1. Introduction
The application of microbial inoculants in agricultural systems is earning more interest in modern agriculture, since these inoculants have the potential to improve plant growth and enhance availability of nutrients in soil [
1,
2]
. It was reported that the application of bio-inoculants could improve plant growth even under unfavorable soil conditions such as acidic soils with low content of available nutrients [
3]. Acidic soil is considered to be major problem in arable lands worldwide [4-6]. Soil acidity could have many negative effects such as reducing soil structure quality, decreasing the availability of essential nutrients such as phosphorus [
7], increasing soil toxicity due to the release and accumulation of toxic elements such as aluminum [
8] and inhibiting beneficial communities of the microorganisms in soil [
9].
Although the plant beneficial role of fungi is in general understudied [
10] it is well established that arbuscular mycorrhizal fungi (AMF), which form a beneficial symbiotic relationship with most crop plants [
11,
12] are able to improve the availability of soil nutrients [
13], reduce nutrient leaching [
14], improve soil structure [
15], and improve plant tolerance to biotic and abiotic stress [
13]. AMF can also increase plant growth and resistance under suboptimal acidic soil conditions [
3].
Plant growth-promoting rhizobacteria (PGPR) are a promising potential tool to sustainable agricultural production [
16,
17]. PGPR are a group of bacteria which can improve growth by different mechanisms [
18,
19]. Many species of PGPR enhance the availability of essential nutrients and improve the efficiency of the applied nutrients [
20,
21], provide growth hormones to the plants [
22,
23] and improve plant resistance against pathogens [
24] and abiotic stress [
25].
Kosakonia radicincitans (formerly
Enterobacter radicincitans) is a bacterium belonging to the PGPR which is able to colonize plant surface and tissues [
26].
K. radicincitans can provide many advantages to plant due to its ability to fix atmospheric N, solubilizing P, producing growth hormones, and inhibiting pathogenic fungi [27-30].
Activities and advantages provided by microorganisms in the soil are highly correlated to the soil conditions such as soil content of the available nutrients and the organic matter (OM). It was reported that the application of organic fertilizer can improve the microbial activity in soil [
31], soil structure soil [
32], and increase soil pH value after application to acidic soil soil [
33].
However, the effect of the application of microbial inoculants in combination with organic fertilizer could be different depending on soil pH and content of available nutrients. The aim of this work is to investigate the differentiation of barley plant responses to the application of microbial inoculants containing AMF and/or PGPR alone or in combination with cattle manure in soils with intermediate and low pH values.
2. Materials and Methods
2.1. Site description
A field experiment was established in Rostock, northern Germany, about 15 km in land from the Baltic Sea. The study area is strongly affected by marine conditions. The climate is temperate with annual rainfall of about 593 mm. The mean annual temperature is 8.1 °C. The soil texture is loamy sand, the dominating soil type on the site is a stagnic cambisol. Two sites with different soil properties were selected with the main characteristics of both sites presented in
Table 1.
2.2. Microbial inoculants
Arbuscular mycorrhizal fungi
The AMF preparation used was a commercial product (Mycorrhiza granulates from the company INOQ GmbH in Germany). The AMF product was a mix of three Glomus species (Glomus etunicatum, G. intraradices and G. claroideum) with spore concentration of 105 l-1. The carrier material was expanded clay with grain size of 2 to 4 mm with a pH value of 7.5. According to the manufacturer's instructions, 100 ml of the AMF preparation were applied per square meter of soil.
The plant inoculation process with AMF was different between the two experiments.
In the first experiment, the barley seeds were treated with the fungicide Aagrano (chemical compound Imazalil), and AMF spores were added into the root zones of the young plants four weeks after sowing to avoid the negative effect of the fungicide on AMF growth. Cracks in the soil among the plant rows were made manually using a mattock and then the AMF spores were added into the soil along the rows. Following this, the soil was introduced back over the spores.
In the second experiment, the seeds were not treated with fungicide to avoid the delay of AMF addition. Instead, barley seeds were treated by x-ray in the Fraunhofer Institute for Electron Beam and Plasma Technology in Dresden, Germany. Using this technology, seeds are treated with low energy electrons for seed dressing to inactivate the pathogenic organisms on their surfaces and in the seed coats [
34]. Therefore, the application of AMF was possible without delay. AMF were added using the sowing machine directly before the seeds into the seeding depth.
