1. Glial Scar Formation and Composition
Spinal cord injury (SCI) is a condition that affects between 8.8 and 246 people in a million, according to the World Health Organization. Unlike many other neurological disorders, SCI affects mostly young people, causing deficits in sensory, motor and autonomic functions. Axonal atrophy following injury to the central nervous system (CNS) and inability of central axons to regenerate has been described already by Ramón y Cajal in 1928 (Ramón y Cajal 1928), but to this day, promoting regrowth of injured axons remains one of the main challenges to regain function. Various factors may affect axonal regeneration after SCI, and one of them is the glial scar, which has been proposed by several studies as the main constraint for repair (Reier et al., 1983; Davies et al., 1999; Asher et al., 2001).
The glial scar is a compact mesh structure that surrounds the CNS lesions rapidly after the initial damage, and is fully formed between 2 and 3 weeks post injury (wpi) in rodents. This process is triggered by the breakdown of the blood-brain barrier (BBB) and the infiltration of non-neural cells into the CNS parenchyma (Preston et al., 2001). Early on, this infiltrated parenchyma in the epicenter lesion presents edema, myelin debris and degenerating neurons and glia. As the days go by, the edema is reduced and a fibrotic scar begins to form, occupying the entire lesion epicenter by 8 days post-injury (dpi) in rodents (Maxwell et al., 1990). The fibrotic scar is composed by invading hematogenous cells: lymphocytes, macrophages and leukocytes that infiltrate the spinal cord parenchyma; additionally, days after injury, fibroblasts from the damaged meninges invade the lesion core, where they proliferate and secrete molecules that change the extracellular matrix (ECM) (Kawano et al., 2012). Glial cells are rarely found within this fibrotic tissue, accumulating around it instead and separating the lesion epicenter from the spared nervous tissue by forming the glial scar.
This glial scar is mainly composed of astrocytes, NG2+-glia and microglia, with newly-proliferated astrocytes playing the leading role (Kawano et al., 2012). By 2 dpi in rodents, astrocytes can be identified in the vicinity of the lesion epicenter, where they progressively accumulate to form the dense structure that isolates the lesion epicenter from the spared tissue, with their processes extending towards the lesion (Maxwell et al., 1990). During the first two weeks after SCI, astrocytes, NG2+-glia and microglia all undergo extensive proliferation and their numbers remain elevated during the chronic phase (Zai and Wrathall, 2005). Other than their accumulation and dense organization, these glial cells undergo diverse functional and morphological changes themselves, while also affecting the composition of the extracellular matrix (ECM).
The ECM is an essential, non-cellular scaffold that varies for each tissue type, but is always made up of collagens, fibronectin, laminin, hyaluronan and elastin, added to different proteins, proteoglycans and glycosaminoglycans (Theocharis
et al., 2015). The composition of the ECM can influence cell adhesion, migration, proliferation and differentiation, and therefore, cellular and tissue function (Krishnaswamy
et al., 2019). In the healthy adult CNS specifically, the ECM is more loose than in other tissues, because it is constituted primarily by hyaluronan, sulfated proteoglycans and tenascin-R (Lau
et al., 2013; Rauch, 2007). The molecules comprising the ECM play a role in axonal guidance and synaptogenesis that can vary between regions, ages and animals (Haggerty
et al., 2017; Becker
et al., 2000); for example, tenascin-R inhibits axon growth in mouse cerebellar neurites (Pesheva
et al., 1993), but it promotes growth in embryonic mouse hippocampal neurons (Lochter and Schachner, 1993). In the post-SCI, glial and blood-derived cells in the lesion overexpress matrix metalloproteases (MMPs) (Gaudet and Popovich, 2014), resulting in the degradation of hyaluronan and other ECM components into low molecular weight fragments that can amplify inflammation (Jiang
et al., 2007). Additionally, glial cells modify the ECM composition further by overexpressing sulfated proteoglycans, among them chondroitin sulfate proteoglycans (CSPGs) like NG2. The possible consequences of sulfate proteoglycans overexpression include restriction of axon plasticity, migration of inflammatory cells, sequestering or presenting growth factors, cytokines and chemokines (Reviewed in Gaudet and Popovich, 2014). Dysregulation of these sulfated proteoglycans can have long-lasting effects on the structure and dynamics of the local tissue (Gaudet and Popovich, 2014) and represents one of the major factors inhibiting axonal regeneration (Bradbury
et al., 2002). This is one of the main reasons why the glial scar has been traditionally viewed as detrimental for recovery after SCI. However, a growing number of experiments demonstrate that the glial scar plays a neuroprotective role as well (Sofroniew 2005; Bradbury and Burnside, 2019). In this context, the depletion of the glial scar, or the cells involved in its formation represent interesting approaches to determine whether the glial scar promotes or inhibit axonal regeneration and functional recovery after SCI. Here, we review the studies that specifically ablated each of the glial cell populations involved in the glial scar formation and describe how those manipulations affected recovery after SCI in animal models (
Table 1).
2. Conditional Cell Ablation Strategies
2.1. Genetic approaches for ablation of specific glial cell populations
Several genetic strategies of conditional cell ablation have been described in the literature. Their major principle is the expression of a foreign protein, typically of bacterial or viral origin, whose transcription is regulated by a promoter specific to the targeted cell population. Such protein, be it an enzyme or a receptor, will grant that cell population the unique capacity to break down an administered prodrug into its active form, initiating the cell’s apoptotic death through one of several possible mechanisms.
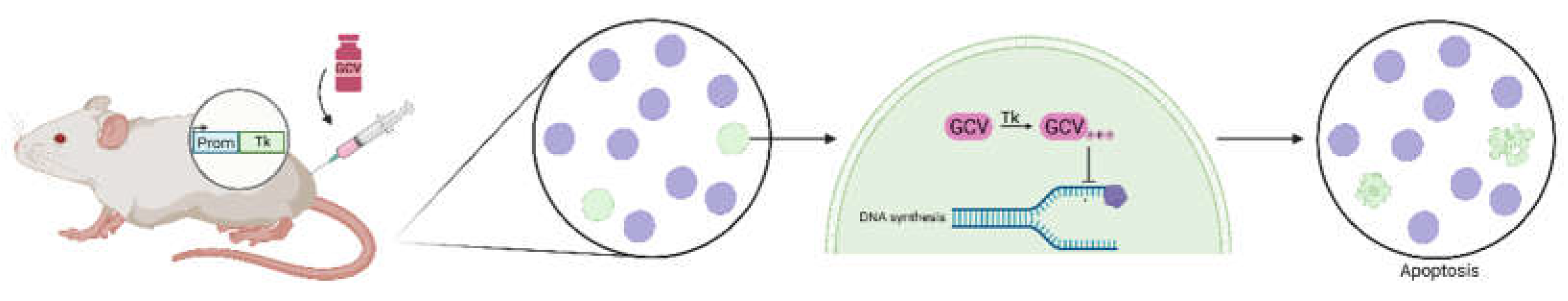
One of these cell ablation strategies is the Tk/GCV system, where the Thymidine kinase (Tk) gene -of the
Herpes Simplex Virus- is expressed under the control of a cell-specific promoter. Then, when the Ganciclovir (GCV) prodrug is applied systemically, the Tk (expressed exclusively in the cells of interest) phosphorylates it to its active form, inhibiting DNA synthesis and leading to apoptosis of the proliferative population of interest, and thus to its elimination (
Figure 1). This system can be used in transgenic animals that express the Tk under the cell-specific promoter of interest or, alternatively, it can be delivered through viral transfection. However, given its mechanism of action, the Tk/GCV system can only ablate proliferating cells.
