Submitted:
26 May 2023
Posted:
29 May 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
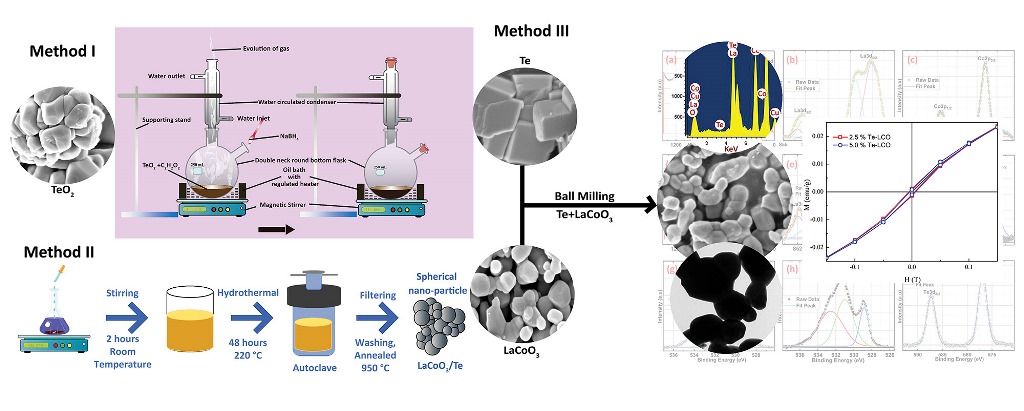
2. Materials and Methods
2.1. Materials
2.1.1. Materials for the preparation of Te (Method 1).
2.1.2. Materials for the preparation of LaCoO 3 (Method 2).
2.1.3. Materials for the preparation of Te (2.5%) impregnated LaCoO3 and Te (5%) impregnated LaCoO3(Method 3).
2.2. Methods
2.2.1. Method 1: The preparation of Te.
2.2.2. Method 2: The preparation of LaCoO3.
2.2.3. Method 3: The preparation of Te impregnated LaCoO3.
2.3. Characterisation Technique
3. Results and Discussion
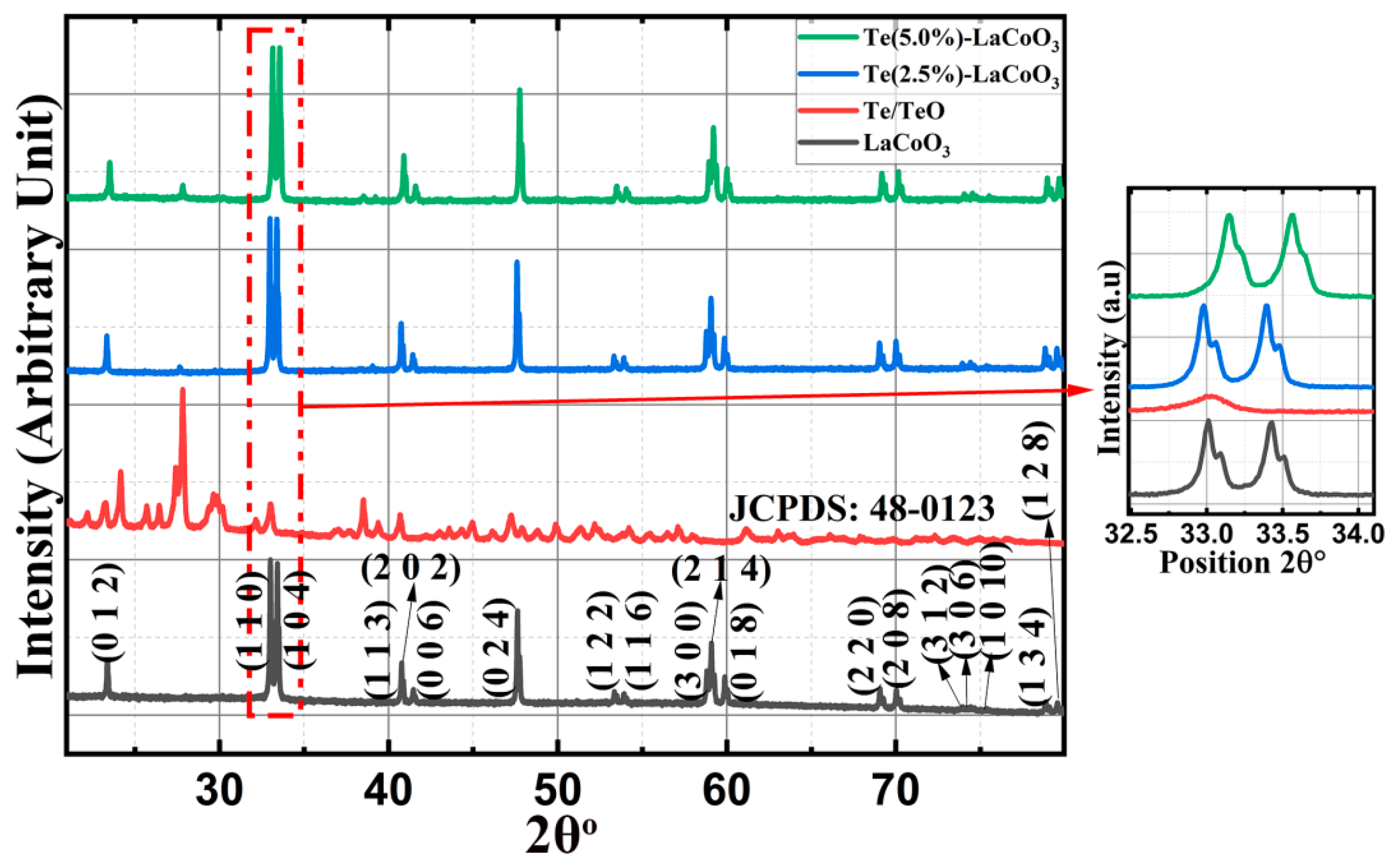
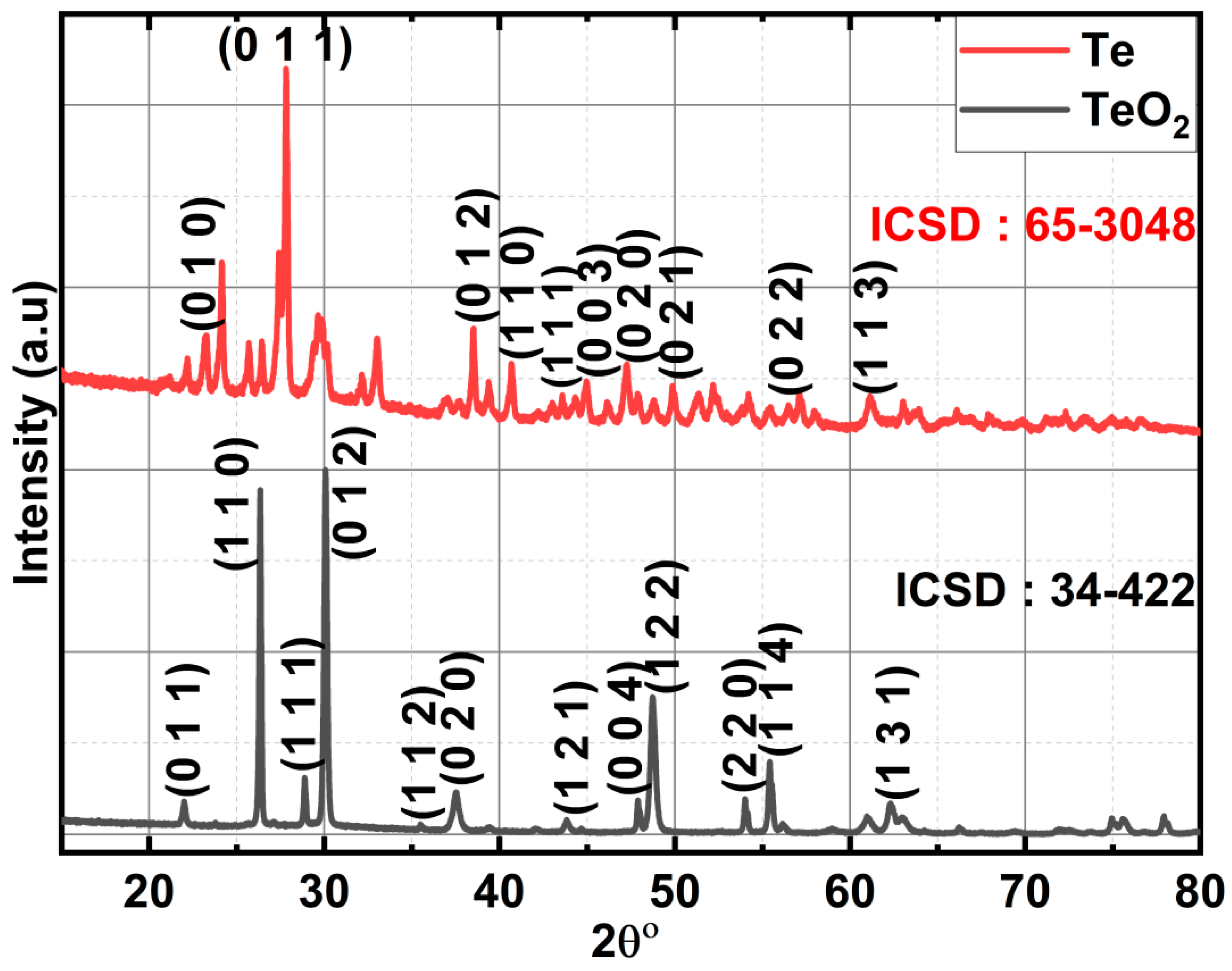
3.1. Powder X-Ray Diffraction (P-XRD) of Te Incorporated LaCoO3
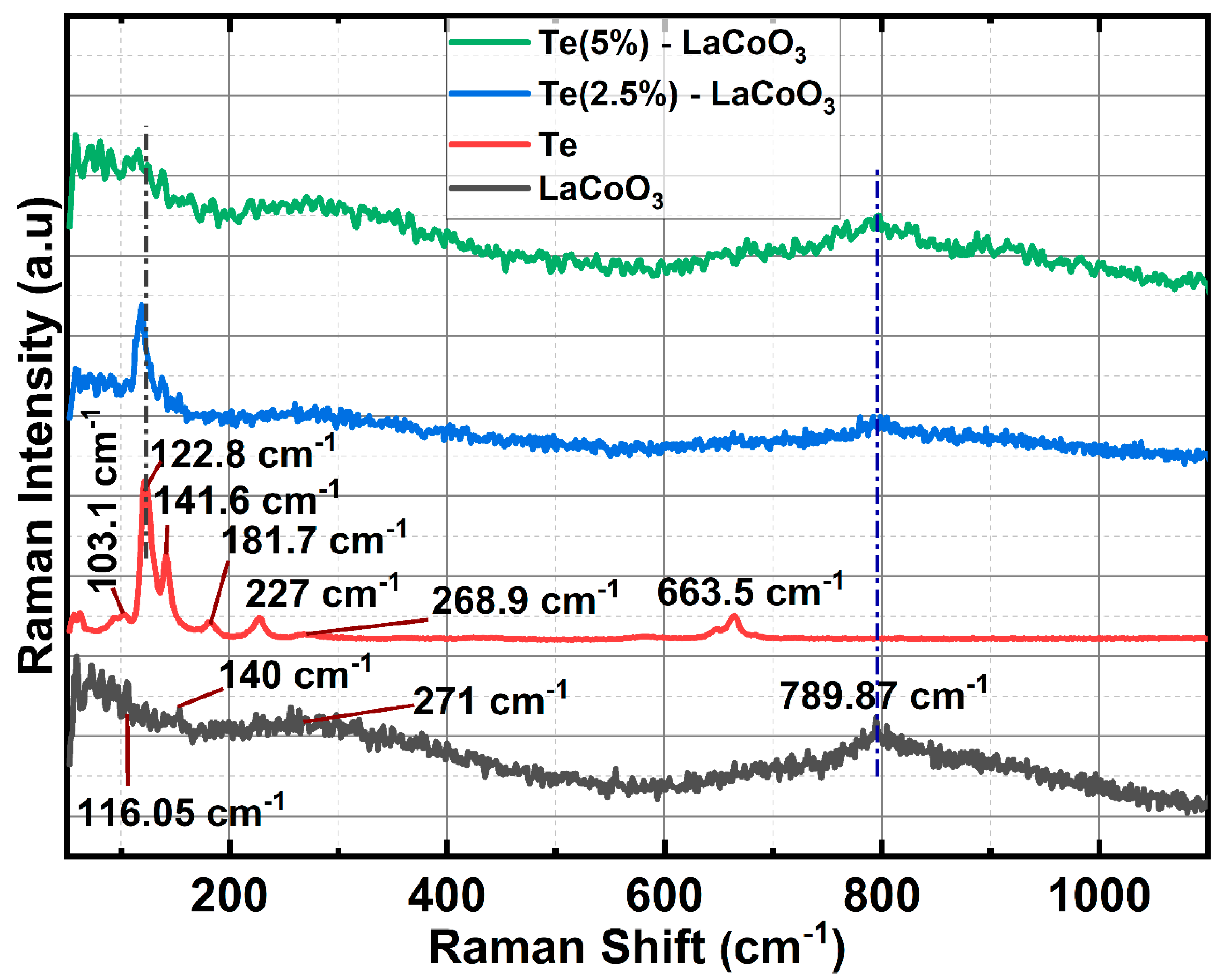
3.2. Raman Spectrum analysis of Te Incorporated LaCoO3
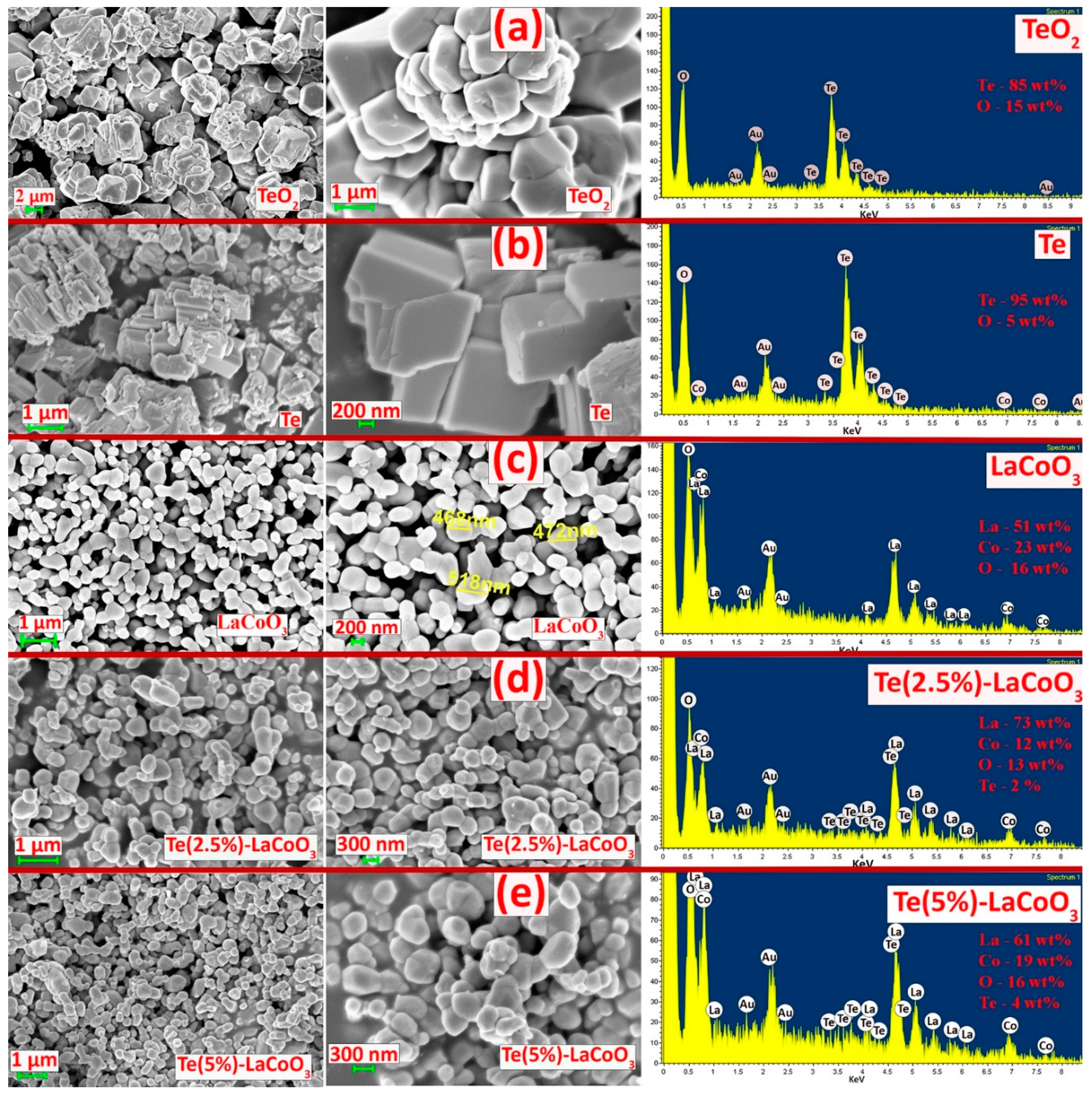
3.3. SEM and Energy-Dispersive X-Ray (EDS) Analysis of Te Incorporated LaCoO3
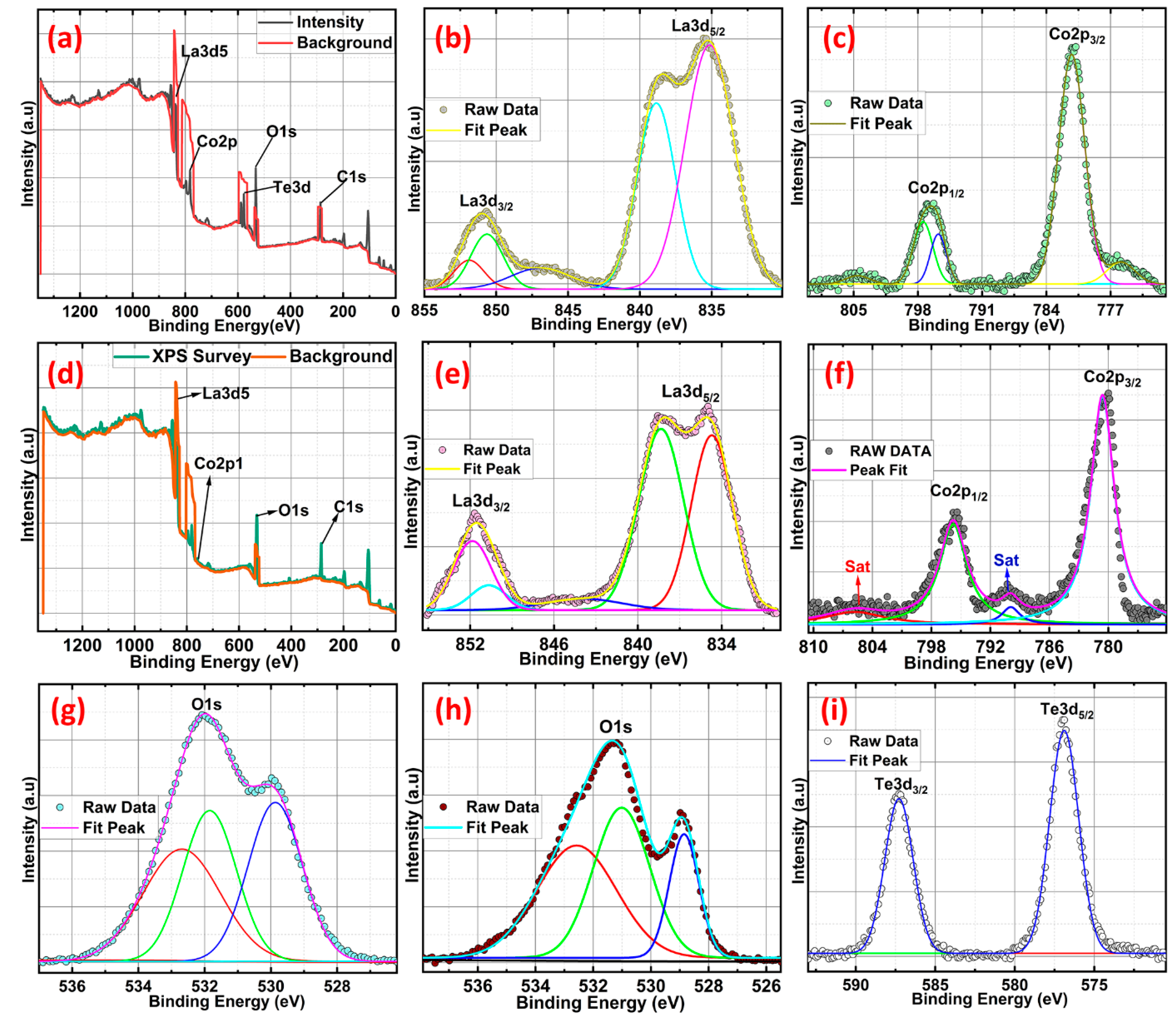
3.4. X-Ray Photoelectron Spectroscopy (XPS) studies of Te Incorporated LaCoO3
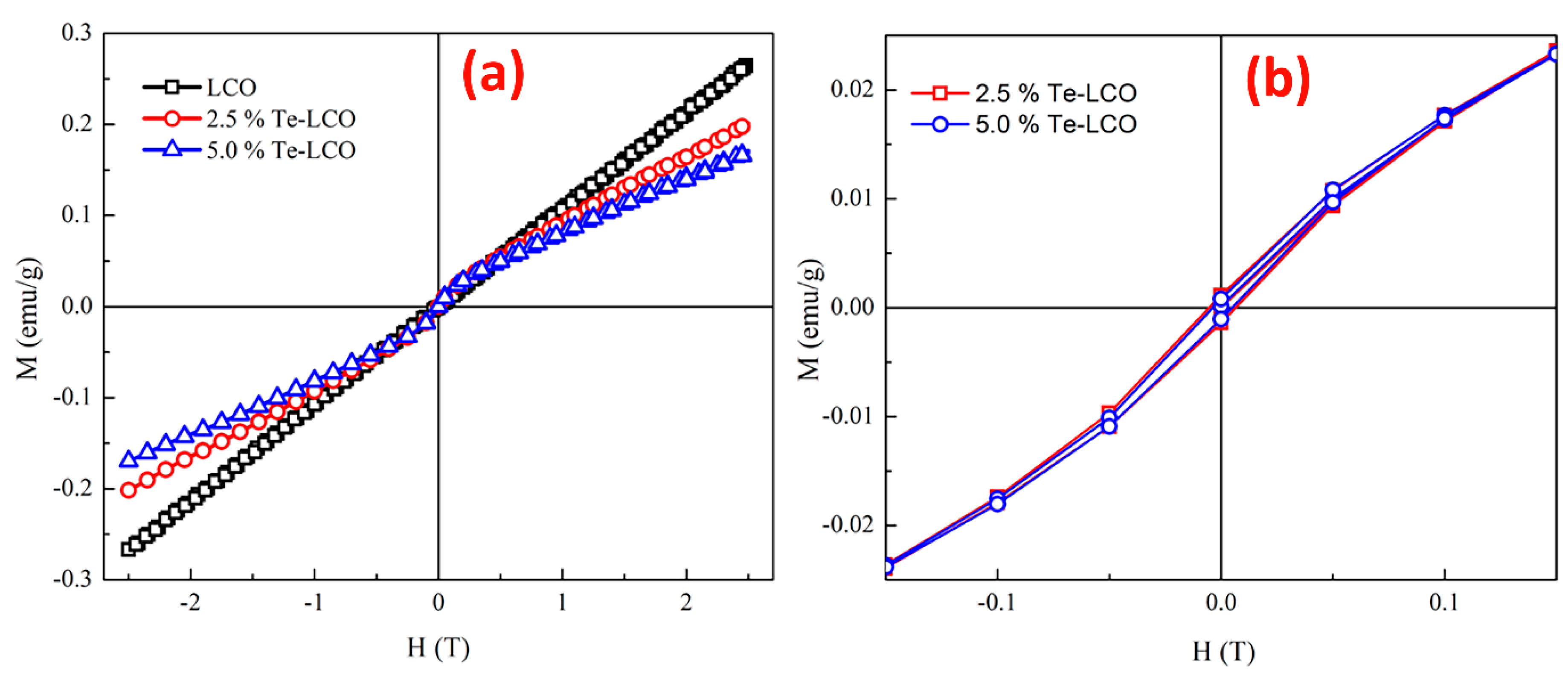
3.4. Magnetic Properties of Te Incorporated LaCoO3
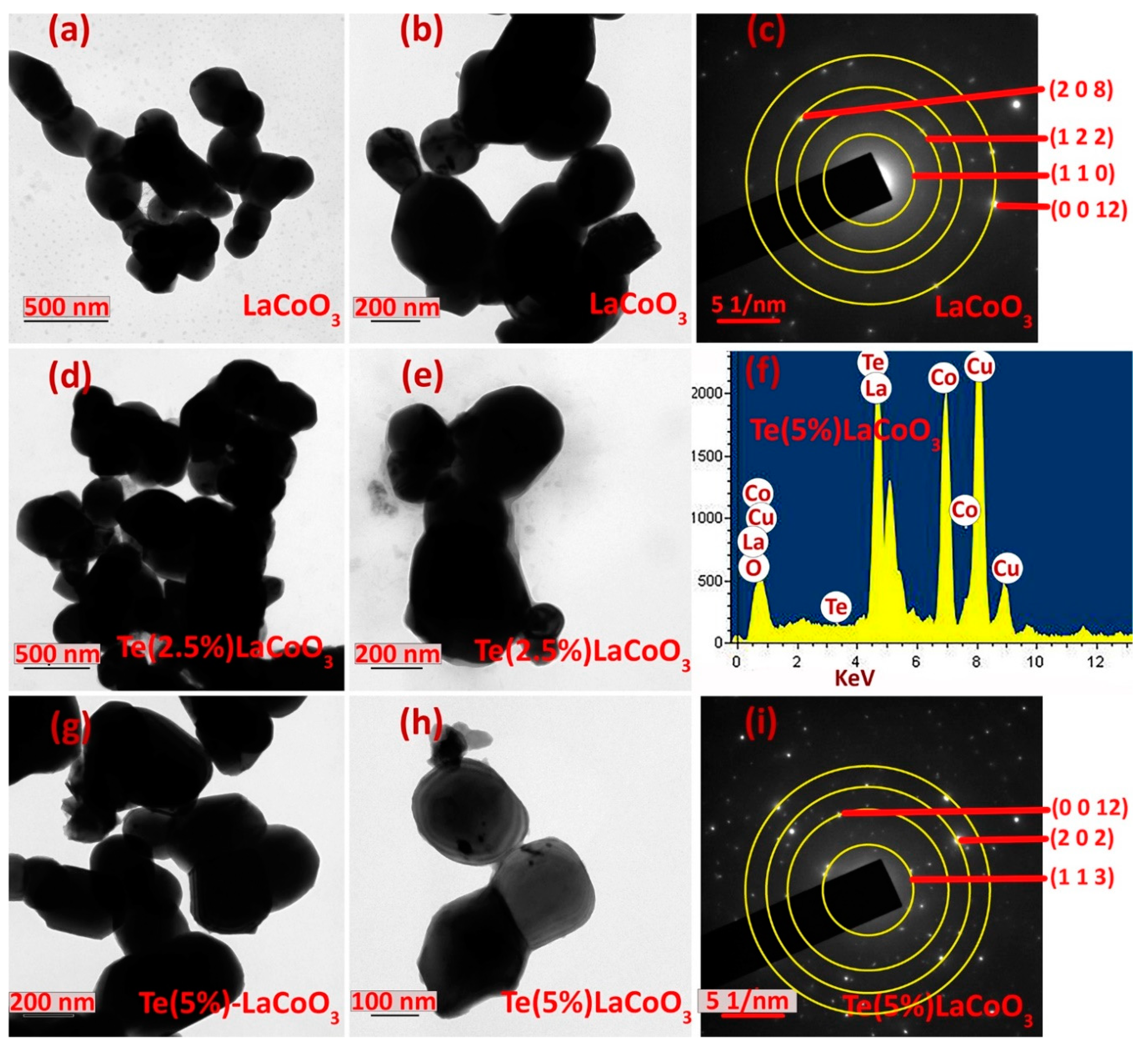
3.5. Transmission analysis of Te Incorporated LaCoO3
4. Conclusion
Data Availability
Acknowledgments
Conflicts of Interest
References
- N Fist, J Dinan, R Stadelmannand N Orlovskaya, (2012) “In situ three point bending device for measurements of vibrational response of ceramics under stress by microRaman spectroscopy,” Advances in Applied Ceramics, vol. 111, no. 7, pp. 433-439, 2012. [CrossRef]
- C.B Samantaray, H Sim, and H Hwang, “Electronic structure and optical properties of barium strontium titanate (BaxSr1−xTiO3) using first-principles method,”Physica B: Condensed Matter, vol. 351, no. 1-2, pp. 158-162, 2004. [CrossRef]