Kosakonia radicincitans
The bacterial inoculant was prepared at the microbiology laboratory of the Leibniz Institute of Vegetable and Ornamental Crops Groβbeeren (IGZ), Germany.
K. radicincitans cells were grown in a standard nutrient solution (MERCK 1) at 29 °C in a rotary incubator at 100 rpm for 48 h [
30].
The seeds were soaked in a bacterial suspension (108 cells mL-1) for 5–10 minutes. Afterwards, they were dried in the dark at room temperature. During the two-leaf growth stage of the plants, additionally the bacterial suspension (108 cells mL-1) was sprayed with a hand pump onto the young plants (1 ml per plant) in all experiments. The aim of the second inoculation was to improve the opportunity for the bacterial cells to colonize and establish on the plants, as well as successfully compete with the native bacterial communities.
2.3. Experimental design
Seven treatments were applied as follows (1) Ctrl (control, without any additions), (2) MF (mineral fertilizer, 120 kg ha
-1 of calcium ammonium nitrate, 27% N, were added in two batches, the first application was 80 kg ha
-1 added directly after sowing, and 40 kg ha
-1 was added five weeks after the first application), (3) KR (
K. radicincitans), (4) AMF, (5) OF (organic fertilizer, 3 L of cattle manure in liquid form were applied per square meter), (6) OF +KR (7) OF + AMF. Characteristics and nutrient content of the organic fertilizer are presented in
Table 2. The manure was analyzed at LUFA laboratory (Agricultural Analysis and Research Institute in Mecklenburg – Vorpommern).
2.4. Plant and soil analysis
Three random soil samples were collected from each plot before sowing. The soil samples were dried at room temperature and then sieved using a 2 mm sieve.
For pH determination, 10 g of the sieved soil were mixed with 25 ml 0.01 N CaCl2 in a flask, then the suspension was stirred with a glass rod, and after 30 minutes, the suspension was stirred again and then filtered. pH value was measured after 1 h, using an electrode (pH Electrode SenTix 81, Sensor technique Meisberg GmbH).
Soil organic matter was determined by drying fine soil at 105°C for 4 h, and then the samples were weighed (w1). Afterwards, the samples were put into a muffle furnace at 550°C for 4 h and weighed again (w2).
Soil organic matter (SOM) was calculated according to the equation:
For P, K, and Mg determination, 10 g of air-dried soil were mixed in 125 ml Doppel-Lactat, the solution was shaken for 1.5 hrs and then filtered. Then, 25 ml of the filtrated soil solution was mixed with 15 ml vanadate-molybdate mixture and 50 ml DL solution in a volumetric flask. After 2 h, P was measured by spectrophotometer at a wavelength of 430 nm (Spekol 11, Carl Zeiss, Jena). Soil-filtrated suspension was also used for K and Mg determination: K was measured by flame photometer (Elex 6361, Eppendorf) and Mg was measured by spectrometer (Epos Analyzer 5060, Com Eppendorf).
Barley plants were harvested 17 weeks after sowing in the first experiment in 2017 and after 16 weeks in the second experiment in 2018.
After harvest, plant seeds were dried for 96 hours at 60 °C; following this, sample of seeds were milled and prepared for chemical analyses. 2 g of the dry milled seeds were put in a muffle furnace at 550 °C for 4 h, then, the ash was digested in 22 ml HCl (25%) in 50 ml volumetric flasks and put on an electric heater for 15–20 minutes. After cooling, the digestion solution was supplemented with distilled water. Later, the solution was filtered into 50 ml flasks. After filtration, 10 ml from the solution was put into 100 ml volumetric flasks and then the flasks were filled up with distilled water. Afterwards, 15 ml from the solution was transferred into 50 ml volumetric flasks and the volume was supplemented with vanadate-molybdate mixture. After 2 hrs, P was measured by spectrophotometer at a wavelength of 430 nm (Spekol 11, Carl Zeiss, Jena). K and Mg were estimated from in the filtered suspension using flame photometer (Elex 6361, Eppendorf) for K and spectrophotometer (Epos Analyzer 5060, Com Eppendorf) for Mg. Nitrogen was analysed as total N using modified Kjeldahl digestion method.
