1. Introduction
The use of extracorporeal membrane oxygenation (ECMO) as a supportive/rescue therapy for refractory cardiac or respiratory failure has increased dramatically over the last decade [
1]. ECMO has shown survival benefits but carries a substantial risk of acute brain injury (ABI), which can lead to increased morbidity and mortality [
2].
The brain is susceptible to injury in response to decreased blood supply due to its high metabolic demand. The term “ABI” includes ischemic stroke, hypoxic-ischemic brain injury (HIBI), intracerebral hemorrhage, subdural hemorrhage, subarachnoid hemorrhage, and brain death. Targeted clinical [
3,
4,
5] and pathologic studies [
6,
7,
8] report a much higher prevalence of ABI in ECMO patients compared to registry studies e.g., that supported by the Extracorporeal Life Support Organization (ELSO). Even non-registry studies underestimate the frequency of ABI due to a lack of standardized neuromonitoring and the challenges of imaging patients on ECMO [
8,
9,
10,
11]. Of note is that ABI in the setting of ECMO approximately doubles an already-high mortality risk [
8,
9]. In a retrospective analysis of brain autopsy in ECMO patients, HIBI was reported as the most common type of ABI in ECMO. HIBI presents as global hypoxic-ischemic encephalopathy associated with widespread brain injury noted more in the cerebral cortices (82%) than in the subcortical and infratentorial areas (i.e., cerebellum 55%, brainstem 36% and basal ganglia 18%) [
12]. In ECMO supported patients, HIBI presents as significant neurological impairment, ranging in severity from mild cognitive deficits, persistent vegetative state to coma [
13,
14].
Despite numerous studies looking at ABI in ECMO patients, specific data pertaining to HIBI is sparse. Gaining a better understanding of the prevalence, mechanisms, and neuromonitoring of HIBI in ECMO might significantly improve our management of these critically ill individuals. In this narrative review, we aim to synthesize the available literature on HIBI in ECMO patients, focusing on the prevalence, risk factors, underlying pathophysiology, outcomes, neuromonitoring strategies, therapeutic techniques and future directions. Our goal is to contribute to the development of effective clinical practices that decrease the burden of HIBI in ECMO patients.
2. Clinical Evidence of HIBI in ECMO
2.1. Epidemiology
ABI is one of the most common complications of ECMO, though prevalence varies significantly by both diagnostic methodology and subpopulation [
9,
15]. In a meta-analysis by Shoskes et al., ABI was reported in 16% of patients on ECMO, half of which were HIBI [
16]. When a standardized neuromonitoring protocol was used in a single retrospective analysis, ABI was diagnosed in 33% of ECMO patients, 35% of which were characterized by HIBI [
17]. Through direct tissue examination, an autopsy study of 25 ECMO patients by Cho et al. identified ABI in 68% of patients, two-thirds of which were characterized by HIBI [
12]. Furthermore, in a detailed analysis comparing the risks with different cannulation strategies, more cases of ABI and HIBI were reported with venoarterial ECMO (VA-ECMO) compared to venovenous ECMO (VV-ECMO), for ABI: 19% vs 10%; (p = 0.002) and for HIBI: 13% vs 1%; (p < 0.001) [
16]. Meanwhile, in a meta-analysis of extracorporeal cardiopulmonary resuscitation (eCPR), Migdady et al. reported ABI in 27% patients, 23% of which was constituted by HIBI [
2]. Although there are inconsistencies in the definition and reporting methodology, the prevalence of ABI and the portion of those patients suffereing from HIBI appears substantial.
2.2. Timing and Etiology of HIBI
In clinical terms, HIBI can be attributed to two primary factors: a diffuse perfusion deficit and/or a global hypoxic insult to the brain[
18]. The timing of HIBI varies, occurring 1) before ECMO cannulation; 2) during ECMO support; or 3) during the decannulation period [
8,
11].
For VA-ECMO patients, the inciting event surrounding cannulation derives from poor oxygen delivery and includes cardiac arrest or severe shock such as that due to post-cardiotomy shock, cardiomyopathy, myocardial infarction, cardiac arrhythmia, fulminant myocarditis, or massive pulmonary embolism [
19,
20,
21]. On the other hand, VV-ECMO can cause HIBI as a result of hypoxic perfusion, not hypoperfusion, and often is associated with acute respiratory distress syndrome (ARDS) and/or progression or exacerbation of underlying chronic pulmonary disease. The underlying causes of ARDS include bacterial, viral or fungal pneumonia, aspiration pneumonitis, or pancreatitis [
22].
While patients are on VA-ECMO support, lack of pulsatile blood flow in and of itself can be associated with ABI. In a recent study[
23], HIBI occurred in 34% of patients with ABI and low pulse pressure was an independent risk factor. The mechanism is thought to be endothelial dysfunction, decreased cerebral oxygen consumption with disruption of cerebral autoregulation [
24]. Differential hypoxia (also known as Harlequin or North-South syndrome) is another etiology for HIBI unique to peripherally cannulated VA-ECMO patients with hypoxic respiratory failure coupled with cardiogenic shock (
Figure 1). It has been reported in 8.8% of all VA-ECMO patients, and represents an avoidable cause of HIBI [
25]. In the context of hypoxic respiratory failure, the ejection of deoxygenated blood from left ventricle (either by the ventricle itself or a percutaneous left ventricular assist device) can lead to hypoxic cerebral perfusion and a global brain injury. The therapeutic options to manage this phenomenon include: optimization of oxygen delivery to the native lung (e.g., through ventilator manipulation), changing to a central cannulation via the ascending aorta or the right subclavian artery, veno-arteriovenous cannulation (VAV)-ECMO, or decannulation versus de-escalation to VV-ECMO if there is sufficient cardiac recovery [
26].
For patients on VV-ECMO support, recirculation and low ECMO-flow-to-cardiac-output ratio are two common underlying etiologies for persistent peripheral systemic hypoxemia [
27] which can result in HIBI. Normally, after passing through the oxygenator, oxygenated blood is directed back into the body at a level at or near the right atrium. From there, it passes through the tricuspid valve and enters the pulmonary circulation. Recirculation occurs when the oxygenated ECMO outflow is drained by the ECMO inflow cannula, preventing it from effectively returning to the right ventricle, leading to futile recirculation of ECMO flow. The sine qua non of recirculation is an abnormally high venous drainage O
2 saturation [
28]. If recirculation is not a concern, appropriate systemic Hb-O
2 saturation is a function of the ratio of ECMO-flow-to-cardiac-output flow. Often the patient’s native output is supranormal while there is a limit to the maximum ECMO flows that can be achieved. An excess in native cardiac output relative to ECMO flow will thus result blood bypassing the ECMO circuit, effectively creating shunt and worsening hypoxemia.
In both VV- and VA-ECMO patients, issues such as significant blood loss, air entrainment, low pump flow or oxygenator thrombus can result in ECMO failure, leading to systemic desaturation or hypoperfusion and increasing the risk of HIBI [
27].
HIBI can also occur in the peri-decannulation period. The decannulation process itself can cause physiological stress and instability. Risk of end-organ hypoperfusion with neurological complications must be guarded against when weaning from ECMO [
29].
2.3. Risk factors
The risk factors for ABI vary due to inherent practice differences across ECMO centers including the devices used, anticoagulation strategies, and individual patient differences [
30,
31]. There are seven studies that have explored risk factors associated with ABI in ECMO, but only four studies specifically address the risk factors for ABI due to HIBI [
16,
17,
23]. (
Table 1)
In patients undergoing VA-ECMO, a history of hypertension is associated with ABI and HIBI, possibly the result of a rightward shift of the cerebral autoregulation curve leading to hypoperfusion at blood pressures normally counteracted by vasodilatory mechanisms. In this setting, higher levels of blood pressure may be required to maintain adequate cerebral perfusion [
6]. Furthermore, high lactate levels on day 1 of ECMO and low pH (acidosis) during this peri-ECMO cannulation period—both of which are markers of systemic hypoperfusion—were other risk factors seen.[
12]. Additional risk factors linked to ABI that might potentially be linked to HIBI are an early low pulse pressure (<20%) or a loss of pulsatility which is associated with endothelial dysfunction, decreased local oxygen consumption, and disruption of cerebral autoregulation, which increases the risk of ABI and HIBI [
23]. A high pre-cannulation PaCO
2 and large peri-cannulation PaCO
2 drop (∆PaCO
2) were also associated with an increased ABI risk [
17]. Acute decreases in PaCO
2 induce vasoconstriction of cerebral vessels, which decreases cerebral blood flow (CBF) and increases the risk of ischemia [
32,
33,
34]. Hypocapnia also increases neuronal excitability and cerebral metabolic demand, exacerbated by any decreased CBF, increasing the risk of ABI [
32,
35,
36]. In a retrospective analysis by Shou et al., severe hyperoxia (≥300 mm Hg) following ECMO initiation was associated with ABI and mortality [
37]. Therefore, optimization of ABG parameters during ECMO has the potential to decrease ABI and improve survival by increasing carbon dioxide clearance.
In patients undergoing VV-ECMO, risk factors for ABI and HIBI include lower pH, hypoxemia during the peri-cannulation period and markers of coagulation disturbances (D-dimer, fibrinogen) [
38].