- C.B. Samantaray, H. Sim, and H. Hwang, “The electronic structures and optical properties of BaTiO3 and SrTiO3 using first-principles calculations,” Microelectronics Journal, vol. 36, no. 8, pp.725-728, 2005. [CrossRef]
- J. G. Bednorz, and K. A. Müller, “Sr1−xCaxTiO3: An XY Quantum Ferroelectric with Transition to Randomness,” Physical Review Letters, vol. 52, pp. 2289-2292, 1984. [CrossRef]
- H. P. R. Frederikse, W. R. Thurber, and W. R. Hosler, “Electronic Transport in Strontium Titanate,” Physical Review Journals Archive, vol. 134, pp. A442-A445, 1964. [CrossRef]
- C. S. Koonce, M. L. Cohen, J. F. Schooley, W. R. Hosler, and E. R. Pfeiffer, “Superconducting Transition Temperatures of Semiconducting SrTiO3,”Physical Review Journals Archive, vol. 163, pp. 380-390, 1967. [CrossRef]
- H. Wang, B. Wang, Q. Li, Z. Zhu, R. Wang, and C. H. Woo, “First-principles study of the cubic perovskites BiMO3 (M=Al, Ga, In, and Sc),” Physical Review B,vol. 75, pp. 245209, 2007. [CrossRef]
- P. Baettig, C.F. Schelle, R. LeSar, U. V. Waghmare, and N.A. Spaldin, “Theoretical prediction of new high-performance lead-free Piezoelectrics,” Chemistry of Materials, vol. 17, pp. 1376–1380, 2005. [CrossRef]
- H. Muta, K. Kurosaki, and S. Yamanaka, “Thermoelectric properties of rare earth doped SrTiO3,” Journal of Alloys and Compounds, vol. 350, no. 1-2, pp. 292-295, 2003. [CrossRef]
- P. Sivaprakash, S.Divya, R.Parameshwari,C. Saravanan, S.Sagadevan, S. Arumugam and S. Esakki Muthu,“Influence of Zn2+ doping towards the structural, magnetic, and dielectric properties of NiFe2O4 composite. Journal of Materials Science: Materials in Electronics,vol. 31, pp. 16369–16378, 2020. [CrossRef]
