1. Introduction
Neonatal apnoea is a life-threatening complication in newborns that might successfully be treated with methylxanthines (aminophylline, theophylline) decreasing its incidence and the need for mechanical ventilation [
1,
2]. Caffeine has become the drug of choice to treat neonatal apnoea due to its efficacy, tolerability, large therapeutic window, and safety margin [
3]. Caffeine is a xanthine exerting many complex and pleiotropic effects on various organ systems mediated by a variety of mechanisms, including antagonism of the adenosine and GABA receptors, inhibition of phosphodiesterase enzymes, and sensitizing of various ryanodine-sensitive calcium release channels [
4,
5]. Its interference with the sympathetic nervous system has long been appreciated [
4] although the results remain controversial [
6]. As for improvement of apnoea, its effects include stimulation of the respiratory drive, enhancement of minute ventilation, increased response to hypercapnia, increased skeletal muscle tone, decreased diaphragmatic fatigue, and relaxation of airway smooth muscle [
2,
7]. Its effects on the cardiovascular system (CVS) are complex, including direct and indirect effects on the vessels and the heart. In the heart, it increases the heart rate (HR), cardiac contractility and stroke volume, consequently increasing cardiac output and mean arterial blood pressure [
6,
8,
9]. As for the vascular system, caffeine exerts vasodilative effects in the pulmonary and most vessels of the systemic circulation but induces vasoconstriction in the cerebral circulation [
8]. Caffeine also increases the metabolic rate, neuromuscular transmission and catecholamine release [
10].
HR is modulated by an interplay between the sympathetic and the parasympathetic branches of the autonomic nervous system (ANS), and is subjected to beat-to-beat variability which could be assessed by measuring the time interval between consecutive heartbeats [
11,
12]. While the effect of the parasympathetic nervous system (PNS) on the HR is expressed quickly, the effect of the sympathetic nervous system (SNS) takes longer for its full expression. These different time frames of the action of the PNS and the SNS on the sinoatrial node may partly be explained by the corresponding neurotransmitter kinetics [
13].
A relevant clinical tool for a non-invasive assessment of the ANS maturation in newborns, as well as the effects of the ANS on newborn's heart, is spectral analysis of heart rate variability (HRV) [
13]. Accordingly, HRV could be separated into typical frequency-spectra, most usually high frequency (HF) and low frequency (LF) spectrum, using various methods. While there is no uniform consensus on the relevant parameters of HRV that might adequately reflect the balance between PNS and SNS, one of the usually applied parameters is the ratio between LF and HF spectrum (LF/HF ratio). Although not unequivocal, higher LF/HF implies higher SNS activity, while larger HF spectrum is suggested to reflect PNS activity [
11,
14,
15,
16].
In general, a higher HRV is suggested to be linked to decreased cardiovascular risk and consequently decreased mortality in adults [
17,
18,
19,
20], and with well-being and decreased mortality in newborns [
21,
22,
23]. Studies performed in newborns found an increased HRV in term compared to preterm newborns [
24,
25,
26], which might point to a more mature ANS, especially the PNS branch, in terms. It has been reported that PNS develops optimally after 37 weeks of postmenstrual age (PMA; the time elapsed between the first day of the last menstrual period and birth (gestational age) plus the time elapsed after birth (chronological age)) [
27,
28,
29]. Moreover, it has been suggested that HRV in newborns is affected by sleeping position. We have previously shown an increased HRV in supine compared to prone position suggesting that supine is more favourable regarding well-being of the newborns [
30].
The effect of caffeine on HRV remains controversial. Studies conducted in healthy adults mostly showed an increase in HRV after caffeine administration [
31,
32,
33,
34]. Only a few studies assessed HRV in newborns; even less is known about potential influence of caffeine on ANS as assessed by spectral analysis of HRV. To this end, the aim of our study was to evaluate the impact of caffeine treatment on HRV in newborns. In addition, we sought for a potential effect of caffeine treatment on breathing frequency (BF), the HR, the arterial oxygen saturation (SaO
2), and the body temperature (T). We correlated the parameters of HRV with PMA, during as well as after caffeine treatment. We hypothesized that due to its known effects on the CVS, caffeine might affect the HRV in newborns.
2. Materials and Methods
Patients
A prospective clinical intervention study was performed in 25 newborns with apnoea who had been admitted to the Neonatal Department of University Medical Centre Ljubljana, Division of Paediatrics and treated with caffeine citrate.
Newborns included in our study had the following (often coexisting) diagnoses: neonatal respiratory distress syndrome, mild dysmorphic features, mild non-optimal neurological signs, congenital heart defects, newborn jaundice, congenital anomalies of the kidney and urinary tract, bronchopulmonary dysplasia, anaemia of prematurity, pneumonia, grade 1 intraventricular haemorrhage, macrosomy, cryptorchidism, hypoglycaemia, intraamniotic bleeding, bilateral pneumothorax.
Exclusion criteria were severe perinatal hypoxia, infection, liver or renal insufficiency, neurological disorders, and congenital anomalies. Newborns whose data could not be used for spectral analysis due to artefacts of the recordings were also excluded from the study.
