1. Introduction
Trigeminal neuralgia (TN) is defined as a neurological disorder, characterized by paroxysmal pain, generally associated with trigger points that correspond to areas innervated by a branch of the trigeminal nerve; this pain can occasionally be manifested continuously, and it is a relevant factor for disability during the course of the disease and the functional limitation associated [
1]. TN can be associated to ipsilateral facial spasm (tic douloureux) and to mild autonomic symptoms, like epiphora and/or ipsilateral conjunctival injection. After a painful paroxysm, generally short, there is a refractory period, during which pain is not triggered [
2]. Routinely, TN can be divided into classical, secondary and idiopathic [
3]. According to the international classification for headache disorders, Beta version (ICHD-3 Beta 2018) for its diagnosis, it should be defined with the presence of unilateral paroxysmal pain, clearly delimited to a division of a trigeminal nerve branch, associated with allodynia, and not explained by an alternate diagnosis [
2].
Once the clinical diagnosis of TN is established, it should be complemented with an MRI, in multi-planar projection with thin slices and SSFP sequence with great T2 weighting, which provides excellent contrast resolution between de CF and adjacent soft tissues. The SSFP sequences are often mentioned by its specific abbreviation given by the provider [
4], FIESTA (fast imaging employing steady state acquisition; GE Healthcare, Milwaukee, WI, United States) or 3D CISS (Three-dimensional constructive interference in steady state; Siemens Healthcare, Erlangen, Germany), to investigate possible secondary aetiologies, mainly vascular compression of the nerve, like the superior cerebellar artery , antero-inferior cerebellar artery, basilar artery and venous complex, as well as other causes like tumoral lesions [
5].
The annual incidence of TN has been reported in 4.7 to 12.6 per 100,000 people[
6]
,[
7], with predominance in females, being 51 years the average age of presentation[
7]. Initial treatment for TN is pharmacological, carbamazepine is the drug of choice, oxcarbazepine, pregabalin, lamotrigine, phenytoin, topiramate and amitriptyline are also useful, requiring in some cases combined schemes to obtain therapeutic results[
8]. When the pharmacological treatment fails, there are a variety of procedures, which include, microvascular decompression (MVD), percutaneous rhizotomy by radiofrequency, percutaneous rhizotomy with glycerol, balloon percutaneous compression and stereotactic radiosurgery [
9].
Traditionally, MVD of the trigeminal nerve has shown a rate of success in the management of pain between 80.3 to 96%, with immediate control of pain and with variable rates of complications that oscillate between 4 to 6% according to the group [
10,
11,
12], performing a conventional approach trough retrosigmoid craniotomy, making a keyhole of 40 mm approximately, with sufficient and wide exposition.
We present the technique for a retrosigmoid approach trough a miniasterional craniotomy as an effective and safe technique, that allows a proper surgical corridor for the upper, middle and lower cerebellar complexes, in case of being required for associated pathologies [
13].
2. Methods and Materials
A total of 22 patients were treated at the Neurosurgery department of the General Hospital of Mexico (Mexico City, Mexico) for the clinical management of TN. The records of patients managed in our clinic were considered (2016-2018). According to the eligibility criteria, all adult patients (18–65 years) of both genders, with TN secondary refractory medical management, anatomic impairment of the fifth nerve (determined through magnetic resonance imaging) were included. Those patients with previous surgical intervention for TN, evidence of multiple sclerosis or other neurological disorders that may mimic TN, contraindications to surgery, pregnancy or lactation were excluded. The data extraction was focused on collecting information on the demographic aspects (age, gender), etiology, anatomical location/distribution of the nervous assault, and affected side. The clinical evaluation of the patients was focused on collecting data corresponding to the pre- and postoperative state of the pain status according to Visual Analogue Scale (VAS), and according to Barrow Neurological Institute Pain Scale (BNIPS). Statistical significance differences between pre-operative and post-operative pain scales were calculated using an ANOVA test and the post-hoc analysis with DMS or C-Dunnett tests, depending on the homogeneity of the variances defined by Levene's test, the effect size was calculated using the Cohen's d, and recalculated considering the correction coefficient for small sample sizes. Data measures were performed using SPSS 25.0 for Windows software (SPSS, Inc., Chicago, IL), where a p-value < 0.05 was considered significant.
