Introduction
Human longevity has increased rapidly since the beginning of the 20th century due to medical advances, societal changes, and improved quality of life, which subsequently has led to delayed childbearing [
1,
2]. Consequently, the past two decades witnessed an increased interest in the investigation of the aging process. However, mechanisms regulating germ cell aging, and how they relate to somatic aging remain incompletely characterized.
Several mechanisms have been implicated in promoting cellular senescence, including telomere shortening [
3] and mitochondrial dysfunction [
4,
5]. Telomeres consist of tandem repeats of 5′-TTAGGG-3′ sequences that are located at the end of the chromosomes and play a crucial role in preserving chromosome stability. During each cell division, 20-50 telomeric base pairs cannot be replicated and are lost at the 5’-end of the newly synthesized DNA strand, leading to telomere shortening [
6]. Decreasing telomere length acts as a “mitotic clock” for cellular senescence and aging. This is because t
elomere shortening that occurs with each round of cell division ultimately leads to chromosome ends becoming exposed and activates a DNA damage response, which results in a permanent mitotic arrest known as replicative senescence [
7]
. Telomere damage may also occur in non-dividing cells, such as oocytes, and may result from oxidative DNA damage to guanine-rich telomeric repeats when exposed to reactive oxygen species (ROS), as well as epigenetic, environmental, dietary, and lifestyle variables [
8,
9].
Mitochondria on the other hand serve a crucial role in energy production, cellular metabolism, regulation of membrane potential, and apoptosis [
10]. More recently, mitochondrial unfolded protein response (mtUPR), which ensures mitochondrial protein hemostasis by sensing mitochondrial (unfolded protein) stress, has been implicated in aging [
11]. Activation of mtUPR contributes to enhanced longevity in experimental models [12-14], while a dysfunctional mtUPR may result in age-related accumulation of damaged proteins, decreased oxidative phosphorylation, and increased ROS (reviewed in [
11]). Multiple mouse models of mitochondrial dysfunction also result in female infertility, further highlighting the the complex relationship between mitochondrial function, aging and reproductive potential [
5,
15,
16].
Caseinolytic peptidase P (CLPP) is a key regulator of mtUPR [17-20]. CLPP cleaves misfolded mitochondrial proteins that are then exported to the cytoplasm where they activate transcription factors that induce mtUPR (reviewed in [
11]). Previous research has shown that global germline
Clpp loss in female mice impairs oocyte maturation and two-cell embryo formation and causes blastocyst development failure, which eventually results in infertility [
5,
21]. In addition, the absence of CLPP causes accelerated ovarian follicle depletion and results in a phenotype similar to diminished ovarian reserve and ovarian aging [
5].
In this study, we aimed to investigate whether the infertility and ovarian follicular depletion phenotype observed in mice with global deletion of Clpp is associated with changes in somatic tissues that suggest accelerated aging. Our findings demonstrate that lack of Clpp causes shorter telomeres in oocytes and a number of somatic tissues of Clpp-/- mice. These changes are associated with decreased expression of genes that regulate telomere length and stability.
Materials and methods
Animals
Clpp+/- mice in a C57BL/6J background (Founder Line # IST13563G11) generated by Texas A&M Institute for Genomic Medicine (TIGM), an institute of AgriLife, and obtained from Georg Auburger, PhD (Goethe University Medical School, Frankfurt am Main, Germany) [
21] were crossbred to obtain
Clpp-/- mice. Mice care, breeding, and experimental procedures were conducted according to the Yale University Animal Research requirements, by using protocols approved by Institutional Animal Care and Use Committee (Protocol #2020-11207). Genotyping was carried out using the methods previously described [
22].
Collection of oocytes and cumulus cells
To obtain immature (germinal vesicle-stage, GV) oocytes, 2-, 6-, and 9-month-old Clpp-/- and wild type (WT) female mice were injected intraperitoneally with 10IU pregnant mare’s serum gonadotropin (PMSG, Sigma, St. Louis, MO). After 44 hours of PMSG injection, ovaries were removed and punctured with a 26-gauge needle in M2 medium (Sigma, St. Louis, MO) supplemented by 10µM milrinone (Sigma, St. Louis, MO) to prevent meiotic resumption.
To collect mature oocytes arrested in the metaphase of the second meiotic divisio (MII), 10IU of human chorionic gonadotropin (hCG; Sigma, St. Louis, MO) was injected 48h hours after the PMSG (Sigma, St. Louis, MO). After 14-16 hours of hCG injection, unfertilized MII oocytes were collected from oviducts. Oocytes were stripped from cumulus cells with a mouth pipette and collected in individual tubes.
Blood and tissue collection
Blood samples (0.5 ml) were collected from each mice (n=5) through intracardiac puncture with a 18-gauge needle. After blood collection, mice were perfused with normal saline through a needle in the left ventricle. After perfusion, tissues (heart, liver, spleen, lung, kidney, uterus, and ovaries) were dissected and washed in Dulbecco’s Phosphate Buffer Saline (DPBS) for 10 seconds. Tissues were stored at -80°C until further experiments.
