Submitted:
25 May 2023
Posted:
26 May 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
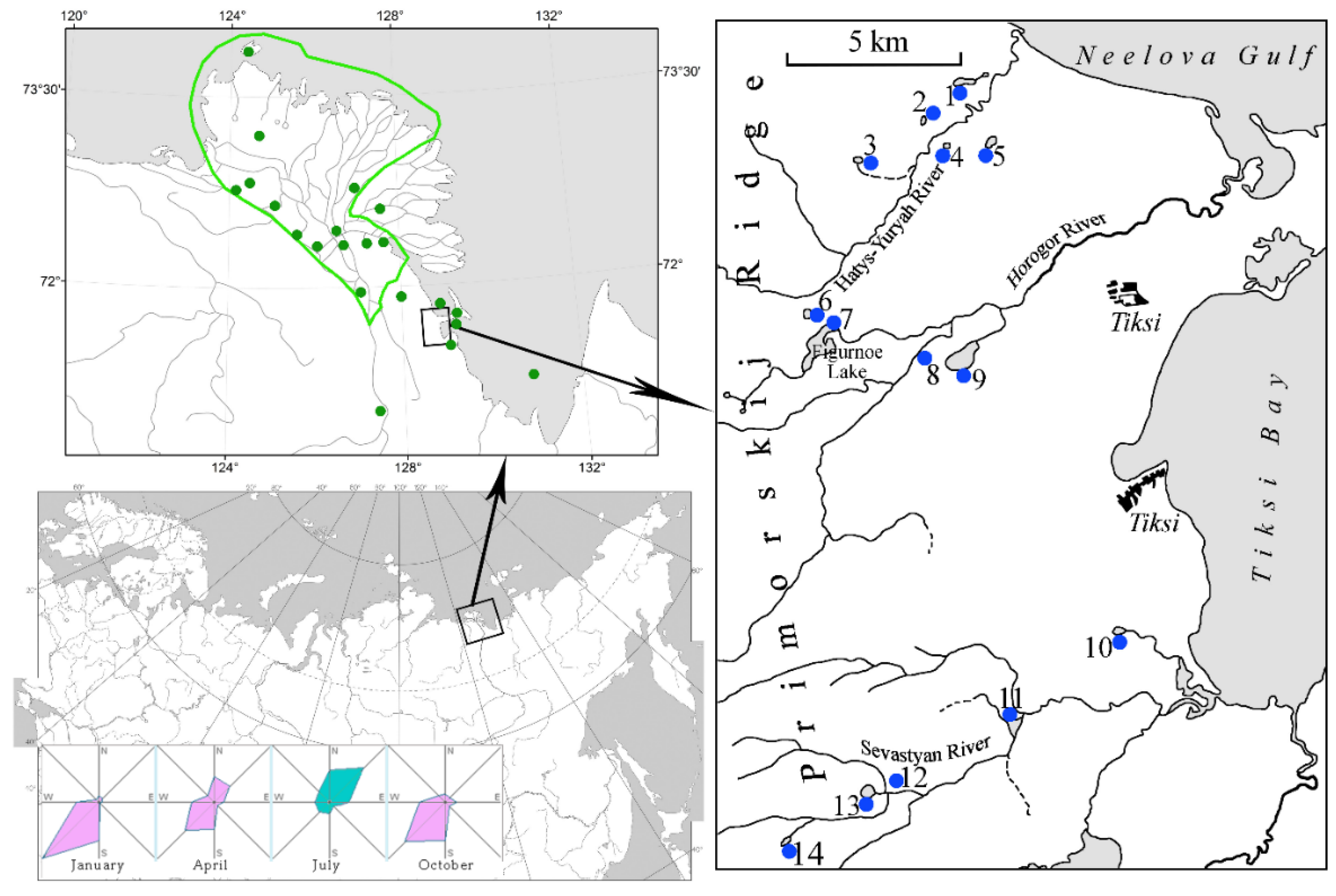
2.1. Description of study area

2.2. Sampling
2.3. Diatom analysis
3. Results
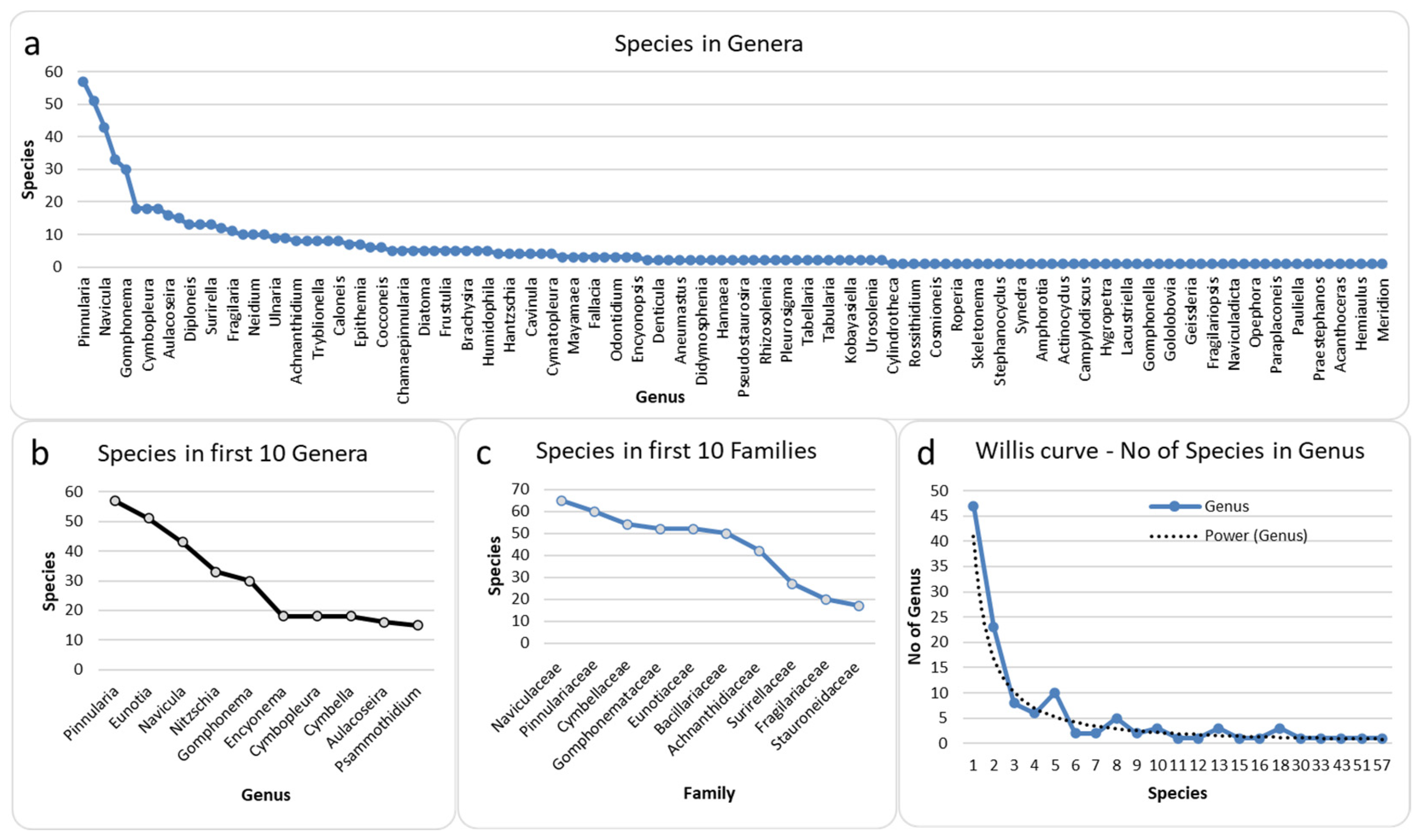
3.1. Taxonomical and floristic analysis of diatoms
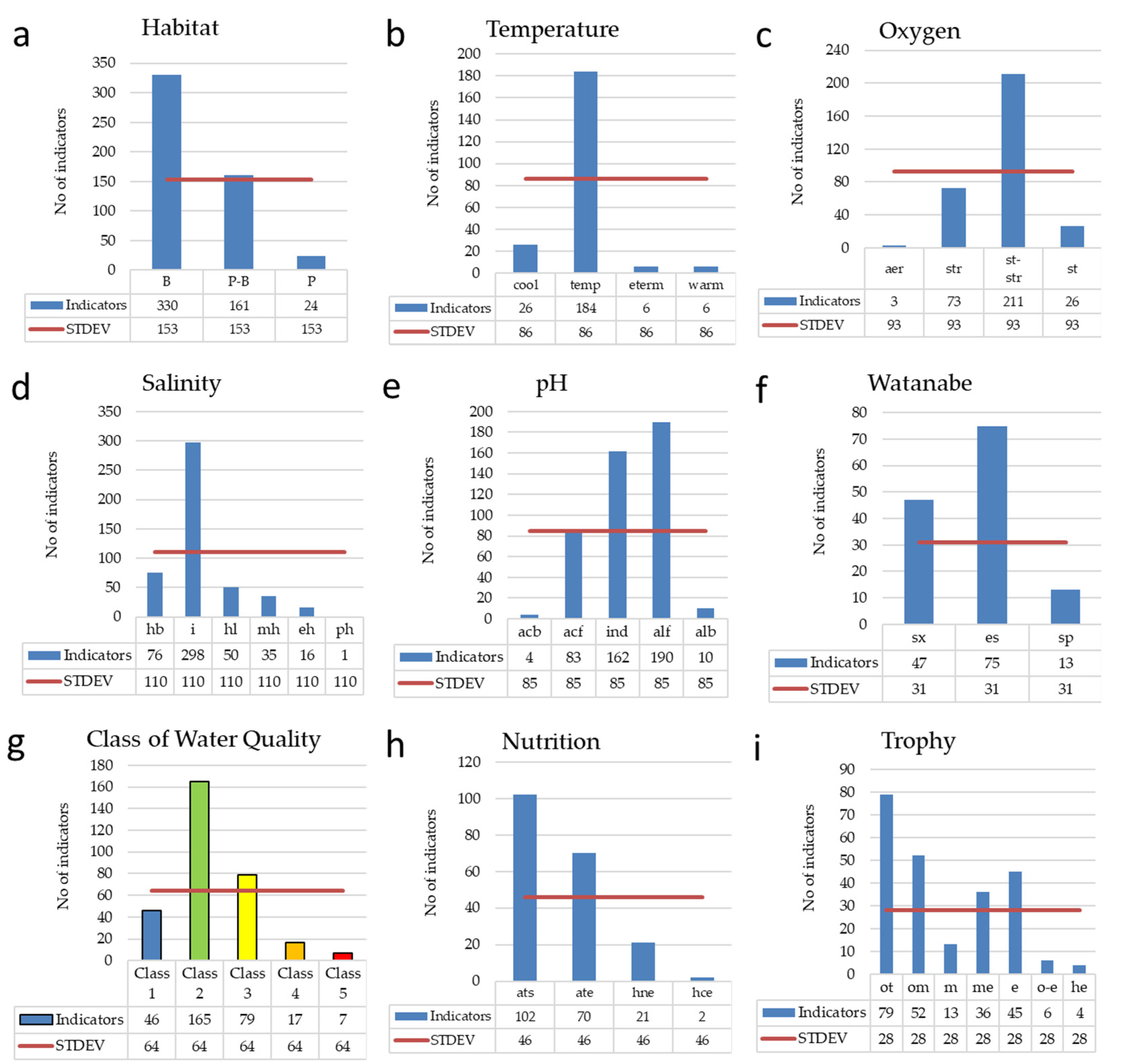
