Submitted:
24 May 2023
Posted:
25 May 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
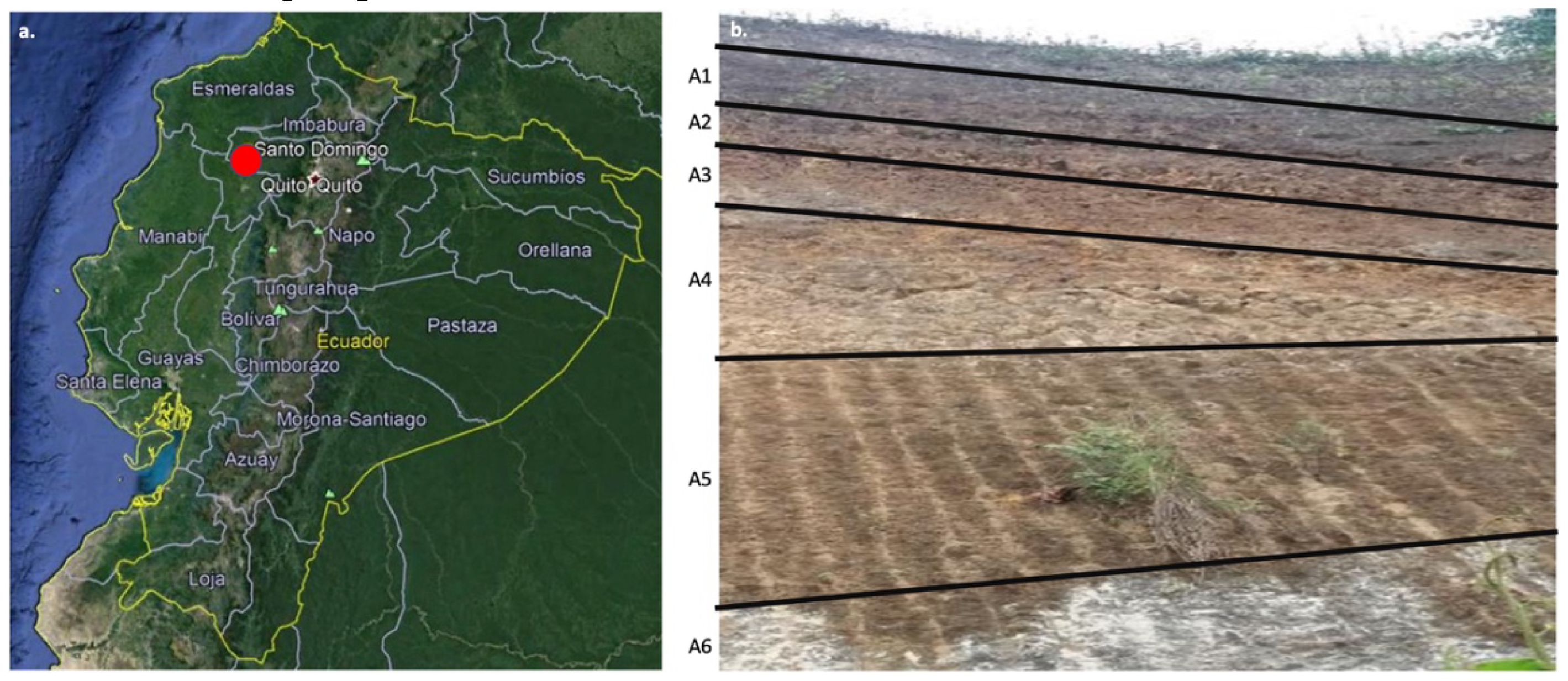
2.1. Clay Samples
2.2. Organic matter and carbonate removal treatment
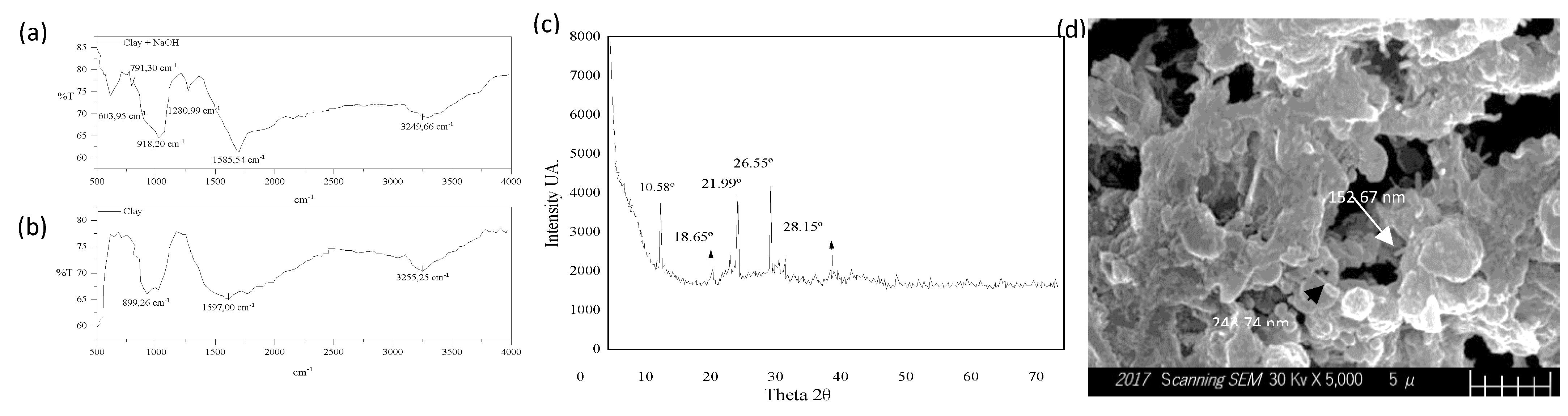
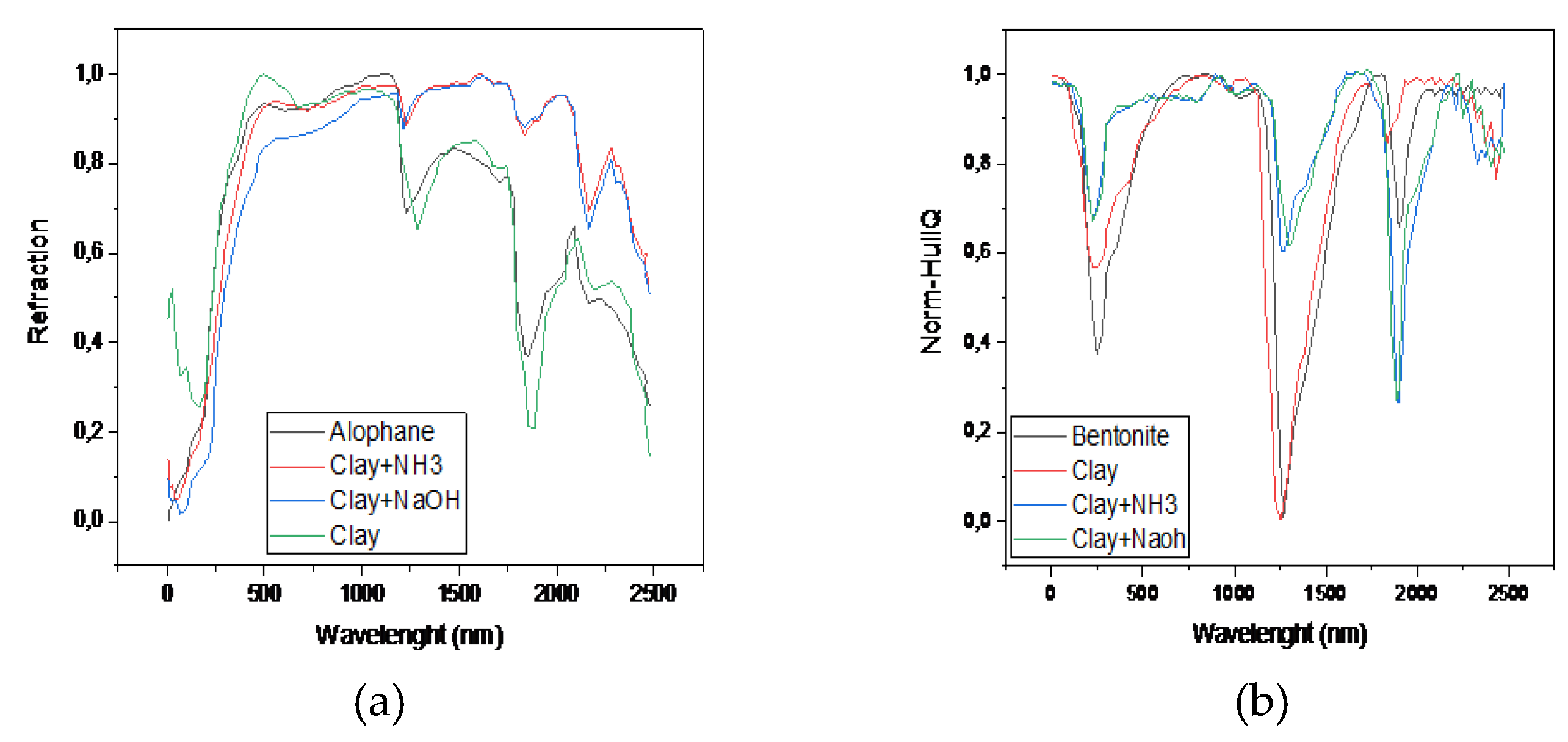
2.3. Infrared Spectrometer, FTIR
2.4. X-ray diffraction, XRD
2.5. X ray fluorescence, XRF
2.6. Scanning electron microscope, SEM
2.7. BET Isotermhemical
2.8. Laser granulometry
2.9. SPECMIN, TSG
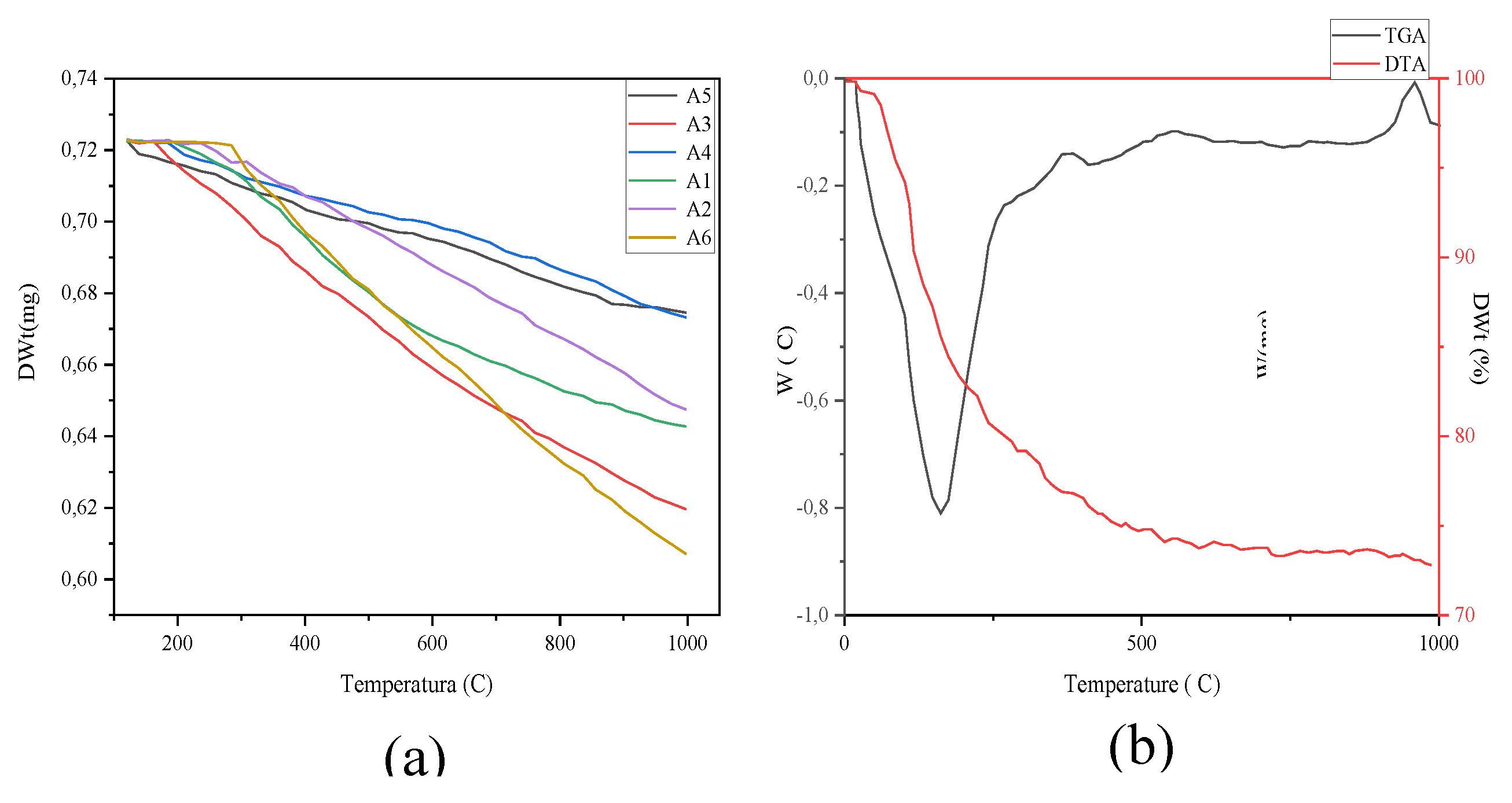
2.10. Thermogravimetry, TGA