Telomere length measurement
GV and MII stage oocytes (collected from 10 mice for each genotype at each timepoint, and pooled as 2 mice per sample) were collected from 2-, 6-, and 9-month-old Clpp-/- mice and compared to WT. DNA extraction from oocytes and cumulus cells was performed using the QIAmp DNA Micro Kit (Qiagen, Valencia, CA), and DNA extraction from white blood cells (WBC) was conducted with DNA Isolation Kit for Mammalian Blood (Roche, Basel, Switzerland) according to manufacturer’s protocol and both concentrations were quantified using Qubit 3.0 (Life Technologies).
Average telomere length was measured from total genomic mouse DNA using a real-time quantitative PCR method previously described [
16]. The average telomere length ratio was obtained by quantifying telomeric DNA with specially designed primer sequences and dividing that amount by the quantity of a single-copy gene, acidic ribosomal phosphoprotein PO (36B4) gene. Forward and reverse telomere and 36B4 primers are shown in Supplementary Table 1. Each reaction included 10 μl iQ™ SYBR
® Green Supermix, (Bio-Rad), 400 nM of each primer, 1 ng genomic DNA, and enough double-distilled H
2O to complete the volume in 20-μl reaction. PCR reactions were performed on the iCycler iQ real-time PCR detection system (Bio-Rad, Hercules, CA). For each PCR reaction, a standard curve was generated by serial dilutions of known amounts of DNA from the same tissues. The telomere signal was normalized to the signal from the single-copy gene to generate a T/S ratio indicative of relative telomere length. The relative input amount of the telomere PCR then was divided by the relative input amount of the 36B4 PCR of the same sample. Each real-time PCR experiment was repeated a minimum of three times.
Quantitative reverse-transcription polymerase chain reaction (qRT-PCR)
Total RNA was obtained from 20 oocytes per mouse using RNAqueous Microkit (Ambion, Austin, TX) and was treated for genomic DNA contamination using DNase I (Ambion). Reverse transcription was performed using the RETROscript kit (Ambion) in two steps: first, template RNA and oligo(dT) primers were incubated at 850C for 3 min to eliminate any secondary structures, and then the buffer and enzyme were added and the reaction was carried out at 420C for 1 h. qPCR was carried out on an iCycler (Bio-Rad Laboratories). Each 10-µl PCR reaction contained 5µl of iQ™ SYBR® Green Supermix (Bio-Rad Laboratories), 3µl of H2O, 0.5µl of each primer, and 1µl of cDNA. The 2-∆∆CT (cycle threshold) method was used to calculate relative expression levels after normalization to β-actin or Gapdh levels. Samples were assayed in triplicate and each experiment was repeated at least three times using individual animals from each genotype. The primers used for real-time PCR reactions are reported in Supplementary Table 1.
Immunofluorescent staining
For immunofluorescent staining, cumulus oophorus complexes (COCs) containing GV stage oocytes were collected and fixed with 4% paraformaldehyde (Sigma, St. Louis, MO) in Dulbecco’s Phosphate Buffer Saline (DPBS) for 5 min, then washed three times in 1X DPBS. They were placed in 0.5% Triton X-100 in DPBS at room temperature for 10 minutes, then washed in DPBS for 5 minutes. After blocking in 3% BSA (Sigma, St. Louis, MO) at room temperature for 1h, COCs were incubated overnight at 4°C with rat anti-TRF1 monoclonal antibody (Abcam, Cambridge, UK Cat# ab192629, 1:100) or mouse anti-H2A.X monoclonal antibody (Sigma-Aldrich, St. Louis, MO Cat# 05-636-25UG 1:100) as a primary antibody. After three washes with 1X DPBS for 10 minutes, COCs were incubated with Alexa Fluor 488-conjugated goat anti-mouse antibody (1:400) or Alexa Fluor 568-conjugated goat anti-rat antibody (1:400) for 1h at room temperature. Finally, they were mounted with 4′, 6-diamidino-2-phenylindole (DAPI;1:1000) (Life Technologies, Carlsbad, CA) on glass slides and stored at 4°C until imaging. Images were captured on Leica SP8 spectral scanning confocal microscope and Image J software was used to quantify the fluorescence intensity.
Discussion
CLPP is essential for mtUPR as it promotes the breakdown of misfolded proteins within the mitochondria and initiates a series of reactions to eliminate the detrimental impact of mitochondrial stressors and to re-establish protein homeostasis [
11]. Studies using knockout mouse models demonstrated that CLPP is required for female fertility [
5,
21]. In addition, global germline deletion of
Clpp results in the activation of the mTOR (mammalian target of rapamycin) pathway and causes accelerated loss of ovarian follicular reserve, highlighting the role of CLPP in female reproductive competence and senescence [
5]. In this study, we asked whether the infertility and ovarian aging phenotype caused by global germline deletion of
Clpp is associated with somatic aging, and tested telomere length in young and aging mice gametes, gonads and somatic tissues. We found shortening of telomeres in both oocytes and somatic tissues at 6 and 9 months. In addition, expression of several genes associated with telomere integrity were decreased, and double strand DNA breaks were increased in telomeric regions. Our findings demonstrate how loss of mitochondrial protein homeostasis may accelerate telomere shortening in oocytes and somatic cells, and provide a link between reproductive and somatic aging.