3.2. Bioindicators and ecological preferences of diatoms
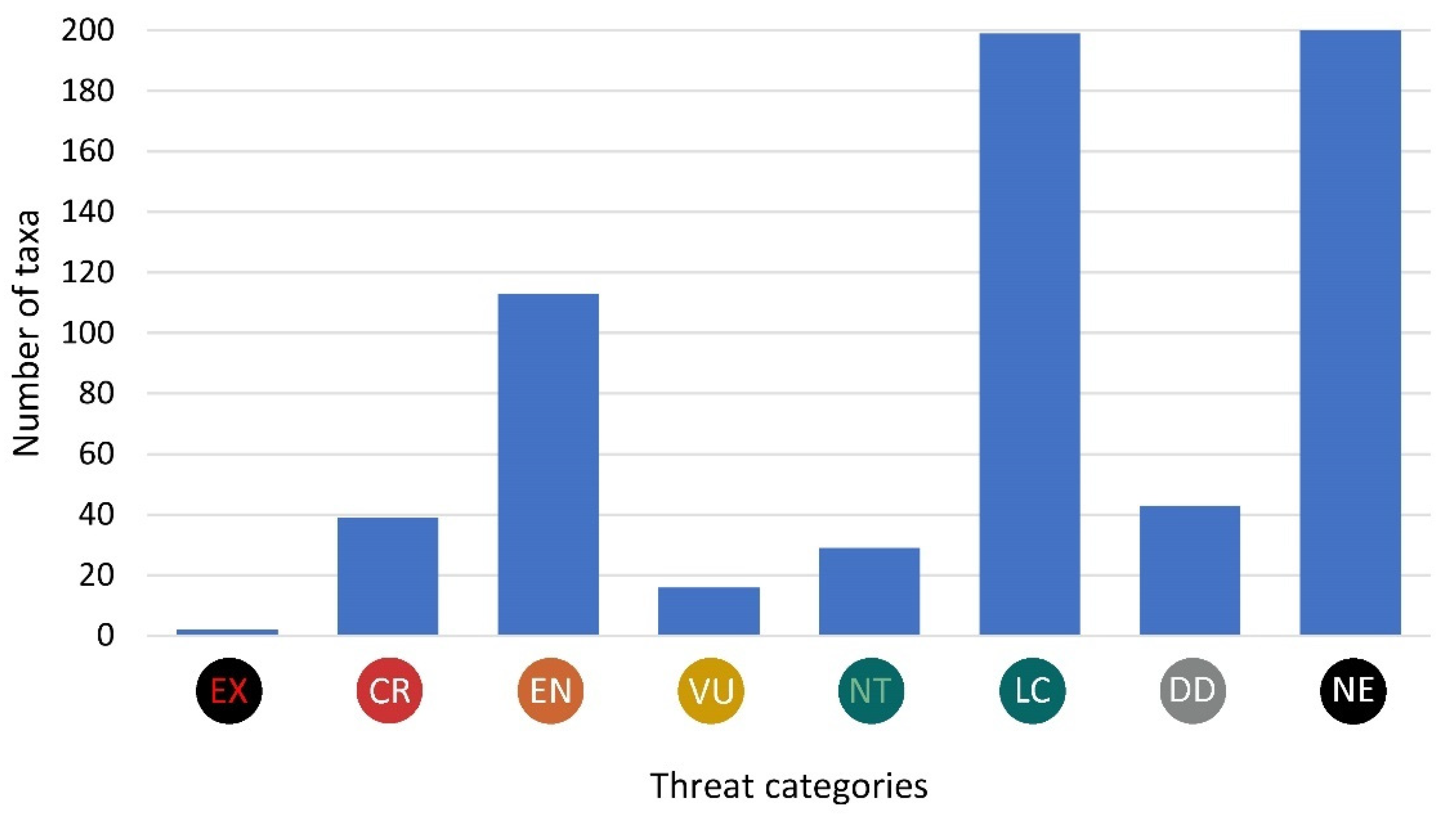
3.3. Species in Threat Categories
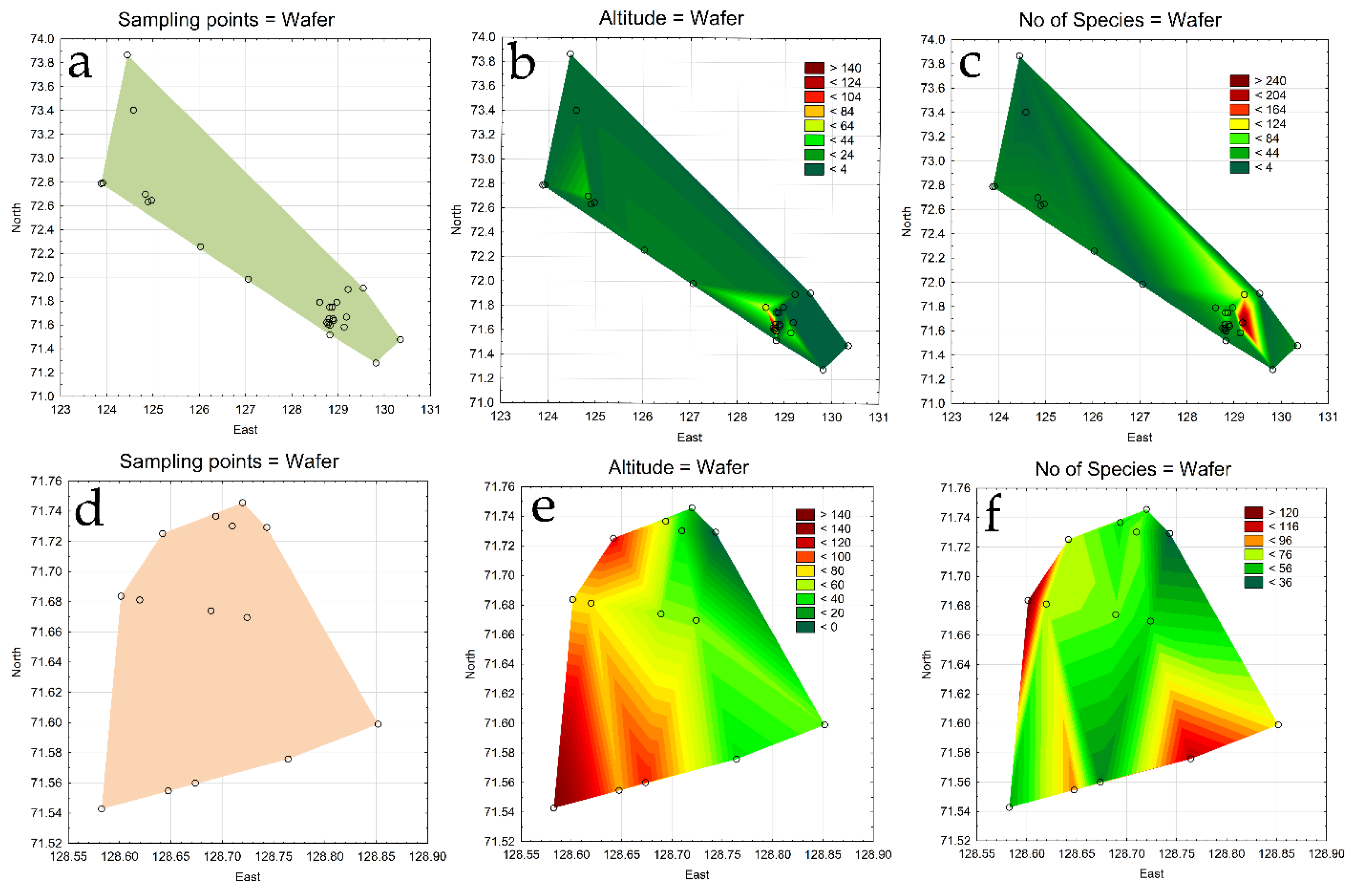
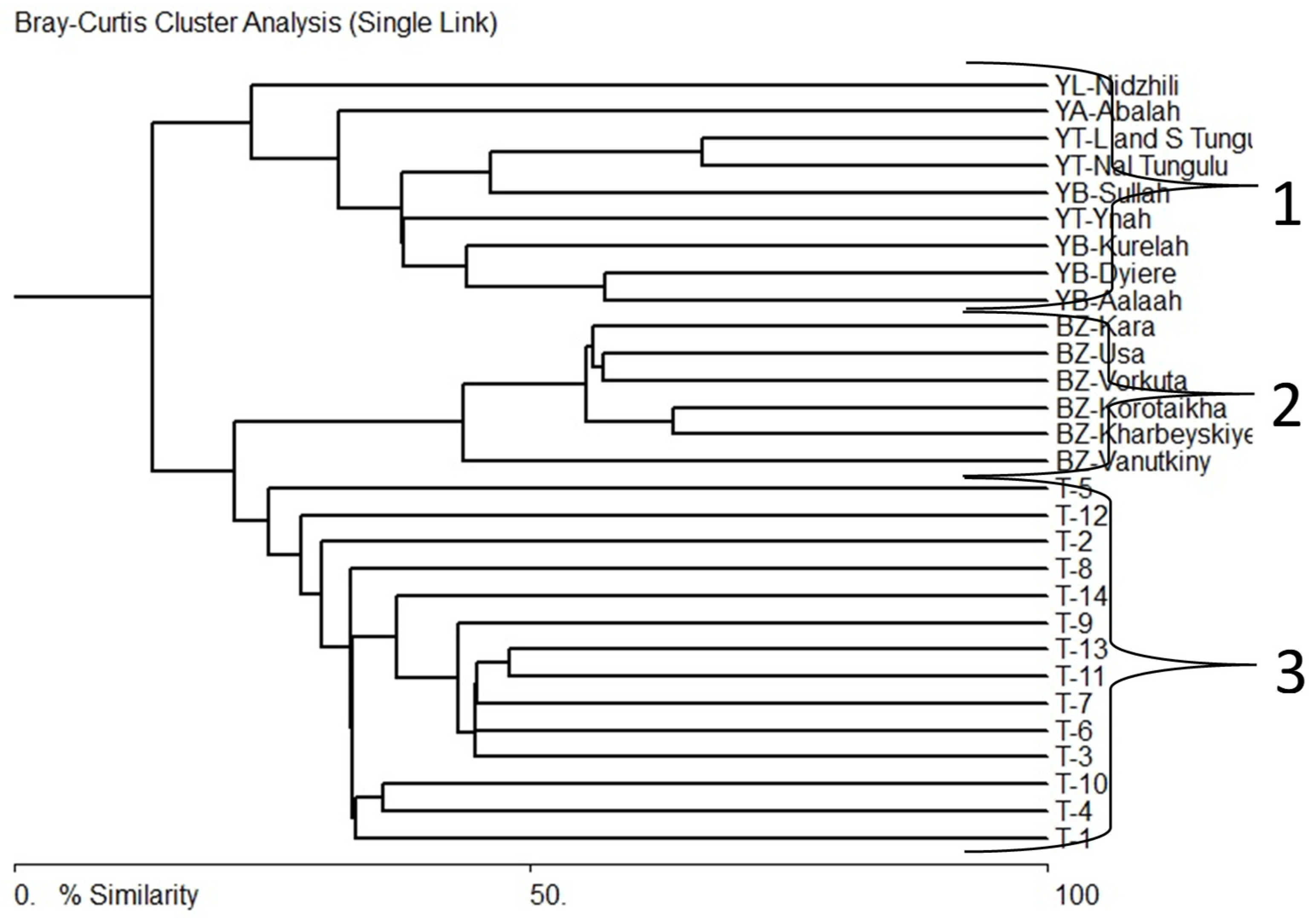
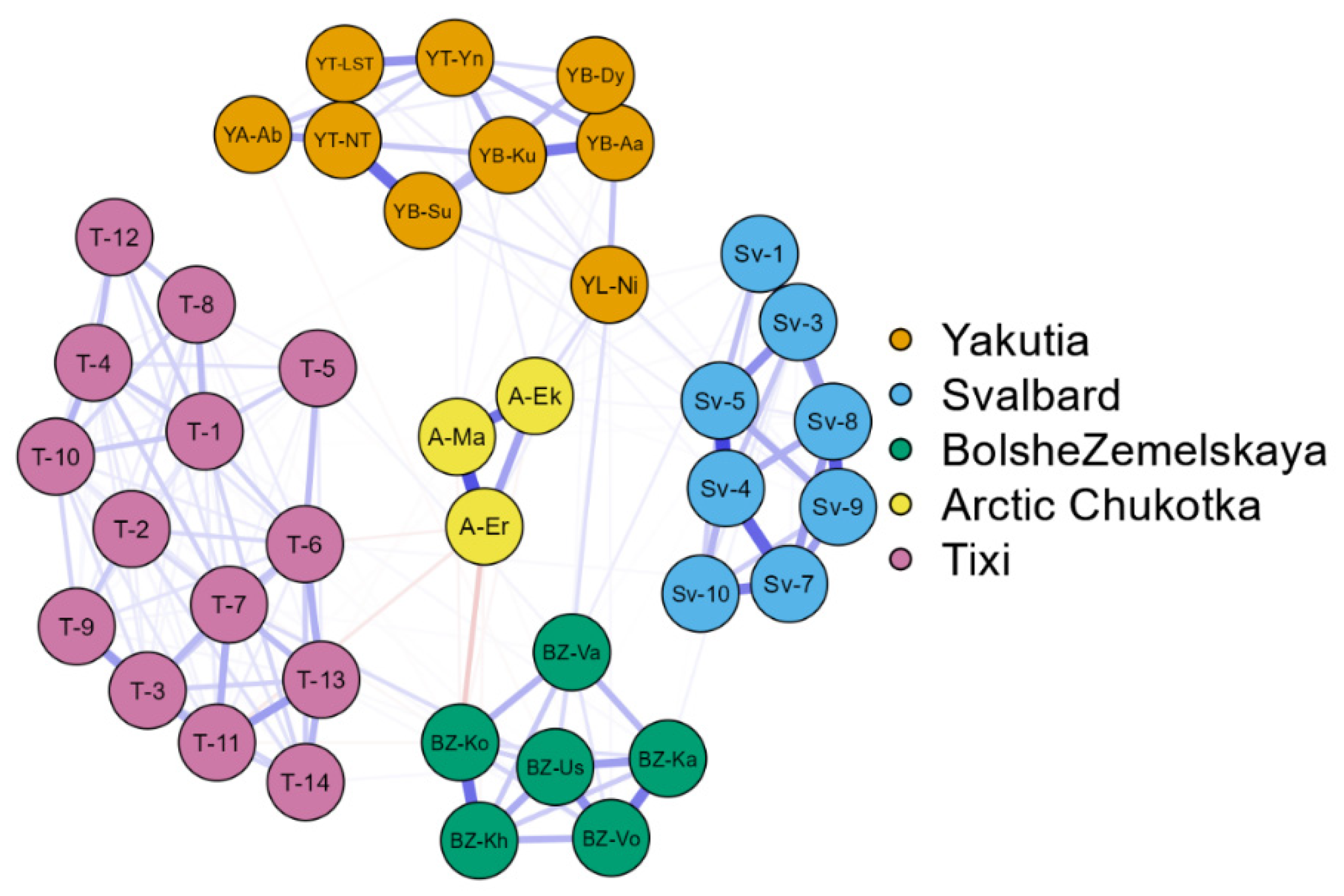
3.4. Comparative floristic
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barinova, S.; Stenina, A. Diatom diversity and ecological variables in the Arctic lakes of the Kostyanoi Nos Cape (Nenetsky Natural Reserve, Russian North). Plant Biosystems - An International Journal Dealing with all Aspects of Plant Biology 2013, 147(2), pp. 397-410. [CrossRef]
- Stenina, A.S. Diatoms (Bacillariophyta) in the lakes of the east of the Bolshezemelskaya tundra; Institute of Biology, Komi Scientific Center, Ural Branch, Russian Academy of Sciences: Syktyvkar, Russia, 2009; 176 p. (In Russian).
- Kharitonov, V.G. Diatoms of the benthos of water bodies of Wrangel Island. News of taxonomy of lower plants 1981, 18, pp. 33–39.