2.11. Differential scanning calorimeter, DSC
2.12. Atmospheric Adsorption Test
3. Results and Discussion
3.1. Characterization of volcanic clays from Santo Domingo de los Tsáchilas
3.2. Application
4. Conclusion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hunt, A. The Oxford handbook of archaeological ceramic analysis. 2017 [cited 2022 Dec 3]; Available from: https://books.google.com.ec/books?hl=es&lr=&id=RQ-DDQAAQBAJ&oi=fnd&pg=PP1&dq=The+Oxford+Handbook+of+Archaeological+Ceramic+Analysis+-+Google+Libros+%5BInternet%5D.+%5Bcited+2022+Nov+30%5D.+Available+from:+https://books.google.es/books%3Fhl%3Des%26lr%3D%26id%3DRQ-DDQAAQBAJ%26oi%3Dfnd%26pg%3DPA148%26dq%3Dclay,%2B%2Braw%2Bmaterial%2Bfor%2Bthe%2Belaboration%2Bof%2Bvarious%2Belements,%2B&ots=jsHe9Rcr-W&sig=tKsqEhJMx6uf-Pq9zhAdA4MnBC4.
- Ross, C.S.; Shannon, E.v. THE MINERALS OF BENTONITE AND RELATED CLAYS AND THEIR PHYSICAL PROPERTIES1. Journal of the American Ceramic Society 1926, 9, 77–96, Available from: https://onlinelibrary.wiley.co. [Google Scholar] [CrossRef]
- Bergaya, F.; Beneke, K.; Lagaly, G. History and Perspectives of Clay Science.