A number of plausible mechanisms could be linking mitochondrial dysfunction and telomere shortening. Oxidative stress causes cell death and/or senescence, and as a result, rapid cell divions occur in the surrounding essential cells as a protective strategy, which leads to telomere shortening [
28,
29]. Reciprocally, in tissues with short telomeres, mitochondrial quantity and oxidative phosphorylation capacity are impaired, resulting in decreased ATP synthesis, decreased metabolic capacity, impaired gluconeogenesis, and elevated ROS levels [
30,
31]. We have previously reported that global knockout of
Clpp resulted in increased ROS levels in GV oocytes [
5], strengthening the association between increased ROS and telomere shortening in this model.
In 6- and 9-month-old
Clpp-deficient mice, we found shorter telomere length in the liver when compared with WT, suggesting that the impaired mitrochondrial function within the liver in
Clpp-/- mice could be triggering hepatic cellular changes that culminate in telomere shortening. Highly proliferative tissues, including the hematopoietic and immune systems exhibit impaired proliferative capacity in later generations of the telomerase RNA component (TERC)-deficient mice [
32,
33]. Telomerase complex gene mutations have been linked to rare human diseases such as Dyskeratosis Congenita (DKC) and Idiopathic Pulmonary Fibrosis [
34], both of which are characterized by accelerated telomere shortening and organ failure. Patients with such diseases had an increased frequency of liver pathologies such as fibrosis and cirrhosis [
35,
36]. Similarly, two studies investigated the frequency of telomerase mutations in patients with sporadic cirrhosis compared to healthy controls and demonstrated mutation missense mutations in the Telomerase Reverse Transcriptase (TERT) and TERC genes in diseased patients [
37].
Global deletion of
Clpp resulted in mitochondrial dysfunction in oocytes by decrasing ATP production, expression levels of Electron Transport Chain (ETC) enzymes and increased levels of ROS [
5]. Besides, the lack of
Clpp affected the reproductive phenotype. Mice demonstrated a decrease in the number of mature oocytes and 2-cell embryos, and no blastocysts, resulting in infertility. In addition, they had accelerated follicular depletion and a phenotype consistent with dimished ovarian reserve [
5]. In the current study, oocyte telomeres are shortened in at 6- and 9-month-old mice models with global deletion of
Clpp. Consistent with our findings, when guanine-rich telomeric repeats are exposed to oxidative stress including ROS, they undergo oxidative DNA damage that shortens telomeres in non-dividing cells like oocytes, thereby potentially accelerating aging [
38].
Global deletion of
Clpp is also associated with spindle abnormalities and decreased ability to complete in vitro maturation of GV stage oocytes [
5]. In the current study, shorter telomeres in 6- and 9-month-old
Clpp-/- mice oocytes suggest that the reproductive phenotype of
Clpp-/- mice may be, in part, due to oocyte telomere shortening. Indeed, telomeres play an important role in the regulation of chromosomal motions during meiosis, including bouquet creation at the leptotene stage, homologous pairing, and interaction with microtubules and spindles [
39,
40]. Short telomeres are linked to infertility, aberrant spindles, and misaligned metaphase chromosomes in oocytes of telomerase-deficient mice [
41]. Similarly, telomere length in human unfertilized oocytes was associated with the morphological quality of the embryos generated from sibling oocytes from the same cohort, and subsequent pregnancy outcomes [
42,
43].
The impact of telomere length on oocyte function has been explored in animal and human studies [42-44]. Meanwhile, the role telomere length in cumulus/granulosa cell function remains to be further characterized, and exisiting data does not suggest this as a key regulatory mechanism. In the context of mtUPR, while global
Clpp knockout was associated with infertility [
5], granulosa cell-specific targeted deletion of
Clpp did not affect reproduction [
45]. In keeping with these findings, we did not find cumulus/granulosa cell telomere length to be shortened in
Clpp-/- mice. This is not surprising as recent studies investigating the telomere length and epigenetic aging markers in human cumulus/granulosa cells did not see a difference when comparing younger versus older reproductive age women or those with good versus poor ovarian response [46-48].
A number of telomere proteins, including TRF1, TRF2, and POT1a, regulate telomere stability and length [
49]. Zhang et al. revealed that mice with targeted deletion of mitochondrial fusion protein Mitofusin 2 (MFN2) have defective oocyte maturation, subfertility, shortened telomeres, and decreased TRF1 expression in oocytes [
16]. They also showed co-localization of TRF1 with DNA repair factor 53BP1, suggesting DNA damage. Our findings are similar in that
Trf1,
Trf2, and
Pot1a expression is decreased in 6-month-old
Clpp-/- mice oocytes, and TRF1 co-localizes with DBA repair factor H2AX. Collectively these two studies show a consistent effect of mitochondrial dysfunction impacting dysregulated telomere mechanisms, thereby leading to DNA damage and telomere shortening.
In this study, we expanded our understanding of the role of Clpp and mtUPR in female reproduction and aging by characterizing telomere shortening and damage in a mouse model with global deletion of Clpp. As reported previously, the lack of Clpp results in functional mitochondrial abnormalities, infertility, and ovarian follicular depletion/aging. The current study demonstrates how this mitochondrial pathway, when impaired, may also promote somatic aging. In addition, our findings provide a preliminary understanding of how mitochondrial and telomeric aging mechansims may interact to accelerate reproductive and somatic aging. Further studies are needed to delineate the intricate interactions between these two aging pathways and to determine whether these could be exploited to delay or reverse ovarian (or somatic) aging.