The caffeine treatment regimen consisted of a loading dose of 20 mg/kg body mass of caffeine citrate (i.e., 10 mg/kg caffeine), followed by a daily maintenance dose of 5 mg/kg of caffeine citrate (i.e., 2.5 mg/kg caffeine) after 24 hours. Recordings on caffeine treatment were collected 48 hours after the loading dose of caffeine. The newborns were treated for ten days on average, either orally or intravenously, regarding their clinical state. It has previously been shown that the route of administration does not affect the pharmacokinetics of caffeine as there is almost complete bioavailability after oral or intravenous administration [
35].
The study was approved by the National Medical Ethics Committee of the Republic of Slovenia (0120-458/2016-3 KME 67/09/16) and complies with the principles of the Declaration of Helsinki, European Convention on Human Rights and Biomedicine, and the Slovenian Code of Medical Deontology. Written parental consent was obtained for all participants. Our trial was retrospectively registered on April 27th, 2021, with a reference number NCT04869176.
Study Setting
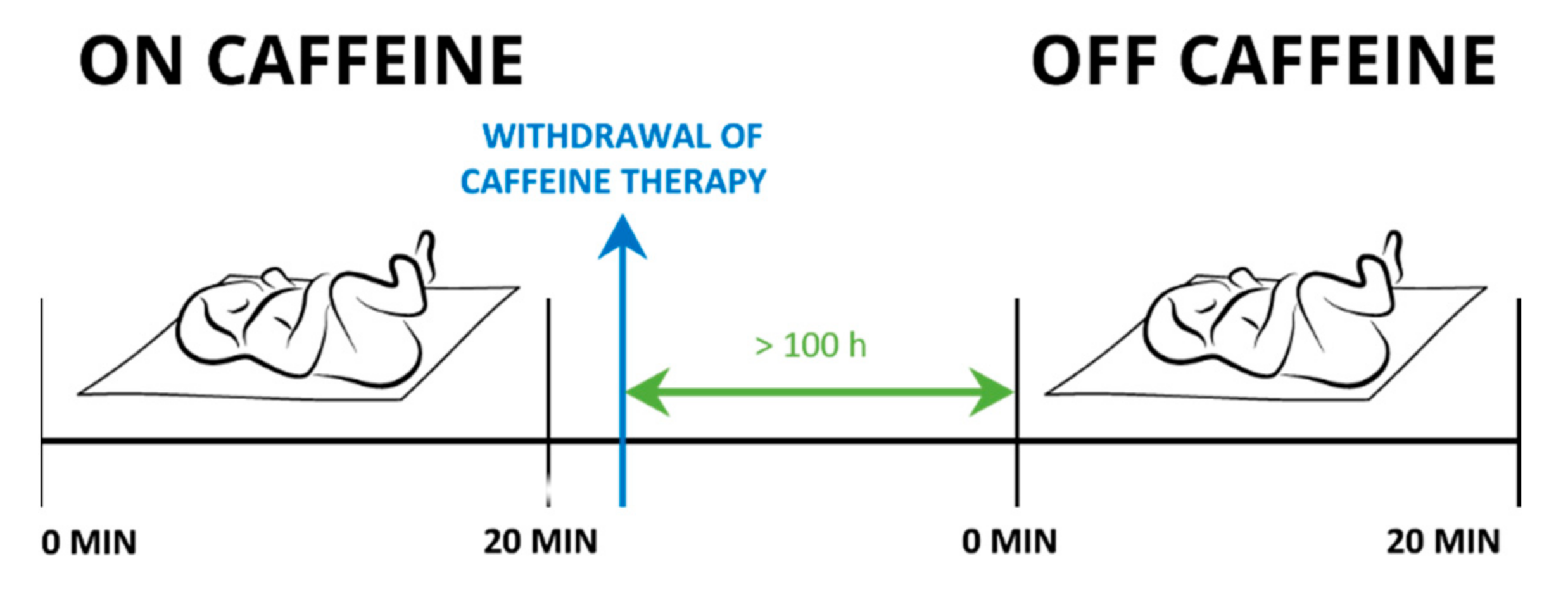
Each newborn underwent two measurement settings: the first set of experiments was performed while receiving the caffeine citrate. The second set of experiments was repeated 100 ± 26 hours after the treatment with caffeine had been withdrawn. These newborns served as controls. In eight newborns, we could not perform the control measurements due to technical difficulties.
Measurements were performed while the newborns were sleeping in a supine position. Their state of alertness was scored one or two as determined according to Prechtl [
36]. We simultaneously assessed newborn’s BF, SaO
2, T, and ECG, in a 20-minute interval (
Figure 1).
BF, SaO2 and T were measured three times during a suitable alertness state of the newborn and an average over three measurements was reported. BF was determined manually by observing the chest movement. SaO2 was measured by a pulse oximeter (IntelliVue MP 50, Philips, Germany) attached to the right hand. T was measured by a frontal non-contact infrared thermometer (Veratemp, USA).
