Submitted:
25 May 2023
Posted:
26 May 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Nanoparticle and Membrane Characterization
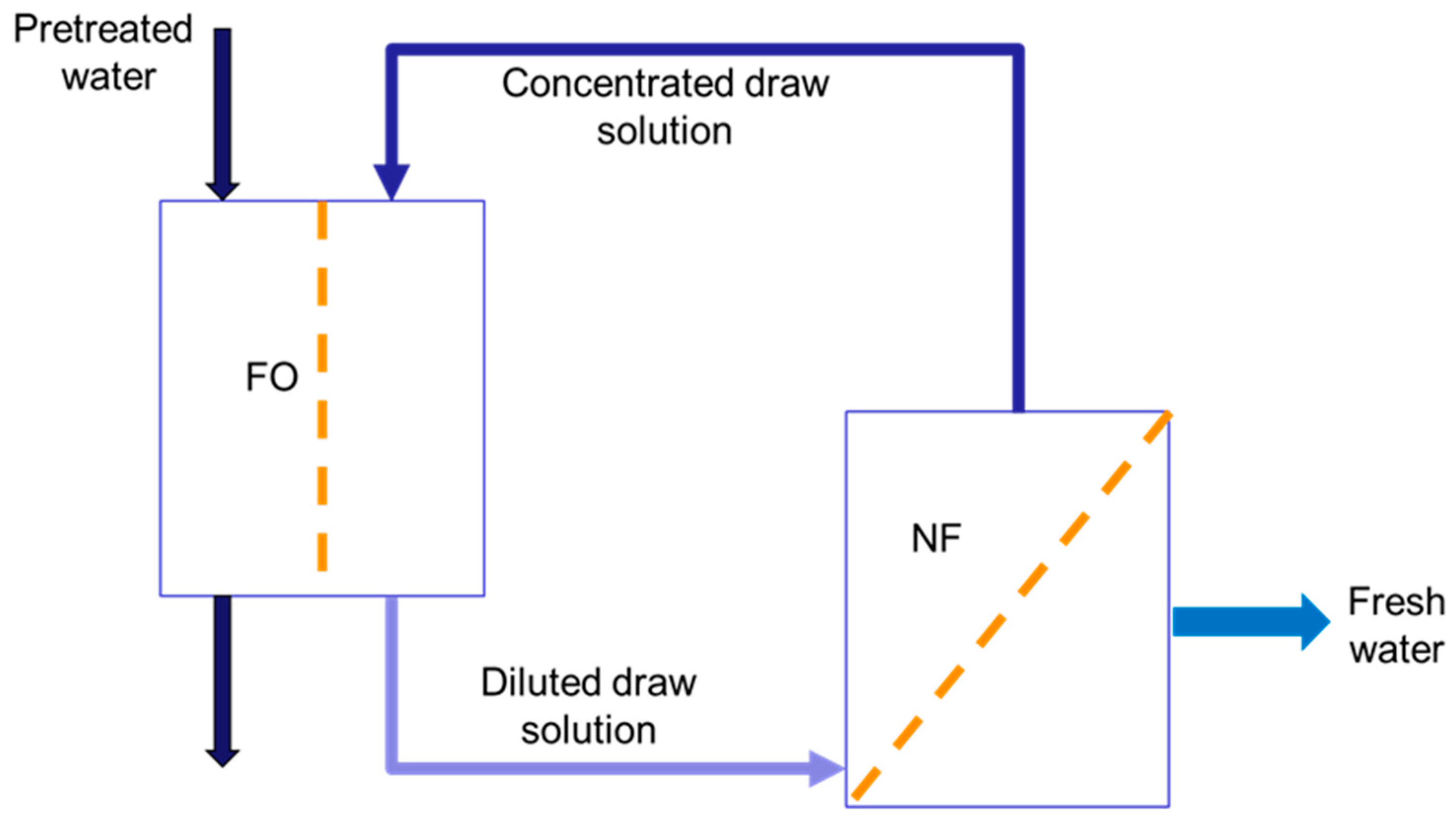
2.3. Forward Osmosis Tests and Data Analysis
2.4. Rejection of heavy metals in NF process
3. Results and Discussion
3.1. Nanoparticle and Membrane Characterization
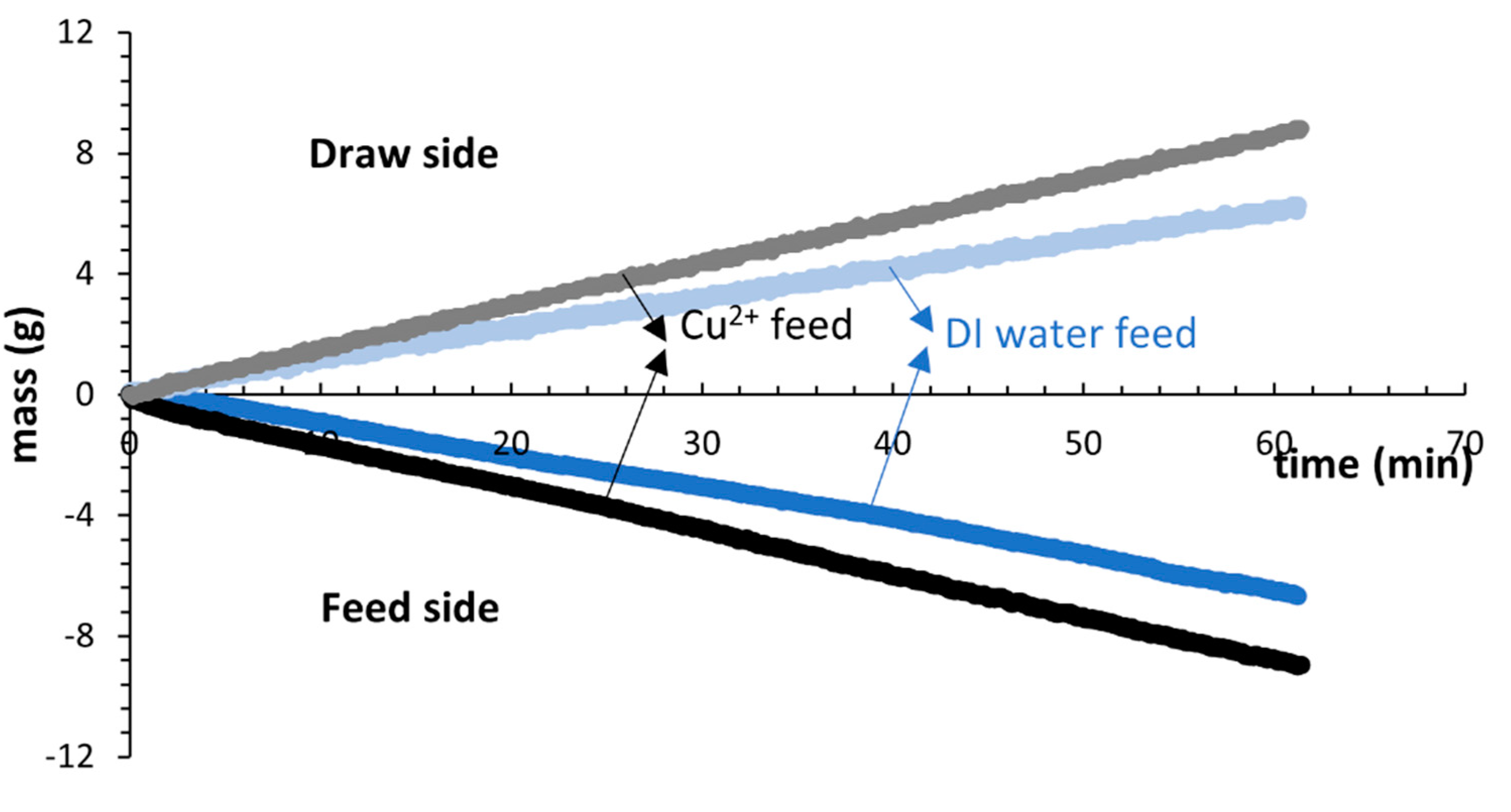
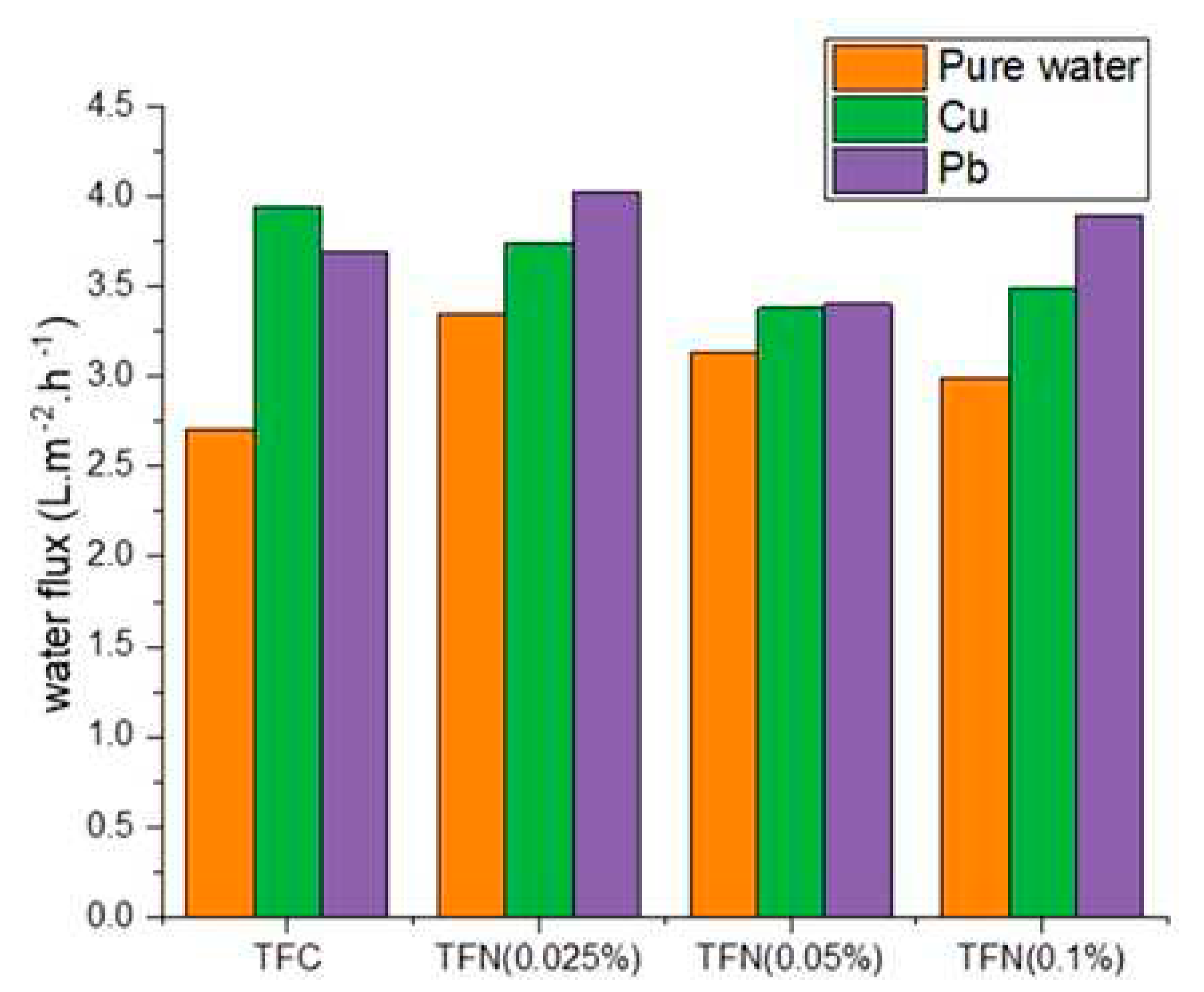
3.2. FO Performance
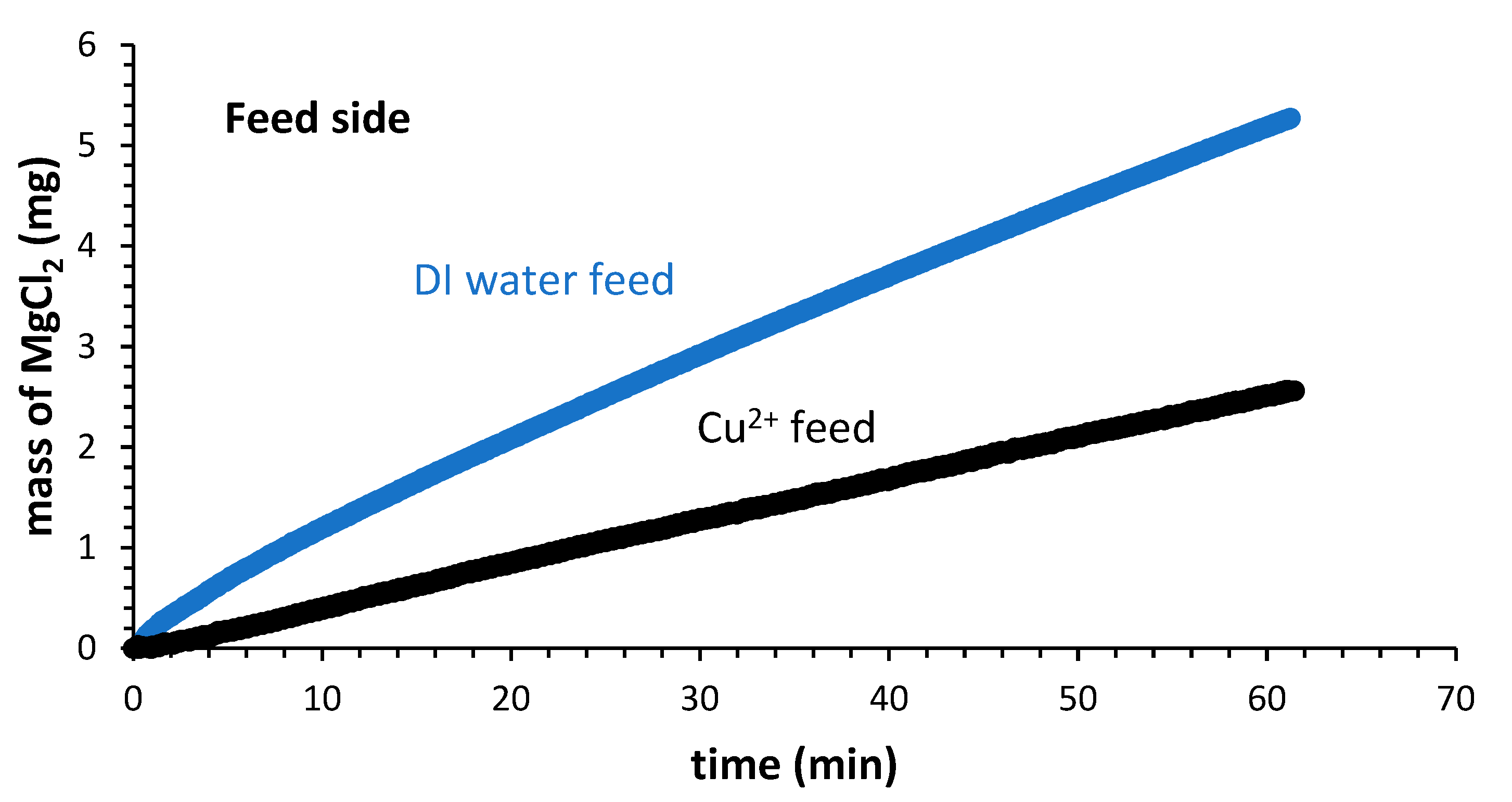
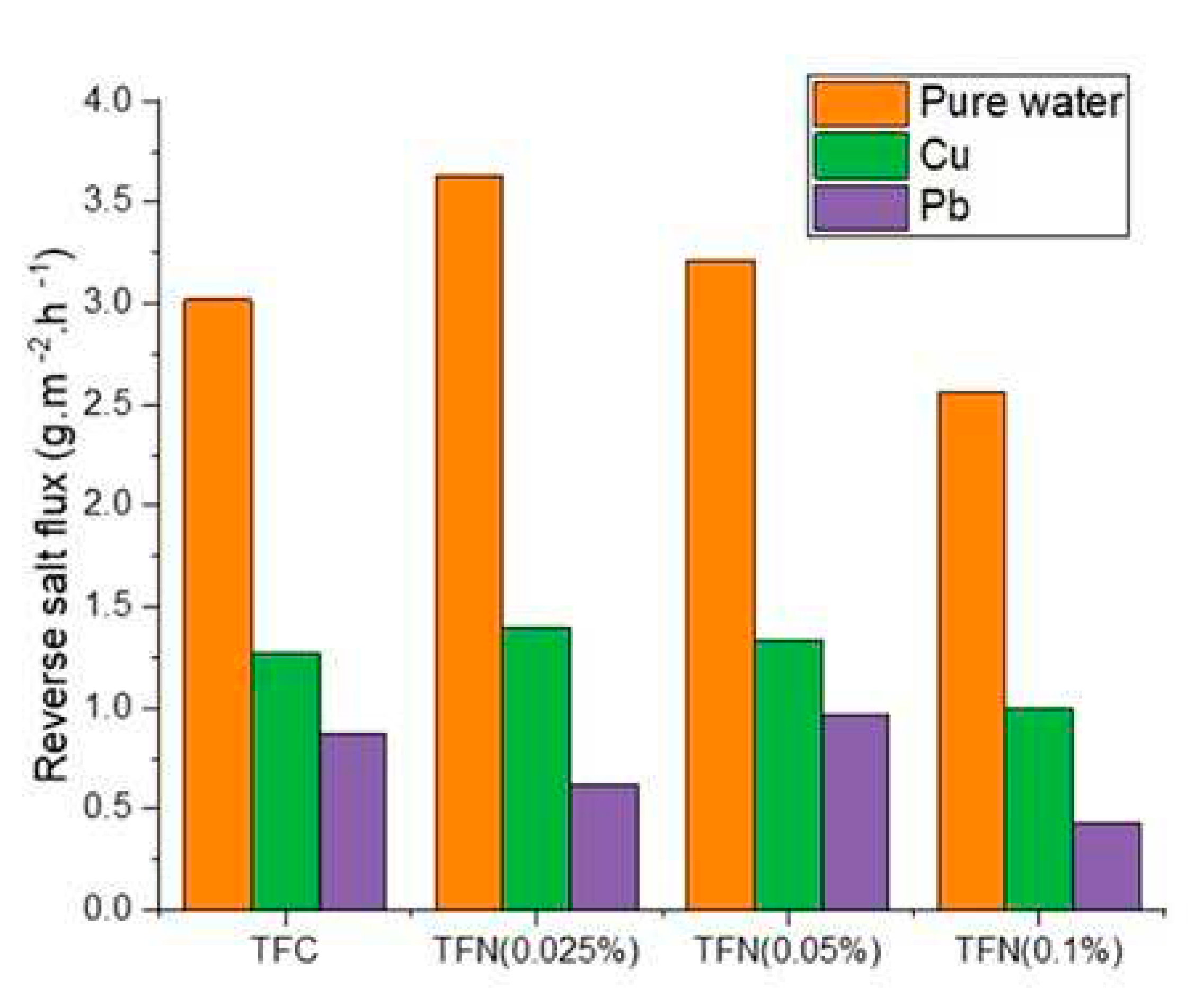
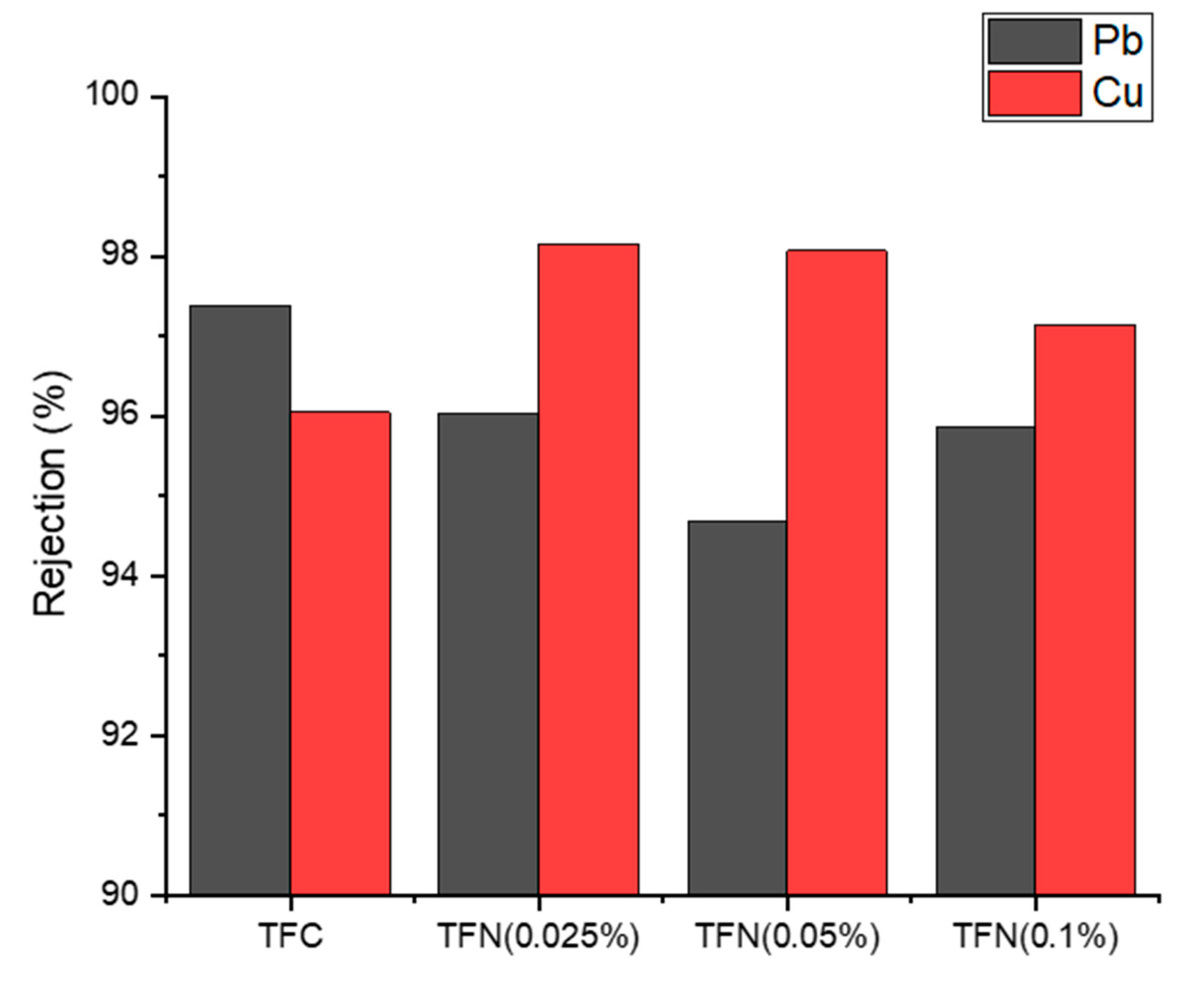
3.3. Rejection of Heavy Metals in FO and NF Processes
4. Conclusions
Acknowledgments
References
- Cheng, X.; Zhang, Y.; Shao, S.; Lai, C.; Wu, D.; Xu, J.; Luo, X.; Xu, D.; Liang, H.; Zhu, X. Highly Permeable Positively Charged Nanofiltration Membranes with Multilayer Structures for Multiple Heavy Metal Removals. Desalination 2023, 548, 116266. [Google Scholar] [CrossRef]
