1. Introduction
Hepatocellular carcinoma (HCC) is the most prevalent type of liver cancer, accounting for 90% of all occurrences of primary liver cancer [
1]. HCC is a recognized inflammation-related cancer form. The immunological microenvironment of the liver plays a crucial role in the etiology of illness [
2]. Infection with the hepatitis B virus or the hepatitis C virus [
3], persistent alcohol consumption, nonalcoholic steatohepatitis, and exposure to aflatoxin B are all variables that might increase the likelihood of developing hepatocellular carcinoma [
4]. When certain infectious pathogens generate persistent inflammation, innate and adaptive immune system modifications influence disease development [
5]. Persistent inflammation promotes the development of HCC by the infiltration of diverse immune cells, including dendritic cells, macrophages, natural killer cells, neutrophils, T cells, and B cells [
6].
T cells are recognized to have a crucial role in the development of HCC. Among the subtypes of T cells, the dysregulation of the function of CD4+ T cells is emerging as a factor in HCC. By secreting cytokines and activating CD8+ T lymphocytes, CD4+ T cells play a role in antitumor immune responses [
7]. Activated CD4+ T cells stimulate CD8+ cytotoxic T lymphocytes (CTLs) via dendritic cells or by directly secreting IL-2. After receiving signals from dendritic cells, CD4+ T cells are activated and polarized to the Th1 phenotype, producing effector cytokines such as IFN and TNF. CD4+ T cells also stimulate the differentiation and maturation of B cells into plasma cells [
8]. After specific cytokine signaling and transcription factor expression, CD4+ T cells may differentiate into several subsets, including T helper 1 (Th1), T-helper 2 (Th2), T-helper 17 (Th17), follicular helper T cells (Tfh), and T-regulatory cells (Tregs) [
9]. The steady decline of CD4+ T lymphocytes is substantially related with increased mortality and a reduced survival time in patients with HCC [
10].
Long noncoding RNAs (lncRNAs) play a significant role in a variety of physiological and pathological processes via several regulatory mechanisms [
11]. Many evidence points to that abnormal expression of certain lncRNAs in HCC contributes to the progression of the illness [
12,
13,
14]. Several studies demonstrated that lncRNAs are essential for T cell development, activation, and pathogen response [
15,
16,
17]. Accumulating evidence indicates that lncRNA and microRNA (miRNA) interact to control gene expression [
18,
19]. LncRNAs may act as miRNA sponges or bind to sites on the target genes of miRNAs to reduce the interaction of miRNA and its target mRNA. On the other hand, miRNAs can regulate the stability and half-life of lncRNA by target lncRNA. In addition, some lncRNAs containing miRNA sequences can be cleaved by Dicer and/or Drosha to produce miRNAs [
20].
There has been no extensive research of the lncRNA–miRNA/mRNA network of circulating CD4+ T cells in HCC. In this work, circulating CD4+ cells from HCC clinical samples were extracted and compared to those from healthy people. The expression landscape of lncRNAs, miRNAs, and mRNAs is analyzed, and the regulatory network of miRNA–mRNA/ lncRNA is created.
2. Materials and Methods
2.1. Study Patient Population
This study was approved by the Institutional Review Board of Chang Gung Memorial Hospital (IRB number: 201900911B0), and all participants provided written informed consent. Blood samples were taken from patients with or without HCC. We employed 10 healthy controls and 10 HCC patients in our NGS study. The demographics of these twenty people are presented in Supplemental Table S1. To validate the expression of discovered genes, one hundred samples of healthy controls and sixty samples of HCC were utilized. The demographics of these individuals from the validation cohort are given in Supplemental Table S2.
2.2. Isolation of Circulating CD4+ Cell and RNA Extraction
PBMC were isolated using density gradient centrifugation and Ficoll-PaqueTM PREMIUM solution (GE Healthcare, 17544202). Anti-human CD4 Particles - DM magnetic nanoparticles (BD IMagTM, 557767) were used to isolate CD4+ cells according to the manufacturer's procedure. The collected CD4+ cells were lysed using 700 ul of QIAzol Lysis Reagent, and RNA was extracted using the miRNeasy Mini Kit (QIAGEN, 217004) per the manufacturer's instructions. Then, 50 l RNase-free water was added to the RNA-isolated samples.
2.3. RNA Quality Determination and NGS
The extracted RNA samples were quantified using a NanoDrop 2000 (Thermo Scientific) spectrophotometer and a Qubit 2.0 Fluorometer (Thermo scientific). Qubit / NanoDrop ratio (1±0.3 assign as well) and Caliper RNA Quality Score (RQS) adopting Caliper LabChip GX Electrophoresis System were utilized to evaluate RNA quality (Caliper Life Sciences). Using the TruSeq® Stranded Total RNA Sample Preparation Instructions, cDNA libraries were constructed (Illumina, 15031048). Equal samples from each library were pooled and sequenced with a NextSeq 500. (Illumina).
For RNA-Seq, the row sequences were assessed for quality using FastQC v0.11.8. and trimmed for primer-adaptor sequences using the RNA-seq alignment tool from BaseSpace (Illumina), followed by alignment to the human reference genome (hg38) with STAR v2.7.3a. The expression levels and differential expression analysis were estimated using DESeq2 v1.24.0. (selection criteria: adjusted P value of <0.05 and |fold change| > 2).
For miRNA-Seq, the sequences were assessed for quality and adapter trimming using Trim_Galore v. 0.4.4, followed by alignment to the human reference genome (hg38) from UCSC with bwa v. 0.7.15. The expression profiles were produced using BCGSC miRNA Profiling Pipeline and differential expression analysis were estimated using DEseq2 v. 1.16.1.
2.4. GO and KEGG Enrichment Analysis
Gene Ontology (GO) and the Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analysis were performed using the R package clusterProfiler (version 3.18.1) to detect the enrichment of signaling pathway. The GO analysis included analyses of biological processes, cellular components, and molecular functions. GO and KEGG terms with p < 0.05 were considered significantly enriched by differentially expressed genes (DEGs).
2.5. Real-Time Quantitative Polymerase Chain Reaction
For measuring the expression of mRNAs and lncRNAs, the transcripts were reverse transcribed to cDNA by High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems, 4368814). 7500 Real-Time PCR System and Power SYBR Green PCR Master Mix (ABI 4367659) were utilized for gene expression investigation (Applied Biosystems). The sequences of the primers used are shown in Supplemental Table S3.
For miRNA, the transcripts were reverse transcribed to cDNA by TaqMan™ Advanced miRNA cDNA Synthesis Kit (Applied Biosystems, A28007). TaqMan Advanced miRNA Assay (Applied Biosystems, A25576) and TaqMan Fast Advanced Master Mix were utilized for the miRNA expression study (Applied Biosystems, 444557).
2.6. The Interaction of miRNA-lncRNA and miRNA-mRNA
The Encyclopedia of RNA Interactomes (ENCORI;
https://starbase.sysu.edu.cn/; vers. 3.0) was used to obtain information on the miRNA-lncRNA or miRNA-mRNA interaction [
21]. ENCORI is an open-source platform for researching the interactions between miRNAs and their targets. At least three miRNA-target prediction databases, including PITA, miRmap, and TargetScan, were utilized to identify the targeted miRNAs of candidate genes in the present work.
2.7. CD4+ T Cells Infiltration Analysis
The association between CD4
+ T cells infiltrates and differential express gene was evaluated using TIMER2.0 website (
http://timer.cistrome.org/), which provides robust estimation of immune infiltration levels using website algorithms, including TIMER, EPIC, xCell, CIBERSORT, and quanTIseq [
22].
2.8. Statistical Analysis
The Windows version of GraphPad Prism 5 was used for all statistical tests (version 5.01). The data were provided as a mean ± standard error (SEM). The Mann–Whitney U test was used to conduct the pairwise comparisons, and the resulting p values were then shown. A value of P 0.05 was used to indicate statistical significance in all two-tailed tests.