As for the ECG tracing, five precordial ECG electrodes (ECG Holter, Vision 5L, Burdick, Milwaukee, USA) were attached to the newborn's chest prior to feeding. After feeding, the newborn was placed supine in a bed and a 20-minute tracing was obtained during the suitable alertness state. During the recordings, the heating was turned off to avoid potential interference with the ECG signal.
Data Analysis
Data were extracted from ECG recordings using programmes Vision Premier ver. 3.4 (Cardiac Science Corp., Waukesha, Wisconsin, USA) and Nevrokard (Nevrokard, Izola, Slovenia). For the analysis of each recording, a 5-minute segment was used. Before and after an ectopic beat, RR intervals (intervals between two subsequent R-waves of the QRS complex) were measured and replaced by two interpolated RR intervals, which were calculated from a proceeding and a succeeding sinus interval. If the programme (Nevrokard) did not correctly determine the R-wave due to the artefact, the exact position of the R wave was determined by the investigator who performed the spectral analysis. Data containing artefacts in more than 1% of the corresponding segment were removed from subsequent analyses.
Interpolated RR intervals were analysed by using fast Fourier transformation, a frequency domain linear method of assessing HRV. Fast Fourier transformation enclosed 1024 points, and a Hamming window was used for the calculation of spectral density. In addition to the total power spectrum (TP), two frequency bands were assessed: one for the LF (in the range of 0.04 – 0.15 Hz) and one for the HF (in the range of 0.15 – 1.0 Hz), LF and HF being represented also as ratio (LF/HF) and in normalized units (LFnu, HFnu) [
11]. We selected a segment which corresponded to the suitable alertness state of each newborn. Mean HR value was obtained from the corresponding analysed segment.
Statistical Analysis
Statistical analysis was performed by Microsoft Excel 2010 and IBM SPSS Statistics 24. Data distribution was tested by the Shapiro-Wilk normality test. Numeric variables are shown either as arithmetic mean and standard deviation (SD) for a normal (HR, BF, T), or median and interquartile range (IQR) for an abnormal distribution (SaO2, HRV parameters), respectively.
We compared variables according to the presence of caffeine ('on-' or 'off-caffeine').
We assessed potential correlation between PMA and HRV parameters regarding the presence of caffeine. We correlated the data from the first measurement (PMA 'on caffeine' and HRV parameters 'on caffeine'). The same comparisons were made for the second measurement (PMA 'off caffeine' and HRV parameters 'off caffeine').
Student's t-test was used for comparisons of normally distributed variables, and Wilcoxon signed-rank test for non-normally distributed data. The correlation between HRV parameters and PMA was tested with the Pearson correlation coefficient. A significance level was set at p ≤ 0.05.
3. Results
The demographic data and baseline characteristics of the newborns are shown in