- Fu, F.; Wang, Q. Removal of Heavy Metal Ions from Wastewaters: A Review. J. Environ. Manage. 2011, 92, 407–418. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, N.; Yusof, N.; Lau, W.J.; Jaafar, J.; Ismail, A.F. Recent Trends of Heavy Metal Removal from Water/Wastewater by Membrane Technologies. J. Ind. Eng. Chem. 2019, 76, 17–38. [Google Scholar] [CrossRef]
- Samavati, Z.; Samavati, A.; Goh, P.S.; Fauzi Ismail, A.; Sohaimi Abdullah, M. A Comprehensive Review of Recent Advances in Nanofiltration Membranes for Heavy Metal Removal from Wastewater. Chem. Eng. Res. Des. 2023, 189, 530–571. [Google Scholar] [CrossRef]
- Suhalim, N.S.; Kasim, N.; Mahmoudi, E.; Shamsudin, I.J.; Mohammad, A.W.; Zuki, F.M.; Jamari, N.L.A. Rejection Mechanism of Ionic Solute Removal by Nanofiltration Membranes: An Overview. Nanomaterials 2022, 12. [Google Scholar] [CrossRef]
- Peydayesh, M.; Mohammadi, T.; Nikouzad, S.K. A Positively Charged Composite Loose Nanofiltration Membrane for Water Purification from Heavy Metals. J. Memb. Sci. 2020, 611, 118205. [Google Scholar] [CrossRef]
