Introduction
The devastating formation of brain metastasis occurs in 10-16 % of breast cancer patients and is usually a late event in the natural course of the disease. The median survival following cerebral seeding is very limited, ranging from only a few months to two years [
1,
2]. Among all breast cancer subtypes, human epidermal growth factor receptor 2 (HER2)-positive - and triple-negative (estrogen- and progesterone receptors- and HER2 protein-negative) breast cancers have a higher risk of developing brain metastases [
3,
4,
5,
6]. The development of cerebral metastases depends primarily on the successful penetration of the blood-brain barrier (BBB) by circulating tumor cells. The underlying mechanisms and molecular pathways utilized by the breast cancer cells to cross the BBB are largely unknown, although several genes and pathways were associated with this event [
7].
Previously, we found that T lymphocyte-related genes are associated with brain seeding by comparing gene expression profiles of primary breast cancer samples of women with and without brain metastasis [
8]. We demonstrated that ER- breast cancer cells that were co-cultured with activated T cells displayed an increased propensity to cross an
in vitro blood-brain barrier (BBB). Moreover, we found specific upregulation of guanylate-binding protein 1 (GBP1) in the tumor cells crossing the BBB and we also demonstrated that GBP-1 was overexpressed in the primary breast cancer samples of those women who had developed brain metastases [
8]. GBP1 is a GTPase in the dynamin superfamily and a downstream target of the interferon-gamma (IFN-γ) pathway. T lymphocytes are known to secrete IFN-γ upon activation and interaction with cancer cells [
9,
10]. So far, neither the particular subset of T cells, nor the T cell derived cytokine(s) that contribute most to the enhanced transmigration of the breast cancer cells through the BBB, have been identified. The aim of the present study was to reveal which T cell subset(s) facilitate BBB transmigration of MDA-MB-231 breast cancer cells most, and verify the involvement of IFN-γ and its signaling pathway in this event.
Results
Activated CD8+ T lymphocytes most strongly stimulate MDA-MB-231 breast cancer cells to pass the BBB
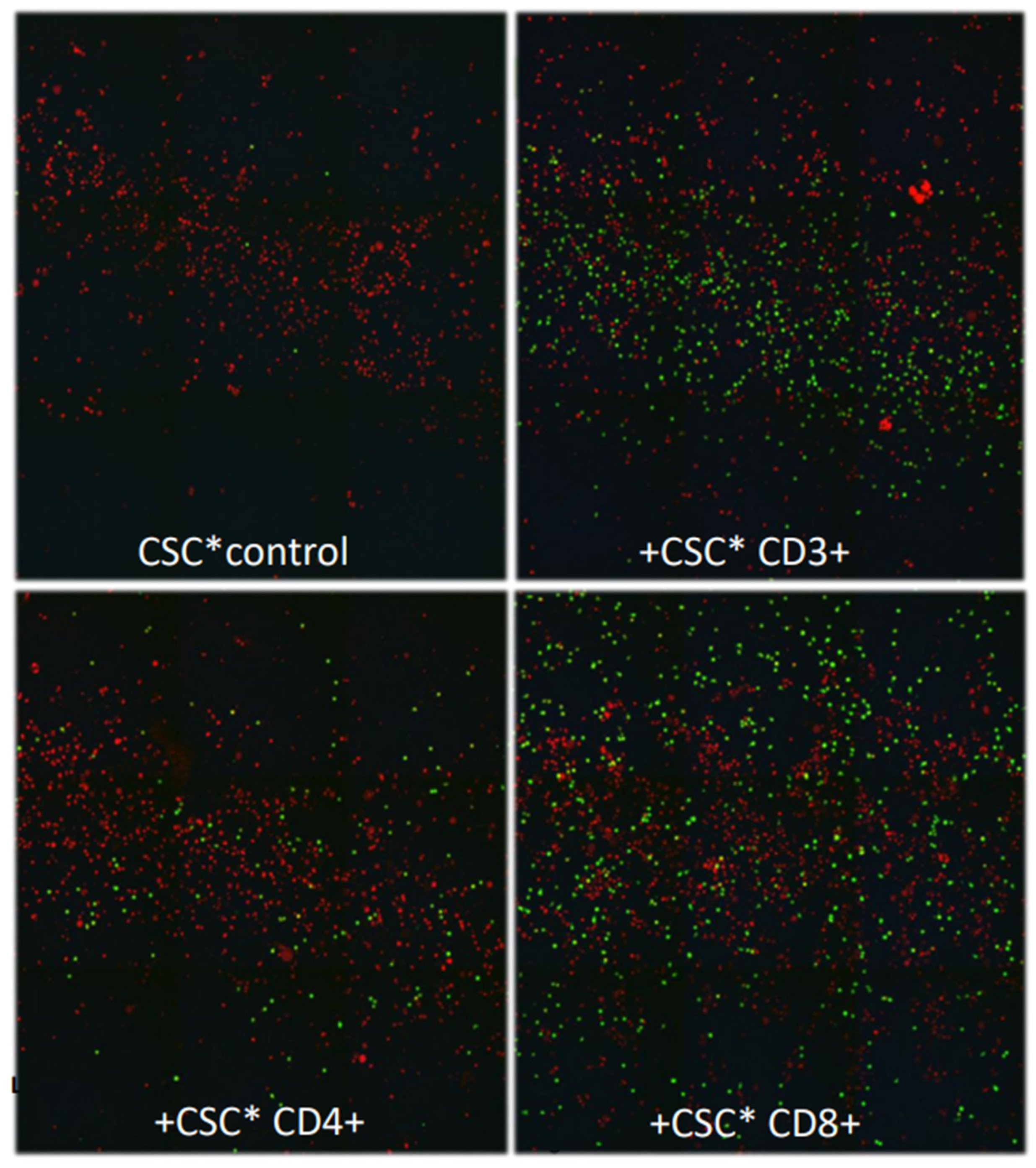
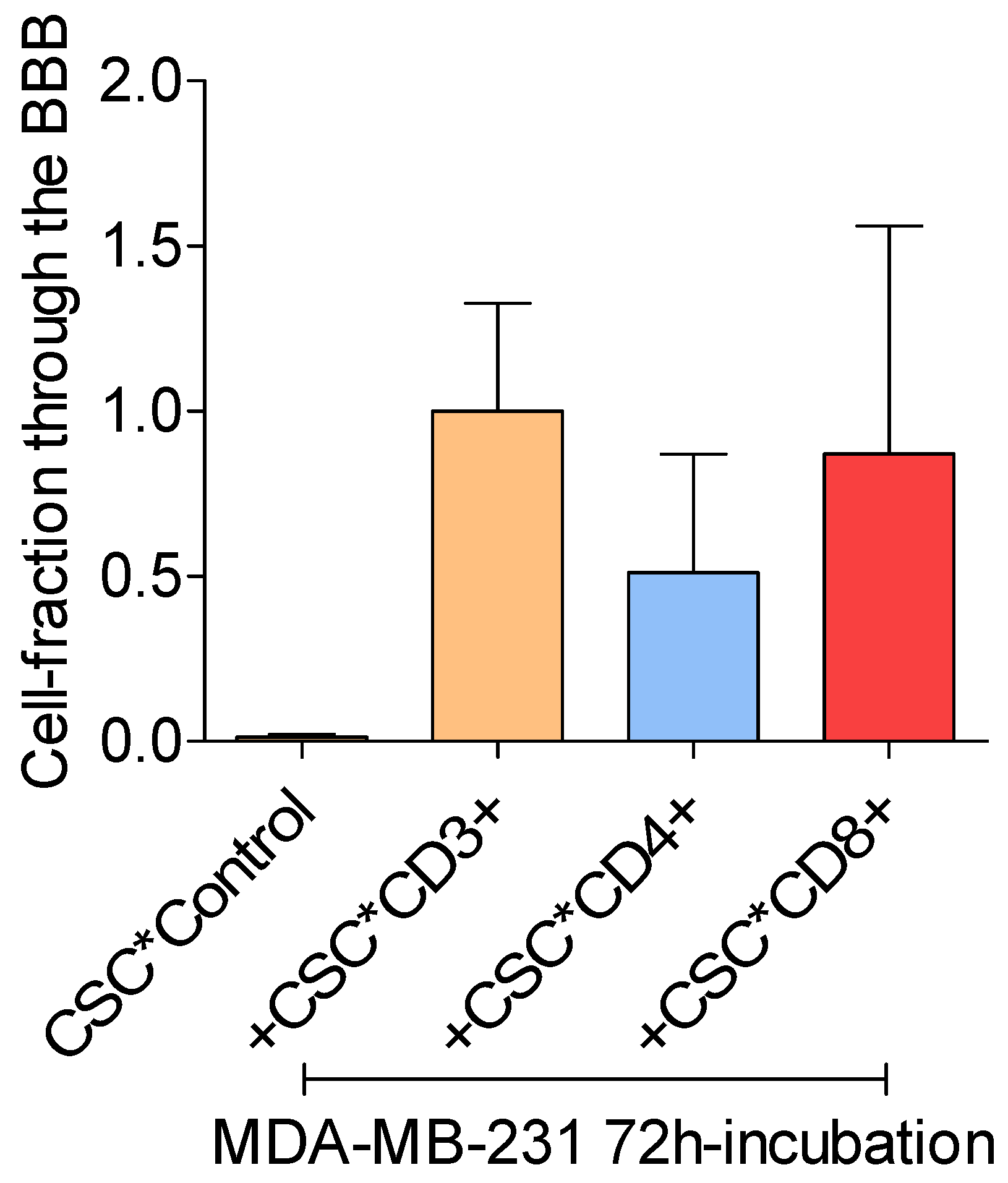
All CSC-activated T lymphocyte fractions increased the ability of MDA-MB-231 breast cancer cells to cross the in vitro BBB. However, the CD8+ T lymphocyte fraction stimulated the BBB-passage of the breast cancer cells more than the CD4+ T-lymphocyte fraction did (p > 0.05). Addition of CSC to the breast cancer cells without activated T lymphocytes did not result in an increased BBB passage (Figure 1b,c).
