Submitted:
25 May 2023
Posted:
29 May 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Study area

2.2. Study species and occurrence data
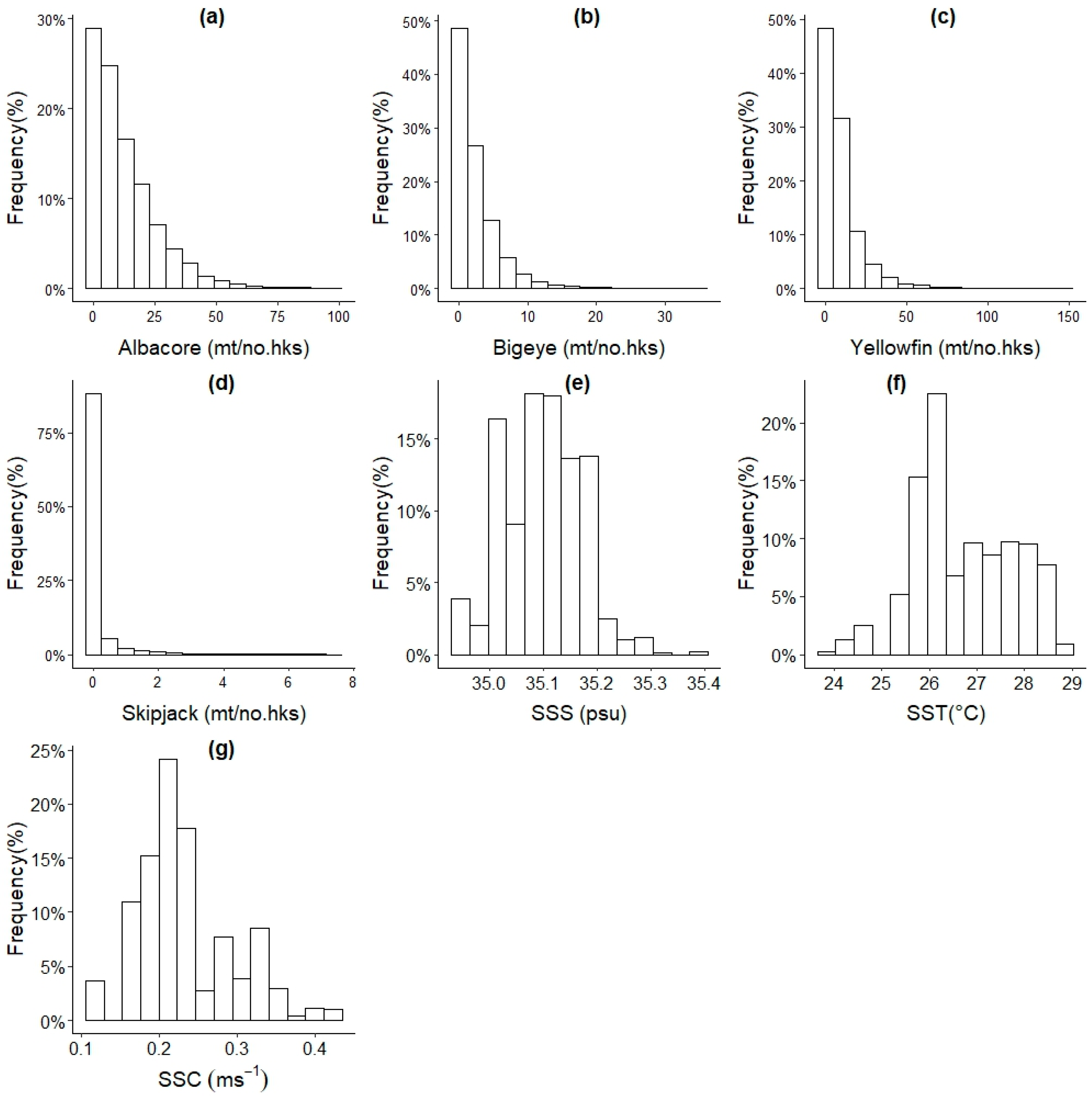
2.3. Predictor variables
| Provider | Variable/code | Resolutions | Units |
|---|---|---|---|
|
Tonga tuna longline fisheries |
Catch per unit effort (CPUE) | Daily, 1 degree2 |
mt/no. hks/ record |
|
Bio–ORACLE version 2.0 dataset, bio-oracle.org |
Sea surface salinity (SSS) | Long term mean, 5 arcmin, ≈ 9.2 km at equator, raster layers |
PSU |
| Sea surface current (SSC) |
ms-1 |
||
| Sea surface temperature (SST) |
°C |
2.4. Species distribution modelling
2.5. Climatic suitable areas
3. Results
3.1. Performances of Species Distribution Modelling
3.2. Relative contribution of predictor variables
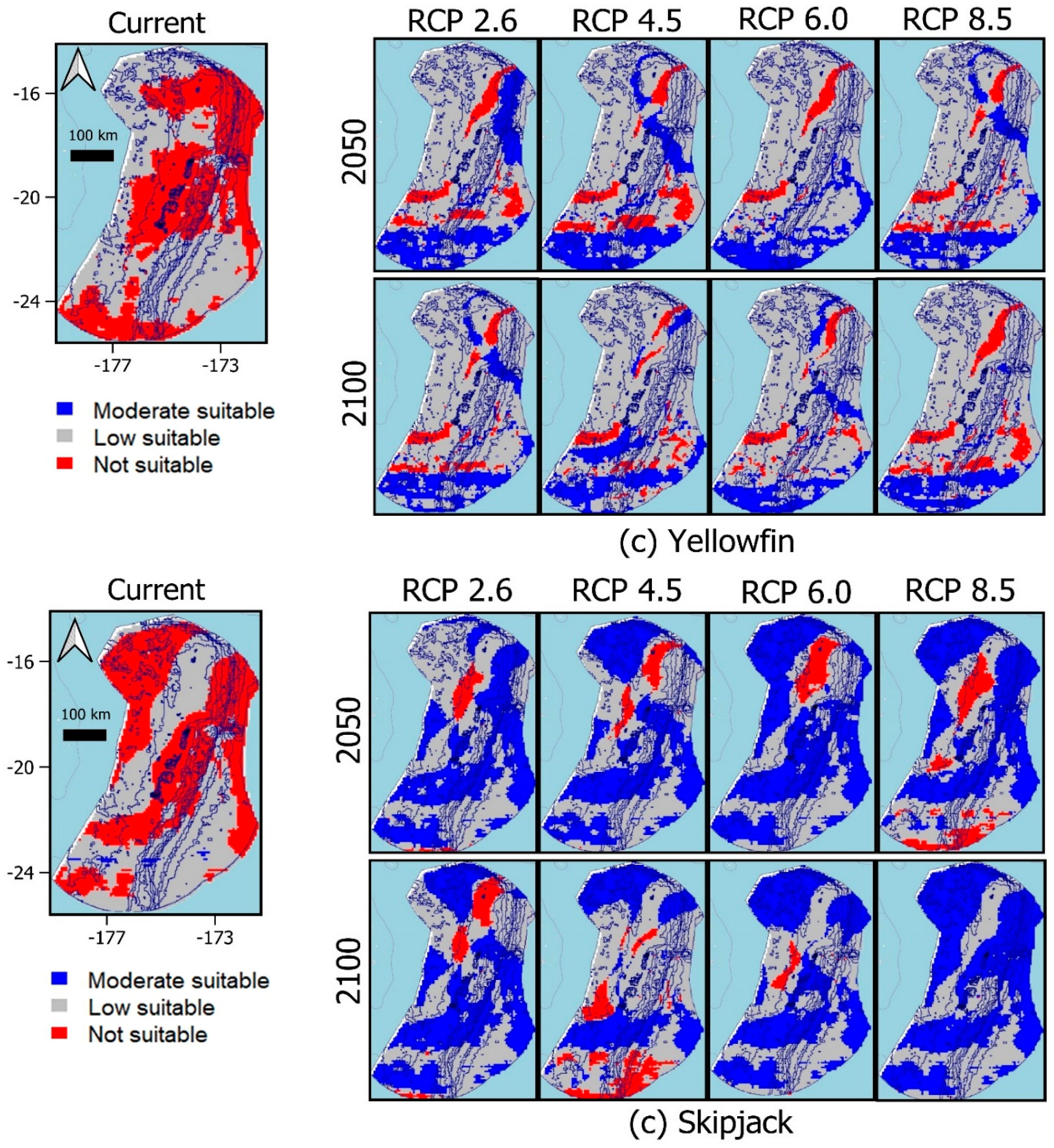
3.3. Predicted suitability habitat
|
Scenario |
Albacore (km2) |
Bigeye (km2) |
Yellowfin (km2) | Skipjack (km2) |
Total (km2) |
|---|---|---|---|---|---|
| Current | 10,222 | 32,876 | 18,503 | 17,350 | 78,951 |
| RCP 2.6/2050 | 11,338 | 39,758 | 20,064 | 22,901 | 94,062 |
| RCP 2.6/2100 | 11,105 | 43,466 | 19,787 | 26,158 | 100,515 |