- Pinseel, E.; Van de Vijver, B.; Kavan, J.; Verleyen, E.; Kopalová, K. Diversity, ecology and community structure of the freshwater littoral diatom flora from Petuniabukta (Spitsbergen). Polar Biol 2017, 40, pp. 533–551. [CrossRef]
- Picińska-Fałtynowicz, J. Freshwater benthic diatoms from the south-western part of the Hornsund fiord area, SW Spitsbergen. Polar Research 1988, 6(1), pp. 19-34. [CrossRef]
- Protected Areas of Russia. Red Books. Red Book of the Republic of Sakha (Yakutia). Developer FGBU "AARI", Laboratory of geoinformation technologies. Available online: http://www.oopt.aari.ru/rbdata/75/plant.
- Kharitonov, V.G. Diatom algae of fresh water bodies. In Ecology of the Basin of the r. Amguema; Russian acad. Sciences, Far Eastern Department, Institute of Biological Problems of the North. Publisher: Vladivostok, Russia, 1993, pp. 47–81. (In Russian).
- Kharitonov, V.G. Diatoms (Bacillariophyseae) in the Sediments of Three Mountain, Oligotrophic Lakes in the Amguema River Basin (Chukotka). Siberian Ecological Journal 2010, 4, pp. 609-622.
- Kharitonov, V.G.; Genkal, S.I. Diatoms of Lake Elgygytgyn and its environs (Chukotka). Publisher: NESC FEB RAS: Magadan, Russia, 2012; pp. 1-402. (In Russian).
- Kopyrina, L.; Pshennikova, E.; Barinova, S. Diversity and ecological characteristic of algae and cyanobacteria of thermokarst lakes in Yakutia (northeastern Russia). Oceanological and Hydrobiological Studies 2020, 49(2), pp. 99-122. [CrossRef]
- Kopyrina L.; Pshennikova E.; Barinova S. Diversity and Ecology of Non-Diatom Algae in a Swampy Mountain Lake of the Suntar-Khayat Ridge (Republic Sakha, Yakutia, Russia). Transylvanian Review of Systematical and Ecological Research 2022, 24(1), pp. 17-34. [CrossRef]
- Barinova, S.; Gabyshev, V.; Gabysheva, O. Climate impact of freshwater biodiversity: general patterns in extreme environments of North-Eastern Siberia (Russia). British Journal of Environment and Climate Change 2014, 4(4), pp. 423-443. [CrossRef]
- Barinova, S.; Gabyshev, V.; Boboev, M.; Kukhaleishvili, L.; Bilous, O. Algal Indication of Climatic Gradients, American Journal of Environmental Protection. Special Issue: Applied Ecology: Problems, Innovations 2015, 4(3-1), pp. 72-77. [CrossRef]
- Gabyshev, V.; Ivanova, A.; Gabysheva, O. Long-term survey of algae of the Lena Delta Wildlife Reserve including data on chemistry of waters. Institute for Biological Problems of Cryolithozone SB RAS. Sampling event dataset 2021, Version 1.2. [CrossRef]
- Barinova S.S.; Gabyshev V.A.; Ivanova A.P.; Gabysheva O.I. Bioindication of the water salinity dynamics by the microalgae communities in the Lena River Delta, Laptev Sea, Russian Arctic. Marine Biological Journal 2021, 6(3), pp. 15–28. [CrossRef]
- Gabysheva, O.I.; Gabyshev, V.A.; Barinova, S. Influence of the Active Layer Thickness of Permafrost in Eastern Siberia on the River Discharge of Nutrients into the Arctic Ocean. Water, 2022, 14(1), pp. 84. [CrossRef]
- Gabyshev, V.; Barinova, S.; Ivanova, A.; Gabysheva, O.; Curtean-Bănăduc, A. The Indicator Role of Algae in Assessing the Organic Pollution in the Lena River Delta, the Russian Arctic. Front. Environ. Sci. 2022, 10, pp. 921819. [CrossRef]
- Barinova, S.; Gabyshev, V.; Genkal, S.; Gabysheva, O. Diatoms of Small Water Bodies as Bioindicators in the Assessment of Climatic and Anthropogenic Impacts on the Coast of Tiksi Bay, Russian Arctic. Water 2023, 15, pp. 1533. [CrossRef]
- Gabyshev, V.; Ivanova, A.; Gabysheva, O. Long-term survey of algae of the Lena Delta Wildlife Reserve including data on chemistry of waters. Institute for Biological Problems of Cryolithozone SB RAS. Sampling event dataset 2021, Version 1.2. [CrossRef]
- Bessudova, A.; Gabyshev, V.; Firsova, A.; Gabysheva, O.; Bukin, Y.; Likhoshway, Y. Diversity variation of silica-scaled chrysophytes related to differences in physicochemical variables in estuaries of rivers in an Arctic watershed. Sustainability 2021, 13, pp. 13768. [CrossRef]
- Komulainen, S.F. Freshwater algae in red data books: state-of-the-art and problems. Transactions of Karelian Research Centre of Russian Academy of Science. Biogeography, Petrozavodsk 2009, 1, pp. 57-61.
- Gvozdetsky, N.A.; Mikhailov, N.I. Physical Geography of the USSR: Asian part, 3rd ed.; Mysl': Moscow, Russia, 1978; 512 p. (In Russian).
- Grosswald, M.G.; Spektor, V.B. Glacial landforms of the Tiksi region (western coast of the Buor-Khaya Bay, Northern Yakutia). Geomorfologiya (Geomorphology RAS) 1993, pp. 72–82.
- Spektor, V.B.; Shestakova, A.A.; Torgovkin, Y.I. New genetic types of landforms and de posits of the coastal part of the western sector of the Laptev Sea and the Lena River Delta. Arctic and Subarctic Natural Resources 2021, 26(2), pp. 81–93. [CrossRef]
- Konstantinov, P.Y.; Tregubov, O.D.; Zhang, M.; Li G. Permafrost temperature dynamics over the last 30 years in certain regions of the Eastern sector of the Russian Arcric. International journal of applied sciences and technologies «Integral» 2022, pp. 260–267. [CrossRef]
- Reference book on the climate of the USSR. Issue. 24: Yakut ASSR. Part 2: Temperature of air and soil / Yakutsk hydrometeorological observatory. Publisher: Gidrometeoizdat: Leningrad, USSR, 1966; pp. 1-398.
- Fedorov, A.N.; Botulu, T.A.; Varlamov. S.P. Permafrost landscapes of Yakutia (explanatory note to the permafrost-landscape map of the Yakut ASSR, scale 1:2500000). GUGK: Novosibirsk, Russia, 1989; 170 p. (In Russian).
- Kirillov, A.F.; Chernyshev, I.A. Annotated listing of pisciforms and fishes of marine and fresh waters of Yakutia. Bulletin of Yakutsk State University 2006, 3(4), pp. 5–13.
- Balonov, I.M. Preparation of diatoms and golden algae for electron microscopy. In: Methods for the study of biogeocenoses of inland waterbodies. Moscow: Nauka, USSR, 1975, pp. 87–89. (In Russian).