2.4. Outcome
No studies have specifically investigated the neurological outcome of HIBI in ECMO. However, there are some studies that have identified factors related to neurological prognosis of patients undergoing ECMO cannulation for cardiac or respiratory arrest, which are known to be the main causes of HIBI. A meta-analysis by Migdady et al. reported that 24% of patients with eCPR achieved good neurologic outcome. This meta-analysis also showed 27% patients had at least one neurologic complication, of which 85% was constituted by HIBI [
2]. In a retrospective analysis, Chambrun et al. reported 27% one-year survival rate among patients treated with VA-ECMO after refractory shock post-cardiac arrest, and all of them achieved a favorable neurologic outcome[
39]. Low hemoglobin or high serum lactic acid levels before ECMO, and prolonged interval from cardiac arrest to ECMO initiation were associated with poor neurological outcomes after successful eCPR [
40]. Poor neurological prognosis is perceived as an important cause of withdrawal of life sustaining therapy (WLST) within 72 hours after ECMO cannulation. In a study by Carlson et al., early WLST was observed in more than half of the eCPR patients. Early WLST prevents a complete understanding of prevalence, timing and mechanism of HIBI and consequent neurological complications in ECMO patients [
41]. Further clinical studies are warranted to define the relationship of HIBI to outcome in ECMO patients.
3. Preclinical models of HIBI in ECMO
A limited number of pre-clinical models that investigate HIBI in ECMO exist (
Table 2). While there are 21 preclinical studies that investigated neurological outcomes in eCPR, we identified three studies that specifically examined HIBI in ECMO. Foerster et al. investigated the effect of anticoagulation during eCPR on neurological outcomes. In this study, 12 pigs (six with anticoagulation, six without anticoagulation) were placed on VA-ECMO after 15 minutes of cardiac arrest. Neurological outcomes were assessed using Neurological Deficit Score (NDS), electroencephalography (EEG), magnetic resonance imaging (MRI), and histological examination. Brain histology after seven days of cardiac arrest revealed moderate hypoxic-ischemic damage in both groups, as evidenced by appearance of dark neurons and eosinophilic neurons in hippocampus, cerebellum, and frontal lobe, however still in the reversible state of brain ischemia [
42]. Although this study demonstrated that eCPR intervention after 15 minutes of cardiac arrest was associated with a moderate degree of HIBI based on histological findings and that anticoagulation did not mitigate HIBI, the study does not allow for distinction between the impact of eCPR versus that of the arrest and associated global hypoxic injury itself on HIBI.
In a study of 14 pigs performed by Putzer et al., low-flow VA-ECMO was initiated after 8 minutes of cardiac arrest. After 10 minutes, adrenaline was continuously infused to achieve mean arterial pressure (MAP) of 40 or 60. Low-flow ECMO prior to adrenaline administration resulted in an inadequate MAP and cerebral perfusion pressure (CPP). This insufficiency contributed to HIBI, as indicated by increased levels of cerebral microdialysis markers (lactate, pyruvate, and lactate to pyruvate ratio). Adrenaline infusion increased MAP, CPP, regional CBF, and cerebral oxygen supply, thereby mitigating hypoxic-ischemic brain damage during low-flow eCPR [
43]. This study showed that after an 8-minute-long cardiac arrest, HIBI could be mitigated by enhancing MAP and CPP through the use of adrenaline during VA-ECMO therapy. More recently, Rozencwajg et al. studied the effect of VA-ECMO flow on brain injury in 6 sheep. After inducing severe cardiorespiratory failure, the animals were randomized into 2 groups—3 with low-flow at 2.5L/min and 3 with high-flow at 4.5L/min. Neurological outcomes were thoroughly evaluated by examining brain hemodynamics, oxygenation, metabolism, and histology after 6 hours. Although both groups exhibited a similar pattern of HIBI on histology, the low-flow group had significantly more severe histological brain injury, characterized by neuronal shrinkage, congestion, and perivascular edema. An increase in cerebral metabolites suggestive of anaerobic metabolism, such as lactate, pyruvate, and the lactate-to-pyruvate ratio further corroborated the presence of HIBI in the low flow group. Inadequate oxygenation, as observed through continuous brain tissue oxygen (PbtO
2) and near-infrared spectroscopy (NIRS), was consistent with hypoxia and hypoperfusion seen in HIBI in the low-flow group [
44].
In summary, preclinical studies investigating HIBI in ECMO appear to demonstrate mitigation of HIBI resulting from the institution of ECMO with adequate flow and blood pressure. Whether ECMO in and of itself contributes to HIBI is unanswered, although its benefits clearly outweigh its drawbacks when looking at oxygen delivery and the overall prevention of an ischemic/hypoxic brain injury. Lacking is the determination of optimal targets for important physiological variables such as pH, O2, CO2, MAP, and temperature. There is a pressing need for more preclinical research that employs multimodal neuromonitoring strategies to evaluate neurological outcomes and improve our understanding of HIBI in the setting of ECMO rescue and support.
4. Pathophysiology
The pathophysiology of HIBI is complex as it depends on the underlying pathology necessitating the use of ECMO along with the risk of HIBI associated with ECMO support itself.
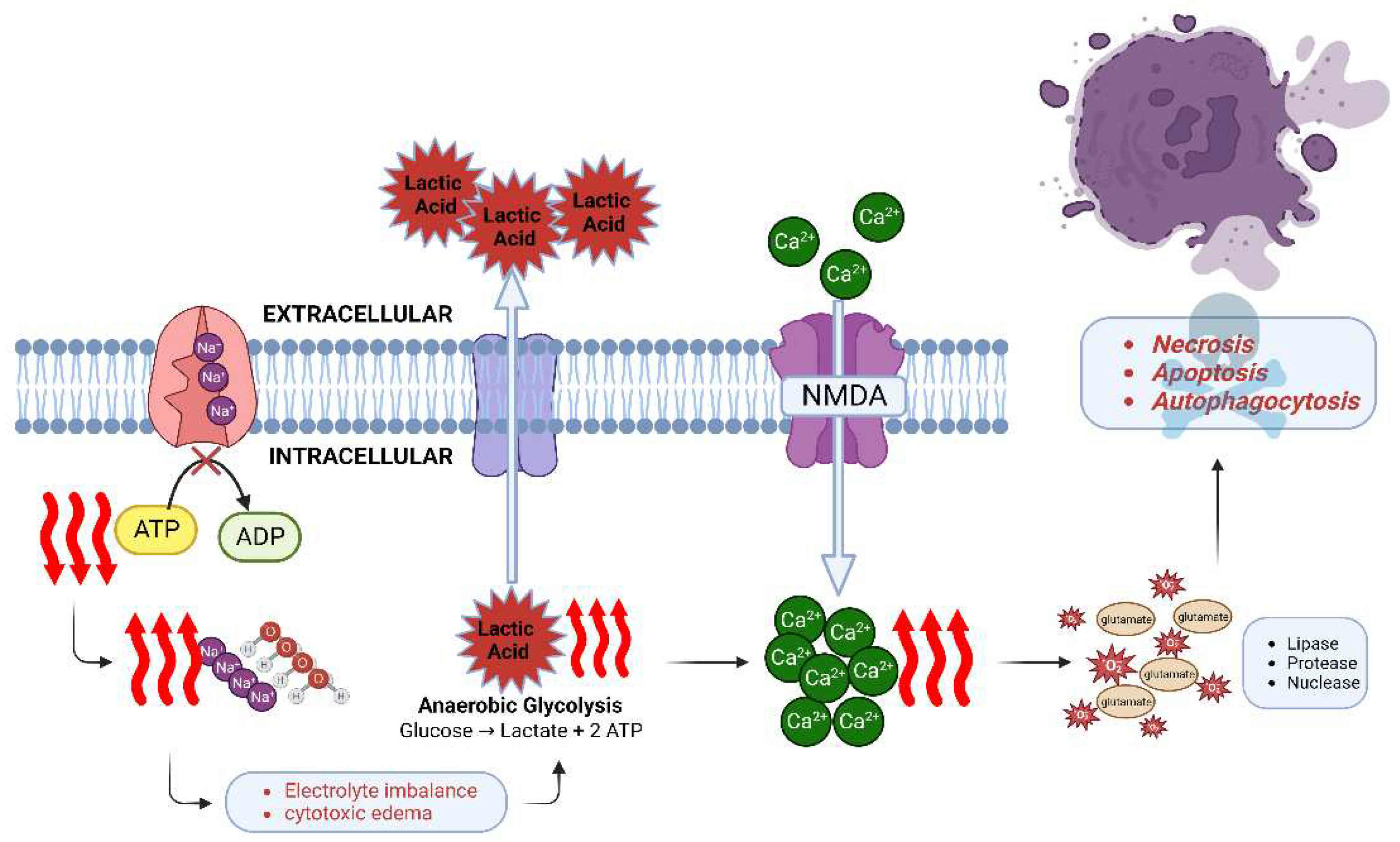
At the molecular cellular level, sudden cessation of CBF initiates the cascade of neuronal ischemia and subsequent cell death within minutes [
45]. Energy production is impaired and adenosine triphosphate (ATP) stores are quickly depleted. The ATP-dependent Na+/K+ ATPase can no longer maintain electrolyte homeostasis, sodium and water begin to accumulate inside the cell leading to cytotoxic edema [
46]. Oxidative phosphorylation shifts to anaerobic glycolysis [
47] with a resultant accumulation of lactate, leading to intracellular and extracellular acidosis and further impairing cell function. Neuronal ischemia activates the N-methyl-D-aspartate (NMDA) receptor which causes an influx of calcium ions. Together with release of excitotoxic neurotransmitter glutamate and formation of reactive oxygen species, numerous enzymes are released and activated, such as lipases, proteases, and nucleases, which accelerate cell death [
48,
49]. Three types of neuronal death have been described [
18]: necrosis, apoptosis and autophagocytosis. Both calcium influx and NMDA receptor activation contribute, and in combination with the production of nitric oxide and free radicals, apoptosis and autophagocytosis occur (
Figure 2).