Technical note:
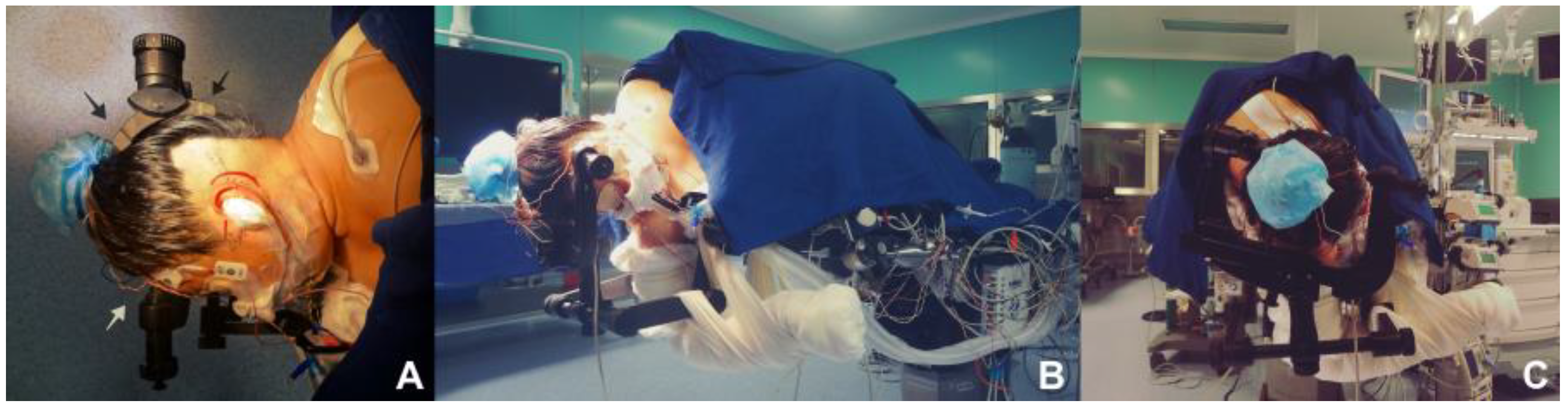
Proper positioning is thought as the corner stone for performing a parasterional burr-hole, since it avoids the retraction of the cerebellum, and prevents contralateral venous return from alteration, being of great help and utility at the moment of the procedure (
Figure 1A–D). A 3 pins cephalic support is placed (Doro-Integra LifeSciences Corporation, Plainsboro, New Jersey, E.U.A.). The patient is positioned in lateral decubitus, contralateral to the area being approached, having the inferior arm outside the surgical table, held over a sling. Chest is elevated approximately 15
o, and the head is placed with a minimum flexion of 5
o and a 10
o inferior rotation, so that the retromastoid plane is parallel to the floor. Park Bench position is preferred over the supine decubitus approach with cephalic rotation, for providing a better venous contralateral return, control of the upper, middle and lower cerebellar complexes and being more comfortable for the neurosurgeon[
14].
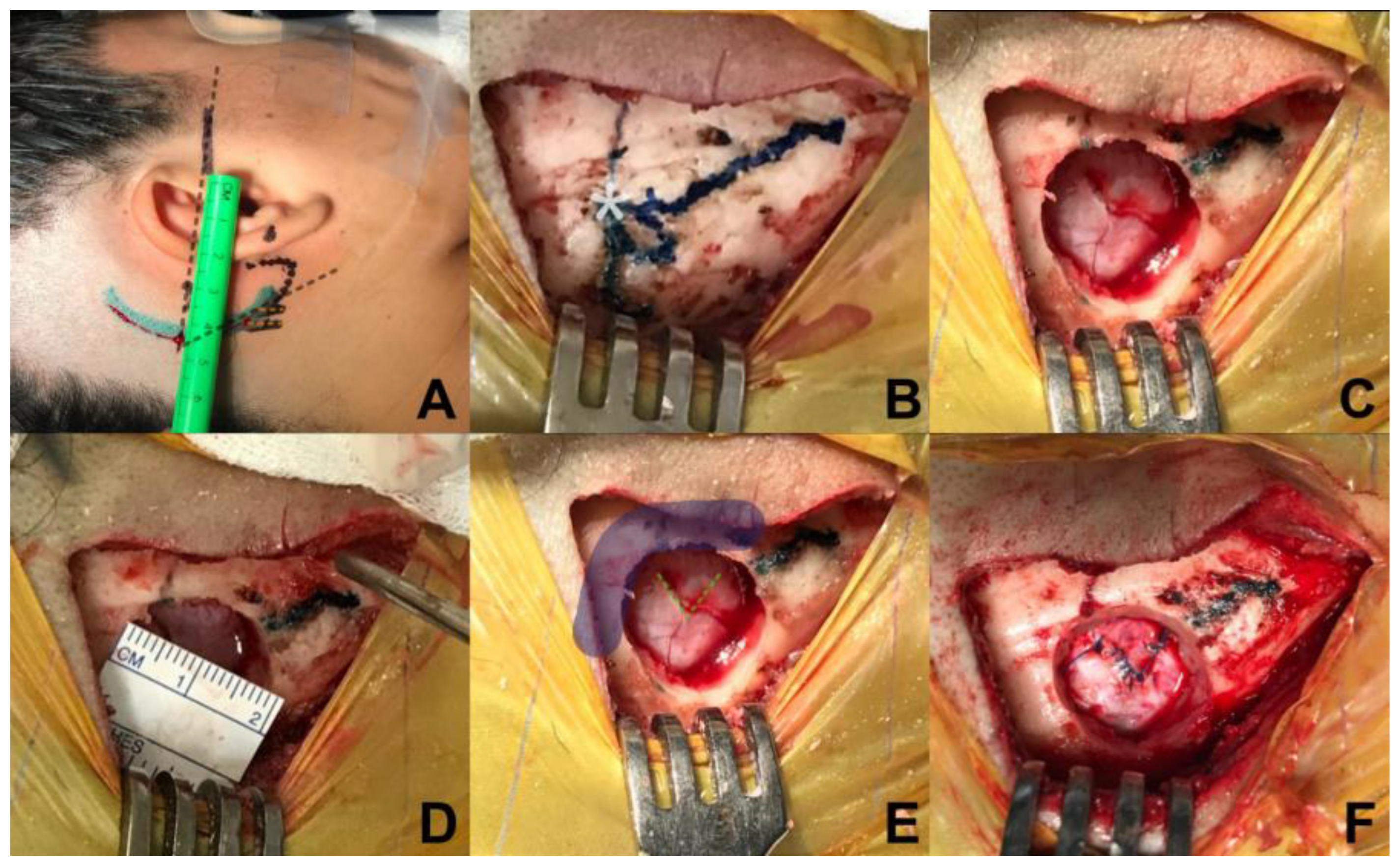
Relative to the incision, a retro-auricular curved incision is performed, 50 mm behind the tragus, starting from the upper portion of the mastoid, 5 mm in front of the digastric cleft and is extended 10 mm above where the asterion has been referred. To locate the position of the asterion, Reid’s orbitomeatal line is projected towards posterior, which corresponds in all cases to the base of the zygoma and the union of the upper third with the medial third of the auricular pavilion, the asterion is located where Reid’s line crosses with a line drawn up to the digastric cleft (
Figure 2A,B). An incision is made, of approximately 50mm long, and subperiostic dissection is made with monopolar, revealing the digastric cleft, the mastoid base towards inferior and asterion towards superior[
15]. Retro mastoid emissary vein is controlled with bone wax and is not considered as an anatomic reference for the craniotomy, given the great variability of its position.[
16]
