1. Introduction
Heart Failure (HF) and Atrial Fibrillation (AF) are two cardiovascular (CV) conditions affecting many people worldwide. In recent years both conditions have increased in prevalence probably due to a combination of increasing life expectancy and increase in co morbidities such as hypertension, diabetes and obesity.AF is the most common arrhythmia and American Heart Association estimated that around 46.3 million people around the globe suffered with AF in 2016 (23.2million males, 23.1 million females) [
1,
2]. Researchers predict that the following decades the number of individuals with AF is going to be higher, reaching 6-16 million in the USA in 2050 and 14 million in Europe in 2060 [
3,
4,
5]. HF is a syndrome affecting many people globally. The prevalence of AF in HF is between 13% to 27% [
6,
7,
8,
9,
10]. In the Framingham Heart Study, among 1470 patients with AF or HF, 383 individuals (26%) suffered from both conditions [
11].There is a connection between these two CV conditions, as they have similar risk factors, they are often concomitant, and each can result in development of the other.
Decreased cardiac output in patients with HF results in neurohormonal activation that leads to cardiac remodeling and fibrosis formation, which plays an important role in AF initiation. Also, in these patients there is an increased Left Ventricular Filling Pressure (LVFP) transmitted to the left atrium that leads to elevated atrial pressure and dilation. A dilated atrium is able to maintain the arrhythmia through multiple wavelets of re-entry [
12]. In patients with AF the increased resting heart rate (HR) and the exaggerated HR response during exercise result in reduced Left Ventricle (LV) filling time, absence of atrial contraction, tachycardia-induced cardiomyopathy and thus in an impaired diastolic and systolic function [
13].This relation between AF and HF explains why treating the one affects the other.
2. AV node in chronic AF and HF patients
The Atrioventricular node (AV node) is a secondary cardiac pacemaker and the only part of the electrical conduction system of the human heart that connects the atria and ventricles. AF is characterized by chaotic and irregular impulses in the atria ranged from 300 to 600 beats per minute (bpm). Rapid ventricular rate is prevented by the AV node, which slows the rate of impulses passing to the ventricles.
Two distinct treatment strategies exist for AF. One is rhythm control and the other rate control. Rhythm control tends to be used in younger patients with paroxysmal AF, while rate control is often the preferred method for older persistent AF patients. Rate control can be achieved by using a combination of beta-blockers, digoxin and non-dihydropyridine calcium channel blockers. All of these are possible options in HFpEF, while only beta-blockers and digoxin can be used in HFrEF. An alternative rate control option to medical therapy is the “pace and ablate” approach which offers a more feasible ventricular rate control and R-R interval adjustment. Current 2020 ESC guidelines recommend AV node ablation in patients with AF who are non-responders or intolerant to pharmacological treatment and are not eligible for left atrial ablation (Class IIa, Level of Evidence B) [
14].Also, 2014 guidelines of American College of Cardiology/American Heart Association (ACC/AHA) endorse AV node ablation with ventricular pacing to control heart rate in patients with HF and AF,who do not tolerate pharmacological therapy or are non-responders (Class IIa, Level of Evidence B) [
15]. In recent years, radiofrequency (RF) catheter ablation of the AV junction is the preferable method.
3. Clinical outcomes of AV node ablation
Although AV node ablation is performed in many patients with chronic drug-refractory AF and HF, only a few randomized trials have been conducted in order to compare the clinical outcomes of this procedure. There are, however several non-randomized studies comparing the patients’ clinical condition at baseline with their condition after a follow-up period (
Table 1).
Table 1.
Clinical outcomes of AV node ablation.
Table 1.
Clinical outcomes of AV node ablation.
| First author |
Year of Publication |
Design |
Number of Patients |
Highlights |
Brignole et al.
[19] |
1994 |
AVNA + pacing vs pacing |
23 |
↓Symptoms
↑QOL
↑LV function |
Brignole et al.
[20] |
1997 |
AVNA + DDDR pacemaker vs pharmaceutical drugs |
43 |
↓Palpitations
↓Effort dyspnea
↓Exercise intolerance
↓Easy fatigue |
Marshall et al.
[21] |
1999 |
AVNA + DDDR/MS pacing vs drug therapy |
56 |
↓Overall Symptoms
↓Palpitations
↓Dyspnea
↑Psychological general well-being |
Brignole et al.
[22] |
1998 |
AVNA + VVIR pacing vs drug therapy |
66 |
↓Palpitations
↓Exercise intolerance
↓Effort dyspnea
↓Chest discomfort
↓Easy fatigue |
Ueng et al.
[18] |
2001 |
AVNA + VVIR pacing vs drug therapy |
50 |
↑QOL
↑Overall activity
↓Overall symptoms
↑LVEF |
| Weerasooriya et al. [23] |
2003 |
AVNA + pacing vs medication |
99 |
↓Peak ventricular rate during exercise and daily activity
↓Symptoms
↑QOL |
Wood et al.
[16] |
2000 |
Meta-analysis |
1181 |
↑QOL
↑Exercise duration
↓Symptoms
↓Healthcare use
↑Cardiac function |
Chatarjee et al.
[17] |
2013 |
Meta-analysis |
7867 |
↑QOL
↓Symptoms
↑LVEF |
| AVNA, Atrioventricular node ablation; QOL, Quality of Life; LVEF, Left Ventricular Ejection Fraction |
A meta-analysis by
Wood et al. included 21 studies (2 randomized trials, 19 non-randomized studies) conducted in a 9-year period (from 1989 to 1998) and 1181 patients with drug-refractory atrial tachyarrhythmia, mainly AF (97%) and evaluated the clinical outcomes of AV node ablation plus pacing [
16]. 15 studies (13 non-randomized, 2 randomized) and 642 patients were included in the analysis of clinical outcomes with an average follow-up duration from 48 days to 2.3 years. This analysis assessed 19 parameters containing QOL, exercise duration, symptoms, healthcare use and cardiac function. There was a significant improvement in all parameters, apart from longitudinal fractional shortening (p=0.08).
A recent meta-analysis by
Chatarjee et al. reviewed the efficacy, effectiveness and safety of AV nodal ablation compared to those of pharmacological rhythm control strategies [
17]. The efficacy analysis included 5 randomized or prospective trials (n=314), while the other two included 11studies (n=810) and 47 studies (n=5632) respectively. AV nodal ablation was related to improvement in QOL and symptoms, while there was no significant difference in exercise duration compared to drug therapy. In patients with impaired systolic function at baseline who underwent ablation, there was a significant increase in EF compared to those treated with medication (+4%; 95% CI 3.11-4.89). Also, all-cause mortality was equivalent in both AV node ablation and drug therapy (RR 1.05; 95% CI 0.29-3.85) and there were low percentages of ablation-related mortality (0.27%) and malignant arrhythmias (0.57%). At an average follow-up of 26.5 months SCD incidence of 2.1% was reported.
Several trials have randomized patients in 2 groups (ablation plus pacemaker implantation group and medical therapy group) to compare the impact of these approaches on clinical outcomes. Findings of these trials were in accordance with the meta-analyses reports and showed that AV node ablation is associated with less symptoms compared to medical therapy. However, one study showed no difference between the 2 groups [
18].