At the functional cellular level, HIBI causes mitochondrial dysfunction which impairs ATP production and increases free radical synthesis. Mitochondrial dysfunction can dismantle cell structures (for example demyelination and cytoskeletal damage), impair the blood brain barrier, and halt synaptic transmission. HIBI can upregulate (e.g., Bcl-2, HSP) or downregulate various genes to protect against or facilitate cell damage caused by mitochondrial dysfunction [
18,
50].
At the tissue level, the ECMO circuit converts the blood into a prothrombotic and proinflammatory state (
Figure 1). Together with the pre-ECMO low flow state, and the subsequent continuous non-pulsatile flow mode, all these can result in endothelial injury, microcirculation disruption and then eventually cerebral autoregulation impairment [
51]. In this context, CBF becomes extremely sensitive to systemic blood pressure and vulnerable to blood pressure changes as a result of loss of cerebral autoregulation. The patient with pre-ECMO essential hypertension is especially at risk given that the cerebral autoregulation curve has already been shifted to the right [
6,
12].
5. Neuromonitoring
5.1. Serial neurologic examination
The serial neurologic examination is essential in evaluating acute neurologic changes. While sedation minimization is frequently a goal, sedation remains a necessity for many patients supported by ECMO, thus a complete neurological examination is not always possible. Noninvasive neuromonitoring may be crucial in these patients with impaired consciousness as it has the ability to rapidly detect ABI [
52,
53]. In a study conducted by Ong et al., an increased detection of ABI (from 23% to 33%) and improved neurological outcomes at discharge (from 30% to 54%) were observed with the introduction of standardized neuromonitoring for ECMO patients[
54].
5.2. EEG
EEG is a noninvasive tool that measures cortical electrical activity with spatial and temporal resolution and is sensitive to changes in brain structure and function[
52]. EEG monitoring can be intermittent or continuously to evaluate changes in either generalized or focal background. In a meta-analysis study by Perera et al., EEG was highly specific for disability and death when status epilepticus, burst suppression, or electrocerebral silence was reported in comatose patients with HIBI secondary to cardiac arrest [
55]. Additionally, continuous EEG (cEEG) indicating moderate encephalopathy (diffuse slowing with reactivity/variability) and seizure or generalized periodic discharges is associated with a poor outcome in cardiac arrest [
56]. Meanwhile, a recent study by Hwang et al. in comatose ECMO patients showed no epileptiform discharges or seizures, even though the cEEG was performed in the absence of confounding sedating medication. The study also found that intact reactivity, present state changes, and fair/good variability may be associated with survival at hospital discharge. This study implies these features are a better approach than relying on the presence of “highly malignant” patterns for neurological prognosis [
57,
58]. According to “The American Clinical Neurophysiology Society Consensus Statement on EEG (cEEG) in Critically Ill Adults and Children”, patients on ECMO requiring sedation and paralysis are designated as a high-risk group that should be monitored by cEEG [
59]. The widespread use of cEEG in comatose ECMO patients has the potential to be beneficial in assessing the degree of brain injury and monitoring seizures and brain function, as well as predict neurological outcomes [
52].
5.3. Cerebral NIRS
Cerebral NIRS noninvasively measures regional oxygen saturation (rSO
2) by determining the relative concentrations of oxygenated and deoxygenated hemoglobin in the cerebral circulation [
60]. Since HIBI is caused by decreased blood flow and oxygen to the brain, NIRS measurement of rSO
2 can be used as a diagnostic tool. Several studies [
61,
62,
63] have shown that NIRS is capable of detecting ABI in ECMO patients. For example, a significant (>25%) drop from baseline, as well as frequency, duration, and burden of desaturations noted on NIRS all have been related to ABI on ECMO. However, lack of standardization of the monitors and variable sensitivity under differing patient conditions, at least at the moment, makes the readings non-specific. Thresholds for intervention are therefore unclear [
64]. With no specific NIRS data on ECMO-associated HIBI, more studies are warranted to define the utility of NIRS in this group of patients.
5.4. SSEP
Somatosensory evoked potentials (SSEPs) are neurophysiological monitoring tool that apply mild electrical stimulation in peripheral nerve and measures the electrical responses along the somatosensory pathway [
65]. Negative peak at 20ms (N20) is the first component recorded from the cortex in median nerve SSEPs [
66]. The European Resuscitation Council (ERC) and the European Society of Intensive Care Medicine (ESICM) guidelines include an absence of the N20 SSEP wave among the most robust predictors to be tested at 72 hr after return of spontaneous circulation (ROSC) in cardiac arrest patients[
67,
68]. Also, bilaterally absent cortical SSEP response is a very reliable predictor of poor neurologic outcome in patients with HIBI. However, SSEP has a high specificity, but a low sensitivity. In other words, even many patients doomed to a poor neurological outcome have a bilaterally present N20 SSEP wave[
69]. To address this limitation, several studies measured amplitude changes in the N20 wave at specific time points or combined bilateral absence of the cortical N20 with N20-P25 threshold amplitude results to obtain better prognostic neurological outcome [
70,
71]. Furthermore, given the sensitivity of SSEP to a wide range of physiologic factors (blood flow, blood pressure, hematocrit, hypoxia, hypercarbia, increased intracranial pressure (ICP) and commonly used analgosedative agents (e.g., midazolam, propofol, opioids, ketamine)[
72,
73], SSEP should be cautiously interpreted in ECMO patients. To date, no studies have utilized SSEP alone to predict neurological outcomes in ECMO population. Given the high potential for SSEP to be used as a neurological outcome predictor in ECMO patients, further studies on this topic are required [
70,
71].
5.5. SSEP and EEG
SSEPs in combination with EEG findings predict neurological outcomes better than SSEP alone. For example, in cardiac arrest patients, the combination of high N20 amplitude with a benign EEG increased the sensitivity of predicting a favorable neurological outcome from 61% to 91% [
74]. A study by Cho et al. analyzed the combined results of SSEP and EEG for predicting neurological outcomes in ECMO patients. ECMO patients with poor neurological outcomes demonstrated a loss of EEG reactivity despite a relatively preserved EEG background and preservation of the SSEP response. This triad of neurophysiological findings may represent a pattern of ECMO-specific brain injury termed “ECMO brain” and suggests that ABI in ECMO patients appears to exhibit a different neurological injury pattern than is seen in other common neurologic injuries [
75].
5.6. Transcranial doppler
Transcranial doppler (TCD) is a traditional neuromonitoring tool that enables the measurement of blood flow velocity in major cerebral arteries. It is commonly utilized in the assessment of cerebrovascular disorders such as ischemic stroke, subarachnoid hemorrhage, and cerebral vasospasm. However, given its mechanism of action, TCD is not applicable to monitoring HIBI patients, in whom the pathology is mainly related to global hypoxia. Instead, TCD is used to monitor ECMO circuit clot, cerebral emboli detection, and cerebral hemodynamics[
76,
77].
5.7. Plasma Biomarkers
Brain injury biomarkers, such as neuron-specific enolase (NSE) and calcium-binding protein B (S-100B), have been commonly examined to evaluate brain injury in post-cardiac arrest patients [
78]. European guidelines suggest NSE as the preferred biomarker in neuroprognostication[
79]. However, hemolysis occurs frequently in ECMO, which can lead to false-positive NSE results [
80]. The half-life for NSE and S100B is approximately 24 hr and 2 hr, respectively. Therefore, it is expected that S-100B peaks earlier in the circulation after HIBI than NSE [
81]. A systematic review by Wang et al. demonstrated that early S100B or late NSE value functioned as reliable prognostic indicators for post-cardiac arrest patients and the specificity was consistently high regardless of the timing of measurement [
82]. Similarly, in children requiring ECMO, a study of six brain injury biomarkers found that NSE and glial fibrillary acidic protein (GFAP) were significantly associated with unfavorable outcomes [
83]. These findings suggest that NSE, S100B, and GFAP can be useful in predicting neurological outcomes. A more recent meta-analysis study by Hoiland et al. identified neurofilament light (Nf-L) had the highest diagnostic accuracy in predicting unfavorable neurological outcome in patients with HIBI following cardiac arrest. Tau, a marker which reflects axonal injury, had greater diagnostic accuracy compared with NSE or S-100B [
84]. The prognostic value of biomarkers varies in different meta-analysis studies, emphasizing the need for caution when interpreting results. Furthermore, the complexity of brain injury in patients receiving ECMO is unlikely to be captured by a single biomarker. Biomarkers may help predict prognosis as part of a multidisciplinary evaluation that includes imaging and clinical assessment to increase sensitivity [
24]. Future studies may need to investigate a panel of biomarkers for each category to better understand the nature of brain injury, or combine multiple biomarkers to improve diagnostic accuracy in the ECMO population. Also, when using biomarkers for neurological prognostication, the timing of biomarker draws should be considered.