3. Results
3.1. NGS Analysis
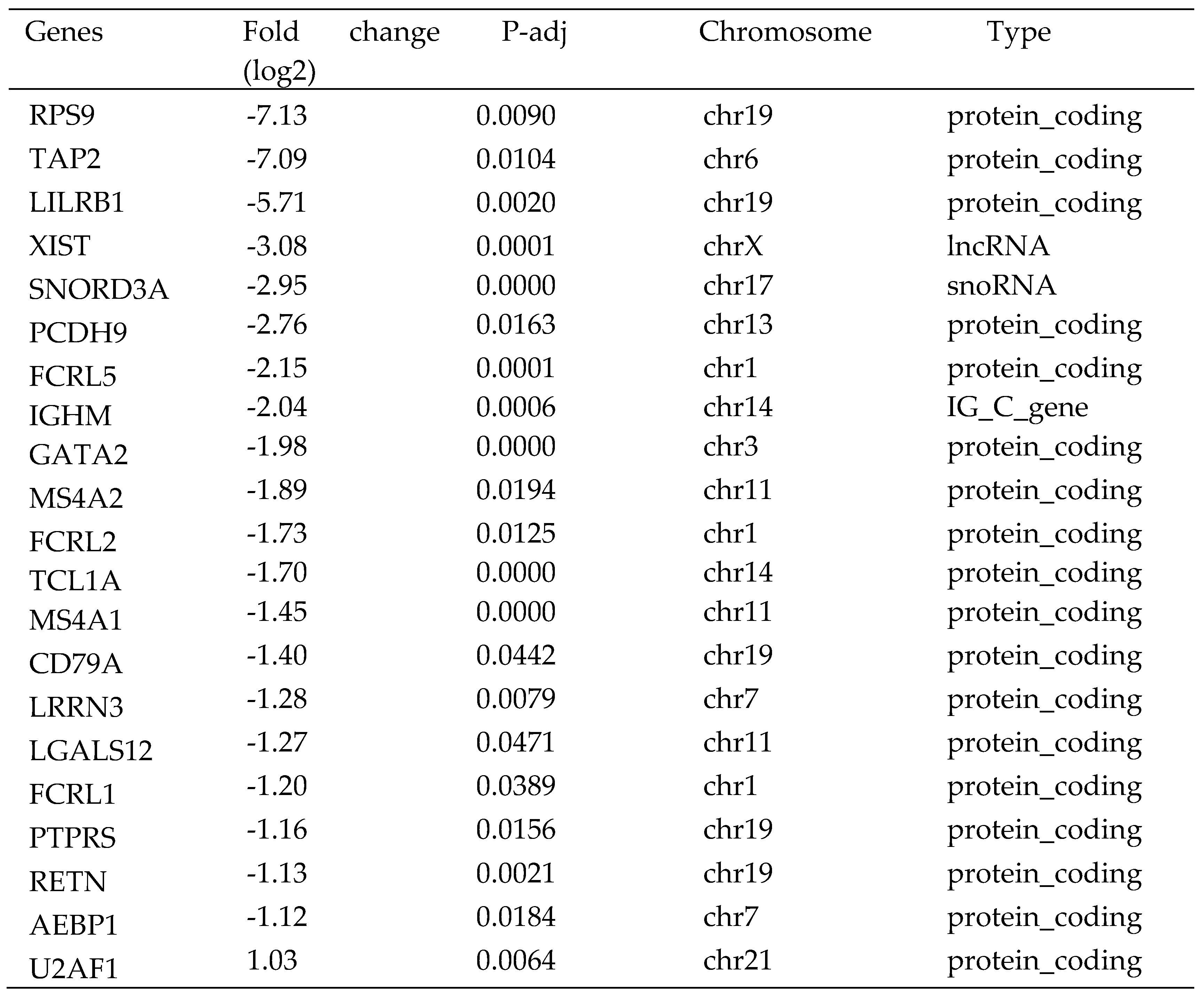
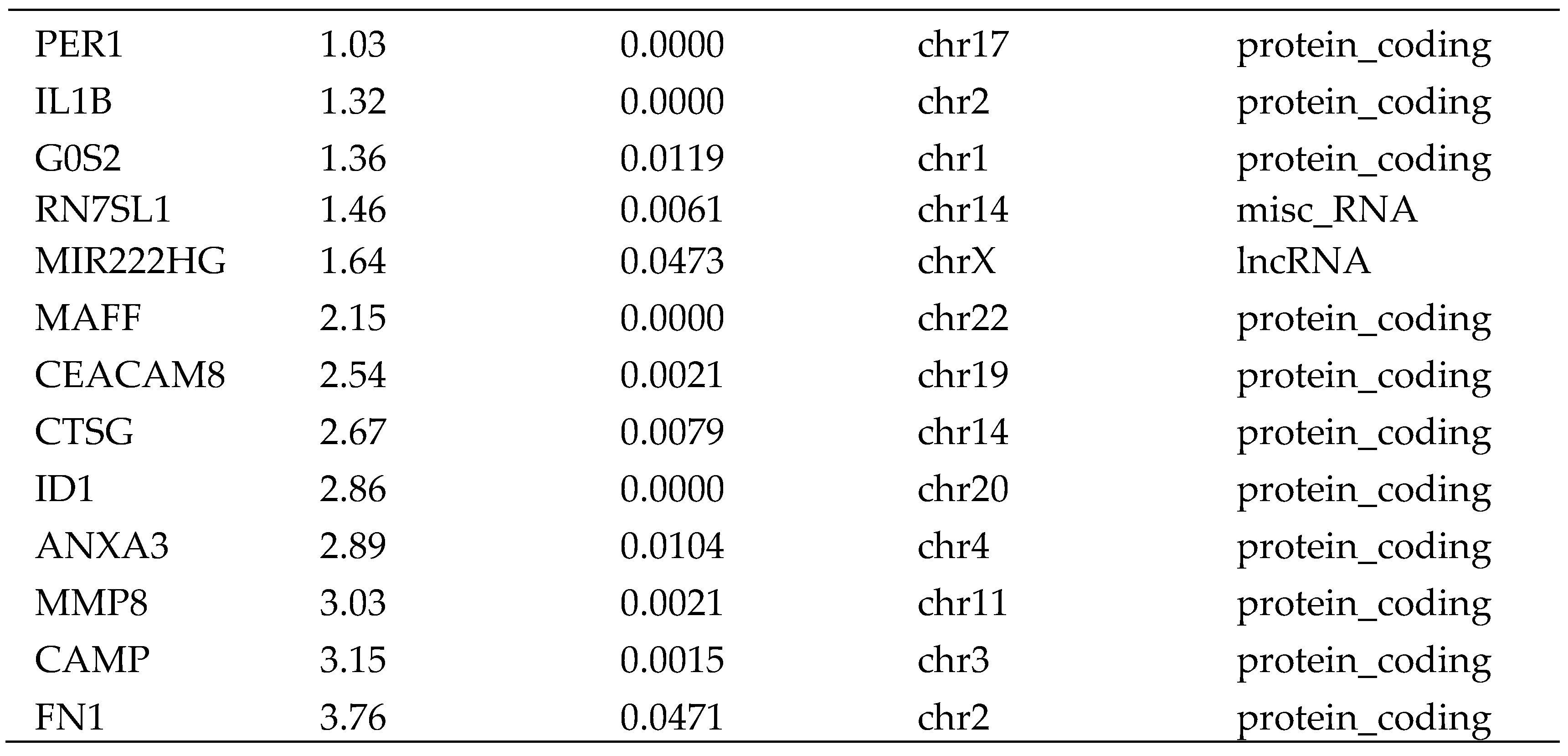
RNA-Seq analysis of circulating CD4
+ cells from 10 HCC patients vs. 10 health control showed that the expression of 34 transcripts were significantly differently expressed in the circulating CD4+ T cells. Two of the 34 transcripts were lncRNAs (XIST and MIR222HG), 29 were mRNAs, and three were of other types of RNAs (
Table 1). Among these two lncRNAs, MIR222HG was up-regulated and XIST was down-regulated in HCC patients. Among the 29 mRNAs, 12 were up-regulated and 17 was down-regulated in HCC patients (
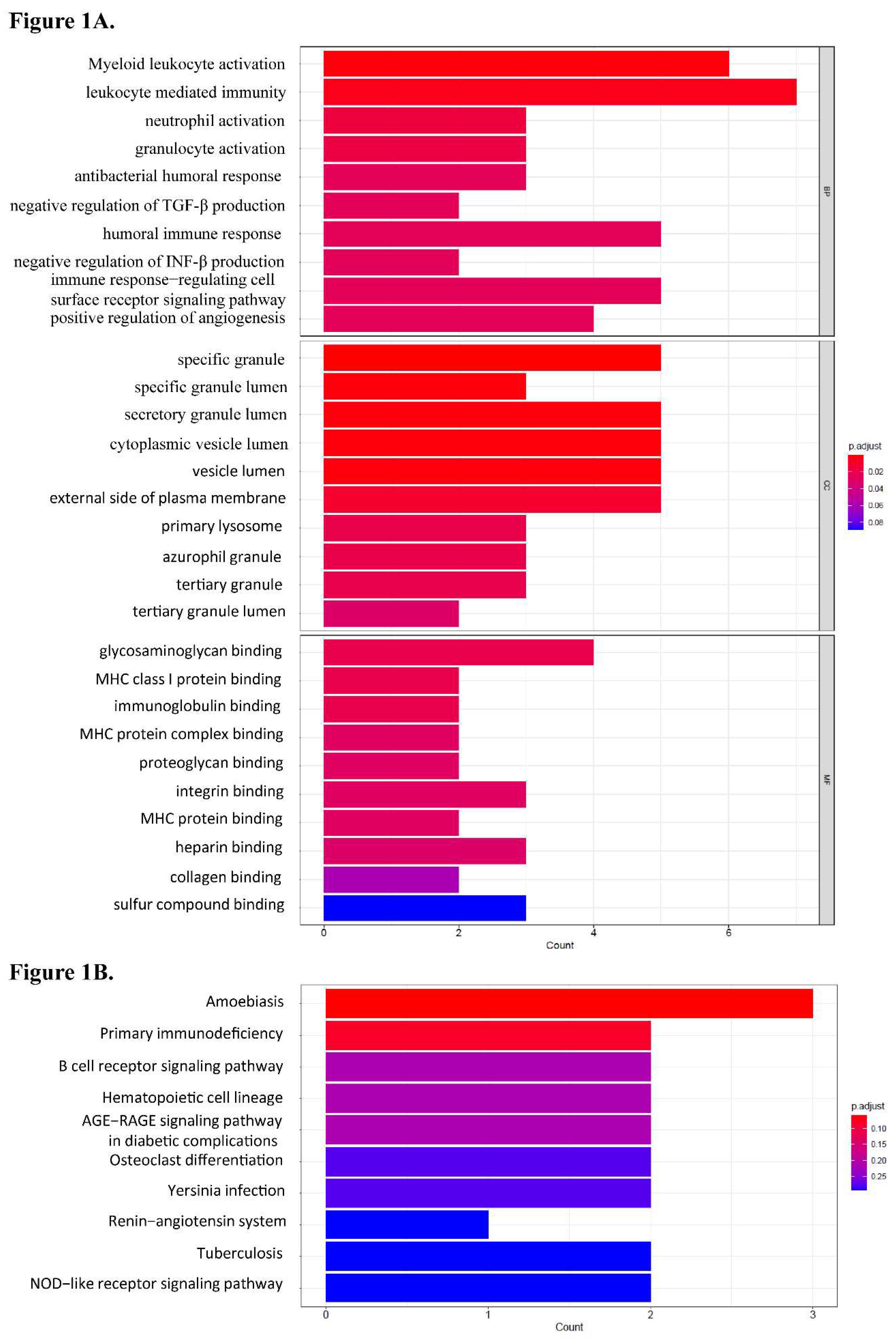
Table 1). The GO analysis revealed that the biological processes of 34 differentially expressed genes (DEGs) were predominantly associated with leukocyte-mediated immunity, myeloid leukocyte activation, neutrophil activation, granulocyte activation, and humoral immune response (
Figure 1A). The cellular component of these DEGs was engaged in granule lumen and vesicle lumen. The molecular function of these DEGs was involved in glycosaminoglycan binding, immunoglobulin binding and MHC protein complex binding. The results of the KEGG pathway enrichment analysis showed that DEGs were mostly implicated in the primary immunodeficiency and B cell receptor signaling pathways (
Figure 1B).