| RCP 4.5/2050 | 11,549 | 40,012 | 20,123 | 24,059 | 95,744 |
| RCP 4.5/2100 | 11,011 | 33,558 | 19,353 | 22,000 | 85,923 |
| RCP 6.0/2050 | 12,317 | 54,573 | 20,143 | 32,725 | 119,759 |
| RCP 6.0/2100 | 11,670 | 41,539 | 19,667 | 29,964 | 102,840 |
| RCP 8.5/2050 | 11,542 | 37,452 | 20,139 | 25,422 | 95,555 |
| RCP 8.5/2100 | 13,095 | 48,053 | 20,015 | 56,682 | 137,845 |
|
Scenario |
% Increase relative to current scenario | ||||
|---|---|---|---|---|---|
| Albacore | Bigeye | Yellowfin | Skipjack | Total | |
| RCP 2.6/2050 | 10.92 | 20.94 | 8.44 | 31.99 | 19.14 |
| RCP 2.6/2100 | 8.65 | 32.21 | 6.94 | 50.76 | 27.31 |
| RCP 4.5/2050 | 12.99 | 21.71 | 8.76 | 38.67 | 21.27 |
| RCP 4.5/2100 | 7.72 | 2.08 | 4.59 | 26.80 | 8.83 |
| RCP 6.0/2050 | 20.50 | 66.00 | 8.86 | 88.61 | 51.69 |
| RCP 6.0/2100 | 14.17 | 26.35 | 6.29 | 72.70 | 30.26 |
| RCP 8.5/2050 | 12.92 | 13.92 | 8.84 | 46.52 | 19.76 |
| RCP 8.5/2100 | 28.11 | 46.16 | 8.17 | 226.69 | 74.60 |
3.4. Biogeographical distribution of species

4. Discussion
5. Conclusion
Supplementary Materials
Author contributions
Data Availability Statement