- Abdullah, W.N.A.S.; Tiandee, S.; Lau, W.; Aziz, F.; Ismail, A.F. Potential Use of Nanofiltration Like-Forward Osmosis Membranes for Copper Ion Removal. Chinese J. Chem. Eng. 2020, 28, 420–428. [Google Scholar] [CrossRef]
- Wang, Y.N.; Goh, K.; Li, X.; Setiawan, L.; Wang, R. Membranes and Processes for Forward Osmosis-Based Desalination: Recent Advances and Future Prospects. Desalination 2018, 434, 81–99. [Google Scholar] [CrossRef]
- Suwaileh, W.A.; Johnson, D.J.; Sarp, S.; Hilal, N. Advances in Forward Osmosis Membranes: Altering the Sub-Layer Structure via Recent Fabrication and Chemical Modification Approaches. Desalination 2018, 436, 176–201. [Google Scholar] [CrossRef]
- Zhu, L.; Ding, C.; Zhu, T.; Wang, Y. A Review on the Forward Osmosis Applications and Fouling Control Strategies for Wastewater Treatment. Front. Chem. Sci. Eng. 2022, 16, 661–680. [Google Scholar] [CrossRef]
- Singh, S.K.; Sharma, C.; Maiti, A. A Comprehensive Review of Standalone and Hybrid Forward Osmosis for Water Treatment: Membranes and Recovery Strategies of Draw Solutions. J. Environ. Chem. Eng. 2021, 9, 105473. [Google Scholar] [CrossRef]
