Submitted:
23 May 2023
Posted:
25 May 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
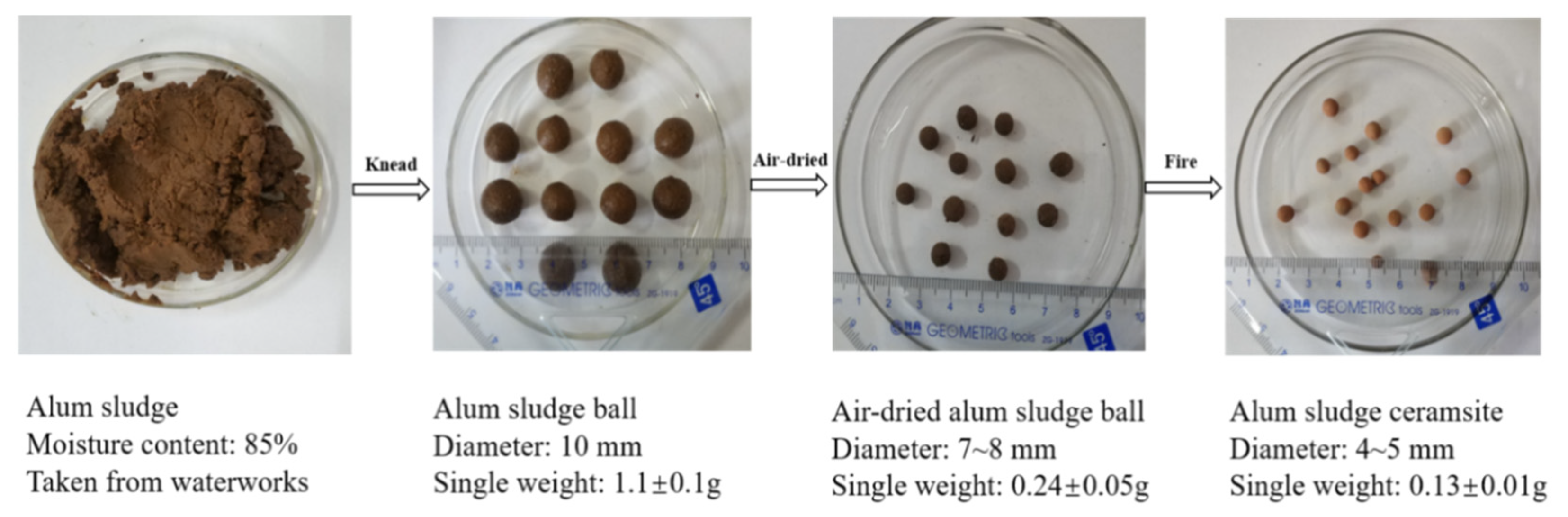
2.1. Preparation of ASC
2.2. P-adsorption tests
2.3. Analytical methods
2.4. P-adsorption models
3. Results
3.1. ASC product and its characterization
3.1.1. Apparent change
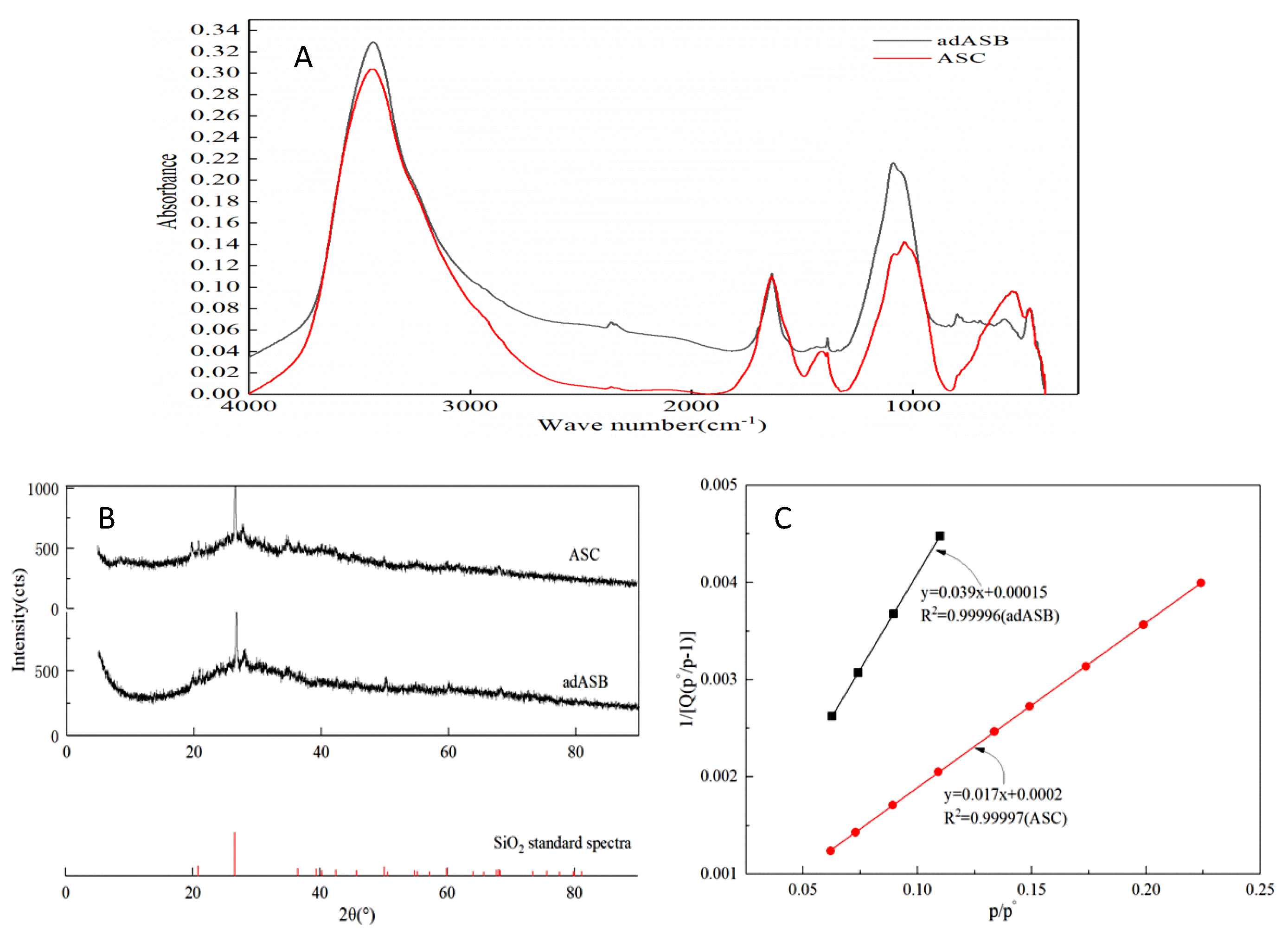
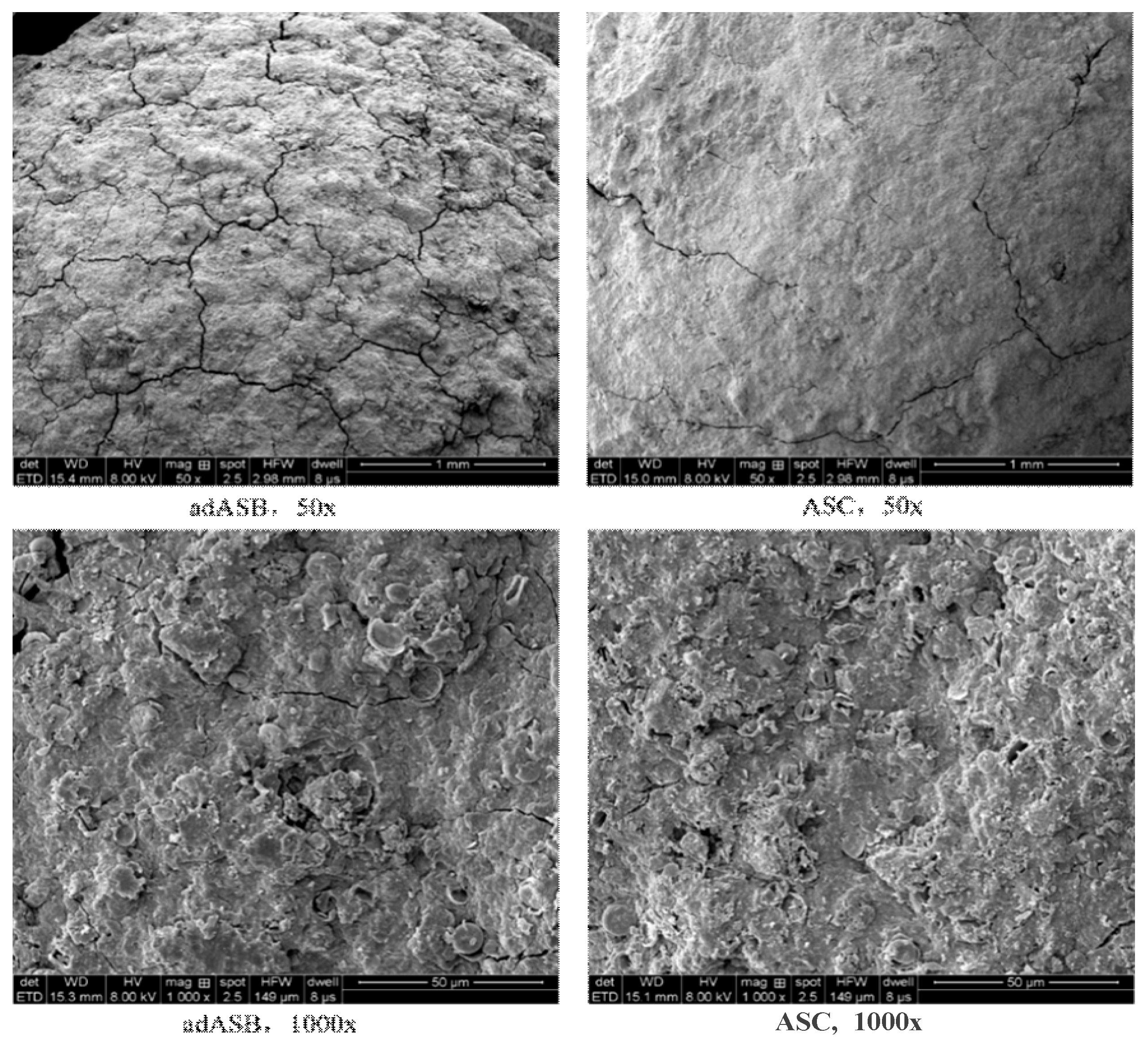
3.1.2. Microstructure of adASB and ASC
3.2. Adsorption behaviors on P removal
3.2.1. Adsorption kinetics
3.2.2. Adsorption isotherm
4. Discussion
4.1. The firing procedure and features of ASC
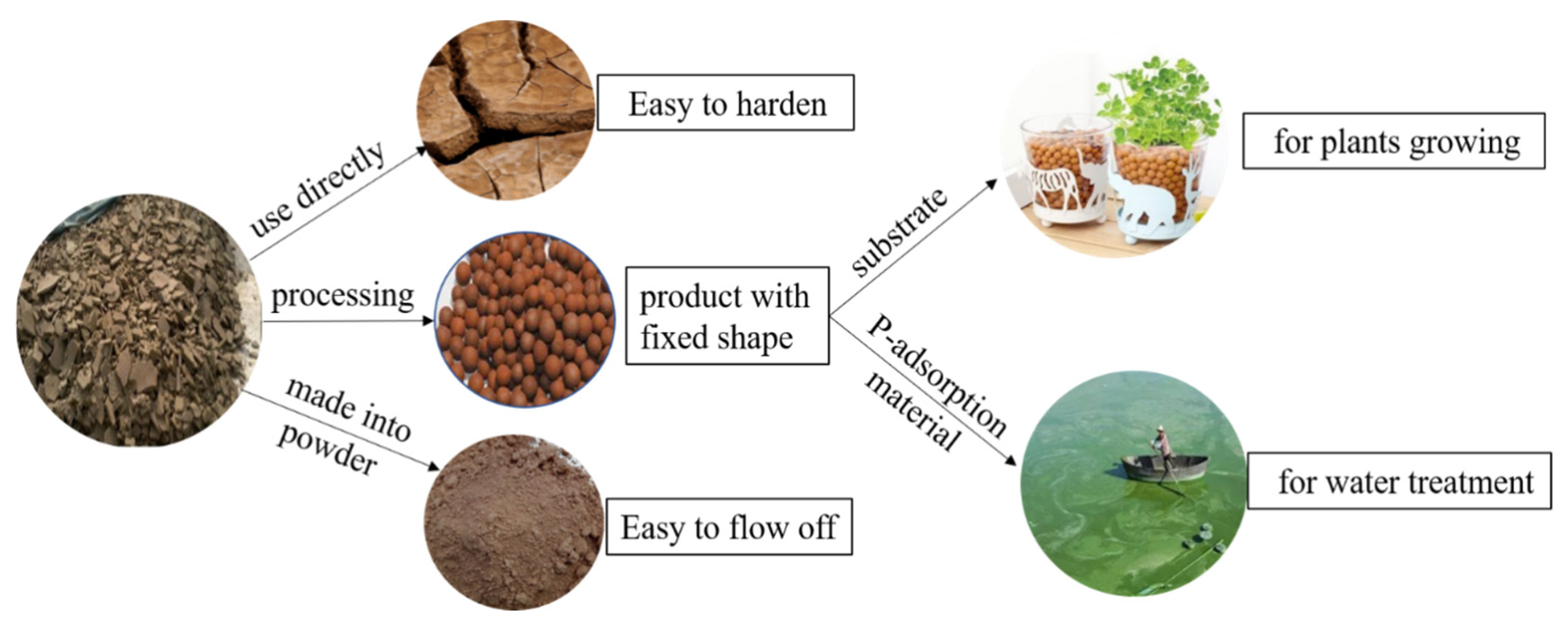
4.2. Prospects of using ASC in China
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Cao W.P., Zhang Y.Q. Removal of nitrogen (N) from hypereutrophic waters by ecological floating beds (EFBs) with various substrates. Ecological Engineering. 2014, 62,148-152. [CrossRef]