Other similar systems that can target non-proliferating cells include (1) the NTR-CB1954, in which the E. coli enzyme nitroreductase (NTR) activates the CB1954 prodrug, inducing DNA interstrand cross-linking and cell death in the cells expressing NTR (Knox et al., 1993; Clark et al., 1997; Drabek et al., 1997); and (2) DTA/DTR, where a Diphtheria toxin receptor (DTR) is expressed under a cell-specific promoter and is harmless until the moment of ligand (Diphtheria toxin A, DTA) administration. When DTA binds the DTR, the inhibition of protein synthesis is triggered in the targeted cells, leading to their apoptosis (Saito et al., 2001).
A disadvantage that is common to these cell ablation strategies is the necessity to obtain and maintain (or even generate new) transgenic mice that express the involved enzyme or receptor, under a cell-specific promoter. This may be expensive and time-consuming. Therefore, alternative available approaches for ablation of glial cells can be considered, e.g. pharmacological.
2.2. Pharmacological approaches for ablation of specific glial cell populations
Pharmacological approaches employ drugs or toxins that affect a specific cell type and can be applied to non-transgenic laboratory animals. However, the risk of using such toxins or drugs is the possible off-target effects. For example, in the case of microglia, it has been shown that these cells are the main source of Colony Stimulating Factor 1 Receptor (CSF-1R) expression in the CNS, and CSF-1R ablation or loss of function leads to microglia depletion (Ginhoux et al., 2010). Thus, it is reasonable to expect that inhibition of CSF-1R can lead to conditional microglial depletion. In fact, several specific kinase inhibitors have been developed and tested in vivo, some efficiently crossing the BBB and ablating microglia (Elmore et al., 2014). Among them PLX3397 and PLX5622, that can be administered orally in the chow, appear the most potent and are widely used in microglia research (Elmore et al., 2014; Bellver-Landete et al., 2019; Spangenber et al., 2019; Green et al., 2020; Fu et al., 2020; Zhou et al., 2023). Moreover, the Food and Drug Administration (FDA) has approved PLX3397 as a therapeutic agent for patients with Tenosynovial giant cell tumor, where blocking the CSF-1/CSF-1R pathway reduces the number of infiltrating monocytes and macrophages, and minimizes tumor growth (Benner et al., 2020).
For specific ablation of astrocytes, the typical toxin is L-alpha-aminoadipate (L-AAA). L-AAA is a naturally-occurring glutamate homolog that constitutes an intermediate in the lysine metabolism (Chang, 1978; 1982). The L-AAA inhibits glutamate synthesis (Tsai et al., 1996) and uptake (Khurgel et al., 1996) in astrocytes, rapidly ablating them without affecting the surrounding cell types, even if administered for extended periods of time; however, it is incapable of crossing the BBB in the adult CNS and must, therefore, be injected into the brain or spinal cord (Khurgel et al., 1996).
Even though there are pharmacological drugs that induce oligodendrocytes death, such as the Clostridium perfringens Epsilon toxin (Linden et al., 2015) and Cuprizone (effect and mechanism of action reviewed in Zirngibl et al., 2022), no drugs for specific ablation of NG2+ oligodendrocyte precursor cells have been reported so far.
3. Astrocytes in the Glial Scar after SCI: What Have We Learned from Ablation Experiments?
Astrocytes are glial cells that have multiple functions in the adult CNS, including regulation of the BBB and CSF-brain barrier formation and function, regulation of blood supply, uptake of glucose, glutamate and lactate supply to neurons, regulation of synaptic transmission and plasticity, pruning and maintenance of synapses, removal of neurotransmitters from the extracellular space; extracellular ions homeostasis and pH control. Embryonic astrocytes also support axonal growth, but this function gets lost with age, until the point when mature astrocytes inhibit axon growth (Bähr et al., 1995).
In the intact adult spinal cord astrocytes barely proliferate, but damage to the CNS triggers an astrocytic response known as astrogliosis. During this process, inflammatory cytokines promote proliferation of adult astrocytes and ependymal cells, giving origin to reactive astrocytes (Barnabé-Heider et al., 2010; Magnus et al., 2008). In rodent models of SCI, first signs of astrogliosis appear as early as 2-7 dpi (Faulkner et al., 2004; Zai and Wrathall, 2005), but reactive astrocytes persist chronically after SCI. These newly-proliferated reactive astrocytes -which acquire a different morphological structure with extended thick processes- are the ones that comprise the glial scar, where their cell density nearly doubles compared to astrocytes in uninjured tissue (Wanner et al., 2013).
One of the main reasons why astrocytes (and the whole glial scar) are viewed as detrimental for axon regrowth is their overexpression of CSPGs after SCI (Jones et al., 2003; Hara et al., 2017), which are associated to inhibition of axonal regeneration (McKeon et al., 1991; 1995). However, other cell types in the scar also express CSPGs (Tan et al., 2005; Asher et al., 2002; Uhlin-Hansen et al., 1993) and astrocyte ablation does not eradicate CSPGs in the lesion (Anderson et al., 2016). Most importantly, some of those CSPGs are axon-growth permissive; therefore, CSPG presence in the glial scar does not prevent axon regrowth in a stimulated environment (Anderson et al., 2016). The role of CSPGs in the glial scar after SCI is just one of the interesting findings resulting from astrocytic ablation experiments.
Several groups have made use of the Tk/GCV system in order to ablate reactive proliferating astrocytes in mouse models of SCI, either by using transgenic GFAP-Tk mouse lines (Faulkner et al., 2004; Anderson et al., 2016) or by lentivirus-mediated gene expression (Gu et al., 2019). Both strategies proved effective at ablating this cell population, although the lentiviral approach did affect a small number of Olig2+ cells (Gu et al., 2019). Ablation of scar-forming astrocytes resulted in extended tissue damage, failure to repair the BBB and the consequent increase in macrophage infiltration of the spinal cord parenchyma, more extensive demyelination and oligodendrocyte death, decreased neuronal survival near the injury, and diminished locomotor function recovery (Faulkner et al., 2004; Gu et al., 2019). These results were observed at different timepoints ranging from 2 to 6 weeks post injury (wpi).