Acknowledgements
Conflict of interest
References
- McMahon S.M, Harrison S.P, Armbruster W.S, Bartlein P.J, Beale, C.M, Edwards M.E, Kattge J, Midgley G, Morin X, and Prentice I.C. Improving assessment and modelling of climate change impacts on global terrestrial biodiversity. Trends in Ecology & Evolution. 2011; 26(5): 249–259. [CrossRef]
- Hattab T, Albouy C., Lasram FBR, Somot S., Le Loc’h F. and Leprieur F. Towards a better understanding of potential impacts of climate change on marine species distribution: a multiscale modelling approach. Global Ecology and Biogeography, 2015;23(12), 1417–1429. [CrossRef]
- Mannocci L, Boustany AM, Roberts JJ, Palacios DM, Dunn DC, Halpin PN, et al. Temporal resolutions in species distribution models of highly mobile marine animals: recommendations for ecologists and managers. Divers Distrib. 2017;23(10): 1098–1109. [CrossRef]
- Booth DJ, Feary D, Kobayashi D, Luiz O, Nakamura Y. Tropical Marine Fishes and Fisheries and Climate Change. In: Phillips BF, Pérez-Ramírez M, eds. Climate Change Impacts on Fisheries and Aquaculture: A Global Analysis. Wiley-Blackwell; 2017: 875–896.