- Yang Y., Zhao Y.Q., Babatunde A.O., Wang L., Ren Y., Han Y. Characteristics and mechanisms of phosphate adsorption on dewatered alum sludge. Separation and Purification Technology. 2006, 51, 193-200. [CrossRef]
- Babatunde A.O., Zhao Y.Q., Burke A.M., Morris M.A., Hanrahan J.P. Characterization of aluminium-based water treatment residual for potential phosphorus removal in engineered wetlands. Environmental Pollution. 2009, 157, 2830-2836. [CrossRef]
- Yang Y., Zhao Y.Q., Kearney P. Influence of ageing on the structure and phosphate adsorption capacity of dewatered alum sludge. Chemical Engineering Journal. 2008, 145, 276-284. [CrossRef]
- Zhao Y.Q., Babatunde A.O., Zhao X.H., Li W.C., Development of alum sludge-based constructed wetland: An innovative and cost effective system for wastewater treatment. Journal of Environmental Science and Health, Part A. 2009, 44, 827-832. [CrossRef]
- Hu Y.S., Zhao Y.Q., Zhao X.H., Kumar J.L.G. Comprehensive analysis of step-feeding strategy to enhance biological nitrogen removal in alum sludge-based tidal flow constructed wetlands. Bioresource Technology.2012, 111, 27-35. [CrossRef]
- Babatunde A.O., Zhao Y.Q. Forms, patterns and extractability of phosphorus retained in alum sludge used as substrate in laboratory-scale constructed wetland systems. Chemical Engineering Journal. 2009, 152, 8-13. [CrossRef]
- Li X., Cui J., Pei Y. Granulation of drinking water treatment residuals as applicable media for phosphorus removal. Journal of Environmental Management. 2018, 213, 36. [CrossRef]
- Zhao G.Y. Screening and adsorption mechanisms on substrates for phosphorus removal in constructed wetlands. Tongji University, 2007.
- Chakravarty S., Mohanty A., Sudha T.N., Upadhyay A.K., Konar J., Sircar J.K., Madhukar A., Gupta K.K. Removal of Pb (II) ions from aqueous solution by adsorption using bael leaves (Aegle marmelos). Journal of Hazardous Materials. 2010, 173, 502-509. [CrossRef]
- Luo L.Q., Tu X., Zhou P.F. Preparation and application of sludge ceramsite prepared from sludge. China Mining Magazine. 2018, 27, 154-160. [CrossRef]
- He J., Wang Q.S., Ren A.L. Technology research on the production of high strength ceramsite by waterworks sewage. Industrial Safety and Environmental Protection. 2010, 36, 51-52. [CrossRef]
- Ren X., Wang J.Q., Guo M.Q., Zhao X.S. Adsorption of Cr6+ in the water by ceramsite made from urban waterworks sludge. Jilin Normal University Journal(Natural Science Edition). 2017, 38, 113-118.