5.8. Imaging
5.8.1. Brain CT
Brain computed tomography (CT) is a valuable diagnostic tool for HIBI in ECMO patients. The greatest benefit is that the examination can be performed even while maintaining ECMO. The test can be conducted immediately upon detection of neurological changes, allowing rapid acquisition and early diagnosis. However, there are certain limitations, including a low resolution, the necessity for transport, radiation exposure, and relative insensitivity to early ischemic injury [
61]. When performing brain CT in ECMO patients, several factors need to be considered due to the differences in the pathophysiology of ECMO patients compared to non-ECMO patients. These factors include incompatibility of ECMO circuit components with MRI, logistics of transport, timing of contrast administration, and interpretation of contrast-enhanced CT imaging in the context of altered blood flow patterns [
85]. Furthermore, brain CT displays only non-specific signs such as cerebral edema, sulci effacement, and decreased gray matter (GM)/white matter (WM) differentiation [
86]. The regions primarily affected by profound hypoxia-ischemia are the deep gray matter nuclei, cortices, hippocampi, and cerebellum [
87]. The pattern or location of the lesion may be helpful in diagnosing HIBI and differentiating it from other diseases with similar patterns. In a study performed by Zotzmann et al., full body CT scans performed within 24 hours of ECPR revealed cerebral edema suggestive of HIBI in 26.2% of patients[
88]. Overall, brain CT scans have certain limitations, such as low resolution and relative insensitivity to early ischemic injury, but can provide important information for diagnosing HIBI in ECMO patients.
5.8.2. Brain MRI
In the diagnosis of HIBI, brain MRI is highly sensitive and shows different changes depending on the timing of the injury [
89,
90]. However, the ECMO circuit has components that are not compatible with the magnetic field of the MRI. Consequently, until decannulation and removal of ECMO pump, MRI is not feasible[
85]. According to the current ELSO guidelines, neuroimaging in the form of brain CT or MRI should be performed before discharge in neonatal and pediatric ECMO patients for any indication[
61]. On the other hand, there is no ELSO guidelines related to brain imaging in adult ECMO patients. Brain imaging is performed as needed when neurological symptoms are present and brain injury is suspected. In the acute phase, diffusion-weighted imaging (DWI) is preferred over T2 weighted imaging for detecting lesions because of the sensitivity to detect the presence of cytotoxic edema earlier. In the late subacute phase, postanoxic leukoencephalopathy and contrast enhancement could be observed. In the chronic phase, atrophic changes over tissue signal changes are more prevalent [
86]. To overcome the limitations of MRI examination, research on low-field portable MRI technology was recently conducted (SAFE MRI ECMO study) [
91,
92]. A study by Cho et al. on 3 ECMO patients reported that imaging using low-field MRI can be conducted without significant changes in MAP, ECMO flow, and SpO
2. Low-field MRI was able to produce high-quality neuroimages superior to brain CT to enable bedside detection of ABI [
91]. The evaluation of brain injury to detect HIBI using low-field portable MRI in 19 cardiac arrest patients showed that 12 patients (63.2%) had findings consistent with the findings on conventional MRI report. Low-field MRI was performed without safety events or disrupting intensive care unit equipment setup, but had lower signal-to-noise ratio compared to conventional MRI, resulting in degraded image quality [
93]. It is anticipated that in the future, early diagnosis of various neurological sequelae of ECMO, including HIBI, will be possible with bedside portable MRI.
6. Therapeutic management
Immediate intervention to reverse hypoxia and ischemia is critical to prevent HIBI, but the exact effects on cerebral perfusion of the circuit and the hemodynamic support achieved by ECMO are unclear. Thus, at the present time, treatment for ECMO-associated HIBI [
94] is similar to that of a typical cardiac arrest and consists of temperature control and management of cerebral edema and elevated ICP.
6.1. Temperature control
Targeted temperature management (TTM), which maintains a set temperature range of 32-36℃, is a widely used neuroprotective intervention in patients after cardiac arrest; however, its benefit in the context of eCPR remains unclear. Several studies have evaluated the impact of TTM on survival and neurological outcomes among eCPR patients and none has found a significant benefit [
95,
96]. Two recent trials about the effect of moderate hypothermia on mortality in patients with cardiogenic shock supported by VA-ECMO [
97], and in patients with cardiac arrest [
98], showed no improvement in survival, however, neurologic outcome were not assessed in either of the studies. Furthermore, 2022 ERC-ESICM guidelines state that there is insufficient evidence to support benefits/risks of using TTM and actively recommend preventing fever after cardiac arrest [
99]. In fact, given the strong basic and preclinical scientific support for temperature control in global ischemia [
100] and the high incidence of HIBI and prolonged absent/low cerebral perfusion in VA-ECMO patients, a well-designed multicenter prospective observational cohort study is necessary for overall VA-ECMO patients as well eCPR. Despite its potential to exacerbate any underlying coagulopathy, TTM should not be dismissed as ineffective in treating HIBI in ECMO.
6.2. Cerebral edema and elevated ICP
Several studies [
101,
102] have been conducted on the premise that ICP may be elevated in HIBI due to brain edema in the setting of an ischemic, hypoxic injury. In a preliminary study by Fergusson et al. on HIBI patients after cardiac arrest, ICP management guided by invasive neuromonitoring was associated with a 6-month favorable neurological outcome [
103]. However, the level of ICP in the ECMO population is unknown because invasive ICP monitoring requires anticoagulation to be discontinued, which has its own risks in ECMO patients. Therefore, ICP management in ECMO patients is reserved for patients who clinically exhibit manifestations of transtentorial herniation, such as acute dilated non-reactive pupil to light. ICP control in patients with HIBI undergoing ECMO can be managed using the following commonly employed strategies [
94] : (a) Head-of-bed elevation > 30 degrees [
79,
104], such as with the Reverse Trendelenburg position; (b) Normocapnia with PaCO2 35-45mmHg is recommended [
105], although hyperventilation can be employed as a tempory measure [
106], which can easily be achieved by utilizing the gas flow across the oxygenator to maintain PaCO
2 levels below 30mmHg; (c) Hyperosmolar therapy with the intermittent administration of hypertonic saline and/or mannitol [
106,
107]; (d) Control of factors that increase ICP, such as pain, seizure, agitation, and fever; (e) Use of effective analgesics and sedatives such as propofol, barbiturates [
106], and midazolam [
102]; and (f) Consideration of neurosurgical intervention, such as decompressive craniectomy, though the benefit of this approach is unknown [
94].
7. Future directions
Numerous studies have reported HIBI as a common type of ABI seen in ECMO. As a result, it is important to deepen our understanding of its pathophysiology in these patients, concentrating on the physiologic parameters over which we have control that affect it. [
50]. The field of ABI and specifically HIBI is wide open to identify and standardize neuromonitoring techniques, new therapies, and understand long-term neurologic and functional outcomes [
108]. New neuroprotective agents such as antioxidants, anti-inflammatory agents, and neurotransmitter modulation all show promise in mitigating the severity of HIBI. Furthermore, advances in ECMO technology, such as new circuit membrane materials, alternative cannulation strategies, and increasing pulsatile flow may mitigate HIBI.
Finally, there has been only minimal research on the risk of psychiatric illness or the level of cognitive impairment that determine quality of life of survivors with HIBI in ECMO-treated patients. The incidence of psychiatric illness including depression, anxiety, insomnia and PTSD, as well as cognitive recovery after ECMO related ABI has not been well described. An approach to caring for these patients that is multidisciplinary will be essential to understand and improve their management.
Supplementary Materials
Not applicable.