Anderson et al. (2016) used the same approach to ablate reactive proliferating astrocytes, but focused their study on axonal regrowth in three fiber tracts: the descending corticospinal tract (CST), the ascending sensory tract (AST) and the descending serotonergic tract (5HT). They report that not only did astrocyte ablation not promote spontaneous axonal regrowth, but in fact it increased the axonal dieback in the AST and CST. The 5HT axons in mice were less affected by the SCI and did not present augmented dieback after astrocytic ablation; still, they failed to spontaneously regrow in the lesion center at 8 wpi.
Seeing that astrocyte ablation in the acute phase of SCI in mice can be detrimental, Anderson et al. (2016) ablated the astrocytes during the chronic phase of SCI (i.e. 5 wpi) making use of a DTA/DTR system. At 10 wpi, the authors observed failure of the axons to spontaneously regrow within all three fiber tracts studied. Interestingly, if Neurotrophin-3 (NT3) and Brain Derived Neurotrophic Factor (BDNF) (factors promoting axon growth) were administered locally after SCI, few AST axons were found to regrow into the lesion core (Anderson et al., 2016). However, administration of these same neurotrophic factors in combination with preconditioning lesions (a process in which the sciatic nerve is transected and ligated one week before SCI) (Richardson et al., 1984; Neumann and Woolf, 1999; Omura et al., 2015) successfully promoted AST axon regrowth through the glial scar, in spite of CSPG presence (Anderson et al., 2016). Tk/GCV ablation of reactive astrocytes in this paradigm completely prevented axon regrowth, indicating that astrocytes are not detrimental for axon regeneration after SCI, but actually support it (Anderson et al., 2016).
Taken together, astrocyte ablation experiments suggest that reactive astrocytes play a beneficial role after SCI, and may be important for recovery at the cellular and at the functional level, even at chronic stages of SCI. Hence, therapies to promote axonal regeneration can be explored in the presence of the astrocytic scar.
4. Microglia Ablation after SCI: How Does It Affect Glial Scar Formation?
Even though for years -and to this day- microglial cells have been categorized as “resting” or “active”, they are in fact the most dynamic cells in the healthy CNS (Tremblay 2011). Even under normal conditions, microglia throughout the whole nervous tissue are always actively sensing their environment (Nimmerjahn et al., 2005) and rapidly responding to its changes (Hanisch and Kettenmann, 2007). Among the key functions of microglia in the healthy adult nervous system are surveillance, phagocytosis of apoptotic neurons, pruning of synapses, neurotransmission regulator and circuit reorganization (reviewed in Tremblay et al., 2011).
In response to focal CNS lesions in rodents, microglia can rapidly reorient their processes towards the lesion, and already after 30 minutes they form a border that seals the lesion and prevents spreading of the damage (Davalos et al., 2005; Nimmerjahn et al., 2005). After SCI, microglial response is complex: cells retract their cytoplasmic processes and become more circular in shape (Bellver-Landete et al., 2019), they proliferate (Schwab et al., 2001), produce pro-inflammatory cytokines (Pineau and Lacroix, 2007) and reactive nitrogen species (Xu et al., 2001), and enter a highly phagocytic state (Bellver-Landete et al., 2019). At 14 dpi in rodents, microglia accumulate in the lesion and can be found between the fibrotic and glial scars -contacting astrocytes-, where they persist at least until 35 dpi (Bellver-Landete et al., 2019).
Microglial ablation in rodent SCI models has been carried out pharmacologically with either PLX5622 (Bellver-Landete et al., 2019) or PLX3397 (Fu et al., 2020; Zhou et al., 2023). In all those experiments, microglia ablation resulted in a transient enlargement of the fibrotic scar area at 7 dpi that was no longer visible from 14 dpi onward. However, Bellver-Landete et al. (2019) found secondary satellite lesions filled with blood-derived myeloid cells and pericytes at 14 and 35 dpi. Other early effects of microglial ablation were reduction in astrocytic proliferation at 7 dpi (Bellver-Landete et al., 2019; Zhou et al., 2023) and decreased locomotor recovery from 7 dpi onward (Bellver-Landete et al., 2019; Fu et al., 2020; Zhou et al., 2023). All these studies agree that after 14 dpi, the astrocytic scar of microglia-ablated mice is less compact and astrocytes are not aligned, but randomly positioned around the lesion center instead. This is accompanied by an exacerbated infiltration of blood-derived cells into the spinal cord parenchyma and a reduced number of neurons near the lesion. In addition to these effects, Bellver-Landete et al. (2019) observed a decrease in the Olig2+/CC1+ oligodendrocyte population around the lesion.
Zhou et al. (2023) quantified proteins after microglial ablation and found increased levels of three pro-inflammatory cytokines, i.e. TNF-α, IL-6, and IL- 1β, which could be responsible for the augmented neuronal cell death after SCI. Fu et al. (2020) analyzed the dieback of CST axons after SCI, in mice with and without microglia ablation. The authors injected biotinylated dextran amine (BDA) –a tracer that allows axon visualization- into the cortex of mice at 6 wpi. Two weeks later (8 wpi) they obtained sections from the injured spinal cords, stained them to see the BDA-labelled axons, and found that mice with microglia ablation presented more extended axonal dieback further away from the lesion site.
Additionally, Zhou et al. (2023) obtained transcriptomes from the lesion and adjacent spinal cord tissue at 7 dpi after SCI in mice treated or untreated with PLX3397, as well as from the sham-operated control animals. They found upregulation of several groups of genes in the SCI group vs. sham-operated controls; specifically, genes involved in inflammation, fibrosis, extracellular matrix organization, cell adhesion, and immune response. The PLX3397 treatment reverted many of these changes after SCI, presenting a genetic expression more similar to uninjured mice; this indicates that microglial ablation prevents the inflammatory and immune responses that are initiated after SCI.
Given the negative effects of microglial depletion on SCI recovery, Bellver-Landete et al. (2019) used an additional interesting strategy to study the role of microglia after SCI. The authors aimed to enhance microglial proliferation at the site of injury by using local injection of a bioresorbable hydrogel with incorporated macrophage-colony stimulating factor (M-CSF). The M-CSF is a cytokine that induces proliferation and maturation of macrophages and microglia in rodents (Yamamoto et al., 2010) and humans (Smith et al., 2013). The proliferative effects of M-CSF are dose-dependent (Yamamoto et al., 2010) and immediate on human cultured microglia (Smith et al., 2013). Although the proportion of proliferative cells may vary, microglial division occurs consistently during treatment (Smith et al., 2013). The hydrogel can sustain drug release for 3-7 days, therefore this system allows to increase the proliferation of microglia during this period of time. Stimulation of microglia proliferation resulted in reduced lesion area at 7 dpi and better locomotor recovery between 7 and 21 dpi, further supporting the idea that microglia exert a protective role after SCI.
Taken together, the extensive evidence gathered by several research groups unequivocally revealed microglia as a cell population whose implication after SCI is purely beneficial.