- J. Millis, Boris I. Shraiman, and R. Mueller, “Dynamic Jahn-Teller Effect and Colossal Magnetoresistance in La1-xSrxMnO3,” Physical Review Letters, vol. 77, 175-178, 1996. [CrossRef]
- S. Divya, P. Sivaprakash, S. Raja, S. Esakki Muthu, Emad M. Eed, S. Arumugam and Tae Hwan Oh, “Temperature-dependent dielectric and magnetic properties of NiFe2O4 nanoparticles,” Applied Nanoscience, 2021. [CrossRef]
- M. Dragan, S. Enache, M. Varlam, and K. Petrov, “Perovskite-Type Lanthanum Cobaltite LaCoO3: Aspects of Processing Route toward Practical Applications,” Cobalt Compounds and Applications, IntechOpen, 2019. [CrossRef]
- H. Hilgenkamp, Ariando, H.J.H. Smilde, D.H.A. Blank, G. Rijnders, H. Rogalla, J.R. Kirtley, and C.C. Tsuel, “Ordering and manipulation of the magnetic moments in large-scale superconducting π-loop arrays,” Nature, vol. 422, pp. 50–53, 2003. [CrossRef]
- P. M. Raccah, and J. B. Goodenough, “First-Order Localized-Electron ⇆ Collective-Electron Transition in LaCoO3,” Physical Review Journals Archive,vol. 155, pp. 932-943, 1967. [CrossRef]
- M.A. Señarís-Rodríguez, and J.B. Goodenough, “Magnetic and Transport Properties of the System La1-xSrxCoO3-δ (0 < x ≤ 0.50),” Journal of Solid State Chemistry,vol. 118, pp. 323–336, 1995. [CrossRef]
- Maignan, D. Flahaut, and S. Hebert, “Sign change of the thermoelectric power in LaCoO3,” The European Physical Journal B - Condensed Matter and Complex Systems, vol. 39, pp. 145–148, 2004. [CrossRef]
- E. J. Guo, R. Desautels, D.Keavney, M. A. Roldan, B. J. Kirby, D. Lee, Z. Liao, T. Charlton, A.Herklotz, T. Z. Ward, M. R. Fitzsimmons, andH. N. Lee, “Nanoscale ferroelastic twins formed in strained LaCoO3 films,” Science advances, vol. 5, pp. 1-5, 2019. [CrossRef]