Figure 1b.
Confocal images taken of the breast cancer cells that reached the bottom of the 24-well chambers by crossing the in vitro BBB. Cancer cells that were incubated with cell stimulation cocktail (CSC) only did not cross the in vitro BBB (control, upper panel left). The number of cancer cells that crossed the in vitro BBB were larger following co-culture with CD8+ than with CD4+ T cells (p > 0.05). (Green dots: breast cancer cells; red dots: nuclei of cells other than breast cancer cells; living cancer cells nuclear-stained with Hoechst 33342).
Figure 1b.
Confocal images taken of the breast cancer cells that reached the bottom of the 24-well chambers by crossing the in vitro BBB. Cancer cells that were incubated with cell stimulation cocktail (CSC) only did not cross the in vitro BBB (control, upper panel left). The number of cancer cells that crossed the in vitro BBB were larger following co-culture with CD8+ than with CD4+ T cells (p > 0.05). (Green dots: breast cancer cells; red dots: nuclei of cells other than breast cancer cells; living cancer cells nuclear-stained with Hoechst 33342).
Figure 1c.
Mean-percentages breast cancer cells that passed the in vitro BBB after incubation with PMA (ctr), and after co-culture with PMA-activated T lymphocyte subtypes. The fractions were calibrated to the fraction of tumor cells co-cultured with activated CD3+ T cells. Mean values ± SD; data from 3 independent experiments. .
Figure 1c.
Mean-percentages breast cancer cells that passed the in vitro BBB after incubation with PMA (ctr), and after co-culture with PMA-activated T lymphocyte subtypes. The fractions were calibrated to the fraction of tumor cells co-cultured with activated CD3+ T cells. Mean values ± SD; data from 3 independent experiments. .
T cell secreted IFN-γ
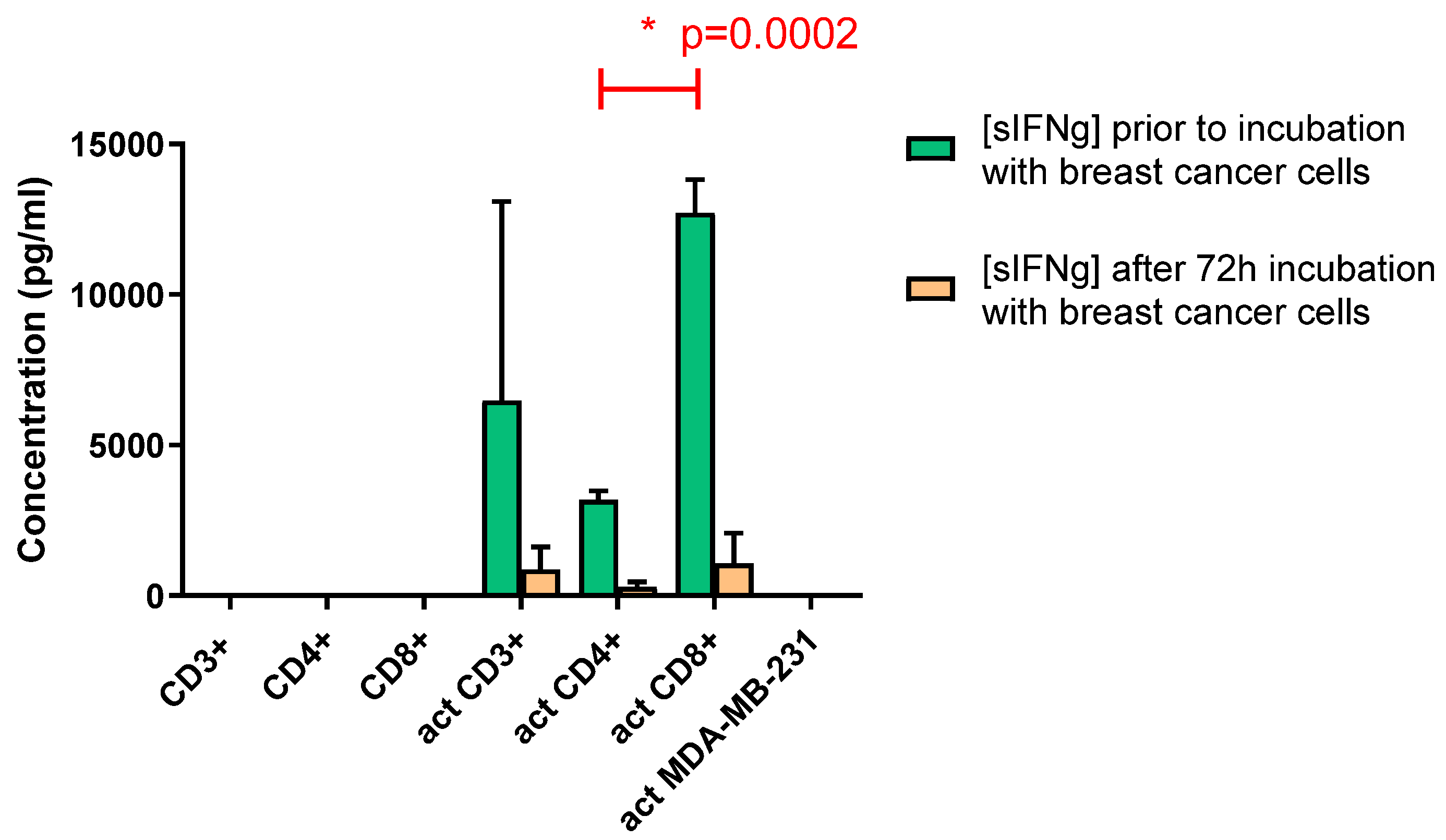
Concentrations of soluble IFN-γ (sIFN-γ) in the media of the CSC activated fractions of T cells were determined. Upon CSC activation and co-culture with the breast cancer cells, the respective T cell fractions secreted variable concentrations of IFN-γ in the media. No IFN-γ was detectable in the media of CSC-exposed breast cancer cells. The CD8+ cells secreted approximately four-fold higher levels of IFN-γ than CD4+ cells did (p < 0.001, Figure 2a). The IFN-γ levels dropped significantly 72 hours after co-culturing with the breast cancer cells for the CD4+ and CD8+ (together CD3+) T lymphocytes (Figure 2a).