Table 1. The newborns had been treated with caffeine after being diagnosed with neonatal apnoea. The treatment was discontinued at 37 ± 2 weeks of PMA. In the subsequent analysis on potential effects of caffeine, we included only 17 newborns in whom the measurements could be repeated after withdrawal of caffeine.
The Effect of Caffeine on the Heart Rate, Breathing Frequency, Arterial Oxygen Saturation and Body Temperature
The BF was significantly higher during caffeine treatment. No significant differences in the HR, SaO
2 or T were found between the treatment ('on caffeine') and post-treatment ('off caffeine') (
Table 2).
The Effect of Caffeine on HRV
No association between caffeine treatment and any of the HRV parameters were found (
Table 3).
The Correlation between Postmenstrual Age and the Parameters of HRV
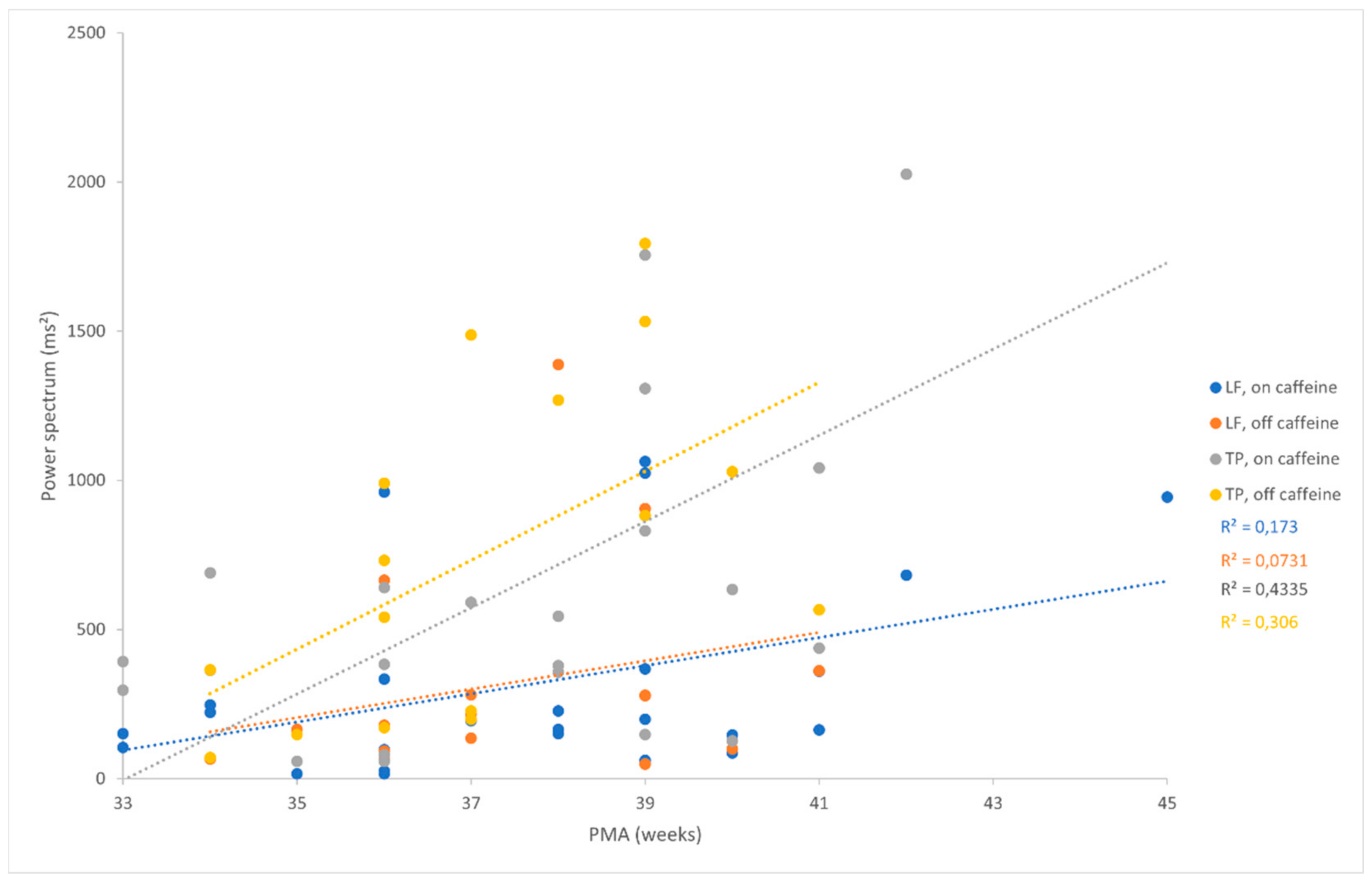
We found a positive correlation between some of the HRV parameters and PMA (
Figure 2). During caffeine treatment, we found a moderate positive correlation between PMA and LF (Pearson correlation coefficient = 0.42; p = 0.039), and between PMA and TP (Pearson correlation coefficient = 0.41; p = 0.044). After the cessation of the caffeine treatment, a positive and moderately strong significant correlation was found between PMA and LF (Pearson correlation coefficient = 0.57; p = 0.017), and between PMA and TP (Pearson correlation coefficient = 0.5; p = 0.041).
4. Discussion
The main finding of our study is that treatment with caffeine does not affect any of the spectral indices of HRV in newborns. Moreover, caffeine did not induce any significant changes in HR, SaO
2 or T but did increase BF. Apparently, the maintenance dose of 2.5 mg/kg body mass to treat apnoea is not sufficient to exert any measurable effects on the above parameters, except for an expected increase in BF. To the best of our knowledge, our study is the first to have assessed the effect of caffeine on HRV in newborns at 37 weeks PMA. The preterm newborns included in the only two available similar studies were significantly younger [
1,
9].
The only parameter that was affected by caffeine was BF. The increase of BF after the caffeine treatment was expected, as caffeine is a known stimulant of the respiratory centre.
In our study, the values of SaO2 were comparable during and after the cessation of caffeine treatment. As the values of SaO2 were mostly in physiological limits, caffeine could have hardly induced an additional increase.
Caffeine reportedly increases the rate of metabolism and could thus potentially induce an increase in T. Nevertheless, the maintenance dose used in our study apparently was not sufficient to induce any measurable effects of caffeine on T since we found no differences in T during versus after the caffeine treatment. In fact, this is a favourable outcome regarding the interpretation of the HRV data; an elevated T namely increases HR [
37] and if this had been the case in our study, it would have interfered with the interpretation of the effects of caffeine on HRV. Moreover, increased HR implies additional burden to the heart as it significantly increases the heart work [
38].