- Jozanikohan, G.; Sahabi, F.; Hossain Norouzi, G.; Memarian, H. Thermal Analysis: A Complementary Method to Study the Shurijeh Clay Minerals. Int J Min & Geo-Eng. 2015, 49, 33–45. [Google Scholar]
- Grim, R.E. Clay Mineralogy. Science (1979). 1962, 135, 890–8, Available from: https://www.science.or. [Google Scholar] [CrossRef] [PubMed]
- Ross, C.S.; Kerr, P.F. The clay minerals and their identity. Journal of Sedimentary Research 1931, 1, 55–65. [Google Scholar]
- Gruner, J.W. The Crystal Structure of Kaolinite. Z Kristallogr Cryst Mater. 1932, 83, 75–88, Available from: https://www.degruyter.com/documen. [Google Scholar] [CrossRef]
- Insley H, of REJ of R of the NB, 1935 undefined. Thermal behavior of the kaolin minerals. nvlpubs.nist.gov 1935, 14. Available from: http://nvlpubs.nist.gov/nistpubs/jres/14/jresv14n5p615_A1b.pdf. [Google Scholar]
- Shaw, B.T. The nature of colloidal clay as reve aled by the electron microscope. Journal of Physical Chemistry 1942, 46, 1032–43, Available from: https://pubs.acs.or. [Google Scholar] [CrossRef]
- Grim, R.E.; Bradley, W.F. A UNIQUE CLAY FROM THE GOOSE LAKE, ILLINOIS, AREA. Journal of the American Ceramic Society 1939, 22, 157–64, Available from: https://www.researchgate.net/publication/229994475_A_unique_clay_from_the_Goose_Lake_Illinois_area. [Google Scholar] [CrossRef]
- Jordan, J.W. Organophilic bentonites. I: Swelling in organic liquids. Journal of Physical and Colloid Chemistry 1949, 53, 294–306, Available from: https://pubs.acs.or. [Google Scholar] [CrossRef]
- Richardson, L.L. Use of bleaching, clays, in processing edible oils. Journal of the American Oil Chemists’ Society 1978, 55, 777–80, Available from: https://link.springer.com/article/10.1007/BF02682647. [Google Scholar] [CrossRef]
- Chauhan, R.S.; Chaturvedi, R.; Gutch, P.K. Polymer-clay Nano Composites. Def Sci, J. 2006, 56, 649. [Google Scholar] [CrossRef]
- Kojima, Y.; Usuki, A.; Kawasumi, M.; Okada, A.; Kurauchi, T.; Kamigaito, O. Synthesis of nylon 6–clay hybrid by montmorillonite intercalated with ϵ-caprolactam. J Polym Sci A Polym Chem 1993, 31, 983–6, Available from: https://onlinelibrary.wiley.co. [Google Scholar] [CrossRef]
- Lepoittevin, B.; Devalckenaere, M.; Pantoustier, N.; Alexandre, M.; Kubies, D.; Calberg, C.; et al. Poly (ε-caprolactone)/clay nanocomposites prepared by melt intercalation: mechanical, thermal and rheological properties. Elsevier 2002, 43. Available from: https://www.sciencedirect.com/science/article/pii/S003238610200229X. [Google Scholar] [CrossRef]
- Deshmane, C.; Yuan, Q. , … RPMS and, 2007 undefined. On striking variation in impact toughness of polyethylene–clay and polypropylene–clay nanocomposite systems: the effect of clay–polymer interaction. Elsevier [cited 2022 Dec 1]; Available online: https://www.sciencedirect.com/science/article/pii/S0921509306027183.
- Kaufhold, S.; Ufer, K.; Kaufhold, A.; Stucki, J.W.; Anastácio, A.S.; Jahn, R.; et al. Quantification of Allophane from Ecuador. Clays and Clay Minerals 2010, 58, 707–16, Available from: https://link.springer.com/article/10.1346/CCMN.2010.0580509. [Google Scholar] [CrossRef]
- EBSCOhost | 54292474 | ENGINEERING PROPERTIES OF NANOCLAY MODIFIED ASPHALT CONCRETE MIXTURES. [Internet]. [cited 2022 Dec 1]. Available online: https://web.p.ebscohost.com/abstract?direct=true&profile=ehost&scope=site&authtype=crawler&jrnl=2193567X&asa=Y&AN=54292474&h=W6omaecdNYnpwIFB3%2fW4riTg%2bUGgqBCwmoeEAfZEI87M5OBOXlt%2bpI7LXD8IbpR%2bME7VUz%2fppQgk%2bxGusTcZEw%3d%3d&crl=c&resultNs=AdminWebAuth&resultLocal=ErrCrlNotAuth&crlhashurl=login.aspx%3fdirect%3dtrue%26profile%3dehost%26scope%3dsite%26authtype%3dcrawler%26jrnl%3d2193567X%26asa%3dY%26AN%3d54292474.