Regarding to craniotomy, a single keyhole is made with a self-blocking drill bit, 14 mm below the union of the sigmoid and transverse sinuses, medial and inferior from the asterion (
Figure 2C,D), identification of the transverse and sigmoid sinuses is necessary for a safe dural opening[
17]. Different from the conventional retrosigmoid approach, we consider a parasterional craniotomy (<20mm) as sufficient for the cerebellopontine angle exploration to handle vascular compressions associated with TN. This approach not only allows manipulation of the upper cerebellar complex, but also provides an excellent surgical corridor for the middle and lower ones[
13]
,[
18]
In relation to dural opening, it was performed in a triangular shape, with a base of 7mm approximately, in direction towards the sigmoid sinus, in order to have only one reference to avoid dural retraction (
Figure 2E,F). This kind of dural opening is more comfortable for closure, minimizes the requirement of autologous graft and reduces the risk of unnoticed injury of the transverse and sigmoid sinuses at the time of closure.
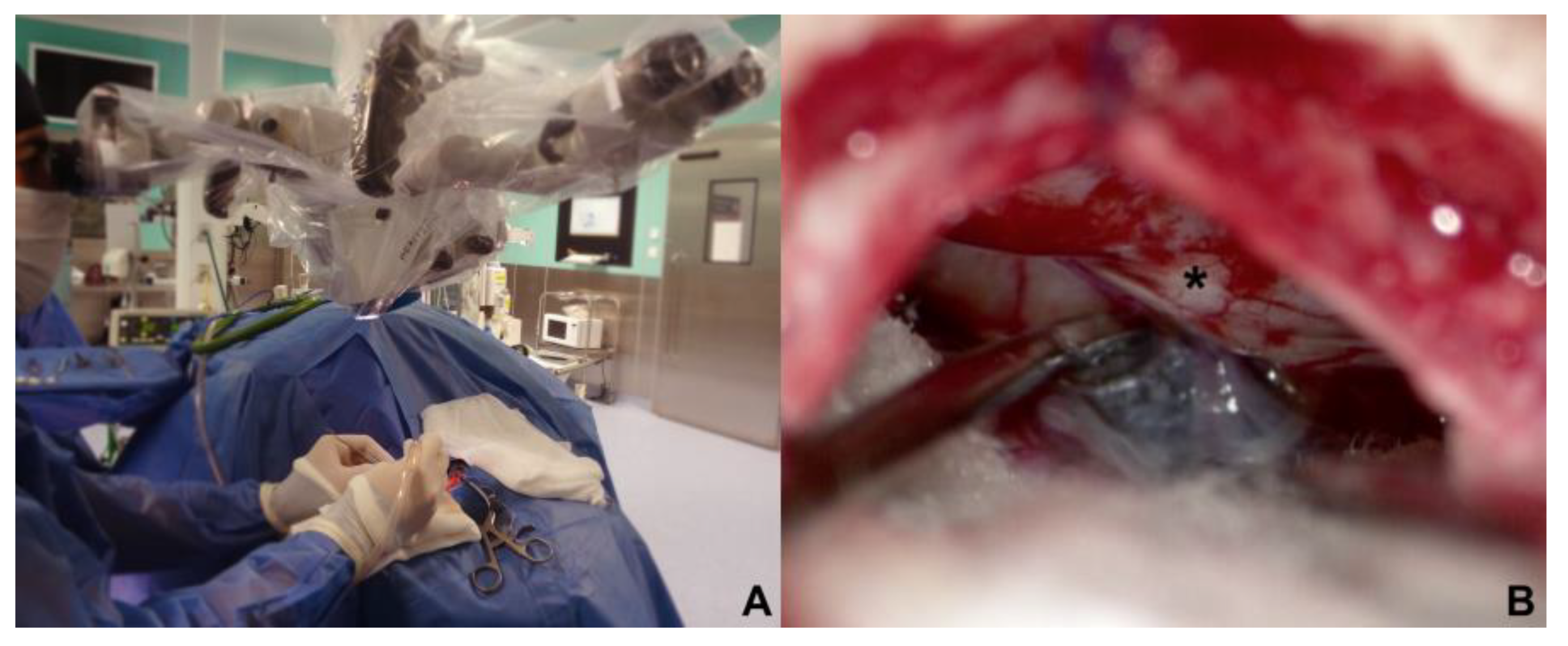
The Approach of the cerebellopontine cistern were performed through a passive drainage of cerebrospinal fluid is permitted, which can be maximized by contralateral rotation, this also allows visualisation of the posterior petrous surface and the pathway to access the cistern of the cerebellopontine angle. Under magnified vision, the petrous surface is followed with the centered microscope and an angle of 110-120
o approximately for and adequate advancement in the angle’s cistern (
Figure 3A,B); liberation of arachnoid adhesions is needed for the cerebellar hemisphere to drop without retraction. The first step consists in identifying the Tübingen line which indicates the suprameatal tubercle projection[
19], demonstrating the subarcuate artery and an arachnoid layer that covers the middle cerebellar complex[
20]. For approaching the trigeminal nerve, an arachnoid opening is made above the middle cerebellar complex. The microscope is angled 20