Dating back to 1994
Brignole et al. randomized 23 patients with resistant to treatment AF or atrial flutter into 2 groups, in order to assess the impact of AV node ablation on quality of life (QOL), LV performance and exercise performance [
19]. In the first group (n=12) patients treated with both RF ablation of AV node and pacing therapy, while in the second one (n=11) they treated only with a pacemaker. After 15 days in the RF group there was a significant decrease in symptoms compared to the control group, leading to an improvement in QOL (palpitations; p=0.004, rest dyspnea; p=NS, effort dyspnea; p=0.03, exercise intolerance; p=0.005, asthenia; p=0.02). Following that, in the control group RF ablation was also performed. After 90 days, an improvement was noticed in the New York Heart Association (NYHA) functional classification (p=0.002) as well as in LV function and exercise performance (34% increase in echocardiographic fractional shortening; p=0.003, 15% increase in exercise time; p=0.001). It is worth to mention that the amelioration of LV performance refers only to patients with a baseline LV dysfunction.
In a subsequent study,
Brignole et al. established the efficacy and superiority of AV node ablation plus pacing over drug therapy in paroxysmal AF [
20]. 43 patients with drug refractory paroxysmal AF were randomly divided into 2 groups, treated either with AV junction ablation plus DDDR mode-switching pacemaker or with pharmaceutical drugs. Patients in the ablation plus DDDR group had notably lower scores in the QOL questionnaires and specific symptoms scales after a 6-month follow-up period compared to those in the second group. This difference was remarkable in the Living with HF questionnaire, palpitations, effort dyspnea, exercise intolerance and easy fatigue (-53%; p=0.0006, -71%; p=0.0000, -36%; p=0.04, -46%; p=0.001 and -51%; p=0.02 respectively), while scores in rest dyspnea, chest discomfort and NYHA functional classification showed a less important decrease (-56%; p=0.13, -50%; p=0.42, -17%; p=0.08 respectively). In another study, 56 symptomatic patients with paroxysmal AF were assigned by
Marshall et al.to constant drug therapy or AV junction ablation plus DDDR/MS pacing, like in the study by
Brignole et al. [
21]. In “pace and ablate” strategy there were more significant differences in scores for overall symptoms (-48% vs -4%, p<0.005), palpitations (-62% vs -5%, p<0.001), dyspnoea (-44% vs -3%, p<0.005) and psychological general well-being (+12% vs +0.5%, p<0.05) than those in pharmacological strategy.
Another study was conducted in 66 patients with chronic AF and HF with a resting HR>90 bpm. In this study there was one group of patients treated with AV node ablation plus VVIR pacemaker and one treated with drugs. After 12 months those in the first group showed significant reduction in palpitations (p=0.000), exercise intolerance (p=0.005), effort dyspnea (p=0.01), chest discomfort (p=0.02) and easy fatigue (p=0.02) compared to those in the second group. QOL and NYHA class did not differ between the 2 groups and also the cardiac performance remained constant [
22].
Uenget al. randomly allocated 50 patients with chronic lone AF in 2 groups [
18]. In the first group (n=21) they underwent AV node ablation plus VVIR pacing and in the other one they received only medication (n=29). After a 12-month follow-up period, the pace & ablate group showed significant improvements in general quality of life (-20%, p<0.001), overall activity scale (-23%, p=0.004) and overall symptoms (-24%, p<0.001) and a significant increase of LVEF (49% ± 5% group 1 vs 44% ± 6% group 2, p=0.02), while there was no statistically significant differences in drug group between enrollment and follow-up.
The Australian Intervention Randomized Control of Rate in Atrial Fibrillation Trial (AIRCRAFT) included 99 patients with mild to moderate symptomatic permanent AF who treated either with AV junction ablation plus pacing or with medication [
23]. At 12-month follow-up, no significant differences were observed between the 2 groups regarding the LVEF or exercise duration on treadmill testing, whereas in the ablation group there was a remarkable reduction in the peak ventricular rate during exercise (112±17 bpm versus 153±36 bpm, p<0.05) as well as during daily activities (117±16 bpm versus 152±37 bpm, p<0.05). Ablation group had less symptoms (RRR 18%, p=0.004) and an improvement in QOL.
To conclude, AV node ablation and pacemaker implantation demonstrate several beneficial effects on clinical outcomes in these patients compared to pharmacological rate control. Data collected by using different kinds of questionnaires showed an improvement in QOL and symptoms. Symptoms were reduced presumably due to ventricular rate adjustment and less side effects of pharmacological therapy. LVEF improvement was associated, mainly, with an improvement in diastolic filling and the cessation of negative inotropic medication, resulting in increasing of cardiac output and exercise duration.
4. Survival analysis
In the meta-analysis by
Wood et al.1073 patients from 16 studies were included in the mortality analysis with a mean follow-up between 3 months- 2.3 years. The estimated monthly and 1-year mortality rates were 1.4% (95% CI 0.04-2.4) and 6.3% (95% CI 5.5-7.2), respectively [
24].
Ozcan et al. assessed the mortality rate in 350 patients in Minnesota [
25].During a mean 36-month follow-up 78 patients died. There was not significant difference in survival rates between the AV node ablation group and the controls (RR 1.14; 95% CI 0.81-1.60; p=0.44). Also, they found that previous MI, history of CHF and medical treatment after ablation were independent factors of death (p<0.001, p=0.02, p=0.03 respectively).
In another study, 359 patients with refractory AF were treated with RF ablation of AV junction plus pacing. 46 of them died within a mean 41-month follow-up. 1-year and 5-year survival probability for all patients were 0.953 and 0.827, respectively. They also found that age ≥ 65 years (HR 1.92; 95% CI 1.00-3.69), presence of HF (HR 3.93; 95% CI 1.87-7.86), diabetes (HR 2.91; 95% CI 1.47-5.77) and fractional shortening ≤20% (HR 5.79; 95% CI 3.00-11.18) are independent predictors of death [
26].
Darpo et al. observed 220 patients with paroxysmal or chronic AF, who underwent ablation of AV junction plus pacing [
27]. 31 deaths were recorded during an average 31-month follow-up period. The annual incidence of sudden death and sudden unexplained death was 1.9% and 1%, respectively.
The Atrioventricular Junction Ablation and Biventricular Pacing for Atrial Fibrillation and Heart Failure (APAF-CRT) trial enrolled patients with symptomatic, permanent AF, narrow QRS complex (≤110ms) and at least one HF hospitalization during the last year prior to the time of inclusion [
28].133 patients were randomized into two groups: AV junction ablation+ CRT group and pharmacological rate control group with a median 29-month follow-up. Atrioventricular junction (AVJ) ablation plus CRT was superior to drug therapy in reducing both all-cause mortality (HR 0.26; 95% CI 0.10-0.65; p=0.004)and the secondary combined endpoint of death of any cause or HF hospitalization (HR 0.40; 95% CI 0.22-0.73; p=0.002).Regarding the primary endpoint of all-cause mortality, both patients with EF > 35% and those with EF≤35% were benefited by the AVJ ablation and biventricular (BiV) pacing (HR 0.27; 95% CI 0.08-0.84; p=0.02 and HR 0.34; 95% CI 0.06-1.92; p=0.22, respectively).