- Brierley AS, Kingsford MJ. Impacts of Climate Change on Marine Organisms and Ecosystems. Current Biology. 2009;19(14): 602–614. [CrossRef]
- Dueri S, Bopp L, Maury O. Projecting the impacts of climate change on skipjack tuna abundance and spatial distribution. Global Change Biology. 2014 Mar;20(3): 742–53. [CrossRef]
- Senina I, Lehodey P, Calmettes B, Dessert M, Hampton J, Smith N, Gorgues T, Aumont O, Lengaigne M, Menkes C, Nicol S. Impact of climate change on tropical Pacific tuna and their fisheries in Pacific Islands waters and high seas areas. 14th Regular Session of the Scientific Committee of the Western and Central Pacific Fisheries Commission, WCPFC-SC14; 2018.
- Lehodey P, Senina I, Sibert J, Bopp L, Calmettes B, Hampton J, Murtugudde R. Preliminary forecasts of Pacific bigeye tuna population trends under the A2 IPCC scenario. Progress in Oceanography. 2010 Jul 1;86(1-2): 302–315. [CrossRef]
- Dell’Apa A, Carney K, Davenport T.M, Carle M.V. Potential Medium-Term Impacts of Climate Change on Tuna and Billfish in the Gulf of Mexico: A Qualitative Framework for Management and Conservation. Marine Environmental Research. 2018;141: 1–11. [CrossRef]
- Marcogliese DJ. The impact of climate change on the parasites and infectious diseases of aquatic animals. Rev Sci Tech. 2008;27(2): 467–84.
- Pörtner HO, Langenbuch M, Michaelidis B. Synergistic effects of temperature extremes, hypoxia, and increases in CO2 on marine animals: from earth history to global change. Journal of Geophysical Research: Oceans. 2005;110(C9).
- Franklin, Janet, Josep M Serra-Diaz, Alexandra D Syphard, and Helen M Regan. "Global Change and Terrestrial Plant Community Dynamics." Proceedings of the National Academy of Sciences 113 (14): 3725–34.
- Ménard F, Lorrain A, Potier M, Marsac F. Isotopic evidence of distinct feeding ecologies and movement patterns in two migratory predators (yellowfin tuna and swordfish) of the western Indian Ocean. Mar Biol. 2007;153(2): 141–52. [CrossRef]
- Richardson DE, Marancik KE, Guyon JR, Lutcavage ME, Galuardi B, Lam CH, Walsh HJ, Wildes S, Yates DA, Hare JA. Discovery of a spawning ground reveals diverse migration strategies in Atlantic bluefin tuna (Thunnus thynnus). Proceedings of the National Academy of Sciences. 2016;113(12): 3299–3304.
- Brouwer S, Pilling G, Hampton J, Williams P, McKechnie S, Tremblay-Boyer L. The Western and Central Pacific Tuna Fishery: 2017 Overview and Status of Stocks. Tuna Fisheries Assessment Report. 2018;18.
- Evans K, Young J.W, Nicol S, Kolody D, Allain V, Bell J, Brown J.N, Ganachaud A, Hobday A.J, Hunt B, Innes J. Optimising fisheries management in relation to tuna catches in the western central Pacific Ocean: A review of research priorities and opportunities. Marine Policy. 2015 Sep 1;59: 94–104. [CrossRef]
- Yeeting AD, Bush SR, Ram-Bidesi V, Bailey M. Implications of new economic policy instruments for tuna management in the western and central Pacific. Mar. Policy. 2016;63: 45–52.
- Gillett R, Tauati M.I. Fisheries of the Pacific Islands: regional and national information. FAO fisheries and aquaculture technical paper. 2018(625): I–400.
- 19. Ministry of Agriculture, Forestry; Fisheries, Fishery Forum Agency, Nuku’alofa, Tonga. Tonga Tuna Fishery Framework 2018–2022.
- Bell JD, Allain V, Sen Gupta A, Johnson J.E, Hampton J, Hobday AJ, Lehodey P. Climate change impacts, vulnerabilities and adaptations: western and central Pacific Ocean marine fisheries. In: Phillips BF, Pérez-Ramírez M, Hall SJ, editors. Impacts of Climate Change on Fisheries and Aquaculture. 2018. 305.
- Brander K.M. Global Fish Production and Climate Change. Proceedings of the National Academy of Sciences. 2007;104(50): 19709–19714.