Soil sampling and microbial analyses
After harvest, three random soil samples were collected from each plot (0–30 cm soil layer). Soil samples were sieved to 2 mm and stored at -20°C until the microbial parameters were measured.
Soil microbial measurements
Substrate-induced respiration (SIR) method was applied to measure the soil microbial biomass and the basal respiration. An infrared gas analyzer is used for the measurements [
35]. The operating principle of the infrared gas analyzer offers an automated system for continuous soil respiration and microbial biomass measurements based on infrared gas analysis. The switching device is controlled by a computer and allows doing measurements each hour of up to 24 samples when switching intervals of 2.5 mins are selected. This allows the use of the SIR method for biomass determination. The system is runned by using a software [
35].
The soil microbial biomass carbon (Cmic) content of soil samples (100 g wet soil, 50 % water holding capacity) was calculated according to the correlation of SIR with the fumigation incubation method. The soil was mixed with glucose (2 mg g−1 soil) and analysed under continuous gas flow at 20 °C ± 1 K. Cmic, which includes all respiratory active soil organisms that are able to metabolize glucose, is expressed as μgCmic g-1dry soil.
Soil basal respiration was measured by the infrared gas analyser without the addition of substrates (20 °C ± 1 K) and expressed as μg CO2-C g-1dry soil h-1.
P-Solubilizing Bacteria
The most probable number of P-solubilizing bacteria was determined using dilution and plating method [
36]. The isolated microorganisms were 5 times tenfold diluted in sterile 0.05 M NaCl. 100 μl of succeeding dilutions were streaked onto solid Muroveć nutrient medium in three replicates and incubated at 29 °C for two weeks [
37]. Muroveć medium consists of (g l
-1) K
2SO
40.2, MgSO
4* 7H
2O 0.4, agar agar 20, glucose 10, L-aspa ragine 1 (both separately filter sterilized and added after autoclaving and cooling down the medium to 60 °C), simultaneously CaCl
2 2.2, Na
3PO
4 x 12H
2O 3.8 were mixed by consistent shaking the medium to precipitate calcium phosphate. After one and two weeks colonies inducing pellucid zones in the medium (zones of P-solubilizing activity) were counted. The number of P-solubilizing bacteria was calculated per g dry soil according the MPN method.