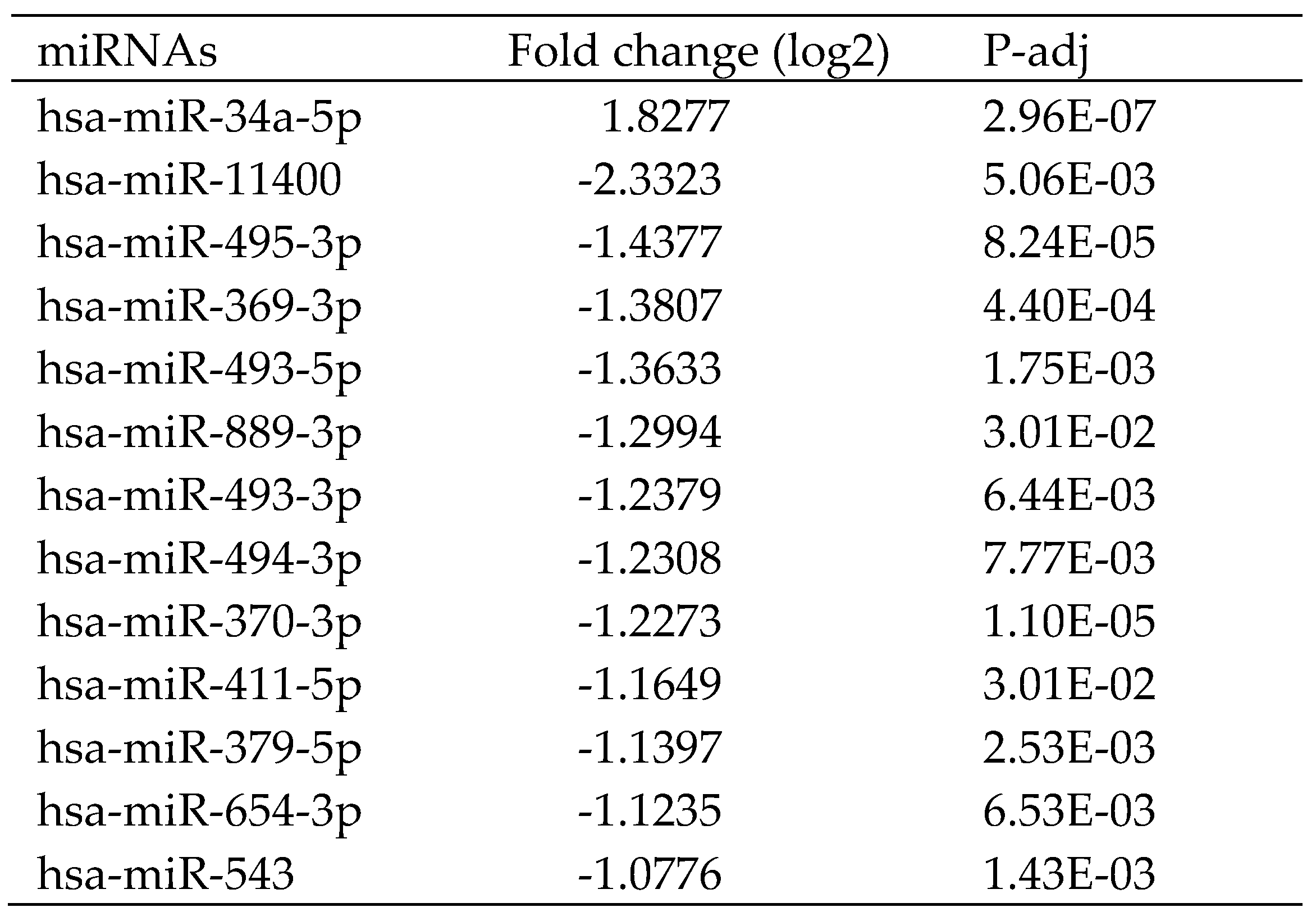
The miRNA-seq study revealed that the expression of 13 miRNAs, one of which was up-regulated and twelve of which were down-regulated, was significantly different between HCC patients and healthy controls (
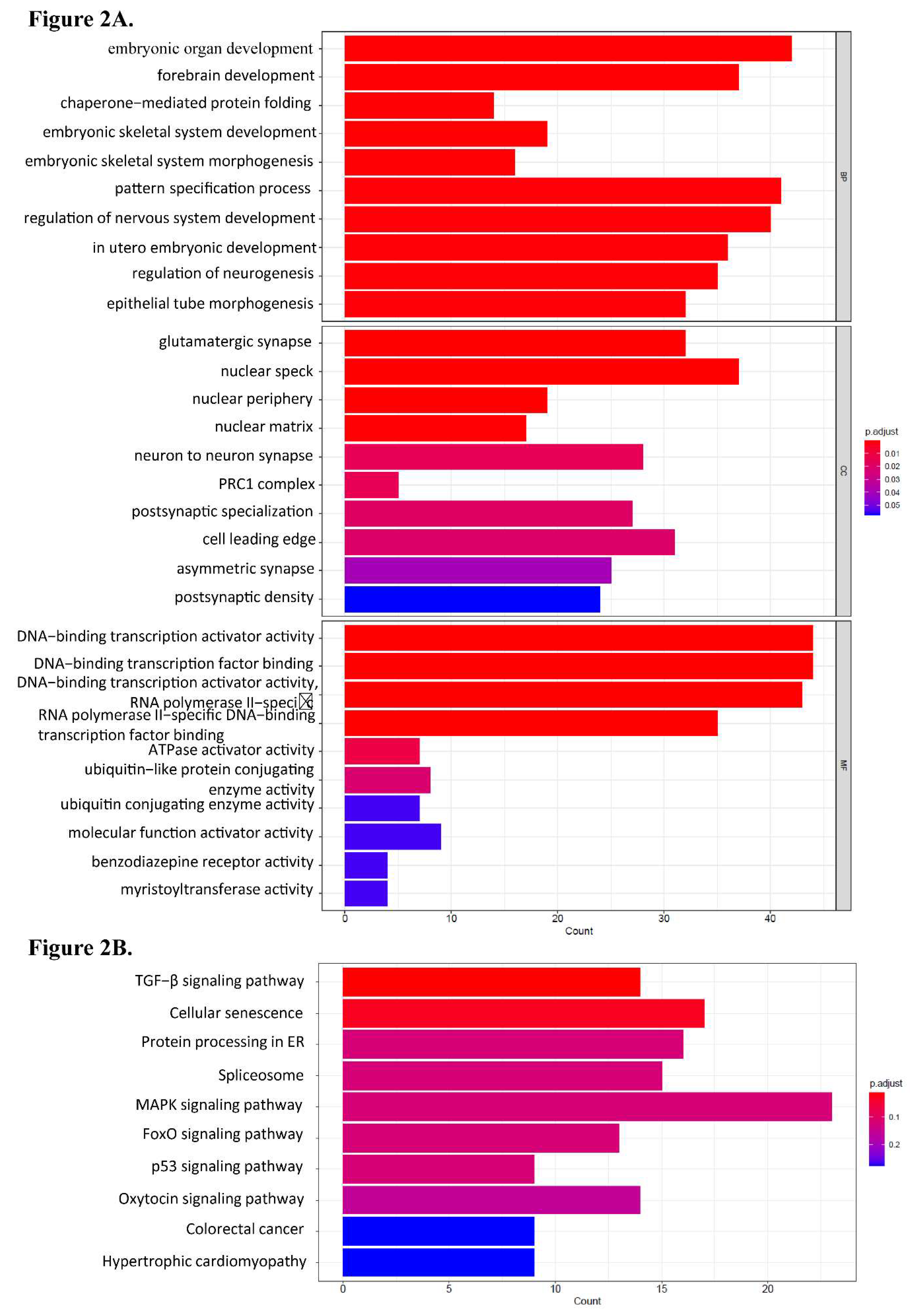
Table 2). The predicted target genes of these miRNAs with differential expression are reported in Table 4. The GO analysis revealed that these miRNA target genes are crucial to tissues development. Protein activity was associated with the biological function of these miRNA target genes. KEGG pathway enrichment result for these predicted target genes was involved in TGF−β signaling pathway, cellular senescence, protein processing in endoplasmic reticulum, spliceosome and several signaling pathway (
Figure 2).