- S. Jhelai, M. Radhakrishnan, N. Padmanathan, S. Esakki Muthu, P. Sivaprakash, and M. Kadiresan, “Effect of Mn substitution on magnetic behaviour of oxygen defective LaCoO3 perovskite oxide,” Materials Science and Engineering: B, vol. 284, pp. 115875, 2022. [CrossRef]
- J.S. Zhou, J.Q. Yan, and J.B. Goodenough, “Bulk modulus anomaly in RCoO3 (R=La, Pr, and Nd),” Physical Review B - Condensed Matter and Materials Physics, vol. 71, pp. 22010, 2005. [CrossRef]
- T. Vogt, J.A. Hriljac, N.C. Hyatt, and P. Woodward, “Pressure-induced intermediate-to-low spin state transition in LaCoO3,” Physical Review B- Condensed Matter and Materials Physics, vol. 67, pp. 140401, 2003. [CrossRef]
- S. Divya, P. Sivaprakash, S. Raja, S. Esakki Muthu, Ikhyun Kim, N. Renuka, S. Arumugam, and Tae Hwan Oh, “Impact of Zn doping on the dielectric and magnetic properties of CoFe2O4 nanoparticles,” Ceramics International, vol. 48,pp. 33208-33218, 2022. [CrossRef]
- Y. Xu, P. Zielke, N. van Nong, S. Pirou, R. Reolon, X. Si, S.B. Simonsen, P. Norby, H. Lühmann, W. Bensch, and R. Kiebach, “Hydrothermal Synthesis, Characterization, and Sintering Behavior of Core-Shell Particles: A PrincipleStudy on Lanthanum Strontium Cobaltite Coated with Nanosized Gadolinium Doped Ceria,” Ceramics. vol.1,pp. 246-260, 2018. [CrossRef]
- M. Ayyob, I. Ahmad, F. Hussain, M. Kashif Bangash, J.A. Awan, and J.N. Jaubert, “A new technique for the synthesis of lanthanum substituted nickel cobaltite nanocomposites for the photo catalytic degradation of organic dyes in wastewater,” Arabian Journal of Chemistry,vol. 13, pp. 6341–6347, 2020. [CrossRef]
- Deeksha, P. Kour, I. Ahmed, K. K.Haldar,and K. Yadav, “Tuning the Morphology of Lanthanum Cobaltite Using the Surfactant-Assisted Hydrothermal Approach for Enhancing Oxygen Evolution Catalysis,” Proceedings of the National Workshop on Recent Advances in Condensed Matter and High Energy Physics, Springer Proceedings in Physics, Springer, Singapore,vol. 278, pp. 15-24, 2022. [CrossRef]