5. Worsening of HF
A “Pace and ablate” strategy was shown to improve cardiac performance and HF status in patients with drug-refractory AF. However, this approach has some drawbacks, especially when is followed by permanent RV apical pacing. AV node ablation has beneficial effects on cardiac function and LVEF, which are counterbalanced by the adverse hemodynamic effects and modification of the LV due to RV apical pacing. RV pacing promotes cardiac dyssynchrony and results in worsening of HF and also intensifies coexistent mitral regurgitation in patients with chronic AF [
29,
30].
In a study by
Vanderheydenet al., 108 patients with refractory AF were treated with AV node ablation. In 8 of them (~7,4%) hemodynamic deterioration was developed and was related to exacerbation of mitral regurgitation [
31]. After 3 to 8 weeks from the procedure, 3 of these 8 patients developed acute pulmonary edema and 5 of them congestive heart failure (CHF). It was found that patients with enlarged LV at the end of diastole and established mitral regurgitation at baseline were at increased risk.
Twidale et al. performed AV node ablation plus pacing in 44 patients with AF and CHF [
32]. 4 patients (~9%) developed worsening of heart failure within 24 hours after the procedure.The authors referred to the observation of
Vanderheyden et al. that mitral regurgitation was a result of ventricular pacing after the AV node ablation and recommended fluid restriction and administration of diuretics.
Björkenheimet al. reported that in 117 who underwent a “Pace and ablate“ approach, during the 58-month follow-up period, 23 of them (~20%) had at least one hospitalization due to HF [
33]. Of those 23 hospitalizations, 13(~57%) had known HF before the procedure.
6. Sudden death and ventricular arrhythmias
Sudden death and ventricular arrhythmias usually occur early after the AV node ablation and pacing and are associated with failure of the pacing system, coexisting heart disease or the procedure itself.
Ozcanet al. observed that 334 patients, approximately 2.7% of them (n=9) had sudden death after the procedure [
34]. In 1.2% of these patients (n=4) sudden death occurred the first 4 days after the ablation and was considered procedure-related. Three other patients (0.9%) died suddenly within 3 months after the procedure and were considered probably related to it. Independent predictors for sudden death included Diabetes, NYHA ≥ 2, preexisting ventricular arrhythmia, valvulopathies and chronic obstructive pulmonary disease. Also, in a recent meta-analysis the incidence of sudden cardiac death after AV junction ablation was 2.1% at an average follow-up of 26.5 months [
17].
The presence of bradycardia has been shown to play an important role in the development of ventricular arrhythmias and especially VT. A case report by
Peters et al.presented a patient with repeated VF which started right after the catheter ablation of AV junction [
35]. This arrhythmia was pause- and bradycardia-dependent and was quelled by pacing at 90 bpm. The authors reported that this serious fatal arrhythmia may be bradycardia-induced and was not related to the ablation procedure. A study by
Geelen et al.included 235 patients with refractory atrial arrhythmias (84% AF) treated with ablation [
36]. 100 patients had a pacing rate of ≤ 70bpm and in 6 of them (6%) VF or sudden death was reported. In the other group of 135 patients, pacing rate was about 90bpm for 1-3 months after the procedure and neither VF nor sudden death occurred. Thus, they suggested that these complications can be prevented by programming of a higher base rate, faster rates right after the ablation. Also, in other 2 studies no early sudden death or ventricular arrhythmias were reported in patients with AF who had a pacing rate at >70bpm for 1-3 weeks after the AV node ablation [
27,
37].In a recent study by
Wang et al., sudden death rate was decreased to 0.2% when pacing was set at a lower rate of 90bpm [
38].
In an ECG ventricular repolarization is depicted by QT interval and changes in this parameter can trigger malignant arrhythmias.
Cellarier et al. compared a group of 11 patients who underwent AV node ablation and received a VVI pacemaker with a control group consisted of 6 patients with chronic complete heart block and a VVI pacemaker [
39]. This study showed an abnormal long QT interval during the first 2 days after the ablation at rates < 75 bpm, which was eliminated the following days. The authors suggested that pacing in these patients should be at ≥ 75 bpm, so to avoid bradycardia-dependent prolongation of QT interval and subsequent arrhythmias and sudden death [
36].
Raj et al. evaluated QT dispersion during an abrupt rate drop from 80 to 40 bpm in 20 patients after ablation of AV junction [
40]. 13 of these patients had normal LV function and 7 of them had impaired LV function. A significant increase in QT dispersion was recorded in patients with LV dysfunction compared to those with normal LV function (p=0.01).
Another key point in pathogenesis of ventricular arrhythmias developed after AV junction ablation is sympathetic nervous system stimulation.
Hamdan et al. evaluated the sympathetic nervous system activation in 10 patients with chronic AF after a successful AV ablation [
41]. They reported an increase in activation of SNS in patients with pacing at 60bpm, while at 90bpm SNS activity was reduced.
7. Risk of permanent AF
Ablation of AV junction and permanent pacing in patients with refractory AF have been linked to more relapses of paroxysmal AF and increased incidence of permanent AF development. A study by
Queiroga et al. involved 114 patients with paroxysmal AF (83%, n=95) and paroxysmal AF/flutter (17%, n=19) who underwent AV node ablation [
42]. During a 72-month period, 52% of patients developed permanent AF, while 48% remained in sinus rhythm without a difference between AF and flutter. From the parameters evaluated at baseline, only age over 80 years seemed to be associated with progression to permanent AF but without significance (p=0.07). According to
Marshall et al., although AV node ablation and pacing had beneficial effects on symptoms compared to drug therapy, “ablate and pace” strategy was associated with development of persistent AF at 6 weeks (12/37 patients, 32% in ablate and pace group versus 0/19 in drug group; p<0.01) [
21]. A study by
Brignole et al. reported similar results to those of Marshall et al. with 5 out of 21 patients (24%) progressing to permanent AF 6 months after the ablation, compared to none of the patients received drug therapy [
20].
The probability of progression to permanent AF after AV node ablation has been shown to rise as time goes on.
Gianfranchiet al. studied 63 patients with refractory paroxysmal AF [
43].These patients were treated with AV junction ablation and dual-chamber pacemaker implantation and 22 of them (35%) developed permanent AF during an average 23-month follow-up. The authors estimated that the actuarial rate of permanent AF was 22%, 40% and 56% at 1,2 and 3 years following ablation.In a study by
Gribbin et al., 42% of patients(n=26/62) with paroxysmal AF, who underwent AV junction ablation developed permanent AF during a 30-month follow-up period [
44]. 75% of patients had developed permanent AF 86 months (~7 years) after the procedure.
Permanent pacing may have deleterious effects on cardiac function and may lead to an increased incidence of AF. RV pacing seems to be related to an increased risk of AF relapses and progression to permanent AF. A post hoc analysis of the Mode Selection Trial (MOST) assessed patients with DDDR or VVIR pacemaker and QRS duration <120ms at baseline [
45]. In this analysis, cumulative percent of ventricular paced (Cum%VP) beats was shown to be a predictive factor of HF hospitalizations in both DDDR and VVIR groups (HR 2.99; 95% CI 1.15-7.75 for Cum%VP> 40% and HR 2.56; 95% CI 1.48-4.43 for Cum%VP> 80%, respectively),as well as the risk of developing AF.