5. NG2+-glia Ablation after SCI: What We Learnt and What Remains Unknown
NG2+-glia constitutes the fourth most abundant glial cell type, and the most widely distributed proliferating cell type, in the normal adult CNS (Dawson et al., 2003). Differentiation of NG2+-glia cells into oligodendrocytes constitutes their canonical function, which is maintained throughout life (Hughes et al., 2018). These cells are capable of proliferating, migrating and differentiating in response to external molecules, which they can rapidly sense with their motile processes (Hill et al., 2014; Hughes et al., 2013). One of the most remarkable features of NG2+-glia is their synaptic communication with neurons (Bergles et al., 2000; Kula et al., 2019), which together with their morphological complexity and persistence in the CNS throughout life, suggests that NG2+-glia may play other roles in the healthy CNS, independently of its capacity to generate oligodendrocytes (Butt et al., 2002).
NG2+-glia show a wide variety of responses to CNS injury, but the functional role of these responses is only partially understood and might vary with different factors, including cause of injury, its location within the CNS, age of the animals, etc. One type of NG2-glia response is injury-induced cell-fate plasticity, where they not only differentiate into oligodendrocytes to promote remyelination of axons (Gensert and Goldman, 1997), but can additionally differentiate into Schwann cells after focal demyelination (Zawadzka et al., 2010), and into astrocytes after SCI (Hackett et al., 2016). Other types of NG2+-glia response to CNS injuries include their activation and participation in the glial scar formation after SCI (Buss et al., 2009), and promoting inflammation after injury to the brain white matter (Seo et al., 2013).
After SCI, NG2+-glial cells migrate towards the injury site (Hughes et al., 2013) and undergo massive proliferation, representing 50% of all proliferating cells (McTigue et al., 2001) and reaching their maximum at 7 dpi (Hesp et al., 2018). Even though their numbers are increased, NG2+-glia are incapable of populating the fibrotic tissue formed in the lesion epicenter, but rather remain within the glial scar arising around the lesion (Tripathi and McTigue, 2007; Hesp et al., 2015; Hackett and Lee, 2016). Being a target of various factors which can influence their fate -such as bone morphogenetic proteins (BMPs, growth factors) produced by reactive astrocytes (Wang et al., 2011) or overexpressed pro-inflammatory cytokines (reviewed in Levine 2014)- NG2+-glial cells may differentiate into astrocytes after SCI (Sellers et al., 2009; Hackett et al., 2018).At the same time, other part of the NG2+-glia population may differentiate into Schwann cells and oligodendrocytes (Assinck et al., 2017). The newly-differentiated Schwann cells and oligodendrocytes play a key role in remyelination, as many oligodendrocytes originally present in the tissue die, while the spared ones are incapable of remyelinating the axons (Keirstead et al., 1998; Crawford et al., 2016). It should be noted that the relevance of remyelination after SCI in mice is still a matter of debate. Several studies have reported improved locomotor recovery after transplantation of neural precursor cells (NPCs) capable of myelination (Cao et al., 2005; Yasuda et al., 2011; Hawryluk et al., 2014), suggesting that remyelination may be important for neuroregeneration after SCI. However, in addition to remyelination, transplanted NPCs may induce change by -for example- differentiating into astrocytes or by providing trophic support to axons; thus, it is possible that the observed recovery is mediated by a combination of these effects, rather than by remyelination itself (Plemel et al., 2014; Duncan et al., 2019). Furthermore, Duncan et al. (2018) analyzed the effect of knocking down the Myelin regulatory factor (Myrf) and found that, even though Myrf is essential to induce remyelination, its knockdown does not affect locomotor recovery. These findings indicate that remyelination is not required for locomotor recovery after SCI in mice.
Another type of response of NG2+-glia after injury is CSPGs overexpression. Although evidence exists that CSPGs (and NG2 among them) are inhibitory for axon regrowth after SCI (Dou and Levine, 1994; Tan et al., 2006; Petrosyan et al., 2013), several studies have observed that regenerating axons in the injured spinal cord appear more frequently in areas of the spinal cord showing NG2 labeling (Jones et al., 2003; McTigue et al., 2006). This suggests that the NG2 proteoglycan may actually provide a permissive environment for axons to grow. However, even if this is the case, NG2+-glia can still entrap the growing axons through the establishment of NG2+-glia-axon synapses, preventing their regrowth after SCI (Di Maio et al., 2011; Filous et al., 2014; Son 2015).
Given the several -and sometimes opposing- roles that the NG2+ glia play after SCI, ablating them in vivo seems a good approach to better define if their overall function is detrimental or beneficial in the post-SCI environment. In order to ablate proliferating NG2+ cells, Hesp et al. (2018) made use of a Tk/GCV system with transgenic NG2-Tk mice and GCV infusion starting immediately after SCI; the animals were sacrificed and the tissue was analyzed at 7, 11 and 21 dpi. The animals sacrificed at 7 and 11 dpi received GCV infusion for 7 and 11 days respectively, while the animals sacrificed at 21 dpi received GCV infusion for 14 days and were kept for an extra week to study the NG2+ cells response after the ablation treatment was stopped (Hesp et al., 2018). Notably, NG2+ is expressed not only by oligodendroglial progenitors but also by pericytes, and the used approach eliminates both cell populations. However, after SCI, NG2+ pericytes occupy the fibrotic scar formed at the epicenter of the lesion, while NG2+ oligodendroglial progenitors occupy the glial scar around the lesion. Therefore, the effects of abolishing each cell type can be studied and distinguished in the respective area of interest. Additionally, the authors performed a time course study to determine the ratio of proliferating NG2+ glia and pericytes throughout the first 14 dpi by staining spinal cord sections with Ki67, NG2 and PDGFRb antibodies (Hesp et al., 2018).
NG2+ ablation resulted in loss of tissue integrity, extended edema and hemorrhage in the lesion site at 7 dpi. The effects were still visible -although less pronounced- at 11 dpi, when lesion expansion was also observed. At 21 dpi, the swelling and the signs of edema were significantly reduced and were similar to sham-operated control mice (Hesp et al., 2018). As to the glial scar formation, NG2+ cells ablation resulted in a more discontinuous and less compact glial scar, with reduced astrocyte numbers and increased macrophage infiltration. Moreover, at 21 dpi (7 days after stopping GCV treatment) presence of NG2+ cells reached control levels and axons were penetrating the lesion epicenter in the Tk/GCV treated mice; these axons were usually observed in contact with NG2+ cells, some even seemingly “traveling along NG2+ processes”. This suggests that acute ablation of NG2+ oligodendroglial progenitors and/or NG2+ pericytes results in a more permissive environment for axonal regrowth afterwards. However, at the same time NG2+ cell ablation negatively affected locomotion, impairing recovery of the forelimb step length in the Tk/GCV treated mice, opposed to the complete recovery observed in control mice with SCI at 21 dpi (Hesp et al., 2018). It would certainly be interesting to extend the period of analysis after stopping GCV treatment in order to see whether the glial and fibrotic scars recover to their full density after a longer NG2+ repopulation period and what that may mean in terms of axon regrowth and locomotor recovery.