- L. Tepech-Carrillo, A. Escobedo-Morales, A. Pérez-Centeno, E. Chigo-Anota, J.F. Sánchez-Ramírez, E. López-Apreza, and J. Gutiérrez-Gutiérrez, “Preparation of Nanosized LaCoO3 through Calcination of a Hydrothermally Synthesized Precursor,” Journal of Nanomaterials,vol. 2016, pp. 7, 2016.
- M. Popa, J. Frantti, and M. Kakihana, “Characterization of LaMeO3 (Me: Mn, Co, Fe) perovskite powders obtained by polymerizable complex method,” Solid State Ionics. Vol. 154–155, pp. 135-141, 2002. [CrossRef]
- M.N. Iliev, and M. V Abrashev, “Raman phonons and Raman Jahn-Teller bands in perovskite-like manganites,” Journal of Raman Spectroscopy, vol. 32, no. 10, pp. 805–811, 2001. [CrossRef]
- N. Orlovskaya, D. Steinmetz, S. Yarmolenko, D. Pai, J. Sankar, and J. Goodenough, “Detection of temperature- and stress-induced modifications of LaCoO3 by micro-Raman spectroscopy,”Physical Review B - Condensed Matter and Materials Physics, vol. 72, pp. 014122, 2005. [CrossRef]
- R.M. Martin, G.I. R.M. Martin, G.I. Ucovsky, and K. Helliwell, “Intermolecnlar bonding and lattice dynamics of Se and Te,” Physical Review B - Condensed Matter and Materials Physics, vol. 13, pp. 1383, 1976. [CrossRef]
- S. Pine and G. Dresselhaus, “Raman Scattering in Paratellurite, TeO2,” Physical Review B - Condensed Matter and Materials Physics, vol. 5, pp. 4087, 1972. [CrossRef]
- . Marini, D. Chermisi, M. Lavagnini, D. Di Castro, C. Petrillo, L. Degiorgi, S. Scandolo, and P. Postorino, “High-pressure phases of crystallite tellurium: A combined Raman and ab initio study,”Physical Review B - Condensed Matter and Materials Physics, vol. 86. Pp. 064103, 2012. [CrossRef]
