistical Package for Social Science SPSS version 23.0 was used to perform all the statistical analyses, establishing the significance at p < 0.05.
RESULTS
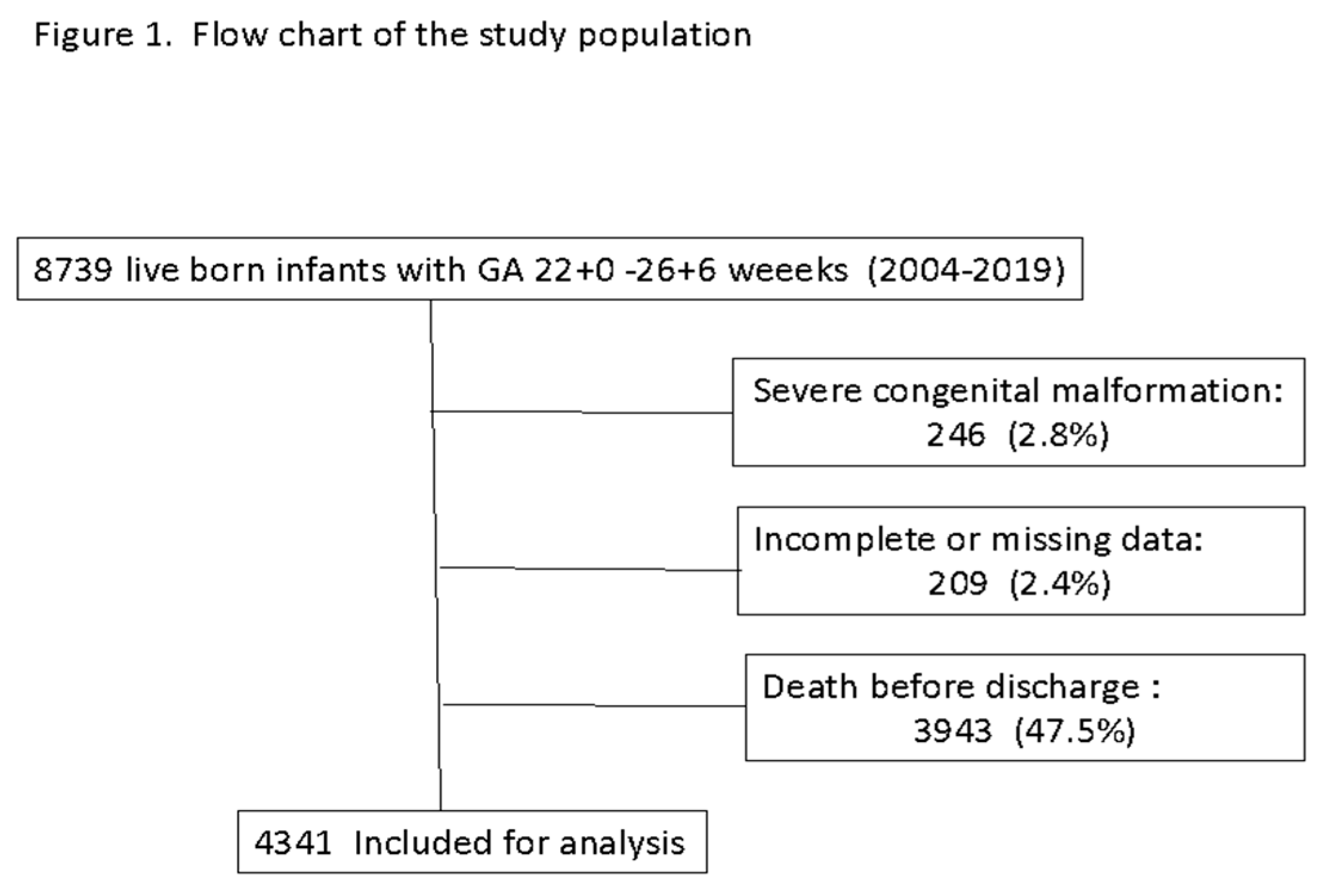
Data from 8,739 infants born at a GA of 22.0 to 26.6 weeks were registered in the database during the study period. Figure 1 shows the flow chart of the study population. After excluding 246 infants with major congenital anomalies, 209 infants with incomplete or missing data, and 3,943 infants who died before discharge, those 4,341 infants who survived to discharge were included for analysis.