Apart from the effects of pacing, other factors play an important role in developing permanent AF.
Brignole et al.randomized 137 patients with symptomatic paroxysmal AF, who treated with AV node ablation and pacing, to either continue antiarrhythmic drug therapy (n=68) or not (n=69) [
46].Although in the drug group there were less patients who developed permanent AF (OR 0.43; 95% CI 0.18-0.98), there was no difference in the outcomes between those progressing to chronic AF and those not. Despite the positive impact of drug therapy on developing permanent AF, patients in the drug group had more episodes of HF and hospitalizations.
8. Biventricular pacing in HF and AF post AV node ablation
The Dual chamber and VVI Implantable Defibrillator (DAVID) trial included 506 patients with LVEF ≤ 40% who had indication for ICD implantation [
47]. Patients randomized to either DDDR (70bpm) or VVI (40bpm) pacing mode. Rates of CHF hospitalization were higher in DDDR group (13.3% VVI versus 22.6% DDDR; relative hazard 1.54; 95% CI 0.97-2.46). This difference was due to loss of ventricular synchrony triggered by RV apical pacing (~3% in VVI, ~56% in DDDR). A
post hoc analysis of the MOST trial demonstrated the deleterious effects of RV pacing on LV function caused by electromechanical dyssynchrony between the two chambers [
45]. Thus, RV pacing results in development or worsening of HF.
Several studies have demonstrated the superiority of LV or biventricular (BiV) pacing to RV pacing in patients who underwent AV node ablation for AF (
Table 2).
Table 2.
Comparison of BiV and RV pacing.
Table 2.
Comparison of BiV and RV pacing.
| First author |
Year of Publication |
Number of Patients |
Design |
Highlights |
Puggioni et al.
[48] |
2004 |
44 |
LV pacing vs RV pacing + AVNA |
↑LVEF
↓MR |
Simantirakis et al.
[49] |
2004 |
12 |
LV-based pacing vs RV pacing + AVNA |
↑LV contractility |
Leon et al.
[50] |
2002 |
20 |
BiV pacing vs RV pacing +AVNA |
↑LVEF
↓LV diastolic diameter
↓End-systolic diameter
↓Number of hospitalizations
NYHA class improved |
Doshi et al.
[51] |
2005 |
184 |
BiV pacing vs RV pacing +AVNA |
↑6-min walk distance
↑LVEF
|
Stavrakis et al.
[52] |
2012 |
68 |
CRT vs RV pacing + AVNA
(Meta-analysis) |
↓HF hospitalizations
↑LVEF |
| LV, Left Ventricle; RV, Right Ventricle; AVNA, Atrioventricular Node Ablation; BiV, Biventricular; CRT, Cardiac Resynchronization Therapy; LVEF, Left Ventricular Ejection Fraction; MR, Mitral Regurgitation; NYHA, New York Heart Association; HF, Heart Failure |
A study by
Puggioni et al. included 44 patients with AF who underwent AV node ablation [
48]. They compared RV to LV pacing performed during the first 24 hours after ablation. LV pacing was associated with a more important increase in EF (17.6% in LV versus 11.2% in RV) and a greater decrease in mitral regurgitation (16.7% in LV versus 0% in RV).
Simantirakis et al. included 12 patients (6 with impaired and 6 with normal LV function) with permanent AF treated with AV node ablation plus pacing in their study [
49]. LV-based pacing (LV free wall and BiV pacing) benefited LV contractility and LV filling in these patients compared to RV apical pacing, while there was no difference between LV free wall and BiV pacing.
Leon et al. studied 20 patients with concomitant CHF and AF who initially underwent AV node ablation and RV pacing and subsequently they received a BiV pacemaker [
50]. Changing to BiV pacing showed a significant increase in EF (30.9 ± 11.5% compared to 21.5 ± 6.9% at baseline; p<0.001) and an improvement in NYHA functional classification (2.4 ± 0.6% compared to 3.4 ± 0.5 at baseline; p<0.001).
The Post-AV Nodal Ablation Evaluation (PAVE) trial randomized 184 patients with AF,who treated with AV node ablation, to take either BiV (n=103) or RV (n=81) pacemaker [
51]. At baseline, patients had a mean LVEF of 46% and 83% of them had known HF with NYHA Class II or III. At 6 months after ablation, there was a significant increase in 6-minute walk distance in BiV group compared to this in RV group. Also, in the BiV group there was no difference in LVEF between 6 months post ablation and baseline (46 ± 13% versus 46 ± 16%, respectively). However, at 6 months post ablation the EF in patients with BiV was significantly higher than the RV group (46 ± 13% versus 41 ± 13% respectively; p=0.03).BiV pacemaker seemed to benefit patients with EF ≤ 45% or NYHA II/III more than those with normal EF or NYHA I regarding the 6-minute walk test.
A recent meta-analysis by
Stavrakis et al.evaluated the impact of CRT and RV pacing on patients with AF who underwent AV node ablation [
52]. This study contained 5 trials with 686 patients (413 patients in CRT group and 273 patients in RV group). CRT was associated with a significant decrease in HF hospitalizations (RR 0.38; 95% CI 0.17-0.85; P=0.02) compared to RV pacing and with a significant change in LVEF (mean change 1.97%; 95% CI 1.52-2.42; p<0.00001).
9.1. Indications of BiV pacing or CRT
Current 2021 ESC guidelines on Heart Failure recommend the use of CRT rather than RV pacing for patients with HFrEF (EF<40%) irrespective of NYHA class or QRS width, who are candidates for ventricular pacing for high degree AV block or AF (Class I, Level of Evidence A) [
53].Also, according to the current 2021 ESC guidelines on cardiac pacing and cardiac resynchronization therapy, CRT should be considered for patients with LVEF ≤35% who remain in NYHA class III/IV despite the optimal medical treatment and have AF with inherent QRS ≥ 130ms (Class IIa, Level of Evidence C) [
54]. In patients with symptomatic AF and uncontrolled heart rate, who are eligible for AVJ ablation, CRT is recommended in patients with HFrEF (Class I, Level of Evidence B) and it should be preferred over RV pacing in patients with HFmrEF (Class IIa, Level of Evidence C). These recommendations differ in patients with HFpEF where there is a Class IIa, Level of Evidence B recommendation for RV pacing and a Class IIb, Level of Evidence C for CRT (
Table 3).
Table 3.
ESC Recommendations for CRT implantation in patients with HF ± AF.
Table 3.