In our study, the HR and HRV parameters obtained during caffeine treatment were comparable to the parameters after discontinuation of treatment. Since cardiogenic effects described in literature in terms of tachycardia only occur when applying toxic doses of caffeine (plasma level exceeding 20 mg/L) [
39], we may conclude that our maintenance dose was not sufficient to impact the HR in newborns. Our results regarding the effect of caffeine on HR and HRV are in accordance with the study of Ulanovsky et al. who also didn’t show any impact of caffeine (applied in a loading dose 15 - 20 mg/kg/day, followed by a maintenance dose of 5 - 10 mg/kg/day) either on the HR or HRV in premature newborns [
1]. Yet, their sample may not be comparable to ours, as the newborns in their study [
1] as well as in the Huvanandana's et al. [
9] were younger than the newborns in our study (gestational age 30.3 ± 2.5 weeks and 27.0 (23.6 - 33.3) weeks compared to 34 ± 5 weeks in our study) and also had significantly lower birth weight (1397 ± 458 g and 934 (552 - 2100) g compared to 2353 ± 914 g in our study) [
1,
9]. On the other hand, Huvanandana et al. who compared the results of the linear and non-linear analyses of HRV in preterm newborns prior to and two hours after a loading dose of caffeine, reported an increased HRV after caffeine administration when using non-linear, but not when using linear modelling, as it was analysed in our study [
9]. They suspected that linear metrics might not adequately capture potentially altered dynamics in the HR control [
9]. Moreover, the influences of caffeine on the heart and the activity of ANS seem controversial as caffeine has been shown to increase the HF component of HRV in adults, apparently increasing the PNS activity [
31,
40]. On the other hand, caffeine has been suggested to modulate the response to stress by increasing the levels of circulating catecholamines and cortisol, both of which strongly impact the heart and the sinoatrial node [
4].
Although all parameters in our study were measured after the newborns were fed, and during the first 20 minutes of sleep, the HRV measurements could also be dependent on the sleep phase which we did not assess. Namely, the sleep onset in newborns corresponds to a REM phase, one sleep cycle lasts for about 50 minutes, and consists of equal consequent proportions of REM and non-REM sleep [
41,
42]. Yiallourou et al., who assessed HRV in preterm and term newborns, found significantly increased values of LF/HF, and both LF and TP spectra during REM compared to non-REM sleep [
25]. Similarly, Takatani et al. showed higher LF, HF and LF/HF during REM than during non-REM in newborns [
26]. To this end, assessing the phase of sleep in our newborns might be valuable for further interpretation.
We found a positive correlation between HRV (LF and TP but not HF or LF/HF) and PMA, irrespective of caffeine treatment. Anyhow, HRV is known to be dependent on mean HR – the lower the mean HR the higher the HRV [
27]. In our study, the HR did not significantly change during the two measurements. A positive correlation between PMA and HRV supports the idea of ANS maturation with increasing age; yet, due to the narrow time frame (100 ± 26 hours) between the consecutive measurements the results should be interpreted with caution. Our results are accordant with our previous study [
30] and with the study of Sahni et al., who showed a significant increase in HRV with increasing PMA in growing low birth weight infants, and implied an important role of ANS maturation in the control of cardiac activity [
43]. Based on our observation, it seems that the maturation of ANS proceeds independently of caffeine. Contrary to our hypothesis and our observation from our previous study [
30], where the study population was on average four weeks older, the HF did not significantly increase with increasing PMA in the present study. The discrepant findings could be due to limitations of the spectral analysis. In the present study, the mean HR was about 139 beats per minute. The Nyquist frequency of our sample was therefore 69.5 per minute, which is approximately 1.16 Hz. The upper limit of HF spectrum, used for our analysis, was 1 Hz. Anything above this value was therefore a part of HF score that fell out of the analysis.
It is also important to note that in humans, if the BF is outside the HF band, the power of HF cannot be considered as a marker of vagal modulation. Although in the developing newborn we may expect that the PNS is still maturating, we probably should use the same rationale for the definition of the HF band. The highest BF measured in our study was 56 beats per minute (with SD of 12) which is borderline for the upper limit of the HF band. This indicates a limitation of the use of the HF band in our sample.
Besides, the influence of respiratory sinus arrhythmia on HRV should be taken into consideration. As PNS has a much shorter delay than SNS on the heart to covariate with respiration, PNS presents the main factor contributing to respiratory sinus arrhythmia. Many reports have confirmed that a considerable part of HRV, affected by PNS, actually arises from the respiratory sinus arrhythmia [
14,
27], yet this phenomenon has not been adequately studied in newborns so far. We can speculate that PNS, not fully developed in newborns, thus contributes less to HRV. It is also possible that HF did not significantly increase with PMA due to higher BF in newborns as compared to BF in adults. Moreover, our newborns presented with neonatal apnoea which is related to respiratory disorder or abnormal breathing patterns; therefore, their breathing pattern might have differently affected the HF than the pattern in healthy newborns.
The limitation of our study is a small sample size with rather heterogenous underlying diagnoses; yet, it was homogenous regarding PMA, haemodynamic and breathing parameters assessed. Another limitation is a rather long half-life of caffeine in preterm newborns – 87 ± 25 hours at 35 weeks PMA [
44]. A prolonged half-life of caffeine could persist up to 38 weeks PMA due to immature liver function [
45]. Accordingly, caffeine concentration might have been elevated in some newborns by the time of the control measurements. Unfortunately, we could not exceed this time frame due to limited duration of hospitalizations; however, by ensuring that 100 hours on average passed between the last dose of caffeine and the second measurement, this option seems rather unlikely. It would be more meaningful to assess the relationship between caffeine and HRV based on caffeine concentrations in blood rather than just on caffeine dose, yet our newborns did not have central venous access and it would therefore be unethical to collect the blood samples for the purposes of our study only.