- Chen Y. Study on preparation ceramsite in water supply sludge and it’s application of phpsphorus removal. Shandong University, 2017.
- Meng P.P. Study of light-weight aggregates-based constructed wetlands for wastewater treatment and development of sludge media. Shandong University, 2017.
- Zheng Y., Yu Y., Li Y., Wu C., Liu W., Liu C. Adsorption of phosphorus or ammonia nitrogen by ceramsite made from waterworks sludge. Chinese Journal of Environmental Engineering. 2015, 9, 756-762.
- Liu Q.D., Zhou Z.M., Zhang H.Z., Fei L., Xie B., Li S., Wan B. Phosphorus removal characteristics of calcined water treatment plant sludge. Environmental Chemistry. 2019, 38, 95-103. [CrossRef]
- Li X.L., Marella T.K., Tao L., Dai L., Peng L., Song C., Li G. The application of ceramsite ecological floating bed in aquaculture: Its effects on water quality, phytoplankton, bacteria and fish production. Water Science and Technology. 2018, 77, 2742-2745. [CrossRef]
- Babatunde A.O., Kumar J. L.G., Zhao Y. Constructed wetlands using aluminium-based drinking water treatment sludge as P-removing substrate: Should aluminium release be a concern? Journal of Environmental Monitoring. 2011, 13, 1775.
- Li Z., Jiang N., Wu F., Zou Z. Experimental investigation of phosphorus adsorption capacity of the waterworks sludges from five cities in China. Ecological Engineering. 2013, 53, 165-172. [CrossRef]
- Chen T. Treatment and utilization of sludge. Science News. 2002, 11.


| Name | Equation | Parameters |
|---|---|---|
| Pseudo-first order kinetic model |
|
qe, Qt: the amounts of adsorbed P at equilibrium and at time t (min), respectively (mg/g); K1: the first-order rate constant (min/L). |
| Pseudo-second order kinetic model | K2: the second-order rate constant (g/mg min). | |
| Intra-particle diffusion model | K3: the intra-particle diffusion rate constant (min-1). | |
| Langmuir Isotherm |
Qe: the mass of P adsorbed on adsorbent at equilibrium (mg/g); Ce: the equilibrium concentration of P solution (mg/L); Qm: the maximum adsorption capacity (mg/g); K: Langmuir constant (l/mg), a measure of the affinity of the adsorbate for the adsorbent. |
|
| Freundlich Isotherm |
Kf : the Freundlich constant (l/g) related to the bonding energy; 1/n: the heterogeneity factor. |
| Materials | p/p°range |
Total pore volumes (cm3/g) |
Average pore diameters (nm) |
BET surface area (m2/g) |
R2 |
|---|---|---|---|---|---|
| adASB | 0.0626-0.1098 | 0.066 | 2.412 | 110.23 | 0.9996 |
| ASC | 0.06209-0.224 | 0.224 | 3.540 | 253.29 | 0.9997 |
| Material | qesj | Pseudo-first-order kinetics | Pseudo-second-order kinetics | Intra-particle diffusion | ||||||
| qe | K1 | R2 | qe | K2 | R2 | R2(first stage) | K3 | R2(second stage) | ||
| adASB | 0.418 | 0.357 | 0.0016 | 0.980 | 0.432 | 0.022 | 0.999 | 0.958 | 0.014 | 0.924 |
| ASC | 0.403 | 0.332 | 0.0014 | 0.978 | 0.418 | 0.020 | 0.999 | 0.952 | 0.014 | 0.921 |
| Material | Langmuir | Freundlich | ||||
| Qm | K | R2 | Kf | 1/n | R2 | |
| adASB | 1.89 | 0.11 | 0.985 | 0.257 | 0.57 | 0.967 |
| ASC | 1.66 | 0.11 | 0.986 | 0.229 | 0.56 | 0.954 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




