Submitted:
26 May 2023
Posted:
29 May 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
- Traditional view of microbial methane formation in humans
- Alternative mechanism(s) of non-microbial methane formation in eukaryotes
- Application of DMSO to humans
- Aims and postulates
2. Materials and Methods
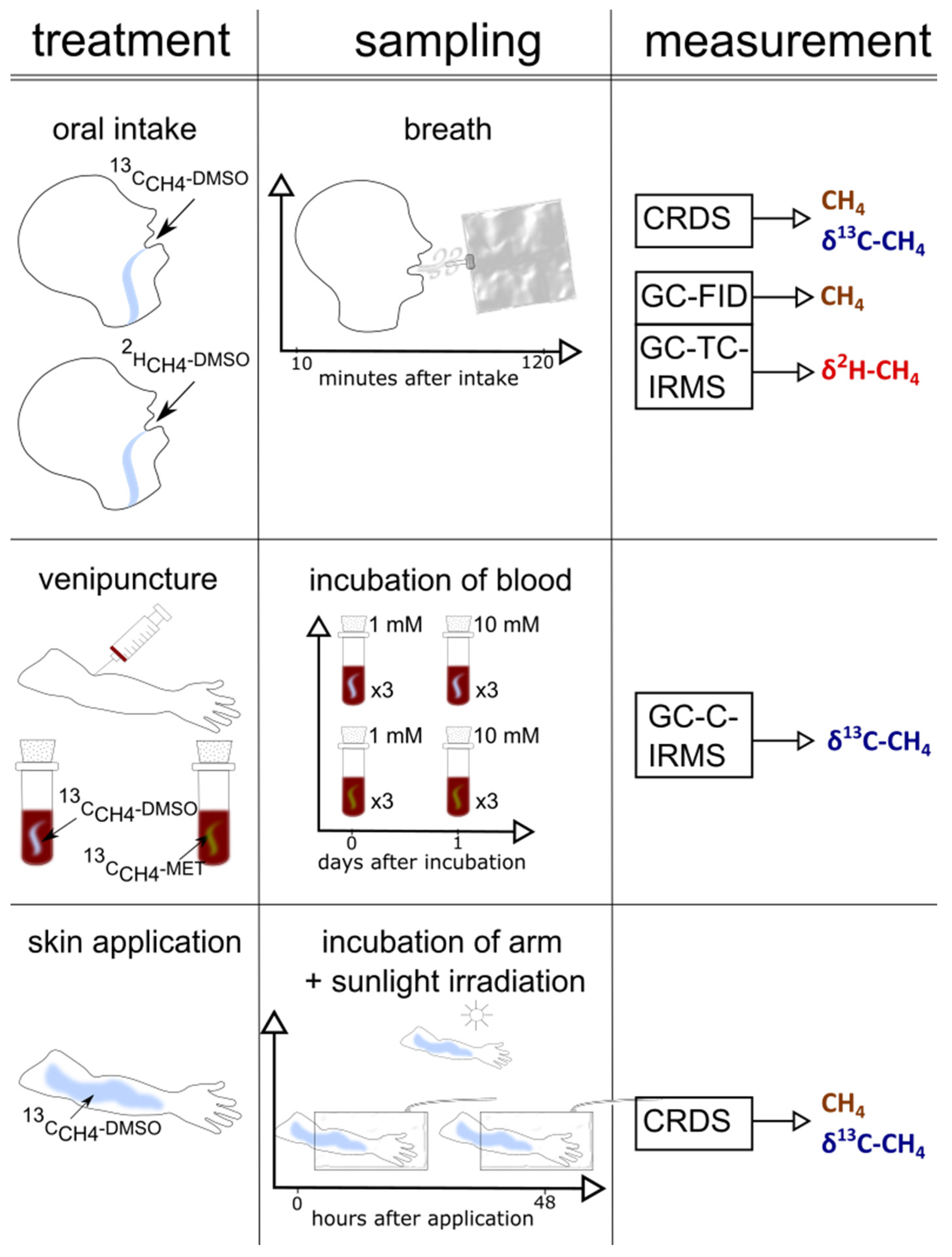
2.1. Subject, materials, experiments, and sampling of air
2.1.1. Subject of the study
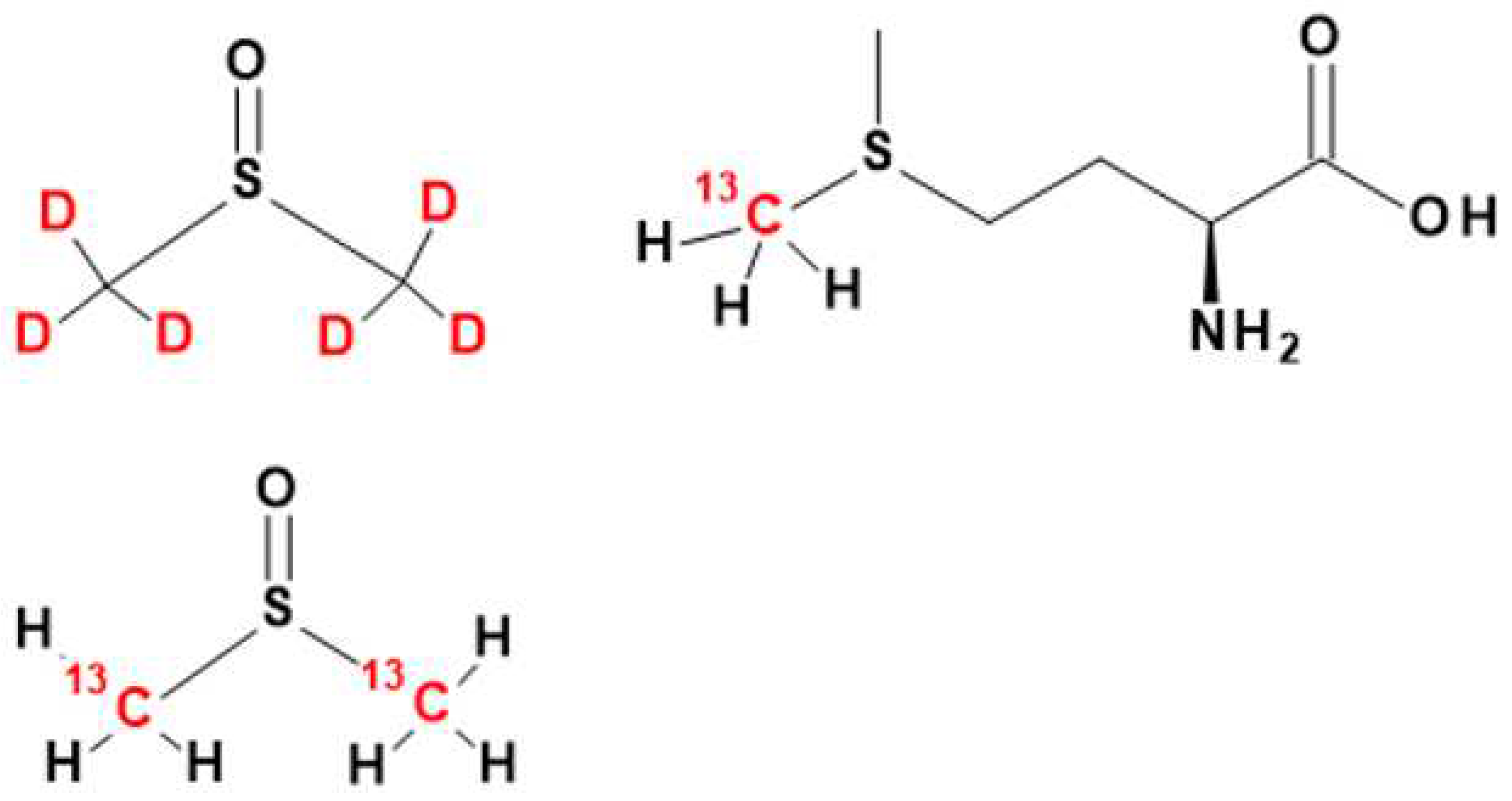
2.1.2. Materials: Position-specific isotopically labeled DMSO and methionine