As stated above, HF values above 1 Hz could not be included in the analyses due to the upper limit of the frequency spectrum, therefore narrowing the available data.
Additional insight into potential effect of caffeine on haemodynamic parameters would be obtained by continuous measurement of arterial blood pressure which was not done in our study. Our study could be improved by continuous measurement of BF, as well as blood pressure monitoring, and by also measuring EEG to determine the phase of sleep which might have impacted the outcome.
Finally, a note should be given on the study design: a more reliable estimation of potential impact of caffeine on HRV would be obtained causally, i.e., by first assessing the parameters before caffeine was applied, and subsequently during the application of caffeine. As our newborns needed a prompt treatment for apnoea, such design was not feasible. Another approach would be to check the effect of caffeine in healthy newborns which would enable a successive regimen of measurements; yet the application of caffeine in healthy newborns is not acceptable from ethical point of view.
5. Conclusions
This is the first study assessing the impact of caffeine on HRV in newborns at 37 weeks PMA. The results of our study did not show any effects of caffeine on any of the parameters of HRV, but increased the BF, as expected. We might speculate that the maintenance dose of caffeine in newborns is too low to affect the HR and HRV. Furthermore, we showed a positive correlation between HRV and PMA, regardless of caffeine intake, pointing to ANS maturation with age, that apparently is not subjected to any alterations induced by a standard treatment dose of caffeine. Considering the data on the physiological effects of caffeine shown in this as well as in some other studies, we may imply suitability of caffeine use in the prescribed dosage in newborns.
Author Contributions
Conceptualization, H.L., E.R., J.F., M.K. and P.F.; Data curation, E.R. and J.F.; Formal analysis, E.R., J.F. and M.K.; Investigation, E.R. and J.F; Methodology, P.F.; Project administration, H.L., E.R., J.F. and P.F.; Resources, M.K. and P.F.; Software, M.K.; Supervision, H.L., M.K. and P.F.; Validation, H.L., E.R., J.F., M.K. and P.F.; Visualization, H.L., E.R., J.F., M.K. and P.F.; Writing – original draft, E.R., J.F. and P.F.; Writing – review & editing, H.L., E.R., J.F., M.K. and P.F.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the National Medical Ethics Committee of the Republic of Slovenia (0120-458/2016-3 KME 67/09/16).
Informed Consent Statement
Written informed parental consent was obtained for all subjects involved in the study.
Acknowledgments
We thank the statistician Mrs Vanja Erčulj for providing thorough statistical analysis.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ulanovsky, I.; Haleluya, N.S.; Blazer, S.; Weissman, A. The Effects of Caffeine on Heart Rate Variability in Newborns with Apnea of Prematurity. J Perinatol 2014, 34, 620–623. [Google Scholar] [CrossRef]
- Nehlig, A.; Daval, J.L.; Debry, G. Caffeine and the Central Nervous System: Mechanisms of Action, Biochemical, Metabolic and Psychostimulant Effects. Brain Res Brain Res Rev 1992, 17, 139–170. [Google Scholar] [CrossRef]
- Long, J.-Y.; Guo, H.-L.; He, X.; Hu, Y.-H.; Xia, Y.; Cheng, R.; Ding, X.-S.; Chen, F.; Xu, J. Caffeine for the Pharmacological Treatment of Apnea of Prematurity in the NICU: Dose Selection Conundrum, Therapeutic Drug Monitoring and Genetic Factors. Front Pharmacol 2021, 12, 681842. [Google Scholar] [CrossRef]
- Echeverri, D.; Montes, F.R.; Cabrera, M.; Galán, A.; Prieto, A. Caffeine’s Vascular Mechanisms of Action. Int J Vasc Med 2010, 2010, 834060. [Google Scholar] [CrossRef]
- Daly, J.W. Caffeine Analogs: Biomedical Impact. Cell Mol Life Sci 2007, 64, 2153–2169. [Google Scholar] [CrossRef]
- Mahmud, A.; Feely, J. Acute Effect of Caffeine on Arterial Stiffness and Aortic Pressure Waveform. Hypertension 2001, 38, 227–231. [Google Scholar] [CrossRef]
- Early Caffeine in Preterm Neonates. Available online: https://clinicaltrials.gov/ct2/show/NCT03086473 (accessed 28th March, 2023).
- Shrestha, B.; Jawa, G. Caffeine Citrate - Is It a Silver Bullet in Neonatology? Pediatr Neonatol 2017, 58, 391–397. [Google Scholar] [CrossRef]
- Huvanandana, J.; Thamrin, C.; McEwan, A.L.; Hinder, M.; Tracy, M.B. Cardiovascular Impact of Intravenous Caffeine in Preterm Infants. Acta Paediatr 2019, 108, 423–429. [Google Scholar] [CrossRef]
- G. Natarajan, J. M. Lopes, and J. V. Aranda, Pharmacologic Treatment of Neonatal Apnea, in Neonatal and Pediatric Pharmacology: Therapeutic Principles in Practice, 4th ed.; S. J. Yaffe and J. V. Aranda Eds; Lippincott Williams & Wilkins: Philadelphia; USA, 2011, ch. 20, pp. 241-251.
- Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Heart Rate Variability: Standards of Measurement, Physiological Interpretation and Clinical Use. Circulation 1996, 93, 1043–1065. [Google Scholar] [CrossRef]
- ChuDuc, H.; NguyenPhan, K.; NguyenViet, D. A Review of Heart Rate Variability and Its Applications. APCBEE Procedia 2013, 7, 80–85. [Google Scholar] [CrossRef]
- Berntson, G.G.; Bigger, J.T.J.; Eckberg, D.L.; Grossman, P.; Kaufmann, P.G.; Malik, M.; Nagaraja, H.N.; Porges, S.W.; Saul, J.P.; Stone, P.H.; et al. Heart Rate Variability: Origins, Methods, and Interpretive Caveats. Psychophysiology 1997, 34, 623–648. [Google Scholar] [CrossRef]
- Appelhans, B.M.; Luecken, L.J. Heart Rate Variability as an Index of Regulated Emotional Responding. Review of General Psychology 2006, 10, 229–240. [Google Scholar] [CrossRef]
- Billman, G.E. The LF/HF Ratio Does Not Accurately Measure Cardiac Sympatho-Vagal Balance. Front Physiol 2013, 4, 26. [Google Scholar] [CrossRef]
- Reyes del Paso, G.A.; Langewitz, W.; Mulder, L.J.M.; van Roon, A.; Duschek, S. The Utility of Low Frequency Heart Rate Variability as an Index of Sympathetic Cardiac Tone: A Review with Emphasis on a Reanalysis of Previous Studies. Psychophysiology 2013, 50, 477–487. [Google Scholar] [CrossRef]
- Billman, G.E. Heart Rate Variability - a Historical Perspective. Front Physiol 2011, 2, 86. [Google Scholar] [CrossRef]
- Soares-Miranda, L.; Sattelmair, J.; Chaves, P.; Duncan, G.E.; Siscovick, D.S.; Stein, P.K.; Mozaffarian, D. Physical Activity and Heart Rate Variability in Older Adults: The Cardiovascular Health Study. Circulation 2014, 129, 2100–2110. [Google Scholar] [CrossRef]
- Lai, S.; Mangiulli, M.; Perrotta, A.M.; Di Lazzaro Giraldi, G.; Testorio, M.; Rosato, E.; Cianci, R.; Gigante, A. Reduction in Heart Rate Variability in Autosomal Dominant Polycystic Kidney Disease. Kidney Blood Press Res 2019, 44, 1142–1148. [Google Scholar] [CrossRef]
- Goldberger, A.L.; Amaral, L.A.N.; Hausdorff, J.M.; Ivanov, P.C.; Peng, C.-K.; Stanley, H.E. Fractal Dynamics in Physiology: Alterations with Disease and Aging. Proc Natl Acad Sci U S A 2002, 99 Suppl 1, 2466–2472. [Google Scholar] [CrossRef]
- Tuzcu, V.; Nas, S.; Ulusar, U.; Ugur, A.; Kaiser, J.R. Altered Heart Rhythm Dynamics in Very Low Birth Weight Infants with Impending Intraventricular Hemorrhage. Pediatrics 2009, 123, 810–815. [Google Scholar] [CrossRef]
- Aarimaa, T.; Oja, R. Transcutaneous PO2, PCO2 and Heart Rate Patterns during Normal Postnatal Adaptation and Respiratory Distress. Early Hum Dev 1988, 16, 3–11. [Google Scholar] [CrossRef]
- Hörnchen, H.; Betz, R.; Kotlarek, F.; Roebruck, P. Microprocessor-Based Long Term Cardiorespirography. II. Status Evaluation in Term and Premature Newborns. J Perinat Med 1983, 11, 32–42. [Google Scholar] [CrossRef] [PubMed]
- Selig, F.A.; Tonolli, E.R.; da Silva, É.V.C.M.; de Godoy, M.F. Heart Rate Variability in Preterm and Term Neonates. Arq. Bras. Cardiol. 2011, 96, 443–449. [Google Scholar] [CrossRef]
- Yiallourou, S.R.; Witcombe, N.B.; Sands, S.A.; Walker, A.M.; Horne, R.S.C. The Development of Autonomic Cardiovascular Control Is Altered by Preterm Birth. Early Hum Dev 2013, 89, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Takatani, T.; Takahashi, Y.; Yoshida, R.; Imai, R.; Uchiike, T.; Yamazaki, M.; Shima, M.; Nishikubo, T.; Ikada, Y.; Fujimoto, S. Relationship between Frequency Spectrum of Heart Rate Variability and Autonomic Nervous Activities during Sleep in Newborns. Brain and Development 2018, 40, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, S.; Silva, M.J.; Guimarães, H. Autonomic Nervous System in Newborns: A Review Based on Heart Rate Variability. Childs Nerv Syst 2017, 33, 1053–1063. [Google Scholar] [CrossRef]