Figure 2a.
Concentrations of sIFN-γ for the T cell fractions before and after co-culturing with the MDA-MB-231 breast cancer cells. No sIFN-γ was detected upon co-culturing non-activated T cells with the breast cancer cells (left three bars). Activated T cells secreted sIFN-γ upon co-culturing with the breast cancer cells. The CD8+ T cells secreted higher concentrations of sIFN-γ than CD4+ cells did (p< 0.001; green bars). There is significant decrease in concentrations of sIFN-γ following 72 hours of incubation with the tumor cells (orange bars). After 72 hours incubation no significant differences in IFN-γ levels were present between the incubations with CD8+ or CD4+ cells (orange bars). Activation of MDA-MB-231 breast cancer cells did not secrete IFN-γ most right column). Graph representative of two independent experiments (act = activated as described in the M&M section).
Figure 2a.
Concentrations of sIFN-γ for the T cell fractions before and after co-culturing with the MDA-MB-231 breast cancer cells. No sIFN-γ was detected upon co-culturing non-activated T cells with the breast cancer cells (left three bars). Activated T cells secreted sIFN-γ upon co-culturing with the breast cancer cells. The CD8+ T cells secreted higher concentrations of sIFN-γ than CD4+ cells did (p< 0.001; green bars). There is significant decrease in concentrations of sIFN-γ following 72 hours of incubation with the tumor cells (orange bars). After 72 hours incubation no significant differences in IFN-γ levels were present between the incubations with CD8+ or CD4+ cells (orange bars). Activation of MDA-MB-231 breast cancer cells did not secrete IFN-γ most right column). Graph representative of two independent experiments (act = activated as described in the M&M section).
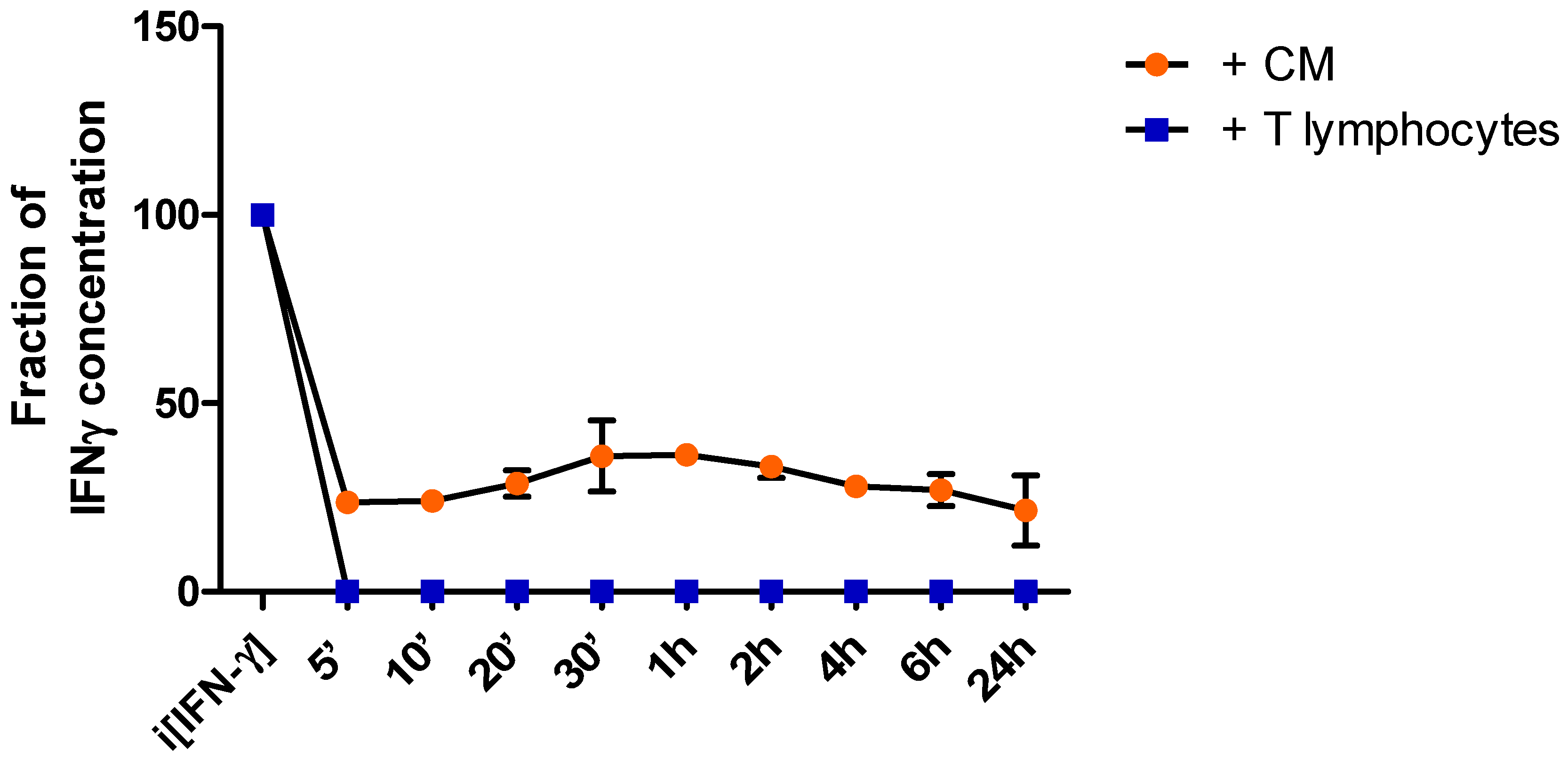
In order to monitor sIFN-γ concentrations in the media over time the supernatant was collected at different time points following incubation of breast cancer cells with either activated T lymphocytes (MDA+T), or with the media conditioned by activated T lymphocytes (MDA+CM). After five minutes of co-culturing the cancer cells with the T cells sIFN-γ was no longer detected (Figure 2b). Upon incubation with the CM without the T cells the sIFN-γ levels dropped to approximately 20% of the initial concentrations and did not change over 24 hours (Figure 2b).
Figure 2b.
sIFN-γ concentration curve. Concentration curve of sIFN-γ following incubation of the cancer cells with CM of activated bulk T cells (orange dots), or co-culture with activated bulk T cells (blue blocks). Within the first 5 minutes a steep drop in IFN-γ is recorded for both conditions . Graph representative of two independent experiments.
Figure 2b.
sIFN-γ concentration curve. Concentration curve of sIFN-γ following incubation of the cancer cells with CM of activated bulk T cells (orange dots), or co-culture with activated bulk T cells (blue blocks). Within the first 5 minutes a steep drop in IFN-γ is recorded for both conditions . Graph representative of two independent experiments.
IFN-γ neutralization and IFNGR1 receptor blocking in the breast cancer cells impairs passing the BBB
In order to investigate whether IFN-γ enhanced BBB passage of the MDA-MB-231 breast cancer cells we used neutralizing antibodies to the IFN-γ molecule (anti-hIFN-γ-IgA), and blocked the INF-γ receptor IFNGR1 by using a monoclonal antibody (human IFNGR1/CD119). Blocking of IFNGR1 resulted in reduction in protein expression of subunit IFNGR2 of the IFNγ receptor (Suppl. Figure 1). Blocking IFNGR1 in the breast cancer cells prior to overnight incubation with CM of activated bulk T lymphocytes resulted in a concentration-dependent decrease in passage of the tumor cells through the in vitro BBB (Figure 3a). Neutralization of IFN-γ in the media of the activated bulk T lymphocytes by using anti-hIFN-γ mAb (10 µg/ml) prior to the incubation with the tumor cells also reduced their passage significantly (80%; p < 0.002, Figure 3b).
