1. Introduction
The human amniotic membrane (AM) has long been used to treat wounds in ophthalmology, dermatology and surgery [
1]. The therapeutic effect of AM allografts originates in the high content of growth factors, cytokines, extracellular matrix components, and the presence of pluripotent stem cells [
2,
3]. AM is typically obtained from the placenta after caesarean section, prepared by manual separation from the underlying chorionic membrane, cleaned, decontaminated, and stored. For transplantation purposes serological tests for potentially transmissible diseases (human immunodeficiency virus, hepatitis B and C viruses, and syphilis) must be negative both at the time of tissue collection and when repeated after 180 days [
4,
5].
Based on the number of papers published on the use of placental membranes for grafting in PubMed database, the most common storage method for AM is cryopreservation [
6,
7,
8]. Reports over the last few years suggest that cryopreservation has been slowly supplanted by freeze-dry methods (lyophilized, vacuum freeze dehydrated), which are particularly common in wound healing treatment [
9,
10,
11]. However, for ophthalmosurgery both storage methods for AM are used equally [
10,
12,
13].
The method of AM processing may affect its therapeutic properties, i.e. induction of granularization and re-epithelialization [
14], reduction of fibrosis [
15], pain [
16,
17], inflammation [
18], antimicrobial [
19] and antiviral [
20] properties and also pro-angiogenic [
21,
22] and anti-angiogenic [
23,
24] features.
Cryopreservation preserves the biological activity and structure of tissue. Before cryopreservation storage, AM is chemically decontaminated and usually placed in a mixture of culture medium and glycerol [
25,
26].
Freeze-dried AM is prepared in a lyophilizer, where the tissue is gently dried using a high vacuum to a final water content of 5–10% [
27]. The crucial advantage of freeze-drying compared to cryopreservation is that AM allografts can be stored and transported at room temperature. Disadvantages include tissue destruction, a decrease of protein levels and activity if gamma-sterilization is used for terminal sterilization [
28,
29,
30,
31]. Recently, radiation has been substituted for a less destructive chemical decontamination technique under clean room conditions. Thus, the AM structure is preserved with no serious structure and composition deterioration [
11,
32].
During clinical studies, apart from accelerating healing, the most outstanding effect of AM application is rapid wound pain alleviation [
17,
33,
34]. The exact mechanism of analgesic action of AM was not known for a long time and no directly acting component was characterized [
35]. Pain relief was thought to be due to the good adhesion of the AM graft to the wound surface, which covers free nerve endings, maintains wound moisture, and releases anti-inflammatory substances that can indirectly relieve pain [
35,
36,
37,
38,
39].
Recently we have detected and analyzed the levels of
N-acylethanolamides (NAEs), particularly palmitoylethanolamide (PEA), oleoylethanolamide (OEA), and anandamide (AEA) in various placental tissues, mainly in amniotic and chorionic membrane [
40]. We suggested that these NAEs are responsible for pain relief and also have an anti-inflammatory effect. These NAEs were represented in AM in the concentration order of PEA ˃ OEA ˃ AEA, and the concentration of all NAEs increased significantly after 24 hours decontamination with antibiotic solution [
40].
NAEs are widely spread endogenous bioactive lipid mediators derived from complex membrane lipids in response to environmental stimuli and play an important role in numerous physiological processes such as immune function, metabolic regulation, pain and inflammation [
41]. It has been proposed that PEA, the most abundant NAE in vertebrates [
41], accumulates in tissues after injury, and exerts anti-inflammatory, neuroprotective, analgesic and anti-nociceptive effects mainly through the PPAR-α receptor [
42,
43]. All these PEA properties have been demonstrated in human clinical trials [
44,
45,
46], including trials on treatment of chronic pain [
45,
47].
Beside PEA, AEA has also been implicated in possessing anti-nociceptive and anti-inflammatory effects [
41,
48,
49,
50]. Similarly, a role for AEA in wound healing also been suggested [
41,
49]. OEA has mostly anorexigenic properties [
51] and its ability to reduce nociceptive responses and inflammation has been shown [
44].
The aim of this study was to monitor dependence of changes in the concentration of PEA, AEA and OEA in AM on the method of tissue storage (cryopreservation and lyophilization) and the duration of storage, in order to find out whether and for how long individual NAEs persist in the tissue prepared for transplantation purposes.
2. Materials and Methods
2.1. Placenta Retrieval, Decontamination and Amniotic Membrane Preparation
The study followed the Ethics Committee's standards of participating institutions, 1st Medical Faculty of Charles University and General Teaching Hospital (GTH), and University Hospital Motol (UHM), all in Prague, and adhered to the tenets set out in the Declaration of Helsinki. Human placentas obtained at elective Caesarean section from normal pregnancies were obtained with informed consent after delivery at UHM. Only healthy donors, screened for hepatitis B and C, syphilis, HIV and C-reactive protein (˂ 10 mg/l) were selected.