3.2. The miRNA-lncRNA or miRNA-mRNA Interaction by ENCORI
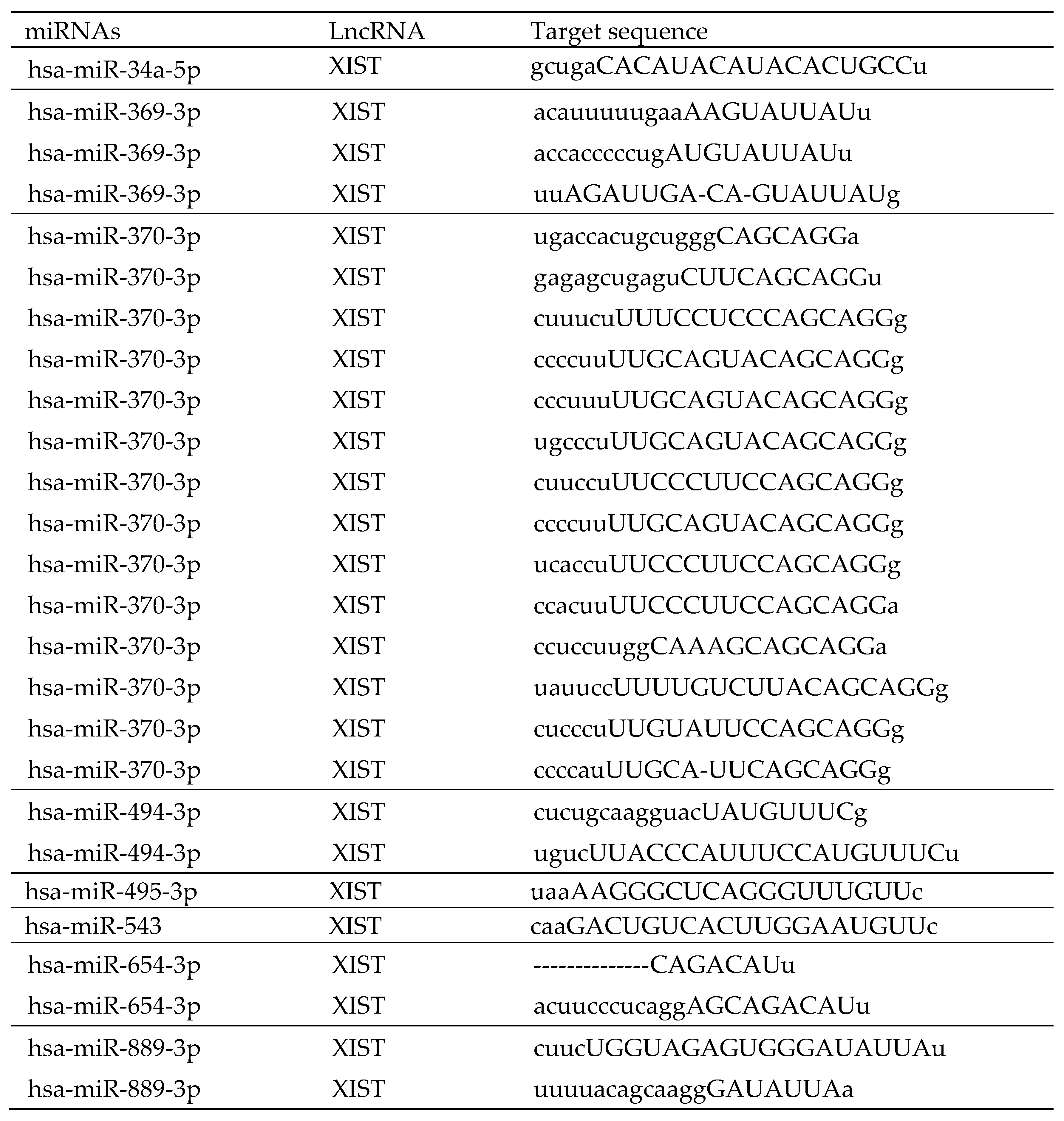
ENCORI was used to study the interaction between miRNA and lncRNA as well as miRNA and mRNA. Based on ENCORI analysis, there are 8 of 13 differential expression miRNAs to XIST (
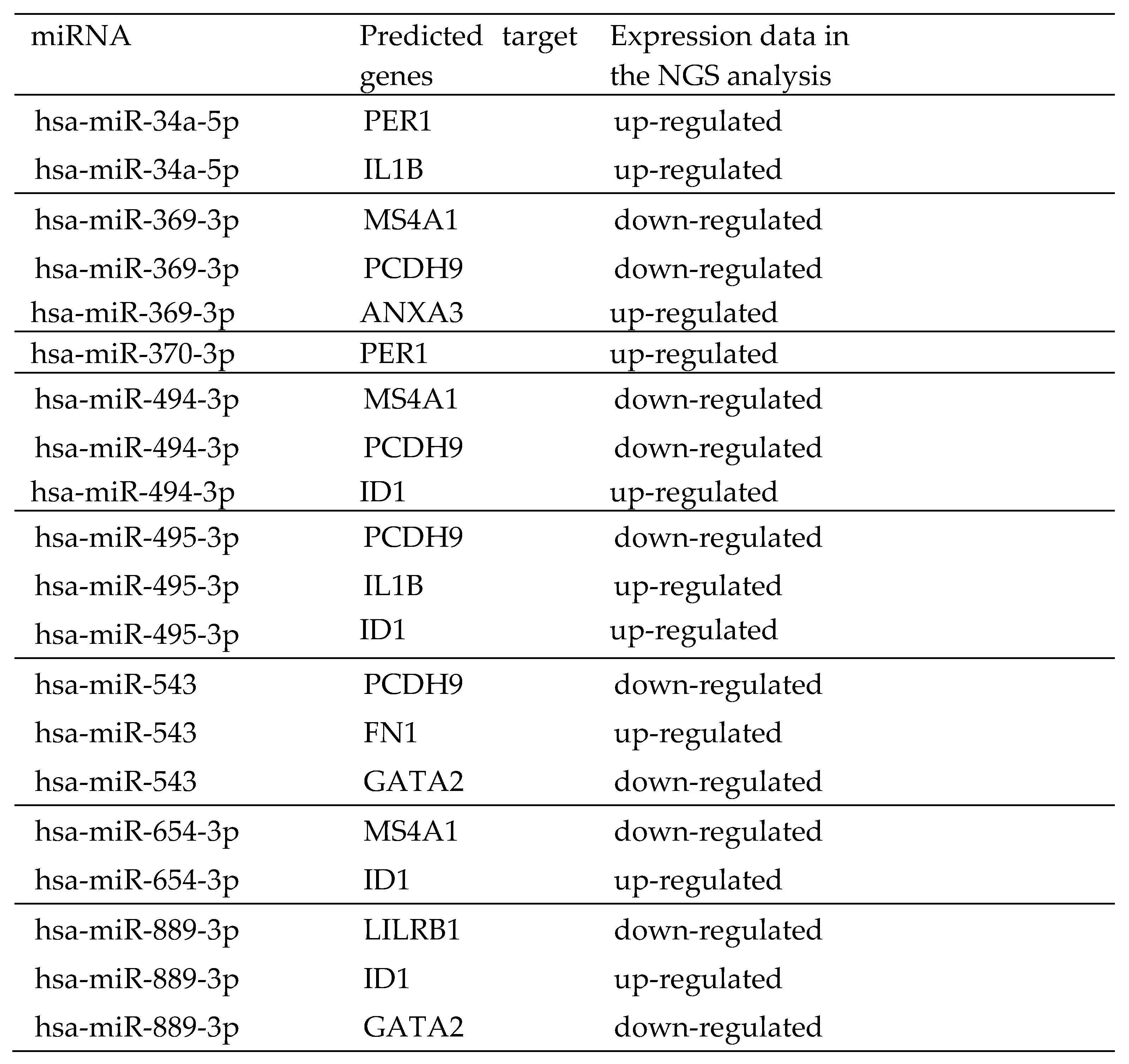
Table 3), including hsa-miR-34a-5p, hsa-miR-369-3p, hsa-miR-370-3p, hsa-miR-494-3p, hsa-miR-495-3p, hsa-miR-543, hsa-miR-654-3p and hsa-miR-889-3p. However, there is no differential expression miRNAs be related to MIR222HG. The target mRNAs of eight XIST-related miRNAs predicted by ENCORI were identified (Supplemental Table S4) and compared to their expression level from the DEGs in this study (
Table 4). Due to the limited number of isolated CD4+ T cells, only those up-regulated mRNAs that are related with down-regulated miRNAs were chosen for further real-time qPCR validation in this work. Five mRNAs were identified: Inhibitor of DNA binding 1 (ID1), Interleukin 1 beta (IL1β), Annexin A3 (ANXA3), Period circadian regulator 1 (PER1), and Fibronectin 1 (FN1).