o lateral to visualise the upper portion. Between the superior petrous venous complex (Dandy’s vein) and the middle cerebellar complex, the trigeminal nerve is identified in its cisternal portion; in our experience, we consider not necessary to handle or coagulate Dandy’s vein in this work angle. Although the incidence of complications due to Dandy’s vein obliteration is low, it can occur, and sequels could be worst for the evolution of the existing pathology[
21,
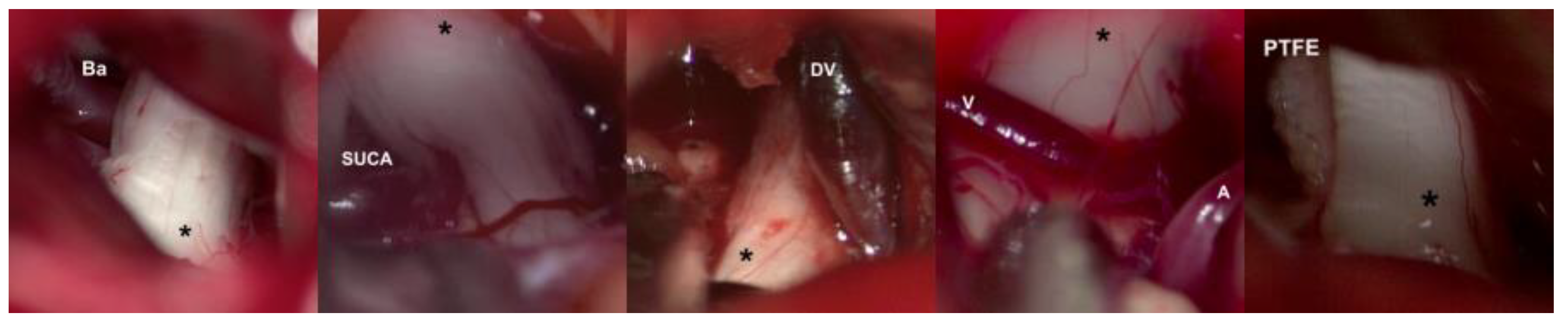
22]. Trigeminal nerve is identified by its larger size compared to the middle cerebellar complex; a motor branch is identified at the nerve’s upper aspect. Release of arachnoid adherences should be performed in an organized manner along the nerve, with special caution in the medial surface, we recommend to always explore from the inferior edge of the nerve to avoid handling the motor branch and to understand the trigeminal nerve with a somatotopic organization, lowering the injury possibility of branches in V1. In cases where a vascular association is not observed as the cause of compression, strong arachnoid reactions can be identified over the nerve, with good results after its complete liberation from the nerve. Superior cerebellar artery has been identified as the most frequent cause of compression, followed by venous compression and the anterior-inferior cerebellar artery (
Figure 4A–D).
Release of the cisternal portion of the trigeminal nerve should be systematic, checking all its aspects according to the detailed planning with the MRI in high resolution sequences. Once achieved the vascular liberation of the trigeminal nerve, we proceed to implant a polytetrafluorethylene interface (PTFE) which can be found in diverse presentations. (
Figure 4E)
Dural Closure were carried out with the dura mater opening in a triangular shape the closure becomes easier, and the risk of fistulae is minimized, since it does not retract, with the point of reference located in the flap apex, closure is commenced, with simple continuous suture. Valsalva mechanisms are performed to document a hermetic closing and the absence of cerebrospinal fluid fistulae.
