1. Introduction
Dementia is an umbrella term for acquired, chronic, progressive, age-related, and functionally impairing neurocognitive decline, encompassing several different and heterogeneous clinical conditions including, most commonly, Alzheimer's disease, vascular dementia, Lewy body disease and frontotemporal dementia [
1]. Dementia is recognized as a burdensome public health issue, both currently and in the decades to come [
2]. Around 47 million people worldwide have dementia, which is predicted to rise to a staggering 132 million by 2050 [
3]. Various clinical dementia staging scales have been developed throughout the years, each with different pros, cons, dissemination, and implementation, including the widely used Clinical Dementia Rating (CDR), Global Deterioration scale (GDS), and Functional Assessment Staging (FAST) [
4,
5,
6,
7].
Nutritional and feeding problems are known to occur throughout the illness, across its different stages, becoming more common as the disease progresses, and negatively impacting the clinical course, outcome, and caregiver burden [
8]. Therefore, it is unsurprising that several studies focus on the nutritional issues of patients with advanced dementia. The use of enteral tube feeding in individuals with severe dementia has been the subject of several studies and systematic reviews [
9,
10,
11,
12,
13] and was addressed in the most recent clinical practice guidelines covering this issue, promoted by the American Geriatric Society [
14] and by the European Society for Clinical Nutrition and Metabolism (ESPEN) [
4]. Both societies advise against the use of enteral tube feeding in these patients (while advocating for careful handfeeding instead), after assuming that it is not associated with longer survival or improvement in nutritional status, that it causes the excessive use of restraints, and that it is not effective in preventing pressure ulcers and aspiration pneumonia [
4,
14]. Nevertheless, some authors expressed concern regarding the quality of the scientific evidence used to formulate the recommendations, arguing that it suffers from bias and inaccurate methodologies, including an inadequate control group and a lack of data on quality of life [
15]. Most of the studies that are used to advocate against tube feeding report short survival periods, with or without enteral tube feeding [
16], contrasting with the more extended survival period of PEG-fed dementia patients seen during the clinical practice of several teams, including our artificial nutrition team. In fact, PEG-feeding has long been advised for dementia patients by teams taking care of those persons [
17,
18,
19].
Additionally, recent data suggest that if there is a medical indication for enteral tube feeding, it should not, a priori, be precluded just because the patient has a dementia diagnosis [
20]. These authors call for a critical revision of the recommendations on enteral tube feeding in patients with advanced dementia and uphold, for the time being, that the decision-making process should be personalized as much as possible, avoiding generalizations [
15]. This conflict of opinions continues to fuel the ongoing controversy regarding whether one should continue hand feeding or initiate enteral tube feeding in this population.
Overall, acknowledging the gaps in research in this field, the complex ethical dilemmas, as well as the heterogeneity within dementia syndromes, one should bear in mind that a "one-size-fits-all" approach may not be suitable for the management of nutritional and feeding problems of citizens with advanced dementia.
Our artificial nutrition team, GENE (Grupo de Estudo de Nutrição Entérica/parentérica), evaluated every patient with dementia, proposed to endoscopic gastrostomy for long-term tube feeding. People with severe dementia also underwent endoscopic gastrostomy if they maintained a close relationship with family, friends, and caregivers, and if a long survival period was expected.
The current study aims to demonstrate that the PEG procedure is safe for "Persons With Severe Dementia" (PWSD) criteria and may contribute to a better nutritional status of PWSD.
Objectives
1. To evaluate PWSD' clinical and nutritional status with at three GENE routine evaluation follow-ups. T0 - at the day of the endoscopic gastrostomy procedure, T1 – 1 month after gastrostomy and T2 – three months after gastrostomy.
Using several easily accessible tools, even with patients who have speech difficulties, namely:
1.1. Anthropometry.
1.2 Laboratory data.
2. To evaluate the survival of PEG-fed PWSD after the gastrostomy procedure.
3. To evaluate the impact of nutritional status on the survival outcome of PWSD patients that underwent endoscopic gastrostomy, using anthropometric and biochemical markers.
4. To evaluate the impact of PEG feeding on the nutritional status and patients outcome, evaluated using anthropometric and biochemical markers.
5. To evaluate the occurrence of major complications from the gastrostomy procedure or PEG-feeding to establish the safety of endoscopic gastrostomy on PWSD.