3.3. Validation of the Expression of Transcripts
Real-time qPCR was used to determine the expression levels of XIST, eight XIST-related miRNAs, and their target mRNAs in 160 validation samples (100 health control and 60 HCC samples).
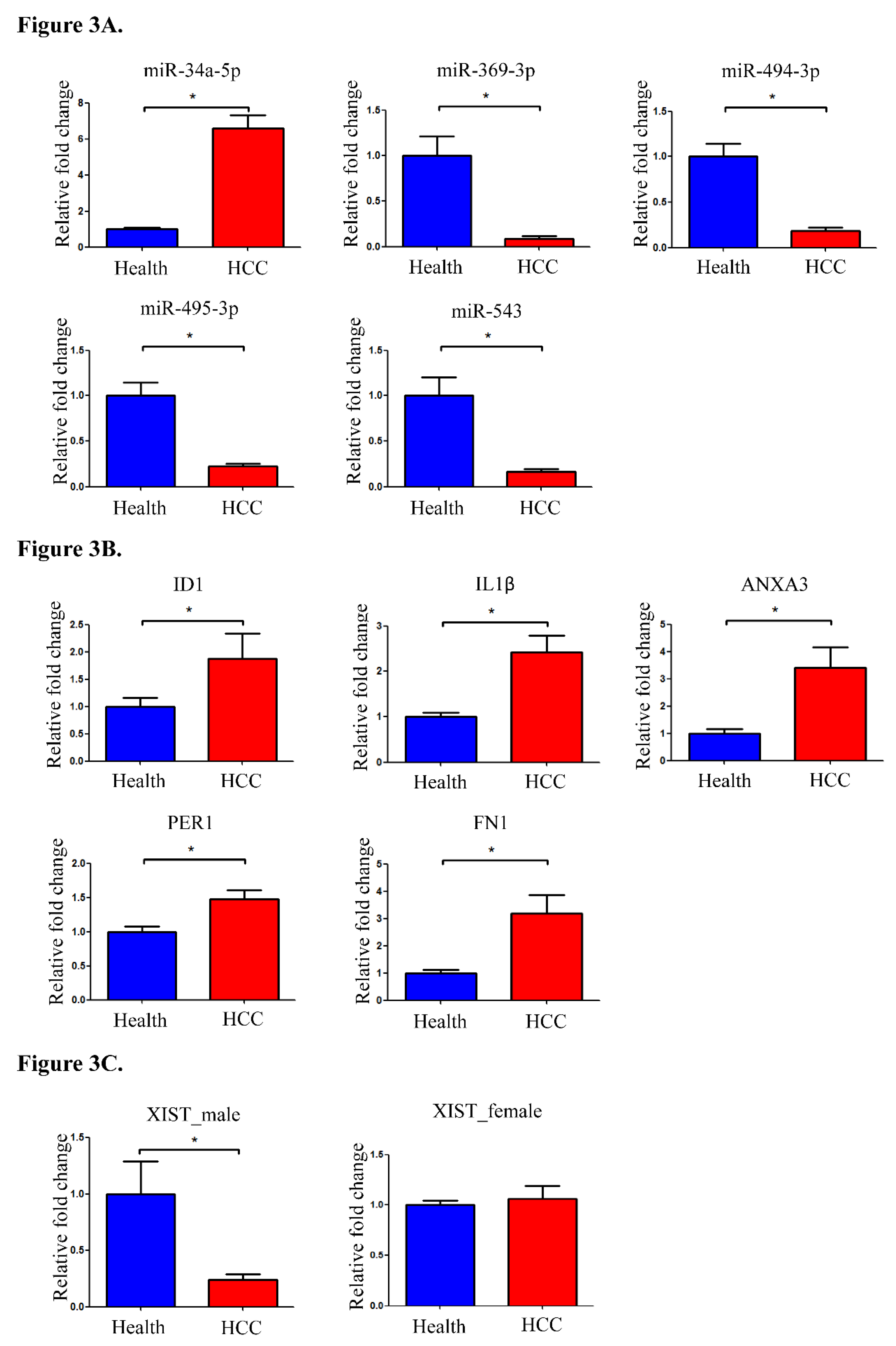
Figure 3A demonstrates that miR-34a-5p was substantially up-regulated while miR-369-3p, miR-494-3p, miR-495-3p, and miR-543 were significantly down-regulated in HCC patients using real-time qPCR. These results are consistent with those disclosed by NGS. Nevertheless, the expression levels of three (miR-370-3p, miR-654-3p, and miR-889-3p) of the eight miRNAs associated with XIST were either modest or undetectable.
We then measure the identified potential target mRNAs utilizing real-time qPCR. These mRNAs were chosen based on the intersection of the target mRNAs of the aforementioned substantially downregulated miRNAs and those presenting increased expression in the NGS experiments. Five mRNAs (ID1, IL1β, ANXA3, PER1, and FN1) were selected for the real time qPCR experiment. ID1, which is a target for miR-494-3p, miR-495-3p, miR-654-3p, and miR-889-3p, was significantly up-regulated in HCC patients. IL1β as a target for miR-34a-5p and miR-495-3p, ANXA3 as a target of miR-369-3p, PER1 as a target for miR-34a-5p and miR-370-3p, and FN1 as a target for miR-543 had significant upregulation in HCC patients than the healthy subjects (
Figure 3B).