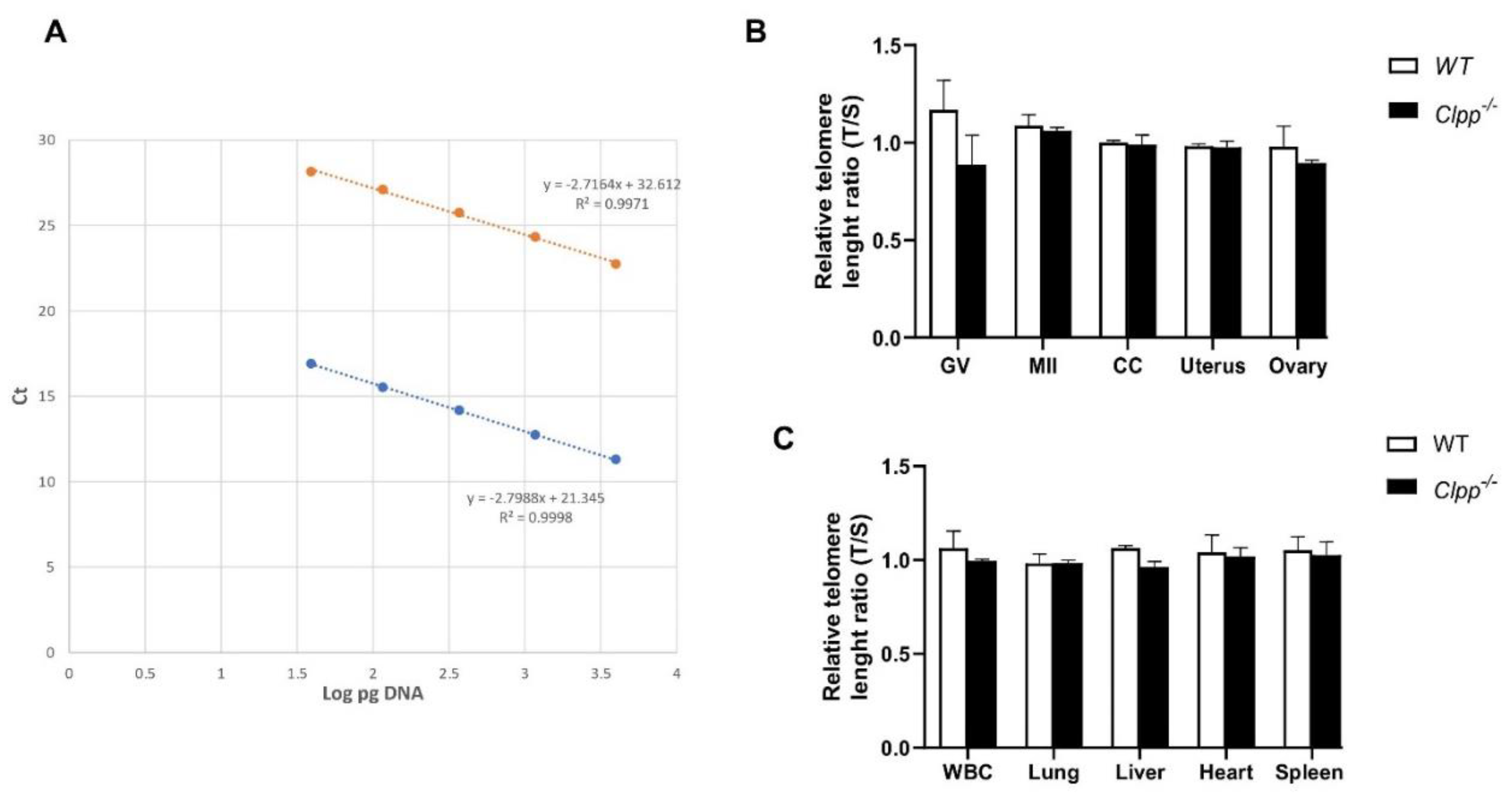
Figure 1.
Telomere length in oocytes, ovaries, and somatic tissues of 2-month-old Clpp-/- and WT mice. (A) The standard curve was generated by serial dilution of known amounts of DNA to calculate relative DNA concentrations (log DNA) from Ct values of the qPCR products. Blue dots: telomere; orange dots: 36B4 single copy gene (control). The correlation regression equation and coefficients (R2) of Ct versus log DNA are shown. (B,C) The relative telomere lengths of GV and MII oocytes, ovaries and somatic cells and tissues are represented as ratios of T/S. GV: Germinal vesicle, CC: Cumulus cells, WBC: White blood cells. Data presented as mean ± SEM. **p < 0.01, *p < 0.05 using t-test.
Figure 1.
Telomere length in oocytes, ovaries, and somatic tissues of 2-month-old Clpp-/- and WT mice. (A) The standard curve was generated by serial dilution of known amounts of DNA to calculate relative DNA concentrations (log DNA) from Ct values of the qPCR products. Blue dots: telomere; orange dots: 36B4 single copy gene (control). The correlation regression equation and coefficients (R2) of Ct versus log DNA are shown. (B,C) The relative telomere lengths of GV and MII oocytes, ovaries and somatic cells and tissues are represented as ratios of T/S. GV: Germinal vesicle, CC: Cumulus cells, WBC: White blood cells. Data presented as mean ± SEM. **p < 0.01, *p < 0.05 using t-test.
