Submitted:
26 May 2023
Posted:
29 May 2023
You are already at the latest version
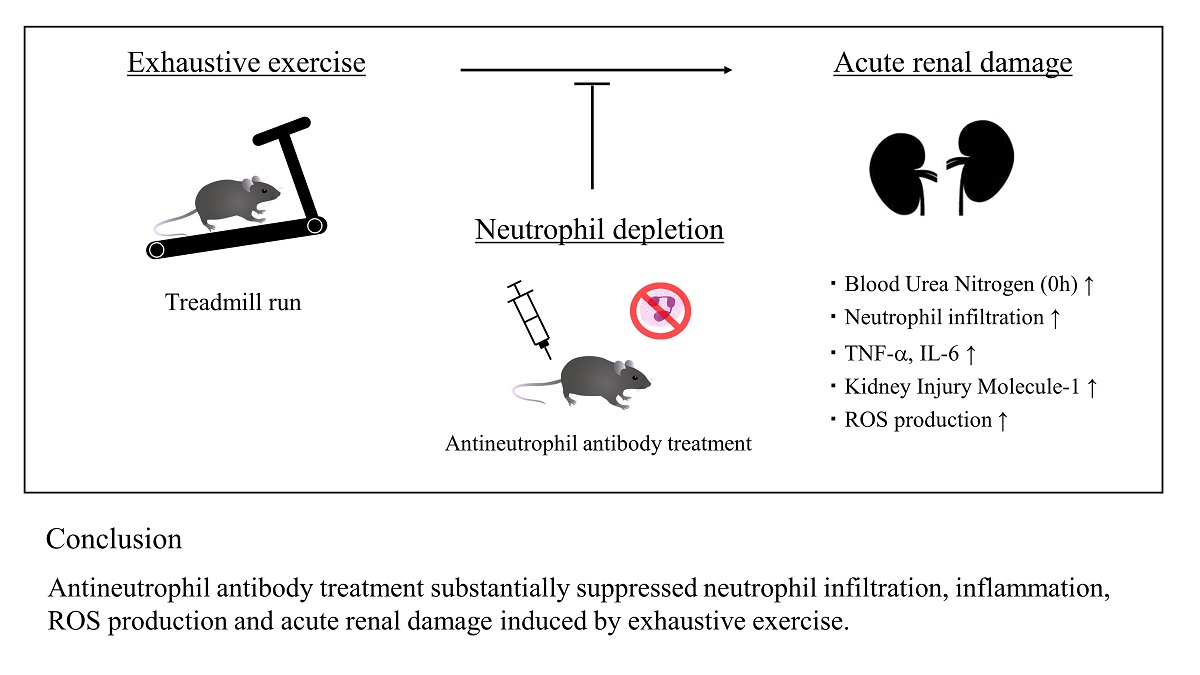
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Injection of antineutrophil antibody
2.3. Exercise protocol
2.4. Blood and kidney sampling
2.5. Assessment of renal function
2.6. Hematoxylin and eosin (H&E) staining
2.7. Immunohistochemical staining
2.8. Terminal deoxynucleotidyl transferase (TdT)-mediated dUTP nick-end labeling (TUNEL) assay
2.9. KIM-1 assay
2.10. Quantitative reverse transcriptase-polymerase chain reaction (RT-PCR)
2.11. Nicotinamide adenine dinucleotide phosphate (NADPH) oxidase activity
2.12. Hydrogen peroxide assay
2.13. Statistical analyses
3. Results
3.1. Running time
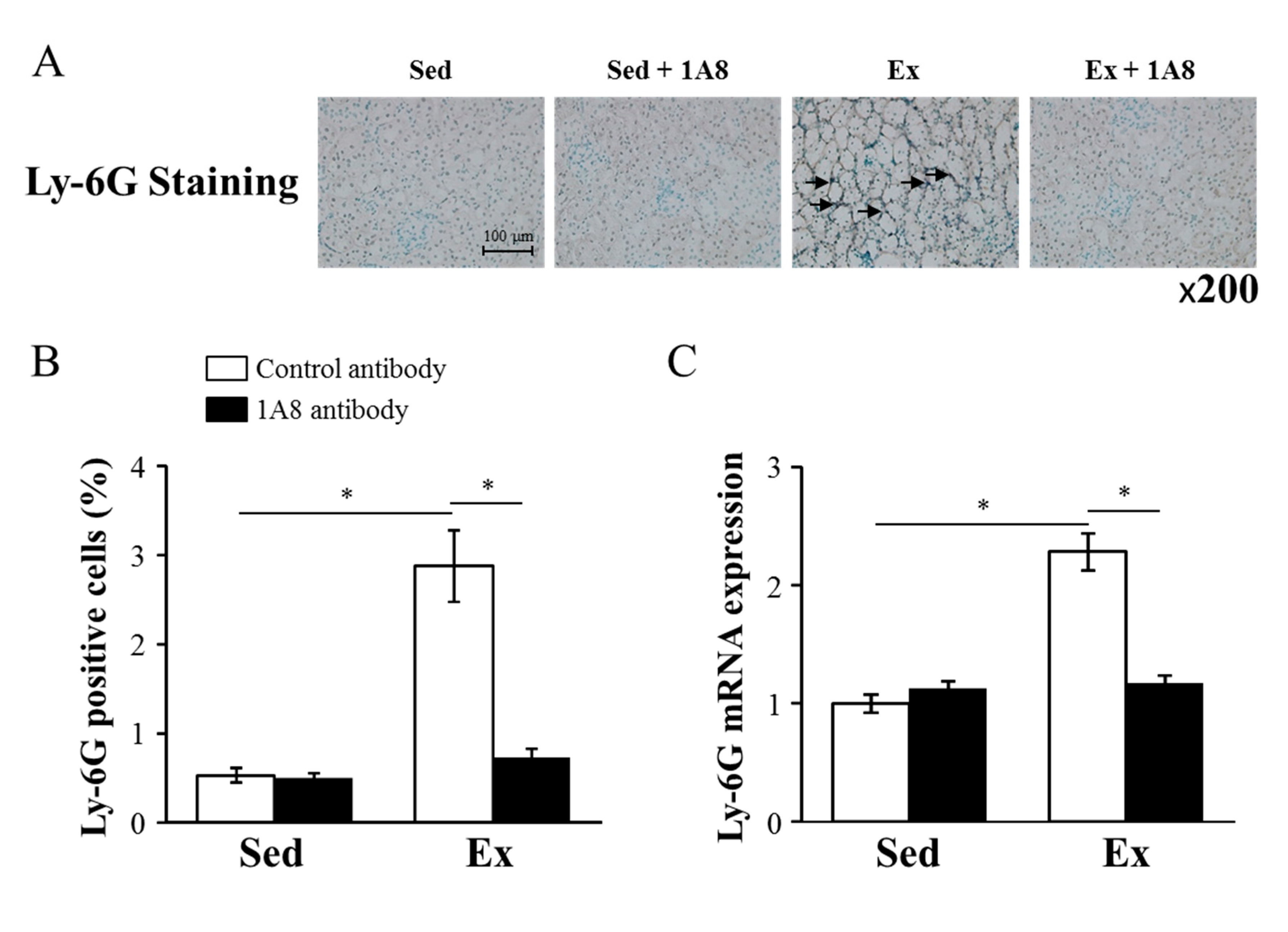
3.2. Neutrophil infiltration into the kidney
3.3. Renal function
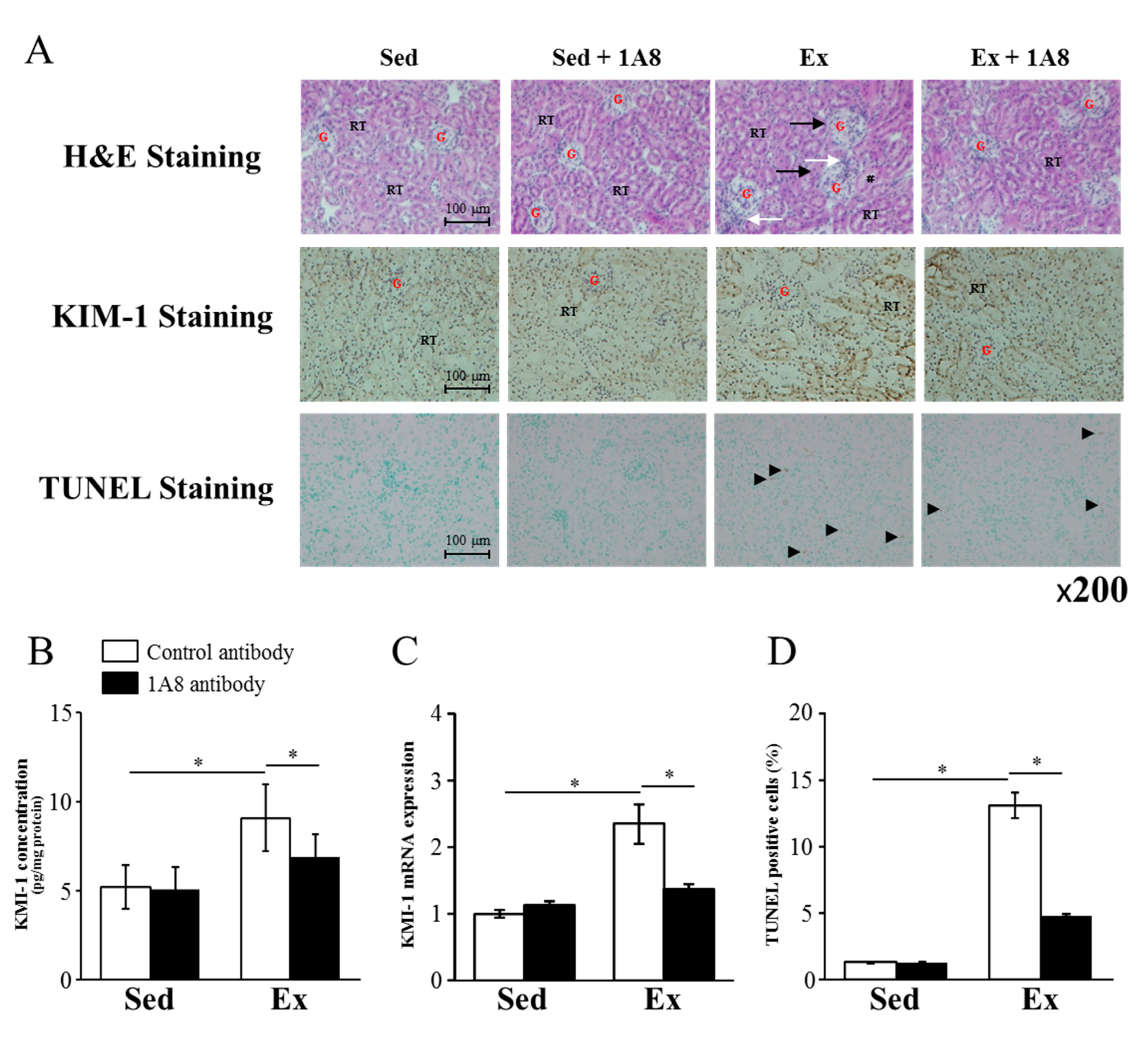
3.4. Renal histology
| Gene | Time | Sed | Sed + 1A8 | Ex | Ex + 1A8 |
|---|---|---|---|---|---|
| BUN (mg/dL) | 0 h | 27.2 ±. 0.9 | 25.4 ± 1.3 | 54.9 ± 1.3* | 45.0 ± 5.0§ |
| 24 h | 25.7 ± 1.0 | 22.6 ± 1.5 | 22.6 ± 1.5 | 19.0 ± 0.8 | |
| CRE (mol/L) | 0 h | 84.0 ± 5.2 | 80.0 ± 7.8 | 87.0 ± 4.2 | 80.0 ± 2.5 |
| 24 h | 76.0 ± 3.0 | 81.0 ± 3.4 | 65.0 ± 3.0 | 69.0 ± 3.4 |
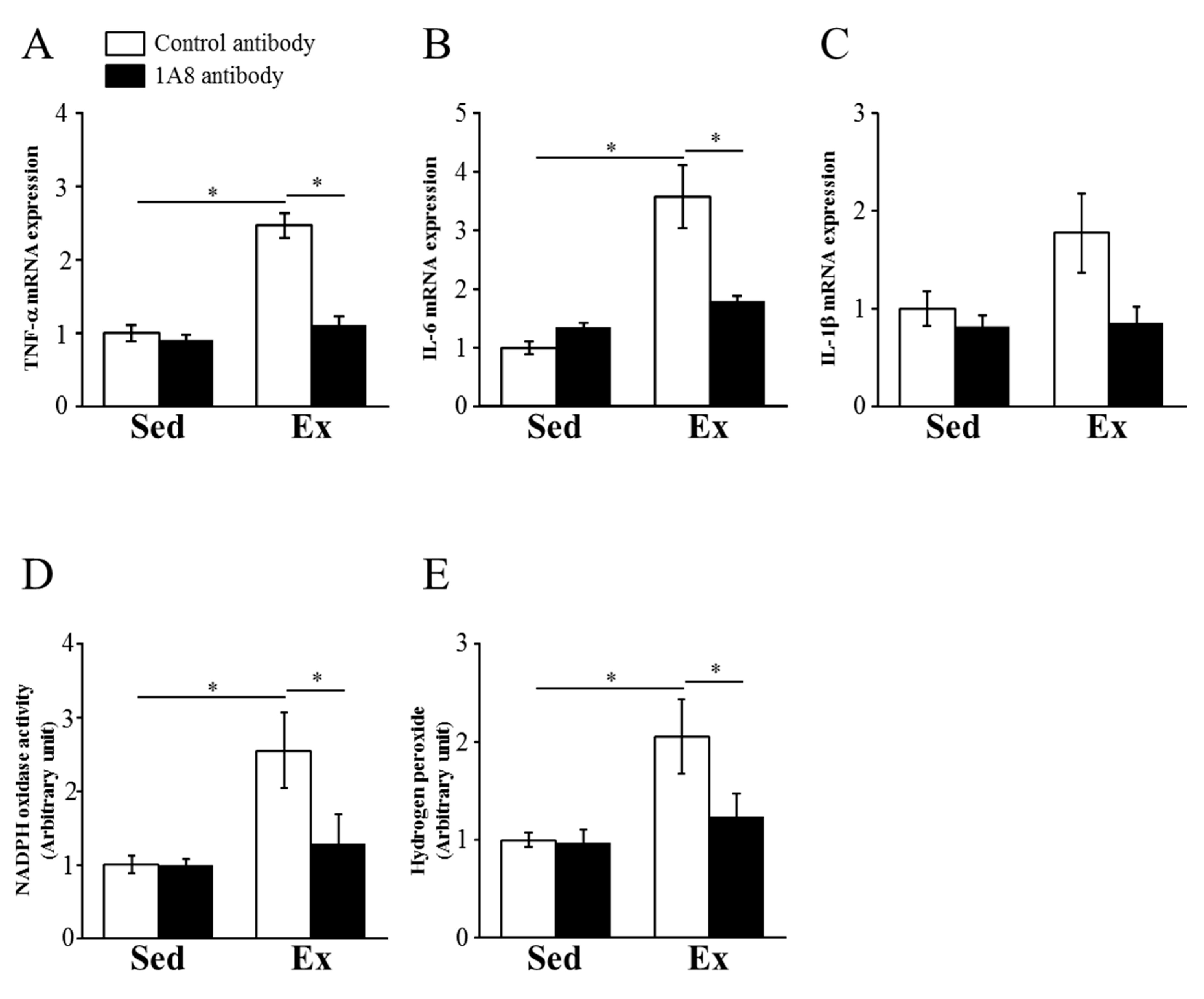
3.5. Inflammatory cytokines and ROS in the kidney
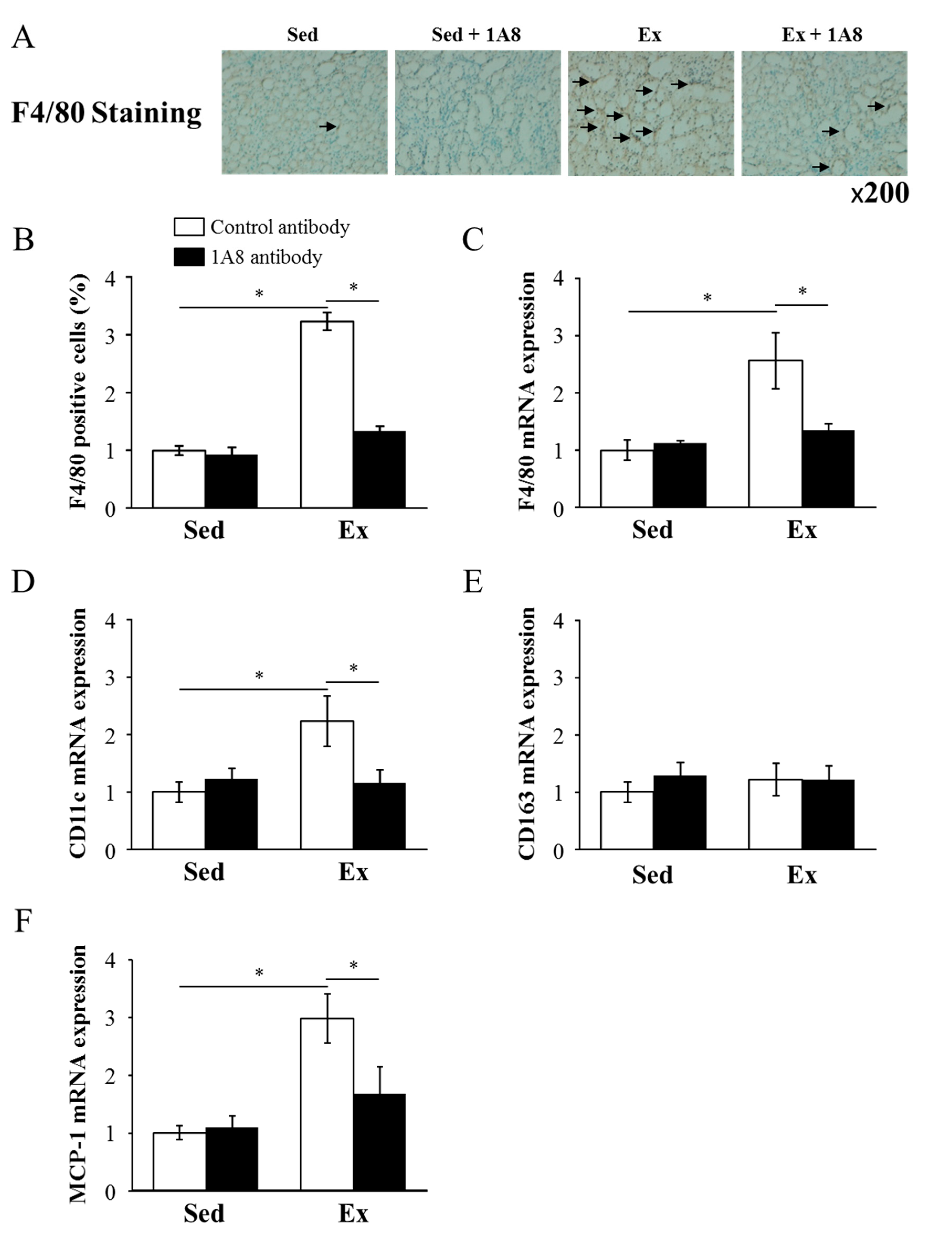
3.6. Macrophage infiltration into the kidney