Given that XIST is a sex-linked lncRNA, the amount of XIST expression for male and female was detected separately by real-time qPCR analyses. XIST expression was considerably suppressed in male HCC patients but not in female HCC patients (
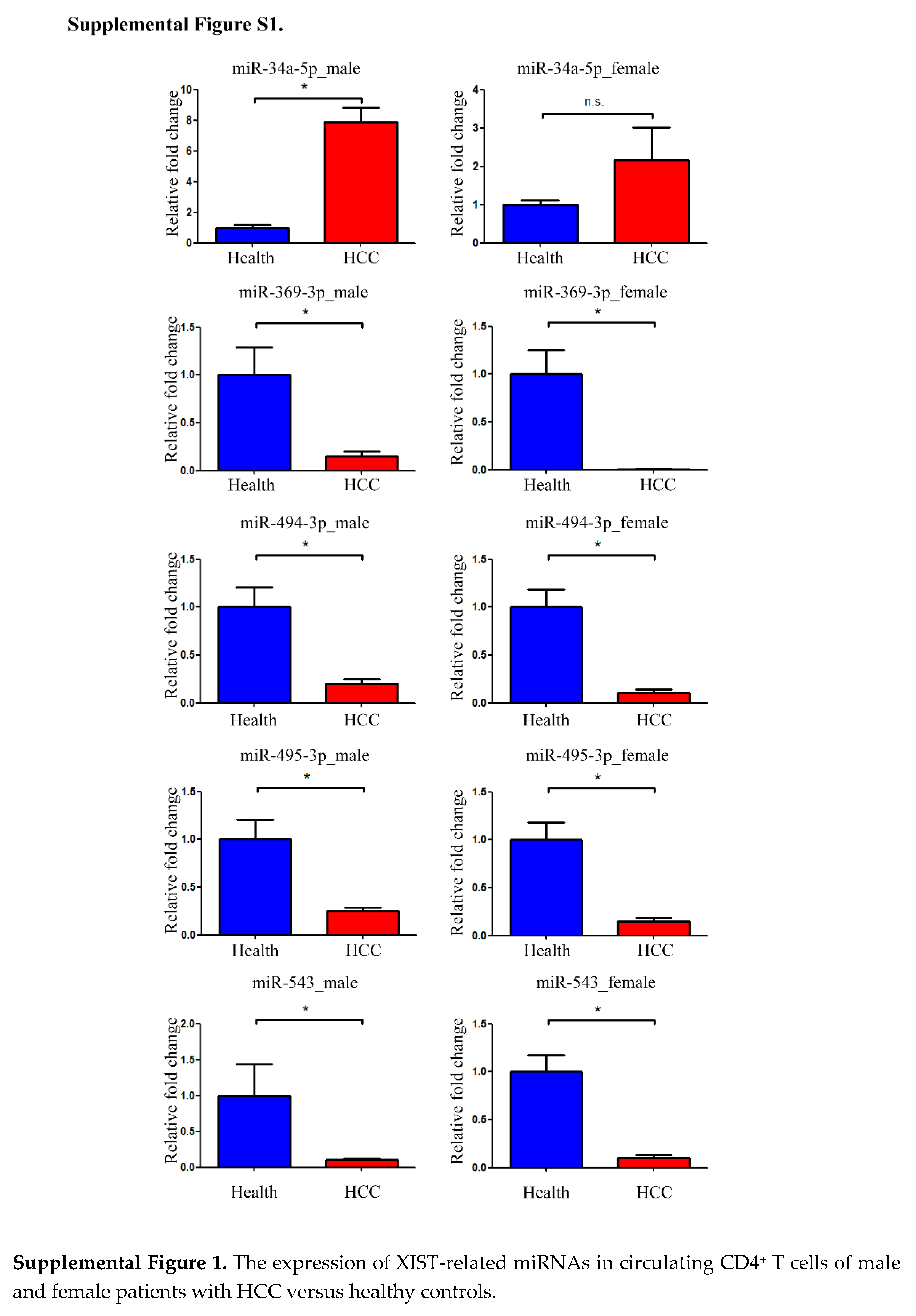
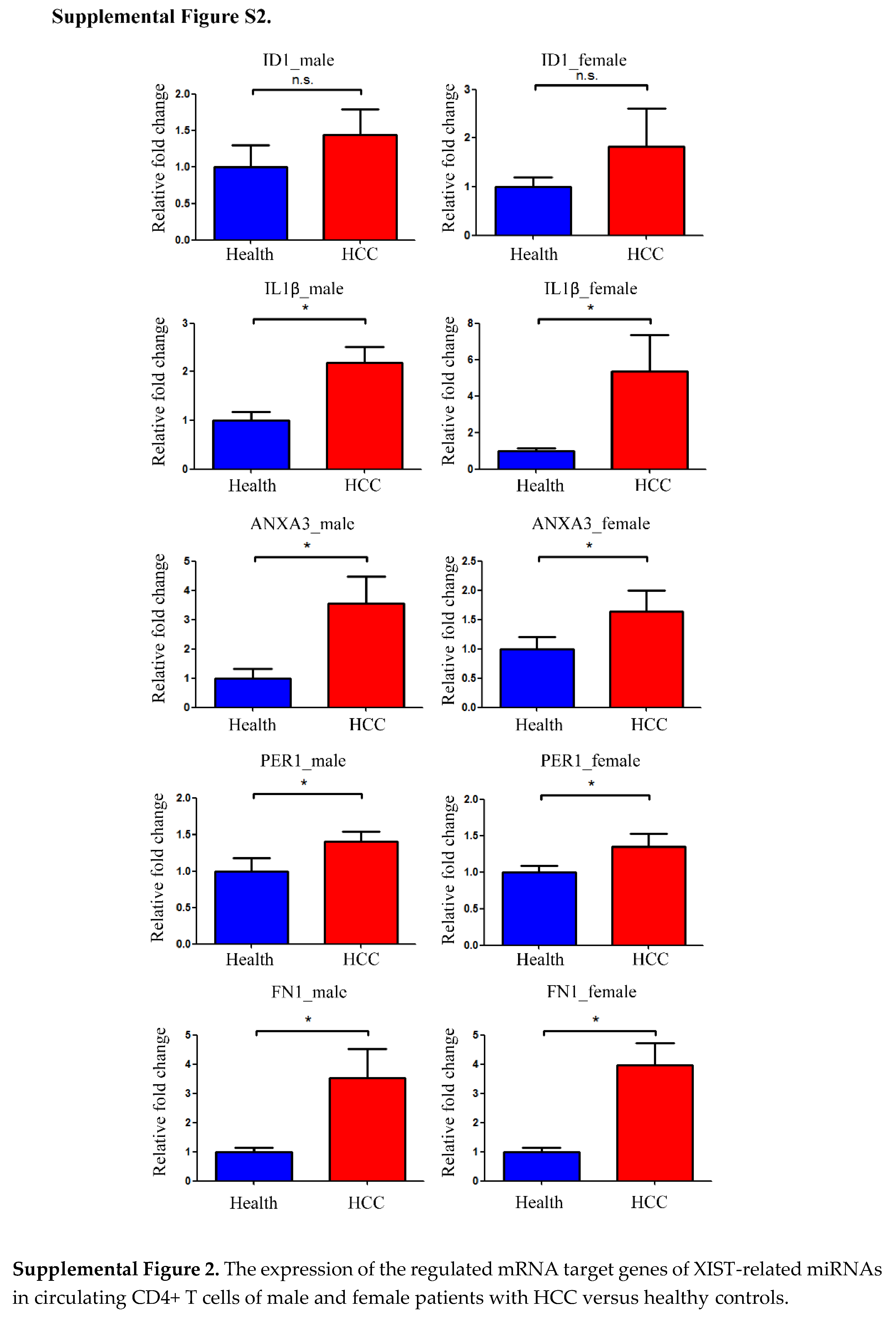
Figure 3C). In this study, the expression levels of XIST-related miRNAs and their regulated mRNAs were evaluated in male and female HCC patients. There were no remarkable difference regarding the expression of miRNAs and the regulated mRNAs between male and female HCC patients (Supplemental Figure S1 and S2).
Figure 3.
miRNA / mRNA / lncRNA expression level verification. Quantification of (A) miRNAs, (B) differentially expressed miRNA-targeted mRNAs and (C) XIST expression by real-time qPCR.
Figure 3.
miRNA / mRNA / lncRNA expression level verification. Quantification of (A) miRNAs, (B) differentially expressed miRNA-targeted mRNAs and (C) XIST expression by real-time qPCR.
