Submitted:
23 May 2023
Posted:
25 May 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Epidemiology
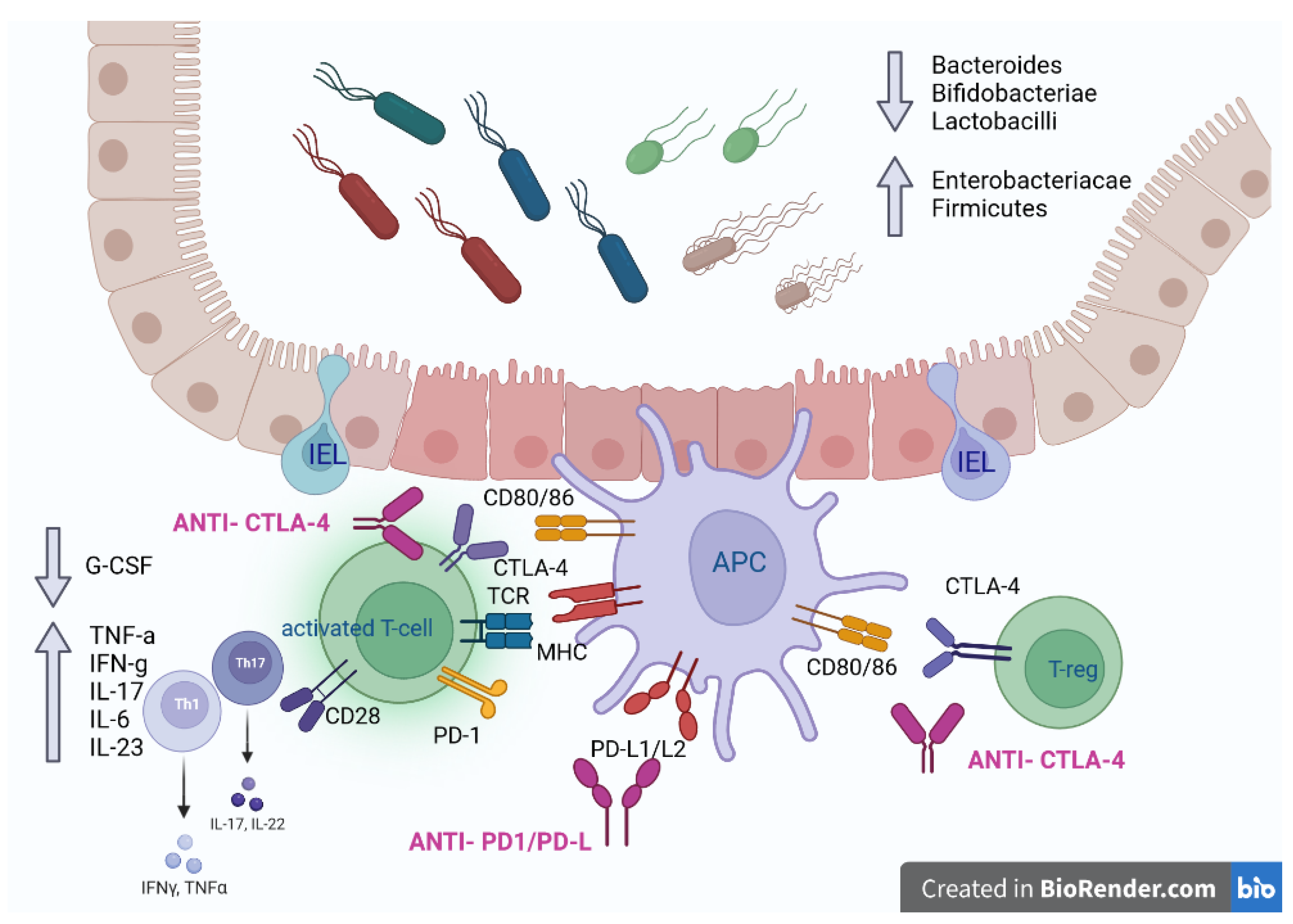
3. Pathogenesis of checkpoint inhibitors colitis (CIC)
3.1. On target effects
3.2. Off target effects
3.3. Host-related factors
3.4. CIC and IBD: shared pathogenesis
4. Diagnosis
4.1. Diagnostic work up
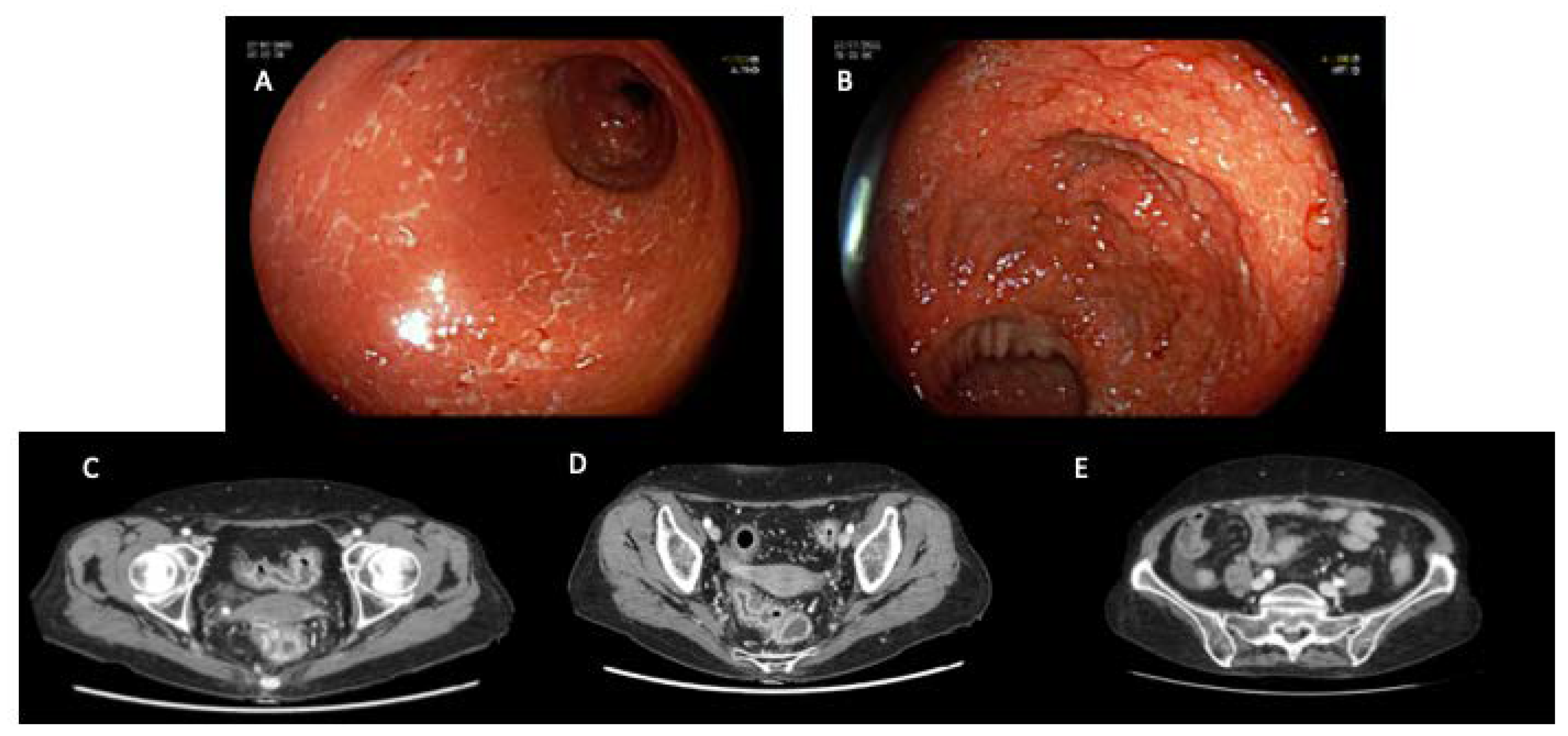
4.2. Histological diagnosis and histological variants
4.3. Imaging
5. Management
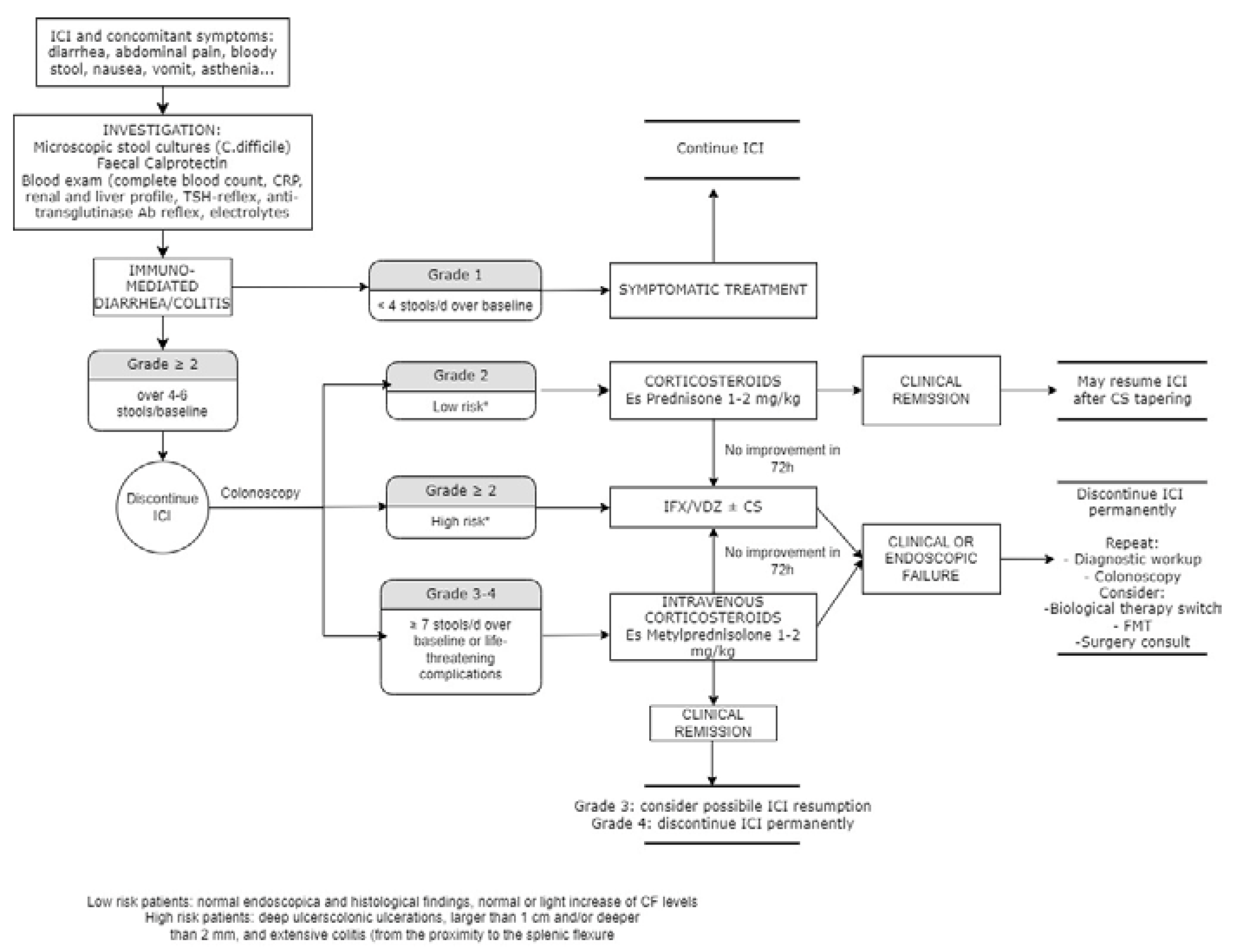
5.1. Rechallenge of ICIs and risk of relapse
5.2. ICIs treatment in IBD patients
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- F. Bray, M. Laversanne, E. Weiderpass, and I. Soerjomataram. The ever-increasing importance of cancer as a leading cause of premature death worldwide. Cancer 2021, 127. [Google Scholar]
- Herbst Daniel Morgensztern & Chris Boshoff Roy, S. The biology and management of non-small cell lung cancer. Nature 2018.
- Y. Zhang and Z. Zhang. The history and advances in cancer immunotherapy: understanding the characteristics of tumor-infiltrating immune cells and their therapeutic implications. Cellular and Molecular Immunology 2020, 17. [Google Scholar]