ESC Recommendations for CRT implantation in patients with HF ± AF.
| Class |
Level |
Recommendation |
| Class I |
A |
CRT rather than RV pacing is recommended for patients with HFrEF (EF<40%) irrespective of NYHA class or QRS width, who are candidates for ventricular pacing for high degree AV block. Patients with AF are also included in this recommendation. |
| B |
CRT is recommended in patients withHFrEF, symptomatic AFand uncontrolled heart rate who are eligible for AVJ ablation. |
| Class IIa |
B |
AVJ ablation should be added in patients with HF and permanent AF who are eligible for CRT, in case of incomplete biventricular pacing (<90-95%) because of conducted AF. |
| B |
RV pacing should be considered in patients with HFpEF, symptomatic AF and uncontrolled heart rate who are going to undergo AVJ ablation. |
| Β |
Upgrading from RV pacing to CRT should be considered in patients with conventional pacemaker or ICD who develop HF with LVEF ≤35% despite the optimal medical treatment and have a significant proportion of RV pacing. |
| C |
CRT rather than standard RV pacing should be considered in patients with HFmrEF, symptomatic AF and uncontrolled heart rate who are candidates for AVJ ablation. |
| C |
CRT should be considered for patients with HF and LVEF ≤35% who remain in NYHA class III/IV despite the optimal medical treatment and have AF with inherent QRS ≥ 130ms. |
| Class IIb |
B |
CRT may be considered in patients with HFpEF, symptomatic AF and uncontrolled heart rate who are candidates for AVJ ablation. |
9.2. His pacing when biventricular pacing fails
Although BiV pacing is superior to RV apical pacing in patients treated with AV node ablation, approximately 30% of them are non-responders. Also, sometimes the implantation of the LV lead(via the coronary sinus) is not possible or unsuccessful.
His-bundle pacing (HBP) offers a physiological approach to ventricular stimulation by using the native pathway of normal conduction, leading to synchronized ventricular contraction in both ventricles. In cases of failed BiV implantation or non-responders to it, HBP was shown to be efficient as an alternative option [
55].
Deshmukh et al. performed permanent HBP for the first time [
56]. HBP was successful in ~60% of patients with AF, dilated myocardiopathy, LVEF <40% and NYHA III/IV and followed by ablation of AV junction. This procedure was associated with better ventricular systolic function.
Huang et al. studied 52 patients with symptomatic AF and concomitant HF who underwent AV node ablation and HBP [
57]. After a 20-month follow-up a significant increase in LVEF was reported (p<0.001). The reduction in HF was more notable in patients with HFrEF (n=20) than in those with HFpEF (n=22). HBP resulted in improvement in NYHA classification in both HFrEF (from 2.9 ± 0.6 at baseline to 1.4 ± 0.4) and HFpEF (from 2.7 ± 0.6 at baseline to 1.4 ± 0.5). Also, the use of diuretics for management of HF was decreased in both HFrEF and HFpEF in patients with HBP.
Patients with AF and HF who underwent AV node ablation and HBP were enrolled in a recent observational study[
58]. LVEF improved from 44.9 ± 14.9%at baseline to 57.6 ± 12.5%during a median follow-up of 3 years (p<0.001). Also, LVEF ≤40%, serum creatinine≥97 μmol/L and pulmonary artery systolic pressure (PASP) ≥40mmHg at baseline were associated with increased heart failure hospitalization and mortality (p<0.05).
Although non-randomized observational studies have shown the feasibility of HBP plus AVNA in these patients, more data are required.
Huang et al. have recently published the results of a multicenter, randomized trial in which a comparison between BiV pacing and HBP after AVNA in patients with persistent AF and reduced LVEF was performed [
59].All patients enrolled received both BiV and HBP after AVNA and then they were randomized into 2 groups in the phase 1 (BiV pacing or HBP the first 9 months). In the next phase patients were switched to the other pacing modality. There was a significant improvement regarding the primary endpoint (change in LVEF) in the HBP group compared with BiV pacing group (phase 1: ΔLVEF
HBP 21.3% and ΔLVEF
BiV pacing 16.7%; phase 2: ΔLVEF
HBP3.5% and ΔLVEF
BiV pacing -2.4%; p=0.015).Significant improvement in LV end-diastolic diameter, NYHA functional class and B-type natriuretic peptide level was noted in both groups compared to baseline. However, there was no significant difference in these secondary endpoints between BiV pacing and HBP group.
HBP as a treatment option in patients who are eligible for CRT, but the coronary sinus lead implantation is unsuccessful,is included in the 2021 ESC guidelines on pacing and CRT (Class IIa, Level of Evidence B) [
54].Finally, there is a Class IIb, Level of Evidence C recommendation for HBP with a ventricular backup lead when a “pace and ablate” strategy is indicated for rapidly conducted supraventricular arrhythmia.
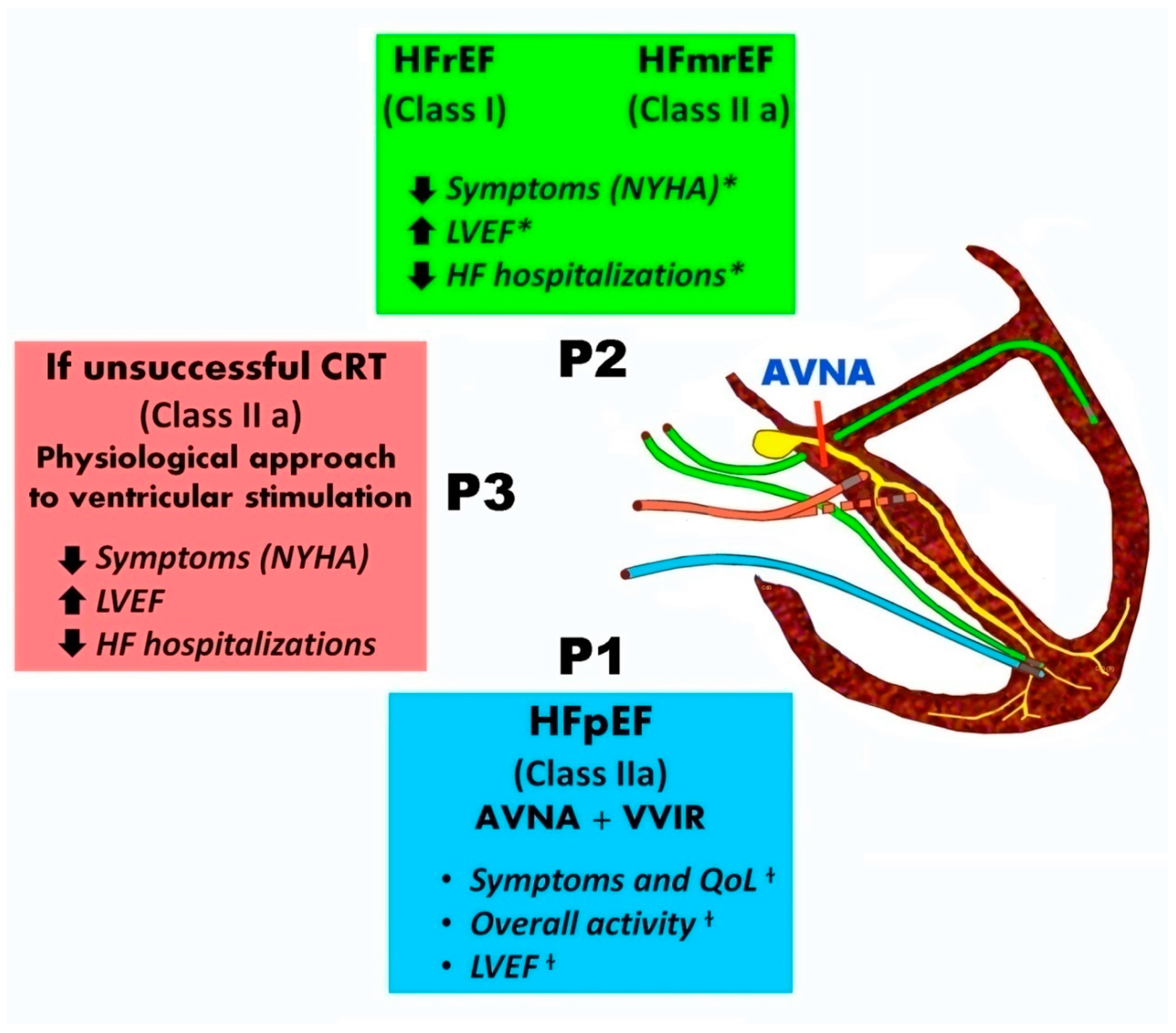
Figure 1 summarizes the indications and benefits of different pacing modalities combined with AV node ablation.