2. Materials and Methods
2.1. Patients
We studied consecutive adult patients with severe dementia who were referred and underwent endoscopic gastrostomy to have PEG nutritional support, for 16 years, from January 2005 to December 2020. Patients were considered eligible if they were referred to PEG as severe dementia by their attending clinicians, whatever dementia staging tool was used. All data are part of the routine evaluation of PEG patients and were collected from GENE clinical files.
All dementia patients in our artificial feeding team files were eligible for the study. The exclusion criteria were:
2.2. Safety
Complications with PEG were a significant concern and our team aimed to ensure this procedure's safety. During the follow-ups, we recorded and evaluated all potential major omplications associated with PEG.
2.3. Clinical Outcome
We collected the survival period (in months) of the PEG dementia patients from the endoscopic gastrostomy procedure until death or until 31st December 2020.
2.4. Anthropometric Evaluation
We recorded clinic and anthropometric data on the day of the endoscopic gastrostomy or the day before (T0), one month after endoscopic gastrostomy (T1) and three months after endoscopic gastrostomy (T2). The anthropometry measurements followed the International Society for the Advancement of Kinanthropometry manual. We obtained three consecutive measurements each time. The clinical file record represents those three measurements' mean.
2.4.1. Body mass index (BMI): BMI was obtained in most patients using the equation
Weight/Height2. If patients were bedridden and could not stand up for weight and height evaluation, BMI was estimated using the mid-upper arm circumference (MUAC) and regression equations described by Powell-Tuck and Hennessy [
21]; this method has been previously used and proved to provide a reliable BMI estimation in PEG patients [
22,
23]. Each patient was classified by the WHO classification according to their age [
24]. (
Table 1)
2.4.2. Mid Upper Arm Circumference (MUAC) was evaluated using an inextensible measuring tape with a 1 mm resolution. MUAC results from evaluating several tissues representing fat and lean mass
2.4.3. Tricipital skinfold (TSF) was measured using a Lange Skinfold caliper with a 1 mm resolution. TSF evaluates the subcutaneous adipose tissue and estimates adipose reserves
2.4.4. The Mid-Arm Muscle Circumference (MAMC) was calculated according to the equation: MAMC = MUAC (cm) - 0.314 × TSF (mm). The MAMC allows us to estimate lean and muscle mass
For each patient, MUAC, MAMC, and TSF were compared with reference values of the National Health and Nutrition Examination Survey (NHANES) through the comparison with the Frisancho reference tables [
25,
26].
Although nutritional evaluation could benefit from sophisticated devices for measuring body composition, such as bioelectrical impedance analysis (BIA) or CT Scan analysis, those devices were not available for all patients. Although less precise, BMI and anthropometry measures are inexpensive and widespread nutritional evaluation tools, classically used to evaluate fat/lean mass [
27] and available everywhere, even in institutions with scarce resources.
2.5. Laboratory Evaluation
A blood sample was obtained from these patients minutes before the endoscopic gastrostomy procedure (T0) and one and three months after the gastrostomy procedure (T1/T2). Blood samples were obtained between 8:00 and 10:00 AM following at least 12 h of fasting. Serum Albumin <3.5 g/dl, serum Transferrin <200 mg/dl, serum Total Cholesterol <160 mg/dl and Hemoglobin (Male <13g/dL, Female <12 g/dL) were considered low values, suggestive of poor prognosis and/or malnutrition [
28,
29,
30]. Nevertheless, laboratory data were always regarded as dependent of several non-nutritional influences.
2.6. Ethical Considerations
All subjects were informed of the procedures of the Artificial Feeding Team for PEG-feeding patients and gave their informed consent. This rectrospective study was approved by the Hospital Garcia de Orta Ethics Committee
2.7. Statistics
We used the SPSS software version 25 (IBM corporation) to compute the descriptive statistics and perform survival analysis. Survival analysis included plotting the Kaplan-Meier patients' survival rate curves, estimating the mean and the median survival time, and evaluating the dependency on survival time of predictor variables by modelling the Cox proportional-hazards regression model. In the model, patient death was the event of interest. Censored data was defined as becoming from patients alive at the study's end. The Walt test evaluated the linear combination of the Cox model estimated parameters, and the Breslow-Day statistic tested for the hazard ratios homogeneity between category variables levels.