- H. Raskov, A. Orhan, J. P. Christensen, and I. Gögenur. Cytotoxic CD8+ T cells in cancer and cancer immunotherapy. British Journal of Cancer 2021, 124, 359–367. [Google Scholar] [CrossRef]
- D. Hanahan and R. A. Weinberg. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar]
- H. Ledford. Melanoma drug wins US approval. Nature 2011, 471. [Google Scholar]
- F. S. Hodi et al. Improved Survival with Ipilimumab in Patients with Metastatic Melanoma. N. Engl. J. Med. 2010, 363, 711–723. [Google Scholar] [CrossRef] [PubMed]
- R. J. Motzer et al. Nivolumab versus Everolimus in Advanced Renal-Cell Carcinoma. N. Engl. J. Med. 2015, 373, 1803–1813. [Google Scholar] [CrossRef]
- S. Bagchi, R. S. Bagchi, R. Yuan, and E. G. Engleman. Immune Checkpoint Inhibitors for the Treatment of Cancer: Clinical Impact and Mechanisms of Response and Resistance. 2021.
- J. R. Brahmer et al. Society for immunotherapy of cancer (sitc) clinical practice guideline on immune checkpoint inhibitor-related adverse events. J. Immunother. Cancer 2021, 9. [Google Scholar]
- M. Ramos-Casals et al. Immune-related adverse events of checkpoint inhibitors. Nature Reviews Disease Primers 2020, 6. [Google Scholar]
- A. N. Tran et al. Immune Checkpoint Inhibitor-associated Diarrhea and Colitis: A Systematic Review and Meta-analysis of Observational Studies.” 2021.