Taken together, although experiments with ablation of NG2-expressing cells brought interesting results, they did not allow to fully understand the role of NG2+-glia in the glial scar formed after SCI. The beneficial effects of NG2+ cell ablation could be linked not only to NG2+-glia, but also to the changes in the fibrotic scar after ablating the NG2+ pericytes. Therefore, finding a way to ablate exclusively the NG2+-glia, without affecting other cell types expressing NG2 could be a major breakthrough to identify the function of the NG2+-glia in the SCI environment. One way to do this may be designing a Split-Cre system (Hirrlinger et al., 2009) under the control of two NG2+-glia specific promoters (such as NG2 and Olig2) that may enable the analysis of NG2+-glia functions without confounding effects.
6. Conclusion
The ablation of specific glial populations in SCI models has proven a powerful approach to discover the overall function of the glial scar and the role of each cell type involved in its formation. It appears that the glial scar plays a protective role after injury despite its inhibitory role regarding axonal regeneration. Hence, therapeutic strategies aiming at glial cell ablation after SCI should be re-evaluated. Reaching a higher understanding of the diverse glial cell responses to injury should enable targeted modifications in gene expression, in order to prevent protective responses from turning into chronic detrimental events, as is the case for inflammation. Still, it should be kept in mind that modifications to the response of one cell population may affect other cell types as, for instance, evidenced by the disorganization of astrocytes when microglia or NG2+-glia are altered (Zhou et al., 2023; Hesp et al., 2018).
Besides glial cells, another interesting player in the injury environment is the ECM, given its capacity to provide biochemical cues and initiate signaling pathways (Rozario and DeSimone, 2010), especially knowing that administration of neurotrophic factors promotes axon regrowth beyond the glial scar after SCI (Anderson et al., 2016). An ultimate challenge will be finding the right balance between a sufficient response, necessary to initiate the protective cascade of events, and a decrease in the excessive positive feedback that prevents functional recovery over the long-term.
Author Contributions
LPG conceived the original idea for this manuscript, created the outline, collected the literature, and wrote the first draft. LPG and MK worked together on the intermediate versions of the manuscript. LPG created the final version of the manuscript, the figure and the table. Both authors approved the submitted version of the manuscript.
Funding
Our work is supported by IKERBASQUE (Basque Foundation for Science), the Spanish Ministry of Science and Innovation (grant PID2019-110195RB-I00), and the Basque Government PIBA Project (PIBA 2020_1_0030).
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
Conflicts of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Anderson, M. A., Burda, J. E., Ren, Y., Ao, Y., O’Shea, T. M., Kawaguchi, R., … Sofroniew, M. V. (2016). Astrocyte scar formation AIDS central nervous system axon regeneration. Nature, 532(7598), 195–200. [CrossRef]
- Asher, R. A., Morgenstern, D. A., Moon, L. D. F., & Fawcett, J. W. (2001). Chondroitin sulphate proteoglycans: inhibitory components of the glial scar. Progress in Brain Research, 132, 611–619. [CrossRef]
- Asher, R. A., Morgenstern, D. A., Shearer, M. C., Adcock, K. H., Pesheva, P., & Fawcett, J. W. (2002). Versican is upregulated in CNS injury and is a product of oligodendrocyte lineage cells. Journal of Neuroscience, 22(6), 2225–2236. [CrossRef]
- Assinck, P., Duncan, G. J., Plemel, J. R., Lee, M. J., Stratton, J. A., Manesh, S. B., … Tetzlaff, W. (2017). Myelinogenic plasticity of oligodendrocyte precursor cells following spinal cord contusion injury. Journal of Neuroscience, 37(36), 8635–8654. [CrossRef]
- Bähr, M., Przyrembel, C., & Bastmeyer, M. (1995). Astrocytes from adult rat optic nerves are nonpermissive for regenerating retinal ganglion cell axons. Experimental Neurology, 131(2), 211–220. [CrossRef]
- Barnabé-Heider, F., Göritz, C., Sabelström, H., Takebayashi, H., Pfrieger, F. W., Meletis, K., & Frisén, J. (2010). Origin of new glial cells in intact and injured adult spinal cord. Cell Stem Cell, 7(4), 470–482. [CrossRef]
- Becker, T., Anliker, B., Becker, C. G., Taylor, J., Schachner, M., Meyer, R. L., & Bartsch, U. (2000). Tenascin-R inhibits regrowth of optic fibers in vitro and persists in the optic nerve of mice after injury. Glia, 29(4), 330–346. [CrossRef]
- Bellver-Landete, V., Bretheau, F., Mailhot, B., Vallières, N., Lessard, M., Janelle, M. E., … Lacroix, S. (2019). Microglia are an essential component of the neuroprotective scar that forms after spinal cord injury. Nature Communications, 10(1). [CrossRef]
- Benner, B., Good, L., Quiroga, D., Schultz, T. E., Kassem, M., Carson, W. E., … Wesolowski, R. (2020). Pexidartinib, a novel small molecule csf-1r inhibitor in use for tenosynovial giant cell tumor: A systematic review of pre-clinical and clinical development. Drug Design, Development and Therapy, 14, 1693–1704. [CrossRef]
- Bergles, D. E., Roberts, J. D. B., Somogyl, P., & Jahr, C. E. (2000). Glutamatergic synapses on oligodendrocyte precursor cells in the hippocampus. Nature, 405(6783), 187–191. [CrossRef]
- Bradbury, E. J., & Burnside, E. R. (2019). Moving beyond the glial scar for spinal cord repair. Nature Communications, 10(1), 1–15. [CrossRef]
- Bradbury, E. J., Moon, L. D. F., Popat, R. J., King, V. R., Bennett, G. S., Patel, P. N., … McMahon, S. B. (2002). Chondroitinase ABC promotes functional recovery after spinal cord injury. Nature, 416(6881), 636–640. [CrossRef]
- Buss, A., Pech, K., Kakulas, B. A., Martin, D., Schoenen, J., Noth, J., & Brook, G. A. (2009). NG2 and phosphacan are present in the astroglial scar after human traumatic spinal cord injury. BMC Neurology, 9, 1–15. [CrossRef]