- Ghanbari, M.; Emadzadeh, D.; Lau, W.J.; Riazi, H.; Almasi, D.; Ismail, A.F. Minimizing Structural Parameter of Thin Film Composite Forward Osmosis Membranes Using Polysulfone/Halloysite Nanotubes as Membrane Substrates. Desalination 2016, 377, 152–162. [Google Scholar] [CrossRef]
- Emadzadeh, D.; Ghanbari, M.; Lau, W.J.; Rahbari-Sisakht, M.; Matsuura, T.; Ismail, A.F.; Kruczek, B. Solvothermal Synthesis of Nanoporous TiO<inf>2</Inf>: The Impact on Thin-Film Composite Membranes for Engineered Osmosis Application. Nanotechnology 2016, 27. [Google Scholar] [CrossRef]
- Abdullah, W.N.A.S.; Lau, W.J.; Aziz, F.; Emadzadeh, D.; Ismail, A.F. Performance of Nanofiltration-Like Forward-Osmosis Membranes for Aerobically Treated Palm Oil Mill Effluent. Chem. Eng. Technol. 2018, 41, 303–312. [Google Scholar] [CrossRef]
- Setiawan, L.; Wang, R.; Li, K.; Fane, A.G. Fabrication of Novel Poly(Amide-Imide) Forward Osmosis Hollow Fiber Membranes with a Positively Charged Nanofiltration-like Selective Layer. J. Memb. Sci. 2011, 369, 196–205. [Google Scholar] [CrossRef]