- N. C. Institute. Common Terminology Criteria for Adverse Events (CTCAE).” 2017.
- L. Khoja, D. Day, T. Wei-Wu Chen, L. L. Siu, and A. R. Hansen. Tumour- and class-specific patterns of immune-related adverse events of immune checkpoint inhibitors: A systematic review. Annals of Oncology 2017, 28. [Google Scholar]
- J. J. Wright, A. C. Powers, and D. B. Johnson. Endocrine toxicities of immune checkpoint inhibitors. Nature Reviews Endocrinology 2021, 17, 389–399. [Google Scholar] [CrossRef] [PubMed]
- P. Arnaud-Coffin et al. A systematic review of adverse events in randomized trials assessing immune checkpoint inhibitors. International Journal of Cancer 2019, 145, 639–648. [Google Scholar] [CrossRef]
- J. A. Seidel, A. Otsuka, and K. Kabashima. Anti-PD-1 and anti-CTLA-4 therapies in cancer: Mechanisms of action, efficacy, and limitations. Frontiers in Oncology 2018.
- A. Tarhini. Immune-Mediated Adverse Events Associated with Ipilimumab CTLA-4 Blockade Therapy: The Underlying Mechanisms and Clinical Management. Scientifica (Cairo). 2013, 2013, 1–19. [Google Scholar]
- J. S. Weber, K. C. Kähler, and A. Hauschild. Management of immune-related adverse events and kinetics of response with ipilimumab. Journal of Clinical Oncology 2012, 30, 2691–2697. [Google Scholar] [CrossRef] [PubMed]
- K. E. Beck et al. Enterocolitis in patients with cancer after antibody blockade of cytotoxic T-lymphocyte-associated antigen 4. J. Clin. Oncol. 2006, 24. [Google Scholar]
- H. Abu-Sbeih, F. S. Ali, and Y. Wang. Immune-checkpoint inhibitors induced diarrhea and colitis: A review of incidence, pathogenesis and management. Current Opinion in Gastroenterology 2020, 36, 25–32. [Google Scholar]
- L. Tang et al. Immune Checkpoint Inhibitor-Associated Colitis: From Mechanism to Management. Frontiers in Immunology 2021, 12. [Google Scholar]
- F. Martins et al. Adverse effects of immune-checkpoint inhibitors: epidemiology, management and surveillance. Nature Reviews Clinical Oncology 2019, 16. [Google Scholar]
- L. Marthey et al. Cancer immunotherapy with anti-CTLA-4 monoclonal antibodies induces an inflammatory bowel disease. J. Crohn’s Colitis 2016, 10. [Google Scholar]
- D. Y. Wang et al. Fatal Toxic Effects Associated With Immune Checkpoint Inhibitors: A Systematic Review and Meta-analysis. JAMA Oncol. 2018, 4, 1721–1728. [Google Scholar] [CrossRef]
- M. S. Hughes et al. Budesonide treatment for microscopic colitis from immune checkpoint inhibitors. J. Immunother. Cancer 2019, 7. [Google Scholar]
- A. Haryal et al. Immune checkpoint inhibitor gastritis is often associated with concomitant enterocolitis, which impacts the clinical course. Cancer 2023, 129, 367–375. [Google Scholar]
- A. Som et al. Immune checkpoint inhibitor-induced colitis: A comprehensive review. World Journal of Clinical Cases 2019, 7. [Google Scholar]
- E. De Martin et al. Characterization of liver injury induced by cancer immunotherapy using immune checkpoint inhibitors. J. Hepatol. 2018, 68, 1181–1190. [Google Scholar] [CrossRef]
- M. Porcu et al. Immune Checkpoint Inhibitor-Induced Pancreatic Injury: Imaging Findings and Literature Review. Targeted Oncology 2020, 15, 25–35. [Google Scholar] [CrossRef]
- R. Barroso-Sousa et al. Incidence of endocrine dysfunction following the use of different immune checkpoint inhibitor regimens a systematic review and meta-analysis. JAMA Oncology 2018, 4. [Google Scholar]
- J. J. Koldenhof and K. P. M. Suijkerbuijk. Diarrhoea during checkpoint blockade, not always colitis. European Journal of Cancer 2017, 87. [Google Scholar]
- “Immunomodulators - Cancer Research Institute (CRI).”.
- L. Spain, S. Diem, and J. Larkin. Management of toxicities of immune checkpoint inhibitors. Cancer Treatment Reviews 2016, 44, 51–60. [Google Scholar]
- C. Li, P. Jiang, S. Wei, X. Xu, and J. Wang. Regulatory T cells in tumor microenvironment: New mechanisms, potential therapeutic strategies and future prospects. Molecular Cancer 2020, 19. [Google Scholar]
- D. Nagorsen, C. Scheibenbogen, F. M. Marincola, A. Letsch, and U. Keilholz. Natural T cell immunity against cancer. Clin. Cancer Res. 2003, 9, 4296–4303. [Google Scholar]
- O. S. Qureshi et al. Trans-endocytosis of CD80 and CD86: A molecular basis for the cell-extrinsic function of CTLA-4. Science 2011, 332. [Google Scholar]
- K. Wing et al. CTLA-4 control over Foxp3+ regulatory T cell function. Science 2008, 322. [Google Scholar]
- J. M. Moreau, M. Velegraki, C. Bolyard, M. D. Rosenblum, and Z. Li. Transforming growth factor-β1 in regulatory T cell biology. Science immunology 2022, 7. [Google Scholar]
- A. H. Sharpe and K. E. Pauken. The diverse functions of the PD1 inhibitory pathway. Nature Reviews Immunology 2018, 18. [Google Scholar]
- E. I. Buchbinder and A. Desai. CTLA-4 and PD-1 pathways similarities, differences, and implications of their inhibition. American Journal of Clinical Oncology: Cancer Clinical Trials 2016, 39. [Google Scholar]
- D. S. Thommen and T. N. Schumacher. T Cell Dysfunction in Cancer. Cancer Cell 2018, 33. [Google Scholar]
- C. H. June, J. T. Warshauer, and J. A. Bluestone. Is autoimmunity the Achilles’ heel of cancer immunotherapy? Nature Medicine 2017, 23. [Google Scholar]
- R. Poto et al. Holistic Approach to Immune Checkpoint Inhibitor-Related Adverse Events. Frontiers in Immunology 2022, 13. [Google Scholar]