3. Results
3.1. Subjects
This study involved 120 patients with severe dementia diagnostic. Of these patients, 20 were excluded for incomplete data. The remaining 100 patients presented all data accessible on clinical records, except Hemoglobine that was only available in 65. From these 100 patients displaying all the criteria, 39 were males, and 61 were females. Ages ranged from 51 to 100 years (mean: 78.4 yr.; median: 80.5 yr.). Most patients (n=88) were older citizans, 65 years old or older. Only 12 were younger adults, less than 65 years old.
Table 2 displays the anthropometry and laboratory serum data subject's characterization.
3.2. Anthropometry
3.2.1. Body mass index (BMI) at T0
BMI was obtained in all 100 patients. For 46 patients, BMI was estimated using the Powell-Tuck and Hennessy regression equations. BMI ranged from 14 Kg/m2 to 41 Kg/m2 (mean: 23.71Kg/m2; median: 22.80 Kg/m2). The WHO classification was used according to age. Subscribing to this classification, 39 patients displayed low BMI. With age inferior of 65, 2 patients displayed low BMI (<18.5 Kg/m2) and with an age of 65 or more, 37 patients displayed a low BMI of <22 Kg/m2(
Table 2)
3.2.2. Mid Upper Arm Circumference (MUAC) at T0
Compared with Frisancho criteria [
25], 77 showed MUAC in the low range.
3.2.3. Tricipital Skinfold (TSF) at T0
In this anthropometric parameter, 84 displayed low TSF.
3.2.4. Mid-Arm Muscle Circumference (MAMC) at T0
In this anthropometric parameter, 52 patients showed MAMC in the low range.
3.3. Laboratory Assessment
3.3.1. Serum Albumin at T0
On the day of gastrostomy, 64 patients presented low serum Albumin.
3.3.2. Serum Transferrin at T0
On the day of gastrostomy, 62 showed low serum Transferrin.
3.3.3. Serum Total Cholesterol at T0
On the day of gastrostomy, 51 displayed low serum Total Cholesterol.
3.3.4. Hemoglobin at T0
On the day of gastrostomy, 43 out of 65, displayed low Hemoglobin.
Table 2.
Characterization of subjects by anthropometry and laboratory serum data.
Table 2.
Characterization of subjects by anthropometry and laboratory serum data.
| |
Total (n = 100) |
Male (n=39) |
Female (n=61) |
Total Mean |
| Anthropometry Results |
| BMI |
39 Low |
13 Low |
26 Low |
|
| 33 Normal |
16 Normal |
17 Normal |
|
| 28 High |
10 High |
18 High |
| MUAC |
77 Low |
31 Low |
46 Low |
| 23 Normal |
8 Normal |
15 Normal |
| TSF |
84 Low |
29 Low |
55 Low |
| 16 Normal |
10 Normal |
6 Normal |
| MAMC |
52 Low |
31 Low |
21 Low |
| 48 Normal |
8 Normal |
40 Normal |
| Laboratory serum data |
| Albumin |
64 Low |
31 Low |
33 Low |
3.25 g/dL |
| 36 Normal |
8 Normal |
28 Normal |
| Transferrin |
62 Low |
35 Low |
45 Low |
170.6 mg/dL |
| 34 Normal |
4 Normal |
16 Normal |
| Total Cholesterol |
51 Low |
28 Low |
23 Low |
164.4 mg/dL |
| 49 Normal |
11 Normal |
38 Normal |
| Hemoglobin* (n=65) |
43 low |
23 Low |
20 Low |
11.3 g/dl |
| 22 Normal |
2 Normal |
20 Normal |
3.4. Safety
During the follow-ups, the detection of problems associated with PEG tube placement was considered. From all dementia patients, no major life-threatening problems were reported or detected.
3.5. Evolution Data According to Follow-Ups (T0 to T2)
Only BMI has increased during follow-ups (T0 to T2). Hemoglobin, Albumin and Transferrin decreased during follow-ups (T0 to T2). However, the only significant change was of Albumin and Transferrin. These two laboratory parameters significantly decreased at the 3 months of evaluation (p=0.001). Total Cholesterol increased during the first month however without statistical significance. (
Table 3).