- Su, J.; Yang, Q.; Teo, J.F.; Chung, T.S. Cellulose Acetate Nanofiltration Hollow Fiber Membranes for Forward Osmosis Processes. J. Memb. Sci. 2010, 355, 36–44. [Google Scholar] [CrossRef]
- Atashgar, A.; Emadzadeh, D.; Akbari, S.; Kruczek, B. Incorporation of Functionalized Halloysite Nanotubes (HNTs) into Thin-Film Nanocomposite (TFN) Nanofiltration Membranes for Water Softening. Membranes (Basel). 2023, 13. [Google Scholar] [CrossRef]
- Shahamati Fard, F.; Akbari, S.; Pajootan, E.; Arami, M. Enhanced Acidic Dye Adsorption onto the Dendrimer-Based Modified Halloysite Nanotubes. Desalin. Water Treat. 2016, 57, 26222–26239. [Google Scholar] [CrossRef]
- Bai, D.; Asempour, F.; Kruczek, B. Can the Time-Lag Method Be Used for the Characterization of Liquid Permeation Membranes? Chem. Eng. Res. Des. 2020, 162, 228–237. [Google Scholar] [CrossRef]
- Bai, D.; Kruczek, B. Effect of Membrane Orientation and Concentration of Draw Solution on the Behavior of Commercial Osmotic Membrane in a Novel Dynamic Forward Osmosis Tests. Membranes (Basel). 2022, 12. [Google Scholar] [CrossRef]
- Asempour, F.; Emadzadeh, D.; Matsuura, T.; Kruczek, B. Synthesis and Characterization of Novel Cellulose Nanocrystals-Based Thin Film Nanocomposite Membranes for Reverse Osmosis Applications. Desalination 2018, 439. [Google Scholar] [CrossRef]
- Tul Muntha, S.; Kausar, A.; Siddiq, M. Advances in Polymeric Nanofiltration Membrane: A Review. Polym. - Plast. Technol. Eng. 2017, 56, 841–856. [Google Scholar] [CrossRef]
- Madaeni, S.S.; Salehi, E. Adsorption of Cations on Nanofiltration Membrane: Separation Mechanism, Isotherm Confirmation and Thermodynamic Analysis. Chem. Eng. J. 2009, 150, 114–121. [Google Scholar] [CrossRef]
- Hurwitz, G.; Guillen, G.R.; Hoek, E.M.V. Probing Polyamide Membrane Surface Charge, Zeta Potential, Wettability, and Hydrophilicity with Contact Angle Measurements. J. Memb. Sci. 2010, 349, 349–357. [Google Scholar] [CrossRef]