- J. He, W. Lv, Y. Chen, K. Wen, C. Xu, W. Zhang, Y. Li, W. Qin, and W. He, “Tellurium-Impregnated Porous Cobalt-Doped Carbon Polyhedra as Superior Cathodes for Lithium-Tellurium Batteries,” ACS Nano,vol. 11, pp. 8144–8152, 2017. [CrossRef]
- J. Haber, and L. Ungier, “On chemical shifts of ESCA and Auger lines in cobalt oxides,”Journal of Electron Spectroscopy and Related Phenomena, vol. 12,pp. 305-312, 1977. [CrossRef]
- N. S. McIntyre, D. D. Johnston, L. L.Coatsworth, R. D. Davidson, and J. R. Brown, “X-ray photoelectron spectroscopic studies of thin film oxides of cobalt and molybdenum,” Surface and Interface Analysis, vol. 15, no. 4, pp. 265-272, 1990. [CrossRef]
- H. Wang, W. Xu, S. Richins, K. Liaw, L. Yan, M. Zhou, and H. Luo, “Polymer-assisted approach to LaCo1-xNixO3 network nanostructures as bifunctional oxygen electrocatalysts,” Electrochimica Acta, vol. 296, pp. 945–953, 2019. [CrossRef]
- D.C. Frost, C.A. McDowell, and I.S. Woolsey, “Evidence for multiplet splitting of 2p photoelectron lines of transition metal complexes,” Chemical Physics Letters, vol. 17, pp. 320-323, 1972. [CrossRef]
- H. Seim, M. Nieminen, L. Niinistö, H. Fjellvåg, and L.S. Johansson, “Growth of LaCoO3 thin films from β-diketonate precursors,” Applied Surface Science, pp. 112, pp. 243–250, 1997. [CrossRef]
- C. V. Ramana, R.S. Vemuri, V. V. Kaichev, V.A. Kochubey, A.A. Saraev, and V. V. Atuchin, “X-ray photoelectron spectroscopy depth profiling of La2O3/Si thin films deposited by reactive magnetron sputtering,” ACS Applied Materialsand Interfaces, vol. 3, pp. 4370–4373, 2011. [CrossRef]
- X. Jiang, Y. Dong, Z. Zhang, J. Li, J. Qian, and D. Gao, “Cation substitution of B-site in LaCoO3 for bifunctional oxygen electrocatalytic activities,” Journalof Alloys and Compounds,vol. 878, pp. 160433, 2021. [CrossRef]