2.1.3. Experiments and sampling of air
- Oral intake of 13C- and 2H-labeled DMSO
- Arm incubations and exposure to solar light
- Blood samples and incubation with DMSO and methionine
2.2. Analytical measurements
2.2.1. Natural abundance of 13C/12C and 2H/1H, definition of δ values, isotopic excess, and Keeling method
2.2.2. Laser absorption spectroscopy - Cavity Ring Down Spectroscopy
- Measurements of CH4 concentrations and stable carbon isotope values
2.2.3. Measurements of CH4 concentrations using gas chromatography flame ionization detection (GC-FID)
2.2.4. Continuous flow isotope ratio mass spectrometry
2.2.4.1. Measurement of δ13C-CH4 values
2.2.4.2. Measurement of δ2H-CH4 values
2.3. Statistics
3. Results
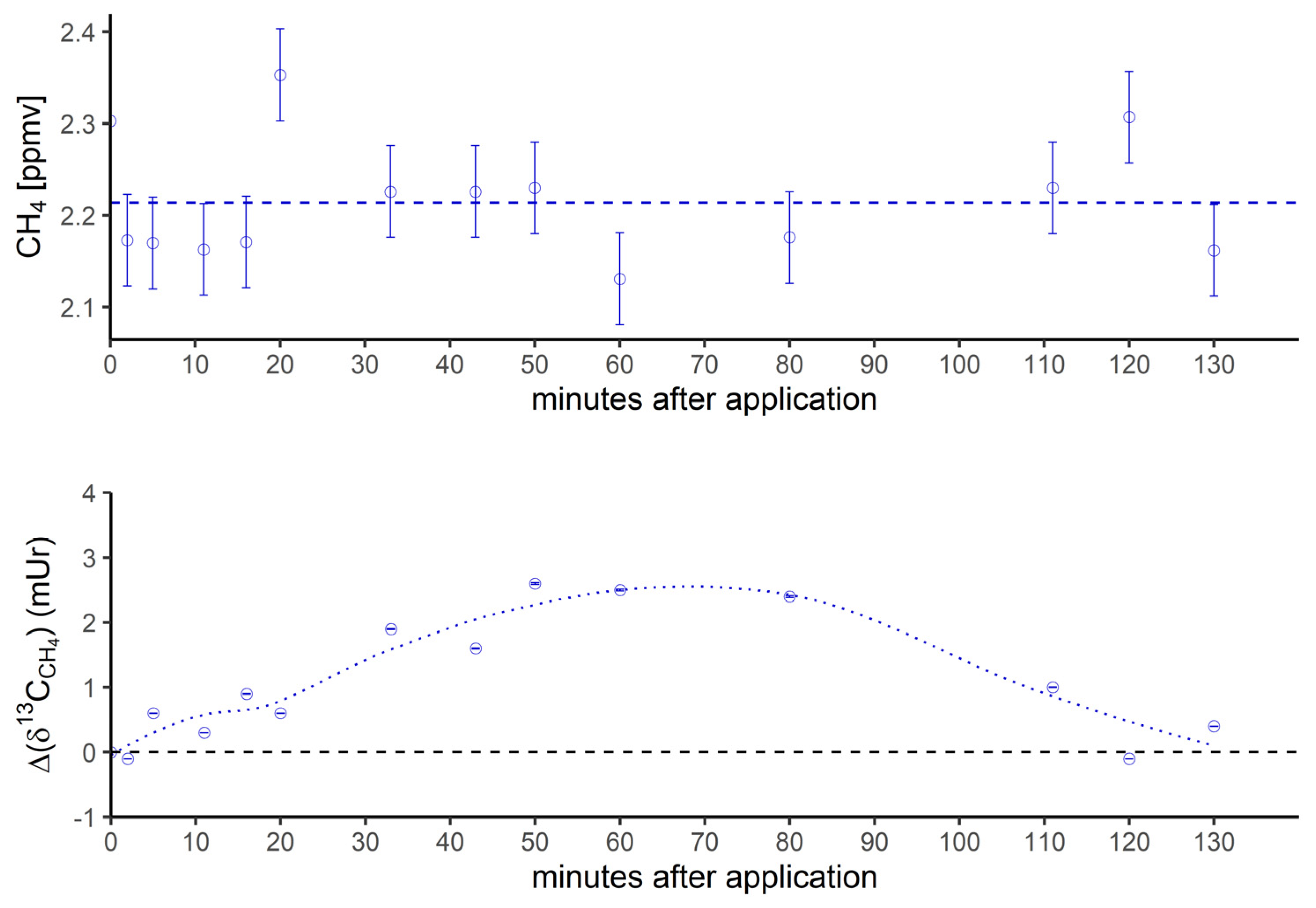
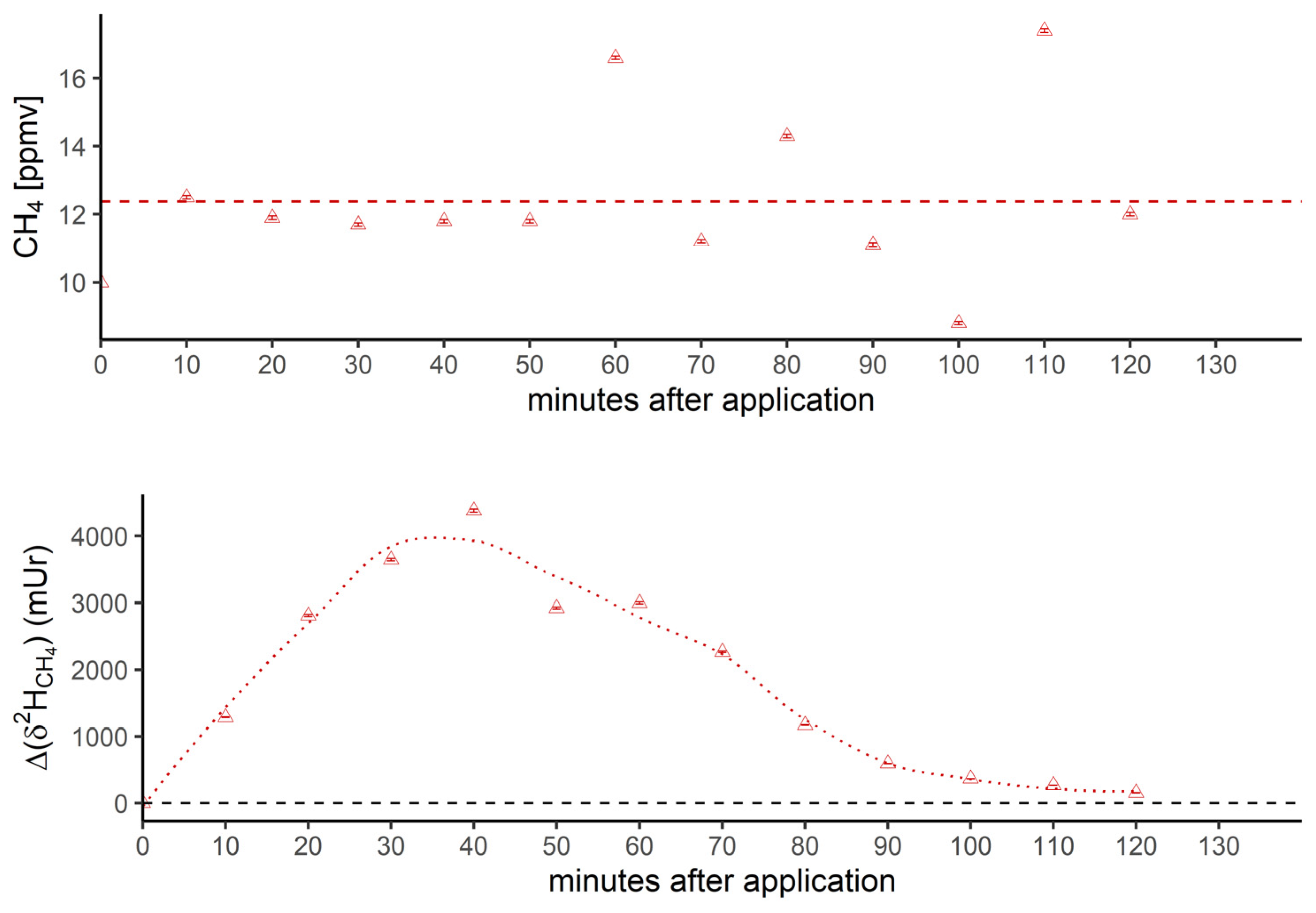
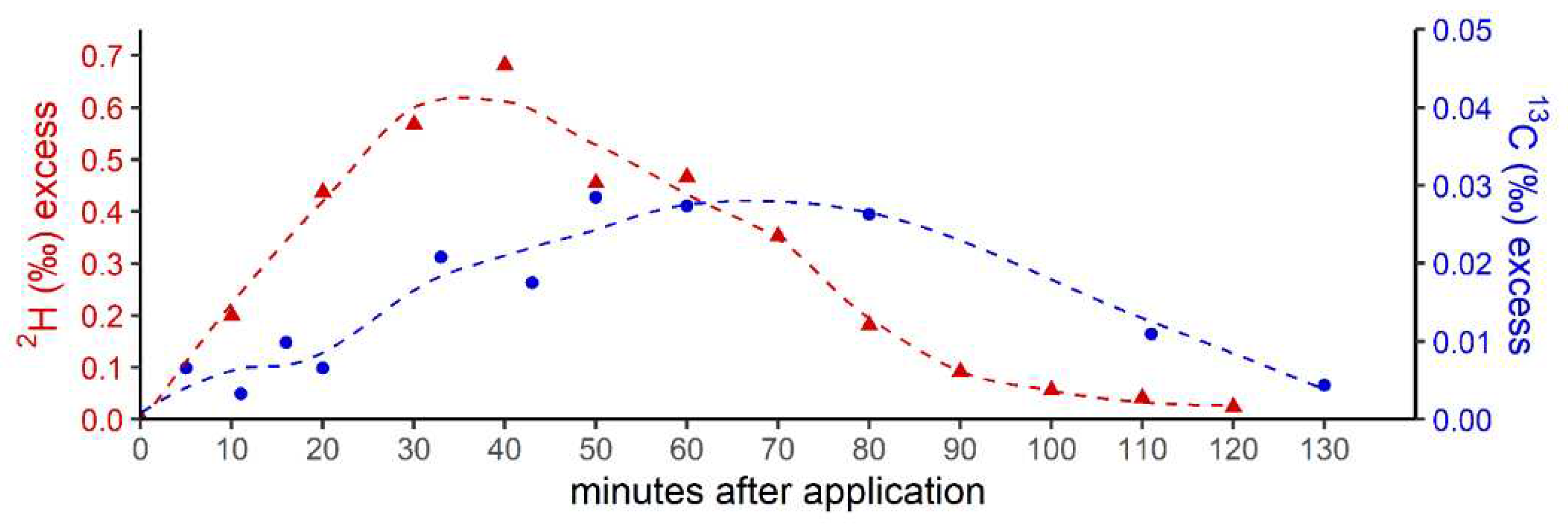