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Suzuki, K.; Hayashida, H. Effect of Exercise Intensity on Cell-Mediated Immunity. Sports (Basel) 2021, 9. [Google Scholar] [CrossRef]
- Clarkson, P.M. Exertional rhabdomyolysis and acute renal failure in marathon runners. Sports Med 2007, 37, 361–363. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.; Nakaji, S.; Yamada, M.; Liu, Q.; Kurakake, S.; Okamura, N.; Kumae, T.; Umeda, T.; Sugawara, K. Impact of a competitive marathon race on systemic cytokine and neutrophil responses. Med Sci Sports Exerc 2003, 35, 348–355. [Google Scholar] [CrossRef] [PubMed]
- Siegel, A.J.; Hennekens, C.H.; Solomon, H.S.; Van Boeckel, B. Exercise-related hematuria. Findings in a group of marathon runners. JAMA 1979, 241, 391–392. [Google Scholar] [CrossRef]
- Sugama, K.; Suzuki, K.; Yoshitani, K.; Shiraishi, K.; Miura, S.; Yoshioka, H.; Mori, Y.; Kometani, T. Changes of thioredoxin, oxidative stress markers, inflammation and muscle/renal damage following intensive endurance exercise. Exerc Immunol Rev 2015, 21, 130–142. [Google Scholar] [PubMed]
- Hodgson, L.E.; Walter, E.; Venn, R.M.; Galloway, R.; Pitsiladis, Y.; Sardat, F.; Forni, L.G. Acute kidney injury associated with endurance events-is it a cause for concern? A systematic review. BMJ Open Sport Exerc Med 2017, 3, e000093. [Google Scholar] [CrossRef]