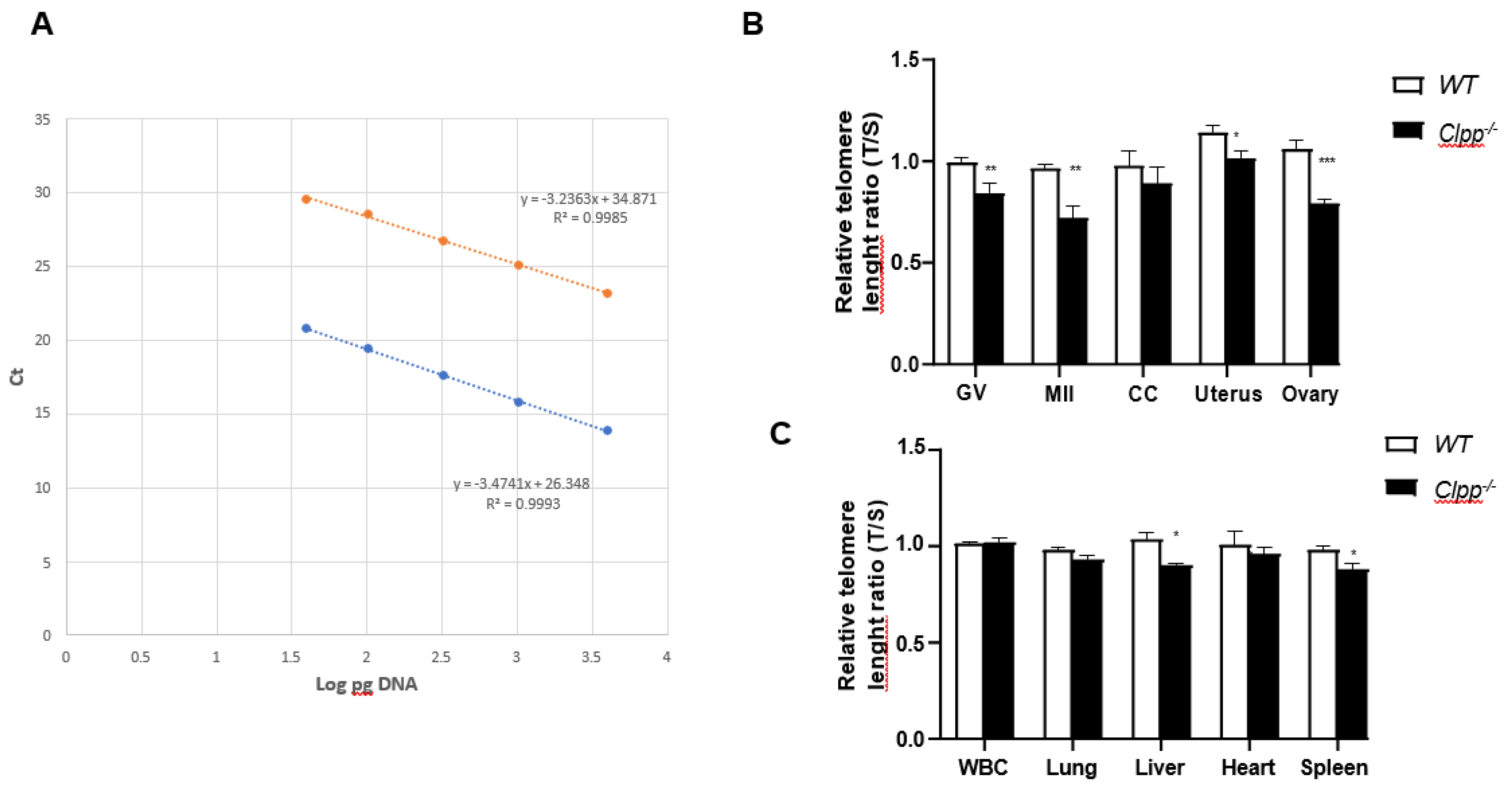
Figure 2.
Telomere length in oocytes, ovaries, and somatic tissues of 6-month-old Clpp-/- and WT mice. (A) The standard curve was generated by serial dilution of known amounts of DNA to calculate relative DNA concentrations (log DNA) from Ct values of the qPCR products. Blue dots: telomere; orange dots: 36B4 single copy gene (control). The correlation regression equation and coefficients (R2) of Ct versus log DNA are shown. (B,C) The relative telomere lengths of GV and MII oocytes, ovaries and somatic cells and tissues are represented as ratios of T/S. GV: Germinal vesicle, CC: Cumulus cells, WBC: White blood cells. Data presented as mean ± SEM. **p < 0.01, *p < 0.05 using t-test.
Figure 2.
Telomere length in oocytes, ovaries, and somatic tissues of 6-month-old Clpp-/- and WT mice. (A) The standard curve was generated by serial dilution of known amounts of DNA to calculate relative DNA concentrations (log DNA) from Ct values of the qPCR products. Blue dots: telomere; orange dots: 36B4 single copy gene (control). The correlation regression equation and coefficients (R2) of Ct versus log DNA are shown. (B,C) The relative telomere lengths of GV and MII oocytes, ovaries and somatic cells and tissues are represented as ratios of T/S. GV: Germinal vesicle, CC: Cumulus cells, WBC: White blood cells. Data presented as mean ± SEM. **p < 0.01, *p < 0.05 using t-test.