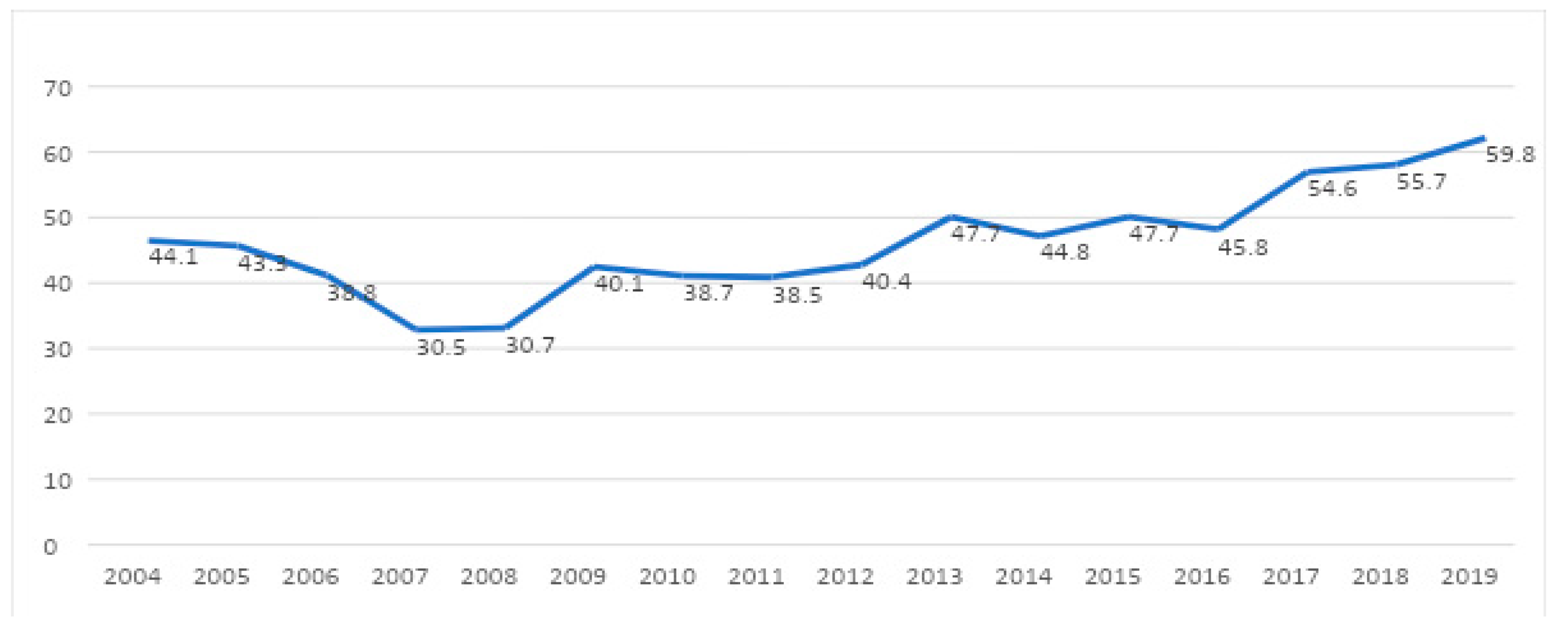
The rate of BPD was 43.7%. A significant increasing trend was observed during the study period (
Figure 2).
Table 1 shows the characteristics of the total population and the differences between those with moderate-severe BPD (n: 1,897, 43.7%) and those with no moderate-severe BPD (n: 2,444, 56.3%). The results of the univariate analysis showed an inverse association of GA, birth weight, and low Apgar score at 1’ and 5’ with moderate-severe BPD. Other independent factors associated with moderate-severe BPD were: male sex, CRIB score, iMV and HFOV, hours of iMV; iNO, surfactant, prophylactic indomethacin, postnatal systemic steroids, LOS, inotropic therapy, NEC and surgical NEC, PDA with medical and surgical treatment, major cerebral lesion and ROP. In addition, the percentage of infants receiving