- Lin, X.; Jiang, C.; Luo, Z.; Qu, S. Protective effect of erythropoietin on renal injury induced in rats by four weeks of exhaustive exercise. BMC Nephrol 2013, 14, 130. [Google Scholar] [CrossRef]
- Mizokami, T.; Shimada, M.; Suzuki, K. Macrophage Depletion Attenuates Acute Renal Damage after Exhaustive Exercise in Mice. Int J Sports Med 2022, 43, 964–970. [Google Scholar] [CrossRef]
- Zuk, A.; Bonventre, J.V. Acute Kidney Injury. Annu Rev Med 2016, 67, 293–307. [Google Scholar] [CrossRef]
- Bonventre, J.V.; Yang, L. Cellular pathophysiology of ischemic acute kidney injury. J Clin Invest 2011, 121, 4210–4221. [Google Scholar] [CrossRef]
- Friedewald, J.J.; Rabb, H. Inflammatory cells in ischemic acute renal failure. Kidney Int 2004, 66, 486–491. [Google Scholar] [CrossRef]
- Day, Y.J.; Huang, L.; Ye, H.; Linden, J.; Okusa, M.D. Renal ischemia-reperfusion injury and adenosine 2A receptor-mediated tissue protection: role of macrophages. Am J Physiol Renal Physiol 2005, 288, F722–F731. [Google Scholar] [CrossRef]
- Oh, D.J.; Dursun, B.; He, Z.; Lu, L.; Hoke, T.S.; Ljubanovic, D.; Faubel, S.; Edelstein, C.L. Fractalkine receptor (CX3CR1) inhibition is protective against ischemic acute renal failure in mice. Am J Physiol Renal Physiol 2008, 294, F264–F271. [Google Scholar] [CrossRef] [PubMed]
- Jo, S.K.; Sung, S.A.; Cho, W.Y.; Go, K.J.; Kim, H.K. Macrophages contribute to the initiation of ischaemic acute renal failure in rats. Nephrol Dial Transplant 2006, 21, 1231–1239. [Google Scholar] [CrossRef] [PubMed]