Figure 1.
Patients with AF undergoing AV node ablation indications and advantages of different pacing modalities .In patients with symptomatic AF and uncontrolled heart rate, who are eligible for AVJ ablation, CRT is recommended in patients with HFrEF (Class I, Level of Evidence B) and it should be preferred over RV pacing in patients with HFmrEF (Class IIa, Level of Evidence C). In patients with HFpEF there is a Class IIa, Level of Evidence B recommendation for RV pacing. HBP as a more physiological treatment option is indicated in patients who are eligible for CRT, but the coronary sinus lead implantation is unsuccessful (Class IIa, Level of Evidence B). P1:VVIR pacing, P2 : CRT pacing, P3:His bundle pacing (HBP) , P3 :His pacing , AVNA=AV node ablation, *CRT beneficial outcomes, + AV node plus VVIR pacing benefits based on trials.
Figure 1.
Patients with AF undergoing AV node ablation indications and advantages of different pacing modalities .In patients with symptomatic AF and uncontrolled heart rate, who are eligible for AVJ ablation, CRT is recommended in patients with HFrEF (Class I, Level of Evidence B) and it should be preferred over RV pacing in patients with HFmrEF (Class IIa, Level of Evidence C). In patients with HFpEF there is a Class IIa, Level of Evidence B recommendation for RV pacing. HBP as a more physiological treatment option is indicated in patients who are eligible for CRT, but the coronary sinus lead implantation is unsuccessful (Class IIa, Level of Evidence B). P1:VVIR pacing, P2 : CRT pacing, P3:His bundle pacing (HBP) , P3 :His pacing , AVNA=AV node ablation, *CRT beneficial outcomes, + AV node plus VVIR pacing benefits based on trials.
9.3. Left bundle branch pacing (LBBP) : a promising modality
In recent years conduction system pacing (CSP) has emerged as it can stimulate the two ventricles simultaneously, providing a more physiological pacing. Except for HBP, LBBP is a new alternativeto CRT which is feasible and safe and has been associated with improved clinical outcomes and echocardiographic parameters [
60].Also, LBBP is a very challenging and rapidly developing modality with a shorter learning curve compared to HBP according to a review conducted at the Royal Brompton Hospital [
61].
A very recent study included patients with HF and refractory AF treated either with BiV (26%) or CSP(HBP 54%, LBBP 20%)plus AV node ablation [
62]. Patients in these three groups had similar baseline characteristics. NYHA class improved in both HBP (p<0.001) and LBBP (p=0.008), but not in the BiV group (p=0.096). Similarly, LVEF improved in HBP (39% to 49%, p<0.001) and LBBP (28% to 40%, p=0.041), but it was not increased in the BiV group (p=0.916). In this study CSP showed significant superiotity compared to BiV in terms of clinical and echocardiographic parameters. However, more studies and clinical trials have to be conducted in order to have more data for patients with AF and HF.
10. Conclusion
HF and AF are two cardiovascular conditions affecting many patients around the globe and they are often concomitant. AV node ablation and pacemaker implantation is performed in patients with AF ± HF who do not respond or are intolerant to medical treatment.
Compared to medical therapy, the “Pace and ablate” approach shows beneficial effects on clinical outcomes. In addition to a significant improvement in symptoms, especially palpitations and exercise tolerance, this procedure leads to enhanced cardiac performance and EF. Although ablation of AV junction is shown to reduce mortality in these patients, some early or late adverse effects and complications may occur, including worsening of HF, sudden death and ventricular arrhythmias and permanent AF. However, efficacy and safety of the procedure has been proven in several studies.
Pacemaker implantation is always performed in patients undergoing AV node ablation, as the destruction of physiological conducting system of the heart makes it mandatory. The majority of studies have demonstrated that CRT is the optimal choice of pacing in these patients and is associated with significant improvement in EF and less hemodynamic deterioration, in contrast with classic RV apical pacing.
In recent years, His bundle pacing is an alternative to CRT in case of failure of CRT or in patients who do not respond to it. This approach exploits the patient’s native conducting system in order to cause synchronous ventricular contraction. Recent studies have revealed several positive effects of HBP on patients’ clinical condition and more data will be provided soon by ongoing clinical trials (NCT02805465, NCT02700425). Beyond His pacing, left bundle pacing may confer longer term benefits also, but as yet no trial data is available.
Author Contributions
Conceptualization I.K, D.V., A.R., N.K, I.I,D.G. ; data collection A.G,M.A, A.A,P.P ;methodology, A.G,C.G,MY.H,T.K.; supervision, I.K, T.K, D.G; writing—original draft, I.K,A.G; writing—review and editing, D.V., A.R., N.K, I.I, T.K, D.G . All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
none.
ABBREVIATIONS
HF-Heart Failure; AF- Atrial Fibrillation; CV-Cardiovascular; LVFP-Left Ventricular Filling Pressure; HFpEF- Heart failure with Preserved Ejection Fraction; HFrEF- Heart failure with Reduced Ejection Fraction; HFmrEF-Heart failure with mildly reduced Ejection Fraction; AV node- Atrioventricular node; QOL-Quality of Life; RR- Relative Risk; SCD- Sudden Cardiac Death; RF- Radiofrequency; LV- Left Ventricle; RRR-Relative Risk Reduction ; CI- Confidence Interval; HR- Heart Rate; LVEF- Left Ventricular Ejection Fraction; VT- Ventricular Tachycardia; VF- Ventricular Fibrillation; ECG-Electrocardiogram; ICD-Implantable Cardioverter Defibrillator; RV- Right Ventricle; BiV- Biventricular; EF- Ejection Fraction; CRT- Cardiac Resynchronization Therapy; ΔLVEFHBP- Difference of LVEF between baseline and HBP; ΔLVEFBiV pacing- Difference of LVEF between baseline and BiV pacing; CSP- Conduction System Pacing; LBBP- Left Bundle Branch Pacing; ESC- European Society of Cardiology.
References
- Chugh SS, Havmoeller R, Narayanan K, et al. Worldwide epidemiology of atrial fibrillation: a Global Burden of Disease 2010 Study. Circulation. 2014;129:837–847.
- Benjamin EJ, Muntner P, Alonso A,et al.; American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee. Heart Disease and Stroke Statistics- 2019 Update: A Report From the American Heart Association. Circulation 2019;139:e56–e528.
- Go AS, Hylek EM, Phillips KA, et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA. 2001;285:2370–2375.