exclusive human milk feeding at discharge was significantly higher in those free of moderate-severe BPD (39.1% versus 30.3%, p<0.001; OR 0.675, 95% CI 0.593-0.768). Also, 63.6% of infants free of moderate-severe BPD received any amount of human milk feeding at discharge versus 58.5% of infants with moderate-severe BPD (p<0.001; OR 0.607, 95% CI 0.537-0.698).
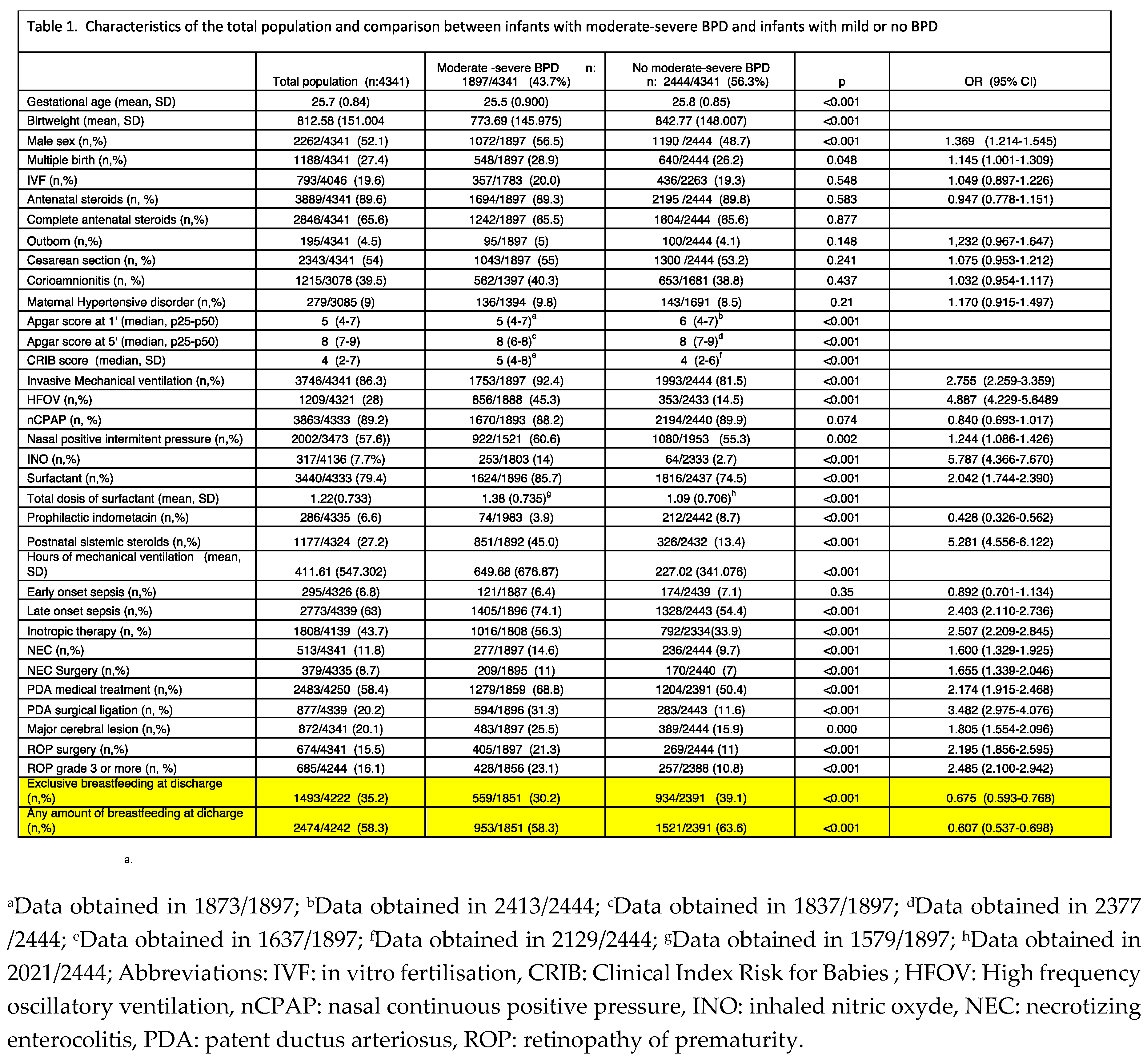
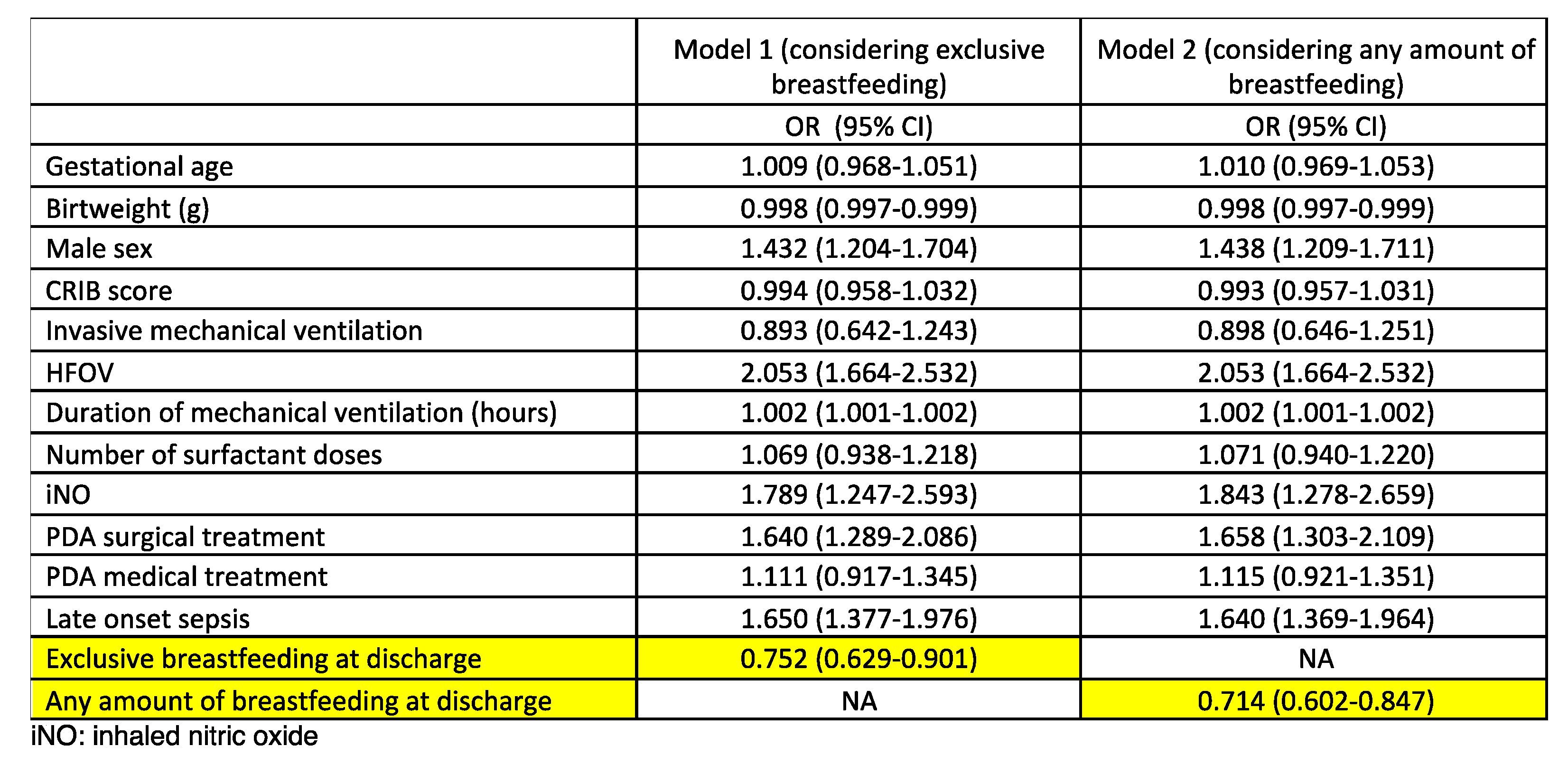
A multivariate analysis was subsequently performed (
Table 2). Exclusive human milk feeding at discharge was associated with a lower incidence of moderate-severe BPD (OR 0.752, 95% CI 0.629-0.901), as any amount of human milk feeding at discharge did (OR 0.714, 95% CI 0.602-0.847). Male sex, HFOV, iNO, PDA with surgical treatment, and LOS were the other variables associated with moderate-severe BPD.