2.5. Statistical Analysis
All statistical analyses were carried out with four replications and the mean values of the four replicates. Soil sampling was done with four replications and with the mixture of three samples of each replicate. The data in all the experiments were subjected to a one-way analysis of variance. One way ANOVA was performed to test the differences among the treatments. The mean values were compared with a post-hoc test followed by a Tukey‘s HSD test at P < 0.05. The data were analysed using Statistica 6.0 (StatSoft 2001) software.
3. Results
3.1. Crop yield and nutrient uptake:
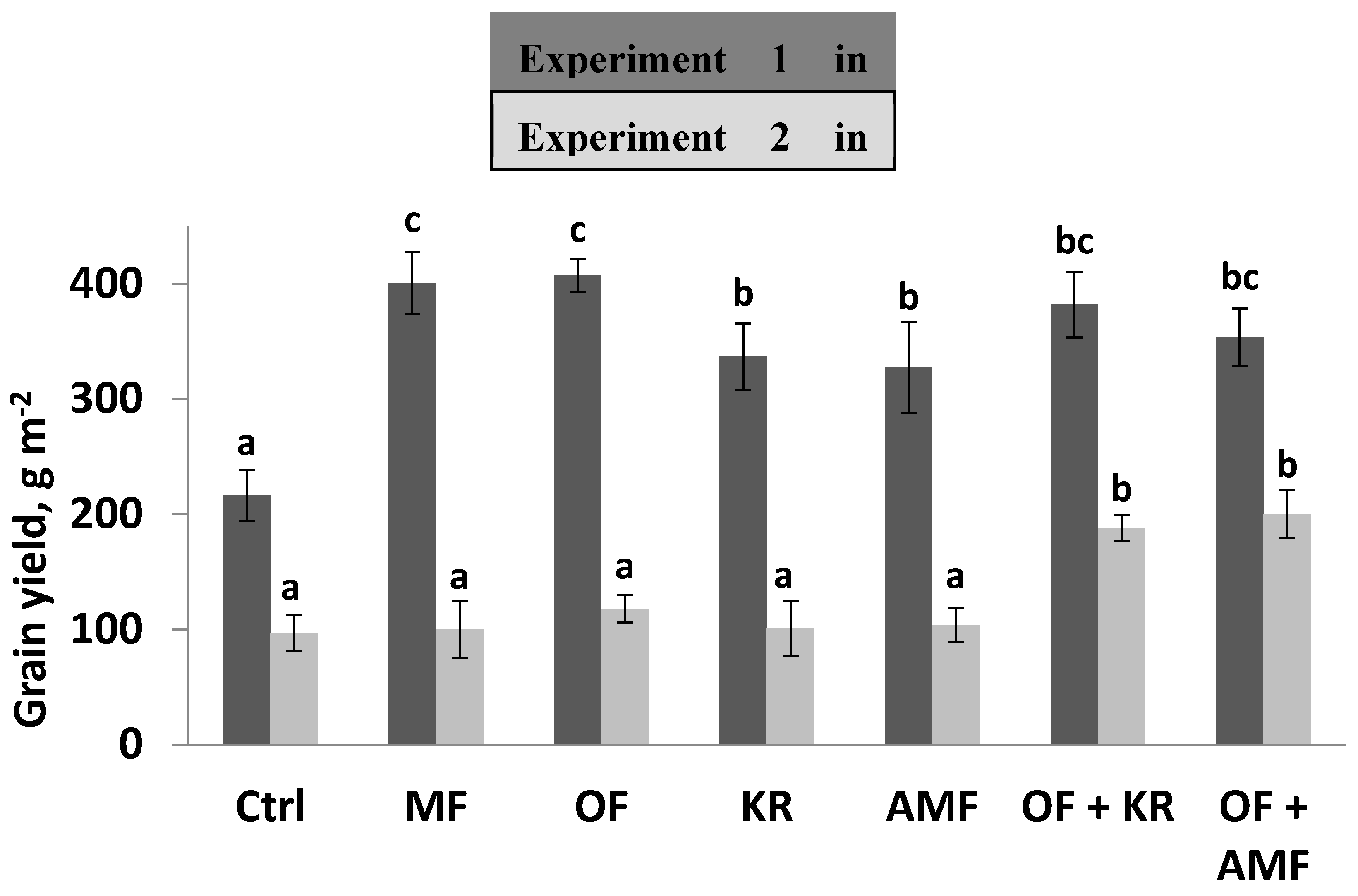
In the first experiment when acidity of soil was medium, the application of single microbial inoculants without other additives enhanced growth and grain yield of barley.
K. radicincitans application increased the yield up to 56% over the control, whereas the yield was increased by 51% over the control by AMF inoculation (
Figure 1). Grain yield was significantly increased in the treatments of the mineral fertilizer or the organic fertilizer comparing to the control treatment in the first experiment (Fig. 1). The single application of the cattle manure enhanced grain yield of barley up to 88% over the non-fertilized control. Similarly, the mineral fertilizer application increased barley yield (85%) compared to the control. The uptake of the nutrients in seeds was significantly affected by the application of the microbial inoculants (
Table 3). The content of P and N was significantly increased after the application of AMF or the
K. radicincitans over the control.
The single application of either mineral or organic fertilizers improved nutrients (P, N, K, and Mg) uptake in comparison to the control in the first experiment (
Table 3). The combined application of the organic fertilizer with AMF or with
K. radicincitans had no significant effect either on grain yield or on nutrient uptake in comparison to the single application of the organic fertilizer, AMF, the
K. radicincitans, or the control in the first experiment (Fig. 1,
Table 3).