- Lange-Bertalot, H.; Moser, G. Brachysira: Monographie der Gattung; wichtige indicator-species für das Gewässer-Monitoring und Naviculadicta nov. gen.; ein Lösungsvorschlag zu dem Problem Navicula sensu lato onhe Navicula sensu stricto. Bibliotheca Diatomologica 1994, 29, pp. 1–212.
- Krammer, K. Die cymbelloiden Diatomeen. Teil 1. Allgemeines und Encyonema part. Bibliotheca Diatomologica 1997, 36, pp. 1–382.
- Krammer, K. Die cymbelloiden Diatomeen. Teil 2. Encyonema part., Encyonopsis und Cymbellopsis. Bibliotheca Diatomologica 1997, 7, pp. 1–469.
- Krammer, K. Pinnularia. In Diatoms of Europe; Lange-Bertalot, H., Eds.; Publisher: Ruggell, Liechtenstein, 2000; 1, pp. 1–703.
- Krammer, K. Cymbella. In Diatoms of Europe; Lange-Bertalot, H., Eds.; Publisher: Ruggell, Liechtenstein, 2002; 3, pp. 1–584.
- Krammer, K. Cymbopleura, Delicata, Navicymbula, Gomphocymbellopsis, Afrocymbella. In Diatoms of Europe; Lange-Bertalot, H., Eds.; Publisher: Ruggell, Liechtenstein, 2003; 4, pp. 1–530.
- Krammer, K.; Lange-Bertalot, H. Bacillariophyceae. Teil 1. Naviculaceae. In Die Süsswasserflora von Mitteleuropa. Publisher: Stuttgart, Germany, 1986; bd 2/1, pp. 1–876.
- Krammer, K.; Lange-Bertalot, H. Bacillariophyceae. Teil 2. Epithemiaceae, Bacillariaceae, Surirellaceae. In Die Süsswasserflora von Mitteleuropa; Publisher: Stuttgart, Germany, 1988; bd. 2/2, pp. 1–536.
- Krammer, K.; Lange-Bertalot, H. Bacillariophyceae. Teil 3. Centrales, Fragilariaceae, Eunotiaceae. In Die Süsswasserflora von Mitteleuropa; H. Ettl, J., Gerloff, H., Eds.; Publisher: Stuttgart, Germany, 1991a; bd. 2/3, pp. 1–576.
- Krammer, K.; Lange-Bertalot, H. Bacillariophyceae. Teil. 4. Achnanthaceae, Kritische Erganzungen zu Navicula (Lineolatae) und Gomphonema. In Die Süsswasserflora von Mitteleuropa; Gustav Fisher Verlag: Stuttgart, Germany, 1991b; bd. 2/4, pp. 1–437.
- Lange-Bertalot, H.; Genkal, S.I. Diatoms of Siberia. I. Iconographia Diatomologica 1999, 6, pp. 7–272.
- Reichardt, E. Zur revision der gattung Gomphonema. Iconographia Diatomologica 1999, 8, pp. 1–203.
- Lange-Bertalot, H. Navicula sensu stricto, 10 genera separated from Navicula sensu lato Frustulia. In Diatoms of Europe; Lange-Bertalot, H., Eds.; A.R.G. Gantner Verlag K.-G.: Ruggell, Liechtenstein, 2001; 2, pp. 1–526.
- Werum, M.; Lange-Bertalot, H. Diatoms in springs from Central Europe and elsewhere under the influence of hydrogeology and anthropogenic impacts. Iconographia Diatomologica 2004, 13, pp. 1-417.
- Levkov, Z. Amphora sensu lato. In Diatoms of Europe; Lange-Bertalot, H., Eds.; A.R.G. Gantner Verlag K.-G.: Ruggell, Liechtenstein, 2009; 5, pp.1–916.
- Genkal, S.I.; Bondarenko, N.A.; Shchur, L.A. Diatoms of lakes in the south and north of Eastern Siberia; Publisher OAO "Rybinsk Press House": Rybinsk, Russia, 2011; 72 p. (In Russian).
- Lange-Bertalot, H.; Bak, M.; Witkowski, A. Eunotia and some related genera. In Diatoms of Europe; A.R.G. Gantner Verlag K.-G.: Ruggell, Liechtenstein, 2011; 6, pp. 1–747.
- Levkov, Z.; Metzeltin, D.; Pavlov, A. Luticola, Luticolopsis. In Diatoms of Europe; Lange-Bertalot, H., Eds.; A.R.G. Gantner Verlag K.-G.: Ruggell, Liechtenstein, 2013; 7, pp. 1–697.
- Levkov, Z.; Mitić-Kopanja, D.; Reichardt, E. The diatom genus Gomphonema from the Republik of Macedonia. In Diatoms of Europe; Lange-Bertalot, H., Eds.; A.R.G. Gantner Verlag K.-G.: Ruggell, Liechtenstein, 2016; 8, pp.1–552.
- Kulikovskiy, M.S.; Glushchtnko, A.M.; Genkal, S.I.; Kuznetsova, I.V. Identification book of diatoms from Russia; Publisher: Filigran': Yaroslavl, Russia, 2016; 804 p. (In Russian).
- Lange-Bertalot, H.; Hofmann, G.; Werum, M.; Cantonati, M. Freshwater benthic diatoms of Central Europe. Koeltz Botanical Books: Schmitten-Oberreifenberg, Germany, 2017; 942 p.
- Beauger, A.; Allain, E.; Voldoire, O.; Blavignac, C.; Rossi, S.; Wetzel, C.; Ector, L. Gomphosphenia vallei (Bacillariophyta), a new diatom species from a stream in the “Réserve Naturelle Nationale de la Vallée de Chaudefour”, Massif Central (France). Phytotaxa 2022, 542(2), pp. 167-179. [CrossRef]
- Guiry, M.D.; Guiry, G.M. AlgaeBase World-Wide Electronic Publication. National University of Ireland, Galway. Available online: http://www.algaebase.org (accessed on 24 June 2019).
- Hofmann, G.; Lange-Bertalot, H.; Werum, M.; Klee, R. Rote Liste und Gesamtartenliste der limnischen Kieselalgen (Bacillariophyta) Deutschlands. In Rote Liste der gefährdeten Tiere, Pflanzen und Pilze Deutschlands; Band 7: Pflanzen. Bonn (Bundesamt für Naturschutz). Naturschutz und Biologische Vielfalt, 2018, 70(7), pp. 601–708.
- IUCN. IUCN Red List Categories and Criteria, Version 3.1, 2nd ed.; IUCN: Gland, Switzerland; Cambridge, UK, 2012; p. 1-32.
- Barinova, S. Essential and practical bioindication methods and systems for the water quality assessment. Int. J. Environ. Sci. Nat. Resour. 2017, 2, pp. 1–11. [CrossRef]
- Barinova, S.S.; Medvedeva, L.A.; Anissimova, O.V. Diversity of Algal Indicators in Environmental Assessment; Pilies Studio Publisher: Tel Aviv, Israel, 2006; 498 p. (In Russian).