- Miyasaka Y, Barnes ME, Gersh BJ, et al. Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation. 2006;114:119–125. [CrossRef]
- Krijthe BP, Kunst A, Benjamin EJ, et al. Projections on the number of individuals with atrial fibrillation in the European Union, from 2000 to 2060. Eur Heart J.2013;34:2746–2751. [CrossRef]
- Middlekauff HR, Stevenson WG, Stevenson LW. Prognostic significance of atrial fibrillation in advanced heart failure: a study of 390 patients. Circulation. 1991;84:40–48. [CrossRef]
- Carson PE, Johnson GR, Dunkman WB, et al. The influence of atrial fibrillation on prognosis in mild to moderate heart failure: the V-HeFT Studies: the V-HeFT VA Cooperative Studies Group. Circulation. 1993;87(suppl):VI-102–VI-110.
- Mahoney P, Kimmel S, DeNofrio D, et al. Prognostic significance of atrial fibrillation in patients at a tertiary medical center referred for heart transplantation because of severe heart failure. Am J Cardiol. 1999;83:1544 –1547. [CrossRef]
- Senni M, Tribouilloy CM, Rodeheffer RJ, et al. Congestive heart failure in the community: a study of all incident cases in Olmsted County, Minnesota, in 1991. Circulation. 1998;98:2282–2289.
- Deedwania PC, Singh BN, Ellenbogen K, et al. Spontaneous conversion and maintenance of sinus rhythm by amiodarone in patients with heart failure and atrial fibrillation: observations from the Veterans Affairs Congestive Heart Failure Survival Trial of Antiarrhythmic Therapy (CHF-STAT): the Department of Veterans Affairs CHF-STAT Investigators. Circulation. 1998;98:2574 –2579.
- Wang TJ, Larson MG, Levy D, et al. Temporal relations of atrial fibrillation and congestive heart failure and their joint influence on mortality: the Framingham Heart Study. Circulation. 2003; 107:2920 –2925.
- Savelieva I., John Camm A. Atrial fibrillation and heart failure: Natural history and pharmacological treatment. Europace. 2004;5(Suppl. 1):S5–S19. [CrossRef]
- Cha Y.M., Redfield M.M., Shen W.K., et al. Atrial fibrillation and ventricular dysfunction: A vicious electromechanical cycle. Circulation. 2004;109:2839–2843.
- Hindricks G, Potpara T, Dagres N, et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association of Cardio-Thoracic Surgery (EACTS). EurHeart J. 2020 Aug 29:ehaa612.
- January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J AmCollCardiol. 2014;64(21):e1–76.
- Wood MA, Brown-Mahoney C, Kay GN, et al. Clinical outcomes after ablation and pacing therapy for atrial fibrillation: a meta-analysis. Circulation 2000;101:1138–44.
- Chatterjee Saurav, SardarPartha, Lichstein Edgar, et al. Pharmacologic rate versus rhythm-control strategies in atrial fibrillation: an updated comprehensive review and meta-analysis. PacingClinElectrophysiol. 2013 Jan;36 (1):122–33.
- Ueng KC, Tsai TP, Tsai CF, et al. Acute and long-term effects of atrioventricular junction ablation and VVIR pacemaker in symptomatic patients with chronic lone atrial fibrillation and normal ventricular response. J Cardiovasc Electrophysiol2001;12:303–9. [CrossRef]
- Brignole M, Gianfranchi L, Menozzi C, et al. Influence of atrioventricular junction radiofrequency ablation in patients with chronic atrial fibrillation and flutter on quality of life and cardiac performance. Am J Cardiol1994;74:242–6. [CrossRef]
- Brignole M, Gianfranchi L, Menozzi C, et al. Assessment of atrioventricular junction ablation and DDDR mode-switching pacemaker versus pharmacological treatment in patients with severely symptomatic paroxysmal atrial fibrillation: a randomized controlled study. Circulation 1997;96:2617–24.
- Marshall HJ, Harris ZI, Griffith MJ, Holder RL, Gammage MD. Prospective randomized study of ablation and pacing versus medical therapy for paroxysmal atrial fibrillation: effects of pacing mode and mode-switch algorithm. Circulation. 1999 Mar 30;99(12):1587-92.
- Brignole M, Menozzi C, Gianfranchi L, et al. Assessment of atrioventricular junction ablation and VVIR pacemaker versus pharmacological treatment in patients with heart failure and chronic atrial fibrillation: a randomized, controlled study. Circulation 1998;98:953–60.
- Weerasooriya R, Davis M, Powell A, et al. The Australian Intervention Randomized Control of Rate in Atrial Fibrillation Trial (AIRCRAFT). J Am Coll Cardiol2003;41:1697–702.
- Wood MA, Brown-Mahoney C, Kay GN, et al. Clinical outcomes after ablation and pacing therapy for atrial fibrillation: a meta-analysis. Circulation 2000;101:1138–44.
- Ozcan C, Jahangir A, Friedman PA, et al. Long-term survival after ablation of the atrioventricular node and implantation of a permanent pacemaker in patients with atrial fibrillation. N. Engl. J. Med. 2001;344 (14):1043– 51.
- Yeung-Lai-Wah JA, Qi A, Uzun O, Humphries K, et al. Long-term survival following radiofrequency catheter ablation of atrioventricular junction for atrial fibrillation: clinical and ablation determinants of mortality. J Interv Card Electrophysiol2002;6:17–23. [CrossRef]
- Darpo B, Walfridsson H, Aunes M, et al. Incidence of sudden death after radiofrequency ablation of the atrioventricular junction for atrial fibrillation. Am J Cardiol1997;80:1174–7. [CrossRef]
- Brignole M, Pentimalli F, Palmisano P, et al. AV junction ablation and cardiac resynchronization for patients with permanent atrial fibrillation and narrow QRS: the APAF-CRT mortality trial. Eur Heart J. 2021 Dec 7;42(46):4731-4739.
- Healey JS, Yee R, Tang A. Right ventricular apical pacing: a necessary evil?.Curr. Opin. Cardiol. 2007;22 (1):33–8. [CrossRef]
- Tops LF, Schalij MJ, Bax JJ. The effects of right ventricular apical pacing on ventricular function and dyssynchrony implications for therapy. J. Am. Coll. Cardiol. 2009;54 (9):764 76. [CrossRef]
- Vanderheyden M, Goethals M, Anguera I, et al. Hemodynamic deterioration following radiofrequency ablation of the atrioventricular conduction system. Pacing ClinElectrophysiol. 1997;20 (10 Pt 1):2422–8.
- Twidale N, McDonald T, Nave K, et al. Comparison of the effects of AV nodal ablation versus AV nodal modification in patients with congestive heart failure and uncontrolled atrial fibrillation. Pacing ClinElectrophysiol. 1998;21 (4 Pt 1):641–51. [CrossRef]
- Björkenheim A, Brandes A, Andersson T, et al. Predictors of hospitalization for heart failure and of all-cause mortality after atrioventricular nodal ablation and right ventricular pacing for atrial fibrillation. Europace. 2014;16 (12):1772–8. [CrossRef]
- Ozcan C, Jahangir A, Friedman PA, et al. Long-term survival after ablation of the atrioventricular node and implantation of a permanent pacemaker in patients with atrial fibrillation. N. Engl. J. Med. 2001;344 (14):1043– 51.