2.2. Sample Preparation
2.2.1. Fresh and Decontaminated Samples
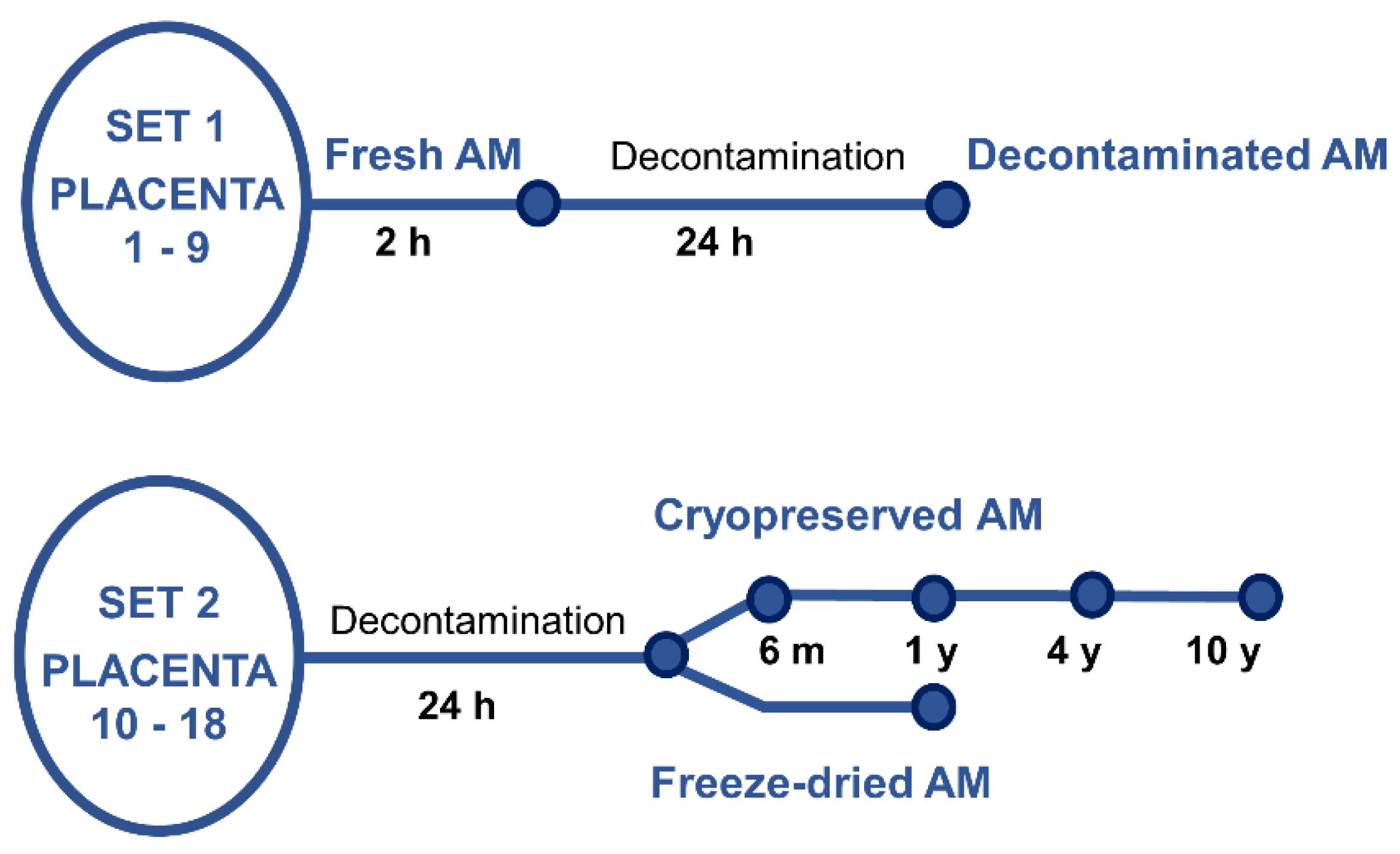
Nine placentas (P1 – P9, set 1),
Figure 1, were used for preparation of fresh and decontaminated samples. Within two hours after the delivery, AM samples were processed and stored using procedures and protocols valid for the preparation of AM for transplantation purposes [
52]. Shortly, AM was prepared by manual dissection in the biohazard cabinet. The tissue was washed using sterile saline (0.9% w/v, Fresenius Kabi, Germany), separated from blood clots. Then part of the AM was stretched on a mesh support (Sanatyl; Tylex, Letovice, Czech Republic), and cut to 2 × 2 cm. These fresh specimens (with no decontamination) were immediately used for NAEs analysis. The other part of AM was placed in decontamination solution BASE 128 (Alchimia srl, Italy) for 24 hours at room temperature. Then the 2x2 cm samples were stretched on a mesh support and immediately used for NAE analysis.
2.2.2. Cryopreserved and Lyophilized Samples
The cryopreserved and freeze-dried samples were obtained from nine other placentas (P10 – P18, set 2),
Figure 1. Immediately after the delivery, each placenta was placed in decontamination solution BASE 128 for 24 hours at room temperature, then the samples were processed as described above, again based on the preparation of AM for transplantation purposes [
52]. The samples were cryopreserved, or freeze-dried (lyophilized).
All specimens for cryopreservation were immersed in 40 ml of 1:1 mixture of Dulbecco's Modified Eagle's Medium (c. n. 32430-027, Gibco Life-Technologies, Invitrogen) /glycerol (Glycerolum 85%, Dr. Kulich Pharma s.r.o., Czech Republic), and stored in sterile containers (S245-J, Medfor Products Ltd, Aldershot, UK) at −80 °C until the date of NAE analyses.
Lyophilized samples were prepared according to a previously published method [
53]. Briefly, AM samples on a mesh carrier (washed with physiological saline) were placed in a sterile Petri dish and freeze-dried (lyophilizer VirTis AdVantage Pro™ Laboratory Benchtop Freeze Dryer, Biotrade Instruments, s. r. o., Prague, Czech Republic). The samples were placed on the pre-frozen lyophilizer shelves (set point: −40 °C) and freezing (thermal treatment) was performed for 30 min after cooling to −20 °C. Then, the sample was dried in six steps: 1) shelf: −20 °C, hold: 360 min.; 2) shelf: 0 °C, hold: 360 min.; 3) shelf: 20 °C, hold: 360 min.; 4–5) shelf: 30 °C, hold: 999 min.; 6) shelf: 30 °C, hold: 762 min. All the six steps had the same ramp (10 minutes) and vacuum (200 mTorr) values. After lyophilization, the samples were placed in secondary packaging and stored at room temperature.
AM cryopreserved specimens were stored for 6 ± 1 month (mean 6.2), 12 ± 2 months (mean 11.9 months), 48 ± 6 months (mean 52 months) and 10 years (± 1 year, mean 10.4), freeze-dried samples were stored for six months (± 1 months, mean 6.1), and for one year (± 2 months, mean 1.1). The freeze-dried samples were stored for one year (± 2 months, mean 1.1).
2.3. Sample Preparation and UHPLC/MS of NAEs
All specimens (size 2 × 2 cm) were used in triplicates. The experiments were performed in duplicates. Fresh and decontaminated AM samples were washed in saline three times for five minutes and removed from mesh support. The same procedure was applied to freeze dried samples, and cryopreserved samples after thawing.
Samples were processed according to a previously published protocol [
40]. Briefly, samples were mechanically homogenized in cold acetonitrile (HiPerSolv CHROMANORM, LC-MS grade, Leuven, Belgium) (cut with scissors, 2 minutes). Then, internal standard solution PEA-d
4 (with ≥ 99% deuterium incorporation, Cayman Chemicals, Ann Arbor, MI, US)) was added (10 µl, 1 µg/ml) to all homogenates. Samples were shaken (4 °C and 800 rpm) and then centrifuged (20 min., 15000 g). Supernatants (extracts) were evaporated to dryness in a vacuum centrifuge set at 0 °C and re-dissolved in 1 ml of 30% (v/v) methanol (HiPerSolv CHROMANORM, LC-MS grade, Leuven, Belgium) and purified by solid phase extraction following a previously published protocol [
40]. Pellets were dried in an evacuated centrifuge (Refrigerated CentriVAp Concentrator, Labconco Corporation, Kansas City, MO, US) and weighed. Samples were stored at -80 °C until UHPLC/MS.