Figure 3.
Telomere length in oocytes, ovaries, and somatic tissues of 9-month-old Clpp-/- and WT mice. (A) The standard curve was generated by serial dilution of known amounts of DNA to calculate relative DNA concentrations (log DNA) from Ct values of the qPCR products. Blue dots: telomere; orange dots: 36B4 single copy gene (control). The correlation regression equation and coefficients (R2) of Ct versus log DNA are shown. (B,C) The relative telomere lengths of GV and MII oocytes, ovaries and somatic cells and tissues are represented as ratios of T/S. GV: Germinal vesicle, CC: Cumulus cells, WBC: White blood cells. Data presented as mean ± SEM. **p < 0.01, *p < 0.05 using t-test.
Figure 3.
Telomere length in oocytes, ovaries, and somatic tissues of 9-month-old Clpp-/- and WT mice. (A) The standard curve was generated by serial dilution of known amounts of DNA to calculate relative DNA concentrations (log DNA) from Ct values of the qPCR products. Blue dots: telomere; orange dots: 36B4 single copy gene (control). The correlation regression equation and coefficients (R2) of Ct versus log DNA are shown. (B,C) The relative telomere lengths of GV and MII oocytes, ovaries and somatic cells and tissues are represented as ratios of T/S. GV: Germinal vesicle, CC: Cumulus cells, WBC: White blood cells. Data presented as mean ± SEM. **p < 0.01, *p < 0.05 using t-test.
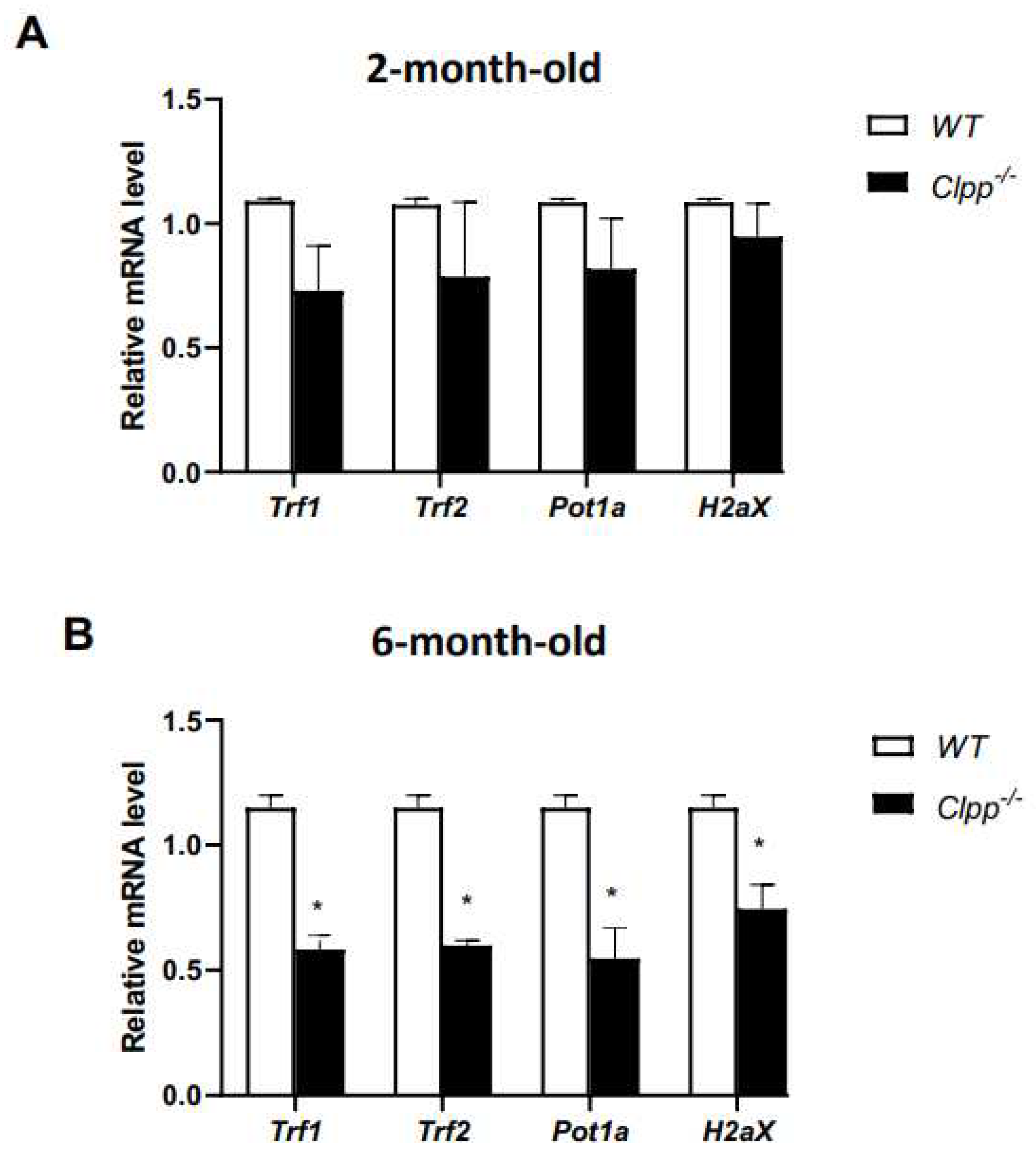
Figure 4.