- Barinova, S.S.; Bilous, O.P.; Tsarenko, P.M. Algal Indication of Water Bodies in Ukraine: Methods and Prospects; Publishing House of Haifa University: Haifa, Kyiv, Israel, 2019; 367 p. (In Russian).
- Love, J.; Selker, R.; Marsman, M.; Jamil, T.; Dropmann, D.; Verhagen, J.A.; Ly, A.; Gronau, F.Q.; Smira, M.; Epskamp, S. et al. JASP: Graphical statistical software for common statistical designs. J. Stat. Softw. 2019, 88, pp. 1–17. [CrossRef]
- McAleece, N., Gage, J.D.G., Lambshead, P.J.D., Paterson, G.L.J. BioDiversity Professional statistics analysis software. Jointly developed by the Scottish Association for Marine Science and the Natural History Museum London, 1997.
- Barinova, S. Systemic Criteria for the Analysis of Alpha- and Gamma-Diversity of Freshwater Algae. Int. J. Environ. Sci. Nat. Res. 2017, 4(2), pp. 555633. [CrossRef]
- Barinova, S. The effect of altitude on distribution of freshwater algae in continental Israel. Current Topics in Plant Biology 2011, 12, pp. 89-95.
- Hustedt, F. Systematisch und Ökologische Untersuchungen über die Diatomeenflora von Java, Bali und Sumatra. Archiv für Hydrobiologie, Suppl. 1938–1939, 15-16, pp. 131–177, 393–506, 638–790, 1–155, 274–394.
- Hustedt, F. Die Diatomeenflora des Flußsystems der Weser im Gebiet der Hansestadt Bremen. Abhandlungen des Naturwissenschaftlichen Vereins zu Bremen 1957, 34, pp. 181–440.
- Watanabe T.; Asai K.; Houki A. Numerical estimation of organic pollution of flowing water by using the epilithic diatom assemblage – Diatom Assemblage Index (DAIpo). Science of Total Environment 1986, 55, pp. 209–218. [CrossRef]
- Sládeček, V. Diatoms as indicators of organic pollution. Acta Hydrochimica et Hydrobiologica 1986, 14, pp. 555–566. [CrossRef]
- Van Dam, H.; Mertens, A.; Sinkeldam, J. A coded checklist and ecological indicator values of freshwater diatoms from the Netherlands. Netherlands Journal of Aquatic Ecology 1994, 28(1), pp. 117–133. [CrossRef]
- Bessudova, A.; Gabyshev, V.; Bukin, Y.; Gabysheva, O.; Likhoshway, Y. V. Species richness of scaled Chrysophytes in arctic waters in the Tiksi Region (Yakutia, Russia). Acta Biologica Sibirica 2022, 8, pp. 431–459. [CrossRef]
- Trifonova, I. Phytoplankton composition and biomass structure in relation to trophic gradient in some temperate and subarctic lakes of north-western Russia and the Prebaltic. Hydrobiologia 1998, 369, pp. 99–108. [CrossRef]
- Knights, D., Piliouras, A., Schwenk, J., Hariharan, J., & Russoniello, C. Seasonal and morphological controls on nitrate retention in Arctic deltas. Geophysical Research Letters 2023, 50, pp. 1-10. [CrossRef]
- Gabyshev, V.A.; Tsarenko, P.M.; Ivanova, A.P. Diversity and Features of the Spatial Structure of Algal Communities of Water Bodies and Watercourses in the Lena River Estuary. Inland Water Biol 12 (Suppl 1) 2019, pp. 1–9. [CrossRef]
- Odum, E.P. Fundamentals of Ecology. Third Edition, W.B. Saunders Co., Philadelphia, 1971; pp. 1-574.
- Sharipova M. Yu. Algae of terraqueous ecotones of the Basu River Valley (South Ural, Russia). International Journal on Algae 2007, 9(2), pp.162-173. [CrossRef]
- Percopo, I.; Siano, R.; Cerino, F.; Sarno, D.; Zingone, A. Phytoplankton diversity during the spring bloom in the northwestern Mediterranean Sea. Botanica Marina, 2011, 54(3), pp. 1-25. [CrossRef]
- Sheath, R.G.; Steinman, A.D. A checklist of freshwater algae of the Northwest Territories, Canada. Can. J. Bot. 1982, 60(10), pp. 1964-1997. [CrossRef]
| No of station | Lake name | Water temperature | Altitude, m | North | East |
|---|---|---|---|---|---|
| 1 | Lake 1 | 16.7 | 25 | 71°44'44" | 128°43'12" |
| 2 | Lake 2 | 15.1 | 66 | 71°44'12" | 128°41'37" |
| 3 | Lake 3 | 15.0 | 109 | 71°43'31" | 128°38'31" |
| 4 | Lake 4 | 20.4 | 38 | 71°43'48" | 128°42'35" |
| 5 | Lake 5 | 20.6 | -4 | 71°43'45" | 128°44'36" |
| 6 | Lake 6 | 16.1 | 76 | 71°41'10" | 128°36'50" |
| 7 | Lake 7 | 14.5 | 76 | 71°40'52" | 128°37'11" |
| 8 | Lake-puddle 8 | 10.1 | 55 | 71°40'26" | 128°41'21" |
| 9 | Lake 9 | 15.1 | 54 | 71°40'10" | 128°43'27" |
| 10 | Lake 10 | 13.3 | 52 | 71°35'56" | 128°51'70" |
| 11 | Lake 11 | 14.0 | 38 | 71°34'33" | 128°45'51" |
| 12 | Swampy lake 12 | - | 105 | 71°33'36" | 128°40'26" |
| 13 | Lake 13 | 14.6 | 85 | 71°33'17" | 128°38'51" |
| 14 | Lake 14 | 14.7 | 154 | 71°32'34" | 128°34'57" |
| IUCN Category | IUCN Code | No of Red List Category | Red List Category | Number of species |
|---|---|---|---|---|
| EXTINCT | EX | 1 | Extinct or Lost | 2 |
| CRITICALLY ENDANGERED | CR | 2, 3 | Threatened with Extinction, Highly Threatened | 40 |
| ENDANGERED | EN | 4, 5 | Threatened, Threat of Unknown Extent | 112 |
| VULNERABLE | VU | 6 | Extremely Rare | 16 |
| NEAR THREATENED | NT | 7 | Near Threatened | 28 |
| LEAST CONCERN | LC | 9 | Not Threatened | 198 |
| DATA DEFICIENT | DD | 8 | Data Deficient | 43 |
| NOT EVALUATED | NE | 0, 10 | Not established | 194 |
| Variable | “Delta region” | “Tiksi region” |
|---|---|---|
| Square, km2 | 14300 | 180 |
| Species with intraspecies | 666 | 385 |
| Taxa without sp. | 631 | 356 |