- L. Armelao, D. Barreca, G. Bottaro, A. Gasparotto, C. Maragno, and E. Tondello, “LaCoO3Nanosystems by a Hybrid CVD/Sol-Gel Route: An XPS Investigation,” Surface Science Spectra,vol. 10, pp. 143–149, 2003. [CrossRef]
- R. P. Vasquez, “X-ray photoemission measurements of La1-xCaxCoO3(x=0, 0.5),” Physical Review B - Condensed Matter and Materials Physics, vol. 54, pp. 14938, 1996. [CrossRef]
- A.J. Ricco, H.S. White, and M.S. Wrighton, “X-ray photoelectron and Auger electron spectroscopic study of the CdTe surface resulting from various surface pretreatments: Correlation of photoelectrochemical and capacitance-potential behavior with surface chemical composition,” Journal of Vacuum Science & Technology A: Vacuum, Surfaces, and Films, vol. 2, pp. 910–915, 1984. [CrossRef]
- A.B. Christie, I. Sutherland, and J.M. Walls, “Studies of the composition, ion-induced reduction and preferential sputtering of anodic oxide films on Hg0.8Cd0.2Te by XPS,” Surface Science, vol. 135, no. 1-3, pp. 225-242, 1983. [CrossRef]
- K. Hidetaka, and Y. Yoshihisa, “Ylide-Metal Complexes. XIV. An X-Ray Photoelectron Spectroscopic Study on Tellurium Complexes of Methylenetriphenylphosphorane,” Bulletin of the Chemical Society of Japan,vol. 61, no. 8, pp. 2990-2992, 1988. [CrossRef]
- W. Branford, M.A. Green, and D.A. Neumann, “Structure and ferromagnetism in Mn4+spinels: AM0.5Mn1.5O4 (A = Li, Cu; M = Ni, Mg),” Chemistry of Materials, vol. 14, no. 4, pp. 1649–1656, 2002. [CrossRef]
- K.P. Thummer, M.C. Chhantbar, K. Modi, and H. Joshi, Effect of Mn4+ substitution on magnetic behaviour of cobalt ferrite, Indian Journal of Physics, vol. 79, pp. 41–45, 2005.
- H. Liang, Y. Hong, C. Zhu, S. Li, Y. Chen, Z. Liu, and D. Ye, “Influence of partial Mn-substitution on surface oxygen species of LaCoO3 catalysts,” Catalysis Today,vol. 201, pp. 98–102, 2013. [CrossRef]