- Di Lorenzo E, Cobb KM, Furtado JC, Schneider N, Anderson BT, Bracco A, Alexander MA, Vimont DJ. Central Pacific El Niño and Decadal Climate Change in the North Pacific Ocean. Nature Geoscience. 2010;3(11): 762–765. [CrossRef]
- Kumar PS, Pillai GN, Manjusha U. El Nino Southern Oscillation (ENSO) impact on tuna fisheries in Indian Ocean. SpringerPlus. 2014;3: 1–13. [CrossRef]
- Asmamaw B, Beyene B, Tessema M, Assefa A. The Impact of Climate Change and Anthropogenic Activities on Fisheries of Lake Logo, South Wello, Ethiopia. 2019.
- Robinson PH. Impacts of manipulating ration metabolizable lysine and methionine levels on the performance of lactating dairy cows: a systematic review of the literature. Livestock Science. 2010;127(2-3): 115–126. [CrossRef]
- Klemas V. Remote sensing of coastal and ocean currents: an overview. J Coast Res. 2012;28(3): 576–586.
- Mahadevan A. The impact of submesoscale physics on primary productivity of plankton. Annu Rev Mar Sci. 2016;8: 161–84. [CrossRef]
- Arı́stegui J, Tett P, Hernández-Guerra A, Basterretxea G, Montero Ma F, Wild K, Sangrá P, et al. The influence of island-generated eddies on chlorophyll distribution: a study of mesoscale variation around Gran Canaria. Deep Sea Research Part I: Oceanographic Research Papers. 1997;44(1):71–96. [CrossRef]
- Berry PM, Dawson TP, Harrison PA, Pearson RG. Modelling Potential Impacts of Climate Change on the Bioclimatic Envelope of Species in Britain and Ireland. Global Ecology and Biogeography. 2002;11(6):453–462.
- Bell JD, Reid C, Batty MJ, Lehodey P, Rodwell L, Hobday AJ, Johnson JE, Demmke A. Effects of Climate Change on Oceanic Fisheries in the Tropical Pacific: Implications for Economic Development and Food Security. Climatic Change. 2013;119(1): 199–212. [CrossRef]
- Koenigstein S, Mark FC, Gößling-Reisemann S, Reuter H, Poertner H-O. Modelling climate change impacts on marine fish populations: process-based integration of ocean warming, acidification and other environmental drivers. Fish Fish. 2016;17(4): 972–1004.
- Muhling BA, Lee SK, Lamkin JT, Liu Y. Predicting the effects of climate change on bluefin tuna (Thunnus thynnus) spawning habitat in the Gulf of Mexico. ICES J Mar Sci. 2011;68(6): 1051–62. [CrossRef]
- Assis J, Tyberghein L, Bosch S, Verbruggen H, Serrão EA, De Clerck O. Bio-ORACLE V2. 0: extending marine data layers for bioclimatic modelling. Global Ecology and Biogeography. 2018;27(3): 277–284. [CrossRef]
- Stone K, Fenner D, LeBlanc D, Vaisey B, Purcell I, Eliason B. Tonga. In: World Seas: An Environmental Evaluation. New York, NY, USA: Academic Press; 2019.
- Martinez LA, Harris WS, Lee DY. Assessing the importance of catch per unit effort in tuna distribution models for effective fisheries management. J Ocean Fish Stud. 2021;37(2): 105–119.
- Smith JR, Johnson MK, Thompson PQ. The role of catch per unit effort in modeling tuna distribution: implications for sustainable fisheries management. Marine Biology and Fisheries Research. 2022;54(3): 321–334.
- Microsoft Corporation. Microsoft Excel. Retrieved from https://office.microsoft.com/excel.
- Naimi B, Hamm NA, Groen TA, Skidmore AK, Toxopeus AG. Where is positional uncertainty a problem for species distribution modelling? Ecography. 2014;37: 191–203.
- R Development Core Team. R: A language and environment for statistical computing. Vienna: R Foundation for Statistical Computing; 2018. Available from: https://www.R-project.org/.