- T. Passat, Y. Touchefeu, N. Gervois, A. Jarry, C. Bossard, and J. Bennouna. Physiopathological mechanisms of immune-related adverse events induced by anti-CTLA-4, anti-PD-1 and anti-PD-L1 antibodies in cancer treatment. Bulletin du Cancer 2018, 105. [Google Scholar]
- A. G. Solimando et al. Immune checkpoint inhibitor-related myositis: From biology to bedside. International Journal of Molecular Sciences 2020, 21. [Google Scholar]
- D. B. Johnson et al. Fulminant Myocarditis with Combination Immune Checkpoint Blockade. N. Engl. J. Med. 2016, 375. [Google Scholar]
- L. Flatz et al. Association of Checkpoint Inhibitor-Induced Toxic Effects with Shared Cancer and Tissue Antigens in Non-Small Cell Lung Cancer. JAMA Oncol. 2019, 5. [Google Scholar]
- K. C. Williams et al. Immune-related adverse events in checkpoint blockade: Observations from human tissue and therapeutic considerations. Frontiers in Immunology 2023, 14. [Google Scholar]
- A. M. Luoma et al. Molecular Pathways of Colon Inflammation Induced by Cancer Immunotherapy. Cell 2020, 182. [Google Scholar]
- T. N. A. I. E. S. S. K. K.Yoshino. Severe colitis after PD-1 blockade with nivolumab in advanced melanoma patients: Potential role of Th1-dominant immune response in immune-related adverse events: Two case reports. BMC Cancer 2019, 19. [Google Scholar]
- C. Coutzac et al. Colon immune-related adverse events: Anti-CTLA-4 and anti-PD-1 blockade induce distinct immunopathological entities. J. Crohn’s Colitis 2017, 11. [Google Scholar]
- K. Klocke, S. Sakaguchi, R. Holmdahl, and K. Wing. Induction of autoimmune disease by deletion of CTLA-4 in mice in adulthood. Proc. Natl. Acad. Sci. U. S. A. 2016, 113. [Google Scholar]
- E. A. Tivol, F. Borriello, A. N. Schweitzer, W. P. Lynch, J. A. Bluestone, and A. H. Sharpe. Loss of CTLA-4 leads to massive lymphoproliferation and fatal multiorgan tissue destruction, revealing a critical negative regulatory role of CTLA-4. Immunity 1995, 3. [Google Scholar]
- G. Kim et al. Spontaneous Colitis Occurrence in Transgenic Mice with Altered B7-Mediated Costimulation. J. Immunol. 2008, 181. [Google Scholar]
- D. Tegtmeyer, M. Seidl, P. Gerner, U. Baumann, and C. Klemann. Inflammatory bowel disease caused by primary immunodeficiencies—Clinical presentations, review of literature, and proposal of a rational diagnostic algorithm. Pediatric Allergy and Immunology 2017, 28. [Google Scholar]
- C. Schwab et al. Phenotype, penetrance, and treatment of 133 cytotoxic T-lymphocyte antigen 4–insufficient subjects. J. Allergy Clin. Immunol. 2018, 142. [Google Scholar]
- K. Okla, D. L. Farber, and W. Zou. Tissue-resident memory T cells in tumor immunity and immunotherapy. Journal of Experimental Medicine 2021, 218. [Google Scholar]
- S. C. Sasson et al. Mucosal-associated invariant T (MAIT) cells are activated in the gastrointestinal tissue of patients with combination ipilimumab and nivolumab therapy-related colitis in a pathology distinct from ulcerative colitis. Clin. Exp. Immunol. 2020, 202. [Google Scholar]
- S. C. Sasson et al. Interferon-Gamma–Producing CD8+ Tissue Resident Memory T Cells Are a Targetable Hallmark of Immune Checkpoint Inhibitor–Colitis. Gastroenterology 2021, 161. [Google Scholar]
- Y. Takahashi et al. CD8+ Lymphocyte Infiltration Is a Specific Feature of Colitis Induced by Immune Checkpoint Inhibitors. Dig. Dis. Sci. 2023, 68. [Google Scholar]
- A. Sharma et al. Anti-CTLA-4 immunotherapy does not deplete Foxp3 þ regulatory T cells (Tregs) in human cancers. Clin. Cancer Res. 2019, 25. [Google Scholar]
- M. Iglesias-Escudero, N. Arias-González, and E. Martínez-Cáceres. Regulatory cells and the effect of cancer immunotherapy. Molecular Cancer 2023, 22. [Google Scholar]
- F. R. Mariotti, L. Quatrini, E. Munari, P. Vacca, and L. Moretta. Innate lymphoid cells: Expression of PD-1 and other checkpoints in normal and pathological conditions. Frontiers in Immunology 2019, 10. [Google Scholar]
- R. G. Domingues and M. R. Hepworth. Immunoregulatory Sensory Circuits in Group 3 Innate Lymphoid Cell (ILC3) Function and Tissue Homeostasis. Frontiers in Immunology 2020, 11. [Google Scholar]
- N. Ghosh, K. K. Chan, B. Jivanelli, and A. R. Bass. Autoantibodies in Patients with Immune-Related Adverse Events from Checkpoint Inhibitors: A Systematic Literature Review. Journal of Clinical Rheumatology 2022, 28. [Google Scholar]
- I. Les et al. Association of immune-related adverse events induced by nivolumab with a battery of autoantibodies. Ann. Med. 2021, 53. [Google Scholar]
- S. Iwama, A. De Remigis, M. K. Callahan, S. F. Slovin, J. D. Wolchok, and P. Caturegli. Pituitary expression of CTLA-4 mediates hypophysitis secondary to administration of CTLA-4 blocking antibody. Sci. Transl. Med. 2014, 6. [Google Scholar]
- J. H. Kang, J. A. Bluestone, and A. Young. Predicting and Preventing Immune Checkpoint Inhibitor Toxicity: Targeting Cytokines. Trends in Immunology 2021, 42. [Google Scholar]
- A. Ceschi, R. Noseda, K. Palin, and K. Verhamme. Immune Checkpoint Inhibitor-Related Cytokine Release Syndrome: Analysis of WHO Global Pharmacovigilance Database. Front. Pharmacol. 2020, 11. [Google Scholar]
- G. Bamias et al. Immunological Characteristics of Colitis Associated with Anti-CTLA-4 Antibody Therapy. Cancer Invest. 2017, 35. [Google Scholar]