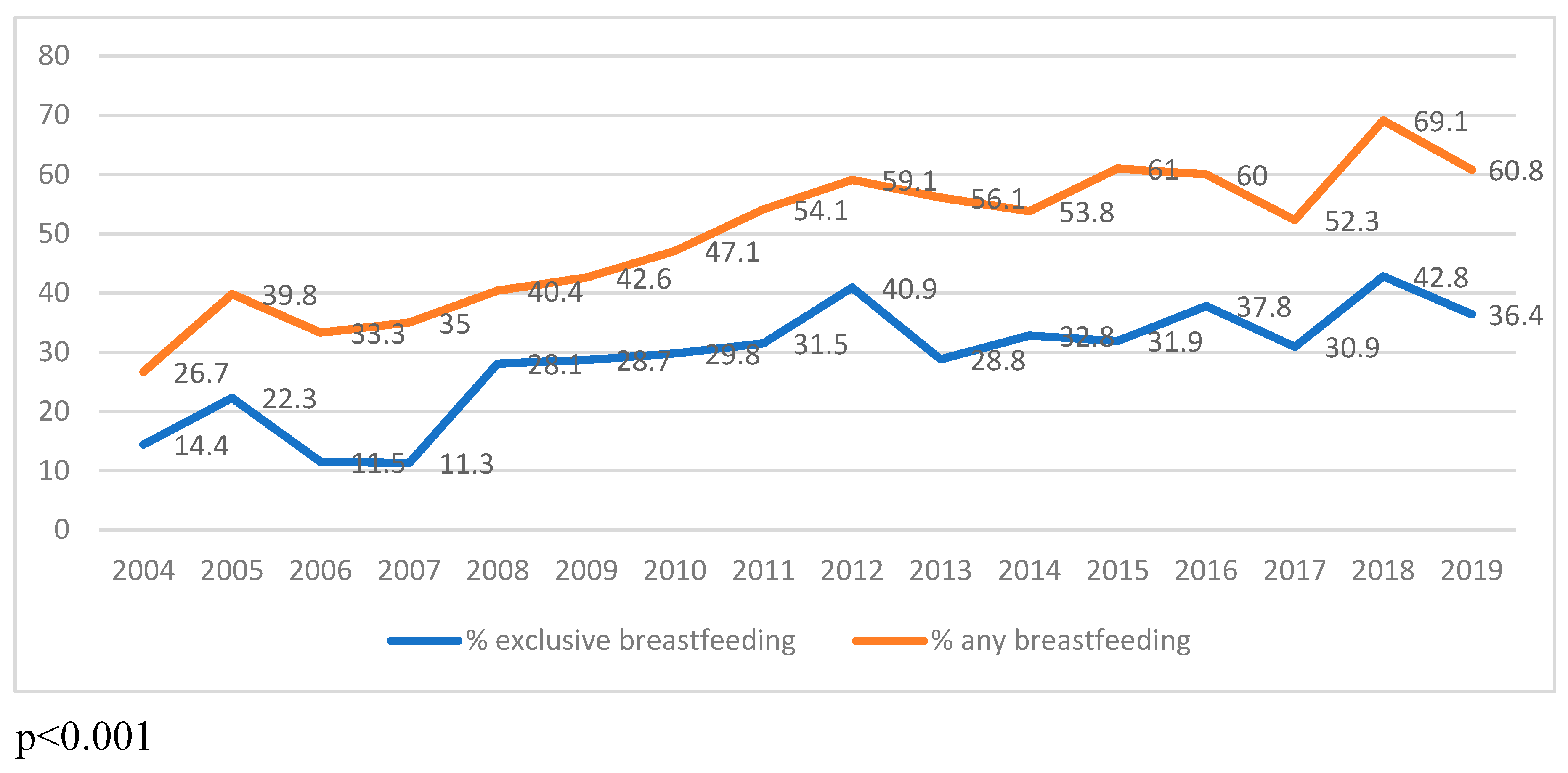
Finally, the temporal trends in human milk feeding were studied. In infants with moderate-severe BPD, exclusive human milk feeding at discharge increased from 14.4% in 2004 to 36.4% in 2019 (p<0.001), as shown in
Figure 3; and any amount of human milk feeding at discharge increased from 26.7% in 2004 to 60.8% in 2019 (p<0.001).
DISCUSSION
In this multicentre national cohort study on the morbidity of extremely preterm infants, we found that those discharged while being fed with human milk were at a lower risk of moderate-severe BPD than those receiving no human milk at discharge.