In contrast to the first experiment, single application of either mineral fertilizer or the manure had no effect on grain yield or nutrient uptake in the second experiment when soil pH value was 4.9 and P content was low (Fig. 1 and
Table 4). Grain yield was significantly higher by the combined application of the organic fertilizer with AMF or
K. radicincitans compared to the single applications of mineral fertilizer, organic fertilizer, AMF,
K. radicincitans, and the control (Fig. 1).
Barley grain yield was significantly enhanced by the combined application of the cattle manure with the microbial inoculants (Fig.1). The combined application of the organic fertilizer and
K. radicincitans increased the grain yield 95% over the control, 60% over the single application of the organic fertilizer, and 86% over the single application of the
K. radicincitan. The combined treatment of the organic fertilizer and AMF improved grain yield by 106% over the non-treated control, 86% over the single application of the manure, and 93% over the single application of AMF (Fig. 1). The combined application of the organic fertilizer with AMF or with
K. radicincitans affected positively nutrient uptake (
Table 4). P, N, K, and Mg increased significantly by the application of the organic fertilizer with
K. radicincitans in comparison to the control or the single application of
K. radicincitans. N and Mg uptake was significantly higher over the single application of the organic fertilizer or the
K. radicincitans. Combined application of the organic fertilizer with AMF enhanced significantly N and Mg uptake in comparison to the control or the single application of the AMF (
Table 4).
Effect on soil microbial parameters
The values of soil microbial parameters were much higher in the first experiment than in the second experiment (
Table 5 and
Table 6). However, the experimental treatments did not significantly affect the microbial basal respiration activity nor the soil microbial biomass content (
Table 5 and
Table 6).
Only under low soil pH conditions the application of cattle manure alone and in combination with AMF and
K. radicincitans induced higher microbial activities and soil microbial biomass, which was not significant due to high variability under field experimental conditions (
Table 6). The number of P solubilizing bacteria significantly increased after the combined application of the cattle manure and
K. radicincitans in the first experiment in comparison to the single applications of the organic manure or the non-inoculated control (
Table 5). In the second experiment, the metabolic quotient was higher after the application of AMF singularly or in combination with the organic fertilizer in comparison to the control or the other treatments, and the lowest value was registered after the combined application of the organic fertilizer and the
K. radicincitans (
Table 6).
4. Discussion
In general, the presence and type of substrates and formulation additives are important factors for the growth, establishment, and activity of plant beneficial microorganisms [
38,
2]. Plant growth and yield usually get many advantages by the application of the mineral fertilizer, organic fertilizers, bio-inoculants, or by the combined application of the organic fertilizers and the bio-inoculants. Our results proved that the combined application of the organic fertilizer with the bio-inoculants, either with AMF or with
K. radicincitans significantly improved grain yield and nutrients uptake under both soil pH and P content conditions. The effect of single applications of the mineral fertilizer, organic fertilizer, AMF, and
K. radicincitans on growth and yield of barley at the unfavorable soil conditions (low pH and content of nutrients) was insignificant. This result could be due to the low soil pH value and low content of nutrients. On the other hand, the single treatments of mineral fertilizer, organic fertilizer, AMF, and
K. radicincitans contributed significantly to grain yield and nutrients uptake at the first experiment when soil pH value was medium, and the P content was higher (except the content of K and Mg with AMF and
K. radicincitans). Soil pH values below 5.0 are considered as a plant growth impediment [
39], since plant growth will be disturbed due to negative effects related to low soil pH such as deficiencies of essential nutrients and mineral toxicity [
39,
40]. The application of organic manure resulted in increase of both soil pH value [
41,
42] and organic matter content [
43] thus improving the conditions (higher pH value and organic matter as an energy source for the microbes) for bio inoculants development. These conditions could explain why the combined application of the manure and AMF or
K. radicincitans in the second experiment increased plant growth more than the single applications. Barley plants showed a positive response to the single inoculation of either AMF or
K. radicincitans when soil was medium acidic and have sufficient content of available P in the first experiment. The application of the bio-inoculants to an arable soil with sufficient content P could improve plant growth under conventional agricultural system, since these soils probably have a poor AMF community [
44] and hence the applied AMF inoculum could be able to improve the diversity of the AMF in soil and to establish symbiotic interactions with plant roots [
45].
Soil pH is a very important factor which could affect microbial activity [
46]. The application of the microbial inoculants affected the soil microbial parameters in both experiments, but in general these parameters were more pronounced in the first experiment compared to the second experiment. This difference could be due to the fact that soil conditions (mainly soil pH) were more suitable for microbial growth and activity in the first experiment than in the second experiment.