- Castenfors, J.; Mossfeldt, F.; Piscator, M. Effect of prolonged heavy exercise on renal function and urinary protein excretion. Acta Physiol Scand 1967, 70, 194–206. [Google Scholar] [CrossRef]
- Poortmans, J.R. Exercise and renal function. Sports Med 1984, 1, 125–153. [Google Scholar] [CrossRef]
- Hellberg, P.O.; Kallskog, T.O. Neutrophil-mediated post-ischemic tubular leakage in the rat kidney. Kidney Int 1989, 36, 555–561. [Google Scholar] [CrossRef]
- Klausner, J.M.; Paterson, I.S.; Goldman, G.; Kobzik, L.; Rodzen, C.; Lawrence, R.; Valeri, C.R.; Shepro, D.; Hechtman, H.B. Postischemic renal injury is mediated by neutrophils and leukotrienes. Am J Physiol 1989, 256, F794–F802. [Google Scholar] [CrossRef]
- Grenz, A.; Kim, J.H.; Bauerle, J.D.; Tak, E.; Eltzschig, H.K.; Clambey, E.T. Adora2b adenosine receptor signaling protects during acute kidney injury via inhibition of neutrophil-dependent TNF-alpha release. J Immunol 2012, 189, 4566–4573. [Google Scholar] [CrossRef]
- Kelly, K.J.; Williams, W.W., Jr.; Colvin, R.B.; Bonventre, J.V. Antibody to intercellular adhesion molecule 1 protects the kidney against ischemic injury. Proc Natl Acad Sci U S A 1994, 91, 812–816. [Google Scholar] [CrossRef]
- Kelly, K.J.; Williams, W.W., Jr.; Colvin, R.B.; Meehan, S.M.; Springer, T.A.; Gutierrez-Ramos, J.C.; Bonventre, J.V. Intercellular adhesion molecule-1-deficient mice are protected against ischemic renal injury. J Clin Invest 1996, 97, 1056–1063. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.L.; Chen, Y.S.; Huang, X.D.; Zhang, L.X. Exhaustive swimming exercise related kidney injury in rats - protective effects of acetylbritannilactone. Int J Sports Med 2012, 33, 1–7. [Google Scholar] [CrossRef]
- Soehnlein, O.; Zernecke, A.; Eriksson, E.E.; Rothfuchs, A.G.; Pham, C.T.; Herwald, H.; Bidzhekov, K.; Rottenberg, M.E.; Weber, C.; Lindbom, L. Neutrophil secretion products pave the way for inflammatory monocytes. Blood 2008, 112, 1461–1471. [Google Scholar] [CrossRef]
- Hoffman, M.D.; Weiss, R.H. Does Acute Kidney Injury From an Ultramarathon Increase the Risk for Greater Subsequent Injury? Clin J Sport Med 2016, 26, 417–422. [Google Scholar] [CrossRef]
- Kao, W.F.; Hou, S.K.; Chiu, Y.H.; Chou, S.L.; Kuo, F.C.; Wang, S.H.; Chen, J.J. Effects of 100-km ultramarathon on acute kidney injury. Clin J Sport Med 2015, 25, 49–54. [Google Scholar] [CrossRef]
- Mansour, S.G.; Verma, G.; Pata, R.W.; Martin, T.G.; Perazella, M.A.; Parikh, C.R. Kidney Injury and Repair Biomarkers in Marathon Runners. Am J Kidney Dis 2017, 70, 252–261. [Google Scholar] [CrossRef] [PubMed]
- Lipman, G.S.; Krabak, B.J.; Waite, B.L.; Logan, S.B.; Menon, A.; Chan, G.K. A prospective cohort study of acute kidney injury in multi-stage ultramarathon runners: the Biochemistry in Endurance Runner Study (BIERS). Res Sports Med 2014, 22, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Poussel, M.; Touze, C.; Allado, E.; Frimat, L.; Hily, O.; Thilly, N.; Rousseau, H.; Vauthier, J.C.; Chenuel, B. Ultramarathon and Renal Function: Does Exercise-Induced Acute Kidney Injury Really Exist in Common Conditions? Front Sports Act Living 2019, 1, 71. [Google Scholar] [CrossRef]
- Suzuki, K.; Totsuka, M.; Nakaji, S.; Yamada, M.; Kudoh, S.; Liu, Q.; Sugawara, K.; Yamaya, K.; Sato, K. Endurance exercise causes interaction among stress hormones, cytokines, neutrophil dynamics, and muscle damage. J Appl Physiol (1985) 1999, 87, 1360–1367. [Google Scholar] [CrossRef]
- Suzuki, K.; Yamada, M.; Kurakake, S.; Okamura, N.; Yamaya, K.; Liu, Q.; Kudoh, S.; Kowatari, K.; Nakaji, S.; Sugawara, K. Circulating cytokines and hormones with immunosuppressive but neutrophil-priming potentials rise after endurance exercise in humans. Eur J Appl Physiol 2000, 81, 281–287. [Google Scholar] [CrossRef]
- Sugama, K.; Suzuki, K.; Yoshitani, K.; Shiraishi, K.; Kometani, T. Urinary excretion of cytokines versus their plasma levels after endurance exercise. Exerc Immunol Rev 2013, 19, 29–48. [Google Scholar] [PubMed]
- Mizokami, T.; Suzuki, K. Neutrophil Depletion Attenuates Acute Liver Stress after Exhaustive Exercise in Mice. Med Sci Sports Exerc 2023, 55, 670–679. [Google Scholar] [CrossRef] [PubMed]