Also, a significant increase in both the rates of BPD and the percentage of human milk feeding at discharge over the period was observed. This improvement in our human milk provision might be related to implementing developmental and family-centered care in Spanish units over the study period.22In the last years of the study, less than half of the infants survived free from moderate-severe BPD. Most studies on temporal trends in respiratory outcomes of extremely preterm infants have reported similar results.5-8 Despite introducing less invasive mechanical ventilation strategies, the rates of BPD have kept unchanged or even worsened in the more immature infants. Several reasons could explain this finding. First, the changes in the definition of BPD and its non-homogenous condition. The classical definition of moderate-severe BPD was based on the need for supplementary oxygen therapy at 36 weeks of PMA. More recently, according to the mode of respiratory support administered at 36 weeks of PMA, regardless of supplemental oxygen use, an updated definition of BPD has been proposed.23 The SEN1500 database started introducing data on any positive pressure ventilation at 36 weeks of PMA in 2010, when nCPAP and high-flow oxygen cannula (HFOC) became generalized in Spanish neonatal intensive care units. Second, an increase in survival of infants born at extreme GA has been suggested to explain the increase in morbidity in survivors.6 Survival of infants born at 23-26.6 weeks has increased over the study period in Spain at all GA, from less than 50% to more than 60%.24 A trend to a more active perinatal approach in those born at 23.0-23.6 weeks has been observed in the last decade, but morbidity has remained unchanged.25 The rate of BPD observed in this cohort (43.7%) is lower compared to the rates of cohorts with a more active approach and more survivors of extreme GA. In a comparative study including the EXPRESS (Sweden), the EPICURE-2 (UK), and the EPIPAGE-2 (France) cohort,26 the first two studies reported BPD rates of 68.6% and 77%, respectively, and the last one, with fewer survivors under 25 weeks, a rate of 39.2%.
The factors associated with a greater risk of BPD encountered in this study have also been described previously, mainly the severity of the respiratory condition during the first days, the significant PDA, and the LOS.27
In recent years there has been a reinforced interest in the two main issues to which this study has been devoted. On the one side, since data are lacking regarding the prognosis of infants born shortly before and after 24 weeks, there is a growing awareness that we do not know how to treat these patients.28,29 Moreover, on the other hand, ongoing research on the pathogenesis of BPD analyses the potential value of human milk to prevent and control this neonatal complication.11,12 Among a small number of preventative targets for BPD, the control of oxidative overload stands out as one of the most promising. When the lungs of extremely premature infants are still developing, hyperoxia increases alveolar epithelial cell death and pulmonary vascular remodelling. Even though oxidative imbalance plays a role in the development of BPD, research on antioxidant molecules in preterm infants is limited. As a result, determining how to administer exogenous antioxidant therapy is a delicate matter.30
Favorably, feeding human milk to preterm infants is not challenging and is aligned with the neonatal antioxidant cycles. Human milk is the safest food for these patients. Human milk delivers several active compounds and stem cells that are thought to minimize oxidative imbalance in the BPD process. We know from last year that xenobiotic metabolites of paracetamol in maternal milk are linked to a 3- to 6-fold increased odds of BPD and ROP in extremely preterm infants.31 Furthermore, given that a single dose of 2g paracetamol or two weeks of therapeutic doses of paracetamol lead to a significant reduction in the total antioxidant capacity of healthy adults,32 this finding further supports the antioxidant protection provided by human milk (as long as it does not contain paracetamol metabolites) to prevent BPD as well as other oxidative stress-related diseases of neonates.
Three systematic reviews have analysed the risk of BPD after human milk intake (MOM or DM, both pasteurized or unpasteurized human milk) in very low birth weight (VLBW) infants.11,12,33 These studies have conducted a comprehensive literature search until 2018. They compared exclusive human milk intake to exclusive formula intake, any milk intake to exclusive formula intake, and the dose-response effect with human milk intake. Seven randomized controlled trials, seventeen cohort studies, and one case-control study in English or Chinese were included. These reviews have reported five improved respiratory outcomes among human milk-fed preterm infants. First, a meta-analysis of three randomized controlled trials discovered that DM supplementation significantly reduced the use of neonatal iMV.12 Second, a meta-analysis of eight observational studies revealed that DM supplementation had a protective effect on BPD and iMV requirements.12 Third, compared to a diet that includes preterm formula and/or bovine milk-based fortifier, an exclusive human milk diet dramatically reduced the risk of BPD. Fourth, it was also discovered that feeding infant with raw human milk instead of pasteurized human milk protected them from BPD.11 Fifth, mother's milk feeds lowered the risk of BPD when used as an exclusive diet.11 And, finally, it is confirmed that exclusive, mainly or any human milk feeding shows a protective effect of BPD.33Since these systematic reviews, studies on the effects of human milk on the occurrence or severity of BPD have continued to be published. A 2020 retrospective study on 1,363 VLBW infants found that those receiving 50 mL/kg/day of human milk during the first four weeks of life had lower odds of BPD, as well as of moderate and severe BPD; in contrast, the authors found no effect of 1-24 mL/kg/day or 25-49 mL/kg/day of human milk on BPD nor on the degree of severity of the disease.34 A 2022 retrospective analysis has reported a new finding; the authors have focused on the first time that premature infants received human milk and showed that the first human milk feeding within 72 hours of birth could reduce the incidence of moderate-severe BPD in VLBW infants and that occurrence of moderate-severe BPD was not linked to human milk aggregates during the hospital stay.35 Additionally, research from 2022 on 4,470 very/extremely low-birth-weight infants showed a 31% reduction in BPD incidence among exclusively human milk-fed preterm neonates.36 Finally, according to a 2023 multivariate analysis, the risk of BPD is 66% lower among VLBW infants fed fresh human milk +/- preterm formula than among their counterparts fed pasteurized human milk +/- preterm formula.37
Since 2011, more than half of infants below 26 weeks of gestation in our study population received some human milk at NICU discharge. This is notable when compared with previous reports of percentages of preterm infants that continue to receive human milk at NICU discharge: 12% of infants below 28 weeks of gestation,38 or 28-50% of VLBW infants in US studies, or 38-66% of VLBW infants in worldwide studies;39,40 or 13–49% of all preterm infants during the hospital stay in developed countries .41
While previous studies have reported rates of human milk feeding at NICU discharge, limited research specifically provided details of the successive stages of human milk discontinuation during the hospital stay of preterm infants. Further, we are unaware of such studies on extremely preterm infants.