3.6. Clinical Outcome
In December 2020, from the 100 patients who fulfilled the included criteria, 11 were still alive, and all alive patients were still PEG-fed and followed at the Artificial Nutrition Outpatients Clinic.
3.7. Kaplan-Meier Survival Analysis
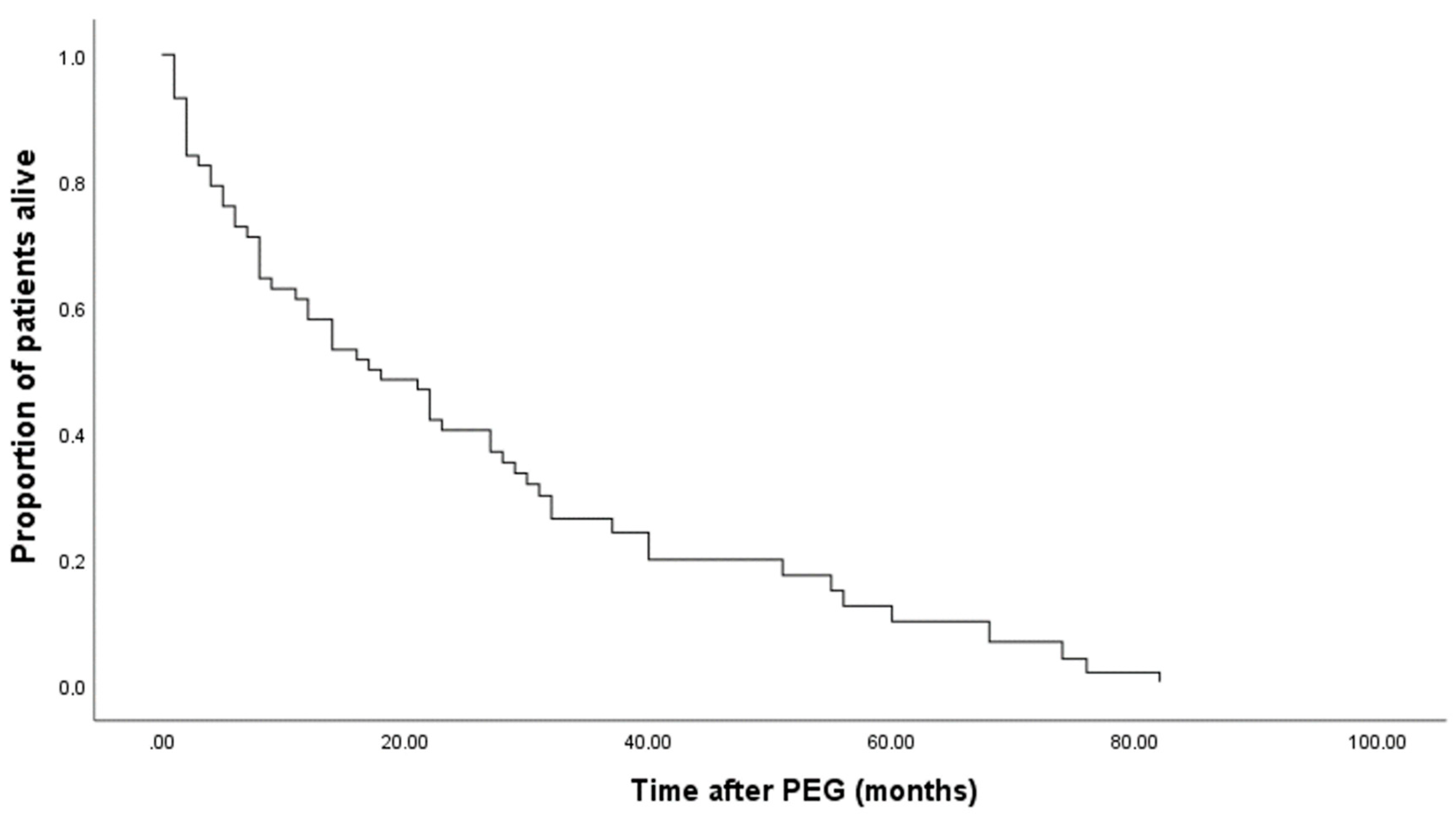
Of 100 patients, 10 decease during the first month (6 Males and 4 females), and 9 decease between the first and the third month (8 males and 1 female). So, after the first three months, only 19 patients decease, and 81 were alive and PEG-fed (Kaplan-Meier Curves in
Figure 1 and
Figure 2).