3. Results
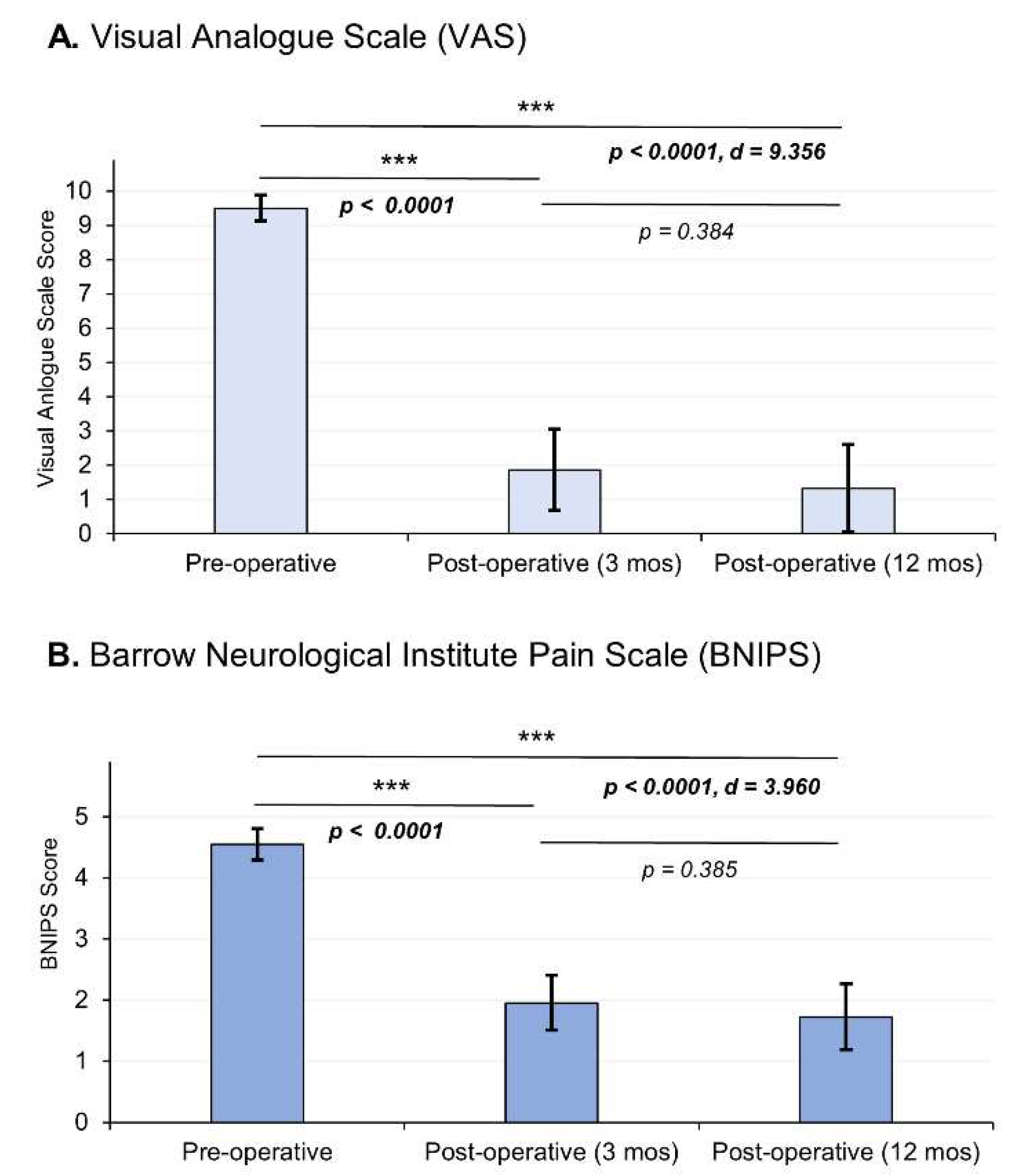
Twenty-two patients were considered in the study, who had a mean age of 54.9 ± 12.73, where 81% women predominated, the most affected was the right side (86%), the most frequent etiology being vascular contact of the superior cerebellar artery (59%), branches V2 and V3 of the trigeminal nerve are predominantly affected (58%) (
Table 1). Regarding the clinical outcomes, we observed that according to the VAS there was a decrease in pain intensity from an average preoperative value of 9.5 ± 0.37 at 12 months to a postoperative value of 1.32 ± 1.28, demonstrating significant changes between the 12-month postoperative evaluation (p < 0.0001) with a considerable effect size (d = 9.356). On the other hand, in relation to the BNIPS scale, a decrease from an average preoperative status of 4.55 ± 0.25, to a postoperative status at 12 months of 1.73 ± 0.54 was also demonstrated, showing significant changes (p < 0.0001, d = 3.960) (
Figure 5). Relative to the distribution of patient’s pain status, sixty-three percent of the population had a VAS score of 10 in the preoperative period, after surgery (12 months after surgery) 68% of the sample presented a VAS equal to 0 (
Table 2). Regarding the BNIPS, 55% of the sample presented a score of V before surgery, which after the intervention and the evaluation at 12 months, showed that most of the patients were found to have a score of I ( 63%) (
Table 3).