A recent report on preterm infants (23-36 weeks of gestation) describes that 5.3% of neonates did not receive any human milk, 48.7% of them received some human milk but stopped prior to NICU discharge, and 46% of them continued some human milk after NICU discharge .42 This raises the question regarding the proportion of human milk and formula fed to preterm infants in our population who were exclusively or mixed human milk-fed at hospital discharge. We still need to collect detailed feeding information to answer this question. However, given that we know that most preterm infants are fed human milk at some point during their stay in the NICU but that about half of them, later in their admission, become exclusively formula-fed, it is not entirely wrong to infer that the three groups of preterm infants in our sample have taken increasing proportions of human milk, depending on whether they never took human milk or stopped taking it during admission, or whether they continued to take some human milk or exclusively human milk at discharge.
Therefore, our results align with previous research that has shown not only how partial feeding with DM or fresh or frozen MOM milk can minimize the occurrence of BPD but also points to a non-linear and inverse dose-response relationship between proportions or amounts of human milk provided and cases of BPD. Our study is unique only in suggesting the beneficial impact of human milk on BPD, specifically for extremely preterm infants under 26 weeks gestation.
The protective role of any amount of breastmilk for infants with BPD continues after discharge. It has been associated with fewer emergency department visits, systemic steroid courses, fewer cough or chest congestion episodes, and a trend toward fewer hospitalizations.43 Hence, if BPD could be prevented to some degree in this group of extremely preterm infants, it is to be expected that after their discharge from the neonatal unit, their respiratory health would be enhanced for years to come.
Limitations
The study has some potential limitations. It is an explicit limitation that this descriptive study was not designed to establish cause-and-effect relationships. There is no intervention; this is a retrospective observational study; therefore, the relationship between human milk and BPD must be regarded as an association. Since the feeding type at discharge was assessed without attention to the specific type of human milk utilized, another limitation of the study is a need for more data on the relative contributions of the MOM milk versus DM. In addition, data about the duration and amount of human milk received from birth to discharge are unavailable. The high drop-out rate of extremely preterm infants from cohorts could affect this study's results. Finally, some infants discharged before 2010 under HFOC without supplemental oxygen at 36 weeks PMA could not have been included in the moderate-severe BPD group. The definition of moderate-severe BPD (or grade 2 or 3) according to the new criteria is the need for nCPAP or HFOC (> 2L/min).23 Most infants in this condition receive an iFO greater than 0.21, so they have probably been categorized as moderate-severe BPD, and the use of HFOC in Spanish NICUs was extremely uncommon before 2010.
Strenghts.
The strengths of the present study are the large number of participating extreme preterm infants, the fact that the study was obtained from a national registry, and that the feeding data have been collected by neonatologists in each NICU through the medical records of each infant after discharge to reduce recall bias.
CONCLUSION
The results of the present study strongly support the role of any amount of human milk in preventing BPD in the most vulnerable population, the extremely preterm infants.
The quality bundles and strategies implemented in neonatal intensive care units to prevent and improve outcomes of infants with BPD should always include the provision of human milk and the promotion of long-term breastfeeding.
Additional research should examine the caregiver effects and medical decision-making that lead to the discontinuance of human milk feeds in extremely preterm infants.
Funding
No specific grant for this research has been received.
Acknowledgements
The authors would like to acknowledge all the investigators of the SEN 1500 for the assistance provided. A list of researchers and hospitals from the Spanish Neonatal Network SEN1500 is provided in supplementary material.
Conflicts of Interest
The authors have no conflicts of interest to declare.
References
- Principi N, Di Pietro GM, Esposito S. Bronchopulmonary dysplasia: clinical aspects and preventive and therapeutic strategies. J. Transl Med. 2018;16:36. [CrossRef]
- Starr MC, Griffin R, Gist KM, Segar JL, Raina R, Guillet R, et al; Neonatal Kidney Collaborative Research Committee. Association of Fluid Balance With Short- and Long-term Respiratory Outcomes in Extremely Premature Neonates. A Secondary Analysis of a Randomized Clinical Trial. JAMA Netw Open. 2022; 5: e2248826. PMID 36580332.
