1. Introduction
The physiological reactions and capacity for stress tolerance of the species significantly impact the chlorophyll content discovered by Zhou research group [
1]. The chlorophyll content is one of the most crucial indicators for determining drought tolerance in tomatoes. Due to metabolic instability caused by drought stress, the amount of chlorophyll in the leaf changes, which lowers light absorption. The quantity of photosynthetic pigments presents overall in leaves affects how much light is absorbed by leaves. The photosynthesis process uses photosynthetic pigments in the photochemical photosystems (PSI, PSII) of leaves to absorb light and transform it into chemical energy. It is possible to anticipate a decrease in photosynthesis and chlorophyll content under drought-stress circumstances [
2]. Drought stress is one of the most severe abiotic stresses that plant face because it reduces the flow of essential nutrients to the root zone and limits water accessibility to cells due to insufficient hydraulic conductance from roots to leaves caused by stomatal closure. Because of the decrease in hydraulic conductivity, the supply of nutrients to the shoot is reduced.
Several studies have found that foliar nitrogen, phosphorus, and potassium applications improve sweet corn nutrient uptake. Foliar feeding is a yield-enhancing corn production technique when combined with a chlorophyll meter [
3]. Another study team discovered that in a closed agricultural system, rates of foliar application of liquid organic fertilizer and nitrogen uptake by sweet corn increase, but not phosphorus and potassium [
4].
The resistance of plant foliage to foliar application varies by plant, according to the reference [
5], as cited in [
6]. Pepper and tomato foliage resistance was comparable (0.48-0.72 Kg of urea sprays 100 L
-1 of water). Furthermore, foliar spraying of magnesium sulfate hydrate (epsomite) in peppers and tomatoes was recommended for maximum product growth, yield, and quality [
7].
Supplemental potassium application reduced fruit weight loss and decay while increasing total soluble content in long sweet pepper; the most efficient sources were potassium sulfate (K
2SO
4) and potassium chloride salt (KCl). The most effective source of increased total phenols was K
2SO
4. Potassium increased total sugar and vitamin C levels, especially when potassium was provided in the form of potassium salt (KCl). The amount of nitrogen and phosphorus in the fruit increased after KCl salt treatments [
8].
Experimented with (Zlaten Medal) pepper plants to investigate and prove the link between nitrogen and pepper plant vegetative growth. Pepper seedlings were grown hydroponically with a nitrogen-free nutrient solution at five days old. The lack of nitrogen in the medium resulted in a decrease in leaf area and accumulation of plant biomass, as well as an increase in the root shoot’s dry weight ratio. When nitrogen was depleted, photosynthetic activity and chlorophyll decreased significantly. Nitrogen is required for the development of the chloroplast structure and, as a result, the photosynthetic strength and productivity of pepper plants, according to research [
9]. According to the scientific studies described above, there is a direct connection between physiological traits related to photosynthesis, vegetative growth, pepper plant production, and nitrogen. The health of vegetative development reflects the health of fruit, growth, and production in general since the leaves are the kitchen that feeds the growth of the pepper fruits.
Higher values for chili pepper leaf area, leaf number, chlorophyll index, nitrogen, potassium, and calcium concentrations in the leaves were obtained because of the foliar application of organic acids of Biomin and Humifolin [
10]. The effects of foliar potassium (K) spray on pepper plants growing in varying irrigation salinity waters were examined in a greenhouse experiment (3000 and 6000 mg L
-1 as compared to tap water with a salinity level of 300 mg L
-1). Irrigation with high salt water decreased plant height, and biomass output, in contrast to irrigation with tap water. When compared to plants subjected to moderate salt irrigation (3000 mg L
-1), plants subjected to extreme salinity irrigation (6000 mg L
-1) showed the most detrimental effects. Thus, 200 mg L
-1 of potassium monophosphate (KMP) applied topically through leaves improved biomass production, and plant development [
11]. Exogenous Alpha-Tocopherol (TOC) foliar spraying increased chlorophyll, enzymatic and non-enzymatic antioxidants, plant development, and salt stress tolerance in pepper plants, enhancing their ability to adjust to soil salinity issues. This might be a result of the staying green effect mediated by cytokinin. The results of this study show that pepper plants may tolerate salt stress better when exogenous spraying of TOC at a concentration of 1 mmol L
-1 boosts the expression of stress response genes [
12]. When sweet pepper plants were subjected to sodium chloride (NaCl) salt stress, foliar silicon spray enhanced the levels of chlorophylls (a and b), mineral nutrients, and moisture status [
13].
Very little calcium (
45Ca) was transported from the treatment site to neighboring tissues when a calcium chloride (
45CaCl
2) solution was sprayed onto the basal and apical leaves of chili pepper. The direct application of repeated calcium spray doses to growing fruiting organs may therefore be the most efficient calcium fertilization technique for raising chili fruit calcium concentrations [
14]. Different calcium formulations, including calcium nitrate of Ca(NO
3)
2, Insol calcium, and Librel calcium, were utilized in the foliar feeding of sweet pepper plants. In comparison to the control, higher concentrations of potassium were found after the application of Ca(NO
3)
2, and higher potassium content was stimulated by the application of Insol calcium. Further, more potassium was discovered in the fruit after feeding with Librel calcium type. The content of phosphorus was lower following the application of both Insol calcium and Librel calcium. Three to five sprays of the tested formulations were applied. Fruits’ average calcium content increased after five treatments by 25.3%, whereas increased after only three treatments by 15.3% over the control [
15]. Prior to harvest, foliar applications of 0.3% or 0.5% calcium chloride (CaCl
2) improve red sweet pepper’s storage quality. Fruits treated with CaCl
2 before harvest experienced a decrease in C
2H
4 generation and respiration rates throughout storage at 7 °C. The ascorbic acid level in the cells was raised by the CaCl
2 treatment. Fruit decay has been greatly delayed as the calcium content has increased [
16].
All potassium humate, potassium chloride, and especially potassium sulfate (K
2SO
4) foliar potassium (K) sources improved the photosynthetic process, pepper plant biomass, chlorophylls (a and b), and endogenous macronutrients (NPK) [
17]. However, Plants treated with iron sulfate (FeSO
4) had the highest vegetative growth indices; foliar iron (Fe) treatment improved total pepper growth significantly. Control plants had the lowest chlorophyll content, whereas FeSO
4 had the highest soil plant analysis development (SPAD) index values of young and old leaves. The iron treatment significantly increased the iron concentration in pepper plant shoots, with the highest concentrations observed in plants sprayed with iron sulfate, intermediate amounts in Fe-EDTA (ferric ethylenediaminetetraacetic acid), and Fe-EDDHA (ethylenediamine di-2-hydroxyphenyl acetate ferric), and the lowest concentrations observed in control plants [
18]. Chitosan-foliar-treated chili pepper plants grew faster than control plants in terms of height, leaf number increments, and chlorophyll content [
19].
Salicylic acid promotes stress tolerance by improving the tomato plant’s physiological response to salt and temperature extremes, as well as altering antioxidant, nutrient, and chlorophyll levels when applied exogenously as a foliar spray [
20]. It was determined that using a copper-based foliar fertilizer with added zinc in conjunction with controlled-release urea can increase tomato plant growth based on a single year’s worth of data. Copper foliar fertilizer and zinc (CFF + Zn) sprayed tomato plants grew taller and had more chlorophyll in their leaves than water-sprayed plants [
21]. However, according to Kaya and Higgs research [
22], foliar zinc application at a low dose (23 mg L
-1) alleviated zinc deficiency in tomatoes while increasing dry matter and chlorophyll content. In the same study, foliar zinc (Zn) treatment at high concentrations (230 mg L
-1) resulted in lower dry matter and chlorophyll content. Increases in dry matter and chlorophyll concentrations were observed in three tomato varieties after foliar application of additional potassium and phosphorus (K and P). Plants grown within high sodium chloride (NaCl) levels had insufficient phosphorus and potassium concentrations, which were remedied by supplemental delivery via leaves [
23].
A lack of calcium, combined with decreased sap movement in the xylem of tomato plants because of a water deficit, can affect xylem tissue production during fruit development. This obstructs the xylem’s movement of calcium into the fruit, and as a result, blossom-end rot may occur, affecting calcium distribution inside the damaged fruit, with healthy fruits providing more soluble calcium than the fruits exhibiting this physiological disorder [
24]. Furthermore, foliar calcium application is required for tomato resistance to bacterial wilt [
25] and fusarium crown rot [
26].
Research cited several reports [
27]. According to one study, foliar phosphorus treatment in greenhouse tomatoes increases chlorophyll, potassium, phosphorus, magnesium, and iron concentrations in the leaves accelerating fruit maturity and increasing marketable production and quality [
28]. Another study found that spraying tomato plants with different calcium salt combinations reduced powdery mildew (
Erysiphe orontii) colony counts on leaves as effectively as elemental sulfur. Powdery mildew development in tomatoes was reduced by foliar calcium treatment due to both osmotic (concentration) and ion-specific effects. Furthermore, higher calcium availability boosts tomato resistance to
Ralstonia solanacearum-caused bacterial wilt, while highly resistant cultivars have high calcium absorption [
27].
A study discovered that foliar calcium nitrate (117 mg kg
-1 nitrogen and 166 mg kg
-1 calcium) and Multifeed (Plaaskem (Pty) Ltd., South Africa) were applied every second week at the indicated application rate of 1 g L
-1, whereas no fertilizer was added to the spray solution in the control treatment (water only). In an open-bag hydroponic system, this letter experiment demonstrated that foliar fertilizer increased the mineral content of tomato fruit (potassium, phosphorus, magnesium, and zinc), as well as the calcium content and total soluble solids of cherry tomatoes [
29]. Tomato crops fertilized via leaves with 6 mmol L
-1 calcium nitrate and 4 mmol L
-1 potassium phosphate had higher quality [
30].
Another research found that foliar feeding a mixture of calcium, boron, zinc, and copper (100 mg L
-1 each), with molybdenum (50 mg L
-1) nutrients resulted in a significant increase in tomato plant height, branches per plant, fruit set, fruits per plant, fruit weight, yield, shelf life, lycopene content, and ascorbic acid when compared to treatments with Ca, B, Zn, Mo, Cu alone or multiplex without calcium [
31]. It was discovered that foliar application of calcium silicate at 1% increased tomato quality parameters such as lycopene and titrable acidity, while potassium silicate at 1% increased the number of flowers, fruits, and yield per plant, while potassium sulfate at 1% increased fruit weight [
32].
The objectives of this communication are to examine what would happen if the user once sprayed 4 mL of each organic solution on the true leaf- saplings developing in simulated ideal environmental conditions for tomatoes and peppers. Salicylic acid at a concentration of 50 mg L-1, sodium bicarbonate 0.52% (w/v), and pure distilled water are the solutions. Indicators relating to the plant’s biochemical makeup will be assessed in this trial to examine how drought-tolerant and non-drought-tolerant cultivars of two crops respond to the treatments (chlorophyll, calcium content, potassium content, and nitrate content). In the end, this trial’s evaluation of plant health will lead the way for researchers to create adequate organic gardening remedies to tackle fungi that cause diseases in the seedlings stage for all varieties.