- Sangeetha, V.H.; Deka, H.; Varghese, T.O.; Nayak, S.K. State of the art and future prospectives of poly(lactic acid) based blends and composites. Polym Compos 2018, 39, 81–101, Available from: https://onlinelibrary.wiley.co. [Google Scholar] [CrossRef]
- Yang, J.; Tighe, S. A Review of Advances of Nanotechnology in Asphalt Mixtures. Procedia Soc Behav Sci. 2013, 96, 1269–76. [Google Scholar] [CrossRef]
- Martinho, F.C.G.; Farinha, J.P.S. An overview of the use of nanoclay modified bitumen in asphalt mixtures for enhanced flexible pavement performances. Road Materials and Pavement Design. 2019, 20, 671–701. [Google Scholar] [CrossRef]
- Kaufhold, S.; Kaufhold, A.; Jahn, R.; Brito, S.; Dohrmann, R.; Hoffmann, R.; et al. A New Massive Deposit of Allophane Raw Material in Ecuador. Clays Clay Miner. 2009, 57, 72–81. [Google Scholar] [CrossRef]
- Clay Minerals in Nature: Their Characterization, Modification and Application - Google Libros [Internet]. [cited 2022 Dec 1]. Available online: https://books.google.com.ec/books?hl=es&lr=&id=S9mgDwAAQBAJ&oi=fnd&pg=PR11&dq=Kaolinites,+Montmorillonites,+Illites+and+Chlorites,+and+their+subdivisions&ots=s6jEafivA0&sig=H0FGP1KpNwgM0PhNt83vpUjBzRs#v=onepage&q&f=false.
- Neall, VE. VOLCANIC SOILS.
- Schaming, D.; Remita, H. Nanotechnology: from the ancient time to nowadays. Foundations of Chemistry 2015, 17, 187–205, Available from: https://link.springer.com/article/10.1007/s10698-015-9235-y. [Google Scholar] [CrossRef]
- Mendoza, R.; Espinoza, A. Guía Técnica para muestreo de suelos. Asa Available online: https://core.ac.uk/download/pdf/151729876.pdf%0Ahttp://repositorio.una.edu.ni/3613/1/P33M539.pdf. 2017, 13–21. [Google Scholar]
- Kaufhold, S.; Ufer, K.; Kaufhold, A.; Stucki, J.W.; Anastácio, A.S.; Jahn, R.; et al. Quantification of allophane from Ecuador. Clays Clay Miner. 2010, 58, 707–16. [Google Scholar] [CrossRef]
- Mondragón, R.; Juliá, J.E.; Barba, A.; Jarque, J.C. Preparación y caracterización de nanofluidos: Influencia de variables sobre su estabilidad, estado de aglomeración y propiedades físicas. Boletin de la Sociedad Espanola de Ceramica y Vidrio. 2014, 53, 101–10. [Google Scholar] [CrossRef]
- el Hafid, K.; Hajjaji, M.; el Hafid, H. Influence of NaOH concentration on microstructure and properties of cured alkali-activated calcined clay. Journal of Building Engineering 2017, 11, 158–65. [Google Scholar] [CrossRef]
- Önal, M.; Sarikaya, Y. Some physicochemical properties of a clay containing smectite and palygorskite. Appl Clay Sci. 2009, 44, 161–5. [Google Scholar] [CrossRef]
- Mishra, A.; Allauddin, S.; Narayan, R. TAC, 2012 undefined. Characterization of surface-modified montmorillonite nanocomposites. Elsevier [Internet]. [cited 2020 May 23]; Available online: https://www.sciencedirect.com/science/article/pii/S027288421100722X.
- Allegretta, I.; Ciasca, B.; Pizzigallo, M.D.R.; Lattanzio, V.M.T.; Terzano, R. A fast method for the chemical analysis of clays by total-reflection x-ray fluorescence spectroscopy (TXRF). Appl Clay Sci. 2019, 180, 105201. [Google Scholar] [CrossRef]
- Bohor, B.F.; Hughes, R.E. Scanning electron microscopy of clays and clay minerals. Clays Clay Miner 1971, 19, 49–54, Available from: https://link.springer.com/article/10.1346/CCMN.1971.0190105. [Google Scholar] [CrossRef]
- Hatch, C.D.; Wiese, J.S.; Crane, C.C.; Harris, K.J.; Kloss, H.G.; BaltrusaitisJ. Water adsorption on clay minerals as a function of relative humidity: Application of BET and Freundlich adsorption models. Langmuir. 2012, 28, 1790–803. [Google Scholar] [CrossRef]
- Dur, J.C.; Elsass, F.; Chaplain, V.; Tessier, D. The relationship between particle-size distribution by laser granulometry and image analysis by transmission electron microscopy in a soil clay fraction. Eur J Soil Sci. 2004, 55, 265–70. [Google Scholar] [CrossRef]
- Percival, J.B.; Bosman, S.A.; Potter, E.G.; Peter, J.M.; Laudadio, A.B.; Abraham, A.C.; et al. Customized spectral libraries for effective mineral exploration: Mining national mineral collections. Clays Clay Miner. 2018, 66, 297–314. [Google Scholar] [CrossRef]
- Fernando Mac-as-Quiroga, I.; Inés Giraldo-Gómez, G.; Roc-o Sanabria-González, N. Characterization of Colombian Clay and Its Potential Use as Adsorbent. hindawi.com [Internet]. 2018 [cited 2020 May 23]; Available online: https://doi.org/10.1155/2018/5969178. [CrossRef]
- Frost, R.L.; Ding, Z. Controlled rate thermal analysis and differential scanning calorimetry of sepiolites and palygorskites [Internet]. Elsevier. 2003 [cited 2020 May 23]. Available online: https://www.sciencedirect.com/science/article/pii/S0040603102002289.