The UHPLC/MS system consisted of an ExionLC UHPLC AD chromatography system and a QTRAP 6500+ mass spectrometer (both Sciex, Foster City, CA, USA) with an electrospray. Recently published UHPLC/MS method was used [
40]. Internal standards PEA-d4 (10 µl at 1 µg/ml) were used to construct minimally seven-point combined NAEs calibration curves (OEA (≥ 98%) and AEA (MaxSpec standard quality) Cayman Chemicals, Ann Arbor, MI, US; PEA (≥ 98%), Merck, Darmstadt, Germany), constructed for the relative signal intensity of the analyte (related to area of the internal standard). Peak integration, calibration curve construction and determination of analyte concentration were performed using Analyst 1.6.3 (Sciex, Darmstadt, Germany). For each AM sample, the concentration of specific NAEs (PEA, OEA and AEA) was related to the weight of extracted material.
2.4. Immunohistochemistry for PPAR α Receptor
Fluorescent indirect immunohistochemistry [
54,
55] was used to detect PPARα receptor in fresh, decontaminated, cryopreserved and freeze-dried AM samples from four placentas. Cryospecimens of human arm skin were used as a positive control [
56]. Specimens were washed three times for five minutes in sterile saline and fixed on a mesh support.
A circle with a diameter of ~1 cm was cut from the 2 × 2 cm samples and placed on a plastic holder in which incubation took place. Samples were fixed using 4% paraformaldehyde, and then cell membranes were permeabilized using 0.33% Triton X-100 (Sigma-Aldrich) diluted in PBS. Primary antibody (mouse monoclonal antibody PPARα, H-2 clone, Santa Cruz Biotechnology sc-398394, diluted 1:500 in 0.1% bovine serum albumin) was applied overnight at room temperature, then samples were washed and secondary antibody Alexa Fluor 488 goat anti-mouse IgG (A11029, Invitrogen, Frederick, Maryland, USA) was applied overnight at 4 °C and washed three times in PBS. The mesh holder was removed and specimens placed on a slide and mounted with VectaShield-PI (Vector Laboratories, Burlingame, CA, USA). The immunostaining was assessed using a fluorescence microscope (Nikon ECLIPSE Ni-U, Nikon) at ×200 and ×400 magnifications. The images were obtained with VDS CD-1300QF (VDS Vosskühler GmbH, Osnabrück, Germany) camera, and for positivity evaluation image-management software (NIS Elements; Laboratory Imaging, Prague, Czech Republic) was used. The percentage of positive cells was calculated in at least 2 000 cells.
2.5. Statistical Analysis