2. Materials and Methods
On September 18, 2021, three tomato cultivars (Mobil, Korall, and Tyking F1) and three sweet pepper cultivars (Carma, Fokusz, and Bobita F1) were planted in a private nursery in Debrecen (Hungary) under ideal and controlled environmental conditions. The emergence began on September 22 at 80% relative humidity and 25°C temperature. 50 seeds of each cultivar were planted in 56 cells of a half-plastic germination tray, with the seeds distributed in 8 rows and 7 columns. Two of the six varieties mentioned were planted in each tray, which was 46 cm long and 28 cm wide.
After emergence, the trays were removed from the nursery, where the tomatoes were in the stage of the second true leaf set and the sweet pepper was in the stage of the first true leaf set. On October 6, 2021, the three trays were placed in a growth incubator (PHCBI Model: MLR-352-PE) at the Institute of Plant Protection. The temperature was set to 25℃. For lighting, three fluorescent lamps were placed (right, left, and opposite the seedlings), each with a power of 37 watts (each lamp has a bright color, Panasonic type, FL40SS, LUMEN/WATT IS 79.6).
The lights are turned on for 10 hours a day (from 8 a.m. to 6 p.m.), then turned off automatically for 14 hours the remainder of the day. The relative humidity was set to 80% using a bucket of water with a capacity of 6 liters of tap water (monitored using a type of sensor Extech 445702).
Under each seedling tray was a flat-water tray or base with 200 mL of tap water. It was discovered that the roots absorb water every 48 hours, requiring the supply to be replenished every two days from October 7 to the end of the experiment on November 14. To assess potting mix characteristics, an average of four samples were collected using two electronic instruments of the type (Pancellent, and Luster leaf 1835). The total dissolved salts (TDS) were 177 ppm (mg L-1) and the pH was 7.
The percentage of emergence was calculated when the seedlings were transferred from the nursery to the growth incubator. Every 10 seeds were counted as a replicate (ANOVA 1-factor of variety) within 5 replicates for each cultivar and within a completely randomized design (Excel 365). Means were separated using the Honestly Significant Difference at a probability level of 0.05 (HSD). To analyze gaps in the means of various groups, the Post Hoc test (Tukey-Kramer a Post Hoc test in Minitab 20) was used.
On October 12th, a Minolta Company tool was used to measure chlorophyll content (SPAD-502, Japan). Tomatoes emerge from the second set of true leaves, while peppers emerge from the first. 5 plants from each variety were chosen at random, and 5 chlorophyll records were collected at random for each plant, with the average record reported. Each record was collected from the upper half of the leaf. Similarly to the preceding parameter, 5 replicates (plants) were chosen at random for each cultivar, and statistics from (ANOVA 1-factor of variety) in (Excel 365) were used within a completely randomized design. At a probability level of 0.05, means were separated using the Honestly Significant Difference method (HSD). The Post Hoc test (Tukey-Kramer a Post Hoc test in Minitab 20) was used to assess gaps in the means of different groups.
Each variety received a spraying treatment 20 days after being transferred to the incubator, on October 25. The following solutions were applied at a rate of 4 mL to each plant:
- 1-
0.52% sodium bicarbonate was sprayed on every 2 rows of seedlings of each tomato and sweet pepper species.
- 2-
2-A spray solution of 50 mg L-1 salicylic acid was sprayed on two rows of each type of tomato and sweet pepper seedlings.
- 3-
Distilled water was sprayed on each seedling’s cultivar of the two plants in two rows of a tray.
After spraying the shoots of each plant, 200 mL of tap water was placed in the base of each tray, and the plants were returned to the growth incubator.
After 2 days, a chlorophyll sensor (SPAD-502) was used to record 5 measurements for each plant from each treatment (48 hours). Similarly, after 8 days of spraying, another chlorophyll content record was taken. The 2 chlorophyll content indices were calculated using the average of the five plants from each treatment. In data collection and statistical analysis (Excel 365), the factorial analysis of two factors was used, with the first variable being the variety and the second being the spray treatment, and each record having five observations. The Post Hoc test (Tukey-Kramer in Minitab 20) was used to assess differences in means between groups.
On October 29, the tomatoes begin to elongate, the cotyledon leaves fall to the ground, the pepper true leaves begin to expand, and the roots of both plants begin to elongate out of the tray. As a result, from October 29 to November 14, the amount of water in the base increased to 300 mL.
The roots of ten normal plants from each treatment were cleaned and dried 40 days after transplantation (November 16, 2021). All parts of ten seedlings were crushed in a blender (SENCOR model) for three minutes. The mixture was pressed in a garlic press to extract plant sap. A total of five samples were collected. The calcium cation (Ca+2), potassium cation (K+), and nitrate (NO3-) contents (mg L-1) in plant sap were measured using three electronic sensors of the type (HORIBA instruments LAQUA-TWIN). where 5 readings were taken for each ion, variety, and treatment. In other words, for each ion in each plant cultivar, 15 readings were taken for three treatments (salicylic acid 50 mg L-1, sodium bicarbonate 0.52%, and control). Before use, sensors were calibrated twice with standard solutions (150 and 2000 ppm, or mg L-1). The data was collected and analyzed using a factorial analysis of two variables (Excel 365), with the first variable being the variety and the second variable being the spray treatment, and each record having five observations. To analyze gaps in the means of different groups, the Post Hoc test (Tukey-Kramer test in Minitab 20) was used.
Correlation and regression analyses (using Microsoft Excel 365) were performed twice for each pair (SPAD after 8 days and nitrate content), (potassium content and calcium content), (nitrate content and calcium content), and finally (nitrate content and potassium content), switching the independent factor and the dependent variable each time for tomato and sweet pepper, respectively. Minitab 20’s regression response optimizer was used to determine the maximum and minimum responses to each plant’s calcium, potassium, nitrate, and chlorophyll content factors, with only genotype and foliar nutrition variables considered (genotype and foliar nutrition interactions discarded due to weak interactions).
The acidity of the organic spray solutions of [salicylic acid (C7H6O3 50 mg L-1, and 100 mg L-1), sodium bicarbonate (NaHCO3 0.52%), Epsom salt (MgSO4.7H2O 0.52%), peroxide (H2O2), and distilled water (H2O)] was tested using an acidity tester (pH meter model: VWR PH/ CO 1030), and four replicates were taken. There were no additives used in the preparation of the raw solutions (without materials of adjuvants adhesiveness, cohesiveness, or distribution). Only three of them were used to check the plant health status of the chemical composition test (C7H6O3 50 mg L-1, NaHCO3 0.52%, and H2O). To calibrate the acidity digital device before each use, the following chemical buffers were used: 4.01, 7, and 10.01. After every measurement, the sensor was cleaned with distilled water and dried. Similarly, the calcium cation (Ca+2), potassium cation (K+), and nitrate (NO3-) contents (mg L-1) in organic foliar sprays were determined using three electronic sensors of the same type (HORIBA instruments LAQUA-TWIN), each with four readings. The latter sensors were calibrated twice with standard solutions prior to use (150 and 2000 ppm, or mg L-1).
3. Results and Discussion
Each tomato variety’s emergence percentage was displayed in (
Table 1). The variety (Tyking F1) was significantly the lowest (86%). Sweet pepper cultivars showed no significant differences (
Table 2).
The emergence percentage expresses the viability of the embryo in seeds produced in a nursery or field. In the lab, the standard germination percentage was tested under suboptimal temperature, humidity, and growing media conditions [
33]. In the nursery, the emergence percentage represents the typical germination percentage. In general, cultivars that are tolerant of abiotic stress appear to have higher percentages of germination (emergence) in the nursery than others.
Before foliar spraying, the chlorophyll content (SAPD index) of tomatoes (
Table 3) and sweet peppers (
Table 4) showed no significant differences between cultivars. In terms of chlorophyll content, the drought-tolerant cultivar (Mobil) outperformed the others. Although (Carma, and Fokusz) had higher chlorophyll content than the non-drought tolerant pepper (Bobita F1), there were no significant differences between them.
The species’ physiological reactions and stress tolerance significantly impact chlorophyll content. The chlorophyll content is an important indicator for determining drought susceptibility in tomatoes [
34]. Because of metabolic instability caused by drought susceptibility, the amount of chlorophyll in the leaf changes, lowering light absorption. The chlorophyll content of the tomato variety (Tyking F1) is expected to be lower than that of other tomato varieties because it is not drought resistant. Previously, close scientific arguments were reported by [
1], and [
2].
At a probability level of 0.05, there was no significant interaction between the effect of plant genotype and foliar nutrition treatment on the SPAD index after two days of foliar nutrition. Tomato foliar nutrition has a positive impact on chlorophyll content for (Mobil and Korall) (
Table 5 and Table 9), and a great increase for (Tyking F1) after 48 hours (
Table 5) and 8 days (Table 9).
There were no significant differences between tomato genotypes (
Table 5) and sweet pepper genotypes (
Table 6). Pepper foliar nutrition increases chlorophyll content slightly in all varieties (
Table 6 and
Table 10), especially after 8 days (Table 10).
Because all aerial plant parts are protected by a hydrophobic cuticle, the bidirectional exchange of water, solutes, and gases between the plant and the atmosphere is restricted. Nutrient solutions, on the other hand, can be absorbed by cracks, stomata, and lenticels [
35]. Stomata are important in the absorption of nutrient solutions applied to leaves and foliage.
Among tomato genotypes, the sodium bicarbonate (0.52%) treatment had a significantly higher SPAD index (after 48 hours of spraying) than the salicylic acid and distilled water treatments (
Table 7). Among sweet pepper genotypes, sodium bicarbonate (0.52%) treatment had a higher SPAD index than salicylic acid treatment (
Table 8). There was a significant difference among sweet pepper genotypes between sodium bicarbonate treatment (0.52%) and distilled water treatment (
Table 8). It is reasonable to conclude that sodium bicarbonate (0.52%) was the primary source of increased chlorophyll content in tomatoes (
Table 7 and Table 11) and sweet pepper varieties (
Table 8 and Table 12). The amount of nitrogen in the leaf will increase the chlorophyll content (as measured by the SPAD value) [
36]. In comparison to other organic solutions, sodium bicarbonate solution had the highest amount of nitrate (NO
3-) (53.5 mg L
-1) (Table 29).
Table 7.
The overall mean for the effect of foliar nutrition on chlorophyll content (SPAD index) after 48 hours of spraying among tomato genotypes.
Table 7.
The overall mean for the effect of foliar nutrition on chlorophyll content (SPAD index) after 48 hours of spraying among tomato genotypes.
| Foliar nutrition |
SPAD index (48 hours) |
| Salicylic Acid 50 mg L-1
|
23.85 B |
| Sodium Bicarbonate 0.52% |
31.02 A |
| Distilled Water |
22.51 B |
Table 8.
The overall mean for the effect of foliar nutrition on chlorophyll content (SPAD index) after 48 hours of spraying among sweet pepper genotypes.
Table 8.