Author Contributions
Conceptualization, Sung-Min Cho, Melissa Ann Vogelsong, Romergryko Geocadin, Glenn Whitman; writing—original draft preparation, Shivalika Khanduja, Jiah Kim, Jin Kook Kang, Chengyuan Alex Feng; writing—review and editing, Shivalika Khanduja, Jiah Kim, Jin Kook Kang, Chengyuan Alex Feng, Melissa Ann Vogelsong, Romergryko Geocadin, Glenn Whitman Sung-Min Cho; visualization, Shivalika Khanduja, Jiah Kim, Jin Kook Kang, Chengyuan Alex Feng, Melissa Ann Vogelsong, Romergryko Geocadin, Glenn Whitman, Sung Min Cho; supervision, Melissa Ann Vogelsong, Romergryko Geocadin, Glenn Whitman, Sung-Min Cho; project administration, Melissa Ann Vogelsong, Romergryko Geocadin, Glenn Whitman, Sung-Min Cho. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
References
- MacLaren, G.; Fisher, D.; Brodie, D. Preparing for the Most Critically Ill Patients With COVID-19: The Potential Role of Extracorporeal Membrane Oxygenation. JAMA 2020, 323, 1245–1246. [Google Scholar] [CrossRef] [PubMed]
- Migdady, I.; Rice, C.; Deshpande, A.; Hernandez, A.V.; Price, C.; Whitman, G.J.; Geocadin, R.G.; Cho, S.-M. Brain Injury and Neurologic Outcome in Patients Undergoing Extracorporeal Cardiopulmonary Resuscitation: A Systematic Review and Meta-Analysis. Critical Care Medicine. [CrossRef]
- Cho, S.-M.; Lee, T.; Starling, R.C.; Thompson, N.R.; Uchino, K. The Impact of Infection and Elevated INR in LVAD-Associated Intracranial Hemorrhage: A Case-Crossover Study. ASAIO journal (American Society for Artificial Internal Organs: 1992) 2019, 65, 545–549. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.; Buletko, A.B.; Matthew, J.; Cho, S.-M. Bloodstream infection is associated with subarachnoid hemorrhage and infectious intracranial aneurysm in left ventricular assist device. Perfusion 2020, 35, 117–120. [Google Scholar] [CrossRef] [PubMed]
- Trachtenberg, B.H.; Cordero-Reyes, A.M.; Aldeiri, M.; Alvarez, P.; Bhimaraj, A.; Ashrith, G.; Elias, B.; Suarez, E.E.; Bruckner, B.; Loebe, M.; et al. Persistent blood stream infection in patients supported with a continuous-flow left ventricular assist device is associated with an increased risk of cerebrovascular accidents. J Card Fail 2015, 21, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Ruland, S.; Aiyagari, V. Cerebral Autoregulation and Blood Pressure Lowering. Hypertension 2007, 49, 977–978. [Google Scholar] [CrossRef] [PubMed]
- Jia, D.; Neo, R.; Lim, E.; Seng, T.C.; MacLaren, G.; Ramanathan, K. Autopsy and clinical discrepancies in patients undergoing extracorporeal membrane oxygenation: a case series. Cardiovascular Pathology 2019, 41, 24–28. [Google Scholar] [CrossRef]
- Cho, S.-M.; Ziai, W.; Mayasi, Y.; Gusdon, A.M.; Creed, J.; Sharrock, M.; Stephens, R.S.; Choi, C.W.; Ritzl, E.K.; Suarez, J.; et al. Noninvasive Neurological Monitoring in Extracorporeal Membrane Oxygenation. ASAIO Journal 2020, 66, 388–393. [Google Scholar] [CrossRef]
- Lorusso, R.; Barili, F.; Mauro, M.D.; Gelsomino, S.; Parise, O.; Rycus, P.T.; Maessen, J.; Mueller, T.; Muellenbach, R.; Belohlavek, J.; et al. In-Hospital Neurologic Complications in Adult Patients Undergoing Venoarterial Extracorporeal Membrane Oxygenation: Results From the Extracorporeal Life Support Organization Registry. Critical Care Medicine 2016, 44, e964–e972. [Google Scholar] [CrossRef]
- Xie, A.; Lo, P.; Yan, T.D.; Forrest, P. Neurologic Complications of Extracorporeal Membrane Oxygenation: A Review. Journal of Cardiothoracic and Vascular Anesthesia 2017, 31, 1836–1846. [Google Scholar] [CrossRef]
- Cho, S.-M.; Ziai, W.; Geocadin, R.; Choi, C.W.; Whitman, G. Arterial-Sided Oxygenator Clot and Transcranial Doppler Ultrasound Emboli in Venoarterial Extracorporeal Membrane Oxygenation. The Annals of Thoracic Surgery 2019, 107, 326–327. [Google Scholar] [CrossRef]
- Cho, S.-M.; Geocadin, R.G.; Caturegli, G.; Chan, V.; White, B.; Dodd-o, J.; Kim, B.S.; Sussman, M.; Choi, C.W.; Whitman, G.; et al. Understanding Characteristics of Acute Brain Injury in Adult Extracorporeal Membrane Oxygenation: An Autopsy Study*. Critical Care Medicine 2020, 48, e532–e536. [Google Scholar] [CrossRef] [PubMed]
- Laver, S.; Farrow, C.; Turner, D.; Nolan, J. Mode of death after admission to an intensive care unit following cardiac arrest. Intensive Care Medicine 2004, 30, 2126–2128. [Google Scholar] [CrossRef] [PubMed]
- Nolan, J.P.; Neumar, R.W.; Adrie, C.; Aibiki, M.; Berg, R.A.; Böttiger, B.W.; Callaway, C.; Clark, R.S.B.; Geocadin, R.G.; Jauch, E.C.; et al. Post-cardiac arrest syndrome: epidemiology, pathophysiology, treatment, and prognostication. A Scientific Statement from the International Liaison Committee on Resuscitation; the American Heart Association Emergency Cardiovascular Care Committee; the Council on Cardiovascular Surgery and Anesthesia; the Council on Cardiopulmonary, Perioperative, and Critical Care; the Council on Clinical Cardiology; the Council on Stroke. Resuscitation 2008, 79, 350–379. [Google Scholar] [CrossRef] [PubMed]
- Lorusso, R.; Gelsomino, S.; Parise, O.; Di Mauro, M.; Barili, F.; Geskes, G.; Vizzardi, E.; Rycus, P.T.; Muellenbach, R.; Mueller, T.; et al. Neurologic Injury in Adults Supported With Veno-Venous Extracorporeal Membrane Oxygenation for Respiratory Failure: Findings From the Extracorporeal Life Support Organization Database. Critical Care Medicine 2017, 45, 1389–1397. [Google Scholar] [CrossRef] [PubMed]
- Shoskes, A.; Migdady, I.; Rice, C.; Hassett, C.; Deshpande, A.; Price, C.; Hernandez, A.V.; Cho, S.-M. Brain Injury Is More Common in Venoarterial Extracorporeal Membrane Oxygenation Than Venovenous Extracorporeal Membrane Oxygenation: A Systematic Review and Meta-Analysis. Critical Care Medicine 2020, 48, 1799–1808. [Google Scholar] [CrossRef] [PubMed]
- Shou, B.L.; Ong, C.S.; Zhou, A.L.; Al-Kawaz, M.N.; Etchill, E.; Giuliano, K.; Dong, J.; Bush, E.; Kim, B.S.; Choi, C.W.; et al. Arterial Carbon Dioxide and Acute Brain Injury in Venoarterial Extracorporeal Membrane Oxygenation. ASAIO Journal 2022, 68, 1501–1507. [Google Scholar] [CrossRef] [PubMed]
- Busl, K.M.; Greer, D.M. Hypoxic-ischemic brain injury: pathophysiology, neuropathology and mechanisms. NeuroRehabilitation 2010, 26, 5–13. [Google Scholar] [CrossRef]
- Ziai, W.C.; Cho, S.M.; Johansen, M.C.; Ergin, B.; Bahouth, M.N. Transcranial Doppler in Acute COVID-19 Infection: Unexpected Associations. Stroke 2021, 52, 2422–2426. [Google Scholar] [CrossRef]
- Le Gall, A.; Follin, A.; Cholley, B.; Mantz, J.; Aissaoui, N.; Pirracchio, R. Veno-arterial-ECMO in the intensive care unit: From technical aspects to clinical practice. Anaesth Crit Care Pain Med 2018, 37, 259–268. [Google Scholar] [CrossRef]
- Loungani, R.S.; Fudim, M.; Ranney, D.; Kochar, A.; Samsky, M.D.; Bonadonna, D.; Itoh, A.; Takayama, H.; Takeda, K.; Wojdyla, D.; et al. Contemporary Use of Venoarterial Extracorporeal Membrane Oxygenation: Insights from the Multicenter RESCUE Registry. J Card Fail 2021, 27, 327–337. [Google Scholar] [CrossRef]
- Wieruszewski, P.M.; Ortoleva, J.P.; Cormican, D.S.; Seelhammer, T.G. Extracorporeal Membrane Oxygenation in Acute Respiratory Failure. Pulm Ther 2023, 9, 109–126. [Google Scholar] [CrossRef] [PubMed]