The mean survival of all patients after the gastrostomy was almost 28 months, while the median was 17 months. In the female group, the mean survival time was significantly higher than in the male group (
Table 4; p<0.001, Breslow-Day test).
On average, the low BMI at T0 were the ones who died first, and the normal/high BMI at T0 were the ones who survived longer; however, these differences were not significative (p=0.716, Breslow-Day test). (
Table 5)
3.8. Survival According to Data Follow-Ups (T0 to T2)
Our Cox regression results show that female sex (p=0.012), PWSD presenting BMI increase during PEG-feeding (p=0.023), and higher baseline Hemoglobin (p=0.005) have a significant effect on reducing risk for death, leading to an increase in survival. However, baseline Hemoglobin shows the weakest effect, with one more unit of Hemoglobin associated with an average of 2% decrease in death risk (
Table 5). Therefore, considering the modifiable factors, BMI recovery at three months was the most important for better survival (
Table 6). For each point more in BMI recovery, there was a 10% significant reduction in the risk of experiencing death (p=0.023) (
Table 6). In arm anthropometry, MUAC and MAMC increase evaluated at the end of 3 months was associated with an increase in survival but not significantly (p=0.172 and p=0.094, respectively) (
Table 6). In fact, MAMC p=0.09 represents a statistical trend that deserves a clinical appraisal.
4. Discussion
Tube feeding in patients with severe dementia is a controversial issue. Some studies and guidelines recommend avoiding tube feeding use in patients with severe dementia because no clear evidence demonstrates the benefits of PEG feeding in nutritional status and survival time. The studies supporting ESPEN guidelines on nutrition in dementia [
8] used on patients in different conditions and institutions, like patients with stroke [
31], and this pathology may have a lower survival time than neurodegenerative disorders. In other studies, they found inconclusive if enteral tube feeding benefits dementia patients, probably due to USA patients being different when compared to European counterparts [
9]. In contrast, PWSD have good medical, nutritional, and family support in our social reality. These three points are the major base for moving towards PEG placement.
Despite advanced dementia criteria, some patients have a strong family relationship that justifies, in ethical terms, the extension of life, probably giving greater comfort and improving the nutritional status.
Our patients with severe dementia had a poor oral intake, translating into a nutritional risk. So, our multidisciplinary team discussed the benefits and disadvantages of different approaches with the patient's families. All patients also had at admission an estimated survival larger than one month; the meantime is suitable for generic PEG placement in patients who do not resume oral feeding according to recommendations [
32].
The present study's patients' average age is 78, so most of our dementia cases are linked to age [
33]. The mean survival of our patients was more than 27 months (median 17 months). Only 19 % of patients died during the first 3 months of PEG feeding. As demonstrated in our previous study [
19], these interesting results reinforce the need to PEG-feed those patients. We suspect that the mean may be influenced by a group of patients with greater longevity (including those still alive). However, a 17 month median is much higher than the survival of the patients described in the studies that support guidelines. We believe some previous studies focused on terminal patients, not the general population of PWSD with advanced disease criteria, who may have a much longer life span.
In this study, we were concerned during the follow-ups about checking for complications related to the PEG. We were careful to explain to caregivers all the precautions they should take to minimize the risk of any complications associated with PEG. In this way, major complications associated with PEG were not detected or reported in our patients.
At admission, we evaluated the BMI in all patients, a worldwide anthropometric parameter validated to assess nutritional status. In our study, mean BMI increases during the two evaluations but not significantly. The three months were probably short to display a more significant BMI improvement, but this is the standard for follow-up appointments. Even though, individual BMI could improve survival by reducing 10% death risk for each point increase in BMI at the end of 3 months. This is a clear demonstration that PEG feeding in these carefully selected patients whit severe dementia can improve nutritional status, and this has a positive impact in survival.
Unlike BMI, Albumin and Transferrin displayed a decrease during the first three months. Since several factors may be involved, this decrease is probably linked to inflammatory, non-nutritional factors. Nevertheless, these laboratory data do not show any impact on the survival of our patients.