Each sample was analyzed in triplicate. The resulting average + SD was calculated from 6–9 mean values (depending on the sample) from each triplicate. Mann-Whitney test was used to compare specific NAEs concentrations between individual AM samples: fresh (control), decontaminated (control for all stored samples), and cryopreserved or freeze-dried samples, see Supplementary data,
Table S1. P-values less than 0.05 were considered statistically significant. Statistical analysis was performed using GraphPad Prism 8.0 software (La Jolla, CA, USA).
3. Results
3.1. NAE Concentrations in Fresh, Cryopreserved and Freeze-Dried Amniotic Membrane
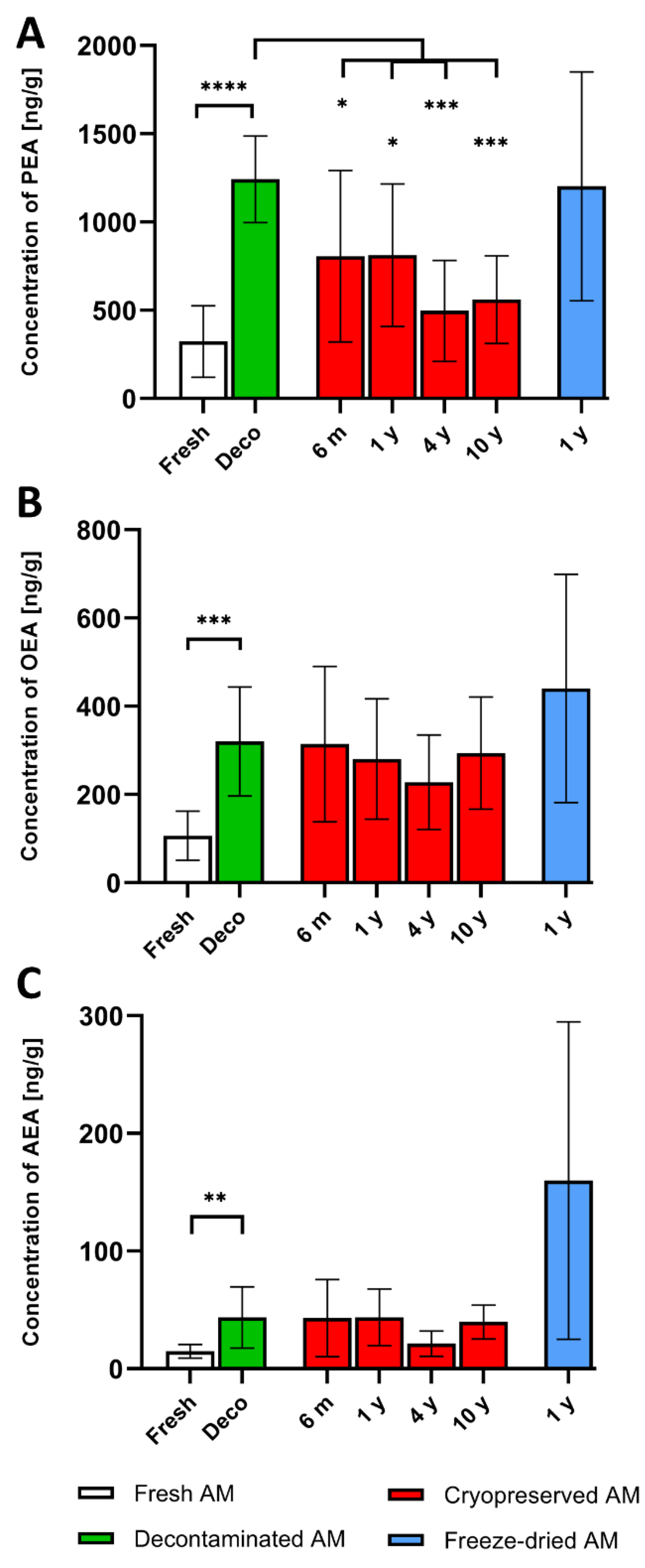
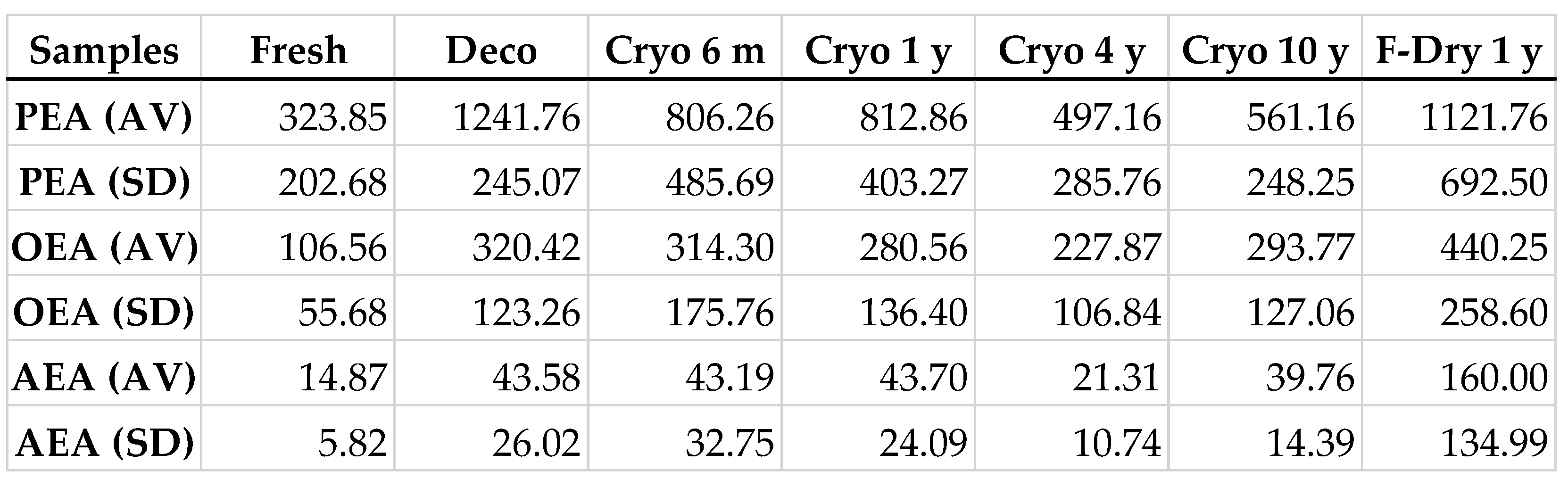
NAEs were detected in all analyzed samples. The highest levels were measured for PEA. Values of individual NAEs in cryopreserved and freeze-dried samples stored for different time periods were compared with values measured after decontamination as all stored AM were decontaminated during tissue processing. A significant increase in all NAEs values compared to fresh samples was detected after 24 h decontamination. These were used as baseline values for comparison with cryopreserved and freeze-dried samples. The average concentrations of PEA, OEA and AEA are shown in
Table 1 and
Figure 2. The mean concentration of PEA in all cryopreserved specimens (six months, one, four and 10 years) was significantly lower compared to decontaminated AM,
Figure 2A. A different situation was observed for OEA, and AEA, where no significant difference was found after cryopreservation,
Figure 2B,C. However, significantly higher values for all NAEs were detected in cryopreserved samples compared to fresh tissue before decontamination, see Supplementary data,
Table S2. The concentration of particular NAEs in freeze-dried AM analyzed after one year were almost identical (PEA, AEA), or even higher (OEA) compared to baseline averages (decontaminated AM), but no significant difference was found,
Figure 2.
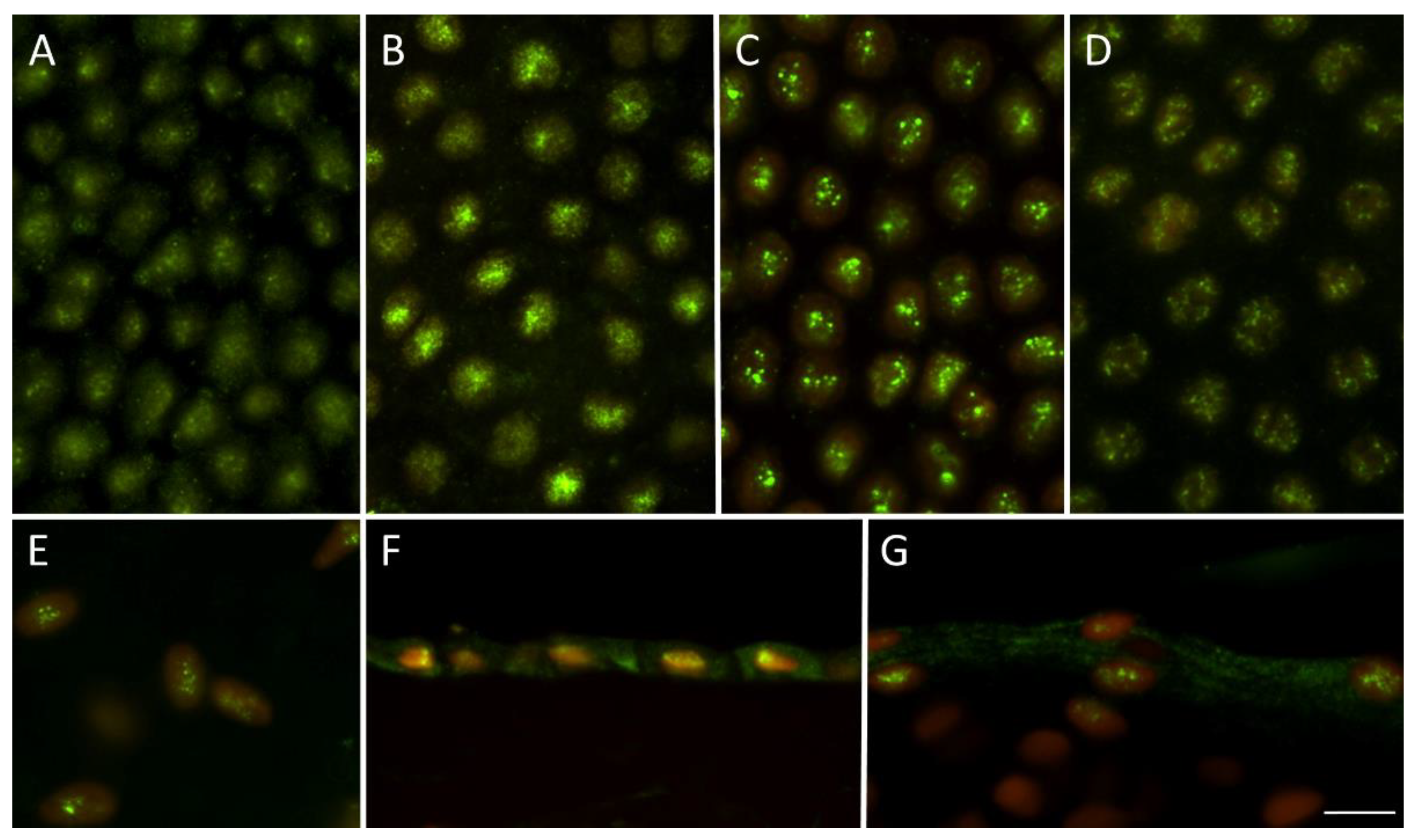
3.2. The Detection of PPAR-α Receptor Using Indirect Immunohistochemistry
The PPAR-α receptor was found in all tested tissue. The strong immunostaining was homogenously present through 98–100% of nuclei in the epithelial layer of AM. The positivity was also observed in mesenchymal cells of the fibroblast layer. The nuclear positivity was more diffused in fresh and decontaminated tissue, while more prominent dot signal was present in cryopreserved and freeze-dried samples, see
Figure 3.