3.4. Analysis of CD4+ T Cells Infiltrates Correlation
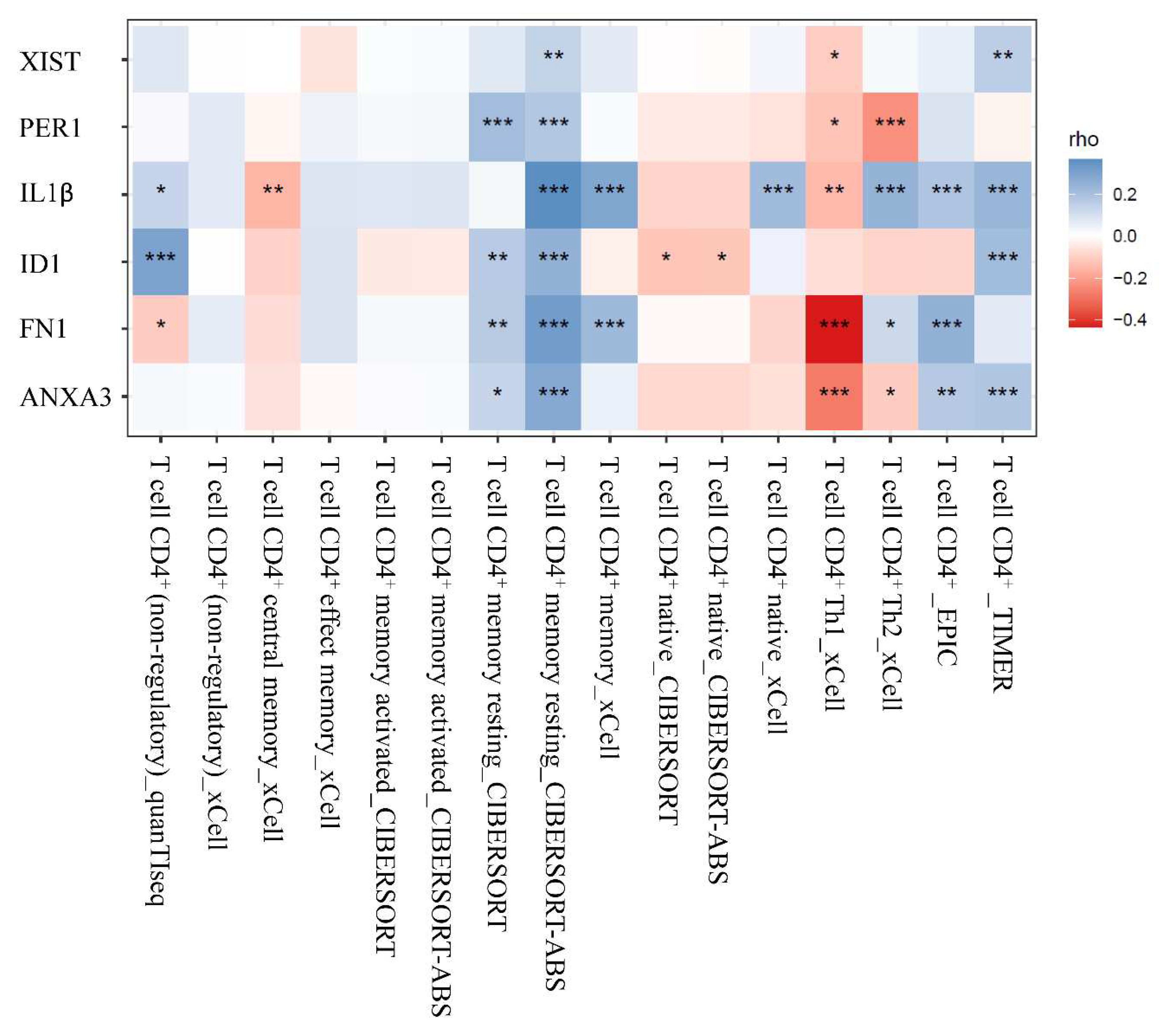
Using the TIMER2.0 website, we investigated the link between the expression of miRNA target genes and the amount of CD4+ T cell infiltration in HCC. The findings revealed a strong correlation between the expression of these miRNAs' target genes and the infiltration of total CD4+ T cells, particularly resting memory CD4+ T cells (
Figure 4). Nevertheless, there was a negative correlation between the infiltration of Th1 CD4+ T cells and the expression of miRNAs' target genes.3.2. Figures, Tables and Schemes
4. Discussion
In this investigation, we discovered circulating CD4+ T cells with differently expressed lncRNAs, miRNAs, and mRNAs in HCC patients. These transcripts are involved in the activation of numerous kinds of white blood cells, including myeloid leukocyte, neutrophil, and granulocyte. A XIST-related lncRNA–miRNA/mRNA network, which contains five miRNAs (hsa-miR-34a-5p, hsa-miR-369-3p, hsa-miR-494-3p, hsa-miR-495-3p, hsa-miR-543) and five miRNA target genes (ID1, IL1β, ANXA3, PER1 and FN1), was rebuilt. This regulatory network associated with XIST may be positively connected with the infiltration of CD4+ T cells, particularly resting memory CD4+ T cells, but negatively correlated with Th1 CD4+ T cells. Our finding indicated that the regulation of circulating CD4+ T cells in HCC patients differs from that of healthy individuals.
XIST plays a role in each stage of X-chromosome inactivation. XIST recruits chromatin complexes that deposit heterochromatic alterations throughout the X, including H3K27me3 and ubiquitin-H2A, leading in transcriptional silence [
23]. In SLE patients, XIST was predominantly up-regulated in activated T cell subsets and was associated by more skewed X-chromosome allelic expression [
24]. Mature nave T lymphocytes exhibit distributed patterns of XIST, but the inactive X lacks epigenetic changes. Activation of mature T cells restores the dormant X gene's XIST transcripts and epigenetic changes [
25]. The expression levels of XIST-related miRNAs and their regulated mRNAs did not change significantly between male and female HCC patients, despite the fact that XIST is a sex-linked lncRNA.
Regarding the XIST-related miRNAs found in this investigation, miR-34a was revealed to target 14 essential components of the NF-κB signaling pathway and to function as a major regulator of NF-κB in T cells [
26]. The rise of miR-34a correlates with CD4+ and CD8+ T cell activation [
27]. Targeting CXCR3, miR-34a-5p inhibits CXCR3 expression in CD4+ and CD8+ T cells. High levels of miR-34a-5p in naïve CD4+ T cells inhibit Th1 cell polarization by downregulating CXCR3, leading in diminished activation of cytotoxic T lymphocytes, natural killer cells, and natural killer T cells, as well as possible lymphopenia [
28]. In addition, miR-369-3p regulates the inflammatory response as the signaling core. The upregulation of miR-369-3p inhibits the LPS-induced inflammatory response, reduces the release of TNF, IL-6, IL-12, IL-1, and IL-1, and increases the production of anti-inflammatory cytokines IL-10 and IL-1RA [
29]. Nuclear translocation of NF-κB is likewise reduced by miR-369-3p [
29]. In viral infections, inhibiting the expression of miR-369-3p dramatically reduces the expression of antiviral response genes, including CCL3, CCL5, CTSS, CXCL11, and IRF3 [
30]. In addition, miR-494-3p has a function in macrophage polarization and activation via activating Wnt signaling to modulate M1/M2 polarization [
31].
The appearance of T cell types may play a crucial role in the development of HCC. For instance, it has been observed that the ratio of Th1 to CD4+ T cells is greater in HCC patients than in healthy controls, and that patients with a higher tumor-infiltrating Th1 cell count had a considerably longer life time (26). In addition, the decrease of CD4+ T cells is linked to a poor prognosis and a high rate of HCC recurrence (10). ID1-expressing CD4+ cells greatly boosted IL-2 production and NF-B activity in response to anti-CD3 stimulation in order to improve proliferation and survival of naïve CD4+ cells (23). IL-1 promotes the proliferation and development of antigen-specific CD4+ T cells toward a proinflammatory Th phenotype via the production of IFN-, IL-17, TNF-, and IL-4 (24,25). Th1 cells have strong ANXA3 expression (26). In the stress hormone signaling pathway, the elevation of PER1 expression inherently suppresses Th1 polarization in naïve CD4+ T cells (27). Local manipulation of FN1 levels may be advantageous for boosting T cell concentration and is essential for T cell interstitial migration (28). These cytokines may contribute to communication between immune cells and affect CD4+ T cell development. Despite indications suggesting these miRNAs are involved in the regulation of T cells, further research is required to determine the effect of the XIST-miRNAs/mRNAs network on the function of circulating CD4+ T cells in HCC.
5. Conclusions
In this work, the circulating CD4+ cells of HCC patients exhibited down-regulation of XIST, dysregulation of five XIST-related miRNAs, and up-regulation of five miRNA target genes, including ID1, IL1β, ANXA3, PER1, and FN1. The explored XIST-miRNA/mRNA network might play a crucial role in controlling the function of T cells.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org, Figure S1: The expression of XIST-related miRNAs in circulating CD4+ T cells of male and female patients with HCC versus healthy controls. Figure S2: The expression of the regulated mRNA target genes of XIST-related miRNAs in circulating CD4+ T cells of male and female patients with HCC versus healthy controls.; Table S1: The clinical data of 20 people who participated in the NGS assessment of differential mRNA, lncRNA, and miRNA gene expression.; Table S2 The clinical data of 160 individuals who had their transcript expression validated using real-time qPCR.; Table S3: The primers sequences used in real-time quantitative polymerase chain reaction. Table S4: The target mRNAs of eight XIST-related miRNAs predicted by ENCORI.
Author Contributions
Conceptualization, T.-M. H. and C.-H. H.; methodology, Y.-W. L.; software, C.-J. W..; validation, C.-Y. H..; formal analysis, P.-C C.; investigation, Y.-C. W; resources, H.-P. L.; data curation, Y.-C. W.; writing—original draft preparation, L.-H. H.; writing—review and editing, C.-S. R.; supervision, T.-M. H. and C.-H. H.; funding acquisition, T.-M. H. and C.-H. H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Chang Gung Memorial Hospital, grant number CMRPG8K1561 and CORPG8L0451.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Institutional Review Board of Chang Gung Memorial Hospital (protocol code 201900911B0 and date of approval 2019/07/15).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data sharing is not applicable to this article.