- Schmidt B, Roberts RS, Davis P, Doyle LW, Barrington KJ, Ohlsson A, Solimano A, Tin W; Caffeine for Apnea of Prematurity Trial Group. Caffeine therapy for apnea of prematurity. N. Engl J Med. 2006;354(20):2112-21. [CrossRef]
- Doyle LW, Cheong JL, Hay S, Manley BJ, Halliday HL. Late (≥ 7 days) systemic postnatal corticosteroids for prevention of bronchopulmonary dysplasia in preterm infants. Cochrane Database Syst Rev. 2021;11:CD001145. [CrossRef]
- Avila-Alvarez A, Zozaya C, Pértega-Diaz S, et al. Temporal trends in respiratory care and bronchopulmonary dysplasia in very preterm infants over a 10-year period in Spain. Arch Dis Child Fetal Neonatal Ed. 2022;107(2):143-149. [CrossRef]
- Stoll BJ, Hansen NI, Bell EF, Walsh MC, Carlo WA, Shankaran S, Laptook AR, et al. Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network. Trends in Care Practices, Morbidity, and Mortality of Extremely Preterm Neonates, 1993-2012. JAMA. 2015;314:1039-1051. [CrossRef]
- Smith VC, Zupancic JA, McCormick MC, Croen LA, Greene J, Escobar GJ, Richardson DK. Trends in severe bronchopulmonary dysplasia rates between 1994 and 2002. J. Pediatr. 2005;146:469-73. [CrossRef]
- Berger TM, Bachmann II, Adams M, Schubiger G. Impact of improved survival of very low-birth-weight infants on incidence and severity of bronchopulmonary dysplasia. Biol. Neonate. 2004;86:124-30. [CrossRef]
- Patel AL, Johnson TJ, Robin B, Bigger HR, Buchanan A, Christian E, Nandhan V, Shroff A, et al. Influence of own mother's milk on bronchopulmonary dysplasia and costs. Arch. Dis Child Fetal Neonatal Ed. 2017;102:F256-F261. [CrossRef]
- Xu Y, Yu Z, Li Q, Zhou J, Yin X, Ma Y, Yin Y, et al. Dose-dependent effect of human milk on Bronchopulmonary dysplasia in very low birth weight infants. BMC. Pediatr. 2020;20:522. [CrossRef]
- Villamor-Martínez E, Pierro M, Cavallaro G, Mosca F, Villamor E. Mother's Own Milk and Bronchopulmonary Dysplasia: A Systematic Review and Meta-Analysis. Front. Pediatr. 2019;7:224. [CrossRef]
- Villamor-Martínez E, Pierro M, Cavallaro G, Mosca F, Kramer BW, Villamor E. Donor Human Milk Protects against Bronchopulmonary Dysplasia: A Systematic Review and Meta-Analysis. Nutrients. 2018;10:238. [CrossRef]
- Hwang JS, Rehan VK. Recent Advances in Bronchopulmonary Dysplasia: Pathophysiology, Prevention, and Treatment. Lung. 2018;196:129-138. [CrossRef]
- Ofman G, Tipple TE. Antioxidants & bronchopulmonary dysplasia: Beating the system or beating a dead horse? Free. Radic Biol Med. 2019;142:138-145. [CrossRef]
- Wang J, Dong W. Oxidative stress and bronchopulmonary dysplasia. Gene. 2018;678:177-183. [CrossRef]
- Asociación Española de Bancos de leche. https://www.aeblh.org/banco-de-leche/bancos-de-leche-en-espana, (last time accessed: April 7,2023).
- San Feliciano L, Moro M, Figueras J, et al. The Spanish Neonatal Network SEN1500: updated information. Pediatr Med. 2022 | https://dx. [CrossRef]
- Jobe AH, Bancalari E: Bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2001; 163: 1723–1729. [CrossRef]
- Ezz-Eldin ZM, Hamid TA, Youssef MR, Nabil Hel-D. Clinical Risk Index for Babies (CRIB II) Scoring System in Prediction of Mortality in Premature Babies. J Clin Diagn Res. 2015 Jun;9(6):SC08-11. [CrossRef]
- Papile LA, Burstein J, Burstein R, et al. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1500g. J Pediatr. 1978;92(4):529–534. [CrossRef]
- Garmer A. An international classification of retinopathy of prematurity. Pediatrics. 1984;74(1):127–133. PMID: 6547526.