4. Discussion
The main goal of this study was to determine whether the analgesic and anti-inflammatory endogenous lipid mediators NAEs, present in AM allografts persist under standard tissue bank storage conditions. We have shown that there is significant decrease of PEA in cryopreserved AM stored from six months to ten years. No further significant decreases in either PEA, AEA or OEA between baseline levels and cryopreserved or freeze-dried AM allografts stored from six months up to the longest tested period were detected.
The presence of NAEs in placental membranes was first described by Marczylo in 2009, who detected anandamid (AEA) in amniochorionic membrane and placenta [
57]. Also, pain relief has been repeatedly described both after the application of AM on the damaged or diseased ocular surface [
58,
59], and also on the surface of various types of wounds [
33,
60]. However, only recently has the presence of NAEs been linked to the analgesic effect of AM allografts [
40]. In addition to the analgesic effect, NAEs can also participate on nociceptive, anti-inflammatory, and neuroprotective effects [
41,
44,
48,
49,
50], which all of them are linked to AM properties receptor [
4,
22,
61].
Previously, we have shown that the content of PEA, AEA and OEA in placental samples is variable, mainly because of the known inter-individual differences between donors, but their concentration can be increased by tissue decontamination [
40]. This effect is probably caused by both the production of NAEs by surviving cells in AM and their release from degrading tissue [
40]. Results from the first set of placentas (1 – 9), confirmed the increase of all NAEs after 24 h of decontamination with an antibiotic solution. For PEA, the average increase was 3.8-fold, for OEA 3-fold, and for AEA 2.9-fold. This indicates that all tested NAEs react to decontamination in a similar way. Since the decontamination was an integral part of the preparation of all placentas that were used for the second part of the experiment (placentas 10 – 18), the mean concentration of particular NAEs in decontaminated samples then served as baselines.
Although cryopreservation resulted in a significant decrease of PEA after all storage periods, OEA and AEA were not significantly changed. On the other hand, the level of PEA in cryopreserved tissue after different storage time periods was significantly higher compared to the average measured in fresh, i.e., in non-decontaminated tissue. The only exception was at four years incubation, where the value was higher, but with no statistical significance, see Supplementary data,
Table S2. For all NAEs, there was no significant decrease between six months to ten-years storage, which indicates that PEA degradation is more dependent on the freezing or melting process rather than the length of cryopreservation. The decrease of PEA concentrations after cryopreservation can be explained by higher sensitivity of this enzyme compared to OEA or AEA. Another possibility is activation of enzymes degrading NAEs in AM [
62].
Due to the six-month quarantine period, after which the allografts are released for transplantation, the most important NAEs values are between the first and fourth year, i.e. in the period during which tissue can be used for grafting before expiration. The cryopreserved and freeze-dried AM allografts are typically stored for between two to five years prior to expiration [
4,
5].
Very interesting values of NAEs were found after twelve-months storage of freeze-dried AM. The measured concentrations were practically identical to decontaminated tissue (PEA), or even higher (OEA, AEA), although the differences were not significant. Since no longer-preserved freeze-dried tissue was available under the conditions of our tissue bank, it is necessary to verify these data on a larger number of AM samples and also after a longer time period.
Since we noticed a decrease in PEA concentration in cryopreserved samples, we wanted to find out if there is any data available on the analgesic effect of AM depending on its type. To the best of our knowledge, no study has compared the analgesic effect between multiple types of AM. Of the studies where pain was assessed using a pain score, analgesic effect was verified in studies using fresh [
16,
17], cryopreserved [
33,
60,
63], and air-dried terminally sterilized (gamma irradiation 25 k Gy) [
34] AM allografts for various wound treatment.
It has been shown, that NAEs exert their actions via several mechanisms [
64], particularly via PPAR-α receptor [
42], which is also expressed in the skin [
49]. Due to the autocrine secretion of NAEs, our aim was also to confirm PPAR-α receptor expression in tested samples. We found that it remains preserved in all types of AM allograft used regardless of the type and length of storage.
5. Conclusions
We analyzed the content of NAEs in long-term stored AM grafts prepared for grafting and have shown that in cryopreserved tissue, PEA, OEA and AEA remain in relatively high concentrations even after four years of storage. This means that the above described features of all three NAEs may be involved in positive analgesic and anti-inflammatory effects of AM allografts during wound healing.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org. Supplementary data, Table S1, Supplementary data, Table S1.
Author Contributions
Conceptualization, K.J. and V.V.; methodology, I.S, V.V., N.S.; investigation, I.S, V.V., N.S., A.S., C.J.J.; resources, K.J., J.B.; statistics: S.S.; writing—original draft preparation, I.S, K.J., N.S., V.V; writing—review and editing, C.J.J.; K.J., V.V; visualization, S.S., N.S.; funding acquisition, K.J. All authors have read and agreed to the published version of the manuscript.
Funding
This work was founded by the Ministry of Health of the Czech Republic, grant number NV18-08-00106, and by Ministry of Education, Youth and Sports the project BBMRI.cz, reg. no. LM202303. Institutional support (Charles University, Prague) was provided by program Cooperatio: Medical Diagnostics and Basic Medical Sciences (KJ).
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committee of the General University Hospital and the First Faculty of Medicine of Charles University, Prague, Czech Republic (No.38/17, Grant AZV VES2018 1. LF UK, 31/05/2017).
Informed Consent Statement
Informed consent was obtained from all placenta donors (No.38/17, Grant AZV VES2018 1. LF UK, 31/05/2017).