- Meldrum, K.K.; Burnett, A.L.; Meng, X.; Misseri, R.; Shaw, M.B.; Gearhart, J.P.; Meldrum, D.R. Liposomal delivery of heat shock protein 72 into renal tubular cells blocks nuclear factor-kappaB activation, tumor necrosis factor-alpha production, and subsequent ischemia-induced apoptosis. Circ Res 2003, 92, 293–299. [Google Scholar] [CrossRef]
- Simone, S.; Rascio, F.; Castellano, G.; Divella, C.; Chieti, A.; Ditonno, P.; Battaglia, M.; Crovace, A.; Staffieri, F.; Oortwijn, B.; et al. Complement-dependent NADPH oxidase enzyme activation in renal ischemia/reperfusion injury. Free Radic Biol Med 2014, 74, 263–273. [Google Scholar] [CrossRef]
- Kashani, K.; Cheungpasitporn, W.; Ronco, C. Biomarkers of acute kidney injury: the pathway from discovery to clinical adoption. Clin Chem Lab Med 2017, 55, 1074–1089. [Google Scholar] [CrossRef] [PubMed]
- Juett, L.A.; James, L.J.; Mears, S.A. Effects of Exercise on Acute Kidney Injury Biomarkers and the Potential Influence of Fluid Intake. Ann Nutr Metab 2020, 76 Suppl 1, 53–59. [Google Scholar] [CrossRef]
- Hewing, B.; Schattke, S.; Spethmann, S.; Sanad, W.; Schroeckh, S.; Schimke, I.; Halleck, F.; Peters, H.; Brechtel, L.; Lock, J.; et al. Cardiac and renal function in a large cohort of amateur marathon runners. Cardiovasc Ultrasound 2015, 13, 13. [Google Scholar] [CrossRef]
- Gordon, S.; Taylor, P.R. Monocyte and macrophage heterogeneity. Nat Rev Immunol 2005, 5, 953–964. [Google Scholar] [CrossRef]
- Mantovani, A.; Sozzani, S.; Locati, M.; Allavena, P.; Sica, A. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol 2002, 23, 549–555. [Google Scholar] [CrossRef]