- Gagnon, R.; Campbell, K.; Hunse, C.; Patrick, J. Patterns of Human Fetal Heart Rate Accelerations from 26 Weeks to Term. Am J Obstet Gynecol 1987, 157, 743–748. [Google Scholar] [CrossRef] [PubMed]
- Engle, W.A. Age Terminology during the Perinatal Period. Pediatrics 2004, 114, 1362–1364. [Google Scholar] [CrossRef]
- Fister, P.; Nolimal, M.; Lenasi, H.; Klemenc, M. The Effect of Sleeping Position on Heart Rate Variability in Newborns. BMC Pediatrics 2020, 20, 156. [Google Scholar] [CrossRef]
- Monda, M.; Viggiano, A.; Vicidomini, C.; Viggiano, A.; Iannaccone, T.; Tafuri, D.; De Luca, B. Espresso Coffee Increases Parasympathetic Activity in Young, Healthy People. Nutr Neurosci 2009, 12, 43–48. [Google Scholar] [CrossRef]
- Notarius, C.F.; Floras, J.S. Caffeine Enhances Heart Rate Variability in Middle-Aged Healthy, But Not Heart Failure Subjects. J Caffeine Res 2012, 2, 77–82. [Google Scholar] [CrossRef]
- Dömötör, Z.; Szemerszky, R.; Köteles, F. Subjective and Objective Effects of Coffee Consumption - Caffeine or Expectations? Acta Physiol Hung 2015, 102, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Yeragani, V.K.; Krishnan, S.; Engels, H.J.; Gretebeck, R. Effects of Caffeine on Linear and Nonlinear Measures of Heart Rate Variability before and after Exercise. Depression and Anxiety 2005, 21, 130–134. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Hady, H.; Nasef, N.; Shabaan, A.E.; Nour, I. Caffeine Therapy in Preterm Infants. World J Clin Pediatr 2015, 4, 81–93. [Google Scholar] [CrossRef] [PubMed]
- Prechtl, H.F. The Behavioural States of the Newborn Infant (a Review). Brain Res 1974, 76, 185–212. [Google Scholar] [CrossRef] [PubMed]
- Davies, P.; Maconochie, I. The Relationship between Body Temperature, Heart Rate and Respiratory Rate in Children. Emerg Med J 2009, 26, 641–643. [Google Scholar] [CrossRef]
- Koskela, J.K.; Tahvanainen, A.; Haring, A.; Tikkakoski, A.J.; Ilveskoski, E.; Viitala, J.; Leskinen, M.H.; Lehtimäki, T.; Kähönen, M.A.; Kööbi, T.; et al. Association of Resting Heart Rate with Cardiovascular Function: A Cross-Sectional Study in 522 Finnish Subjects. BMC Cardiovasc Disord 2013, 13, 102. [Google Scholar] [CrossRef]
- S. B. Ainsworth, Drugs and the body. In Neonatal Formulary 7: Drug Use in Pregnancy and the First Year of Life, 7 ed.; John Wiley & Sons Inc, Chichester, West Sussex, UK, 2015, pp. 12-3.
- Koenig, J.; Jarczok, M.N.; Kuhn, W.; Morsch, K.; Schäfer, A.; Hillecke, T.K.; Thayer, J.F. Impact of Caffeine on Heart Rate Variability: A Systematic Review. Journal of Caffeine Research 2013, 3, 22–37. [Google Scholar] [CrossRef]
- Normal Human Sleep at Different Ages: Infants to Adolescents: https://www.researchgate.net/publication/267856125_Normal_Human_Sleep_at_Different_Ages_Infants_to_Adolescents (accessed on 25th March 2023).
- T. F. Anders, A. Sadeh, and V. Appareddy, Normal sleep in neonates and children. In Principles and Practice of Sleep Medicine in the Child, 2 ed. R. Ferber and M. Kryger Eds. Philadelphia: W. B. Saunders, Philadelphia, USA, 1995; pp. 7-18.
- Sahni, R.; Schulze, K.F.; Kashyap, S.; Ohira-Kist, K.; Myers, M.M.; Fifer, W.P. Body Position, Sleep States, and Cardiorespiratory Activity in Developing Low Birth Weight Infants. Early Hum Dev 1999, 54, 197–206. [Google Scholar] [CrossRef]
- Doyle, J.; Davidson, D.; Katz, S.; Varela, M.; Demeglio, D.; DeCristofaro, J. Apnea of Prematurity and Caffeine Pharmacokinetics: Potential Impact on Hospital Discharge. J Perinatol 2016, 36, 141–144. [Google Scholar] [CrossRef]
- Aranda, J.V.; Cook, C.E.; Gorman, W.; Collinge, J.M.; Loughnan, P.M.; Outerbridge, E.W.; Aldridge, A.; Neims, A.H. Pharmacokinetic Profile of Caffeine in the Premature Newborn Infant with Apnea. J Pediatr 1979, 94, 663–668. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).