Acknowledgments
The authors are thankful to Dr. Denisa Nemetova, Mrs. Dagmar Hrabankova (Department of Transplantation and Tissue Bank, Motol University Hospital), MD. Viera Vesela and MD. Joao Victor Cabral (Institute of Biology and Medical Genetics, Charles University, Prague) for technical assistance with amniotic membrane preparation.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Nejad, A.R.; Hamidieh, A.A.; Amirkhani, M.A.; Sisakht, M.M. Update review on five top clinical applications of human amniotic membrane in regenerative medicine. Placenta 2021, 103, 104–119. [Google Scholar] [CrossRef]
- Liu, Q.-W.; Huang, Q.-M.; Wu, H.-Y.; Zuo, G.-S.-L.; Gu, H.-C.; Deng, K.-Y.; Xin, H.-B. Characteristics and Therapeutic Potential of Human Amnion-Derived Stem Cells. Int. J. Mol. Sci. 2021, 22. [Google Scholar] [CrossRef]
- Weidinger, A.; Poženel, L.; Wolbank, S.; Banerjee, A. Sub-Regional Differences of the Human Amniotic Membrane and Their Potential Impact on Tissue Regeneration Application. Front. Bioeng. Biotechnol. 2021, 8. [Google Scholar] [CrossRef]
- Malhotra, C.; Jain, A.K. Human amniotic membrane transplantation: Different modalities of its use in ophthalmology. World J. Transpl. 2014, 4, 111–121. [Google Scholar] [CrossRef]
- Jirsova, K.; Jones, G.L.A. Amniotic membrane in ophthalmology: Properties, preparation, storage and indications for grafting—A review. Cell Tissue Bank. 2017, 18, 193–204. [Google Scholar] [CrossRef]
- Anselmo, D.S.; McGuire, J.B.; Love, E.; Vlahovic, T. Application of Viable Cryopreserved Human Placental Membrane Grafts in the Treatment of Wounds of Diverse Etiologies: A Case Series. Wounds 2018, 30, 57–61. [Google Scholar]
- Farivar, B.S.; Toursavadkohi, S.; Monahan, T.S.; Sharma, J.; Ucuzian, A.A.; Kundi, R.; Sarkar, R.; Lal, B.K. Prospective study of cryopreserved placental tissue wound matrix in the management of chronic venous leg ulcers. J. Vasc. Surg. Venous Lymphat. Disord. 2019, 7, 228–233. [Google Scholar] [CrossRef]
- Lopez Martinez, J.A.; Rodriguez Valiente, M.; Fuente-Mora, C.; Garcia-Hernandez, A.M.; Canovas Sanchis, S.; Fernandez Pascual, C.J. Use of cryopreserved human amniotic membrane in the treatment of skin ulcers secondary to calciphylaxis. Dermatol. Ther. 2021, 34, e14769. [Google Scholar] [CrossRef]
- Game, F.; Gray, K.; Davis, D.; Sherman, R.; Chokkalingam, K.; Connan, Z.; Fakis, A.; Jones, M. The effectiveness of a new dried human amnion derived membrane in addition to standard care in treating diabetic foot ulcers: A patient and assessor blind, randomised controlled pilot study. Int. Wound J. 2021, 18, 692–700. [Google Scholar] [CrossRef]
- Mimouni, M.; Trinh, T.; Sorkin, N.; Cohen, E.; Santaella, G.; Rootman, D.S.; Slomovic, A.R.; Chan, C.C. Sutureless dehydrated amniotic membrane for persistent epithelial defects. Eur. J. Ophthalmol. 2021, 11206721211011354. [Google Scholar] [CrossRef]
- Serena, T.E.; Orgill, D.P.; Armstrong, D.G.; Galiano, R.D.; Glat, P.M.; Carter, M.J.; Kaufman, J.P.; Li, W.W.; Zelen, C.M. A Multicenter, Randomized, Controlled, Clinical Trial Evaluating Dehydrated Human Amniotic Membrane in the Treatment of Venous Leg Ulcers. Plast. Reconstr. Surg. 2022, 150, 1128–1136. [Google Scholar] [CrossRef]
- Allen, C.L.; Clare, G.; Stewart, E.A.; Branch, M.J.; McIntosh, O.D.; Dadhwal, M.; Dua, H.S.; Hopkinson, A. Augmented dried versus cryopreserved amniotic membrane as an ocular surface dressing. PLoS ONE 2013, 8, e78441. [Google Scholar] [CrossRef]
- Morkin, M.I.; Hamrah, P. Efficacy of self-retained cryopreserved amniotic membrane for treatment of neuropathic corneal pain. Ocul. Surf. 2018, 16, 132–138. [Google Scholar] [CrossRef]
- Insausti, C.L.; Alcaraz, A.; García-Vizcaíno, E.M.; Mrowiec, A.; López-Martínez, M.C.; Blanquer, M.; Piñero, A.; Majado, M.J.; Moraleda, J.M.; Castellanos, G.; et al. Amniotic membrane induces epithelialization in massive posttraumatic wounds. Wound Repair. Regen. 2010, 18, 368–377. [Google Scholar] [CrossRef]
- Mao, Y.; Hoffman, T.; Dhall, S.; Singal, A.; Sathyamoorthy, M.; Danilkovitch, A.; Kohn, J. Endogenous viable cells in lyopreserved amnion retain differentiation potential and anti-fibrotic activity in vitro. Acta Biomater. 2019, 94, 330–339. [Google Scholar] [CrossRef]
- Alsina-Gibert, M.; Pedregosa-Fauste, S. Amniotic membrane transplantation in the treatment of chronic lower limb ulcers. Actas Dermosifiliogr. 2012, 103, 608–613. [Google Scholar] [CrossRef]
- Eskandarlou, M.; Azimi, M.; Rabiee, S.; Seif Rabiee, M.A. The Healing Effect of Amniotic Membrane in Burn Patients. World J. Plast. Surg. 2016, 5, 39–44. [Google Scholar]
- Navas, A.; Magaña-Guerrero, F.S.; Domínguez-López, A.; Chávez-García, C.; Partido, G.; Graue-Hernández, E.O.; Sánchez-García, F.J.; Garfias, Y. Anti-Inflammatory and Anti-Fibrotic Effects of Human Amniotic Membrane Mesenchymal Stem Cells and Their Potential in Corneal Repair. STEM CELLS Transl. Med. 2018, 7, 906–917. [Google Scholar] [CrossRef]
- King, A.E.; Paltoo, A.; Kelly, R.W.; Sallenave, J.M.; Bocking, A.D.; Challis, J.R.G. Expression of Natural Antimicrobials by Human Placenta and Fetal Membranes. Placenta 2007, 28, 161–169. [Google Scholar] [CrossRef]
- Franco, G.R.; de Carvalho, A.F.; Kroon, E.G.; Lovagie, S.; Werenne, J.; Golgher, R.R.; Ferreira, P.C.P.; Bonjardim, C.A. Biological Activities of a Human Amniotic Membrane Interferon. Placenta 1999, 20, 189–196. [Google Scholar] [CrossRef]