- A. A. Tarhini et al. Baseline circulating IL-17 predicts toxicity while TGF-β1 and IL-10 are prognostic of relapse in ipilimumab neoadjuvant therapy of melanoma. J. Immunother. cancer 2015, 3, 39. [Google Scholar]
- K. Tyan et al. Cytokine changes during immune-related adverse events and corticosteroid treatment in melanoma patients receiving immune checkpoint inhibitors. Cancer Immunol. Immunother. 2021, 70. [Google Scholar]
- J. Lo et al. P001 Immune checkpoint inhibitor-induced colitis is mediated by polyfunctional lymphocytes and is dependent on the IL23/IFNg axis. J. Crohn’s Colitis 2022, 16. [Google Scholar]
- Y. Zhou et al. Intestinal toxicity to CTLA-4 blockade driven by IL-6 and myeloid infiltration. J. Exp. Med. 2023, 220. [Google Scholar]
- S. Khan et al. Immune dysregulation in cancer patients developing immune-related adverse events. Br. J. Cancer 2019, 120. [Google Scholar]
- R. Tokunaga et al. CXCL9, CXCL10, CXCL11/CXCR3 axis for immune activation – A target for novel cancer therapy. Cancer Treatment Reviews 2018, 63. [Google Scholar]
- M. F. Neurath. Targeting immune cell circuits and trafficking in inflammatory bowel disease. Nature Immunology 2019, 20. [Google Scholar]
- K. P. Shah et al. Demographic factors associated with toxicity in patients treated with anti-programmed cell death-1 therapy. Cancer Immunol. Res. 2020, 8. [Google Scholar]
- K. Tyan et al. Association of vitamin D intake with decreased risk of immune checkpoint inhibitor-induced colitis. J. Clin. Oncol. 2020, 38. [Google Scholar]
- H. K. Akturk, D. Kahramangil, A. Sarwal, L. Hoffecker, M. H. Murad, and A. W. Michels. Immune checkpoint inhibitor-induced Type 1 diabetes: a systematic review and meta-analysis. Diabetic Medicine 2019, 36. [Google Scholar]
- J. Luo et al. Immunotherapy-Mediated Thyroid Dysfunction: Genetic Risk and Impact on Outcomes with PD-1 Blockade in Non–Small Cell Lung Cancer. Clin. Cancer Res. 2021, 27. [Google Scholar]
- C. Campbell, M. R. Kandalgaonkar, R. M. Golonka, B. S. Yeoh, M. Vijay-Kumar, and P. Saha. Crosstalk between Gut Microbiota and Host Immunity: Impact on Inflammation and Immunotherapy. Biomedicines 2023, 11. [Google Scholar]
- W. Li, Y. Deng, Q. Chu, and P. Zhang. Gut microbiome and cancer immunotherapy. Cancer Letters 2019, 447. [Google Scholar]
- N. Chaput et al. Baseline gut microbiota predicts clinical response and colitis in metastatic melanoma patients treated with ipilimumab. Ann. Oncol. 2017, 28. [Google Scholar]
- K. Dubin et al. Intestinal microbiome analyses identify melanoma patients at risk for checkpoint-blockade-induced colitis. Nat. Commun. 2016, 7. [Google Scholar]
- O. Oey, Y.-Y. Liu, A. F. Sunjaya, D. M. Simadibrata, M. A. Khattak, and E. Gray. Gut microbiota diversity and composition in predicting immunotherapy response and immunotherapy-related colitis in melanoma patients: A systematic review. World J. Clin. Oncol. 2022, 13. [Google Scholar]
- T. Sakurai et al. Integrative analysis of gut microbiome and host transcriptomes reveals associations between treatment outcomes and immunotherapy-induced colitis. Mol. Oncol. 2022, 16. [Google Scholar]
- M. Yuksel et al. A novel ‘humanized mouse’ model for autoimmune hepatitis and the association of gut microbiota with liver inflammation. Hepatology 2015, 62. [Google Scholar]
- M. Saresella et al. Immunological and clinical effect of diet modulation of the gut microbiome in multiple sclerosis patients: A pilot study. Front. Immunol. 2017, 8. [Google Scholar]
- X. Liu et al. Role of the Gut Microbiome in Modulating Arthritis Progression in Mice. Sci. Rep. 2016, 6. [Google Scholar]
- T. Wang et al. Probiotics Lactobacillus reuteri Abrogates Immune Checkpoint Blockade-Associated Colitis by Inhibiting Group 3 Innate Lymphoid Cells. Front. Immunol. 2019, 10. [Google Scholar]
- Q. Mu, V. J. Tavella, and X. M. Luo. Role of Lactobacillus reuteri in human health and diseases. Frontiers in Microbiology 2018, 9. [Google Scholar]
- M. Vétizou et al. Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science 2015, 350. [Google Scholar]
- S. Dasgupta, D. Erturk-Hasdemir, J. Ochoa-Reparaz, H. C. Reinecker, and D. L. Kasper. Plasmacytoid dendritic cells mediate anti-inflammatory responses to a gut commensal molecule via both innate and adaptive mechanisms. Cell Host Microbe 2014, 15. [Google Scholar]
- A. Sivan et al. Commensal Bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy. Science 2015, 350. [Google Scholar]
- F. Wang, Q. Yin, L. Chen, and M. M. Davis. Bifidobacterium can mitigate intestinal immunopathology in the context of CTLA-4 blockade. Proc. Natl. Acad. Sci. U. S. A. 2018, 115. [Google Scholar]
- B. Routy, V. Gopalakrishnan, R. Daillère, L. Zitvogel, J. A. Wargo, and G. Kroemer. The gut microbiota influences anticancer immunosurveillance and general health. Nature Reviews Clinical Oncology 2018, 15. [Google Scholar]
- N. Arpaia et al. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature 2013, 504. [Google Scholar]
- H. R. Wardill, R. J. Chan, A. Chan, D. Keefe, S. P. Costello, and N. H. Hart. Dual contribution of the gut microbiome to immunotherapy efficacy and toxicity: supportive care implications and recommendations. Supportive Care in Cancer 2022, 30. [Google Scholar]
- A. Shirwaikar Thomas, S. Hanauer, and Y. Wang. Immune Checkpoint Inhibitor Enterocolitis vs Idiopathic Inflammatory Bowel Disease. Clinical Gastroenterology and Hepatology 2023.