- Shou, B.L.; Wilcox, C.; Florissi, I.; Kalra, A.; Caturegli, G.; Zhang, L.Q.; Bush, E.; Kim, B.; Keller, S.P.; Whitman, G.J.R.; et al. Early Low Pulse Pressure in VA-ECMO Is Associated with Acute Brain Injury. Neurocrit Care, 1007. [Google Scholar] [CrossRef]
- Wilcox, C.; Choi, C.W.; Cho, S.-M. Brain injury in extracorporeal cardiopulmonary resuscitation: translational to clinical research. J Neurocrit Care 2021, 14, 63–77. [Google Scholar] [CrossRef]
- Rupprecht, L.; Lunz, D.; Philipp, A.; Lubnow, M.; Schmid, C. Pitfalls in percutaneous ECMO cannulation. Heart Lung Vessel 2015, 7, 320–326. [Google Scholar] [PubMed]
- Haldenwang, P.L.; Baumann, A.; Elghannam, M.; Schlomicher, M.; Buchwald, D.; Klak, K.; Strauch, J.T. Two-stage weaning strategy using veno-veno-arterial perfusion for patients on extracorporeal membrane oxygenation following cardiogenic shock. Perfusion 2019, 34, 689–695. [Google Scholar] [CrossRef] [PubMed]
- Patel, B.; Arcaro, M.; Chatterjee, S. Bedside troubleshooting during venovenous extracorporeal membrane oxygenation (ECMO). J Thorac Dis 2019, 11, S1698–s1707. [Google Scholar] [CrossRef]
- Schmidt, M.; Tachon, G.; Devilliers, C.; Muller, G.; Hekimian, G.; Bréchot, N.; Merceron, S.; Luyt, C.E.; Trouillet, J.L.; Chastre, J.; et al. Blood oxygenation and decarboxylation determinants during venovenous ECMO for respiratory failure in adults. Intensive Care Med 2013, 39, 838–846. [Google Scholar] [CrossRef]
- Bishop, M.A.; Moore, A. Extracorporeal Membrane Oxygenation Weaning. In StatPearls; StatPearls Publishing LLC.: Treasure Island (FL), 2023. [Google Scholar]
- Sutter, R.; Tisljar, K.; Marsch, S. Acute Neurologic Complications During Extracorporeal Membrane Oxygenation: A Systematic Review. Critical Care Medicine 2018, 46, 1506–1513. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Xu, J.; Yang, X.; Zou, X.; Shu, H.; Liu, Z.; Shang, Y. Narrative Review of Neurologic Complications in Adults on ECMO: Prevalence, Risks, Outcomes, and Prevention Strategies. Frontiers in Medicine 2021, 8, 713333. [Google Scholar] [CrossRef]
- Godoy, D.A.; Seifi, A.; Garza, D.; Lubillo-Montenegro, S.; Murillo-Cabezas, F. Hyperventilation Therapy for Control of Posttraumatic Intracranial Hypertension. Frontiers in Neurology 2017, 8, 250. [Google Scholar] [CrossRef]
- Harper, A.M.; Bell, R.A. The effect of metabolic acidosis and alkalosis on the blood flow through the cerebral cortex. J Neurol Neurosurg Psychiatry 1963, 26, 341–344. [Google Scholar] [CrossRef]
- Rout, M.W.; Lane, D.J.; Wollner, L. Prognosis in acute cerebrovascular accidents in relation to respiratory pattern and blood gas tensions. Br Med J 1971, 3, 7–9. [Google Scholar] [CrossRef] [PubMed]
- Heffner, J.E.; Sahn, S.A. Controlled hyperventilation in patients with intracranial hypertension. Application and management. Arch Intern Med 1983, 143, 765–769. [Google Scholar] [CrossRef] [PubMed]
- Dulla, C.G.; Dobelis, P.; Pearson, T.; Frenguelli, B.G.; Staley, K.J.; Masino, S.A. Adenosine and ATP Link PCO2 to Cortical Excitability via pH. Neuron 2005, 48, 1011–1023. [Google Scholar] [CrossRef] [PubMed]
- Shou, B.L.; Ong, C.S.; Premraj, L.; Brown, P.; Tonna, J.E.; Dalton, H.J.; Kim, B.S.; Keller, S.P.; Whitman, G.J.R.; Cho, S.M. Arterial oxygen and carbon dioxide tension and acute brain injury in extracorporeal cardiopulmonary resuscitation patients: Analysis of the extracorporeal life support organization registry. J Heart Lung Transplant 2023, 42, 503–511. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.-M.; Canner, J.; Caturegli, G.; Choi, C.W.; Etchill, E.; Giuliano, K.; Chiarini, G.; Calligy, K.; Rycus, P.; Lorusso, R.; et al. Risk Factors of Ischemic and Hemorrhagic Strokes During Venovenous Extracorporeal Membrane Oxygenation: Analysis of Data From the Extracorporeal Life Support Organization Registry. Critical Care Medicine 2021, 49, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Pineton de Chambrun, M.; Bréchot, N.; Lebreton, G.; Schmidt, M.; Hekimian, G.; Demondion, P.; Trouillet, J.L.; Leprince, P.; Chastre, J.; Combes, A.; et al. Venoarterial extracorporeal membrane oxygenation for refractory cardiogenic shock post-cardiac arrest. Intensive Care Med 2016, 42, 1999–2007. [Google Scholar] [CrossRef]
- Ryu, J.A.; Cho, Y.H.; Sung, K.; Choi, S.H.; Yang, J.H.; Choi, J.H.; Lee, D.S.; Yang, J.H. Predictors of neurological outcomes after successful extracorporeal cardiopulmonary resuscitation. BMC Anesthesiol 2015, 15, 26. [Google Scholar] [CrossRef] [PubMed]
- Carlson, J.M.; Etchill, E.; Whitman, G.; Kim, B.S.; Choi, C.W.; Tonna, J.E.; Geocadin, R.; Cho, S.M. Early withdrawal of life sustaining therapy in extracorporeal cardiopulmonary resuscitation (ECPR): Results from the Extracorporeal Life Support Organization registry. Resuscitation 2022, 179, 71–77. [Google Scholar] [CrossRef]
- Foerster, K.; D’Inka, M.; Beyersdorf, F.; Benk, C.; Nguyen-Thanh, T.; Mader, I.; Fritsch, B.; Ihling, C.; Mueller, K.; Heilmann, C.; et al. Prolonged cardiac arrest and resuscitation by extracorporeal life support: favourable outcome without preceding anticoagulation in an experimental setting. Perfusion 2013, 28, 520–528. [Google Scholar] [CrossRef]
- Putzer, G.; Martini, J.; Spraider, P.; Abram, J.; Hornung, R.; Schmidt, C.; Bauer, M.; Pinggera, D.; Krapf, C.; Hell, T.; et al. Adrenaline improves regional cerebral blood flow, cerebral oxygenation and cerebral metabolism during CPR in a porcine cardiac arrest model using low-flow extracorporeal support. Resuscitation 2021, 168, 151–159. [Google Scholar] [CrossRef]
- Rozencwajg, S.; Heinsar, S.; Wildi, K.; Jung, J.S.; Colombo, S.M.; Palmieri, C.; Sato, K.; Ainola, C.; Wang, X.; Abbate, G.; et al. Effect of flow change on brain injury during an experimental model of differential hypoxaemia in cardiogenic shock supported by extracorporeal membrane oxygenation. Sci Rep 2023, 13, 4002. [Google Scholar] [CrossRef] [PubMed]
- Kirino, T. Ischemic tolerance. J Cereb Blood Flow Metab 2002, 22, 1283–1296. [Google Scholar] [CrossRef] [PubMed]
- Liang, D.; Bhatta, S.; Gerzanich, V.; Simard, J.M. Cytotoxic edema: mechanisms of pathological cell swelling. Neurosurg Focus 2007, 22, E2. [Google Scholar] [CrossRef] [PubMed]
- Solaini, G.; Baracca, A.; Lenaz, G.; Sgarbi, G. Hypoxia and mitochondrial oxidative metabolism. Biochim Biophys Acta 2010, 1797, 1171–1177. [Google Scholar] [CrossRef]
- Kiessling, M.; Stumm, G.; Xie, Y.; Herdegen, T.; Aguzzi, A.; Bravo, R.; Gass, P. Differential transcription and translation of immediate early genes in the gerbil hippocampus after transient global ischemia. J Cereb Blood Flow Metab 1993, 13, 914–924. [Google Scholar] [CrossRef]
- Kristian, T.; Siesjo, B.K. Calcium in ischemic cell death. Stroke 1998, 29, 705–718. [Google Scholar] [CrossRef]
- Sekhon, M.S.; Ainslie, P.N.; Griesdale, D.E. Clinical pathophysiology of hypoxic ischemic brain injury after cardiac arrest: a “two-hit” model. 90.
- Cho, S.M.; Farrokh, S.; Whitman, G.; Bleck, T.P.; Geocadin, R.G. Neurocritical Care for Extracorporeal Membrane Oxygenation Patients. 1773-1781.