- López Maestro M, Melgar Bonis A, de la Cruz-Bertolo J, Perapoch López J, Mosqueda Peña R, Pallás Alonso C. Cuidados centrados en el desarrollo. Situación en las unidades de neonatología de España [Developmental centered care. Situation in Spanish neonatal units]. An Pediatr (Barc). 2014 Oct;81(4):232-40. Spanish. Epub 2013 Dec 2. PMID: 24290892. [CrossRef]
- Jensen EA, Dysart K, Gantz MG, et al. The Diagnosis of Bronchopulmonary Dysplasia in Very Preterm Infants. An Evidence-based Approach. Am J Respir Crit Care Med. 2019;200(6):751-759. [CrossRef]
- Porta R, García-Muñoz Rodrigo F, Avila-Alvarez A, et al. Active approach in delivery room and survival of infants born between 22 and 26 gestational weeks are increasing in Spain. Acta Paediatr. 2023;112(3):417-423. [CrossRef]
- Porta R, Ventura PS, Ginovart G, et al. Changes in perinatal management and outcomes in infants born at 23 weeks of gestational age during the last decade in Spain. J Matern Fetal Neonatal Med. 2022;35(26):10296-10304. [CrossRef]
- Morgan AS, Zeitlin J, Källén K, et al. Birth outcomes between 22 and 26 weeks' gestation in national population-based cohorts from Sweden, England and France. Acta Paediatr. 2022;111(1):59-75. [CrossRef]
- Sucasas Alonso A, Pértega Diaz S, Sáez Soto R, Avila-Alvarez A. Epidemiology and risk factors for bronchopulmonary dysplasia in preterm infants born at or less than 32 weeks of gestation. An Pediatr (Engl Ed). 2022;96(3):242-251. [CrossRef]
- Rysavy MA, Mehler K, Oberthür A, Ågren J, Kusuda S, McNamara PJ, Giesinger RE, Kribs A, Normann E, Carlson SJ, Klein JM, Backes CH, Bell EF. An Immature Science: Intensive Care for Infants Born at ≤23 Weeks of Gestation. J Pediatr. 202;233:16-25.e1. [CrossRef]
- Fanczal E, Berecz B, Szijártó A, Gasparics Á, Varga P. The Prognosis of Preterm Infants Born at the Threshold of Viability: Fog Over the Gray Zone - Population-Based Studies of Extremely Preterm Infants. Med Sci Monit. 2020;26:e926947. [CrossRef]
- Yang X, Jiang S, Deng X, Luo Z, Chen A, Yu R. Effects of Antioxidants in Human Milk on Bronchopulmonary Dysplasia Prevention and Treatment: A Review. Front Nutr. 2022;9:924036. [CrossRef]
- Santoro KL, Yakah W, Singh P, Ramiro-Cortijo D, Medina-Morales E, Freedman SD, Martin CR. Acetaminophen and Xenobiotic Metabolites in Human Milk and the Development of Bronchopulmonary Dysplasia and Retinopathy of Prematurity in a Cohort of Extremely Preterm Infants. J Pediatr. 2022;244:224-229.e3. [CrossRef]
- Nuttall SL, Khan JN, Thorpe GH, Langford N, Kendall MJ. The impact of therapeutic doses of paracetamol on serum total antioxidant capacity. J Clin Pharm Ther. 2003;28(4):289-94. [CrossRef]
- Huang J, Zhang L, Tang J, Shi J, Qu Y, Xiong T, Mu D. Human milk as a protective factor for bronchopulmonary dysplasia: a systematic review and meta-analysis. Arch Dis Child Fetal Neonatal Ed. 2019;104(2):F128-F136. [CrossRef]
- Xu Y, Yu Z, Li Q, Zhou J, Yin X, Ma Y, Yin Y, Jiang S, Zhu R, Wu Y, Han L, Gao Y, Xue M, Qiao Y, Zhu L, Tu W, Wu M, Wan J, Wang W, Deng X, Li S, Wang S, Chen X, Zhou Q, Wang J, Cheng R, Wang J, Han S. Dose-dependent effect of human milk on Bronchopulmonary dysplasia in very low birth weight infants. BMC Pediatr. 2020;20(1):522. [CrossRef]
- Zhu Y, Chen X, Zhu J, Jiang C, Yu Z, Su A. Effect of First Mother's Own Milk Feeding Time on the Risk of Moderate and Severe Bronchopulmonary Dysplasia in Infants With Very Low Birth Weight. Front Pediatr. 2022;10:887028. [CrossRef]
- Peng W, Han J, Li S, Zhang L, Yang C, Guo J, Cao Y. The Association of Human Milk Feeding With Short-Term Health Outcomes Among Chinese Very/Extremely Low Birth Weight Infants. J Hum Lact. 2022;38(4):670-677. [CrossRef]
- Huang J, Zheng Z, Zhao XY, Huang LH, Wang L, Zhang XL, Lin XZ. Short-term effects of fresh mother's own milk in very preterm infants. Matern Child Nutr. 2023;19(1):e13430. [CrossRef]
- Briere CE, McGrath JM, Cong X, Brownell E, Cusson R. Direct-Breastfeeding Premature Infants in the Neonatal Intensive Care Unit. J Hum Lact. 2015;31(3):386-92. [CrossRef]
- Fleurant E, Schoeny M, Hoban R, Asiodu IV, Riley B, Meier PP, Bigger H, Patel AL. Barriers to Human Milk Feeding at Discharge of Very-Low-Birth-Weight Infants: Maternal Goal Setting as a Key Social Factor. Breastfeed Med. 2017;12(1):20-27. [CrossRef]
- Chauhan A, Kumar M, Tripathi S, Singh SN, Singh VK. Breastfeeding rates at discharge for very low birthweight neonates and their determinants: An observational study from a tertiary care neonatal intensive care unit in India. J Paediatr Child Health. 2022;58(9):1653-1660. [CrossRef]
- Jiang X, Jiang H. Factors associated with post NICU discharge exclusive breastfeeding rate and duration amongst first time mothers of preterm infants in Shanghai: a longitudinal cohort study. Int Breastfeed J. 2022 May 2;17(1):34. PMID: 35501877; PMCID: PMC9063107. [CrossRef]
- Seshadri N, Kim LY, McGrath-Morrow SA, Collaco JM. Human Milk Cessation in the NICU in Infants with Bronchopulmonary Dysplasia. Am J Perinatol. 2021 Dec 29. Epub ahead of print. PMID: 34753184. [CrossRef]
- Kim LY, McGrath-Morrow SA, Collaco JM. Impact of breast milk on respiratory outcomes in infants with bronchopulmonary dysplasia. Pediatr Pulmonol. 2019;54(3):313-318. Epub 2019 Jan 4. PMID: 30609293; PMCID: PMC6518393. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).