- Butt, A. M., Kiff, J., Hubbard, P., & Berry, M. (2002). Synantocytes: New functions for novel NG2 expressing glia. Journal of Neurocytology, 31(6–7), 551 ppl= – 565. [CrossRef]
- Chang, Y. -F. (1978). Lysine Metabolism in the Rat Brain: the Pipecolic Acid-Forming Pathway. Journal of Neurochemistry, 30(2), 347–354. [CrossRef]
- Chang, Y. F. (1982). Lysine metabolism in the human and the monkey: Demonstration of pipecolic acid formation in the brain and other organs. Neurochemical Research, 7(5), 577–588. [CrossRef]
- Clark, A. J., Iwobi, M., Cui, W., Crompton, M., Harold, G., Hobbs, S., … Gusterson, B. (1997). Selective cell ablation in transgenic mice expressing E. coli nitroreductase. Gene Therapy, 4(2), 101–110. [CrossRef]
- Crawford, A. H., Tripathi, R. B., Foerster, S., McKenzie, I., Kougioumtzidou, E., Grist, M., … Franklin, R. J. M. (2016). Pre-existing mature oligodendrocytes do not contribute to remyelination following toxin-induced spinal cord demyelination. American Journal of Pathology, 186(3), 511–516. [CrossRef]
- Davalos, D., Grutzendler, J., Yang, G., Kim, J. V., Zuo, Y., Jung, S., … Gan, W. B. (2005). ATP mediates rapid microglial response to local brain injury in vivo. Nature Neuroscience, 8(6), 752–758. [CrossRef]
- Davies, S. J. A., Goucher, D. R., Doller, C., & Silver, J. (1999). Robust regeneration of adult sensory axons in degenerating white matter of the adult rat spinal cord. Journal of Neuroscience, 19(14), 5810–5822. [CrossRef]
- Dawson, M. R. L., Polito, A., Levine, J. M., & Reynolds, R. (2003). NG2-expressing glial progenitor cells: An abundant and widespread population of cycling cells in the adult rat CNS. Molecular and Cellular Neuroscience, 24(2), 476–488. [CrossRef]
- Di Maio, A., Skuba, A., Himes, B. T., Bhagat, S. L., Hyun, J. K., Tessler, A., … Son, Y. J. (2011). In vivo imaging of dorsal root regeneration: Rapid immobilization and presynaptic differentiation at the CNS/PNS border. Journal of Neuroscience, 31(12), 4569–4582. [CrossRef]
- Dou, C. L., & Levine, J. M. (1994). Inhibition of neurite growth by the NG2 chondroitin sulfate proteoglycan. Journal of Neuroscience, 14(12), 7616–7628. [CrossRef]
- Drabek, D., Guy, J., Craig, R., & Grosveld, F. (1997). The expression of bacterial nitroreductase in transgenic mice results in specific cell killing by the prodrug CB1954. Gene Therapy, 4(2), 93–100. [CrossRef]
- Duncan, G. J., Manesh, S. B., Hilton, B. J., Assinck, P., Liu, J., Moulson, A., … Tetzlaff, W. (2018). Locomotor recovery following contusive spinal cord injury does not require oligodendrocyte remyelination. Nature Communications, 9(1). [CrossRef]
- Elmore, M. R. P., Najafi, A. R., Koike, M. A., Dagher, N. N., Spangenberg, E. E., Rice, R. A., … Green, K. N. (2014). Colony-stimulating factor 1 receptor signaling is necessary for microglia viability, unmasking a microglia progenitor cell in the adult brain. Neuron, 82(2), 380–397. [CrossRef]
- Faulkner, J. R., Herrmann, J. E., Woo, M. J., Tansey, K. E., Doan, N. B., & Sofroniew, M. V. (2004). Reactive Astrocytes Protect Tissue and Preserve Function after Spinal Cord Injury. Journal of Neuroscience, 24(9), 2143–2155. [CrossRef]
- Filous, A. R., Tran, A., James Howell, C., Busch, S. A., Evans, T. A., Stallcup, W. B., … Silver, J. (2014). Entrapment via synaptic-like connections between NG2 proteoglycan + cells and dystrophic axons in the lesion plays a role in regeneration failure after spinal cord injury. Journal of Neuroscience, 34(49), 16369–16384. [CrossRef]
- Fu, H., Zhao, Y., Hu, D., Wang, S., Yu, T., & Zhang, L. (2020). Depletion of microglia exacerbates injury and impairs function recovery after spinal cord injury in mice. Cell Death and Disease, 11(7). [CrossRef]
- Gaudet, A. D., & Popovich, P. G. (2014). Extracellular matrix regulation of inflammation in the healthy and injured spinal cord. Experimental Neurology, 258, 24–34. [CrossRef]
- Gensert, J. A. M., & Goldman, J. E. (1997). Endogenous progenitors remyelinate demyelinated axons in the adult CNS. Neuron, 19(1), 197–203. [CrossRef]
- Ginhoux, F., Greter, M., Leboeuf, M., Nandi, S., See, P., Gokhan, S., … Merad, M. (2010). Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science, 330(6005), 841–845. [CrossRef]
- Green, K. N., Crapser, J. D., & Hohsfield, L. A. (2020). To Kill a Microglia: A Case for CSF1R Inhibitors. Trends in Immunology, 41(9), 771–784. [CrossRef]
- Gu, Y., Cheng, X., Huang, X., Yuan, Y., Qin, S., Tan, Z., … Su, Z. (2019). Conditional ablation of reactive astrocytes to dissect their roles in spinal cord injury and repair. Brain, Behavior, and Immunity (Vol. 80). Elsevier Inc. [CrossRef]
- Hackett, A. R., Lee, D. H., Dawood, A., Rodriguez, M., Funk, L., Tsoulfas, P., & Lee, J. K. (2016). STAT3 and SOCS3 regulate NG2 cell proliferation and differentiation after contusive spinal cord injury. Neurobiology of Disease, 89, 10–22. [CrossRef]
- Hackett, A. R., & Lee, J. K. (2016). Understanding the NG2 glial scar after spinal cord injury. Frontiers in Neurology, 7(NOV), 1–10. [CrossRef]
- Hackett, A. R., Yahn, S. L., Lyapichev, K., Dajnoki, A., Lee, D. H., Rodriguez, M., … Lee, J. K. (2018). Injury type-dependent differentiation of NG2 glia into heterogeneous astrocytes. Experimental Neurology, 308, 72–79. [CrossRef]
- Haggerty, A. E., Marlow, M. M., & Oudega, M. (2017). Extracellular matrix components as therapeutics for spinal cord injury. Neuroscience Letters, 652, 50–55. [CrossRef]
- Hanisch, U. K., & Kettenmann, H. (2007). Microglia: Active sensor and versatile effector cells in the normal and pathologic brain. Nature Neuroscience, 10(11), 1387–1394. [CrossRef]
- Hara, M., Kobayakawa, K., Ohkawa, Y., Kumamaru, H., Yokota, K., Saito, T., … Okada, S. (2017). Interaction of reactive astrocytes with type i collagen induces astrocytic scar formation through the integrin-N-cadherin pathway after spinal cord injury. Nature Medicine, 23(7), 818–828. [CrossRef]
- Hesp, Z. C., Goldstein, E. A., Miranda, C. J., Kaspar, B. K., & McTigue, D. M. (2015). Chronic oligodendrogenesis and remyelination after spinal cord injury in mice and rats. Journal of Neuroscience, 35(3), 1274–1290. [CrossRef]