- Peters RH, Wever EF, Hauer RN, et al. Bradycardia dependent QT prolongation and ventricular fibrillation following catheter ablation of the atrioventricular junction with radiofrequency energy. Pacing ClinElectrophysiol1994;17:108–12. [CrossRef]
- eelen P, Brugada J, Andries E, et al. Ventricular fibrillation and sudden death after radiofrequency catheter ablation of the atrioventricular junction. Pacing Clin Electrophysiol1997;20:343–8. [CrossRef]
- Jensen SM, Bergfeldt L, Rosenqvist M. Long-term follow-up of patients treated by radiofrequency ablation of the atrioventricular junction. Pacing Clin Electrophysiol1995;18:1609– 14. [CrossRef]
- Wang RX, Lee HC, Hodge DO, et al. Effect of pacing method on risk of sudden death after atrioventricular node ablation and pacemaker implantation in patients with atrial fibrillation. Heart Rhythm. 2013;10 (5):696–701. [CrossRef]
- Cellarier G, Deharo JC, Chalvidan T, et al. Prolonged QT interval and altered QT/RR relation early after radiofrequency ablation of the atrioventricular junction. Am J Cardiol1999;83:1671–4, A7. [CrossRef]
- Raj SR, Gillis AM, Mitchell B, et al. Paced QT dispersion and QT morphology after radiofrequency atrioventricular junction ablation: impact of left ventricular function. Pacing Clin Electrophysiol2003;26:662–8.
- Hamdan MH, Page RL, Sheehan CJ, et al. Increased sympathetic activity after atrioventricular junction ablation in patients with chronic atrial fibrillation. J Am Coll Cardiol2000;36:151–8. [CrossRef]
- Queiroga A,Marshall HJ, Clune M, et al. Ablate and pace revisited: Long-term survival and predictors of permanent atrial fibrillation. Heart. 2003 Sep;89(9):1035-8. [CrossRef]
- Gianfranchi L, Brignole M, Menozzi C, et al. Progression of permanent atrial fibrillation after atrioventricular junction ablation and dual-chamber pacemaker implantationin patients with paroxysmal atrial tachyarrhythmias. AmJCardiol1998;81:351–4.
- Gribbin GM, Bourke JP, McComb JM. Predictors of atrial rhythm after atrioventricular node ablation for the treatment of paroxysmal atrial arrhythmias. Heart 1998;79:548–53. [CrossRef]
- Sweeney MO, Hellkamp AS, Ellenbogen KA, et al. Adverse effect of ventricular pacing on heart failure and atrial fibrillation among patients with normal baseline QRS duration in a clinical trial of pacemaker therapy for sinus node dysfunction. Circulation 2003;107:2932–7. [CrossRef]
- Brignole M, Menozzi C, Gasparini M, et al. An evaluation of the strategy of maintenance of sinus rhythm by antiarrhythmic drug therapy after ablation and pacing therapy in patients with paroxysmal atrial fibrillation. Eur Heart J2002;23:892–900. [CrossRef]
- Wilkoff BL, Cook JR, Epstein AE, et al. Dual-chamber pacing or ventricular backup pacing in patients with an implantable defibrillator: the Dual Chamber and VVI Implantable Defibrillator (DAVID) Trial. JAMA 2002;288:3115–23.
- Puggioni E, Brignole M, Gammage M, et al. Acute comparative effect of right and left ventricular pacing in patients with permanent atrial fibrillation. J Am Coll Cardiol2004;43:234–8.
- Simantirakis EN, Vardakis KE, Kochiadakis GE, et al. Left ventricular mechanics during right ventricular apical or left ventricular-based pacing in patients with chronic atrial fibrillation after atrioventricular junction ablation. J Am CollCardiol2004;43:1013–8. [CrossRef]
- Leon AR, Greenberg JM, Kanuru N, et al. Cardiac resynchronization in patients with congestive heart failure and chronic atrial fibrillation: effect of upgrading to biventricular pacing after chronic right ventricular pacing. J Am Coll Cardiol2002;39:1258–63.
- Doshi RN, Daoud EG, Fellows C, et al. Left ventricular-based cardiac stimulation post AV nodal ablation evaluation (the PAVE study). J. Cardiovasc. Electrophysiol. 2005;16 (11):1160–5. [CrossRef]
- Stavrakis S, Garabelli P, Reynolds DW. Cardiac resynchronization therapy after atrioventricular junction ablation for symptomatic atrial fibrillation: a meta-analysis. Europace. 2012;14 (10):1490–7. [CrossRef]
- McDonagh TA, Metra M, Adamo M, et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021 Sep 21;42(36):3599-3726. [CrossRef]
- Glikson M, Nielsen JC, Kronborg MB, et al. 2021 ESC Guidelines on cardiac pacing and cardiac resynchronization therapy. Eur Heart J. 2021 Sep 14;42(35):3427-3520.
- Sharma PS, Dandamudi G, Herweg B, et al. Permanent His-bundle pacing as an alternative to biventricular pacing for cardiac resynchronization therapy: A multicenter experience. Heart Rhythm. 2018; 15: 413-420. [CrossRef]
- Deshmukh P, Casavant DA, Romanyshyn M, et al. Permanent, direct His-bundle pacing: a novel approach to cardiac pacing in patients with normal His-Purkinje activation. Circulation 2000;101:869–77.
- Huang W, Su L, Wu S, et al. Benefits of permanent His bundle pacing combined with atrioventricular node ablation in atrial fibrillation patients with heart failure with both preserved and reduced left ventricular ejection fraction. J Am Heart Assoc. 2017;6(4):e005309. [CrossRef]
- Su L, Cai M, Wu S, Wang S, Xu T, et al. Long-term performance and risk factors analysis after permanent His-bundle pacing and atrioventricular node ablation in patients with atrial fibrillation and heart failure. Europace. 2020 Dec 26;22(Suppl_2):ii19-ii26. [CrossRef]
- Huang W, Wang S, Su L, et al. His-bundle pacing vs biventricular pacing following atrioventricular nodal ablation in patients with atrial fibrillation and reduced ejection fraction: A multicenter, randomized, crossover study-The ALTERNATIVE-AF trial. Heart Rhythm. 2022 Dec;19(12):1948-1955. [CrossRef]
- Vijayaraman P, Ponnusamy S, Cano Ó, et al. Left Bundle Branch Area Pacing for Cardiac Resynchronization Therapy: Results From the International LBBAP Collaborative Study Group. JACC Clin Electrophysiol. 2021 Feb;7(2):135-147.
- O’Connor M, Shi R, Kramer DB, Riad O, Hunnybun D, Jarman JWE, Foran J, Cantor E, Markides V, Wong T. Conduction system pacing learning curve: Left bundle pacing compared to His bundle pacing. Int J Cardiol Heart Vasc. 2023 Jan 10;44:101171. [CrossRef]
- Ivanovski M, Mrak M, Mežnar AZ, et al. Biventricular versus Conduction System Pacing after Atrioventricular Node Ablation in Heart Failure Patients with Atrial Fibrillation. J Cardiovasc Dev Dis. 2022 Jul 1;9(7):209.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).