The overall mean for the effect of foliar nutrition on chlorophyll content (SPAD index) after 48 hours of spraying among sweet pepper genotypes.
| Foliar nutrition |
SPAD index (48 hours) |
| Salicylic Acid 50 mg L-1
|
27.5 AB |
| Sodium Bicarbonate 0.52% |
31.21 A |
| Distilled Water |
23.05 B |
At a probability level of 0.05, there was no significant interaction between the effect of plant genotype and foliar nutrition treatment on SPAD index (after 8 days). The tomato variety (Tyking F1) outperformed all other tomato varieties (Mobil, and Korall). Both (Mobil and Korall) were not significantly different (
Table 9). There were no significant differences between sweet pepper genotypes (
Table 10). This is due to the sodium bicarbonate solution’s primary effect on the non-drought-tolerant tomato variety (Tyking F1).
Table 9.
Impact of tomato genotypes on the SPAD index after foliar nutrition (8 days).
Table 9.
Impact of tomato genotypes on the SPAD index after foliar nutrition (8 days).
| Tomato genotypes |
SPAD index (8 days) |
| Mobil |
28.38 B |
| Korall |
25.80 B |
| Tyking F1 |
32.50 A |
Table 10.
Impact of sweet pepper genotypes on SPAD index after foliar nutrition (8 days).
Table 10.
Impact of sweet pepper genotypes on SPAD index after foliar nutrition (8 days).
| Sweet pepper genotypes |
SPAD index (8 days) |
| Carma |
31.85 A |
| Fokusz |
33.13 A |
| Bobita F1 |
28.23 A |
Among tomato genotypes, the sodium bicarbonate (0.52%) treatment had a significantly higher SPAD index (after 8 days of spraying), followed by the salicylic acid treatment and distilled water treatment (
Table 11). Sodium bicarbonate (0.52%) and salicylic acid (50 mg L
-1) treatments, on the other hand, have a significantly higher SPAD index than distilled water treatment among sweet pepper genotypes (
Table 12). Nitrate is a nitrogen-rich nitric acid salt that plants can use. Nitrite (NO
2-), on the other hand, is a nitric acid salt in which nitrogen is not readily available to plants [
37]. It can be concluded that sodium bicarbonate is a superior source of nitrate (NO
3-) to improve plant health through increasing chlorophyll content. according to (Table 29) It is worthwhile to experiment with Epsom salt in the future.
Table 11.
The overall mean for the effect of foliar nutrition on chlorophyll content (SPAD index) after 8 days of spraying among tomato genotypes.
Table 11.
The overall mean for the effect of foliar nutrition on chlorophyll content (SPAD index) after 8 days of spraying among tomato genotypes.
| Foliar nutrition |
SPAD index (8 days) |
| Salicylic Acid 50 mg L-1
|
28.34 B |
| Sodium Bicarbonate 0.52% |
32.23 A |
| Distilled Water |
26.10 B |
Table 12.
The overall mean for the effect of foliar nutrition on chlorophyll content (SPAD index) after 8 days of spraying among sweet pepper genotypes.
Table 12.
The overall mean for the effect of foliar nutrition on chlorophyll content (SPAD index) after 8 days of spraying among sweet pepper genotypes.
| Foliar nutrition |
SPAD index (8 days) |
| Salicylic Acid 50 mg L-1
|
34.29 A |
| Sodium Bicarbonate 0.52% |
35.35 A |
| Distilled Water |
23.57 B |
For tomatoes’ calcium cation content (Ca
+2), the interaction between variety and foliar nutrition treatment was not significant. Among foliar nutrition treatments, tomato (Mobil) had a significantly higher calcium cation content (mg L
-1) than both (Korall and Tyking F1) varieties (
Table 13).
Foliar nutrition treatments did not differ in calcium cation content for all tomato genotypes except for sodium bicarbonate, which had the lowest significant calcium cation content (mg L
-1) among tomato genotypes (
Table 14).
Returning to (Table 29), the calcium content in both salicylic acids (50 mg L-1) (16.5 mg L-1) and distilled water (10.25 mg L-1) solutions was the highest (salicylic acid (100 mg L-1) was not used in this experiment). The calcium content of salicylic acid (50 mg L-1) or distilled water may be the cause of tomato (Mobil) plant sap calcium excellence.
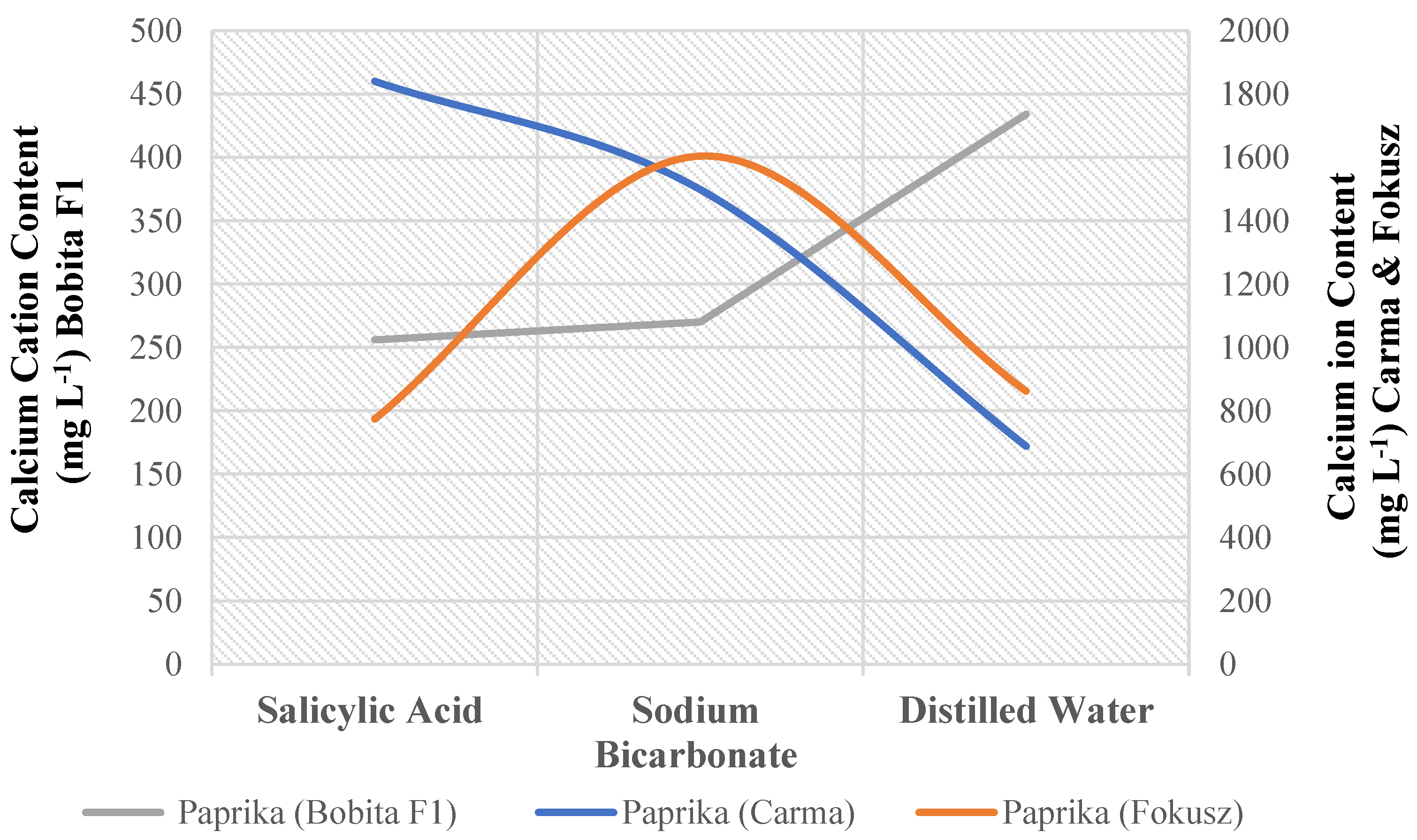
For calcium cation content in sweet pepper, there was a significant interaction between variety and foliar treatment (Appendix A). Two of the nine possible combinations of three sweet pepper genotypes and three foliar nutrition treatments outperform the others significantly. The scores of (Carma) genotype plants under salicylic acid treatment were higher than those of (Fokusz) plants under sodium bicarbonate treatment (
Figure 1). (Bobita F1) has the lowest scores regardless of treatment (
Table 15).
| Source of Variation |
SS |
Df |
MS |
F |
P-value |
F crit |
| Sweet Pepper Genotypes |
8453640 |
2 |
4226820 |
18.2884216 |
3.3043E-06*
|
3.259446306 |
| Foliar Nutrition |
1646413.333 |
2 |
823206.6667 |
3.56181493 |
0.038776574*
|
3.259446306 |
| Interaction |
4030506.667 |
4 |
1007626.667 |
4.359755394 |
0.005613109*
|
2.633532094 |
| Within |
8320320 |
36 |
231120 |
|
| |
| Total |
22450880 |
44 |
|
| *P ≤ 0.05: Significant. |
Salicylic acid (50 mg L
-1) is the most likely cause of the high calcium content in premium varieties; (Mobil) tomato and sweet pepper (Carma). The calcium content of plant sap in this experiment supports a previously published hypothesis by (Massimi and Radócz) [
33]. Seedlings were studied under simulated environmental stresses such as non-ideal temperatures, low humidity, close spacing, minimum light dose, nutrient-deficient water, and salicylic acid lower dosage (50 mg L
-1). Several seedling vigor indices were used to assess the seedling’s growth. Seedlings of tomato (Mobil) and sweet pepper (Carma) outperform other varieties, possibly due to the variety’s vigor under different stress conditions [
33]. These findings show that tomato (Mobil) and sweet pepper (Carma) had a positive impact on development and could be raised in nurseries under optimal conditions before being transferred to open-air environmental and biological exposure conditions in Hungary [
33]. Spraying (Carma) seedlings with an appropriate dose of salicylic acid before transplanting improves stress tolerance by improving the plant’s physiological response to drought, salt, and temperature extremes, as well as changing antioxidants, and nutrients. Salicylic acid’s beneficial effect on tomato plant stem diameter could also be attributed to accelerated cell division [
33]. This acid, like the plant growth regulator auxin, has a stimulatory effect that promotes cell elongation. Pectin is formed with the help of calcium. As a result, a lack of this element will result in the collapse of the cell walls. Plant cell walls are composed of pectin, which has an acidic sugar backbone and neutral sugar side chains. It helps with cell adhesion and cell wall formation [
38]. As a result, it is possible to deduce that salicylic acid plays an indirect role in cell wall formation during division and elongation.
The high temperature raised the relative humidity in the plastic-covered growing facility, influencing transpiration and nutrient transport. Ca
+2 ion uptake was hampered in sweet pepper plants by a long-term root zone temperature of 29 °C [
39]. More calcium is required by young pepper plant cells during the fruit setting stage. This explains the appearance of blossom end rot. Blossom end rot is a serious pepper and tomato disease caused by an environmental problem, most commonly uneven watering (drought conditions) and calcium deficiency [
37]. Thus, it is worth trying salicylic acid (100 mg L
-1) in the future, which had calcium of (51 mg L
-1) (Table 29).