- Hesp, Z. C., Yoseph, R. Y., Suzuki, R., Jukkola, P., Wilson, C., Nishiyama, A., & McTigue, D. M. (2018). Proliferating NG2-cell-dependent angiogenesis and scar formation alter axon growth and functional recovery after spinal cord injury in mice. Journal of Neuroscience, 38(6), 1366–1382. [CrossRef]
- Hill, R. A., Patel, K. D., Goncalves, C. M., Grutzendler, J., & Nishiyama, A. (2014). Modulation of oligodendrocyte generation during a critical temporal window after NG2 cell division. Nature Neuroscience, 17(11), 1518–1527. [CrossRef]
- Hirrlinger, J., Scheller, A., Hirrlinger, P. G., Kellert, B., Tang, W., Wehr, M. C., … Kirchhoff, F. (2009). Split-Cre complementation indicates coincident activity of different genes in vivo. PLoS ONE, 4(1), 1–10. [CrossRef]
- Hughes, E. G., Kang, S. H., Fukaya, M., & Bergles, D. E. (2013). Oligodendrocyte progenitors balance growth with self-repulsion to achieve homeostasis in the adult brain. Nature Neuroscience, 16(6), 668–676. [CrossRef]
- Hughes, E. G., Orthmann-Murphy, J. L., Langseth, A. J., & Bergles, D. E. (2018). Myelin remodeling through experience-dependent oligodendrogenesis in the adult somatosensory cortex. Nature Neuroscience, 21(5), 696–706. [CrossRef]
- Jiang, D., Liang, J., & Noble, P. W. (2007). Hyaluronan in tissue injury and repair. Annual Review of Cell and Developmental Biology, 23, 435–461. [CrossRef]
- Jones, L. L., Margolis, R. U., & Tuszynski, M. H. (2003). The chondroitin sulfate proteoglycans neurocan, brevican, phosphacan, and versican are differentially regulated following spinal cord injury. Experimental Neurology, 182(2), 399–411. [CrossRef]
- Kawano, H., Kimura-Kuroda, J., Komuta, Y., Yoshioka, N., Li, H. P., Kawamura, K., … Raisman, G. (2012). Role of the lesion scar in the response to damage and repair of the central nervous system. Cell and Tissue Research, 349(1), 169–180. [CrossRef]
- Khurgel, M., Koo, A. C., & Ivy, G. O. (1996). Selective ablation of astrocytes by intracerebral injections of α- aminoadipate. Glia, 16(4), 351–358. [CrossRef]
- Knox, R. J., Friedlos, F., & Boland, M. P. (1993). The bioactivation of CB 1954 and its use as a prodrug in antibody-directed enzyme prodrug therapy (ADEPT). Cancer and Metastasis Reviews, 12(2), 195–212. [CrossRef]
- Krishnaswamy, V. R., Benbenishty, A., Blinder, P., & Sagi, I. (2019). Demystifying the extracellular matrix and its proteolytic remodeling in the brain: structural and functional insights. Cellular and Molecular Life Sciences, 76(16), 3229–3248. [CrossRef]
- Kula, B., Chen, T. J., & Kukley, M. (2019). Glutamatergic signaling between neurons and oligodendrocyte lineage cells: Is it synaptic or non-synaptic? Glia, 67(11), 2071–2091. [CrossRef]
- Lau, L. W., Cua, R., Keough, M. B., Haylock-Jacobs, S., & Yong, V. W. (2013). Pathophysiology of the brain extracellular matrix: A new target for remyelination. Nature Reviews Neuroscience, 14(10), 722–729. [CrossRef]
- Linden, J. R., Ma, Y., Zhao, B., Harris, J. M., Rumah, K. R., Schaeren-Wiemers, N., & Vartanian, T. (2015). Clostridium perfringens epsilon toxin causes selective death of mature oligodendrocytes and central nervous system demyelination. MBio, 6(3), 1–13. [CrossRef]
- Lochter, A., & Schachner, M. (1993). Tenascin and extracellular matrix glycoproteins: From promotion to polarization of neurite growth in vitro. Journal of Neuroscience, 13(9), 3986–4000. [CrossRef]
- Magnus T, Carmen J, Deleon J, Xue H, Pardo AC, Lepore AC, Mattson MP, Rao MS, M. N. (2008). Adult glial precursor proliferation in mutant SOD1G93A mice. Glia, 56, 200–208.
- Maxwell, W.L.; Follows, R; Ashhurst, D.E.; Berry, M. (1990). The response of the cerebral hemisphere of the rat to injury. I. The mature rat. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences, 328, 479–500.
- McKeon, R. J., Schreiber, R. C., Rudge, J. S., & Silver, J. (1991). Reduction of neurite outgrowth in a model of glial scarring following CNS injury is correlated with the expression of inhibitory molecules on reactive astrocytes. Journal of Neuroscience, 11(11), 3398–3411. [CrossRef]
- McKeon RJ, Höke A, S. J. (1995). Injury-induced proteoglycans inhibit the potential for laminin-mediated axon growth on astrocytic scars. Experimental Neurology, 136, 32–43.
- McTigue, D. M., Tripathi, R., & Wei, P. (2006). NG2 colocalizes with axons and is expressed by a mixed cell population in spinal cord lesions. Journal of Neuropathology and Experimental Neurology, 65(4), 406–420. [CrossRef]
- McTigue, D. M., Wei, P., & Stokes, B. T. (2001). Proliferation of NG2-positive cells and altered oligodendrocyte numbers in the contused rat spinal cord. Journal of Neuroscience, 21(10), 3392–3400. [CrossRef]
- Nimmerjahn, A., Kirchhoff, F., & Helmchen, F. (2005). Neuroscience: Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science, 308(5726), 1314–1318. [CrossRef]
- Pesheva, P., Gennarini, G., Goridis, C., & Schachner, M. (1993). The F3/11 cell adhesion molecule mediates the repulsion of neurons by the extracellular matrix glycoprotein J1-160/180. Neuron, 10(1), 69–82. [CrossRef]
- Petrosyan, H. A., Hunanyan, A. S., Alessi, V., Schnell, L., Levine, J., & Arvanian, V. L. (2013). Neutralization of inhibitory molecule NG2 improves synaptic transmission, retrograde transport, and locomotor function after spinal cord injury in adult rats. Journal of Neuroscience, 33(9), 4032–4043. [CrossRef]
- Pineau, I., & Lacroix, S. (2007). Proinflammatory cytokine synthesis in the injured mouse spinal cord: Multiphasic expression pattern and identification of the cell types involved. Journal of Comparative Neurology, 500(2), 267–285. [CrossRef]
- Preston, E., Webster, J., & Small, D. (2001). Characteristics of sustained blood-brain barrier opening and tissue injury in a model for focal trauma in the rat. Journal of Neurotrauma, 18(1), 83–92. [CrossRef]
- Ramón y Cajal, S. (1928). Degeneration and Regeneration of the Nervous System. Clarendon Press.