- Koob, T.J.; Lim, J.J.; Massee, M.; Zabek, N.; Rennert, R.; Gurtner, G.; Li, W.W. Angiogenic properties of dehydrated human amnion/chorion allografts: Therapeutic potential for soft tissue repair and regeneration. Vasc. Cell 2014, 6. [Google Scholar] [CrossRef]
- Duan-Arnold, Y.; Uveges, T.E.; Gyurdieva, A.; Johnson, A.; Danilkovitch, A. Angiogenic Potential of Cryopreserved Amniotic Membrane Is Enhanced Through Retention of All Tissue Components in Their Native State. Adv Wound Care (New Rochelle) 2015, 4, 513–522. [Google Scholar] [CrossRef]
- Shao, C.; Sima, J.; Zhang, S.X.; Jin, J.; Reinach, P.; Wang, Z.; Ma, J.-x. Suppression of Corneal Neovascularization by PEDF Release from Human Amniotic Membranes. Invest. Ophthalmol. Vis. Sci. 2004, 45. [Google Scholar] [CrossRef]
- Yin, L.; Pi, Y.L. Effect of amnion membrane transplantation on corneal neovascularization in 10 patients with alkali burn. Int. J. Ophthalmol. 2011, 4, 110–111. [Google Scholar] [CrossRef]
- Kim, J.C.; Tseng, S.C.G. The effects on inhibition of corneal neovascularization after human amniotic membrane transplantation in severely damaged rabbit corneas. Korean J. Ophthalmol. 1995, 9. [Google Scholar] [CrossRef]
- Lee, S.H.; Tseng, S.C. Amniotic membrane transplantation for persistent epithelial defects with ulceration. Am. J. Ophthalmol. 1997, 123, 303–312. [Google Scholar] [CrossRef]
- Wang, W. Lyophilization and development of solid protein pharmaceuticals. Int. J. Pharm. 2000, 203, 1–60. [Google Scholar] [CrossRef]
- Lim, L.S.; Poh, R.W.; Riau, A.K.; Beuerman, R.W.; Tan, D.; Mehta, J.S. Biological and ultrastructural properties of acelagraft, a freeze-dried gamma-irradiated human amniotic membrane. Arch. Ophthalmol. 2010, 128, 1303–1310. [Google Scholar] [CrossRef]
- Paolin, A.; Trojan, D.; Leonardi, A.; Mellone, S.; Volpe, A.; Orlandi, A.; Cogliati, E. Cytokine expression and ultrastructural alterations in fresh-frozen, freeze-dried and γ-irradiated human amniotic membranes. Cell Tissue Bank. 2016, 17, 399–406. [Google Scholar] [CrossRef]
- McQuilling, J.P.; Vines, J.B.; Kimmerling, K.A.; Mowry, K.C. Proteomic Comparison of Amnion and Chorion and Evaluation of the Effects of Processing on Placental Membranes. Wounds 2017, 29, E36–E40. [Google Scholar]
- Johnson, A.; Gyurdieva, A.; Dhall, S.; Danilkovitch, A.; Duan-Arnold, Y. Understanding the Impact of Preservation Methods on the Integrity and Functionality of Placental Allografts. Ann. Plast. Surg. 2017, 79, 203–213. [Google Scholar] [CrossRef]
- DiDomenico, L.A.; Orgill, D.P.; Galiano, R.D.; Serena, T.E.; Carter, M.J.; Kaufman, J.P.; Young, N.J.; Jacobs, A.M.; Zelen, C.M. Use of an aseptically processed, dehydrated human amnion and chorion membrane improves likelihood and rate of healing in chronic diabetic foot ulcers: A prospective, randomised, multi-centre clinical trial in 80 patients. Int. Wound J. 2018, 15, 950–957. [Google Scholar] [CrossRef]
- Kadkhoda, Z.; Tavakoli, A.; Chokami Rafiei, S.; Zolfaghari, F.; Akbari, S. Effect of Amniotic Membrane Dressing on Pain and Healing of Palatal Donor Site: A Randomized Controlled Trial. Int. J. Organ. Transpl. Med. 2020, 11, 55–62. [Google Scholar]
- Vaheb, M.; Kohestani, B.M.; Karrabi, M.; Khosrojerdi, M.; Khajeh, M.; Shahrestanaki, E.; Sahebkar, M. Evaluation of Dried Amniotic Membrane on Wound Healing at Split-Thickness Skin Graft Donor Sites: A Randomized, Placebo-Controlled, Double-blind Trial. Adv. Ski. Wound Care 2020, 33, 636–641. [Google Scholar] [CrossRef]
- Tseng, S.C. HC-HA/PTX3 Purified From Amniotic Membrane as Novel Regenerative Matrix: Insight Into Relationship Between Inflammation and Regeneration. Invest. Ophthalmol. Vis. Sci. 2016, 57, 1–8. [Google Scholar] [CrossRef]
- Talmi, Y.P.; Finkelstein, Y.; Zohar, Y. Use of human amniotic membrane as a biologic dressing. Eur. J. Plast. Surg. 1990, 13. [Google Scholar] [CrossRef]
- Kesting, M.R.; Wolff, K.D.; Hohlweg-Majert, B.; Steinstraesser, L. The role of allogenic amniotic membrane in burn treatment. J. Burn. Care Res. 2008, 29, 907–916. [Google Scholar] [CrossRef]
- Liu, J.; Sheha, H.; Fu, Y.; Liang, L.; Tseng, S.C. Update on amniotic membrane transplantation. Expert. Rev. Ophthalmol. 2010, 5, 645–661. [Google Scholar] [CrossRef]
- ElHeneidy, H.; Omran, E.; Hieneedy, H.; Halwagy, A.; Al-Inany, H.; Al-Ansary, M.; Gad, A. Amniotic membrane can be a valid source for wound healing. Int. J. Women's Health 2016, 8, 225–231. [Google Scholar] [CrossRef]
- Svobodova, A.; Vrkoslav, V.; Smeringaiova, I.; Jirsova, K. Distribution of an analgesic palmitoylethanolamide and other N-acylethanolamines in human placental membranes. PLoS ONE 2023, 18, e0279863. [Google Scholar] [CrossRef]
- Esposito, E.; Cuzzocrea, S. Palmitoylethanolamide is a new possible pharmacological treatment for the inflammation associated with trauma. Mini Rev. Med. Chem. 2013, 13, 237–255. [Google Scholar]
- Lo Verme, J.; Fu, J.; Astarita, G.; La Rana, G.; Russo, R.; Calignano, A.; Piomelli, D. The nuclear receptor peroxisome proliferator-activated receptor-alpha mediates the anti-inflammatory actions of palmitoylethanolamide. Mol. Pharmacol. 2005, 67, 15–19. [Google Scholar] [CrossRef]
- Esposito, E.; Cuzzocrea, S. Palmitoylethanolamide in Homeostatic and Traumatic Central Nervous System Injuries. CNS Neurol. Disord. - Drug Targets 2013, 12, 55–61. [Google Scholar] [CrossRef]