5. Conclusions
As a conclusion, this study demonstrated that the effect of bio-fertilizers and organic fertilizer is depended on the soil conditions. The combined application of the bio-inoculants with organic fertilizer helped plants to grow under conditions of acid soils. The single application of the mineral fertilizer, the organic fertilizer, AMF and K. radicincitans had no significant effect when soil pH and content of nutrients were low, while the combination of the organic fertilizer either with AMF or with K. radicincitans could significantly improve yield and content of nutrients. The effect of the same applications was different under moderate soil pH since the single application of the mineral fertilizer, organic fertilizer or the bio-inoculants increased yield and content of nutrients, but the combined application of the bio inoculants with the organic fertilizer did not further synergistically enhance this effect.
The preliminary results of this study gave the first indication of possible soil quality impacts on microbial plant interactions, which however should be proven with formulated microorganisms.
Author Contributions
Conceptualization and methodology MA, BEL, SR; writing—original draft preparation, MA; writing—MA, SR, NV. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding. The work of NV was related to the European Union’s Horizon 2020 Research and Innovation Program, project EXCALIBUR under grant agreement No. 817946 and partly by the EU project SUSTAINABLE, EU grant agreement no. 101007702.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Araújo, A.; Leite; L. , Iwata, B.; Lira Junior, M.; Xavier, G.; Figueiredo, M. Microbiological process in agroforestry systems. A review. Agron. Sustain. Dev. 2012, 32, 215–226. [Google Scholar] [CrossRef]
- Vassileva, M.; Flor-Peregrin, E.; Malusa, E.; Vassilev, N. Towards Better Understanding of the Interactions and Efficient Application of Plant Beneficial Prebiotics, Probiotics, Postbiotics and Synbiotics. Front. Plant Sci. 2020, 11, 1068. [Google Scholar] [CrossRef] [PubMed]
- Clark, C.; Zeto, S.; Zobel, R. Arbuscular mycorrhizal fungal isolate effectiveness on growth and root colonization of Panicum virgatum in acidic soil. Soil. Biol. Biochem. 1999, 31, 1757–1763. [Google Scholar] [CrossRef]
- Haug, A. Molecular aspects of aluminum toxicity. CRC Crit. Rev. Plant. Sci. 1983, 1, 345–373. [Google Scholar] [CrossRef]
- Eswaran, H.; Reich, P.; Beinroth, F. Global distribution of soils with acidity. In: Moniz AC et al. (Ed) Plant-Soil Interactions at Low pH. Brazilian Soil Science Society 1997, pp. 159-164.
- Bian, M.; Zhou, M.; Sun, D.; Li, C. Molecular Approaches Unravel the Mechanism of Acid Soil Tolerance in Plants. Crop J. 2013, 1, 91–104, (Chapter 1). [Google Scholar] [CrossRef]
- Schroth, G. , Sinclair, F. Trees, Crops and Soil Fertility Concepts and Research Methods. CABI Publishing. 2003, 451 pp. UK.
- Foy, C. Physiological effects of hydrogen, aluminium and manganese toxicities in acid soil. In: Pearson RW, Adams F (eds). Soil acidity and liming, 2nd edn. Wisconsin: American Society of Agronomy, 1984, 57–97.
- Hollier, C.; Reid, M. Agriculture notes, Acid Soils, 2005. ISSN 1329–8062.
- Vassileva, M.; Mendes, G.d.O.; Deriu, M.A.; Benedetto, G.d.; Flor-Peregrin, E.; Mocali, S.; Martos, V.; Vassilev, N. Fungi, P-Solubilization, and Plant Nutrition. Microorganisms 2022, 10, 1716. [Google Scholar] [CrossRef]
- Harley, J.; Smith, S. Mycorrhizal Symbiosis. 1983, Academic Press, Toronto.
- Barber, N. , Kiers, E., Theis, N., Hazzard, R., Adler, L. Linking agricultural practices, mycorrhizal fungi, and traits mediating plant-insect interactions. Ecol. Appl. 2013, 23, 1519–1530. [Google Scholar] [CrossRef]
- Lehnert, H. , Serfling, A., Ordon, F. Impact of a vesicular arbuscular mycorrhiza symbiosis on biotic and abiotic stress tolerance of wheat. Berichte aus dem Julius Kühn-Institut, Young Scientists Meeting 2012.