4. Discussion
Neuropathic pain is the main indication for MVD in TN. According to a recent systematic review published by Holste (2019) et al. 46 articles were included from 1988 to 2018 in which only 28 % (13 studies) reported the pain using a standardized clinical scale.23 The most frequent scales applied where BNIPS, and VAS. The rest of the articles used a non-standardized tool to measure the outcomes of their patients, and some omitted them.23
In the beginning of the TN surgery, Dandy used to made big craniectomies to approach the cerebellopontine angle (≈ 7 x 5 cm).24 Nowadays the use of smaller incisions are becoming more popular in decompression of TN.25 Nevertheless, there is a confusion in the terminology used for the denomination of the bone removal such as the terms “craniotomy”,“craniectomy”,“burr hole”, “key hole”, are commonly applied to refer the approach, and most of the times without define the size of this aperture. The current trends of surgical management for TN are to perform smaller hole, including endoscopic techniques. The use of reduced approaches has the propose of reduce the damage on the scalp of the patient and still have the appropriate amount of space for the neurosurgeon to perform the surgery without inconvenience.
According to a systematic review focused in hearing loss after MVD published by Bartindale in 2017. There are 35 studies that reported the phenomenon of hearing loss by applying minimal invasive procedures.26 Between the reports of the both systematic reviews, one focused on pain (Holste, 2019) and the other on hearing loss (Bartindale, 2017) we found the next results: Only 28% (23 studies of 81) of the studies reported the size of the bone removal, the term most commonly used to report the surgical approach was “Craniectomy” in 30% of the articles (25 studies of 81) and the average size of the bone removal was not always the same in the studies. The range size reported for the term “craniectomy” according to this literature was from 15 to 30 mm, for this reason the term craniectomy is misused because in the rest of the literature it refers for approaches bigger than 40 mm.23,25.
The use of the terminology to describe the surgical approach previously mentioned, has the propose of describing a small hole on the scalp of the patient, but the area applied should be standardized with the aim of establishing relations between the space of the surgery and the clinical improvement. However, a craniectomy is not an adequate term for the procedure, because an article that evaluates the terms trephinations, trephines and craniectomies, found that a “craniectomy” is a term used to refer a huge cranial opening or window of variable shape and that may include a size bigger than 4 cm that correspond at the size of a threpan.26 For this reason, it was decided to refer to this technique as a Retrosigmoid/Para-asterional burr hole, because the description of a burr hole has an average size of a dime (approximately 17.9 mm), the same dimensions used in the patients included in the present study (18 mm).27
TN is a high incidence pathology, and with high health costs. MVD of the trigeminal nerve is established as a therapeutic strategy, with complete resolution of symptoms and low remission in 10 years follow up[
12]. Historically, the first case of MVD was performed by Gardner and Miklos, using a transtentorial subtemporal approach
28. Later in 1967, Janneta
29, from microsurgical observations, developed the pathophysiological bases that support the arterial compression of the trigeminal nerve, and as a result establishing MVD trough a retrosigmoid approach as the standard treatment[
16]. The minimally invasive modification of the technique has been popularized by Revuelta et al
30,31. More recently, pure or combined endoscopic approaches have been described for treating TN, showing superiority in the microsurgical technique with visual improvement as well as better illumination of nervous and vascular structures, also for being minimally invasive
32. Nevertheless, the size of craniotomy proposed by these groups, is using a 2.7 mm endoscope, so, with the use of a 4mm conventional endoscope, the cranial opening is like the one we propose
33. According to our knowledge, there is no description of the technique for a minimally invasive approach for MVD of the trigeminal nerve. retrosigmoid parasterional burr-hole approach is proposed as an effective surgical option for the management of this pathology, minimizing cerebellar manipulation, and with low complication rates, microsurgical training is recommended for avoiding unnecessary injuries of vital structures, having the expertise of the cerebellopontine angle surgical anatomy. MVD must correspond to a safe surgery, with results dependent on a proper trigeminal nerve release, without forgetting any step that guarantees good surgical outcomes.
We consider the parasterional burr-hole as an adequate pathway for the management of the cerebellopontine angle in a posterior normal-tension fossa, this, at the expense that successful MVD is based in the release of the cisternal portion of the trigeminal nerve from the arterial or venous aberrant or ectopic vessel causing the pathology. Thus, a clear anatomy of the area allows for a rapid identification of the work pathway, avoiding conflictive areas as the superior petrous venous plexus and the facial nerve, shortening surgical time. It must be emphasized that for performing a successful MVD with this approach, an adequate positioning of the head is needed for attaining the vision angles required for not retracting the cerebellum, which could be associated with unnoticed postoperative complications.