Expression of telomere-associated genes in Clpp -/- and WT oocytes. Expression of telomere-associated genes was assessed using qRT-PCR in GV oocytes collected from 2-month-old (A) and 6-month-old (B) Clpp-/- and WT mice. Data presented as mean ± SEM with t-test (**p < 0.01, *p < 0.05).
Figure 4.
Expression of telomere-associated genes in Clpp -/- and WT oocytes. Expression of telomere-associated genes was assessed using qRT-PCR in GV oocytes collected from 2-month-old (A) and 6-month-old (B) Clpp-/- and WT mice. Data presented as mean ± SEM with t-test (**p < 0.01, *p < 0.05).
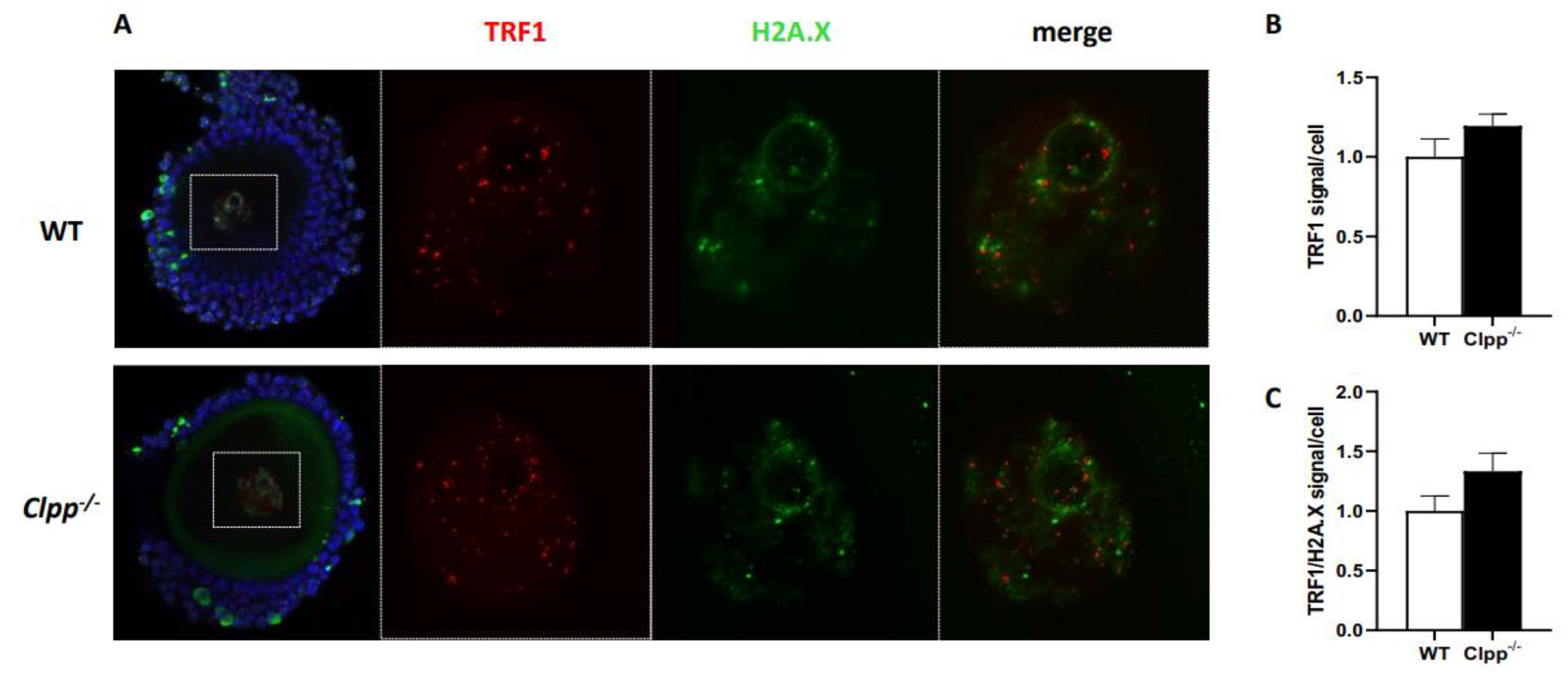
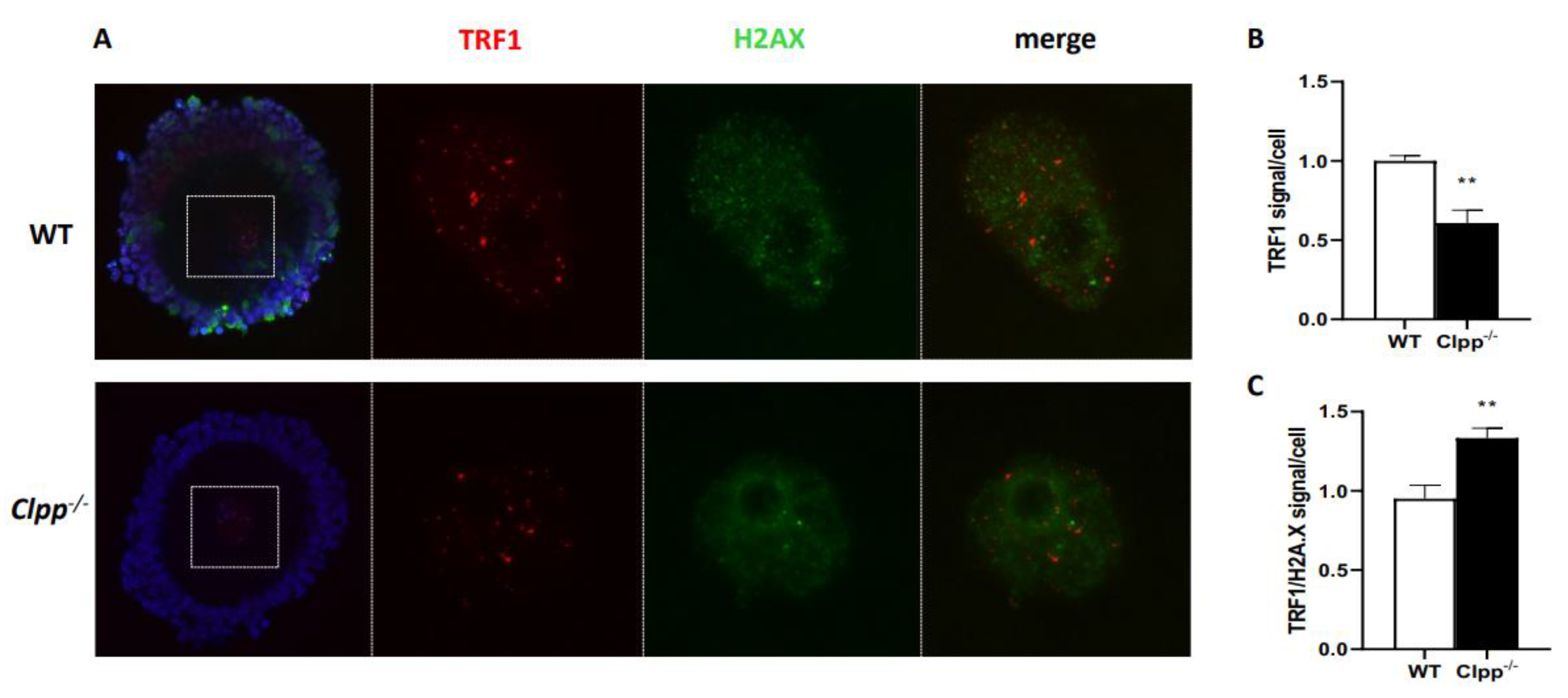
Figure 5.
TRF1 expression and TRF/H2AX co-localization in 2-month-old Clpp -/- and WT mice oocytes. (A) Immunofluorescence double staining of TRF1 (red) and H2AX (green) in cumulus oophorus complexes of 2-month-old Clpp-/- and WT mice. DAPI was used to stain nuclei (blue). (B) Quantitative analysis of TRF1 immunofluorescence in Clpp-/- and WT GV oocytes. (C) Quantitative analysis of co-localization of H2AX and TRF1 in Clpp-/- and WT GV oocytes. Data presented as mean ± SEM with t-test (**p < 0.01, *p < 0.05).
Figure 5.