Acknowledgments
We thank Chang Gung Medical Foundation Kaohsiung Chang Gung Memorial Hospital Biobank and Tissue Bank Core Lab(CLRPG8L0083) for excellent technical support. We are grateful for the support provided by the Biostatistics Center, Kaohsiung Chang Gung Memorial Hospital.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sia, D.; Villanueva, A.; Friedman, S.L.; Llovet, J.M. Liver Cancer Cell of Origin, Molecular Class, and Effects on Patient Prognosis. Gastroenterology 2017, 152, 745–761. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Gea, V.; Toffanin, S.; Friedman, S.L.; Llovet, J.M. Role of the microenvironment in the pathogenesis and treatment of hepatocellular carcinoma. Gastroenterology 2013, 144, 512–527. [Google Scholar] [CrossRef]
- Llovet, J.M.; Kelley, R.K.; Villanueva, A.; Singal, A.G.; Pikarsky, E.; Roayaie, S.; Lencioni, R.; Koike, K.; Zucman-Rossi, J.; Finn, R.S. Hepatocellular carcinoma. Nature reviews. Disease primers 2021, 7, 6. [Google Scholar] [CrossRef]
- Yapali, S.a.T., N. Epidemiology and viral risk factors for hepatocellular carcinoma in the Eastern Mediterranean countries. Hepatoma Research 2018, 4. [Google Scholar] [CrossRef]
- Lawal, G.; Xiao, Y.; Rahnemai-Azar, A.A.; Tsilimigras, D.I.; Kuang, M.; Bakopoulos, A.; Pawlik, T.M. The Immunology of Hepatocellular Carcinoma. Vaccines 2021, 9. [Google Scholar] [CrossRef] [PubMed]
- Hong, G.Q.; Cai, D.; Gong, J.P.; Lai, X. Innate immune cells and their interaction with T cells in hepatocellular carcinoma. Oncology letters 2021, 21, 57. [Google Scholar] [CrossRef]
- Zheng, X.; Jin, W.; Wang, S.; Ding, H. Progression on the Roles and Mechanisms of Tumor-Infiltrating T Lymphocytes in Patients With Hepatocellular Carcinoma. Frontiers in immunology 2021, 12, 729705. [Google Scholar] [CrossRef]
- Tay, R.E.; Richardson, E.K.; Toh, H.C. Revisiting the role of CD4(+) T cells in cancer immunotherapy-new insights into old paradigms. Cancer gene therapy 2021, 28, 5–17. [Google Scholar] [CrossRef]
- Luckheeram, R.V.; Zhou, R.; Verma, A.D.; Xia, B. CD4(+)T cells: differentiation and functions. Clinical & developmental immunology 2012, 2012, 925135. [Google Scholar] [CrossRef]
- Fu, J.; Zhang, Z.; Zhou, L.; Qi, Z.; Xing, S.; Lv, J.; Shi, J.; Fu, B.; Liu, Z.; Zhang, J.Y.; et al. Impairment of CD4+ cytotoxic T cells predicts poor survival and high recurrence rates in patients with hepatocellular carcinoma. Hepatology 2013, 58, 139–149. [Google Scholar] [CrossRef]
- Long, Y.; Wang, X.; Youmans, D.T.; Cech, T.R. How do lncRNAs regulate transcription? Science advances 2017, 3, eaao2110. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Zhou, J.K.; Peng, Y.; He, W.; Huang, C. The role of long noncoding RNAs in hepatocellular carcinoma. Molecular cancer 2020, 19, 77. [Google Scholar] [CrossRef] [PubMed]
- Ghafouri-Fard, S.; Gholipour, M.; Hussen, B.M.; Taheri, M. The Impact of Long Non-Coding RNAs in the Pathogenesis of Hepatocellular Carcinoma. Frontiers in oncology 2021, 11, 649107. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, L.; Gu, J.; Zhang, H.; Yuan, J.; Lian, Q.; Lv, G.; Wang, S.; Wu, Y.; Yang, Y.T.; et al. Recurrently deregulated lncRNAs in hepatocellular carcinoma. Nature communications 2017, 8, 14421. [Google Scholar] [CrossRef]
- West, K.A.; Lagos, D. Long Non-Coding RNA Function in CD4(+) T Cells: What We Know and What Next? Non-coding RNA 2019, 5. [Google Scholar] [CrossRef]
- Hirsova, P.; Bamidele, A.O.; Wang, H.; Povero, D.; Revelo, X.S. Emerging Roles of T Cells in the Pathogenesis of Nonalcoholic Steatohepatitis and Hepatocellular Carcinoma. Frontiers in endocrinology 2021, 12, 760860. [Google Scholar] [CrossRef] [PubMed]
- Plasek, L.M.; Valadkhan, S. lncRNAs in T lymphocytes: RNA regulation at the heart of the immune response. American journal of physiology. Cell physiology 2021, 320, C415–C427. [Google Scholar] [CrossRef]
- Shi, T.; Morishita, A.; Kobara, H.; Masaki, T. The Role of Long Non-Coding RNA and microRNA Networks in Hepatocellular Carcinoma and Its Tumor Microenvironment. International journal of molecular sciences 2021, 22. [Google Scholar] [CrossRef]
- El-Araby R, E.G., Saad A, Elamrosy H, Adel R, Elshafie S, Helal H, Adel M, Roshdy F. LncRNA-miRNA crosstalk in HCC cases on top of HCV infection. Europe PMC 2022.
- Fernandes, J.C.R.; Acuna, S.M.; Aoki, J.I.; Floeter-Winter, L.M.; Muxel, S.M. Long Non-Coding RNAs in the Regulation of Gene Expression: Physiology and Disease. Non-coding RNA 2019, 5. [Google Scholar] [CrossRef]
- Li, J.H.; Liu, S.; Zhou, H.; Qu, L.H.; Yang, J.H. starBase v2.0: decoding miRNA-ceRNA, miRNA-ncRNA and protein-RNA interaction networks from large-scale CLIP-Seq data. Nucleic Acids Res 2014, 42, D92–D97. [Google Scholar] [CrossRef]
- Li, T.; Fu, J.; Zeng, Z.; Cohen, D.; Li, J.; Chen, Q.; Li, B.; Liu, X.S. TIMER2.0 for analysis of tumor-infiltrating immune cells. Nucleic acids research 2020, 48, W509–W514. [Google Scholar] [CrossRef] [PubMed]
- Syrett, C.M.; Paneru, B.; Sandoval-Heglund, D.; Wang, J.; Banerjee, S.; Sindhava, V.; Behrens, E.M.; Atchison, M.; Anguera, M.C. Altered X-chromosome inactivation in T cells may promote sex-biased autoimmune diseases. JCI insight 2019, 4. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, X.; Gibson, A.; Edberg, J.; Kimberly, R.P.; Absher, D.M. Skewed allelic expression on X chromosome associated with aberrant expression of XIST on systemic lupus erythematosus lymphocytes. Human molecular genetics 2020, 29, 2523–2534. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Syrett, C.M.; Kramer, M.C.; Basu, A.; Atchison, M.L.; Anguera, M.C. Unusual maintenance of X chromosome inactivation predisposes female lymphocytes for increased expression from the inactive X. Proceedings of the National Academy of Sciences of the United States of America 2016, 113, E2029–E2038. [Google Scholar] [CrossRef] [PubMed]
- Hart, M.; Walch-Rückheim, B.; Friedmann, K.S.; Rheinheimer, S.; Tänzer, T.; Glombitza, B.; Sester, M.; Lenhof, H.P.; Hoth, M.; Schwarz, E.C.; et al. miR-34a: a new player in the regulation of T cell function by modulation of NF-κB signaling. Cell Death Dis 2019, 10, 46. [Google Scholar] [CrossRef]
- Hart, M.; Walch-Ruckheim, B.; Friedmann, K.S.; Rheinheimer, S.; Tanzer, T.; Glombitza, B.; Sester, M.; Lenhof, H.P.; Hoth, M.; Schwarz, E.C.; et al. miR-34a: a new player in the regulation of T cell function by modulation of NF-kappaB signaling. Cell death & disease 2019, 10, 46. [Google Scholar] [CrossRef]
- Hart, M.; Nickl, L.; Walch-Rueckheim, B.; Krammes, L.; Rheinheimer, S.; Diener, C.; Taenzer, T.; Kehl, T.; Sester, M.; Lenhof, H.P.; et al. Wrinkle in the plan: miR-34a-5p impacts chemokine signaling by modulating CXCL10/CXCL11/CXCR3-axis in CD4(+), CD8(+) T cells, and M1 macrophages. Journal for immunotherapy of cancer 2020, 8. [Google Scholar] [CrossRef]
- Scalavino, V.; Liso, M.; Cavalcanti, E.; Gigante, I.; Lippolis, A.; Mastronardi, M.; Chieppa, M.; Serino, G. miR-369-3p modulates inducible nitric oxide synthase and is involved in regulation of chronic inflammatory response. Sci Rep 2020, 10, 15942. [Google Scholar] [CrossRef]
- Lenart, M.; Dzialo, E.; Kluczewska, A.; Weglarczyk, K.; Szaflarska, A.; Rutkowska-Zapala, M.; Surmiak, M.; Sanak, M.; Pituch-Noworolska, A.; Siedlar, M. miRNA Regulation of NK Cells Antiviral Response in Children With Severe and/or Recurrent Herpes Simplex Virus Infections. Frontiers in immunology 2020, 11, 589866. [Google Scholar] [CrossRef]
- van Ingen, E.; Foks, A.C.; Woudenberg, T.; van der Bent, M.L.; de Jong, A.; Hohensinner, P.J.; Wojta, J.; Bot, I.; Quax, P.H.A.; Nossent, A.Y. Inhibition of microRNA-494-3p activates Wnt signaling and reduces proinflammatory macrophage polarization in atherosclerosis. Molecular therapy. Nucleic acids 2021, 26, 1228–1239. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).