- Vyazovkin, S.; Dranca, I. A DSC study of α- and β-relaxations in a PS-clay system. Journal of Physical Chemistry, B. 2004, 108, 11981–7. [Google Scholar] [CrossRef]
- Abdullah, M.; Zamhari, K. , … MHJ of C, 2016 undefined. High temperature characteristics of warm mix asphalt mixtures with nanoclay and chemical warm mix asphalt modified binders. Elsevier [Internet]. [cited 2020 Aug 11]; Available online: https://www.sciencedirect.com/science/article/pii/S0959652616001785.
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of Gases in Multimolecular Layers. J Am Chem Soc. 1938, 60, 309–19. [Google Scholar] [CrossRef]
- Ahmed, A.; Chaker, Y.; Belarbi, E.H.; Abbas, O.; Chotard, J.N.; Abassi, H.B.; et al. XRD and ATR/FTIR investigations of various montmorillonite clays modified by monocationic and dicationic imidazolium ionic liquids. J Mol Struct. 2018, 1173, 653–64. [Google Scholar] [CrossRef]
- Nayak, P.S.; Singh, B.K. Instrumental characterization of clay by XRF, XRD and FTIR. Bulletin of Materials Science 2007, 30, 235–8, Available from: https://link.springer.com/article/10.1007/s12034-007-0042-5. [Google Scholar] [CrossRef]
- Ural, N. The significance of scanning electron microscopy (SEM) analysis on the microstructure of improved clay: An overview. Open Geosciences 2021, 13, 197–218, Available from: https://www.degruyter.com/documen. [Google Scholar] [CrossRef]
- Sagar Nayak, P.; Singh, B.K. Instrumental characterization of clay by XRF, XRD and FTIR. Vol. 30, Bull. Mater. Sci. 2007. [Google Scholar]
- Occelli, M.; Olivier, J.; Perdigon-Melon, J.; Langmuir, A.A. 2002 undefined. Surface area, pore volume distribution, and acidity in mesoporous expanded clay catalysts from hybrid density functional theory (DFT) and adsorption microcalorimetry. ACS Publications [Internet]. [cited 2020 May 23]; Available online: https://pubs.acs.org/doi/abs/10.1021/la020567o.
- Sears, G.W. Determination of Specific Surface Area of Colloidal Silica by Titration With Sodium Hydroxide. Anal Chem. 1956, 28, 1981–3. [Google Scholar] [CrossRef]
- Dur, J.C.; Elsass, F.; Chaplain, V.; Tessier, D. The relationship between particle-size distribution by laser granulometry and image analysis by transmission electron microscopy in a soil clay fraction. Eur J Soil Sci 2004, 55, 265–70, Available from: https://onlinelibrary.wiley.co. [Google Scholar] [CrossRef]
- Percival, J.B.; Bosman, S.A.; Potter, E.G.; Peter, J.M.; Laudadio, A.B.; Abraham, A.C.; et al. Customized spectral libraries for effective mineral exploration: Mining national mineral collections. Clays Clay Miner. 2018, 66, 297–314. [Google Scholar] [CrossRef]
- Ptáček, P.; Šoukal, F.; Opravil, T.; Havlica, J.; Technology, J.B.P. 2011 undefined. The kinetic analysis of the thermal decomposition of kaolinite by DTG technique. Elsevier [Internet]. [cited 2020 May 23]; Available online: https://www.sciencedirect.com/science/article/pii/S0032591010006273.
- Marchuk, A.; Rengasamy, P.; McNeill, A. Influence of organic matter, clay mineralogy, and pH on the effects of CROSS on soil structure is related to the zeta potential of the dispersed clay. Soil Research 2013, 51, 34, Available from: http://www.publish.csiro.au/?paper=SR13012. [Google Scholar] [CrossRef]
- Alujas, A.; Fernández, R.; Quintana, R.; Scrivener, K.L.; Martirena, F. Pozzolanic reactivity of low grade kaolinitic clays: Influence of calcination temperature and impact of calcination products on OPC hydration. Applied Clay Science 2015, 108, 94–101. [Google Scholar] [CrossRef]
- Viswabaskaran, V.; Gnanam, F.D.; Balasubramanian, M. Mullitisation behaviour of calcined clay-alumina mixtures. Ceram Int. 2003, 29, 561–71. [Google Scholar] [CrossRef]
- Todor, D.; Todor, D. Thermal Analysis Of Minerals Dumitru N Todor [Internet]. 1976 [cited 2020 May 23]. Available online: https://pdfs.semanticscholar.org/fe19/5d62df6714083573eb4ab7e4c3bd172fc3cf.pdf.