Recently, in our hospital, this modification of the technique was adopted, and was performed in the last 30 cases, obtaining a pain control rate of 97%, complications rate less than 4% and in none of the cases coagulation of Dandy’s vein was performed. The average surgical time was of 60 min, from the incision up to the dural closure, average hospital stay was of 1.5 days and the mean postoperative recovery period was of 5 days, with a direct impact in patient’s quality of life, with immediate suspension of medications. Evaluating this, we consider the retrosigmoid parasterional burr-hole approach, along with the modification of the dural opening, as a versatile and effective technique for the management of vascular compression of the trigeminal nerve, with favourable results, reduction in hospital stay and postoperative recovery and minimal aesthetic alteration. Placing this technique above the traditional retrosigmoid approach that entails a wide craniotomy, with unnecessary exposure of the cerebellar cortex.
5. Conclusion
MVD of the trigeminal nerve trough a retrosigmoid parasterional burr-hole approach is feasible and can be a safe and effective technique for the management of the upper, middle and lower cerebellar complexes without associated complications. A detailed knowledge of the anatomy and landmarks is required for its implementation. Even though this technique is well known, we believe that the relevance of the article lies in the consideration of clinimetric scales for the assessment of pain.
Author Contributions
Conceptualization, CRJD. and CRJC; Methodology, DMJA; Software, ASA; Validation, AVE and NOJL; Formal Analysis VCF; Investigation CRJD and CRJC; Resources DMJA; Data Curation AVE; Writing – Original Draft Preparation CRFX; Writing – Review & Editing CRJD, CRJC and ASA; Visualization ASA; Supervision CRJD; Project Administration CRJD.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Institutional Review Board and Ethics Committee of the Hospital General de México “Dr. Eduardo Liceaga”.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data associated with the paper are not publicy available but are available from corresponding author on reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Maarbjerg S, Di Stefano G, Bendtsen L, Cruccu G. Trigeminal neuralgia - Diagnosis and treatment. Cephalalgia. 2017, 37, 648–657. [Google Scholar] [CrossRef] [PubMed]
- Jes Olesen, Denmark, Lars Bendtsen, David Dodick, Anne Ducros, Stefan Evers, Michael First, Peter J Goadsby, Andrew Hershey, Zaza Katsarava, Morris Levin, Julio Pascual, Michael B Russell, Todd Schwedt, Timothy J Steiner, Cristina Tassorelli, Gisela M Ter S-JW. The International Classification of Headache Disorders, 3rd edition. Cephalalgia. 2018, 38, 1–211. [Google Scholar] [CrossRef]
- Cruccu G, Gronseth G, Alksne J, et al. AAN-EFNS guidelines on trigeminal neuralgia management. European Journal of Neurology 2008, 15, 13–1028. [Google Scholar]
- Hughes MA, Frederickson AM, Branstetter BF, Zhu X, Sekula RF. MRI of the trigeminal nerve in patients with trigeminal neuralgia secondary to vascular compression. American Journal of Roentgenology 2016, 206, 595–600. [CrossRef]
- Graff-Radford S, Gordon R, Ganal J, Tetradis S. Trigeminal Neuralgia and Facial Pain Imaging. Current Pain and Headache Reports. 2015, 19. [Google Scholar]
- Katusic S, Willaims DB, Beard CM, Bergstralh EJ, Kurland LT.| Epidemiology and clinical features of idiopathic trigeminal neuralgia and glossopharyngeal neuralgia: Similarities and differences, rochester, minnesota, 1945-19841. Neuroepidemiology. 1991, 10, 276–281.
- Koopman JSHA, Dieleman JP, Huygen FJ, de Mos M, Martin CGM, Sturkenboom MCJM. Incidence of facial pain in the general population. Pain. 2009, 147, 122–127. [Google Scholar] [CrossRef]
- Ariyawardana A, Pallegama R, Sitheeque M, Ranasinghe A. Use of single- and multi-drug regimens in the management of classic (idiopathic) trigeminal neuralgia: an 11-year experience at a single Sri Lankan institution. Journal of Investigative and Clinical Dentistry 2012, 3, 98–102. [Google Scholar] [CrossRef]
- Bick SKB, Eskandar EN. Surgical Treatment of Trigeminal Neuralgia. Neurosurgery Clinics of North America 2017, 28, 429–438. [CrossRef] [PubMed]
- Barker FG, Jannetta PJ, Bissonette DJ, Larkins M V, Jho HD. The long-term outcome of microvascular decompression for trigeminal neuralgia. New England Journal of Medicine 1996, 334, 1077–1084. [CrossRef]
- Sarsam Z, Garcia-Fiñana M, Nurmikko TJ, Varma TRK, Eldridge P. The long-term outcome of microvascular decompression for trigeminal neuralgia. British Journal of Neurosurgery, 2010, 24, 18–25. [CrossRef]
- Günther T, Gerganov VM, Stieglitz L, Ludemann W, Samii A, Samii M. Microvascular decompression for trigeminal neuralgia in the elderly: Long-term treatment outcome and comparison with younger patients. Neurosurgery. 2009, 65, 477–482. [Google Scholar] [CrossRef]
- Revuelta-Gutiérrez R, Morales-Martínez A, Mejías-Soto C, Martínez-Anda J, Ortega-Porcayo L. Microvascular decompression for glossopharyngeal neuralgia through a microasterional approach: A case series. Surgical Neurology International. 2016, 51.