- Van der Heijden, M.G. Mycorrhizal fungi reduce nutrient loss from model grassland ecosystems. Ecology, 2010, 91, 1163–71. [Google Scholar] [CrossRef]
- Qiang-Sheng, W. , Ren-Xue, X., Ying-Ning, Z. Osmotic solute responses of mycorrhizal citrus (Poncirus trifoliata) seedlings to drought stress. Act. Physiol. Planta., 2007, 29, 543–549. [Google Scholar]
- Figueiredo, M. , Seldin, L., Araujo, F., Mariano, R. Plant growth promoting rhizobacteria: fundamentals and applications. In: Maheshwari DK (Ed.), Plant Growth and Health Promoting Bacteria. Springer-Verlag, Berlin, Heidelberg, 2011, pp. 21– 42.
- Shilev, S.; Azaizeh, H.; Vassilev, N.; Georgiev, D.; Babrikova, I. “Interactions in soil-microbe-plant system: adaptation to stressed agriculture,” in Microbial Interventions in Agriculture and Environment, eds D. Singh, V. Gupta, and R. Prabha (Singapore: Springer), 2019, 131–171. [CrossRef]
- Gupta, A.; Gopal, M.; Tilak, K. Mechanism of plant growth promotion by rhizobacteria. Indian J. Exp. Biol. 2000, 38, 856–862. [Google Scholar]
- Glick, B. Plant Growth-Promoting Bacteria: Mechanisms and Applications. Scientifica, Volume 2012, Article ID 96 3401, 2012, 15 pages. [Google Scholar] [CrossRef]
- Khalid, A.; Arshad, M.; Shaharoona, B.; Mahmood, T. Plant growth promoting rhizobacteria and sustainable agriculture. In: Microbial Strategies for Crop Improvement. MS Khan et al. (ed). Springer-Verlag Berlin Heidelberg. 2009, pp.133.
- Ramanjaneyulu, A; Giri, G. ; Kumar, S. Biofertilizers, nitrogen and phosphorus on yield and nutrient economy in forage sorghum affected by nutrient management in preceding mustard. Biores. Managem., 2010, 1, 66–68. [Google Scholar]
- Glick, B.; Todorovic, B.; Czarny, J.; Cheng, Z.; Duan, J.; McConkey, B. Promotion of plant growth by bacterial ACC deaminase. Critical Review in Plant Science, 2005, 26:227–242.
- Etesami, H. , Hosseini, H.M.; Alikhani, H.A. () Bacterial biosynthesis of 1-aminocyclopropane-1-caboxylate (ACC) deaminase, a useful trait to elongation and endophytic colonization of the roots of rice under constant flooded conditions. Physiol. Mol. Biol. Plants 2014, 20, 425–434. [Google Scholar] [CrossRef] [PubMed]
- Kloepper, J. , Ryu, C., Zhang, S. Induced systemic resistance and promotion of plant growth by Bacillus species. Phytopathology 2004, 94, 1259–1266. [Google Scholar] [CrossRef]
- Glick, B. Phytoremediation: synergistic use of plants and bacteria to clean up the environment. Biotechnol. Adv. 2003, 21, 383–393. [Google Scholar] [CrossRef]
- Remus, R.; Ruppel, S.; Jacob, H-J; Hecht-Buchholz, C. ; Merbach, W. Colonization behaviour of two enterobacterial strains on cereals. Biol. Fert. Soils, 2000, 30, 550–557. [Google Scholar] [CrossRef]
- Scholz-Seidel, C. , Ruppel, S. Nitrogenase- and phytohormone activities of Pantoea agglomerans in culture and their reaction in combination with wheat plants. Zbl. Mikrobiol. 1992, 147, 319–328. [Google Scholar]
- Ruppel, S.; Merbach, W. Effects of different nitrogen sources on nitrogen fixation and bacterial growth of Pantoea agglomerans and Azospirillum spp. in bacterial pure culture: an investigation using 15N2 and acetylene incubation. Microbiol. Res. 1995, 150, 1–10. [Google Scholar] [CrossRef]
- Schilling, G.; Gransee, A.; Deubel, A.; Lezovic, G.; Ruppel, S. Phosphorous availability, root exudates, and microbial activity in the rhizosphere. J. Plant Nutr. Soil Sci. 1998, 161, 465–478. [Google Scholar] [CrossRef]
- Ruppel, S. , Rühlmann, J., Merbach, W. Quantification and localization of bacteria in plant tissues using quantitative real-time PCR and online emission fingerprinting. Plant Soil, 2006, 286, 21–35. [Google Scholar] [CrossRef]
- Lazcano, C.; Gómez-Brandón, M.; Revilla, P.; Domínguez, J. Short-term effects of organic and inorganic fertilizers on soil microbial community structure and function. A field study with sweet corn. Biol. Fert. Soils, 2013, 49, 723–733. [Google Scholar] [CrossRef]
- Li, J.; Zhong, X.; Wang, F.; Zhao, Q. () Effect of poultry litter and livestock manure on soil physical and biological indicators in a rice–wheat rotation system", Plant Soil Environ–UZEI, 2011, 57, p.351-356.