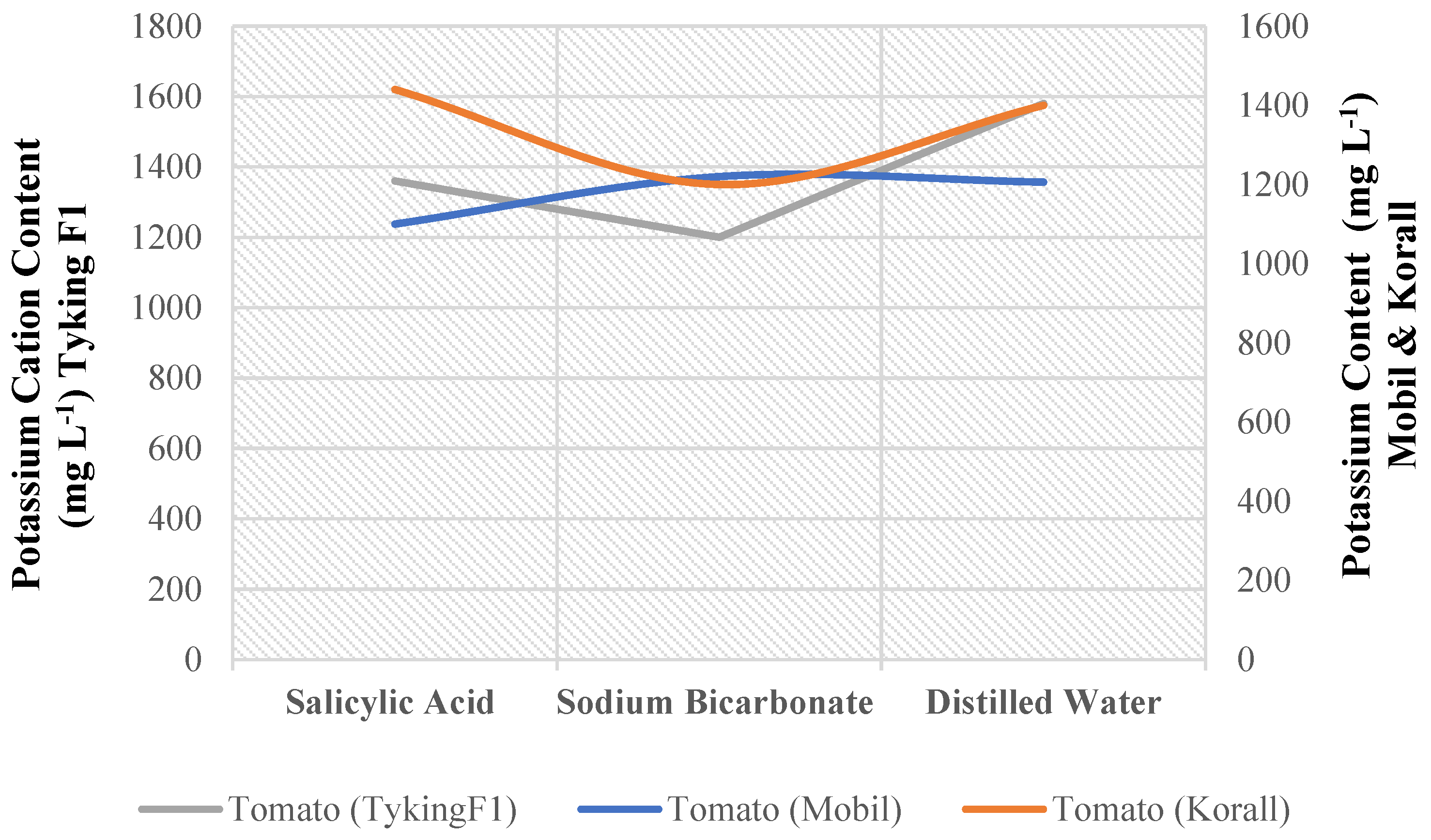
There was a significant interaction between variety and foliar treatment for potassium cation content in tomatoes (Appendix B). One of the nine possible combinations of three tomato genotypes and three foliar nutrition treatments significantly outperformed the others (
Figure 2). (Tyking F1) genotype plants perform well under distilled water spray treatment. Plants treated with salicylic acid (Mobil) performed the worst (
Table 16).
| Source of Variation |
SS |
Df |
MS |
F |
P-value |
F crit |
| Tomato Genotypes |
361773.3333 |
2 |
180886.6667 |
17.01484114 |
6.286E-06*
|
3.259446306 |
| Foliar Nutrition |
266973.3333 |
2 |
133486.6667 |
12.5562291 |
7.29641E-05*
|
3.259446306 |
| Interaction |
305413.3333 |
4 |
76353.33333 |
7.182065217 |
0.000233057*
|
2.633532094 |
| Within |
382720 |
36 |
10631.11111 |
|
| |
| Total |
1316880 |
44 |
|
| *P ≤ 0.05: Significant |
For potassium cation content, no significant interactions were found between the sweet pepper genotype and foliar treatment. There were no significant differences in sweet pepper genotype (
Table 17) or foliar nutrition treatment (
Table 18). The potassium cation content (mg L
-1) in the foliar nutrition treatment of sodium bicarbonate (0.52%) was higher but not significantly different from the salicylic acid and distilled water treatments (
Table 18).
There is no potassium in any of the solutions used on the plants in this experiment. Even in distilled water, there is no potassium (Table 29). It is obvious to generalize that all types of spraying have no effect on the potassium content change in tomato and sweet pepper plants’ sap. This does not change the fact that non-drought-tolerant cultivars had the highest potassium levels when compared to the other cultivars, this is due maybe to the genetic makeup of these varieties (
Table 16 and
Table 17). If diseases or pests are expected because of potassium deficiency, the results may be useful in selecting these varieties. Gray leaf spots, for example, are caused by a fungus (
Stemphylium solani) that is harmful to pepper plants [
40]. This disease in tomatoes and peppers may be caused by a complex combination of potassium deficiency and drought stress. Tomato blotchy ripening is the second disorder, in which unripe patches of the flesh are randomly distributed, hard, yellow, or green. Dry soil, high temperatures, and a lack of potassium are thought to be major contributors [
37]. As previously stated, these cultivars (tomato: Tyking F 1 and sweet pepper: Bobita F1) are drought-sensitive, even though they contain potassium in relatively adequate quantities. Consequently, relying on these varieties is fraught with controversy and error.
The relationship between tomato variety and foliar nutrition treatment was not significant for nitrate levels. Among the foliar nutrition treatments, both tomato varieties (Mobil and Tyking F1) had significantly higher plant sap nitrate content (mg L
-1) than the (Korall) genotype (
Table 19). The relationship between variety and foliar nutrition treatment for nitrate levels in sweet pepper was not significant. The sweet pepper (Fokusz) genotype had significantly higher plant sap nitrate content (mg L
-1) than the genotype (Carma) among foliar nutrition treatments (
Table 20). When compared to the other genotypes, the (Bobita F1) genotype had a non-significant difference in nitrate content.
Plants can use nitrate, a nitrogen-rich nitric acid salt. Nitrite (NO
2-) is a nitric acid salt with inaccessible nitrogen to plants [
37]. The SPAD index accurately predicts the total nitrogen content of plant succulents in all forms (NH
4+, NO
3-, or NO
2-). Nitrate content alone cannot adequately describe vegetative growth vigor and chlorophyll content. Especially if nitrates were converted to amino acid building blocks while the parameters were being recorded. Tomatoes (Mobil and Tyking F1) outperformed Korall in terms of nitrate content. This result is consistent with the SPAD index after 8 days of foliar spraying, where (Mobil and Tyking F1) outperform the variety (Korall). After 8 days of foliar nutrition, the sweet pepper variety (Fokusz) also had the highest nitrate and SPAD index.
Foliar nutrition treatments, on the other hand, had no effect on the nitrate content (mg L
-1) of any tomato genotype (
Table 21) or sweet pepper genotype (
Table 22).
Soil microbiologists have previously shown a link between soil acidity and plant nitrate nitrogen availability. In alkaline environments, nitrates are typically more abundant [
41]. Returning to (Table 29), both salicylic acid (50 mg L
-1) and sodium bicarbonate (0.52%) have a slightly basic acidity. Distilled water, on the other hand, has a neutral acidity. Is the researcher now curious about the plant sap nitrate content when other acidic solutions, such as (salicylic 100 mg L
-1) and peroxide (50%), are used instead? Would the results be the same or better if Epsom salt (pH = 8.0025) was used?
As shown in (
Table 23), salicylic acid 50 mg L
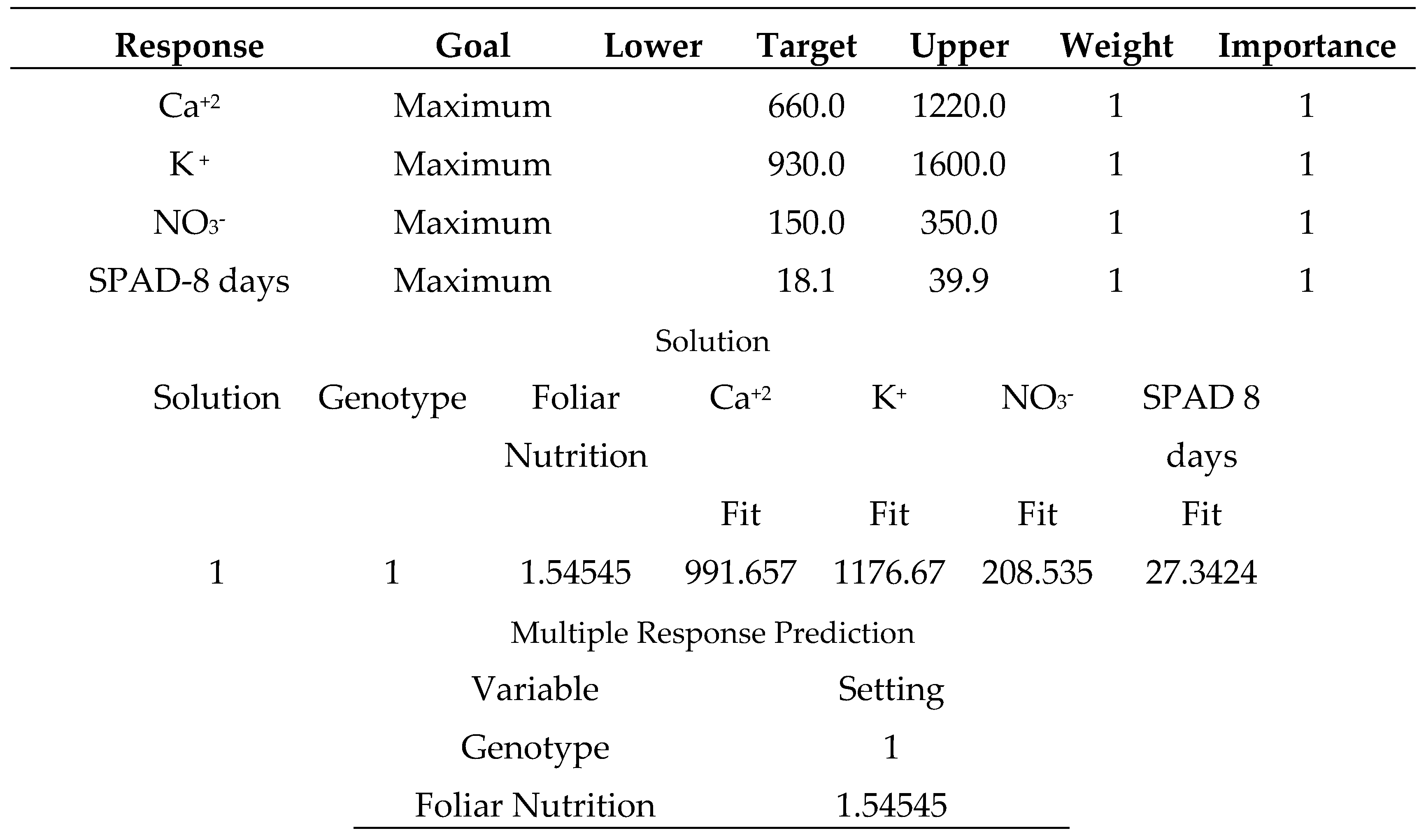
-1 produces the strongest multiple responses, particularly for (Tyking F1, and Korall) tomato cultivars, where the varieties were coded in the following order (1: Mobil, 2: Korall, 3: Tyking F1) and the foliar nutrition was coded in the following order (1: salicylic acid 50 mg L
-1, 2: sodium bicarbonate 0.52%, 3: distilled water).