- Singh, G. Positioning in Neurosurgery. Essentials of Neuroanesthesia. 2017, 183–205. [Google Scholar]
- Campero Á, Herrera D, Ajler P. Abordaje retrosigmoideo. Revista Argentina de Neurocirugia. 2014, 28, 114–119. [Google Scholar]
- Jannetta PJ, McLaughlin MR, Casey KF. Technique of microvascular decompression. Technical note. Neurosurgery Focus. 2005, 18, E5. [Google Scholar]
- Campero A, Ajler P, Campero AA. Microvascular decompresion for trigeminal neuralgia, report of 36 cases and literature review. Surgical Neurology International 2014, 5, 441. [CrossRef]
- Hitotsumatsu T, Matsushima T, Inoue T, et al. Microvascular Decompression for Treatment of Trigeminal Neuralgia, Hemifacial Spasm, and Glossopharyngeal Neuralgia: Three Surgical Approach Variations: Technical Note. Neurosurgery. 2003, 53, 1436–1443. [Google Scholar] [CrossRef]
- Campero A, Martins C, Rhoton A, Tatagiba M. Dural landmark to locate the internal auditory canal in large and giant vestibular schwannomas: The Tübingen line. Operative Neurosurgery. 2011, 69, ons99–ons102. [Google Scholar] [CrossRef]
- Rhoton, J. The cerebellopontine angle and posterior fossa cranial nerves by the retrosigmoid approach. Neurosurgery. 2000, 47 (Suppl. 3), S93–S129. [Google Scholar] [CrossRef] [PubMed]
- Narayan V, Savardekar AR, Patra DP, et al. Safety profile of superior petrosal vein (the vein of Dandy) sacrifice in neurosurgical procedures: a systematic review. Neurosurgical Focus. 2018, 45, E3. [Google Scholar] [CrossRef] [PubMed]
- Zhong J, Li S-T, Xu S-Q, Wan L, Wang X. Management of petrosal veins during microvascular decompression for trigeminal neuralgia. Neurological Research. 2008, 30, 697–700. [Google Scholar] [CrossRef] [PubMed]
- Holste K, Chan AY, Rolston JD, Englot DJ. Pain Outcomes Following Microvascular Decompression for Drug-Resistant Trigeminal Neuralgia: A Systematic Review and Meta-Analysis. Neurosurgery 2020, 86, 182–190. [CrossRef] [PubMed]
- Patel SK, Markosian C, Choudhry OJ, Keller JT, Liu JK. The historical evolution of microvascular decompression for trigeminal neuralgia: from Dandy's discovery to Jannetta's legacy. Acta Neurochir (Wien). 2020, 162, 2773–278. [Google Scholar] [CrossRef] [PubMed]
- Carrillo-Ruiz JD, Muratti-Molina EB, Cojuc-Konigsberg G, Carrillo-Márquez JR. Trephinations, trephines, and craniectomies: contrast between global ancient civilizations and pre-hispanic mexican cultures [published online ahead of print, 2023 Mar 24]. World Neurosurg, 2023; S1878-8750(23)00412-6.
- Bartindale M, Kircher M, Adams W, et al. Hearing Loss following Posterior Fossa Microvascular Decompression: A Systematic Review. Otolaryngol Head Neck Surg. 2018, 158, 62–75. [Google Scholar] [CrossRef]
- Elayouty, A.E.D., AbdelFatah, M.A. Inner membrane opening during the burr-hole evacuation of a chronic subdural hematoma: risk-adding or recurrence-preventing? Egypt J Neurosurg. 2018, 33.
- Gardner WJ, Miklos M V. Response of trigeminal neuralgia to “decompression” of sensory root: Discussion of cause of trigeminal neuralgia. Journal of the American Medical Association. 1959, 170, 1773. [Google Scholar] [CrossRef] [PubMed]
- Jannetta, PJ. Arterial Compression of the Trigeminal Nerve at the Pons in Patients with Trigeminal Neuralgia. Journal of Neurosurgery. 1967, 26, 159–162. [Google Scholar] [CrossRef]
- Revuelta-Gutierrez, Rogelio; Beltran-Rochin, Jose; Escobedo-Rios, Francisco; Florez-Orozco J. Microcraniectomía Asterional: Una Opción Quirúrgica Para la Patología del Angulo Ponto-Cerebeloso. Revista Ecuatoriana de Neurología, 1999; 2–6.
- Revuelta-Gutierrez R, Martinez-Anda JJ, Coll JB, Campos-Romo A, Perez-Peña N. Efficacy and safety of root compression of trigeminal nerve for trigeminal neuralgia without evidence of vascular compression. World Neurosurgery. 2013, 80, 385–389. [Google Scholar] [CrossRef]
- Piazza M, Lee JYK. Endoscopic and Microscopic Microvascular Decompression. Neurosurgery Clinics of North America. 2016, 27, 305–313. [Google Scholar] [CrossRef]
- Bohman L-E, Pierce J, Stephen JH, Sandhu S, Lee JYK. Fully endoscopic microvascular decompression for trigeminal neuralgia: technique review and early outcomes. Neurosurgical Focus. 2014, 37, E18. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).