- Suardiaz, M.; Estivill-Torrus, G.; Goicoechea, C.; Bilbao, A.; Rodriguez de Fonseca, F. Analgesic properties of oleoylethanolamide (OEA) in visceral and inflammatory pain. Pain. 2007, 133, 99–110. [Google Scholar] [CrossRef]
- Gatti, A.; Lazzari, M.; Gianfelice, V.; Di Paolo, A.; Sabato, E.; Sabato, A.F. Palmitoylethanolamide in the treatment of chronic pain caused by different etiopathogenesis. Pain. Med. 2012, 13, 1121–1130. [Google Scholar] [CrossRef]
- Di Paola, R.; Impellizzeri, D.; Fusco, R.; Cordaro, M.; Siracusa, R.; Crupi, R.; Esposito, E.; Cuzzocrea, S. Ultramicronized palmitoylethanolamide (PEA-um((R))) in the treatment of idiopathic pulmonary fibrosis. Pharmacol. Res. 2016, 111, 405–412. [Google Scholar] [CrossRef]
- Paladini, A.; Fusco, M.; Cenacchi, T.; Schievano, C.; Piroli, A.; Varrassi, G. Palmitoylethanolamide, a Special Food for Medical Purposes, in the Treatment of Chronic Pain: A Pooled Data Meta-analysis. Pain. Physician 2016, 19, 11–24. [Google Scholar]
- Di Marzo, V. 'Endocannabinoids' and other fatty acid derivatives with cannabimimetic properties: Biochemistry and possible physiopathological relevance. Biochim. Biophys. Acta 1998, 1392, 153–175. [Google Scholar] [CrossRef]
- Correia-Sa, I.B.; Carvalho, C.M.; Serrao, P.V.; Loureiro, A.I.; Fernandes-Lopes, C.; Marques, M.; Vieira-Coelho, M.A. A new role for anandamide: Defective link between the systemic and skin endocannabinoid systems in hypertrophic human wound healing. Sci. Rep. 2020, 10, 11134. [Google Scholar] [CrossRef]
- Ruhl, T.; Corsten, C.; Beier, J.P.; Kim, B.S. The immunosuppressive effect of the endocannabinoid system on the inflammatory phenotypes of macrophages and mesenchymal stromal cells: A comparative study. Pharmacol. Rep. 2021, 73, 143–153. [Google Scholar] [CrossRef]
- Fu, J.; Gaetani, S.; Oveisi, F.; Lo Verme, J.; Serrano, A.; Rodriguez De Fonseca, F.; Rosengarth, A.; Luecke, H.; Di Giacomo, B.; Tarzia, G.; et al. Oleylethanolamide regulates feeding and body weight through activation of the nuclear receptor PPAR-alpha. Nature 2003, 425, 90–93. [Google Scholar] [CrossRef]
- Svobodova, A.; Horvath, V.; Smeringaiova, I.; Cabral, J.V.; Zemlickova, M.; Fiala, R.; Burkert, J.; Nemetova, D.; Stadler, P.; Lindner, J.; et al. The healing dynamics of non-healing wounds using cryo-preserved amniotic membrane. Int. Wound J. 2022, 19, 1243–1252. [Google Scholar] [CrossRef]
- Dhall, S.; Sathyamoorthy, M.; Kuang, J.Q.; Hoffman, T.; Moorman, M.; Lerch, A.; Jacob, V.; Sinclair, S.M.; Danilkovitch, A. Properties of viable lyopreserved amnion are equivalent to viable cryopreserved amnion with the convenience of ambient storage. PLoS ONE 2018, 13, e0204060. [Google Scholar] [CrossRef]
- Merjava, S.; Neuwirth, A.; Tanzerova, M.; Jirsova, K. The spectrum of cytokeratins expressed in the adult human cornea, limbus and perilimbal conjunctiva. Histol. Histopathol. 2011, 26, 323–331. [Google Scholar] [CrossRef]
- Trosan, P.; Cabral, J.V.; Smeringaiova, I.; Studeny, P.; Jirsova, K. Interleukin-13 increases the stemness of limbal epithelial stem cells cultures. PLoS ONE 2022, 17, e0272081. [Google Scholar] [CrossRef]
- Trivedi, N.R.; Cong, Z.; Nelson, A.M.; Albert, A.J.; Rosamilia, L.L.; Sivarajah, S.; Gilliland, K.L.; Liu, W.; Mauger, D.T.; Gabbay, R.A.; et al. Peroxisome proliferator-activated receptors increase human sebum production. J. Invest. Dermatol. 2006, 126, 2002–2009. [Google Scholar] [CrossRef]
- Lam, P.M.; Marczylo, T.H.; Konje, J.C. Simultaneous measurement of three N-acylethanolamides in human bio-matrices using ultra performance liquid chromatography-tandem mass spectrometry. Anal. Bioanal. Chem. 2010, 398, 2089–2097. [Google Scholar] [CrossRef]
- Georgiadis, N.S.; Ziakas, N.G.; Boboridis, K.G.; Terzidou, C.; Mikropoulos, D.G. Cryopreserved amniotic membrane transplantation for the management of symptomatic bullous keratopathy. Clin. Exp. Ophthalmol. 2008, 36, 130–135. [Google Scholar] [CrossRef]
- Sharma, N.; Singh, D.; Maharana, P.K.; Kriplani, A.; Velpandian, T.; Pandey, R.M.; Vajpayee, R.B. Comparison of Amniotic Membrane Transplantation and Umbilical Cord Serum in Acute Ocular Chemical Burns: A Randomized Controlled Trial. Am. J. Ophthalmol. 2016, 168, 157–163. [Google Scholar] [CrossRef]
- Loeffelbein, D.J.; Rohleder, N.H.; Eddicks, M.; Baumann, C.M.; Stoeckelhuber, M.; Wolff, K.D.; Drecoll, E.; Steinstraesser, L.; Hennerbichler, S.; Kesting, M.R. Evaluation of human amniotic membrane as a wound dressing for split-thickness skin-graft donor sites. Biomed. Res. Int. 2014, 2014, 572183. [Google Scholar] [CrossRef]
- Maskin, S.L. Successful reversal of neuropathic eye pain by treatment of occult ocular surface disease: Case series and implications. Am. J. Ophthalmol. Case Rep. 2022, 27, 101662. [Google Scholar] [CrossRef]
- Park, B.; Gibbons, H.M.; Mitchell, M.D.; Glassa, M. Identification of the CB1 cannabinoid receptor and fatty acid amide hydrolase (FAAH) in the human placenta. Placenta 2003, 24, 473–478. [Google Scholar] [CrossRef]
- Zidan, S.M.; Eleowa, S.A.; Nasef, M.A.; Abd-Almoktader, M.A.; Elbatawy, A.M.; Borhamy, A.G.; Aboliela, M.A.; Ali, A.M.; Algamal, M.R. Maximizing the safety of glycerol preserved human amniotic membrane as a biological dressing. Burns 2015, 41, 1498–1503. [Google Scholar] [CrossRef]
- Rankin, L.; Fowler, C.J. The Basal Pharmacology of Palmitoylethanolamide. Int. J. Mol. Sci. 2020, 21. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).