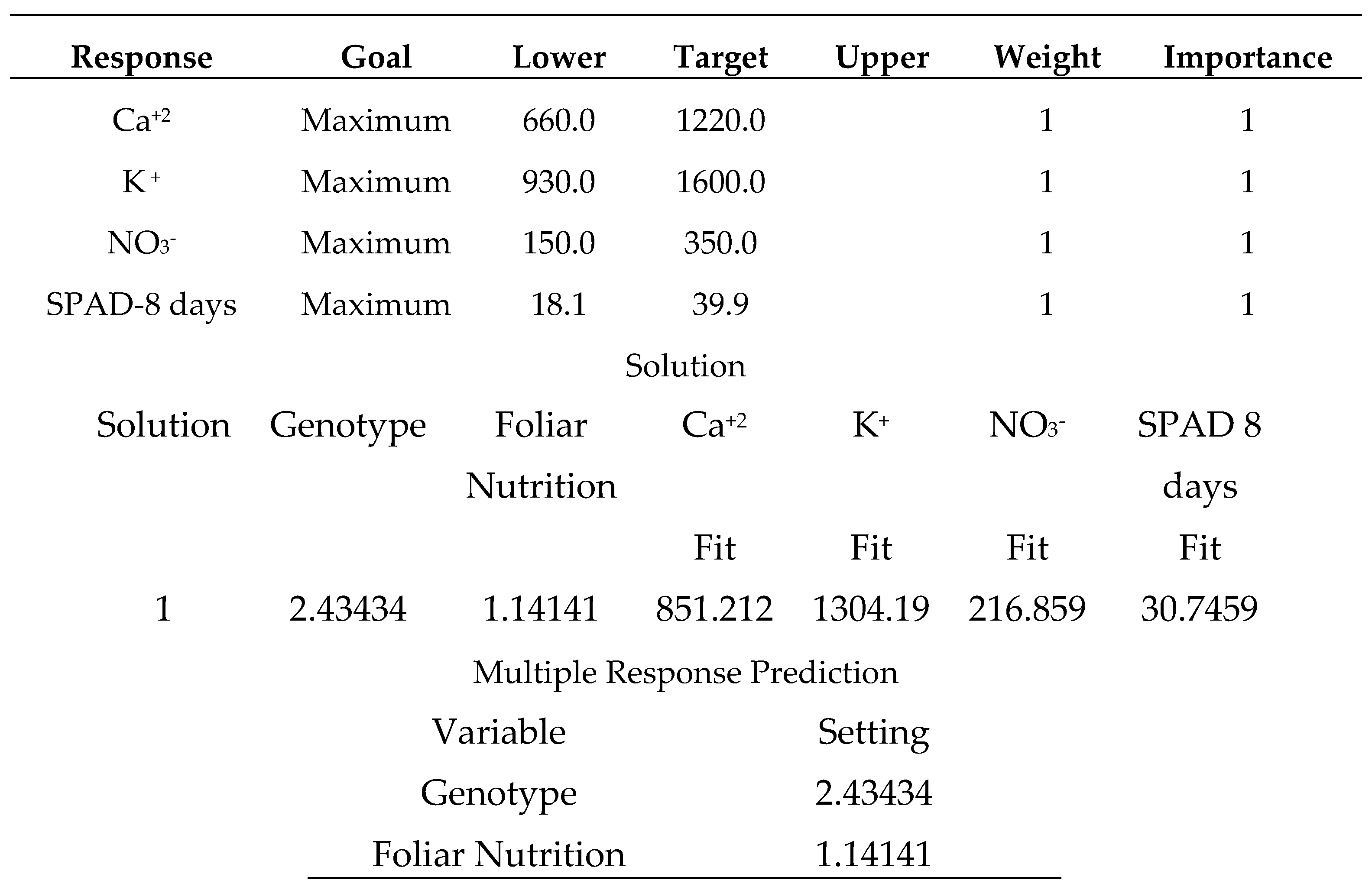
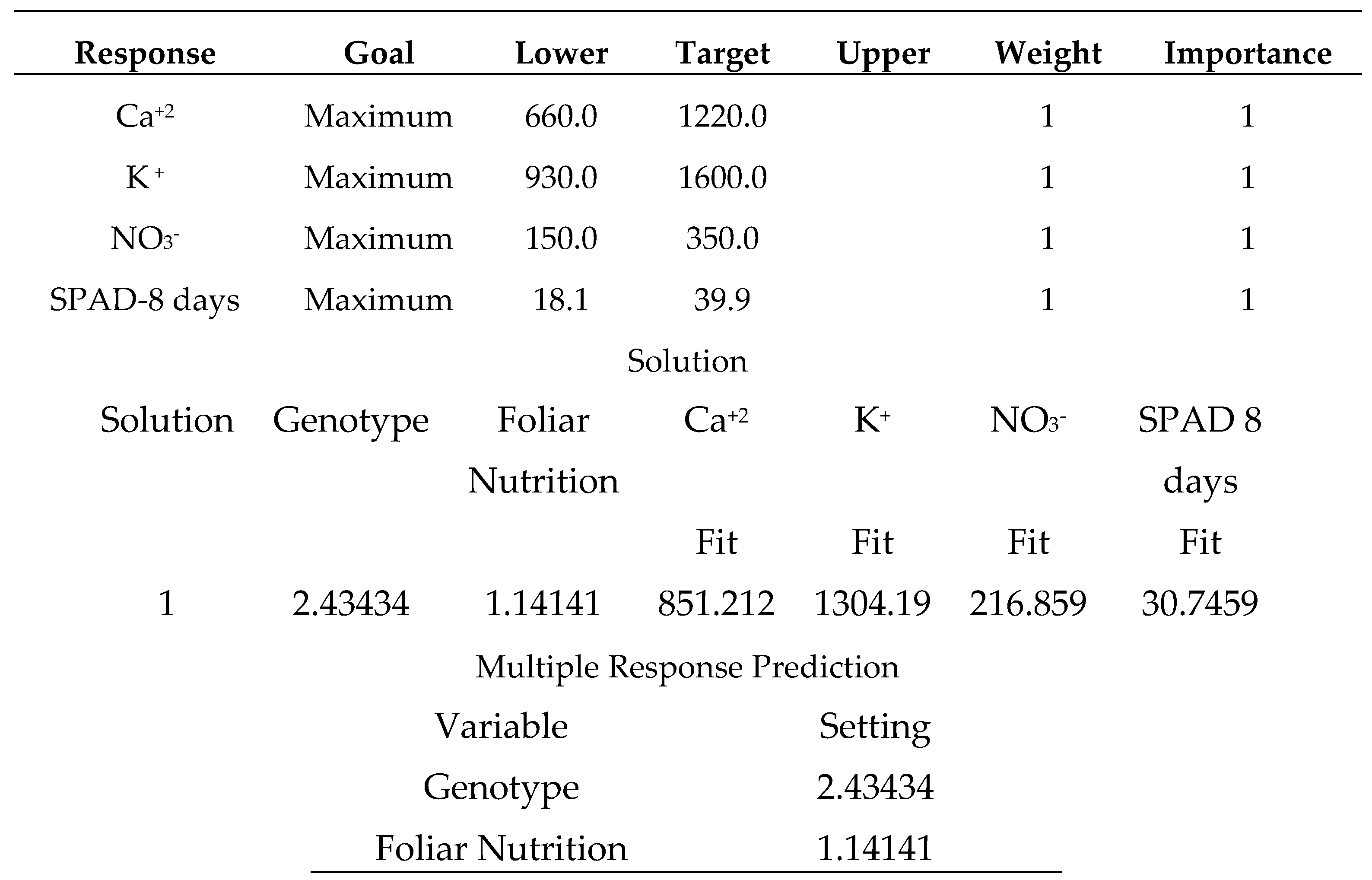
Salicylic acid 50 mg L
-1, as shown in (
Table 24), produces the strongest multiple responses, particularly for the (Carma) sweet pepper cultivar. where the varieties were coded in the following order (1: Carma, 2: Fokusz, 3: Bobita F1) and the foliar nutrition (1: salicylic acid 50 mg L
-1, 2: sodium bicarbonate 0.52%, 3: distilled water).