- P. Xiao, J. Zhu, H. Li, W. Jiang, T. Wang, Y. Zhu, Y. Zhao, and J. Li, “Effect of textural structure on the catalytic performance of LaCoO3 for CO oxidation,”ChemCatChem, vol. 6, no. 6,pp. 1774–1781, 2014. [CrossRef]
- R. Schmidt, J. Wu, C. Leighton, and I. Terry, Dielectric response to the low-temperature magnetic defect structure and spin state transition in polycrystalline LaCoO3,” Physical Review B - Condensed Matter and Materials Physics,vol. 79, pp. 125105, 2009. [CrossRef]
- K. Tomiyasu, M. Sato, S.I. Koyama, T. Nojima, R. Kajimoto, S. Ji, and K. Iwasa, “Magnetic properties of electron-doped LaCoO3,” Journal of the Physical Society of Japan, vol. 86, no. 9, pp. 094706, 2017. [CrossRef]
- D. Gignoux, Etienne Du Tremolet De Lacheisserie, M. Schlenker, “Magnetism: Materials and Applications,” Springer. 0-387-23063-7 (2005).
- R. Wang, C. Ye, H. Wang, and F. Jiang, “Z-Scheme LaCoO3/g-C3N4 for Efficient Full-Spectrum Light-Simulated Solar Photocatalytic Hydrogen Generation,” ACS Omega. vol. 5, no. 47, pp. 30373–30382, 2020. [CrossRef]
| Composition | Crystal System | Space Group | Crystallite size (nm) | Lattice Parameter (Å) | ||
| a | b | c | ||||
| TeO2 | Tetragonal | P41212 | 26.80514 | 5.40681 | 5.40865 | 13.20149 |
| Te | Hexagonal | P3121 | 28.33724 | 4.19097 | 4.19123 | 5.98099 |
| LaCoO3 | Rhombohedral | Rc | 43.42469 | 5.41768 | 5.39366 | 13.13084 |
| 2.5% Te/LCO | Rhombohedral | Rc | 31.59925 | 5.42428 | 5.39256 | 13.1579 |
| 5% Te/LCO | Rhombohedral | Rc | 31.59925 | 5.42681 | 5.39434 | 13.20149 |
| Composition | HC(T) | M (emu/g) @300K | MR(emu/g) |
| LCO | -- | --- | ----- |
| 2.5% Te-LCO | 0.0065 | 0.20166 | 0.00128 |
| 5% Te-LCO | 0.0049 | 0.17051 | 0.00094 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