- M. Y. Song et al. Protective effects of Fc-fused PD-L1 on two different animal models of colitis. Gut 2015, 64. [Google Scholar]
- J. Robertson et al. Intestinal APCs of the endogenous nanomineral pathway fail to express PD-L1 in Crohn’s disease. Sci. Rep. 2016, 6. [Google Scholar]
- M. Zhang et al. Association of CTLA-4 variants with susceptibility to inflammatory bowel disease: A meta-analysis. Human Immunology 2014, 75. [Google Scholar]
- B. L. Adler et al. Histopathological and immunophenotypic features of ipilimumab-associated colitis compared to ulcerative colitis. J. Intern. Med. 2018, 283. [Google Scholar]
- X. C. Morgan et al. Dysfunction of the intestinal microbiome in inflammatory bowel disease and treatment. Genome Biol. 2012, 13. [Google Scholar]
- A. Swidsinski, J. Weber, V. Loening-Baucke, L. P. Hale, and H. Lochs. Spatial organization and composition of the mucosal flora in patients with inflammatory bowel disease. J. Clin. Microbiol. 2005, 43. [Google Scholar]
- D. Knights et al. Complex host genetics influence the microbiome in inflammatory bowel disease. Genome Med. 2014, 6. [Google Scholar]
- H. Li, Z. Y. Fu, M. E. Arslan, H. Lee, and D. Cho. Differential diagnosis and management of immune checkpoint inhibitor-induced colitis: A comprehensive review. World Journal of Experimental Medicine 2021, 11. [Google Scholar]
- M. Dougan, Y. Wang, A. Rubio-Tapia, and J. K. Lim. AGA Clinical Practice Update on Diagnosis and Management of Immune Checkpoint Inhibitor Colitis and Hepatitis: Expert Review. Gastroenterology 2021, 160. [Google Scholar]
- M. H. Geukes Foppen et al. Immune checkpoint inhibition-related colitis: Symptoms, endoscopic features, histology and response to management. ESMO Open 2018, 3. [Google Scholar]
- B. J. Schneider et al. Management of Immune-Related Adverse Events in Patients Treated with Immune Checkpoint Inhibitor Therapy: ASCO Guideline Update. Journal of Clinical Oncology 2021, 39. [Google Scholar]
- N. Powell et al. British Society of Gastroenterology endorsed guidance for the management of immune checkpoint inhibitor-induced enterocolitis. Lancet Gastroenterol. Hepatol. 2020, 5, 679–697. [Google Scholar] [CrossRef]
- J. Ni, X. Zhang, and L. Zhang. Opportunistic bowel infection after corticosteroid dosage tapering in a stage IV lung cancer patient with tislelizumab-related colitis. Thorac. Cancer 2020, 11. [Google Scholar]
- C. Zhou, Y. Klionsky, M. E. Treasure, and D. S. Bruno. Pembrolizumab-Induced Immune-Mediated Colitis in a Patient with Concurrent Clostridium Difficile Infection. Case Rep. Oncol. 2019, 12. [Google Scholar]
- C. Vuillamy et al. Clostridium difficile infection and immune checkpoint inhibitor–induced colitis in melanoma: 18 cases and a review of the literature. Melanoma Res. 2023, 33, 192–198. [Google Scholar]
- A. Hoadley, N. Sandanayake, and G. V. Long. Atrophic exocrine pancreatic insufficiency associated with anti-PD1 therapy. Annals of Oncology 2017, 28. [Google Scholar]
- Y. R. Badran et al. Immune checkpoint inhibitor-associated celiac disease. J. Immunother. Cancer 2020, 8. [Google Scholar]
- A. R. Abolhassani, G. Schuler, M. C. Kirchberger, and L. Heinzerling. C-reactive protein as an early marker of immune-related adverse events. J. Cancer Res. Clin. Oncol. 2019, 145. [Google Scholar]
- Y. Fujisawa et al. Fluctuations in routine blood count might signal severe immune-related adverse events in melanoma patients treated with nivolumab. J. Dermatol. Sci. 2017, 88. [Google Scholar]
- Y. Nakamura et al. Correlation between blood cell count and outcome of melanoma patients treated with anti-PD-1 antibodies. Jpn. J. Clin. Oncol. 2019, 49. [Google Scholar]
- G. Manson, J. Norwood, A. Marabelle, H. Kohrt, and R. Houot. Biomarkers associated with checkpoint inhibitors. Ann. Oncol. 2016, 27. [Google Scholar]
- K. Schindler et al. Correlation of absolute and relative eosinophil counts with immune-related adverse events in melanoma patients treated with ipilimumab. J. Clin. Oncol. 2014, 32. [Google Scholar]
- S. Meshkibaf, A. J. Martins, G. T. Henry, and S. O. Kim. Protective role of G-CSF in dextran sulfate sodium-induced acute colitis through generating gut-homing macrophages. Cytokine 2016, 78, 69–78. [Google Scholar] [CrossRef]
- H. Abu-Sbeih, F. S. Ali, W. Luo, W. Qiao, G. S. Raju, and Y. Wang. Importance of endoscopic and histological evaluation in the management of immune checkpoint inhibitor-induced colitis. J. Immunother. Cancer 2018, 6. [Google Scholar]
- F. Zou et al. Fecal calprotectin concentration to assess endoscopic and histologic remission in patients with cancer with immune-mediated diarrhea and colitis. J. Immunother. Cancer 2021, 9. [Google Scholar]
- Y. Wang et al. Endoscopic and Histologic Features of Immune Checkpoint Inhibitor-Related Colitis. Inflamm. Bowel Dis. 2018, 24. [Google Scholar]
- A. P. Wright, M. S. Piper, S. Bishu, and R. W. Stidham. Systematic review and case series: flexible sigmoidoscopy identifies most cases of checkpoint inhibitor-induced colitis. Alimentary Pharmacology and Therapeutics 2019, 49. [Google Scholar]
- J. D. Herlihy et al. Flexible Sigmoidoscopy Rather than Colonoscopy Is Adequate for the Diagnosis of Ipilimumab-Associated Colitis. South. Med. J. 2019, 112. [Google Scholar]
- C. Ma et al. Recommendations for standardizing biopsy acquisition and histological assessment of immune checkpoint inhibitor-associated colitis. J. Immunother. Cancer 2022, 10. [Google Scholar]
- V. Desmedt et al. Position statement on the management of the immune checkpoint inhibitor-induced colitis via multidisciplinary modified Delphi consensus. Eur. J. Cancer 2023, 187, 36–57. [Google Scholar] [CrossRef]
- J. A. Thompson et al. Management of immunotherapy-related toxicities, version 1.2020 featured updates to the NCCN guidelines. JNCCN J. Natl. Compr. Cancer Netw. 2020, 18. [Google Scholar]
- K. Kubo, M. Kato, and K. Mabe. Nivolumab-Associated Colitis Mimicking Ulcerative Colitis. Clin. Gastroenterol. Hepatol. 2017, 15. [Google Scholar]
- E. Bellaguarda and S. Hanauer. Checkpoint Inhibitor–Induced Colitis. Am. J. Gastroenterol. 2020, 115, 202–210. [Google Scholar] [CrossRef]
- L. Spain et al. Patterns of steroid use in diarrhoea and/or colitis (D/C) from immune checkpoint inhibitors (ICPI). Ann. Oncol. 2016, 27. [Google Scholar]