- Naimi B, Araújo MB. SDM: a reproducible and extensible r platform for species distribution modelling. Ecography. 2016;39(4): 368–75. [CrossRef]
- Liu J, Liu M, Tian H, Zhuang D, Zhang Z, Zhang W, et al. Spatial and temporal patterns of China’s cropland during 1990–2000: an analysis based on Landsat TM data. Remote Sens Environ. 2005;98(4): 442–56. [CrossRef]
- Pearce W, Holmberg K, Hellsten I, Nerlich B. Climate change on Twitter: topics, communities and conversations about the 2013 IPCC Working Group 1 report. PloS one. 2014;9(4): e94785. [CrossRef]
- Cossarizza A, Chang H-D, Radbruch A, Acs A, Adam D, Adam-Klages S, Agace W.W, et al. Guidelines for the Use of Flow Cytometry and Cell Sorting in Immunological Studies. European Journal of Immunology. 2019;49(10): 1457–1973. [CrossRef]
- NOAA National Centers for Environmental Information. (2021). Dataset title. Publication or report title. Date. URL: https://www.gebco.net/data_and_products/gridded_bathymetry_data/.
- Loukos H, Monfray P, Bopp L, Lehodey P. Potential changes in skipjack tuna (Katsuwonus pelamis) habitat from a global warming scenario: modelling approach and preliminary results. Fish Oceanogr. 2003;12(4-5): 474–82. [CrossRef]
- Yen KW, Su NJ, Teemari T, Lee MA, Lu HJ. Predicting the catch potential of skipjack tuna in the western and central Pacific Ocean under different climate change scenarios. J. Mar. Sci. Technol. 2016;24(6): 2.
- Báez JC, Barbosa AM, Pascual P, Ramos ML, Abascal F. Ensemble modeling of the potential distribution of the whale shark in the Atlantic Ocean. Ecology and Evolution. 2020;10(1): 175–184. [CrossRef]
- Dell J, Wilcox C, Hobday A.J. Estimation of Yellowfin Tuna (Thunnus Albacares) Habitat in Waters Adjacent to Australia’s East Coast: Making the Most of Commercial Catch Data. Fisheries Oceanography. 2011;20(5): 383–396. [CrossRef]
- MAFF, FFA. Tonga Tuna Fishery Framework 2013 - 2017. Ministry of Agriculture, Forestry; Fisheries, Fishery Forum Agency, Nuku’alofa, Tonga; 2013.
- Esser LF., Saraiva DD, Jarenkow JA. 2019. Future uncertainties for the distribution and conservation of Paubrasilia echinata under climate change. Acta Botanica Brasilica, 33, 770–776. [CrossRef]
- Köhler M, Esser LF, Font F, Souza-Chies TT, Majure LC. Beyond endemism, expanding conservation efforts: What can new distribution records reveal?. Perspect Plant Ecol Evol Syst. 2020;45: 125543. [CrossRef]
- Eustace A, Esser LF, Mremi R, Malonza PK, Mwaya RT. Protected areas network is not adequate to protect a critically endangered East Africa Chelonian: Modelling distribution of pancake tortoise, Malacochersus tornieri under current and future climates. Plos one. 2021 Jan 20;16(1): e0238669. [CrossRef]
- Llopiz JK, Hobday AJ. A global comparative analysis of the feeding dynamics and environmental conditions of larval tunas, mackerels, and billfishes. Deep Sea Res Part II Top Stud Oceanogr. 2015;113: 113–24. [CrossRef]
- Lan KW, Shimada T, Lee MA, Su NJ, Chang Y. Using remote-sensing environmental and fishery data to map potential yellowfin tuna habitats in the tropical Pacific Ocean. Remote Sens. 2017;9(5): 444. [CrossRef]
- Itoh T, Tsuji S, Nitta A. Migration patterns of young Pacific bluefin tuna (Thunnus orientalis) determined with archival tags. 2003; 12(3), 141–151.
- Reglero P, Ciannelli L, Alvarez-Berastegui D, Balbı́n R, López-Jurado JL, Alemany F. Geographically and environmentally driven spawning distributions of tuna species in the western Mediterranean Sea. Marine Ecology Progress Series. 2012; 463:273–284. [CrossRef]
- Arrizabalaga H, Dufour F, Kell L, Merino G, Ibaibarriaga L, Chust G, Irigoien X. Global habitat preferences of commercially valuable tuna. Deep Sea Research Part II: Topical Studies in Oceanography. 2015;113:102–112.