Most anthropometric data displayed levels related to poor nutrition, so PEG placement could be a tool for improvement since nutritional apport could be better than oral intake only. Arm anthropometry (MUAC, TSF, and MAMC) data showed malnutrition in over eighty per cent of the patients. Estimation of fat and fat-free reserves also revealed a poor nutritional status. TSF recognized more malnourished patients than MAMC, which suggests that fat tissue is more depleted and muscle mass is more preserved in our patients' conditions. Also, MAMC is an independent outcome predictor, highlighting the importance of lean mass in patient survival, probably reflecting more prolonged survival compared to other studies and may be linked to better health and family care. MUAC and MAMC showed a possible influence on survival since for every point increased at the end of three months, there was around 9 and 12% lower risk of death, respectively. Most anthropometric data improved significantly (even without statistical significance) over time and gradually reduced as the disease progressed. Interestingly, MAMC shows an increase over the months of PEG feeding, with a trend to significance (p=0.094), and increasing MAMC reduces the risk of death, highlighting the importance of PEG feeding for recovering lean body mass and improving survival.
BMI, Hemoglobin and female gender were the only three parameters with statistical significance on survival obtained at the beginning of PEG feeding on the day of the gastrostomy procedure. In our experience, the female gender is generally associated with slightly better nutritional status at the beginning of the PEG feeding. We empirically believe this is linked to cultural factors favoring earlier acceptance of PEG tube placing in women more than men. We have seen trend for early acceptance of PEG and better initial nutritional status in women, in PEG patients with other neurological disorders or with head or neck cancer. Our team believes that this reflects sociologic differences of attitude between genders, males being less likely to accept PEG.
Our study has some limitations. One is the nonexistence of a control group to compare our results. We have chosen patients with good family support and believe that having a control group in this situation is unsuitable and unethical. Some missing data also limited our results. We completed processing patient data in December 2020 due to the COVID-19 pandemic. Several patients did not continue their follow-up (refusing to go to the hospital), and some records were incomplete. Also, a wider group could allow more solid evidence, but these careful selected PWSD are scarce and multiceter studies would be necessary for enrolling a larger group.
5. Conclusions
Our team selected PWSD with strong family support and adequate medical and nutritional care, which differs from PWSD patients with a terminal condition in several studies. We demonstrate that, in these selected patients, PEG is a safe procedure, and PEG-feeding can improve anthropometric data, leading to more prolonged survival. Female gender, baseline Hemoglobin and BMI, as well as BMI improvement at the end of 3 months, were markers for better outcomes. One unit increment of BMI recovery was associated with less 10% death risk. In our study, a mean of 27 months survival (median of 17 months) was better than most studies and probably reflects the benefit of a gastrostomy in PWSD. Based on our results, we recommend that PEG should be considered in patients with severe dementia with strong family support when risk factors related to malnutrition are present.
Author Contributions
D.S.-C.—data curation; formal analysis; investigation; and writing. P.M.—formal analysis; writing and reviewing. C.O.—formal analysis; investigation. M.G.—formal analysis; investigation; writing and reviewing. C.A.S.—data curation; formal analysis; investigation; writing and reviewing. J.F.—conceptualization; data curation; formal analysis; methodology; writing and reviewing. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the local health research ethics boards—Garcia de Orta Ethical Committee, Garcia de Orta Centre. All patients gave their informed consent to participate.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the first author.
Acknowledgments
This work is financed by national funds through the FCT—Foundation for Science and Technology, I.P., under the project UIDB/04585/2020. The researchers would like to thank the Centro de Investigação Interdisciplinar Egas Moniz (CiiEM) for the support provided for the publication of this article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Arvanitakis, Z.; Shah, R. C.; Bennett, D. A. Diagnosis and Management of Dementia: Review. JAMA 2019, 322 (16), 1589–1599. [CrossRef]
- Dementia: a public health priority. Who.int. https://www.who.int/publications/i/item/dementia-a-public-health-priority. (accessed on 6 January 2020).
- Prince, M.; Wimo, A.; Guerchet, M.; Ali, G.-C.; Wu, Y.-T.; Prina, M.; Kit, Y.; Xia, Z. Alzint.org. https://www.alzint.org/u/WorldAlzheimerReport2015.pdf (accessed 2020-01-06).