Figure 3.
Blocking of the IFN-γ pathway in MDA-MB-231 breast cancer cells impairs passage through the BBB model. a) Fractions of transmigrated cancer cells through the in vitro BBB model after one hour incubation with increasing concentrations of human IFNGR1/CD119 mAb and subsequent overnight incubation with equal amounts of CM of activated bulk T cells. The fractions are relative to 10 µg/ml IgG1 isotype control. (ctr, MDA-MB-231 control cells; CM, conditioned medium; IgG1, hIgG1 isotype control). Graph representative of three independent functional experiments. b) Fractions of transmigrated cancer cells after neutralizing the sIFN-γ in the media of activated T cells. The fractions are relative to 10 µg/ml anti-IgA2 control. Graph representative of two independent experiments. Human IgA2 isotype control was used as control. c) Western blots of phosphorylated STAT1 (p-STAT1) , total STAT1 (t-STAT1), IFNGR2 and GBP1 protein expression levels in MDA-MB-231 samples after blocking IFNGR1 (experiment described in a.) or after neutralizing the sIFN-γ in the media of activated T cells. Actin was used as loading control for both membranes. A concentration-dependent reduction of p-STAT1, t-STAT1, IFNGR2 and GBP1 proteins was observed by using increased concentrations of human IFNGR1/CD119 monoclonal antibody. Western blots representative of two independent experiments. d) Normalized expression of t-STAT1 and p-STAT1 in the cancer cells following concentration-dependent IFNGR1 blocking in the cancer cells, or after neutralizing the sIFN-γ in the media of activated T cells. The level of p-STAT1 is increased by the addition of CM to the breast cancer cells and step-wise curtailed until it matched the p-STAT1 level observed in MDA-MB-231 control cells, synchronous with IFN-γ pathway blocking. t-STAT1 and p-STAT1 expression levels were calculated in the cancer cells after background deduction and normalization against actin and normalized t-STAT, respectively. (ctr, MDA-MB-231 control cells; CM, conditioned medium; IgG1, anti hIgG1 isotype control; IFN-γ, anti-hIFN-γ-IgA; IgA2, anti hIgA2 isotype control. Graph representative of two independent Western blots).
Figure 3.
Blocking of the IFN-γ pathway in MDA-MB-231 breast cancer cells impairs passage through the BBB model. a) Fractions of transmigrated cancer cells through the in vitro BBB model after one hour incubation with increasing concentrations of human IFNGR1/CD119 mAb and subsequent overnight incubation with equal amounts of CM of activated bulk T cells. The fractions are relative to 10 µg/ml IgG1 isotype control. (ctr, MDA-MB-231 control cells; CM, conditioned medium; IgG1, hIgG1 isotype control). Graph representative of three independent functional experiments. b) Fractions of transmigrated cancer cells after neutralizing the sIFN-γ in the media of activated T cells. The fractions are relative to 10 µg/ml anti-IgA2 control. Graph representative of two independent experiments. Human IgA2 isotype control was used as control. c) Western blots of phosphorylated STAT1 (p-STAT1) , total STAT1 (t-STAT1), IFNGR2 and GBP1 protein expression levels in MDA-MB-231 samples after blocking IFNGR1 (experiment described in a.) or after neutralizing the sIFN-γ in the media of activated T cells. Actin was used as loading control for both membranes. A concentration-dependent reduction of p-STAT1, t-STAT1, IFNGR2 and GBP1 proteins was observed by using increased concentrations of human IFNGR1/CD119 monoclonal antibody. Western blots representative of two independent experiments. d) Normalized expression of t-STAT1 and p-STAT1 in the cancer cells following concentration-dependent IFNGR1 blocking in the cancer cells, or after neutralizing the sIFN-γ in the media of activated T cells. The level of p-STAT1 is increased by the addition of CM to the breast cancer cells and step-wise curtailed until it matched the p-STAT1 level observed in MDA-MB-231 control cells, synchronous with IFN-γ pathway blocking. t-STAT1 and p-STAT1 expression levels were calculated in the cancer cells after background deduction and normalization against actin and normalized t-STAT, respectively. (ctr, MDA-MB-231 control cells; CM, conditioned medium; IgG1, anti hIgG1 isotype control; IFN-γ, anti-hIFN-γ-IgA; IgA2, anti hIgA2 isotype control. Graph representative of two independent Western blots).
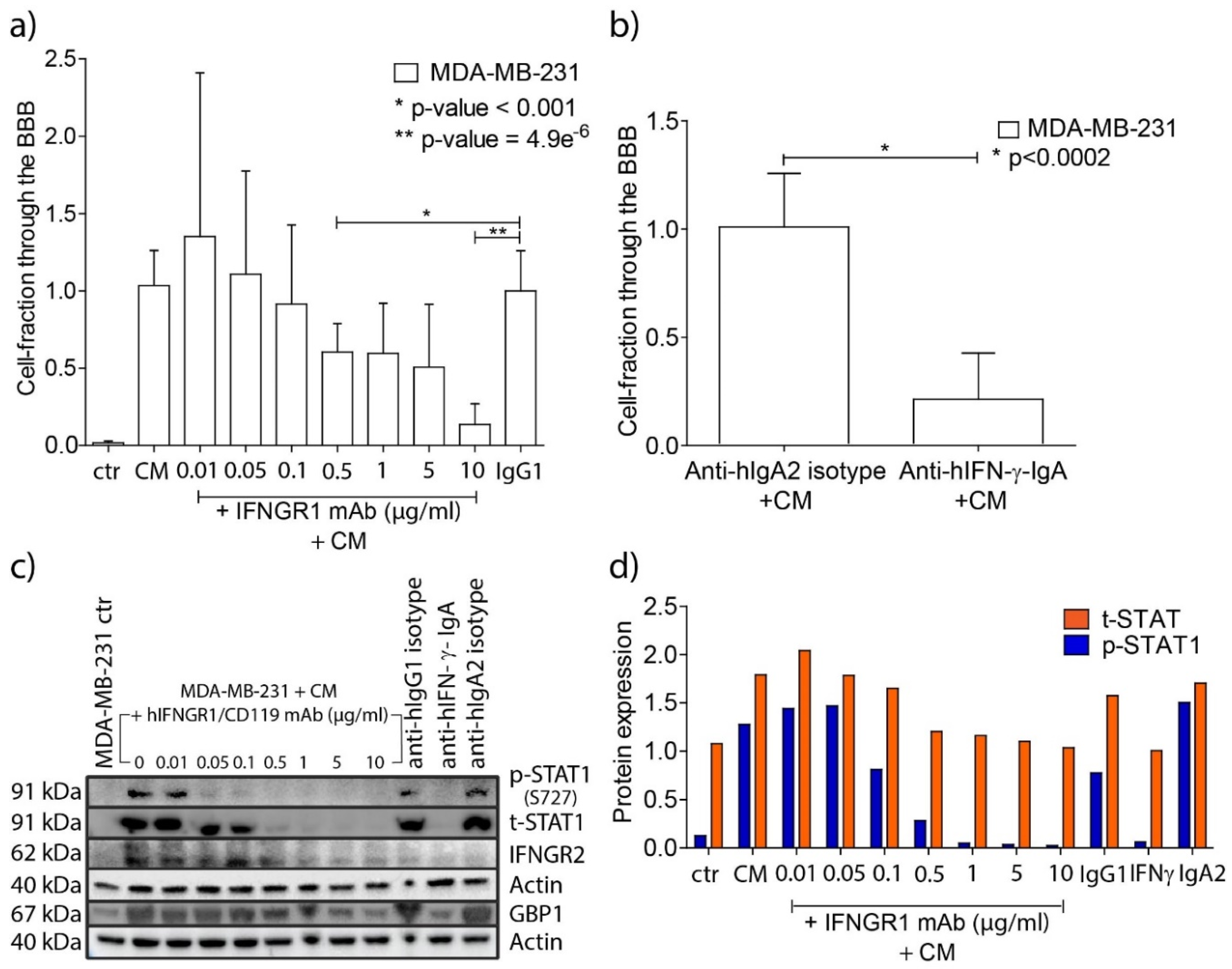
To ascertain the effects of blocking the IFN-γ pathway in the cancer cells, activation of the downstream signaling molecule STAT1 was monitored by measuring phosphorylated STAT1 (p-STAT1). Concentration-dependent reduction of p-STAT1 was observed along with increasing concentrations of the blocking antibody to IFNGR2 (
Figure 3c,d). In addition, we monitored the expression of GBP1 that was found to be upregulated in breast cancer cells upon co-culturing with activated T lymphocytes in our previous work [
8]. Expression of both IFNGR2 and GBP1 was reduced upon IFNGR1 blockage (
Supplemental Figures 1 and 2).