- Akharame, M.O.; Fatoki, O.S.; Opeolu, B.O. Regeneration and Reuse of Polymeric Nanocomposites in Wastewater Remediation: The Future of Economic Water Management; Springer Berlin Heidelberg, 2019; Vol. 76; ISBN 0123456789.
- Qiu, M.; He, C. Efficient Removal of Heavy Metal Ions by Forward Osmosis Membrane with a Polydopamine Modified Zeolitic Imidazolate Framework Incorporated Selective Layer. J. Hazard. Mater. 2019, 367, 339–347. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liu, Y.; Fan, K.; Cheng, P.; Xia, H.; Xia, S. Utilization of Carboxyl Group-Grafted Molybdenum Disulfide for Enhancing the Performance of Thin-Film Nanocomposite Nanofiltration Membranes. Desalination 2023, 548, 116283. [Google Scholar] [CrossRef]
- Huo, H.Q.; Mi, Y.F.; Yang, X.; Lu, H.H.; Ji, Y.L.; Zhou, Y.; Gao, C.J. Polyamide Thin Film Nanocomposite Membranes with In-Situ Integration of Multiple Functional Nanoparticles for High Performance Reverse Osmosis. J. Memb. Sci. 2023, 669, 121311. [Google Scholar] [CrossRef]