- Song LM, Zhang YU, Xu LX, Jiang WX, Wang JQ. Environmental preferences of longlining for yellowfin tuna (Thunnus albacares) in the tropical high seas of the Indian Ocean. Fisheries Oceanography. 2008 Jul;17(4): 239–253. [CrossRef]
- Nataniel A, Lopez J, Soto M. Modelling seasonal environmental preferences of tropical tuna purse seine fisheries in the Mozambique Channel. Fish Res. 2021;243: 106073. [CrossRef]
- Bakare AG, Kour G, Akter M, Iji PA. Impact of climate change on sustainable livestock production and existence of wildlife and marine species in the South Pacific Island Countries: a review. International Journal of Biometeorology. 2020;64(8):1 409–1421. [CrossRef]
- Asch RG, Cheung WL, Reygondeau G. Future marine ecosystem drivers, biodiversity, and fisheries maximum catch potential in Pacific Island countries and territories under climate change. Marine Policy. 2018;88: 285–294. [CrossRef]
- Holland KN, Grubbs RD. Fish visitors to seamounts: tunas and bill fish at seamounts. Seamounts: ecology, fisheries & conservation. 2007 Jan 1: 189–201.
- Johnson M, Brown A, Lee H. Geophysical features of the fishing ground and their influence on tuna abundance. Deep Sea Res Part I Oceanogr Res Pap. 2016;111: 84-94.
- Zhou C, He P, Xu L, Bach P, Wang X, Wan R, Tang H, Zhang Y. The effects of mesoscale oceanographic structures and ambient conditions on the catch of albacore tuna in the South Pacific longline fishery. Fish. Oceanogr. 2020;29(3): 238-251. [CrossRef]
- Williams AJ, Allain V, Nicol SJ, Evans KJ, Hoyle SD, Dupoux C, Vourey E, Dubosc J. Vertical behavior and diet of albacore tuna (Thunnus alalunga) vary with latitude in the South Pacific Ocean. Deep Sea Res. Part II Top. Stud. Oceanogr. 2015;113: 154–169. [CrossRef]
- Lee H, Brown A. Nutrient-rich upwelling and its impact on tuna distribution in the eastern Pacific. Fish Oceanogr. 2014;23(5): 375–384.
- Garcia M, Perez A. Influence of seafloor characteristics on the spatial distribution of Yellowfin tuna in the western Indian Ocean. Acta Oceanologica Sinica, 2018;37(10), 95–101.
- Suzuki K, Okochi M, Nakano H. Influence of pelagic prey species on the distribution and abundance of tuna in the western Pacific Ocean. Fish. Sci. 2014;80(3): 511–519.
- Miyake S, Nishida T, Tsukamoto Y. Effect of anchovy abundance on the movement and aggregation of skipjack tuna schools. Fish Res. 2018;205: 82–91.
- Ito Y, Takahashi M, Okamura H. Environmental factors affecting the distribution and abundance of tuna and their prey in the western North Pacific Ocean. Deep Sea Res Part II Top Stud Oceanogr. 2016;140: 261–270.
- Schaefer KM, Fuller DW, Block BA, Halsey LG. Performance of pop-up satellite archival tags. Marine Ecology Progress Series. 2006;329: 287–298.
- Muhling BA, Liu Y, Lee SK, Lamkin JT, Roffer MA, Muller-Karger F, Walter JF 3rd. Potential impact of climate change on the intra-Americas Sea: part 2. Implications for Atlantic bluefin tuna and skipjack tuna adult and larval habitats. J Mar Syst. 2015;148: 1–13.
- Allain V, Kirby D, Kerandel J-A. Seamount Research Planning Workshop Final Report. In: Report of the Seamount Research Planning Workshop Held at the Secretariat of the Pacific Community, Noumea, New Caledonia, 2006; 20–21.
- Dubroca L, Chassot E, Floch L, Demarcq H, Assan C, de Molina AD. Seamounts and tuna fisheries: tuna hotspots or fishermen habits?. In 2012 Inter-sessional meeting of the tropical tuna species group 2014;2087–2102.
- Smith J, Jones R. Limitations of short time series data in research studies. Journal of Research Methodology. 2010;15(2): 75–87.
- Brown A, Smith B, Jones C. Factors influencing travel costs in the fishing industry. Journal of Maritime Economics. 2010;12(2): 97–115.
- Ward D, Lee H, Johnson M. Distance to fishing grounds and travel costs in the tuna fishery. Mar. Policy. 2015;58: 85–93.
- Smith J, Jones R. Vessel efficiency and its impact on travel costs in the tuna fishery. Fisheries Research. 2018;203: 67–74.