Discussion
Based on expressional differences between tumors with and without brain metastases we discovered a relation between T lymphocytes invading primary breast cancers and their potency of seeding to brain. In additional
in vitro and
in vivo studies we found that T cells affect the passage of breast cancer cells through a modelled BBB [
8]. We also described the specific upregulation of GBP1 in the tumor cells as consequence of T cell interactions. In general, the effects of an inflammatory infiltrate on tumor progression and prognosis of the patients are numerous and complex [
17,
18,
19]. Although the influence of T cell infiltrates on clinical behavior of breast cancers has been noticed in the past, a specific brain metastasis-promoting effect was not described previously [
20,
21,
22]. Two important questions, namely the particular T cell subset that promotes the brain seeding most, and the role of interferon-γ, being a prominent secreted cytokine of T cells, remained unanswered.
In the present study we found that CD8+ T lymphocytes affect the passage of MDA-MB-231 breast cancer cells through the BBB strongest, and that T-lymphocyte-derived IFN-γ plays a prominent role in this phenomenon. It has long been recognized that CD8+ T lymphocytes may not purely function as cytotoxic killer cells, but also have important regulatory functions through differential cytokine production [
23,
24,
25]. CD8+ lymphocytes are known to be more efficient producers of IFN-γ than CD4+ T lymphocytes [
26], a finding corroborated by the significant higher IFN-γ levels secreted in the media of activated CD8+ T cells in this study. Following incubation of CM of the activated T cells with the breast cancer cells, the levels of IFN-γ lowered quickly, indicative of utilization by the cancer cells. The differences in residual levels of sIFN-γ following co-culturing with the activated T cells, or incubation with their CM, may be a sequel of re-uptake by an upregulation of the IFN receptor of the T cells [
27].
To test whether an active IFN-γ signaling pathway in MDA-MB-231 breast cancer cells would be crucial for their successful passage across the
in vitro BBB, we impaired the IFN-γ pathway in breast cancer cells by either blocking the IFN-γ receptor on the MDA-MB-231 breast cancer cells, or by neutralizing the sIFN-γ in the CM of activated T lymphocytes. Both interventions strongly reduced the ability of the breast cancer cells to cross the BBB. In order to validate the blocking effect on the IFN-γ pathway we monitored phosphorylation of the downstream signaling protein STAT1. Our data show that at a concentration of ~0.5 μg/ml IFNGR1 mAb, the protein expression levels of both total STAT1 and phosphorylated STAT1 (p-STAT1) decreased to levels comparable to those observed in unstimulated MDA-MB-231 breast cancer cells. These results are in line with data from literature: blocking of IFN-γ is beneficial to reduce metastasis formation of tumors of various lineages [
28,
29]. Specifically, the IFN-γ signaling pathway plays a role in growth and metastasis of triple-negative breast cancer [
30]. IFN-γ is a potent molecule that regulates the expression of over 100 genes acting in various pathways [
31]. A major cluster of those genes includes chemokines and their receptors, which - among other functions - are involved in the recruitment and directional migration of specific cell types [
32]. In previous work we found upregulation of GBP1 in breast cancer cells following co-culturing with activated T lymphocytes [
8]. The expression of GBP1 is under control of IFN-γ [
33,
34] and blocking of the IFN-γ pathway with inhibitor doses ≥ 5 µg/ml significantly decreased GBP1 expression (
Supplemental figure 2) and significantly decreased passage of breast cancer cells through our
in vitro BBB model (Fig 3a). These data are in line with the assumption that GBP1 expression, dependent on IFN-γ, is linked with increased motility of the cells [
35,
36]. However, blocking of the IFN-γ pathway did not result in a complete transmigration arrest (~80% maximal achieved inhibition). We conclude that the IFN-γ pathway is not exclusively responsible for effective BBB-passage, and that pathways other than that of IFN-γ play additional roles in the transmigration of the tumor cells. Obviously, their elucidation is a necessity for successful prevention of brain metastasis in patients.
CXCL9, also referred to as monokine induced by IFN-γ (MIG) and induced by IFN-γ [
37], appeared to be the highest overexpressed component of the CXCL9, -10, -11/CXCR3 axis found in the primary breast cancer samples that developed brain metastasis. CXCL9 acts as a homing chemokine and is constitutively expressed in brain [
37]. In addition, the CXCL9,-10,-11/CXCR3 signaling axis takes part in intracellular communication and plays a crucial role during the pathogenesis of various central nervous system (CNS) diseases [
38,
39,
40,
41]. Human brain endothelial cells and astrocytes modulate IFN-γ-inducible chemokines and are involved in the invasion of the CNS by immune cells [
37] and likely also circulating cancer cells. CXCL9 is overexpressed in human astrocytes compared to human brain endothelial cells [
37], suggestive of a continuous overexpression of IFN-γ-inducible chemokines on the brain-side of the BBB, and establishing a concentration gradient that attracking metastasizing cells. The cross-talk between the tumor cells and brain cells in this context warrants further studies.
Materials and Methods
T lymphocyte fluorescence-activated flowcytometric cell sorting
Total T lymphocytes (CD45+ CD3+), CD4+ T lymphocytes (CD45+ CD3+ CD4+) and CD8+ T lymphocytes (CD45+ CD3+ CD8+) were sorted using FACSAria III™ (BD Biosciences) from peripheral blood mononuclear cells (PBMC) collected from three healthy donors. (approved by the local medical ethical committee; MEC-2016-202). Peripheral blood samples were collected in lithium heparin tubes (BD Biosciences) and the PBMCs were isolated by using Ficoll-based density separation. PBMCs were incubated for 15 minutes at room temperature (RT) with an antibody mixture consisting of CD45-PerCP (clone: 2D1; BD Biosciences), CD3-FITC (SK7; BD Biosciences), CD4-PB (RPA-T4; BD Biosciences), CD8-PE-Cy7 (SFCI21Thy2D3; Beckman Coulter). The various T cell populations were isolated (purity > 95%). Sorted T cell subpopulations were transferred to RPMI-Hepes media, supplemented with 6% human serum albumin (HSA, Sigma-Aldrich), 1% penicillin-streptomycin (P/S) and 1 µg/ml IL-2 (360 IU/mL, Chiron, Amsterdam, The Netherlands) within 1 hour.
Activation and Expansion of T lymphocytes
The sorted T cell fractions were cultured in RPMI supplemented with 10% fetal bovine serum (FBS, ScienCell) and 1% antibiotics (penicillin and streptomycin) and stimulated overnight with a commercially available phorbol 12-myristate 13-acetate (PMA) and ionomycin cell stimulation cocktail (CSC, eBioscience™, Invitrogen), according to manufacturer’s instructions.