- Duong, L.v. The morphology and structure of intercalated and pillared clays. 2008. [Google Scholar]
- Sapag K, and SMC and SAP, 2001 undefined. Synthesis and characterization of micro-mesoporous solids: pillared clays. Elsevier [Internet]. [cited 2020 May 23]; Available online: https://www.sciencedirect.com/science/article/pii/S0927775701006173.
- Faı¨za Bergaya F, Lagaly G, Boston A•, Heidelberg •, London •, New •, et al. Handbook of Clay Science Second Edition Techniques and Applications. Handbook of Clay Science: Techniques and Applications [Internet]. 2013 [cited 2020 May 23];5B. Available online: http://dx.doi.org/10.1016/B978-0-08-098259-5.00005-6. 23 May. [CrossRef]
- Corazzari, I.; Nisticò, R.; Turci, F. ; … MFPD, 2015 undefined. Advanced physico-chemical characterization of chitosan by means of TGA coupled on-line with FTIR and GCMS: Thermal degradation and water adsorption capacity. Elsevier [Internet]. [cited 2020 Nov 12]; Available online: https://www.sciencedirect.com/science/article/pii/S014139101400439X.
- Brindley, G.W.; Robinson, K. The structure of kaolinite. Mineralogical Magazine and Journal of the Mineralogical Society 1946, 27, 242–53, Available from: https://www.cambridge.org/core/journals/mineralogical-magazine-and-journal-of-the-mineralogical-society/article/abs/structure-of-kaolinite/F8F0F9B622878457EB175E3722C8D51F. [Google Scholar] [CrossRef]
- Duong, L. The morphology and structure of intercalated and pillared clays. 2008; Available online: http://eprints.qut.edu.au/17707. 1770. [Google Scholar]
- Elimbi, A.; Tchakoute, H.K.; Njopwouo, D. Effects of calcination temperature of kaolinite clays on the properties of geopolymer cements. Constr Build Mater. 2011, 25, 2805–12. [Google Scholar] [CrossRef]
- Chotoli, F.F.; Quarcioni, V.A.; Lima, S.S.; Ferreira, J.C.; Ferreira, G.M. Clay activation and color modification in reducing calcination process: Development in lab and industrial scale. RILEM Bookseries. 2015, 10, 479–86. [Google Scholar]
- Trümer, A.; Ludwig, H.M.; Schellhorn, M.; Diedel, R. Effect of a calcined Westerwald bentonite as supplementary cementitious material on the long-term performance of concrete. Appl Clay Sci 2019, 168, 36–42. [Google Scholar] [CrossRef]