- Whalen, J. , Chang, C., Clayton, G., Carefoot, J. Cattle manure amendments can increase the pH of acid soils. Soil Sci. Soc. America J., 2000, 64, 962–966. [Google Scholar] [CrossRef]
- Eschrig, U.; Stahl, M.; Delincee, H. ; Jürgen Schaller, H; Röder, O. Electron seed dressing of barley-aspects of its verification. Eur. Food Res. Technol 2007, 224, 489–497. [Google Scholar] [CrossRef]
- Heinemeyer, O.; Insam, H.; Kaiser, E.; Walenzik, G. Soil microbial biomass and respiration measurements - an automated technique based on infrared gas-analysis. Plant Soil, 1989, 116, 191–195. [Google Scholar] [CrossRef]
- Bast, E. Mikrobiologische Arbeitsmethoden: Eine Einführung in grundlegende Arbeitstechniken, Spektrum, 1999, Akad. Verl. GmbH, Heidelberg, Berlin.
- Deubel, A. Einfluss wurzelbürtiger organischer Kohlenstoffverbindun-gen auf Wachstum und Phosphatmobilisierungsleistung verschiedener Rhizosphärenbakterien. Aachen: Shaker, 1996, 114 p.
- Vassilev, N.; Malusá, E.; Requena, A.R.; Martos, V.; López, A.; Maksimovic, I.; Vassileva, M. Potential Application of Glycerol in the Production of Plant Beneficial Microorganisms. J. Ind. Microbiol. Biotechnol., 2017, 44, 735–743. [Google Scholar] [CrossRef]
- Marschner, H. Mechanisms of adaptation of plants to acid soils. Plant Soil 1991, 134, 1–24. [Google Scholar] [CrossRef]
- Kidd, P, Proctor, J. Why plants grow poorly on very acid soils: are ecologists missing the obvious? J. Exp. Bot., 2001, 52, 791–799. [Google Scholar] [CrossRef]
- Warren, S. , Fonteno, W. () Changes in physical and chemical properties of a loamy sand soil when amended with composted poultry litter. J. Environ. Hortic. 1993, 11, 186–190. [Google Scholar] [CrossRef]
- Eghball, B. , Wienhold, B., Woodbury, B., Eigenberg, R. Plant availability of phosphorus in swine slurry and cattle feedlot manure. Agron. J. 2005, 97, 542–548. [Google Scholar] [CrossRef]
- Aguilera, P.; Demanet, R.; Palma, G. Effect of liquid cow manure on chemical and biological properties in an andisol, R. C. Suelo Nutr. Veg. 2010, 10(2), 158–169. [Google Scholar] [CrossRef]
- Daniell,T. ; Husband, R.; Fitter, A.; Young, J. () Molecular diversity of arbuscular mycorrhizal fungi colonising arable crops. FEMS Microbiol. Ecol., 2001, 36, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Eason, W.; Scullion, J.; Scott, E. Soil parameters and plant responses associated with arbuscular mycorrhizas from contrasting grassland management regimes. Agricul., Ecosys. Environ. 1999, 73, 245–255. [Google Scholar] [CrossRef]
- Rousk, J.; Brookes, P.; Bååth, E. Contrasting soil pH effects on fungal and bacterial growth suggests functional redundancy in carbon mineralisation. Appl. Environ. Microbiol. 2009, 75, 1589–1596. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).