- Chiarini, G.; Cho, S.M.; Whitman, G.; Rasulo, F.; Lorusso, R. Brain Injury in Extracorporeal Membrane Oxygenation: A Multidisciplinary Approach. Semin Neurol 2021, 41, 422–436. [Google Scholar] [CrossRef]
- Peluso, L.; Rechichi, S.; Franchi, F.; Pozzebon, S.; Scolletta, S.; Brasseur, A.; Legros, B.; Vincent, J.L.; Creteur, J.; Gaspard, N.; et al. Electroencephalographic features in patients undergoing extracorporeal membrane oxygenation. Crit Care 2020, 24, 629. [Google Scholar] [CrossRef]
- Ong, C.S.; Etchill, E.; Dong, J.; Shou, B.L.; Shelley, L.; Giuliano, K.; Al-Kawaz, M.; Ritzl, E.K.; Geocadin, R.G.; Kim, B.S.; et al. Neuromonitoring detects brain injury in patients receiving extracorporeal membrane oxygenation support. J Thorac Cardiovasc Surg, 1016. [Google Scholar] [CrossRef]
- Perera, K.; Khan, S.; Singh, S.; Kromm, J.; Wang, M.; Sajobi, T.; Jette, N.; Wiebe, S.; Josephson, C.B. EEG Patterns and Outcomes After Hypoxic Brain Injury: A Systematic Review and Meta-analysis. Neurocrit Care 2022, 36, 292–301. [Google Scholar] [CrossRef]
- Lamartine Monteiro, M.; Taccone, F.S.; Depondt, C.; Lamanna, I.; Gaspard, N.; Ligot, N.; Mavroudakis, N.; Naeije, G.; Vincent, J.L.; Legros, B. The Prognostic Value of 48-h Continuous EEG During Therapeutic Hypothermia After Cardiac Arrest. Neurocrit Care 2016, 24, 153–162. [Google Scholar] [CrossRef]
- Hwang, J.; Geocadin, R.; Ritzl, E.K.; Cho, S.M. Continuous EEG in patients with extracorporeal membrane oxygenation support: Clinical need in multidisciplinary collaboration and standardized monitoring. Clin Neurophysiol 2022, 142, 273–274. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.; Bronder, J.; Martinez, N.C.; Geocadin, R.; Kim, B.S.; Bush, E.; Whitman, G.; Choi, C.W.; Ritzl, E.K.; Cho, S.M. Continuous Electroencephalography Markers of Prognostication in Comatose Patients on Extracorporeal Membrane Oxygenation. Neurocrit Care 2022, 37, 236–245. [Google Scholar] [CrossRef] [PubMed]
- Herman, S.T.; Abend, N.S.; Bleck, T.P.; Chapman, K.E.; Drislane, F.W.; Emerson, R.G.; Gerard, E.E.; Hahn, C.D.; Husain, A.M.; Kaplan, P.W.; et al. Consensus statement on continuous EEG in critically ill adults and children, part I: indications. J Clin Neurophysiol 2015, 32, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Maldonado, Y.; Singh, S.; Taylor, M.A. Cerebral near-infrared spectroscopy in perioperative management of left ventricular assist device and extracorporeal membrane oxygenation patients. Curr Opin Anaesthesiol 2014, 27, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Said, A.S.; Guilliams, K.P.; Bembea, M.M. Neurological Monitoring and Complications of Pediatric Extracorporeal Membrane Oxygenation Support. Pediatr Neurol 2020, 108, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.; Rehan, M.; Parikh, G.; Zammit, C.; Badjatia, N.; Herr, D.; Kon, Z.; Hogue, C.; Mazzeffi, M. Regional Cerebral Oximetry as an Indicator of Acute Brain Injury in Adults Undergoing Veno-Arterial Extracorporeal Membrane Oxygenation-A Prospective Pilot Study. Front Neurol 2018, 9, 993. [Google Scholar] [CrossRef] [PubMed]
- Hunt, M.F.; Clark, K.T.; Whitman, G.; Choi, C.W.; Geocadin, R.G.; Cho, S.M. The Use of Cerebral NIRS Monitoring to Identify Acute Brain Injury in Patients With VA-ECMO. J Intensive Care Med 2021, 36, 1403–1409. [Google Scholar] [CrossRef] [PubMed]
- Andresen, B.; Greisen, G.; Hyttel-Sorensen, S. Comparison of INVOS 5100C and Nonin SenSmart X-100 oximeter performance in preterm infants with spontaneous apnea. Pediatr Res 2020, 87, 1244–1250. [Google Scholar] [CrossRef]
- Waldman, S.D. CHAPTER 221—Evoked Potential Testing. In Pain Review, Waldman, S.D., Ed. W.B. P: Saunders, 2009. [Google Scholar] [CrossRef]
- Uk, M.; Al, E. Clinical Neurophysiology (2Nd Edition); Elsevier (A Divisionof Reed Elsevier India Pvt. Limited): 2010.
- Nolan, J.P.; Soar, J.; Cariou, A.; Cronberg, T.; Moulaert, V.R.; Deakin, C.D.; Bottiger, B.W.; Friberg, H.; Sunde, K.; Sandroni, C. European Resuscitation Council and European Society of Intensive Care Medicine Guidelines for Post-resuscitation Care 2015: Section 5 of the European Resuscitation Council Guidelines for Resuscitation 2015. Resuscitation 2015, 95, 202–222. [Google Scholar] [CrossRef]
- Sandroni, C.; D’Arrigo, S.; Nolan, J.P. Prognostication after cardiac arrest. Crit Care 2018, 22, 150. [Google Scholar] [CrossRef]
- Horn, J.; Tjepkema-Cloostermans, M.C. Somatosensory Evoked Potentials in Patients with Hypoxic-Ischemic Brain Injury. Semin Neurol 2017, 37, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Scarpino, M.; Lolli, F.; Lanzo, G.; Carrai, R.; Spalletti, M.; Valzania, F.; Lombardi, M.; Audenino, D.; Contardi, S.; Grazia Celani, M.; et al. Do changes in SSEP amplitude over time predict the outcome of comatose survivors of cardiac arrest? Resuscitation 2022, 181, 133–139. [Google Scholar] [CrossRef] [PubMed]
- van Soest, T.M.; van Rootselaar, A.F.; Admiraal, M.M.; Potters, W.V.; Koelman, J.; Horn, J. SSEP amplitudes add information for prognostication in postanoxic coma. Resuscitation 2021, 163, 172–175. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.; Kurfess, J.; Treggiari, M.M. Basics of Neuromonitoring and Anesthetic Considerations. Anesthesiol Clin 2021, 39, 195–209. [Google Scholar] [CrossRef] [PubMed]
- Singh, G. Somatosensory evoked potential monitoring. Journal of Neuroanaesthesiology and Critical Care 2018, 03, S97–S104. [Google Scholar] [CrossRef]
- Scarpino, M.; Lolli, F.; Lanzo, G.; Carrai, R.; Spalletti, M.; Valzania, F.; Lombardi, M.; Audenino, D.; Contardi, S.; Celani, M.G.; et al. SSEP amplitude accurately predicts both good and poor neurological outcome early after cardiac arrest; a post-hoc analysis of the ProNeCA multicentre study. Resuscitation 2021, 163, 162–171. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.M.; Choi, C.W.; Whitman, G.; Suarez, J.I.; Martinez, N.C.; Geocadin, R.G.; Ritzl, E.K. Neurophysiological Findings and Brain Injury Pattern in Patients on ECMO. Clin EEG Neurosci 2021, 52, 462–469. [Google Scholar] [CrossRef]
- Caturegli, G.; Zhang, L.Q.; Mayasi, Y.; Gusdon, A.M.; Ergin, B.; Ponomarev, V.; Kim, B.S.; Keller, S.; Geocadin, R.G.; Whitman, G.J.R.; et al. Characterization of Cerebral Hemodynamics with TCD in Patients Undergoing VA-ECMO and VV-ECMO: a Prospective Observational Study. Neurocrit Care, 1007; -6. [Google Scholar] [CrossRef]
- Caturegli, G.; Kapoor, S.; Ponomarev, V.; Kim, B.S.; Whitman, G.J.R.; Ziai, W.; Cho, S.M. Transcranial Doppler microemboli and acute brain injury in extracorporeal membrane oxygenation: A prospective observational study. JTCVS Tech 2022, 15, 111–122. [Google Scholar] [CrossRef]
- Petermichl, W.; Philipp, A.; Hiller, K.A.; Foltan, M.; Floerchinger, B.; Graf, B.; Lunz, D. Reliability of prognostic biomarkers after prehospital extracorporeal cardiopulmonary resuscitation with target temperature management. Scand J Trauma Resusc Emerg Med 2021, 29, 147. [Google Scholar] [CrossRef]
- Nolan, J.P.; Sandroni, C.; Bottiger, B.W.; Cariou, A.; Cronberg, T.; Friberg, H.; Genbrugge, C.; Haywood, K.; Lilja, G.; Moulaert, V.R.M.; et al. European Resuscitation Council and European Society of Intensive Care Medicine Guidelines 2021: Post-resuscitation care. Resuscitation 2021, 161, 220–269. [Google Scholar] [CrossRef]
- Ferraro, S.; Braga, F.; Luksch, R.; Terenziani, M.; Caruso, S.; Panteghini, M. Measurement of Serum Neuron-Specific Enolase in Neuroblastoma: Is There a Clinical Role? Clin Chem 2020, 66, 667–675. [Google Scholar] [CrossRef] [PubMed]