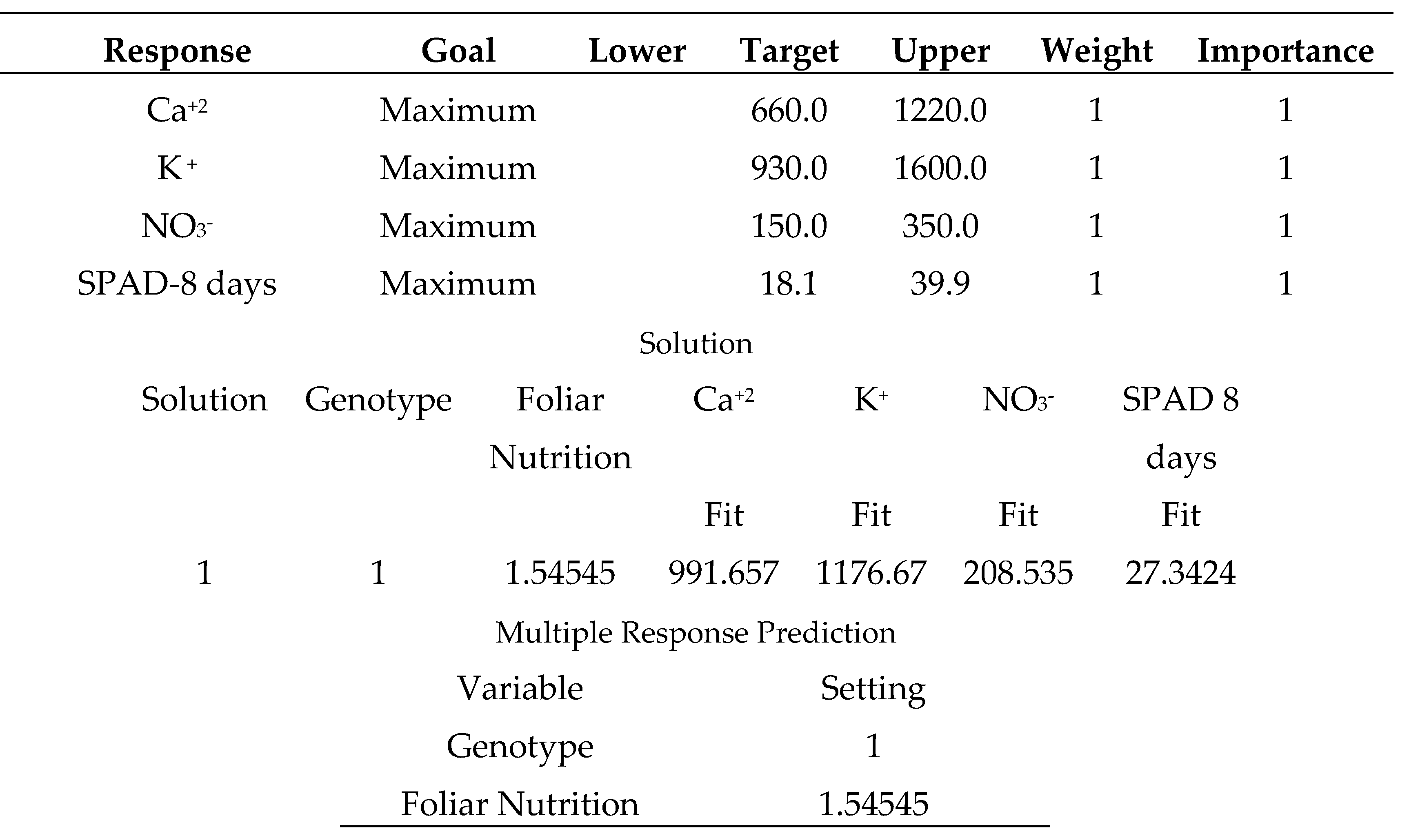
The multiple minimum responses for the variety (Mobil), which outperformed seedling vigor in suboptimal environmental conditions [
33], are shown in (
Table 25). Spraying the same variety with salicylic acid (50 mg L
-1) and sodium bicarbonate (0.52%) produced disappointing results. Finally, it is recommended that growers grow (Mobil) without any supportive spraying treatment. Table (26) displays the multiple minimum responses for the variety (Bobita F1) that demonstrated non-significantly decreased seedling vigor in poor environmental conditions [
33]. When the same type was treated with distilled water, the results were unsatisfactory. Finally, gardeners should consider growing (Carma) with a supportive spraying application of salicylic acid 50 mg L
-1.
This is a compromise or statistical asymptote to eliminate individual comparisons and focus on group comparisons -maximum performance- for general plant health recommendations based on genotype selection and foliar nutrition treatment. One of the primary goals here is that spraying is a preventive measure -within the integrated pest management framework or philosophy- rather than a treatment for pests. In other words, it is a simulation protocol that aims to produce healthy seedlings in optimal nursery conditions prior to growing transplants in a permanent open field, greenhouse, or field tunnel.
Spraying the tomato (Mobil) variety with salicylic acid (50 mg L
-1) or sodium bicarbonate (0.52%) produced disappointing results (
Table 25). Finally, it is recommended that growers grow (Mobil) without any supportive spraying treatment. It has already been demonstrated that tomato (Mobil) produced – significantly - better seedlings vigor under multiple abiotic stresses than other types (Korall and Tyking F1) [
33]. However, almost (Korall) variety will thrive when treated with salicylic acid (50 mg L
-1) and in a better performance than (Tyking F1) (
Table 23).
The sweet pepper variety (Bobita F1) produced multiple minimum responses of all plant health status attributes. The results were disappointing when this variety was treated with distilled water (
Table 26). Finally, gardeners should consider growing (Carma) with a supportive spraying application of salicylic acid (50 mg L
-1) (
Table 24). All sweet pepper types were not significantly different, despite the sweet pepper variety (Carma) having a numerical advantage in the two examined measures of seedling vigor [
33].
According to Table (27), the effect of chlorophyll content on nitrate content was not significant across all tomato genotypes and foliar treatments. There was no discernible effect of potassium on calcium level or nitrate on potassium content. Where nitrate content significantly affects calcium content (
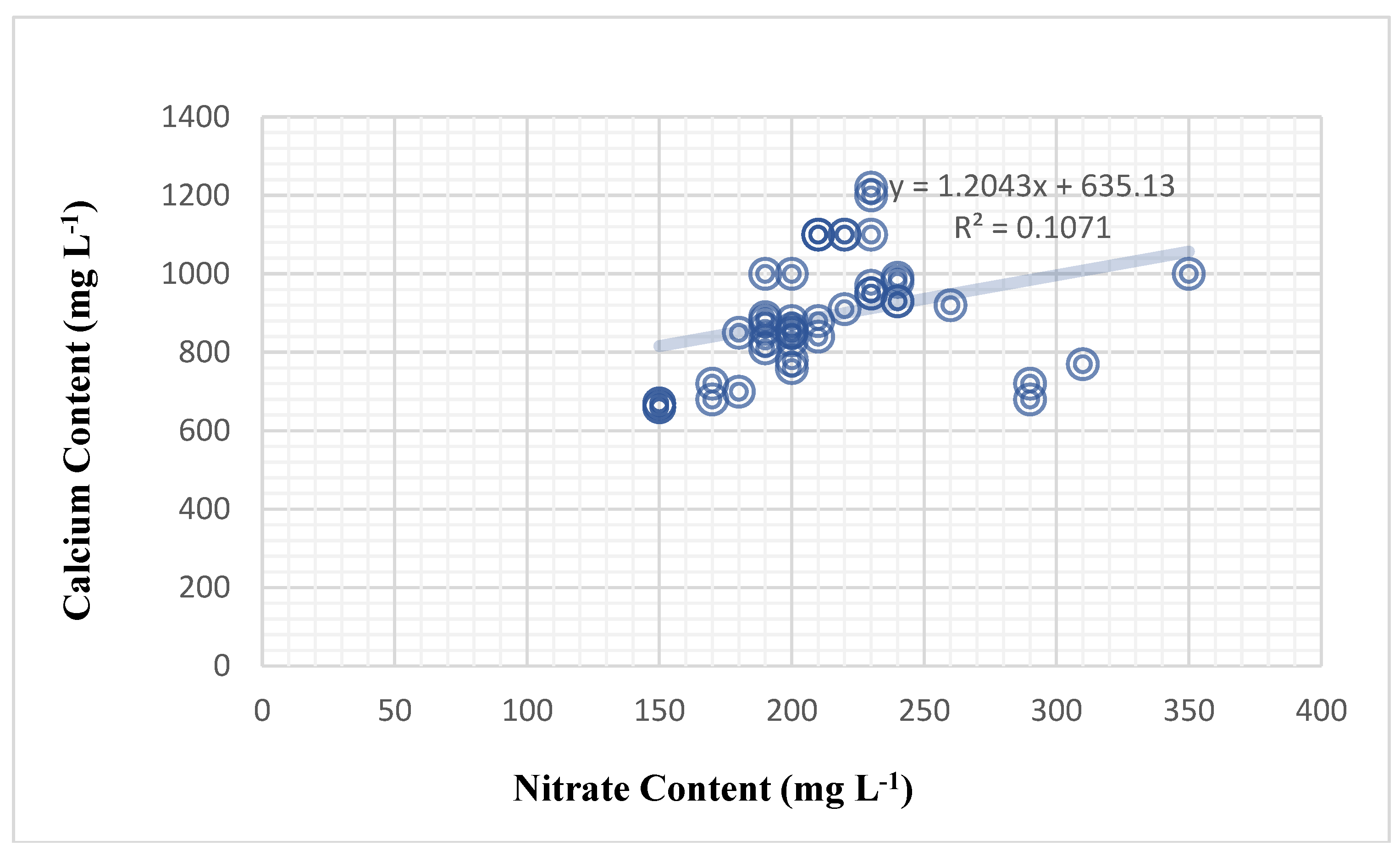
Figure 3), the correlation was weak and positive by 32.7%.
The challenge of supplying nutrients to the soil solution to nourish the plant through its roots brought to light the issue of antagonism, which is described as an abundance of one element or nutrient that results in a decrease in absorption for another [
38]. Calcium availability and potassium absorption are negatively impacted by antagonistic factors such as increasing the soil’s nitrogen content. The intake of potassium is influenced by calcium availability, and excess potassium affects calcium and nitrogen uptake. Nevertheless, synergism is when the amount of one nutrient improves the likelihood that another nutrient will be absorbed.
However, contrasting results in soil fertilization were observed in this foliar nutrition test and through regression and correlation analysis (
Table 27 and
Table 28). This analysis demonstrates the mathematical relationship as well as its strength. As the mathematical model is synonymous with the (antagonism) in soil nutrition, the negative regression relationship appeared in the foliar nutrition of tomatoes. Only in the tomato plant (
Table 27) does the negative relationship (antagonism) appear for the pairs of variables: (potassium content influences calcium content availability, and nitrate content inhibits potassium uptake) (
Table 27). Previous findings (
Tables 16, 17, and 18) revealed that foliar nutrition had no effect on plant sap potassium content. The negative relationships that collect potassium in one of its axes will be avoided in the discussion. Also, it was previously stated that the chlorophyll content of a plant (SPAD index) does not only refer to its nitrate content. As a result, the mathematical relationship between chlorophyll content (SPAD index) and nitrate content will be overlooked (
Table 27 and
Table 28).
The most significant discovery is a significant relationship between nitrate and calcium in both plants. The results show that the plant’s nitrate content had a significant effect on the calcium content of the plant sap. Nitrate content significantly influenced calcium content in the tomato plant in a weak positive relationship (correlation of 32.7%), and the mathematical forecasting relationship deduced was (Y = 1.2043X + 635.13) (
Figure 3).