Bulk T lymphocytes were activated as previously described [
8]. In short, PBMCs were co-cultured with gamma-irradiated (40 Gy) allogeneic PBMCs, Epstein-Barr Virus (EBV)-transformed B-lymphoblast cell lines BSM (also known as GM06821, GLC
neg/HLA-A2
pos) and APD (also known as GM06817, EAD
neg/HLA-A1
pos) cells, in combination with Phytohemagglutinin-L (PHA-L, Sigma) and IL-2 in a 96-well flat-bottomed plate for 6-7 days at 37°C in a humidified incubator with 5% CO
2. After incubation, cells were harvested, centrifuged, and cultured in RPMI-Hepes medium supplemented with HSA and IL-2.
All T cell activations were performed prior to co-culturing experiments.
Cell line culture procedure
Human astrocytes (ScienCell) were cultured in astrocyte medium (AM, ScienCell) supplemented with 1% astrocyte growth factors (AGS, ScienCell), 2% FBS and 1% P/S. Human umbilical vein endothelial cells (HUVECs, ScienCell) were cultured in endothelial cell medium (ECM, ScienCell) supplemented with 1% endothelial cell growth factors (ECGS, ScienCell), 5% FBS and 1% P/S. Human astrocytes and HUVECs were used between passage 2 and 5. MDA-MB-231 breast cancer cell line (ER-, human breast cancer cell line known to metastasize to brain and other organs [
11]), was used and cultured in RPMI-1640 with L-glutamine (BioWhittaker®) medium supplemented with 10% FBS and 1% P/S.
Construction of the in vitro blood-brain-barrier (BBB) model
Details of the BBB
in vitro model were described previously [
8,
12]. In short, twenty-four-well polyethylene terephthalate (PET) hanging cell culture inserts (membrane surface area 0.3 cm
2, pore size 3.0 μm, Merck Millipore) were coated with 2% gelatin, placed upside-down and ~1x10
5 human astrocytes were seeded at the bottom side of the inserts. The cells were allowed to adhere for 3 hours at 37˚C in a humidified incubator with 5% CO
2 and were supplemented with new astrocyte medium every 15 to 30 minutes. After 3 hours, inserts were flipped and placed in 24-well plates. One mL of astrocyte medium was added to the lower chamber. Approximately 5x10
4 endothelial cells in 500 μL of endothelial cell medium were plated to the upper chamber of the inserts and the cultures were given three days to grow, at 37˚C in a humidified incubator with 5% CO
2.
Measuring the transmigration of breast cancer cells through the in vitro BBB
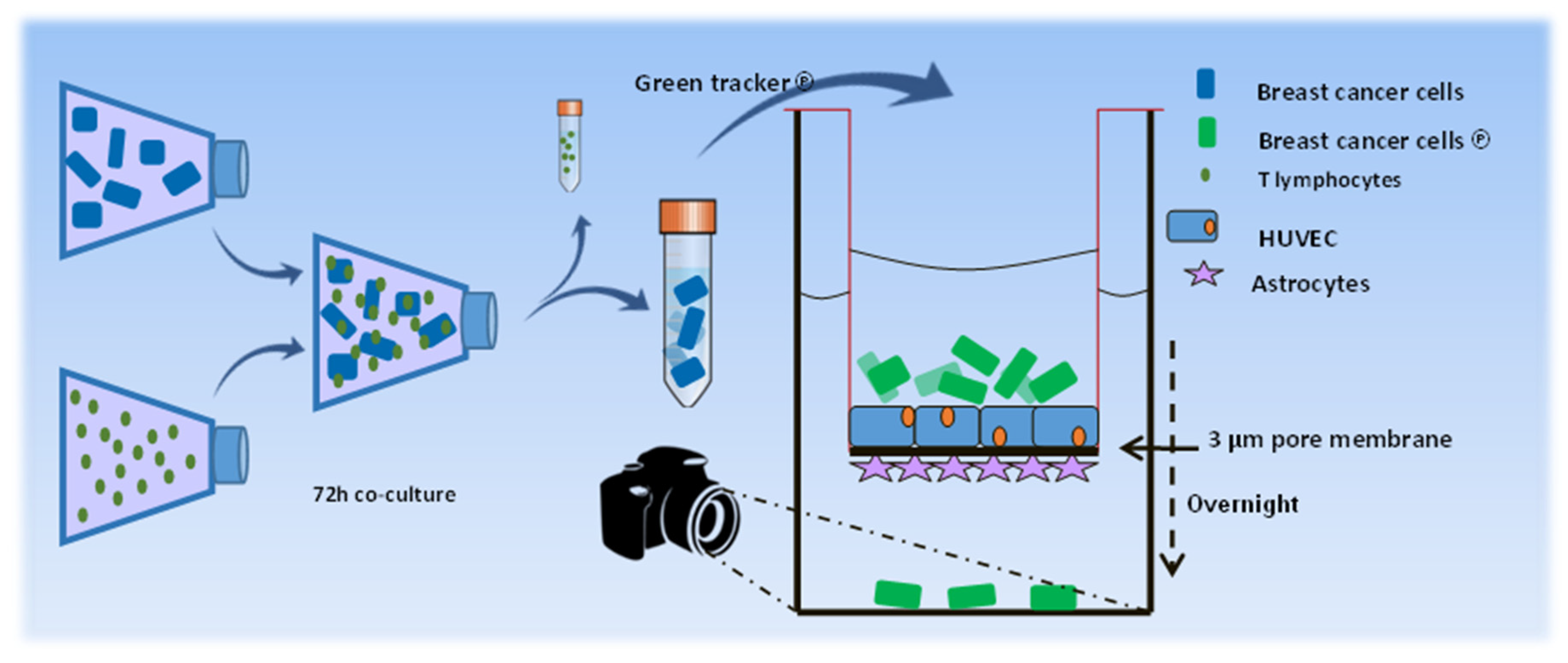
To investigate the influence of T lymphocytes on the ability of the breast cancer cells to cross the BBB, MDA-MB-231 cells were co-cultured for 72 hours with previously activated T lymphocytes (sorted subsets or CD3+ bulk T lymphocytes), at 37˚C in a humidified incubator with 5% CO2. As control, MDA-MB-231 cells were used alone, or in the presence of cell stimulation cocktail. To investigate the influence of the T-lymphocyte-secreted factors in the absence of the T cells the conditioned medium (CM) of activated T cells was added to the cancer cells for 3 consecutive days. The co-culture ratios of the cancer cells / T lymphocytes, and of volume RPMI / CM of T lymphocytes was 3:1. After 3 days of co-culture the T cells were removed by three-step PBS washings. The breast cancer cells were trypsinized and labelled with 5 μM CFMDA cell tracker green (Invitrogen) in serum-free medium, for 45 minutes, at 37˚C. The breast cancer cells were collected, PBS-washed and re-suspended in full-serum medium. Approximately 1.5x105 cancer cells were seeded in the upper chamber of the BBB model and incubated overnight at 37˚C in a humidified incubator with 5% CO2. Subsequently, the transwells were removed and the living cancer cells adhered to the bottom of the 24-well chamber were nuclear-stained with Hoechst 33342 (Invitrogen) and recorded by confocal microscopy. Confocal images were obtained using a Zeiss LSM510 confocal laser-scanning microscope equipped with a 488 nm argon-laser, a 405 nm Diode and a Plan-Neofluar 20× objective with NA 0.5 (Zeiss, Oberkochen, Germany). Pictures were submitted to ImageJ software version 1.49S (http ://www.fiji .sc) and used to calculate the number of cells per mm2. A schematic overview of the experimental procedures is shown in Figure 1a.
Figure 1a.
Schematic presentation of the in vitro experiments using the BBB model. Scheme of in vitro experimental design. MDA-MB-231 breast cancer cells were co-cultured with activated T lymphocytes. Following removal of the T lymphocytes the breast cancer cells were labeled with fluorescent green dye and added to the upper chamber of the BBB model. Transmigration of the cells was monitored using a confocal laser-scanning microscope.