- Böttiger, B.W.; Möbes, S.; Glätzer, R.; Bauer, H.; Gries, A.; Bärtsch, P.; Motsch, J.; Martin, E. Astroglial protein S-100 is an early and sensitive marker of hypoxic brain damage and outcome after cardiac arrest in humans. Circulation 2001, 103, 2694–2698. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.H.; Chang, W.T.; Su, K.I.; Huang, C.H.; Tsai, M.S.; Chou, E.; Lu, T.C.; Chen, W.J.; Lee, C.C.; Chen, S.C. Neuroprognostic accuracy of blood biomarkers for post-cardiac arrest patients: A systematic review and meta-analysis. Resuscitation 2020, 148, 108–117. [Google Scholar] [CrossRef] [PubMed]
- Bembea, M.M.; Rizkalla, N.; Freedy, J.; Barasch, N.; Vaidya, D.; Pronovost, P.J.; Everett, A.D.; Mueller, G. Plasma Biomarkers of Brain Injury as Diagnostic Tools and Outcome Predictors After Extracorporeal Membrane Oxygenation. Crit Care Med 2015, 43, 2202–2211. [Google Scholar] [CrossRef] [PubMed]
- Hoiland, R.L.; Rikhraj, K.J.K.; Thiara, S.; Fordyce, C.; Kramer, A.H.; Skrifvars, M.B.; Wellington, C.L.; Griesdale, D.E.; Fergusson, N.A.; Sekhon, M.S. Neurologic Prognostication After Cardiac Arrest Using Brain Biomarkers: A Systematic Review and Meta-analysis. JAMA Neurol 2022, 79, 390–398. [Google Scholar] [CrossRef]
- Illum, B.; Odish, M.; Minokadeh, A.; Yi, C.; Owens, R.L.; Pollema, T.; LaBuzetta, J.N. Evaluation, Treatment, and Impact of Neurologic Injury in Adult Patients on Extracorporeal Membrane Oxygenation: a Review. Curr Treat Options Neurol 2021, 23, 15. [Google Scholar] [CrossRef]
- Gutierrez, L.G.; Rovira, A.; Portela, L.A.; Leite Cda, C.; Lucato, L.T. CT and MR in non-neonatal hypoxic-ischemic encephalopathy: radiological findings with pathophysiological correlations. Neuroradiology 2010, 52, 949–976. [Google Scholar] [CrossRef]
- Huang, B.Y.; Castillo, M. Hypoxic-ischemic brain injury: imaging findings from birth to adulthood. Radiographics 2008, 28, 417–439. [Google Scholar] [CrossRef]
- Zotzmann, V.; Rilinger, J.; Lang, C.N.; Duerschmied, D.; Benk, C.; Bode, C.; Wengenmayer, T.; Staudacher, D.L. Early full-body computed tomography in patients after extracorporeal cardiopulmonary resuscitation (eCPR). Resuscitation 2020, 146, 149–154. [Google Scholar] [CrossRef]
- Barrett, K.M.; Freeman, W.D.; Weindling, S.M.; Brott, T.G.; Broderick, D.F.; Heckman, M.G.; Crook, J.E.; Divertie, G.D.; Meschia, J.F. Brain injury after cardiopulmonary arrest and its assessment with diffusion-weighted magnetic resonance imaging. Mayo Clin Proc 2007, 82, 828–835. [Google Scholar] [CrossRef]
- Greer, D.; Scripko, P.; Bartscher, J.; Sims, J.; Camargo, E.; Singhal, A.; Furie, K. Serial MRI changes in comatose cardiac arrest patients. Neurocrit Care 2011, 14, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.M.; Wilcox, C.; Keller, S.; Acton, M.; Rando, H.; Etchill, E.; Giuliano, K.; Bush, E.L.; Sair, H.I.; Pitts, J.; et al. Assessing the SAfety and FEasibility of bedside portable low-field brain Magnetic Resonance Imaging in patients on ECMO (SAFE-MRI ECMO study): study protocol and first case series experience. Crit Care 2022, 26, 119. [Google Scholar] [CrossRef] [PubMed]
- Sheth, K.N.; Mazurek, M.H.; Yuen, M.M.; Cahn, B.A.; Shah, J.T.; Ward, A.; Kim, J.A.; Gilmore, E.J.; Falcone, G.J.; Petersen, N.; et al. Assessment of Brain Injury Using Portable, Low-Field Magnetic Resonance Imaging at the Bedside of Critically Ill Patients. JAMA Neurol 2020, 78, 41–47. [Google Scholar] [CrossRef]
- Beekman, R.; Crawford, A.; Mazurek, M.H.; Prabhat, A.M.; Chavva, I.R.; Parasuram, N.; Kim, N.; Kim, J.A.; Petersen, N.; de Havenon, A.; et al. Bedside monitoring of hypoxic ischemic brain injury using low-field, portable brain magnetic resonance imaging after cardiac arrest. Resuscitation 2022, 176, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.M.; Farrokh, S.; Whitman, G.; Bleck, T.P.; Geocadin, R.G. Neurocritical Care for Extracorporeal Membrane Oxygenation Patients. Crit Care Med 2019, 47, 1773–1781. [Google Scholar] [CrossRef]
- Huang, M.; Shoskes, A.; Migdady, I.; Amin, M.; Hasan, L.; Price, C.; Uchino, K.; Choi, C.W.; Hernandez, A.V.; Cho, S.M. Does Targeted Temperature Management Improve Neurological Outcome in Extracorporeal Cardiopulmonary Resuscitation (ECPR)? J Intensive Care Med 2022, 37, 157–167. [Google Scholar] [CrossRef]
- Kim, Y.S.; Cho, Y.H.; Sung, K.; Ryu, J.A.; Chung, C.R.; Suh, G.Y.; Yang, J.H.; Yang, J.H. Target Temperature Management May Not Improve Clinical Outcomes of Extracorporeal Cardiopulmonary Resuscitation. J Intensive Care Med 2019, 34, 790–796. [Google Scholar] [CrossRef]
- Levy, B.; Girerd, N.; Amour, J.; Besnier, E.; Nesseler, N.; Helms, J.; Delmas, C.; Sonneville, R.; Guidon, C.; Rozec, B.; et al. Effect of Moderate Hypothermia vs Normothermia on 30-Day Mortality in Patients With Cardiogenic Shock Receiving Venoarterial Extracorporeal Membrane Oxygenation: A Randomized Clinical Trial. JAMA 2022, 327, 442–453. [Google Scholar] [CrossRef]
- Dankiewicz, J.; Cronberg, T.; Lilja, G.; Jakobsen, J.C.; Levin, H.; Ullén, S.; Rylander, C.; Wise, M.P.; Oddo, M.; Cariou, A.; et al. Hypothermia versus Normothermia after Out-of-Hospital Cardiac Arrest. N Engl J Med 2021, 384, 2283–2294. [Google Scholar] [CrossRef]
- Nolan, J.P.; Sandroni, C.; Andersen, L.W.; Böttiger, B.W.; Cariou, A.; Cronberg, T.; Friberg, H.; Genbrugge, C.; Lilja, G.; Morley, P.T.; et al. ERC-ESICM guidelines on temperature control after cardiac arrest in adults. Resuscitation 2022, 172, 229–236. [Google Scholar] [CrossRef]
- Laptook, A.R.; Corbett, R.J. The effects of temperature on hypoxic-ischemic brain injury. Clin Perinatol 2002, 29, 623–649. [Google Scholar] [CrossRef] [PubMed]
- Balu, R.; Rajagopalan, S.; Baghshomali, S.; Kirschen, M.; Amurthur, A.; Kofke, W.A.; Abella, B.S. Cerebrovascular pressure reactivity and intracranial pressure are associated with neurologic outcome after hypoxic-ischemic brain injury. Resuscitation 2021, 164, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Sekhon, M.S.; Griesdale, D.E.; Ainslie, P.N.; Gooderham, P.; Foster, D.; Czosnyka, M.; Robba, C.; Cardim, D. Intracranial pressure and compliance in hypoxic ischemic brain injury patients after cardiac arrest. Resuscitation 2019, 141, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Fergusson, N.A.; Hoiland, R.L.; Thiara, S.; Foster, D.; Gooderham, P.; Rikhraj, K.; Grunau, B.; Christenson, J.; Ainslie, P.N.; Griesdale, D.E.G.; et al. Goal-Directed Care Using Invasive Neuromonitoring Versus Standard of Care After Cardiac Arrest: A Matched Cohort Study. Crit Care Med 2021, 49, 1333–1346. [Google Scholar] [CrossRef]
- Schulz-Stübner, S.; Thiex, R. Raising the head-of-bed by 30 degrees reduces ICP and improves CPP without compromising cardiac output in euvolemic patients with traumatic brain injury and subarachnoid haemorrhage: a practice audit. Eur J Anaesthesiol 2006, 23, 177–180. [Google Scholar] [CrossRef] [PubMed]
- Callaway, C.W.; Donnino, M.W.; Fink, E.L.; Geocadin, R.G.; Golan, E.; Kern, K.B.; Leary, M.; Meurer, W.J.; Peberdy, M.A.; Thompson, T.M.; et al. Part 8: Post-Cardiac Arrest Care: 2015 American Heart Association Guidelines Update for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation 2015, 132, S465–482. [Google Scholar] [CrossRef] [PubMed]
- Carney, N.; Totten, A.M.; O’Reilly, C.; Ullman, J.S.; Hawryluk, G.W.; Bell, M.J.; Bratton, S.L.; Chesnut, R.; Harris, O.A.; Kissoon, N.; et al. Guidelines for the Management of Severe Traumatic Brain Injury, Fourth Edition. Neurosurgery 2017, 80, 6–15. [Google Scholar] [CrossRef]
- Farrokh, S.; Cho, S.M.; Suarez, J.I. Fluids and hyperosmolar agents in neurocritical care: an update. Curr Opin Crit Care 2019, 25, 105–109. [Google Scholar] [CrossRef]
- Meloni, B.P. Pathophysiology and Neuroprotective Strategies in Hypoxic-Ischemic Brain Injury and Stroke. Brain Sci 2017, 7. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).