- Lee, S.; Huen, S.; Nishio, H.; Nishio, S.; Lee, H.K.; Choi, B.S.; Ruhrberg, C.; Cantley, L.G. Distinct macrophage phenotypes contribute to kidney injury and repair. J Am Soc Nephrol 2011, 22, 317–326. [Google Scholar] [CrossRef]
- Kezic, A.; Stajic, N.; Thaiss, F. Innate Immune Response in Kidney Ischemia/Reperfusion Injury: Potential Target for Therapy. J Immunol Res 2017, 2017, 6305439. [Google Scholar] [CrossRef] [PubMed]
- Furuichi, K.; Wada, T.; Iwata, Y.; Kitagawa, K.; Kobayashi, K.; Hashimoto, H.; Ishiwata, Y.; Asano, M.; Wang, H.; Matsushima, K.; et al. CCR2 signaling contributes to ischemia-reperfusion injury in kidney. J Am Soc Nephrol 2003, 14, 2503–2515. [Google Scholar] [CrossRef] [PubMed]
- Bonavia, A.; Singbartl, K. A review of the role of immune cells in acute kidney injury. Pediatr Nephrol 2018, 33, 1629–1639. [Google Scholar] [CrossRef] [PubMed]
| Gene | Forward | Reverse |
|---|---|---|
| 18s ribosomal RNA | CGGCTACCACATCCAAGGA | AGCTGGAATTACCGCGGC |
| Ly-6G | TGGACTCTCACAGAAGCAAAG | GCAGAGGTCTTCCTTCCAACA |
| TNF- | TCTTCTCATTCCTGCTTGTGG | GAGGCCATTTGGGAACTTCT |
| IL-6 | AACGATGATGCACTTGCAGA | TGGTACTCCAGAAGACCAGAGG |
| IL-1 | GGGCCTCAAAGGAAAGAATC | TTGCTTGGGATCCACACTCT |
| F4/80 | CTTTGGCTATGGGCTTCCAGTC | GCAAGGAGGACAGAG-TTTATCGTG |
| MCP-1 | CTTCTGGGCCTGCTGTTCA | CCAGCCTACTCATTGGGATCA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