- Rikkert, M. G. M. O.; Tona, K. D.; Janssen, L.; Burns, A.; Lobo, A.; Robert, P.; Sartorius, N.; Stoppe, G.; Waldemar, G. Validity, Reliability, and Feasibility of Clinical Staging Scales in Dementia: A Systematic Review: A Systematic Review. Am. J. Alzheimers. Dis. Other Demen. 2011, 26 (5), 357–365. [CrossRef]
- Burke, W. J.; Houston, M. J.; Boust, S. J.; Roccaforte, W. H. Use of the Geriatric Depression Scale in Dementia of the Alzheimer Type. J. Am. Geriatr. Soc. 1989, 37 (9), 856–860. [CrossRef]
- Eisdorfer, C.; Cohen, D.; Tang, M. X. An Empirical Evaluation of the Global Deterioration Scale for Staging Alzheimer's Disease: Correction. Am J Psychiatry 1992, 149 (6). [CrossRef]
- Sclan, S. G.; Reisberg, B. Functional Assessment Staging (FAST) in Alzheimer's Disease: Reliability, Validity, and Ordinality. Int. Psychogeriatr. 1992, 4 Suppl 1, 55–69. [CrossRef]
- Volkert, D.; Chourdakis, M.; Faxen-Irving, G.; Frühwald, T.; Landi, F.; Suominen, M. H.; Vandewoude, M.; Wirth, R.; Schneider, S. M. ESPEN Guidelines on Nutrition in Dementia. Clin. Nutr. 2015, 34 (6), 1052–1073. [CrossRef]
- Sampson, E. L.; Candy, B.; Jones, L. Enteral Tube Feeding for Older People with Advanced Dementia. Cochrane Database Syst. Rev. 2009, No. 2, CD007209. [CrossRef]
- Goldberg, L. S.; Altman, K. W. The Role of Gastrostomy Tube Placement in Advanced Dementia with Dysphagia: A Critical Review. Clin. Interv. Aging 2014, 9, 1733–1739. [CrossRef]
- Salomon, R.; Garbi Novaes, C. Outcomes of Enteral Nutrition for Patients with Advanced Dementia: A Systematic Review. J Nutr Health Aging 2015, 19 (11), 169–177. [CrossRef]
- Brooke, J.; Ojo, O. Enteral Nutrition in Dementia: A Systematic Review. Nutrients 2015, 7 (4), 2456–2468. [CrossRef]
- Douglas, J. W.; Lawrence, J. C.; Turner, L. W. Social Ecological Perspectives of Tube-Feeding Older Adults with Advanced Dementia: A Systematic Literature Review. J. Nutr. Gerontol. Geriatr. 2017, 36 (1), 1–17. [CrossRef]
- American Geriatrics Society Ethics Committee and Clinical Practice and Models of Care Committee. American Geriatrics Society Feeding Tubes in Advanced Dementia Position Statement. J Am Geriatr Soc 2014, 62 (14), 1590–1593. [CrossRef]
- Orlandoni, P.; Jukic Peladic, N.; Cherubini, A. Enteral Nutrition in Advanced Dementia: An Unresolved Dilemma in Clinical Practice. Eur. Geriatr. Med. 2020, 11 (2), 191–194. [CrossRef]
- Goldberg, L.; Altman, K. The Role of Gastrostomy Tube Placement in Advanced Dementia with Dysphagia: A Critical Review. Clin. Interv. Aging 2014, 1733. [CrossRef]
- Sanders, D. S.; Anderson, A. J.; Bardhan, K. D. Percutaneous Endoscopic Gastrostomy: An Effective Strategy for Gastrostomy Feeding in Patients with Dementia. Clin. Med. 2004, 4 (3), 235–241. [CrossRef]
- Sanders, D. S.; Leeds, J. S.; Drew, K. The Role of Percutaneous Endoscopic Gastrostomy in Patients with Dementia. Br. J. Nurs. 2008, 17 (9), 588–594. [CrossRef]
- Nunes, G.; Santos, C. A.; Santos, C.; Fonseca, J. Percutaneous Endoscopic Gastrostomy for Nutritional Support in Dementia Patients. Aging Clin Exp Res 2016, 28 (5), 983–989. [CrossRef]
- Orlandoni, P.; Peladic, N. J.; Di Rosa, M.; Venturini, C.; Fagnani, D.; Sparvoli, D.; Giorgini, N.; Basile, R.; Cola, C. The Outcomes of Long Term Home Enteral Nutrition (HEN) in Older Patients with Severe Dementia. Clin. Nutr. 2019, 38 (4), 1871–1876. [CrossRef]
- Powell-Tuck J, Hennessy EM. A comparison of mid upper arm circumference, body mass index and weight loss as indices of undernutrition in acutely hospitalized patients. Clin. Nutr. 2003, 22(3):307-312. [CrossRef]
- Pereira, M.; Santos, C.; Fonseca, J. Body Mass Index Estimation on Gastrostomy Patients Using the Mid Upper Arm Circumference. The Journal of Aging Research & Clinical Practice 2010, 1, 252–255.