- Rauch, U. (2007). Brain matrix: Structure, turnover and necessity. Biochemical Society Transactions, 35(4), 656–660. [CrossRef]
- Reier, PJ; Stensaas, LJ; Guth, L. (1983). The Astrocytic Scar as an Impediment to Regeneration in the Central Nervous System. Spinal Cord Reconstruction, 163–195.
- Rozario, T., & DeSimone, D. W. (2010). The extracellular matrix in development and morphogenesis: A dynamic view. Developmental Biology, 341(1), 126–140. [CrossRef]
- Saito, M., Iwawaki, T., Taya, C., Yonekawa, H., Noda, M., Inui, Y., … Kohno, K. (2001). Diphtheria toxin receptor-mediated conditional and targeted cell ablation in transgenic mice. Nature Biotechnology, 19(8), 746–750. [CrossRef]
- Schwab, J. M., Frei, E., Klusman, I., Schnell, L., Schwab, M. E., & Schluesener, H. J. (2001). AIF-1 expression defines a proliferating and alert microglial/macrophage phenotype following spinal cord injury in rats. Journal of Neuroimmunology, 119(2), 214–222. [CrossRef]
- Sellers, D. L., Maris, D. O., & Horner, P. J. (2009). Postinjury niches induce temporal shifts in progenitor fates to direct lesion repair after spinal cord injury. Journal of Neuroscience, 29(20), 6722–6733. [CrossRef]
- Seo, J. H., Miyamoto, N., Hayakawa, K., Pham, L. D., Maki, T., Ayata, C., … Arai, K. (2013). OPCs induce early blood-brain barrier opening. The Journal of Clinical Investigation, 123(2), 782–786. [CrossRef]
- Smith, A. M., Gibbons, H. M., Oldfield, R. L., Bergin, P. M., Mee, E. W., Curtis, M. A., … Dragunow, M. (2013). M-CSF increases proliferation and phagocytosis while modulating receptor and transcription factor expression in adult human microglia. Journal of Neuroinflammation, 10, 1–15. [CrossRef]
- Sofroniew, M. V. (2005). Reactive astrocytes in neural repair and protection. Neuroscientist, 11(5), 400–407. [CrossRef]
- Son, Y. J. (2015). Synapsing with NG2 cells (polydendrocytes), unappreciated barrier to axon regeneration? Neural Regeneration Research, 10(3), 346–348. [CrossRef]
- Spangenberg, E., Severson, P. L., Hohsfield, L. A., Crapser, J., Zhang, J., Burton, E. A., … Green, K. N. (2019). Sustained microglial depletion with CSF1R inhibitor impairs parenchymal plaque development in an Alzheimer’s disease model. Nature Communications, 10(1), 1–21. [CrossRef]
- Tan, A. M., Colletti, M., Rorai, A. T., Skene, J. H. P., & Levine, J. M. (2006). Antibodies against the NG2 proteoglycan promote the regeneration of sensory axons within the dorsal columns of the spinal cord. Journal of Neuroscience, 26(18), 4729–4739. [CrossRef]
- Tan, A. M., Zhang, W., & Levine, J. M. (2005). NG2: A component of the glial scar that inhibits axon growth. Journal of Anatomy, 207(6), 717–725. [CrossRef]
- Theocharis, A. D., Skandalis, S. S., Gialeli, C., & Karamanos, N. K. (2016). Extracellular matrix structure. Advanced Drug Delivery Reviews, 97, 4–27. [CrossRef]
- Tremblay, M. É. (2012). The role of microglia at synapses in the healthy CNS: Novel insights from recent imaging studies. Neuron Glia Biology, 7(1), 67–76. [CrossRef]
- Tremblay, M. È., Stevens, B., Sierra, A., Wake, H., Bessis, A., & Nimmerjahn, A. (2011). The role of microglia in the healthy brain. Journal of Neuroscience, 31(45), 16064–16069. [CrossRef]
- Tripathi, Richa; McTigue, D. M. (2007). Prominent oligodendrocyte genesis along the border of spinal contusion lesions. Glia, 55, 697–711.
- Tsai, M. J., Chang, Y. F., Schwarcz, R., & Brookes, N. (1996). Characterization of L-α-aminoadipic acid transport in cultured rat astrocytes. Brain Research, 741(1–2), 166–173. [CrossRef]
- Uhlin-Hansen, L., Wik, T., Kjellen, L., Berg, E., Forsdahl, F., & Kolset, S. O. (1993). Proteoglycan metabolism in normal and inflammatory human macrophages. Blood, 82(9), 2880–2889. [CrossRef]
- Wanner, I. B., Anderson, M. A., Song, B., Levine, J., Fernandez, A., Gray-Thompson, Z., … Sofroniew, M. V. (2013). Glial scar borders are formed by newly proliferated, elongated astrocytes that interact to corral inflammatory and fibrotic cells via STAT3-dependent mechanisms after spinal cord injury. Journal of Neuroscience, 33(31), 12870–12886. [CrossRef]
- Xu, J., Kim, G. M., Chen, S., Yan, P., Ahmed, S. H., Ku, G., … Hsu, C. Y. (2001). INOS and nitrotyrosine expression after spinal cord injury. Journal of Neurotrauma, 18(5), 523–532. [CrossRef]
- Yamamoto, S., Nakajima, K., & Kohsaka, S. (2010). Macrophage-colony stimulating factor as an inducer of microglial proliferation in axotomized rat facial nucleus. Journal of Neurochemistry, 115(4), 1057–1067. [CrossRef]
- Zai, L. J., & Wrathall, J. R. (2005). Cell proliferation and replacement following contusive spinal cord injury. Glia, 50(3), 247–257. [CrossRef]
- Zawadzka, M., Rivers, L. E., Fancy, S. P. J., Zhao, C., Tripathi, R., Jamen, F., … Franklin, R. J. M. (2010). CNS-Resident Glial Progenitor/Stem Cells Produce Schwann Cells as well as Oligodendrocytes during Repair of CNS Demyelination. Cell Stem Cell, 6(6), 578–590. [CrossRef]
- Zhou, Z. L., Xie, H., Tian, X. B., Xu, H. L., Li, W., Yao, S., & Zhang, H. (2023). Microglial depletion impairs glial scar formation and aggravates inflammation partly by inhibiting STAT3 phosphorylation in astrocytes after spinal cord injury. Neural Regeneration Research, 18(6), 1325–1331. [CrossRef]
- Zirngibl, M., Assinck, P., Sizov, A., Caprariello, A. V., & Plemel, J. R. (2022). Oligodendrocyte death and myelin loss in the cuprizone model: an updated overview of the intrinsic and extrinsic causes of cuprizone demyelination. Molecular Neurodegeneration, 17(1), 1–28. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).