There are no negative relationships between variables in the sweet pepper plant (
Table 28). The effect of chlorophyll concentration on nitrate content was not significant across all sweet pepper genotypes and foliar nutrition treatments, as demonstrated in (
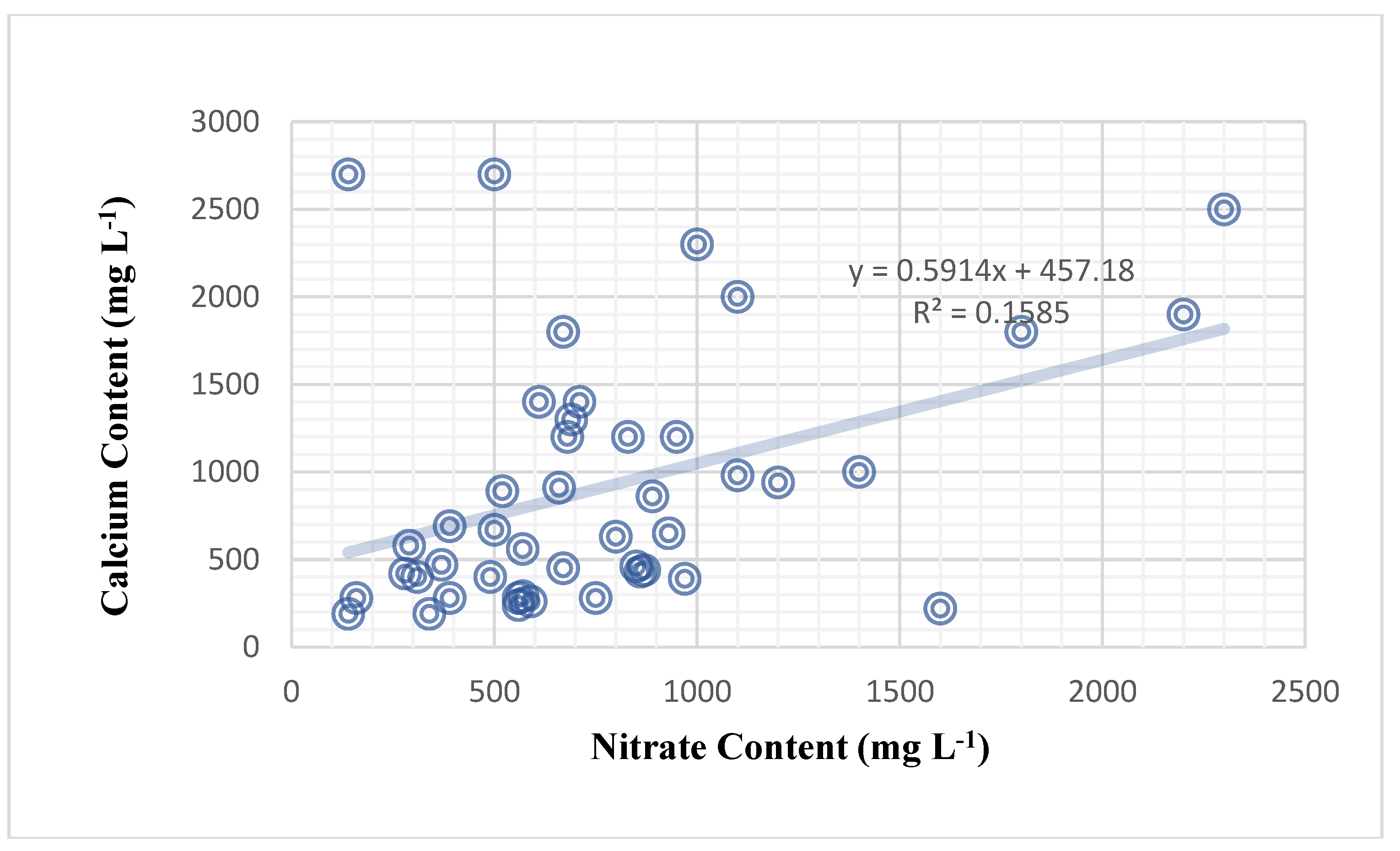
Table 28). The amount of potassium cations in plant sap has no effect on calcium levels. However, nitrate had a significant impact on calcium content (correlation of 39.8%) (
Figure 4). It was discovered that the plant’s nitrate content had a significant effect on the plant’s calcium content. Nitrate content significantly influenced calcium content in the sweet pepper plant in a weak positive relationship (correlation of 39.8%) (
Table 28), and the mathematical forecasting relationship deduced was (Y = 0.5914X + 457.18) (
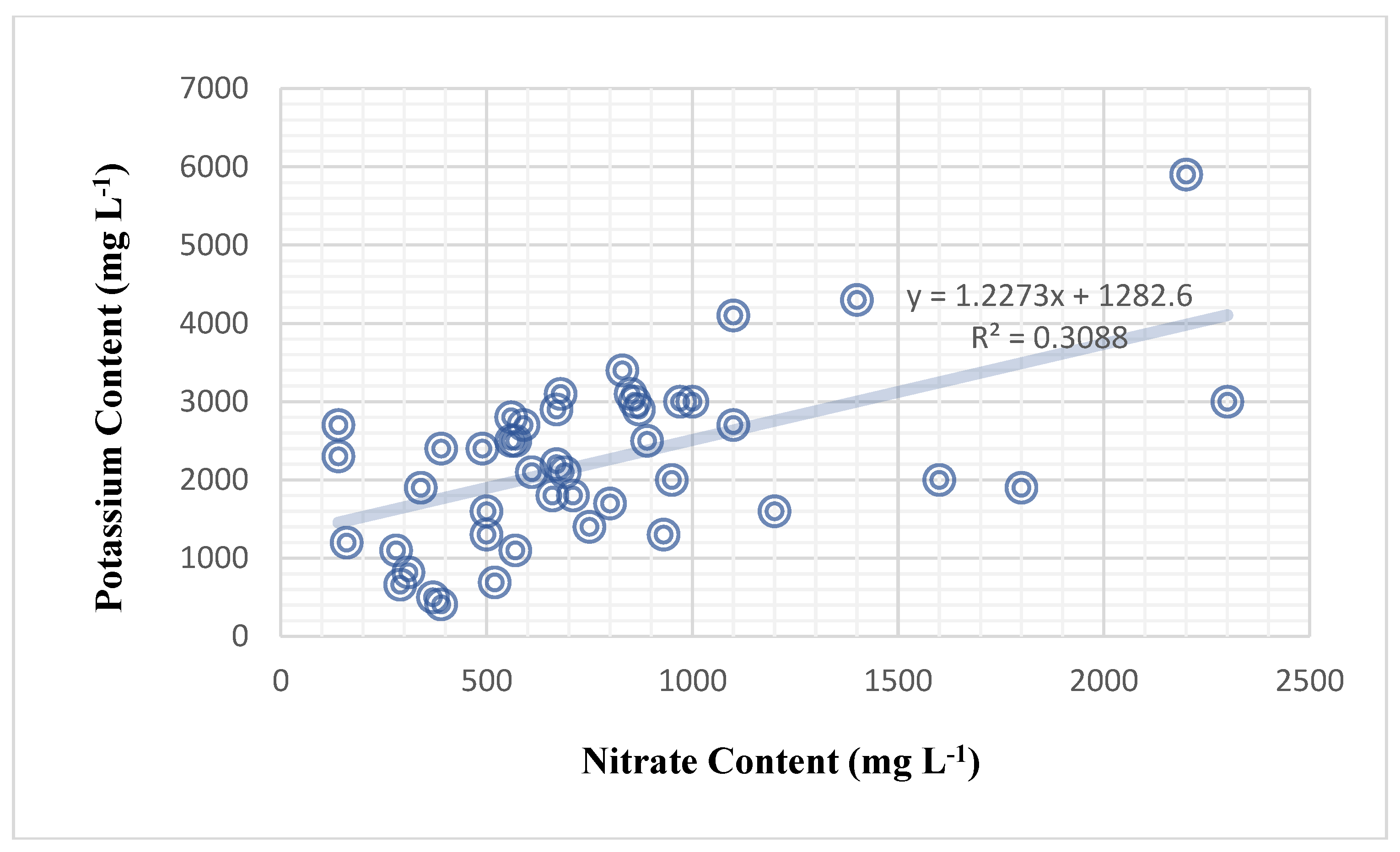
Figure 4). Furthermore, nitrate content significantly influenced potassium content, with a medium and positive correlation of 55.6% (
Figure 5). Previous findings in this article found that the genetic makeup of varieties influences the potassium content of plant sap alone. The mathematical relationship between nitrate and potassium in sweet peppers is beyond the scope of this study.
This study demonstrates the significance of the foliar nutrition technique. The relationship between nitrogen (nitrate ion form) and calcium cation taken up by plants through the foliage appears to be positive rather than inverse. It can be concluded that foliar sprays containing nitrates are preferable for improving calcium uptake in tomato and sweet pepper seedlings. This necessitates the presence of alkaline solutions, which are commonly used in foliar nutrition. Calcium abundance needs basic media [
38] and nitrates [
41]. Foliar nutrition, according to a group of researchers, cannot be used for the three macronutrients (nitrogen, phosphorus, and potassium) [
35]. The findings of this study are both motivating and encouraging because they concern the ionic form of nitrogen (nitrate rather than pure nitrogen element) and calcium as a secondary nutrient.
All raw materials are inexpensive, readily available, and completely impure (
Table 29). Distilled water was the primary solvent used in the preparation of all organic solutions. In certain proportions, adding the active ingredient to distilled water raises nitrates, calcium, or both. These contents decreased in other solutions due to deposition or binding. Calcium levels in baking soda and hydrogen peroxide were reduced to zero. In any case, the acidity of each solution varies.
All spray solutions prepared for foliar nutrition testing were basic (alkaline) unless distilled water (neutral). The salicylic acid (100 mg L
-1) solution and peroxide were also acidic (
Table 29). In this article trial, however, only distilled water, salicylic acid (50 mg L
-1), and sodium bicarbonate (0.52%) were sprayed. The remainder were prepared for future experiments on fungal pathogen control.
Salicylic acid (100 mg L-1) had the highest calcium cation (mg L-1) content, while baking soda and peroxide lacked. It was discovered that the potassium content in all tested solutions was zero. Further, sodium bicarbonate solution has the highest content of nitrate (mg L-1).
Figure 1.
Calcium cation content (mg L-1) combined for 3 sweet pepper genotypes and 3 foliar nutrition treatments.
Figure 1.
Calcium cation content (mg L-1) combined for 3 sweet pepper genotypes and 3 foliar nutrition treatments.
Figure 2.
Potassium cation content (mg L-1) combined for 3 tomato genotypes and 3 foliar nutrition treatments.
Figure 2.
Potassium cation content (mg L-1) combined for 3 tomato genotypes and 3 foliar nutrition treatments.
Figure 3.
Nitrate content (mg L-1) impact on calcium cation content (mg L-1) among 3 tomato genotypes and 3 foliar nutrition treatments.
Figure 3.
Nitrate content (mg L-1) impact on calcium cation content (mg L-1) among 3 tomato genotypes and 3 foliar nutrition treatments.
Figure 4.
Nitrate content (mg L-1) impact on calcium cation content (mg L-1) among 3 sweet pepper genotypes and 3 foliar nutrition treatments.
Figure 4.
Nitrate content (mg L-1) impact on calcium cation content (mg L-1) among 3 sweet pepper genotypes and 3 foliar nutrition treatments.
Figure 5.
Nitrate content (mg L-1) impact on potassium cation content (mg L-1) among 3 sweet pepper genotypes and 3 foliar nutrition treatments.
Figure 5.
Nitrate content (mg L-1) impact on potassium cation content (mg L-1) among 3 sweet pepper genotypes and 3 foliar nutrition treatments.
Table 1.
Impact of tomato genotypes on the emergence (%).
Table 1.
Impact of tomato genotypes on the emergence (%).
| Tomato genotypes |
Emergence (%) |
| Mobil |
98 A |
| Korall |
92 AB |
| Tyking F1 |
86 B |
Table 2.