- Barosa, R.; Roque Ramos, L.; Santos, C. A.; Pereira, M.; Fonseca, J. Mid Upper Arm Circumference and Powell-Tuck and Hennessy's Equation Correlate with Body Mass Index and Can Be Used Sequentially in Gastrostomy Fed Patients. Clin. Nutr. 2018, 37 (5), 1584–1588. [CrossRef]
- World Health Organization—Europe. Nutrition—Body Mass Index—BMI. Available online: https://www.euro.who.int/en/health topics/disease-prevention/nutrition/a-healthylifestyle/body-mass-index-bmi~ (accessed on 6 January 2020 ).
- Frisancho, A. R. New Standards of Weight and Body Composition by Frame Size and Height for Assessment of Nutritional Status of Adults and the Elderly. Am. J. Clin. Nutr. 1984, 40 (4), 808–819. [CrossRef]
- Fonseca, J.; Santos, C. A. Clinical anatomy: anthropometry for nutritional assessment of 367 adults who underwent endoscopic gastrostomy. Acta Med. Port. 2013, 26 (3), 212–218.
- Sultan, S.; Nasir, K.; Qureshi, R.; Dhrolia, M.; Ahmad, A. Assessment of the Nutritional Status of the Hemodialysis Patients by Anthropometric Measurements. Cureus 2021, 13 (10), e18605. [CrossRef]
- Kuzuya, M.; Izawa, S.; Enoki, H.; Okada, K.; Iguchi, A. Is Serum Albumin a Good Marker for Malnutrition in the Physically Impaired Elderly? Clin. Nutr. 2007, 26 (1), 84–90. [CrossRef]
- Fonseca, J.; Adriana Santos, C.; Brito, J. Predicting Survival of Endoscopic Gastrostomy Candidates Using the Underlying Disease, Serum Cholesterol, Albumin and Transferrin Levels. Nutr. Hosp. 2013, 28 (4), 1280–1285. [CrossRef]
- Vyroubal, P.; Chiarla, C.; Giovannini, I.; Hyspler, R.; Ticha, A.; Hrnciarikova, D.; Zadak, Z. Hypocholesterolemia in Clinically Serious Conditions--Review. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc Czech. Repub. 2008, 152 (2), 181–189. [CrossRef]
- Callahan, C. M.; Haag, K. M.; Weinberger, M.; Tierney, W. M.; Buchanan, N. N.; Stump, T. E.; Nisi, R. Outcomes of Percutaneous Endoscopic Gastrostomy among Older Adults in a Community Setting. J. Am. Geriatr. Soc. 2000, 48 (9), 1048–1054. [CrossRef]
- Volkert, D.; Berner, Y. N.; Berry, E.; Cederholm, T.; Coti Bertrand, P.; Milne, A.; Palmblad, J.; Schneider, S.; Sobotka, L.; Stanga, Z.; DGEM (German Society for Nutritional Medicine); Lenzen-Grossimlinghaus, R.; Krys, U.; Pirlich, M.; Herbst, B.; Schütz, T.; Schröer, W.; Weinrebe, W.; Ockenga, J.; Lochs, H.; ESPEN (European Society for Parenteral and Enteral Nutrition). ESPEN Guidelines on Enteral Nutrition: Geriatrics. Clin. Nutr. 2006, 25 (2), 330–360. [CrossRef]
- American Psychiatric Association (2000) Delirium, dementia, and amnestic and olther cognitive disorders. In: Diagnostic and statistical manual of mental disorders text revision, 4th edn. Washington, pp 135–180.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).