- Thabo, B.; Okoli, B.J.; Modise, S.J.; Nelana, S. Rejection Capacity of Nanofiltration Membranes for Nickel, Copper, Silver and Palladium at Various Oxidation States. Membranes (Basel). 2021, 11, 1–14. [Google Scholar] [CrossRef]
- Abedi, F.; Dubé, M.A.; Emadzadeh, D.; Kruczek, B. Improving Nanofiltration Performance Using Modified Cellulose Nanocrystal-Based TFN Membranes. J. Memb. Sci. 2023, 670. [Google Scholar] [CrossRef]
- Ang, W.L.; Wahab Mohammad, A.; Johnson, D.; Hilal, N. Forward Osmosis Research Trends in Desalination and Wastewater Treatment: A Review of Research Trends over the Past Decade. J. Water Process Eng. 2019, 31, 100886. [Google Scholar] [CrossRef]
| Membrane | Initial zeta potential (mV) | Final zeta potential (mV) | Adsorbed Cu (μg) | ||
|---|---|---|---|---|---|
| DI water feed | Cu2+ in feed | DI water feed | Cu2+ in feed | ||
| TFC | -17.8 | -10.2 | -8.72 | 1.27 | 8.83 |
| TFN(025%) | -20.1 | -15.5 | -14.5 | 8.39 | 10.41 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