Figure 1a.
Schematic presentation of the in vitro experiments using the BBB model. Scheme of in vitro experimental design. MDA-MB-231 breast cancer cells were co-cultured with activated T lymphocytes. Following removal of the T lymphocytes the breast cancer cells were labeled with fluorescent green dye and added to the upper chamber of the BBB model. Transmigration of the cells was monitored using a confocal laser-scanning microscope.
IFN-γ signaling pathway blocking and inhibition experiments
The IFN-γ receptor consists of IFNGR1 (CD119 or subunit α), responsible for binding ligand in a species-specific manner, and IFNGR2, (AF-1 or subunit β), required for induction of biologic responses. Because IFNGR2 is constitutively expressed at low levels and is up-regulated by external stimuli [
13,
14], this subunit is used for monitoring successful blockade of the IFN-γ pathway [
13,
14] (
Supplemental Figure 1).
To block the IFN-γ receptor, MDA-MB-231 breast cancer cells were pre-incubated for 1h with a concentration range (0.01-10 µg/ml) of human IFNGR1/CD119 monoclonal antibody (clone 92101; R&D Systems) or 10 ug/ml Mouse IgG1 isotype control (clone 11711; R&D Systems). Subsequently, the cancer cells were incubated overnight with CM from activated bulk T lymphocytes. In order to study the effect of soluble IFN-γ (sIFN-γ), anti-hIFN-γ-IgA (clone H7WM120; InvivoGen, San Diego, CA, USA) or human IgA2 isotype control (anti-βGal, clone T9C6; InvivoGen, San Diego, CA, USA) was added to the CM of activated bulk T lymphocytes for 1h, prior to overnight incubation with the cancer cells. Culture supernatants were collected and MDA-MB-231 breast cancer cells were harvested for functional studies and protein Western blot analysis.
IFN-γ signaling induces phosphorylation of two STAT1 residues: tyrosine 701 (Y701), which facilitates dimerization, nuclear translocation and DNA binding; and Serine 727 (S727) enabeling maximal STAT1 transcriptional activity [
15]. Serine 727 phosphorilation grants nearly 80% of IFN-γ-induced transcriptional activity [
16].
Supernatants from: a) non-activated and overnight activated T lymphocyte subsets, before and after 72h incubation time with MDA-MB-231 breast cancer cells; b) 5, 10, 20 or 30 minutes or one, two, four, six or 24h MDA-MB-231 breast cancer cell culture, alone or incubated with CM from activated bulk T lymphocytes, or after co-culture with T lymphocyte; c) 5, 15, 30 and 60 minutes, and 24h MDA-MB-231 breast cancer cell culture, alone or incubated with 10 ng/ml rIFN-γ, were collected and IFN-γ levels were measured (DuoSet ELISA, R&D Systems, Minneapolis, MN, USA). ELISA was performed following the manufacturer’s instructions, and each sample was measured in duplo.
Reverse transcription-polymerase chain reaction (RT-PCR)
Total RNA was extracted from previously snap-frozen cell pellets using the RNeasy Plus Micro kit (Qiagen), quantitated using NanoDrop 1000 (NanoDrop Technologies) and reversely transcribed into cDNA using the RevertAid H-Minus first-strand cDNA synthesis kit (Thermo Scientific) according to the manufacturer’s protocol. Quantitative real-time PCR was performed using TaqMan Master Mix (Applied Biosystems) on the 7500 Real-Time PCR system, v.2.3 (Applied Biosystems). The following commercially available exon-spanning TaqMan Gene Expression Assays (Applied Biosystems) were used: IFN-γ, exon 3-4 (Hs00989291_m1), HPRT1, exon 2-3 (Hs02800695_m1) and HMBS, exon 13-14 (Hs00609296_g1). HPRT1 and HMBS were used as reference genes. The relative quantification of target gene expression was performed using the 2-ΔΔCt comparative method and the threshold cycle value was defined by the point at which there was a statistically significant detectable increase in fluorescence.
Western blot
Cells were washed twice with ice-cold PBS and scraped in RIPA Buffer (ThermoScientific, Rockford, USA). A protease and phosphatase inhibitor cocktail (Halt™, ThermoScientific, Rockford, USA) was added to RIPA lysis buffer before use. Cell lysates were centrifuged (1000g for 15 minutes). The protein content of the cleared lysates was determined (Pierce™ BCA Protein Assay Kit; ThermoScientific, Rockford, USA). Protein lysates were boiled in SDS-sample buffer and separated by SDS-PAGE (12.5% acrylamide). Proteins were blotted onto nitrocellulose membranes (BIO- RAD; Bio- Rad Laboratories, Hercules, CA, USA) and probed with the following antibodies: phospho-STAT1 (Ser727, PSM1, 1:1000, Thermo Fisher Scientific), total-STAT1 (SM1, 1:1000; Thermo Fisher Scientific), IFN-γ R2 (1:2000, R&D Systems), GBP1 (1B1, 1:200, Santa Cruz) and actin (C-2, 1:1000, Santa Cruz). Visualization was achieved by the chemiluminescence kit (BM Chemiluminescence Western Blotting Substrate (POD), Roche) and a maximum sensitivity substrate (SuperSignal West Femto Maximum Sensitivity Substrate, Thermo Scientific), according to the manufacturers’ instructions. Western blot images were acquired and analyzed through a digital western blot scanner Amersham™ Imager 600 (GE Healthcare Bio-Sciences AB, Uppsala, Sweden). Densities of proteins of interest were measured and normalized towards the structural control protein actin or, in the case of phosphorylated proteins, to its matched total protein.
Re-analysis of the gene expression profile data
To check upregulations of additional genes involved in the IFN-γ pathway, the previously generated gene expression data of primary breast cancers from patients with (n=13) and without (n=9) brain metastasis were analyzed [
8]. Morphological assessment, RNA expression profiling and relevant clinical data regarding this discovery cohort were previously provided [
8].
Statistical analysis
Prism 5.0 (GraphPad Software) was used to perform statistical tests. A two-tailed Student’s t-test was used to determine differences in the sample means. Data are presented as means ± SD. In all statistical analyses p-values of < 0.05 were considered statistically significant. Unless otherwise stated, all in vitro experiments were repeated independently three times.
Conclusions
We proved the involvement of T lymphocyte-mediated activation of IFN-γ signaling in breast cancer cells facilitating their migration through an in vitro BBB model. Our results offer directions for further investigations of the mechanisms underlying the formation of brain metastasis, which will be helpfull in future therapy design for the prevention of cancer cells crossing the BBB in patients with breast cancers.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org.
Author Contributions
Conceptualization and design of experiments, R.M.S.M.P., J.M.K. and D.A.M.M.; methodology, B.S., C.B., R.B.M. and P.J.M.L.; resources, R.D., W.A.D. and C.C.H.J.v.E.; writing and original draft preparation, R.M.S.M.P., J.M.K. and D.A.M.M., supervision, J.M.K. and D.A.M.M. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Department of Pathology of the Erasmus University Medical Center and by Support Casper Foundation: www.supportcasper.nl.
Ethical review and approval, informed consent, were not applicable for this study because it did not involve humans or animals.