| Element | Sample 1 | Sample 2 | Sample 3 |
|---|---|---|---|
| Si | 0,3077 | 0,3357 | 0,3246 |
| Al | 0,2987 | 0,3259 | 0,3151 |
| Fe | 0,1186 | 0,1294 | 0,1251 |
| Na | 0,0316 | 0,0344 | 0,0333 |
| Ca | 0,0138 | 0,015 | 0,0145 |
| Ti | 0,0116 | 0,0127 | 0,0123 |
| Mg | 0,0111 | 0,0121 | 0,0117 |
| K | 0,0041 | 0,0045 | 0,0043 |
| Mn | 0,0015 | 0,0017 | 0,0016 |
| P | 0,0013 | 0,0014 | 0,0014 |
| S | 0,001 | 0,0011 | 0,0011 |
| TOC | 0,199 | 0,126 | 0,155 |
| Overall | 1 | 1 | 1 |
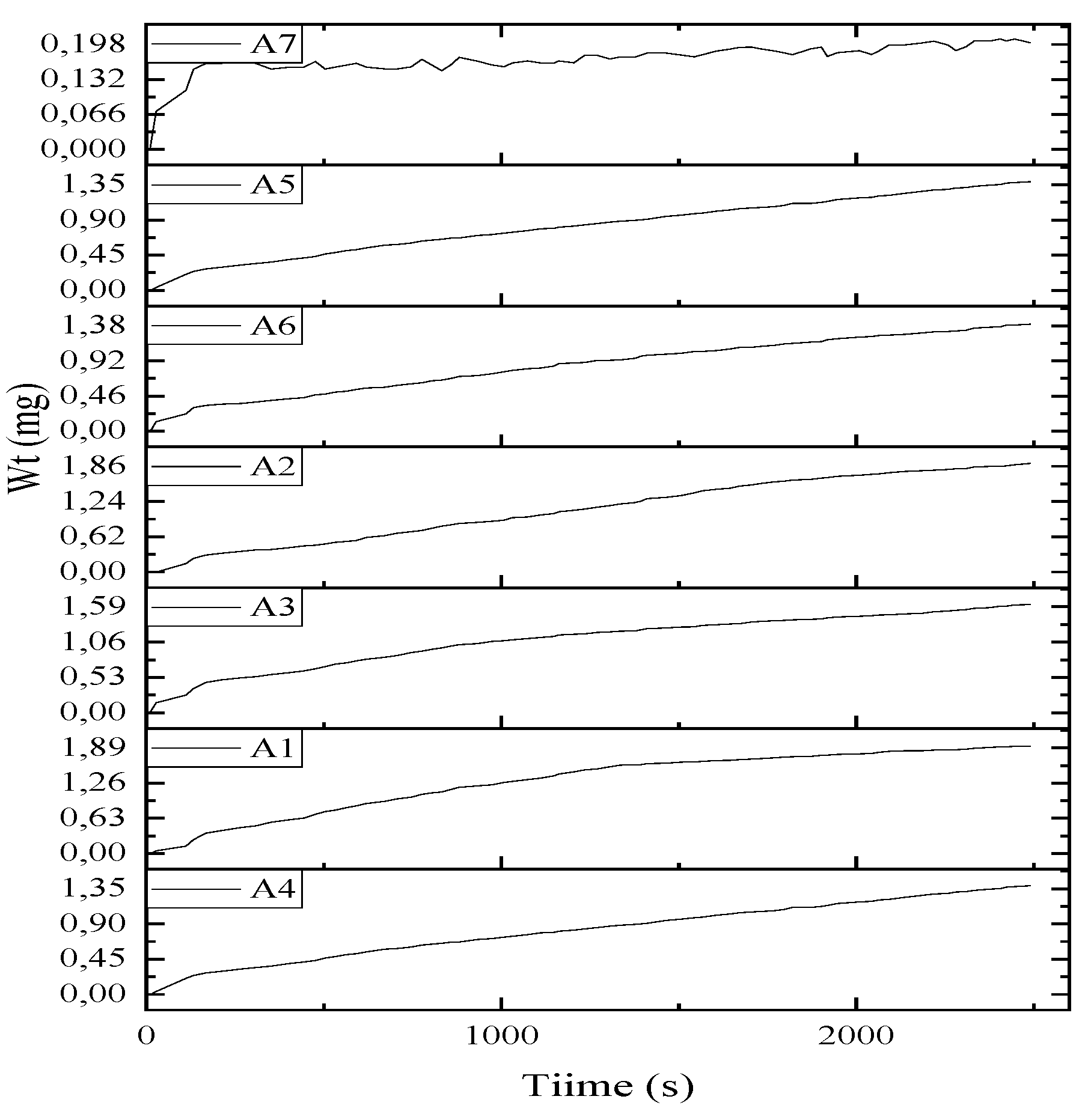
| Weight Wt., g | ||||||
| a) | b) | c) | d) | e) | ||
| A1 | 0,0722 | 0,0307 | 0,0415 | 42,5% | ||
| A2 | 0,0722 | 0,0252 | 0.047 | 34,9% | ||
| A3 | 0,0722 | 0,0291 | 0.0431 | 40,3% | ||
| A4 | 0,0722 | 0,0156 | 0.0566 | 21,6% | ||
| A5 | 0,0722 | 0,0171 | 0.0551 | 23,7% | ||
| A6 | 0,0722 | 0,012 | 0,0602 | 16,6% | ||
| (a) | |||||
| To °C | |||||
| A1 | A2 | A3 | A4 | A5 | A6 |
| 178 | 212 | 192 | 213 | 167 | 258 |
| b) Tm °C | |||||
| A1 A2 | A3 A4 | A5 A6 | |||
| 328 | 339 | 238 | 282 | 347 | 302 |
| 544 | 578 | 682 | 416 | 701 | 697 |
| 656 | 800 | 654 | 871 | ||
| 723 | |||||
| (b) | |||||
| a) Initial Wt. @ T °C | |||||
| Initiation A1 | A2 | A3 | |||
| x | y | x | y | x | y |
| 0.00% | 0.00% | 0.00% | |||
| b) In progress, Wt. @ T°C | |||||
| A1 | A2 | A3 | |||
| 13.20% | 31.00% | 5.40% | 15.50% | 4.00% | 10.00% |
| 29.80% | 70.10% | 16.90% | 48.40% | 30.70% | 76.30% |
| 36.80% | 86.70% | 28.10% | 80.60% | ||
| 39.20% | 92.20% | ||||
| c) Overall , Wt. @ T °C | |||||
| A1 | A2 | A3 | |||
| 42.50% | 100.00% | 34.90% | 100.00% | 40.30% | 100.00% |
| a) Initial, Wt. @ T °C | |||||
| A4 | A5 | A6 | |||
| x | y | x | y | x | y |
| 0.00% | 0.00% | 0.00% | |||
| b) In progress, Wt. @ T °C | |||||
| A4 | A5 | A6 | |||
| 2.20% | 10.30% | 6.50% | 27.50% | 1.50% | 9.20% |
| 6.00% | 27.60% | 15.90% | 67.20% | 10.90% | 65.90% |
| 11.60% | 53.90% | 23.30% | 98.20% | ||
| c) Overall , Wt. @ T °C | |||||
| A4 | A5 | A6 | |||
| 21.60% | 100.00% | 23.70% | 100.00% | 16.60% | 100.00% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