- K. Choi et al. Can Immune Checkpoint Inhibitors Induce Microscopic Colitis or a Brand New Entity? Inflamm. Bowel Dis. 2019, 25. [Google Scholar]
- B. Baroudjian et al. Anti-PD1-induced collagenous colitis in a melanoma patient. Melanoma Res. 2016, 26. [Google Scholar]
- M. J. Mooradian et al. Mucosal inflammation predicts response to systemic steroids in immune checkpoint inhibitor colitis. J. Immunother. Cancer 2020, 8. [Google Scholar]
- P. A. Patil and X. Zhang. Pathologic manifestations of gastrointestinal and hepatobiliary injury in immune checkpoint inhibitor therapy. Arch. Pathol. Lab. Med. 2021, 145. [Google Scholar]
- M. L. Zhang, A. Neyaz, D. Patil, J. Chen, M. Dougan, and V. Deshpande. Immune-related adverse events in the gastrointestinal tract: diagnostic utility of upper gastrointestinal biopsies. Histopathology 2020, 76. [Google Scholar]
- U. N. Shivaji et al. Immune checkpoint inhibitor-associated gastrointestinal and hepatic adverse events and their management. Therapeutic Advances in Gastroenterology 2019, 12. [Google Scholar]
- D. M. Karamchandani and R. Chetty. Immune checkpoint inhibitor-induced gastrointestinal and hepatic injury: Pathologists’ perspective. J. Clin. Pathol. 2018, 71. [Google Scholar]
- S. Sakellariou, D. N. Zouki, D. C. Ziogas, D. Pouloudi, H. Gogas, and I. Delladetsima. Granulomatous colitis in a patient with metastatic melanoma under immunotherapy: a case report and literature review. BMC Gastroenterol. 2021, 21. [Google Scholar]
- J. H. Chen, M. K. Pezhouh, G. Y. Lauwers, and R. Masia. Histopathologic features of colitis due to immunotherapy With Anti-PD-1 antibodies. Am. J. Surg. Pathol. 2017, 41. [Google Scholar]
- P. Bavi, M. Butler, S. Serra, and R. Chetty. Immune modulator-induced changes in the gastrointestinal tract. Histopathology 2017, 71. [Google Scholar]
- R. A. Isidro et al. Medication-specific variations in morphological patterns of injury in immune check-point inhibitor-associated colitis. Histopathology 2021, 78. [Google Scholar]
- A. García-Varona, R. D. Odze, and F. Makrauer. Lymphocytic colitis secondary to ipilimumab treatment. Inflammatory Bowel Diseases 2013, 19. [Google Scholar]
- P. E. S. Romain, A. K. S. Salama, N. Lynn Ferguson, and R. A. Burbridge. Ipilimumab-Associated lymphocytic colitis: A case report. Transl. Gastroenterol. Hepatol. 2016, 5. [Google Scholar]
- A. Gallo et al. Collagenous colitis and atezolizumab therapy: an atypical case. Clin. J. Gastroenterol. 2021, 14. [Google Scholar]
- R. S. Gonzalez, S. N. Salaria, C. D. Bohannon, A. R. Huber, M. M. Feely, and C. Shi. PD-1 inhibitor gastroenterocolitis: case series and appraisal of ‘immunomodulatory gastroenterocolitis. Histopathology 2017, 70. [Google Scholar]
- R. Del Sordo, V. Lougaris, G. Bassotti, A. Armuzzi, and V. Villanacci. Therapeutic agents affecting the immune system and drug-induced inflammatory bowel disease (IBD): A review on etiological and pathogenetic aspects. Clin. Immunol. 2022, 234, 108916. [Google Scholar] [CrossRef]
- V. Villanacci et al. Histopathology of IBD colitis. A practical approach from the pathologists of the Italian group for the study of the gastrointestinal tract (GIPAD). Pathologica 2021, 113. [Google Scholar]
- R. Yamauchi et al. The characteristics of nivolumab-induced colitis: An evaluation of three cases and a literature review. BMC Gastroenterol. 2018, 18. [Google Scholar]
- R. Celli, H. M. Kluger, and X. Zhang. Anti-PD-1 Therapy-Associated Perforating Colitis. Case Rep. Gastrointest. Med. 2018, 2018. [Google Scholar]
- V. T. F. Cheung et al. Immune checkpoint inhibitor-related colitis assessment and prognosis: can IBD scoring point the way? Br. J. Cancer 2020, 123. [Google Scholar]
- B. Gosangi et al. Imaging features of toxicities associated with immune checkpoint inhibitors. Eur. J. Radiol. Open 2022, 9. [Google Scholar]
- A. R. Barina, M. R. Bashir, B. A. Howard, B. A. Hanks, A. K. Salama, and T. A. Jaffe. Isolated recto-sigmoid colitis: a new imaging pattern of ipilimumab-associated colitis. Abdom. Radiol. 2016, 41. [Google Scholar]
- M. Garcia-Neuer et al. Diagnostic comparison of CT scans and colonoscopy for immune-related colitis in ipilimumab-treated advanced melanoma patients. Cancer Immunol. Res. 2017, 5. [Google Scholar]
- A. C. Shieh et al. Imaging and clinical manifestations of immune checkpoint inhibitor-related colitis in cancer patients treated with monotherapy or combination therapy. Abdom. Radiol. 2020, 45. [Google Scholar]
- S. M. Durbin et al. Diagnostic utility of CT for suspected immune checkpoint inhibitor enterocolitis. J. Immunother. cancer 2020, 8. [Google Scholar]
- N. Pisuchpen et al. Multi-detector computed tomography (MDCT)–based severity score as a prognostic tool in patients with suspected immune checkpoint inhibitor therapy associated colitis. Eur. Radiol. 2021, 31. [Google Scholar]
- M. H. G. Foppen et al. Immune checkpoint inhibition-related colitis: Symptoms, endoscopic features, histology and response to management. ESMO Open 2018, 3. [Google Scholar]
- Z. Gong and Y. Wang. Immune Checkpoint Inhibitor-Mediated Diarrhea and Colitis: A Clinical Review.” 2020.
- J. Weber et al. A randomized, double-blind, placebo-controlled, phase II study comparing the tolerability and efficacy of ipilimumab administered with or without prophylactic budesonide in patients with unresectable stage III or IV melanoma. Clin. Cancer Res. 2009, 15, 5591–5598. [Google Scholar]
- H. Abu-Sbeih et al. Impact of antibiotic therapy on the development and response to treatment of immune checkpoint inhibitor-mediated diarrhea and colitis. J. Immunother. Cancer 2019, 7. [Google Scholar]
- J. L. Alexander et al. Oral beclomethasone dipropionate is an effective treatment for immune checkpoint inhibitor induced colitis. J. Immunother. Cancer 2022, 10. [Google Scholar]
- J. Burla et al. Retrospective Analysis of Treatment and Complications of Immune Checkpoint Inhibitor-Associated Colitis: Histological Ulcerations as Potential Predictor for a Steroid-Refractory Disease Course. Inflamm. Intest. Dis. 2020, 5. [Google Scholar]
- M. Ding et al. Treatment and outcomes of immune checkpoint inhibitors-associated colitis/diarrhea: A systematic review and meta-analysis. Digestive and Liver Disease. Elsevier B.V., 2023.