References
- Leone, J.P. and B.A. Leone, Breast cancer brain metastases: the last frontier. Exp Hematol Oncol, 2015. 4: p. 33. [CrossRef]
- Sperduto, P.W., et al., Effect of tumor subtype on survival and the graded prognostic assessment for patients with breast cancer and brain metastases. Int J Radiat Oncol Biol Phys, 2012. 82(5): p. 2111-7. [CrossRef]
- Tham, Y.L., et al., Primary breast cancer phenotypes associated with propensity for central nervous system metastases. Cancer, 2006. 107(4): p. 696-704. [CrossRef]
- Kaplan, M.A., et al., Biological subtypes and survival outcomes in breast cancer patients with brain metastases (study of the Anatolian Society of Medical Oncology). Oncology, 2012. 83(3): p. 141-50. [CrossRef]
- Nam, B.H., et al., Breast cancer subtypes and survival in patients with brain metastases. Breast Cancer Res, 2008. 10(1): p. R20. [CrossRef]
- Sperduto, P.W., et al., The effect of tumor subtype on the time from primary diagnosis to development of brain metastases and survival in patients with breast cancer. J Neurooncol, 2013. 112(3): p. 467-72. [CrossRef]
- Pedrosa, R., et al., Breast cancer brain metastasis: molecular mechanisms and directions for treatment. Neuro Oncol, 2018. 20(11): p. 1439-1449. [CrossRef]
- Mustafa, D.A.M., et al., T lymphocytes facilitate brain metastasis of breast cancer by inducing Guanylate-Binding Protein 1 expression. Acta Neuropathol, 2018. [CrossRef]
- Prakash, B., et al., Structure of human guanylate-binding protein 1 representing a unique class of GTP-binding proteins. Nature, 2000. 403(6769): p. 567-71. [CrossRef]
- Kim, B.H., et al., Interferon-induced guanylate-binding proteins in inflammasome activation and host defense. Nat Immunol, 2016. 17(5): p. 481-9. [CrossRef]
- Riaz, M., et al., Growth and metastatic behavior of molecularly well-characterized human breast cancer cell lines in mice. Breast Cancer Res Treat, 2014. 148(1): p. 19-31. [CrossRef]
- Eugenin, E.A. and J.W. Berman, Chemokine-dependent mechanisms of leukocyte trafficking across a model of the blood-brain barrier. Methods, 2003. 29(4): p. 351-61. [CrossRef]
- Farrar, M.A. and R.D. Schreiber, The molecular cell biology of interferon-gamma and its receptor. Annu Rev Immunol, 1993. 11: p. 571-611. [CrossRef]
- Bach, E.A., M. Aguet, and R.D. Schreiber, The IFN gamma receptor: a paradigm for cytokine receptor signaling. Annu Rev Immunol, 1997. 15: p. 563-91. [CrossRef]
- Barnholt, K.E., et al., Adenosine blocks IFN-gamma-induced phosphorylation of STAT1 on serine 727 to reduce macrophage activation. J Immunol, 2009. 183(10): p. 6767-77. [CrossRef]
- Decker, T. and P. Kovarik, Serine phosphorylation of STATs. Oncogene, 2000. 19(21): p. 2628-37. [CrossRef]
- Aaltomaa, S., et al., Lymphocyte infiltrates as a prognostic variable in female breast cancer. Eur J Cancer, 1992. 28A(4-5): p. 859-64. [CrossRef]
- Carlomagno, C., et al., Prognostic significance of necrosis, elastosis, fibrosis and inflammatory cell reaction in operable breast cancer. Oncology, 1995. 52(4): p. 272-7. [CrossRef]
- Lee, A.H., et al., Different patterns of inflammation and prognosis in invasive carcinoma of the breast. Histopathology, 2006. 48(6): p. 692-701. [CrossRef]
- Marrogi, A.J., et al., Study of tumor infiltrating lymphocytes and transforming growth factor-beta as prognostic factors in breast carcinoma. Int J Cancer, 1997. 74(5): p. 492-501. [CrossRef]
- Mahmoud, S.M., et al., Tumor-infiltrating CD8+ lymphocytes predict clinical outcome in breast cancer. J Clin Oncol, 2011. 29(15): p. 1949-55. [CrossRef]
- Hammerl, D., et al., Breast cancer genomics and immuno-oncological markers to guide immune therapies. Seminars in Cancer Biology, 2018. 52: p. 178-188. [CrossRef]
- Morvan, P.Y., et al., Distinct pattern of IL-2 and IFN-gamma gene expression in CD4 and CD8 T cells: cytofluorometric analysis at a single cell level using non-radioactive probes. Cell Mol Biol (Noisy-le-grand), 1995. 41(7): p. 945-57.
- Seder, R.A., et al., CD8+ T cells can be primed in vitro to produce IL-4. J Immunol, 1992. 148(6): p. 1652-6. [CrossRef]
- Erard, F., et al., Switch of CD8 T cells to noncytolytic CD8-CD4- cells that make TH2 cytokines and help B cells. Science, 1993. 260(5115): p. 1802-5. [CrossRef]
- Pearce, E.L., et al., Control of effector CD8+ T cell function by the transcription factor Eomesodermin. Science, 2003. 302(5647): p. 1041-3. [CrossRef]
- Schroder, K., et al., Interferon-gamma: an overview of signals, mechanisms and functions. J Leukoc Biol, 2004. 75(2): p. 163-89. [CrossRef]
- Ni, C., et al., Accelerated tumour metastasis due to interferon-gamma receptor-mediated dissociation of perivascular cells from blood vessels. J Pathol, 2017. 242(3): p. 334-346. [CrossRef]
- Xiao, M., et al., IFNgamma promotes papilloma development by up-regulating Th17-associated inflammation. Cancer Res, 2009. 69(5): p. 2010-7. [CrossRef]
- Singh, S., et al., Loss of ELF5-FBXW7 stabilizes IFNGR1 to promote the growth and metastasis of triple-negative breast cancer through interferon-gamma signalling. Nat Cell Biol, 2020. 22(5): p. 591-602. [CrossRef]
- Der, S.D., et al., Identification of genes differentially regulated by interferon alpha, beta, or gamma using oligonucleotide arrays. Proc Natl Acad Sci U S A, 1998. 95(26): p. 15623-8. [CrossRef]
- Rossi, D. and A. Zlotnik, The biology of chemokines and their receptors. Annu Rev Immunol, 2000. 18: p. 217-42. [CrossRef]
- Lew, D.J., T. Decker, and J.E. Darnell, Jr., Alpha interferon and gamma interferon stimulate transcription of a single gene through different signal transduction pathways. Mol Cell Biol, 1989. 9(12): p. 5404-11. [CrossRef]
- Darnell, J.E., Jr., I.M. Kerr, and G.R. Stark, Jak-STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science, 1994. 264(5164): p. 1415-21. [CrossRef]
- Zhao, J., et al., Oncogenic Role of Guanylate Binding Protein 1 in Human Prostate Cancer. Front Oncol, 2019. 9: p. 1494. [CrossRef]
- Bai, S., T. Chen, and X. Deng, Guanylate-Binding Protein 1 Promotes Migration and Invasion of Human Periodontal Ligament Stem Cells. Stem Cells Int, 2018. 2018: p. 6082956. [CrossRef]
- Salmaggi, A., et al., Expression and modulation of IFN-gamma-Inducible chemokines (IP-10, Mig, and I-TAC) in human brain endothelium and astrocytes: Possible relevance for the immune invasion of the central nervous system and the pathogenesis of multiple sclerosis. Journal of Interferon and Cytokine Research, 2002. 22(6): p. 631-640. [CrossRef]
- Banisadr, G., et al., Chemokines and brain functions. Curr Drug Targets Inflamm Allergy, 2005. 4(3): p. 387-99. [CrossRef]
- Muller, M., et al., Review: The chemokine receptor CXCR3 and its ligands CXCL9, CXCL10 and CXCL11 in neuroimmunity--a tale of conflict and conundrum. Neuropathol Appl Neurobiol, 2010. 36(5): p. 368-87. [CrossRef]
- Ramesh, G., A.G. MacLean, and M.T. Philipp, Cytokines and Chemokines at the Crossroads of Neuroinflammation, Neurodegeneration, and Neuropathic Pain. Mediators of Inflammation, 2013. [CrossRef]
- Weenink, B., et al., Low-grade glioma harbors few CD8 T cells, which is accompanied by decreased expression of chemo-attractants, not immunogenic antigens. Sci Rep, 2019. 9(1): p. 14643. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).