| Species only | 569 | 337 |
| Sp./Area | 0.05 | 2.14 |
| Ssp./Sp. | 1.11 | 1.14 |
| No of station | Lake name | Lake surface area, km2 | Coastline length, m | No of Species | Sp./Area |
|---|---|---|---|---|---|
| 1 | Lake 1 | 0.075 | 1618.42 | 66 | 881.601 |
| 2 | Lake 2 | 0.038 | 894.56 | 62 | 1635.603 |
| 3 | Lake 3 | 0.031 | 763.29 | 76 | 2460.437 |
| 4 | Lake 4 | 0.008 | 359.76 | 72 | 8891.117 |
| 5 | Lake 5 | 0.067 | 1188.51 | 33 | 493.171 |
| 6 | Lake 6 | 0.042 | 902.78 | 137 | 3252.128 |
| 7 | Lake 7 | 0.586 | 5716.74 | 75 | 128.074 |
| 8 | Lake-puddle 8 | - | - | 69 | - |
| 9 | Lake 9 | 0.486 | 3167.26 | 59 | 121.281 |
| 10 | Lake 10 | 0.077 | 1153.85 | 85 | 1101.333 |
| 11 | Lake 11 | 0.124 | 1800.36 | 117 | 946.742 |
| 12 | Swampy lake 12 | - | - | 43 | - |
| 13 | Lake 13 | 0.158 | 1639.88 | 96 | 609.365 |
| 14 | Lake 14 | 0.023 | 712.85 | 50 | 2175.773 |
| Species in Threat Categories | Hab | T | Oxy | Sal | pH | D | Index S | Sap | Aut-Het | Tro |
|---|---|---|---|---|---|---|---|---|---|---|
| EXTINCT (Extinct or Lost) | ||||||||||
| Cymbopleura designata (Krammer) Bahls | B | temp | st-str | i | alf | - | 1.8 | o-a | ats | m |
| Encyonema latens (Krasske) D.G.Mann | B | - | - | - | - | - | 1.0 | o | ats | ot |
| CRITICALLY ENDANGERED (Threatened with Extinction) | ||||||||||
| Eunotia elegans Østrup | P-B | - | st-str | hb | acf | - | 0.2 | x | ats | ot |
| Eunotia faba Ehrenberg | B | temp | st-str | - | alf | - | - | o | ats | ot |
| Frustulia krammeri Lange-Bertalot & Metzeltin | B | - | - | - | acf | - | - | - | - | e |
| Navigeia thingvallae (Østrup) Bukhtiyarova | B | - | - | - | - | - | - | - | - | - |
| Tetracyclus glans (Ehrenberg) F.W.Mills | P-B | temp | - | i | acf | - | 1.0 | x-o | - | ot |
| CRITICALLY ENDANGERED (Highly Threatened) | ||||||||||
| Boreozonacola hustedtii Lange-Bertalot, Kulikovskiy & Witkowski | - | - | - | - | - | - | - | - | - | - |
| Brachysira calcicola Lange-Bertalot | B | - | - | - | - | - | 1.0 | o | - | - |
| Brachysira procera Lange-Bertalot & Gerd Moser | B | - | - | - | acf | - | - | - | - | - |
| Brachysira styriaca (Grunow) R.Ross | B | temp | - | i | ind | es | 1.0 | o | - | ot |
| Cymbella affinis Kützing | B | temp | st-str | i | alf | sx | 1.1 | b | ats | e |
| Cymbella carassius Skvortzov | - | - | - | - | - | - | - | - | - | - |
| Cymbellafalsa diluviana (Krasske) Lange-Bertalot & Metzeltin | B | - | - | i | alf | - | 1.0 | o | - | ot |
| Cymbopleura tynnii (Krammer) Krammer | B | - | - | - | - | - | - | - | - | - |
| Diploneis ovalis (Hilse) Cleve | B | - | st-str | i | alf | - | 0.9 | x-b | ate | m |
| Eucocconeis alpestris (Brun) Lange-Bertalot | B | temp | str | hb | ind | - | - | - | - | - |
| Eucocconeis austriaca (Hustedt) Lange-Bertalot | B | - | - | i | alf | - | 0.2 | x | ats | ot |
| Eucocconeis flexella (Kützing) F.Meister | B | temp | str | mh | ind | - | - | - | - | - |
| Eunotia bigibba Kützing | B | - | str | i | acf | - | 0.4 | x-o | - | ot |
| Eunotia flexuosa (Brébisson ex Kützing) Kützing | B | temp | st-str | i | acf | - | 0.4 | x-o | - | - |
| Eunotia praerupta Ehrenberg | P-B | cool | st-str | hb | acf | - | 0.3 | x | - | - |
| Eunotia triodon Ehrenberg | B | temp | - | hb | acf | - | 1.0 | o | - | ot |
| Gomphonema helveticum Brun | B | - | - | i | ind | - | - | - | - | - |
| Gomphonema lagerheimii A.Cleve | B | - | str | hb | acf | - | - | - | - | m |
| Gomphonema ventricosum W.Gregory | B | cool | str | i | ind | - | 1.0 | o | - | - |
| Iconella curvula (W.Smith) Ruck & Nakov | B | - | str | hb | acf | - | 2.0 | b | - | me |
| Kobayasiella subtilissima (Cleve) Lange-Bertalot | B | temp | st-str | i | acb | - | 1.6 | b-o | ats | me |
| Mayamaea disjuncta (Hustedt) J.Y. Li & Y.Z.Qi | B | - | str | i | ind | sp | 3.0 | a | ate | he |
| Navicula angusta Grunow | B | - | st-str | i | ind | - | 1.0 | o | - | - |
| Navicula gottlandica Grunow | P-B | - | - | hl | alf | es | 2.5 | b-a | ate | e |
| Navicula mediocostata E.Reichardt | B | - | - | oh | alf | es | 3.0 | a | ate | e |
| Navicula notha J.H.Wallace | B | - | str | i | acf | - | - | - | - | - |
| Neidium iridis (Ehrenberg) Cleve var. iridis | B | temp | st-str | hb | ind | - | - | - | - | - |
| Nitzschia frigida Grunow | - | - | - | - | - | - | - | - | - | - |
| Placoneis opportuna (Hustedt) Chudaev & Gololobova | B | - | - | - | - | es | - | - | - | - |
| Psammothidium kryophilum (J.B.Petersen) E.Reichardt | P-B | - | str | i | ind | sx | 0.5 | x-o | ats | ot |
| Psammothidium levanderi (Hustedt) Bukhtiyarova & Round | B | temp | str | i | ind | sx | 2.0 | b | ats | om |
| Psammothidium rechtense (Leclercq) Lange-Bertalot | B | - | str | hb | alf | - | 1.0 | o | ats | ot |
| Psammothidium rossii (Hustedt) Bukhtiyarova & Round | B | - | str | hb | ind | - | 1.0 | o | ats | ot |
| Psammothidium scoticum (R.J.Flower & V.J.Jones) Bukhtiyarova & Round | B | temp | - | - | - | - | - | - | - | - |
| Psammothidium ventrale (Krasske) Bukhtiyarova & Round | B | - | str | hb | acf | - | 2.0 | b | ats | om |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