- Johnson L, Lee S. The effect of travel costs on tuna catch: a meta-analysis. Rev Fish Sci. 2012;20(4): 258–266.
- Garcia M, Perez A. Nonlinear effects of travel costs on tuna catch: evidence from the eastern Pacific. Fishery Bulletin, 2000;98(3), 540–546.
- Martínez ML, Intralawan A, Vázquez G, Pérez-Maqueo O, Sutton P, Landgrave R. The coasts of our world: ecological, economic and social importance. Ecol Econ. 2007;63(2-3): 254–72. [CrossRef]
- Ferreira AM, Marques JC, Seixas S. "Integrating marine ecosystem conservation and ecosystems services economic valuation: Implications for coastal zones governance." Ecological Indicators, 2017;77, 114–122. [CrossRef]
- Fromentin JM, Powers JE. Atlantic bluefin tuna: population dynamics, ecology, fisheries and management. Fish and fisheries, 2005;6(4), 281–306. [CrossRef]
- Murua H, Rodriguez-Marin E, Neilson JD, Farley JH, Juan-Jordá MJ. Fast versus slow growing tuna species: age, growth, and implications for population dynamics and fisheries management. Rev Fish Biol Fish. 2017;27: 733–73. [CrossRef]
- Jansen T, Watson R. Global marine yield halved as fishing intensity redoubles. Fish Fish. 2013;14(4): 493–503.
- Metian M, Pouil S, Boustany A, Troell M. Farming of Bluefin tuna–reconsidering global estimates and sustainability concerns. Rev Fish Sci Aquac. 2014;22(3): 184–92. [CrossRef]
- Newsome SD, Martinez del Rio C, Bearhop S, Phillips DL. A niche for isotopic ecology. Front Ecol Environ. 2007;5(8): 429–36. [CrossRef]
- Young JW, Lansdell MJ, Campbell RA, Cooper A, Cappo M, Dunning M, Barnes P. Integrating trophic relationships into models for ecosystem-based fisheries management. Rev. Fish Biol. Fish. 2015;25(4): 607–646.
- Block BA, Teo SL, Walli A, Boustany A, Stokesbury MJ, Farwell CJ, Weng KC. Electronic tagging and population structure of Atlantic bluefin tuna. Nature. 2005;434(7037): 1121–1127. [CrossRef]
- Le Pape O, Bonhommeau S. Environmental forcing and Southern Bluefin Tuna. PloS one. 2015;10(5): e0127008.
- Sumaila UR, Cheung WW, Lam VW, Pauly D, Herrick S. Climate change impacts on the biophysics and economics of world fisheries. Nat. Clim. Chang. 2011;1(9): 449–456. [CrossRef]
- Salas S, Chuenpagdee R, Seijo JC, Charles AT, Armijo M. Challenges in the assessment and management of small-scale fisheries in Latin America and the Caribbean. Fish and Fisheries. 2017;18(3): 526–538. [CrossRef]
- Zainuddin M, Saitoh K, Saitoh SI. Albacore (Thunnus alalunga) fishing ground in relation to oceanographic conditions in the western North Pacific Ocean using remotely sensed satellite data. Fish. Oceanogr. 2008;17(2): 61–73. [CrossRef]
- Howell EA, Hawn DR, Polovina JJ. Spatiotemporal variability in bigeye tuna (Thunnus obesus) dive behavior in the central North Pacific Ocean. Prog Oceanogr. 2010;86(1-2): 81–93. [CrossRef]
- Lumban-Gaol J, Leben RR, Vignudelli S, Mahapatra K, Okada Y, Nababan B, et al. Variability of satellite-derived sea surface height anomaly, and its relationship with bigeye tuna (Thunnus obesus) catch in the eastern Indian Ocean. Eur J Remote Sens. 2015;48(1): 465–77.
- Martı́nez-Freirı́a F, Tarroso P, Rebelo H, Brito JC. Contemporary niche contraction affects climate change predictions for elephants and giraffes. Divers Distrib. 2016;22(4): 432–444. [CrossRef]
- Kininmonth S, Blenckner T, Niiranen S, et al. Is Diversity the Missing Link in Coastal Fisheries Management? Diversity. 2022;14(2): 90. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