- H. Abu-Sbeih et al. Early introduction of selective immunosuppressive therapy associated with favorable clinical outcomes in patients with immune checkpoint inhibitor-induced colitis. J. Immunother. Cancer 2019, 7. [Google Scholar]
- H. Abu-Sbeih and Y. Wang. Management Considerations for Immune Checkpoint Inhibitor-Induced Enterocolitis Based on Management of Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2020, 26, 662–668. [Google Scholar] [CrossRef] [PubMed]
- D. H. Johnson et al. Infliximab associated with faster symptom resolution compared with corticosteroids alone for the management of immune-related enterocolitis. J. Immunother. Cancer 2018, 6. [Google Scholar]
- Y. Wang et al. Immune-checkpoint inhibitor-induced diarrhea and colitis in patients with advanced malignancies: Retrospective review at MD Anderson. J. Immunother. Cancer 2018, 6. [Google Scholar]
- S. T. van Turenhout et al. Cytomegalovirus in Steroid-Refractory Immune Checkpoint Inhibition–Related Colitis. Journal of Thoracic Oncology 2020, 15, e15–e20. [Google Scholar] [CrossRef]
- H. Abu-Sbeih et al. Outcomes of vedolizumab therapy in patients with immune checkpoint inhibitor-induced colitis: A multi-center study. J. Immunother. Cancer 2018, 6. [Google Scholar]
- F. Zou et al. Efficacy and safety of vedolizumab and infliximab treatment for immune-mediated diarrhea and colitis in patients with cancer: A two-center observational study. J. Immunother. Cancer 2021, 9. [Google Scholar]
- E. Zhang, C. Kiely, N. Sandanayake, and S. Tattersall. Calcineurin inhibitors in steroid and anti-TNF-alpha refractory immune checkpoint inhibitor colitis. JGH Open 2021, 5, 558–562. [Google Scholar] [CrossRef]
- A. S. Thomas, W. Ma, and Y. Wang. Ustekinumab for Refractory Colitis Associated with Immune Checkpoint Inhibitors. N. Engl. J. Med. 2021, 384, 581–583. [Google Scholar] [CrossRef] [PubMed]
- R. B. Holmstroem et al. Tofacitinib and faecal microbiota transplantation in treating checkpoint inhibitor-induced enterocolitis: case report. BMJ Open Gastroenterol. 2022, 9, e000989. [Google Scholar] [CrossRef] [PubMed]
- M. Chen et al. Fecal Microbiota Transplantation Effectively Cures a Patient With Severe Bleeding Immune Checkpoint Inhibitor-Associated Colitis and a Short Review. Frontiers in Oncology 2022, 12. [Google Scholar]
- Y. Wang et al. Fecal microbiota transplantation for refractory immune checkpoint inhibitor-associated colitis. Nat. Med. 2018, 24, 1804–1808. [Google Scholar] [CrossRef] [PubMed]
- T. K. T. T. T. N. Keiichi Mitsuyama Ryosuke Yamauchi. Characteristic of Immune Checkpoint Inhibitor-Induced Colitis: A Systematic review. Kurume Med. Jounal 2021.
- C. Dolladille et al. Immune Checkpoint Inhibitor Rechallenge after Immune-Related Adverse Events in Patients with Cancer. JAMA Oncol. 2020, 6, 865–871. [Google Scholar] [CrossRef]
- A. Simonaggio et al. Evaluation of Readministration of Immune Checkpoint Inhibitors after Immune-Related Adverse Events in Patients with Cancer. JAMA Oncol. 2019, 5, 1310–1317. [Google Scholar] [CrossRef]
- Q. Zhao et al. Safety and Efficacy of the Rechallenge of Immune Checkpoint Inhibitors After Immune-Related Adverse Events in Patients With Cancer: A Systemic Review and Meta-Analysis. Frontiers in Immunology 2021, 12.
- H. Abu-Sbeih et al. Resumption of Immune Checkpoint Inhibitor Therapy After Immune-Mediated Colitis. J Clin Oncol 2019, 37, 2738–2745. [Google Scholar] [CrossRef]
- H. Abu-Sbeih et al. Immune Checkpoint Inhibitor Therapy in Patients With Preexisting Inflammatory Bowel Disease. J Clin Oncol 2019, 38, 576–583. [Google Scholar]
- J. Meserve, A. Facciorusso, A. K. Holmer, V. Annese, W. J. Sandborn, and S. Singh. Systematic review with meta-analysis: safety and tolerability of immune checkpoint inhibitors in patients with pre-existing inflammatory bowel diseases. Alimentary Pharmacology and Therapeutics 2021, 53, 374–382. [Google Scholar] [CrossRef] [PubMed]
- J. Sleiman, W. Wei, R. Shah, M. S. Faisal, J. Philpott, and P. Funchain. Incidence of immune checkpoint inhibitor-mediated diarrhea and colitis (imDC) in patients with cancer and preexisting inflammatory bowel disease: A propensity score-matched retrospective study. J. Immunother. Cancer 2021, 9. [Google Scholar]
- S. Grimsdottir, M. Attauabi, E. Kristine Dahl, J. Burisch, and J. B. Seidelin. Systematic Review with Meta-analysis: The Impact of Cancer Treatments on the Disease Activity of Inflammatory Bowel Diseases. J. Crohn’s Colitis 2023.
- A. Amiot et al. Management of immune checkpoint inhibitor in patients with cancer and pre-existing inflammatory bowel disease: Recommendations from the GETAID. Digestive and Liver Disease 2022, 54. [Google Scholar]
| Immune checkpoint inhibitor | Target | Date of Approval (year) | Indications (by FDA/EMA) [33] | Immune-related Adverse events [16,34] | Colitis [12,16,34] |
|---|---|---|---|---|---|
| Ipilimumab | CTLA-4 | 2010 | In combination with nivolumab: Previously treated MSI-H/dMMR Metastatic CRC, HCC, intermediate and poo-risk advanced RCC, malignant pleural mesothelioma, Mycosis fungoides, Sezary Syndrome, NSCLC Alone: late-stage melanoma |
Diarrhea 36% (31-41) Rash 23% Hepatitis 5% Hyperthiroidism 4% Hypophisitis 4% Hypothiroidism 3% Pneumonitis 1% |
All grade: 8% (6-10) Grade 3-4: 5% (4-6) |
| Nivolumab | PD-1 | 2015 | In combination with ipilimumab: late-stage melanoma, NSCLC, RCC, CRC, malignant pleural mesothelioma, HCC Alone: late-stage melanoma, NSCLC, RCC, HNCC, Classic Hodgkin Lymphoma, Esophageal Cancer, gastroesophageal cancer, urothelial carcinoma |
Diarrhea 11% Rash 10% Hypothyroidism 8% Hepatitis 5% Hyperthyroidism 5% Pneumonitis 4% Hypohysitis 1% |
All grade: 1% Grade 3-4: 1% |
| Pembrolizumab | PD-1 | 2016 | Late-stage melanoma, NSCLC, CRC, HCC, RCC, HNCC, Cervical Cancer, endometrial cancer, Classic Hodgkin lymphoma, large B-cell lymphoma, Esophageal Cancer, Gastric cancer, urothelial carcinoma, MSI-H/dMMR/TMB-H cancers, CSCC, Merkel Cell Carcinoma, BC | ||
| Cemiplimab | PD-1 | 2018 | CSCC, BCC, NSCLC | ||
| Atezolizumab | PD-L1 | 2016 | Melanoma, NSCLC, SCLC, HCC, Urothelial carcinoma, BC | ||
| Durvalumab | PD-L1 | 2016 | HCC, biliary tract, NSCLC, SCLC, urothelial carcinoma | ||
| Avelumab | PD-L1 | 2017 | RCC, Urothelial carcinoma, Merkel Cell carcinoma |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