Impact of sweet pepper genotypes on the emergence (%).
Table 2.
Impact of sweet pepper genotypes on the emergence (%).
| Sweet pepper genotypes |
Emergence (%) |
| Carma |
90 A |
| Fokusz |
90 A |
| Bobita F1 |
88 A |
Table 3.
Impact of tomato genotypes on the SPAD index before foliar nutrition.
Table 3.
Impact of tomato genotypes on the SPAD index before foliar nutrition.
| Tomato genotypes |
SPAD index |
| Mobil |
26.38 A |
| Korall |
20.14 A |
| Tyking F1 |
19.82 A |
Table 4.
Impact of sweet pepper genotypes on SPAD index before foliar nutrition.
Table 4.
Impact of sweet pepper genotypes on SPAD index before foliar nutrition.
| Sweet pepper genotypes |
SPAD index |
| Carma |
27.94 A |
| Fokusz |
26.50 A |
| Bobita F1 |
25.46 A |
Table 5.
Impact of tomato genotypes on the SPAD index after foliar nutrition (48 hours).
Table 5.
Impact of tomato genotypes on the SPAD index after foliar nutrition (48 hours).
| Tomato genotypes |
SPAD index (48 hours) |
| Mobil |
24.07 A |
| Korall |
24.91 A |
| Tyking F1 |
28.41 A |
Table 6.
Impact of sweet pepper genotypes on SPAD index after foliar nutrition (48 hours).
Table 6.
Impact of sweet pepper genotypes on SPAD index after foliar nutrition (48 hours).
| SPAD index (48 hours) |
Sweet pepper genotypes |
| Carma |
27.21 A |
| Fokusz |
28.66 A |
| Bobita F1 |
25.89 A |
Table 13.
Impact of tomato genotypes on calcium cation content (mg L-1).
Table 13.
Impact of tomato genotypes on calcium cation content (mg L-1).
| Tomato genotypes |
Calcium cation content (Ca+2)
(mg L-1) |
| Mobil |
1042.7 A |
| Korall |
787.33 C |
| Tyking F1 |
845.33 B |
Table 14.
Impact of foliar nutrition treatment on calcium cation content (mg L-1).
Table 14.
Impact of foliar nutrition treatment on calcium cation content (mg L-1).
| Foliar nutrition |
Calcium cation content (Ca+2)
(mg L-1) |
| Salicylic Acid 50 mg L-1
|
955 A |
| Sodium Bicarbonate 0.52% |
770 B |
| Distilled Water |
950 A |
Table 15.
Effect of sweet pepper genotype and foliar nutrition treatment on calcium cation content (mg L-1).
Table 15.
Effect of sweet pepper genotype and foliar nutrition treatment on calcium cation content (mg L-1).
| Genotype |
Salicylic Acid
50 mg L-1
|
Sodium Bicarbonate0.52%
|
Distilled Water |
| Carma |
1840 A |
1498 AB |
688 BC |
| Fokusz |
774 BC |
1604 AB |
862 ABC |
| Bobita F1 |
256 C |
270 C |
434 C |
Table 16.
Effect of tomato genotype and foliar nutrition treatment on potassium cation content (mg L-1).
Table 16.
Effect of tomato genotype and foliar nutrition treatment on potassium cation content (mg L-1).
| Genotype |
Salicylic Acid
50 mg L-1
|
Sodium Bicarbonate
0.52% |
Distilled Water |
| Mobil |
1100 D |
1220 CD |
1206 CD |
| Korall |
1440 AB |
1200 CD |
1400 ABC |
| TykingF1 |
1360 BC |
1200 CD |
1580 A |
Table 17.
Impact of sweet pepper genotypes on potassium cation content (mg L-1).
Table 17.
Impact of sweet pepper genotypes on potassium cation content (mg L-1).
| Sweet pepper genotypes |
Potassium cation content (K+)
(mg L-1) |
| Carma |
1892.7 A |
| Fokusz |
2252.7 A |
| Bobita F1 |
2546.7 A |
Table 18.
Impact of foliar nutrition treatment on potassium cation content (mg L-1).
Table 18.
Impact of foliar nutrition treatment on potassium cation content (mg L-1).
| Foliar nutrition |
Potassium cation content (K+)
(mg L-1) |
| Salicylic Acid 50 mg L-1
|
2413.3 A |
| Sodium Bicarbonate 0.52% |
2430.7 A |
| Distilled Water |
1848.0 A |
Table 19.
Impact of tomato genotypes on nitrate ion content (mg L-1).
Table 19.
Impact of tomato genotypes on nitrate ion content (mg L-1).
| Tomato genotypes |
Nitrate ion content (NO3-)
(mg L-1) |
| Mobil |
222.67 A |
| Korall |
183.33 B |
| Tyking F1 |
233.33 A |
Table 20.
Impact of sweet pepper genotypes on nitrate ion content (mg L-1).
Table 20.
Impact of sweet pepper genotypes on nitrate ion content (mg L-1).
| Sweet pepper genotypes |
Nitrate ion content (NO3-)
(mg L-1) |
| Carma |
575.33 B |
| Fokusz |
1061.33 A |
| Bobita F1 |
680.67 AB |
Table 21.
Impact of foliar nutrition treatment on nitrate ion content (mg L-1) among tomato genotypes.
Table 21.
Impact of foliar nutrition treatment on nitrate ion content (mg L-1) among tomato genotypes.
| Foliar nutrition |
Nitrate ion content (NO3-)
(mg L-1) |
| Salicylic Acid 50 mg L-1
|
214.67 A |
| Sodium Bicarbonate 0.52% |
213.33 A |
| Distilled Water |
211.33 A |
Table 22.
Impact of foliar nutrition treatment on nitrate ion content (mg L-1) among sweet pepper genotypes.
Table 22.
Impact of foliar nutrition treatment on nitrate ion content (mg L-1) among sweet pepper genotypes.
| Foliar nutrition |
Nitrate ion content (NO3-)
(mg L-1) |
| Salicylic Acid 50 mg L-1
|
755.33 A |
| Sodium Bicarbonate 0.52% |
849.33 A |
| Distilled Water |
712.67 A |
Table 23.
Multiple maximum response optimization for (Ca+2, K+, NO3-, SPAD-8 days) parameters in tomato.
Table 23.
Multiple maximum response optimization for (Ca+2, K+, NO3-, SPAD-8 days) parameters in tomato.
Table 24.
Multiple maximum response optimization for (Ca+2, K+, NO3-, SPAD-8 days) parameters in sweet pepper.
Table 24.
Multiple maximum response optimization for (Ca+2, K+, NO3-, SPAD-8 days) parameters in sweet pepper.
Table 25.
Multiple minimum response optimization for (Ca+2, K+, NO3-, SPAD-8 days) parameters in tomato.
Table 25.
Multiple minimum response optimization for (Ca+2, K+, NO3-, SPAD-8 days) parameters in tomato.
Table 26.
Multiple minimum response optimization for (Ca+2, K+, NO3-, SPAD-8 days) parameters in sweet pepper.
Table 26.
Multiple minimum response optimization for (Ca+2, K+, NO3-, SPAD-8 days) parameters in sweet pepper.
Table 27.
Correlation and regression analyses for pairs among tomato genotypes and foliar nutrition treatments.
Table 27.
Correlation and regression analyses for pairs among tomato genotypes and foliar nutrition treatments.
| Statistics |
Independent Variable |
Dependent variable |
| Factors |
SPAD -8 days |
Nitrate content |
| Regression |
0.0345 |
| Regression model |
Y =1.3908X + 172.93 |
| Correlation |
0.186 |
| Interpretation |
Very weak and positive |
| Significant F |
0.221540953 (Not significant) |
| |
| Factors |
Potassium content |
Calcium content |
| Regression |
0.0321 |
| Regression model |
Y =-0.1555X + 1094 |
| Correlation |
0.179 |
| Interpretation |
Very weak and negative |
| Significant F |
0.239261839 (Not significant) |
| |
| Factors |
Nitrate content |
Calcium content |
| Regression |
0.107 |
| Regression model |
Y = 1.2043X + 635.13 |
| Correlation |
0.327 |
| Interpretation |
Weak and positive |
| Significant F |
0.028174712 *
|
| |
| Factors |
Nitrate content |
Potassium content |
| Regression |
0.0161 |
| Regression model |
Y = -0.5383X +1415.4 |
| Correlation |
0.127 |
| Interpretation |
Very weak and negative |
| Significant F |
0.405557908 (Not significant) |
Table 28.
Correlation and regression analyses for pairs among sweet pepper genotypes and foliar nutrition treatments.
Table 28.
Correlation and regression analyses for pairs among sweet pepper genotypes and foliar nutrition treatments.
| Statistics |
Independent
variable |
Dependent variable |
| Factors |
SPAD -8 days |
Nitrate content |
| Regression |
0.0066 |
| Regression model |
Y =4.33X + 637.92 |
| Correlation |
0.081 |
| Interpretation |
Very weak and positive |
| Significant F |
0.596893587 (Not Significant) |
| |
| Factors |
Potassium content |
Calcium content |
| Regression |
0.055 |
| Regression model |
Y = 0.1578X + 562.02 |
| Correlation |
0.235 |
| Interpretation |
Weak and positive |
| Significant F |
0.120812311 (Not Significant) |
| |
| Factors |
Nitrate content |
Calcium content |
| Regression |
0.1585 |
| Regression model |
Y = 0.5914X + 457.18 |
| Correlation |
0.398 |
| Interpretation |
Weak and positive |
| Significant F |
0.006758211 *
|
| |
| Factors |
Nitrate content |
Potassium content |
| Regression |
0.3088 |
| Regression model |
Y = 1.2273X +1282.6 |
| Correlation |
0.556 |
| Interpretation |
Medium and positive |
| Significant F |
0.0000742078233112152 *
|
Table 29.
The overall mean for the acidity, calcium content (mg L-1), and nitrate content (mg L-1) of organic spray solutions.
Table 29.
The overall mean for the acidity, calcium content (mg L-1), and nitrate content (mg L-1) of organic spray solutions.
| Raw material |
Major active Ingredient |
Acidity pH of
final solution ± SEM |
Calcium cation content
(Ca+2)
(mg L-1) |
Nitrate ion content
(NO3-)
(mg L-1) |
| Distilled Water |
(H2O) |
7.0225 ± 0.263134 |
10.25 |
8.5 |
Salicylic Acid
(50 mg L-1) |
(C7H6O3) |
7.4125 ± 0.087595 |
16.5 |
6.5 |
Salicylic Acid
(100 mg L-1) |
(C7H6O3) |
6.27 ± 0.04916 |
51 |
7.5 |
Baking Soda
(0.52% w/v) |
(NaHCO3) |
7.63 ± 0.073258 |
0 |
53.5 |
Epsom Salt
(0.52 % w/v) |
(MgSO4.7H2O) |
8.0025 ± 0.078991 |
6.75 |
17.75 |
Hydrogen Peroxide (0.03)
(50% v/v) |
(H2O2) |
4.32 ± 0.007071 |
0 |
11.25 |