TRF1 expression and TRF/H2AX co-localization in 2-month-old Clpp -/- and WT mice oocytes. (A) Immunofluorescence double staining of TRF1 (red) and H2AX (green) in cumulus oophorus complexes of 2-month-old Clpp-/- and WT mice. DAPI was used to stain nuclei (blue). (B) Quantitative analysis of TRF1 immunofluorescence in Clpp-/- and WT GV oocytes. (C) Quantitative analysis of co-localization of H2AX and TRF1 in Clpp-/- and WT GV oocytes. Data presented as mean ± SEM with t-test (**p < 0.01, *p < 0.05).
Figure 6.
TRF1 expression and TRF/H2AX co-localization in 6-month-old Clpp -/- and WT mice oocytes. (A) Immunofluorescence double staining of TRF1 (red) and H2AX (green) in cumulus oophorus complexes of 6-month-old Clpp-/- and WT mice. DAPI was used to stain nuclei (blue). (B) Quantitative analysis of TRF1 immunofluorescence in Clpp-/- and WT GV oocytes. (C) Quantitative analysis of co-localization of H2AX and TRF1 in Clpp-/- and WT GV oocytes. Data presented as mean ± SEM with t-test (**p < 0.01, *p < 0.05).
Figure 6.
TRF1 expression and TRF/H2AX co-localization in 6-month-old Clpp -/- and WT mice oocytes. (A) Immunofluorescence double staining of TRF1 (red) and H2AX (green) in cumulus oophorus complexes of 6-month-old Clpp-/- and WT mice. DAPI was used to stain nuclei (blue). (B) Quantitative analysis of TRF1 immunofluorescence in Clpp-/- and WT GV oocytes. (C) Quantitative analysis of co-localization of H2AX and TRF1 in Clpp-/- and WT GV oocytes. Data presented as mean ± SEM with t-test (**p < 0.01, *p < 0.05).