3.1. Oral intake of isotopically labeled DMSO and measurements of breath air
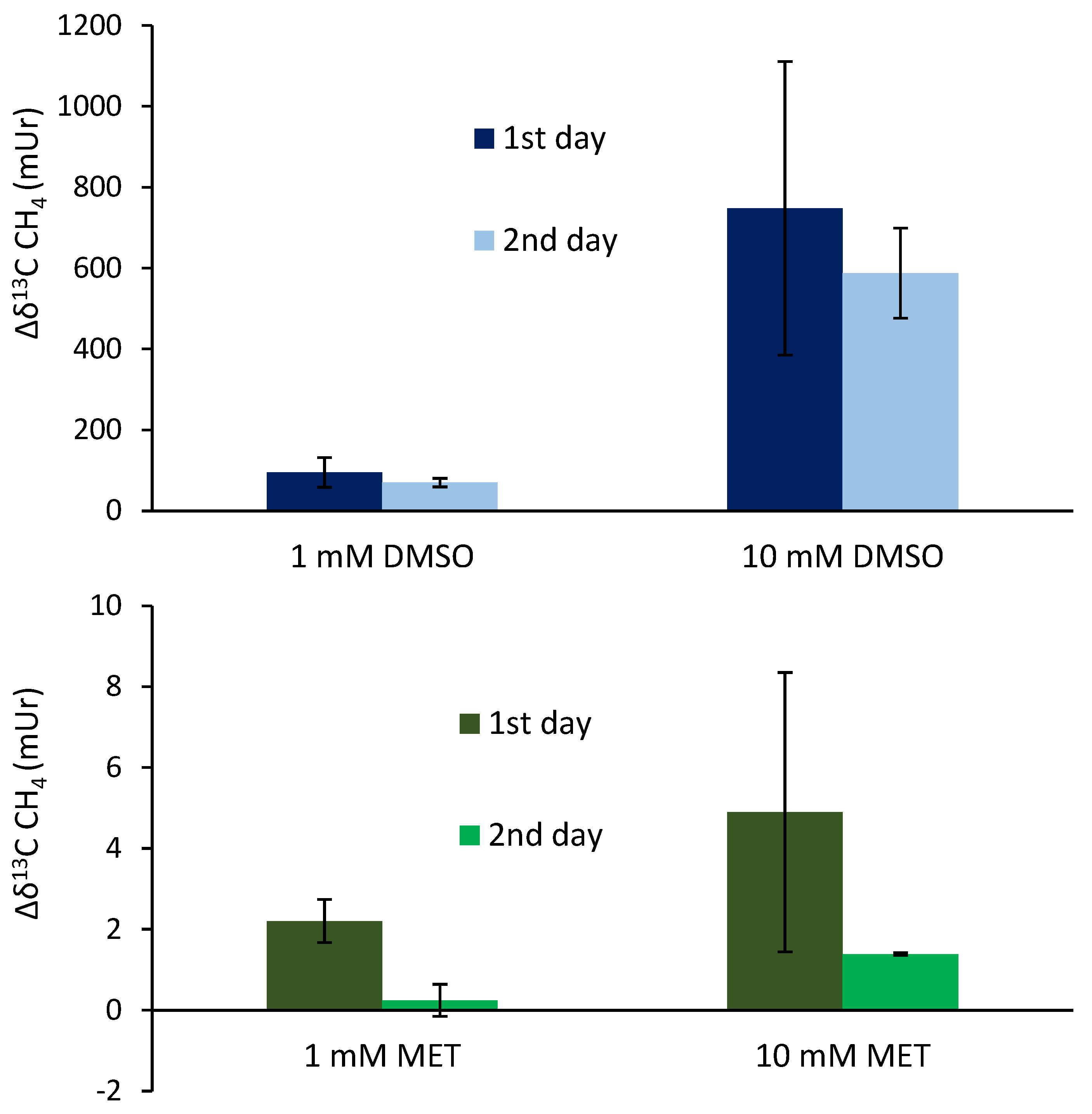
3.2. Blood samples and addition of isotopically labeled DMSO and methionine
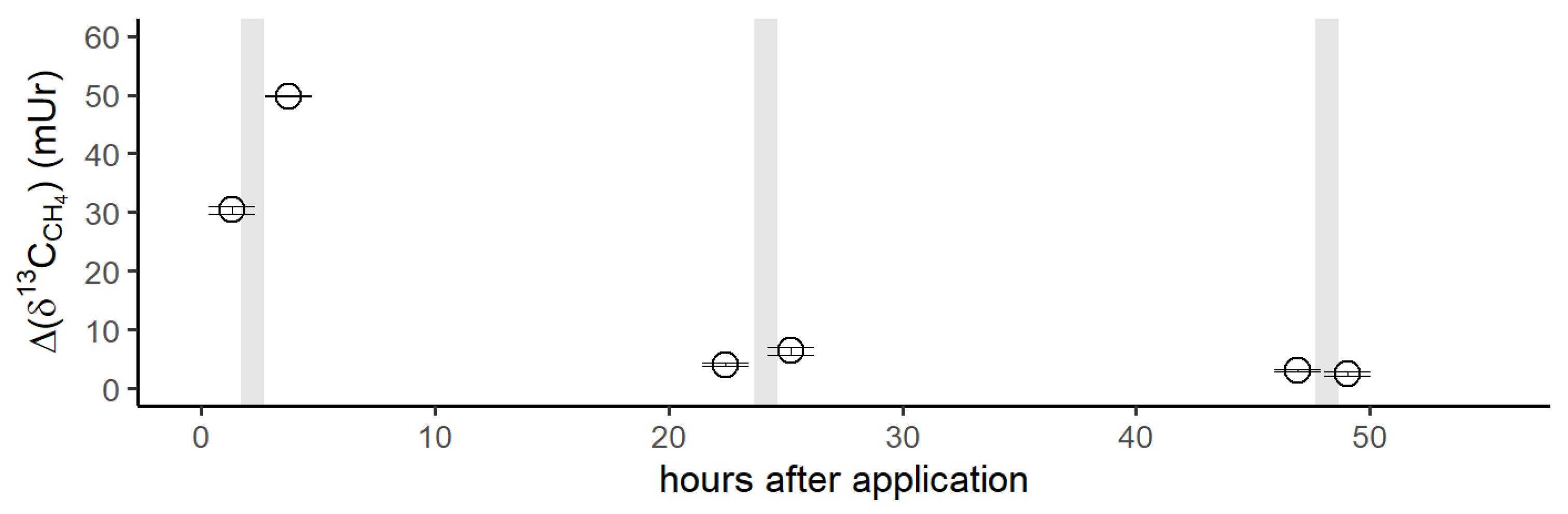
3.3. Skin application of isotopically labeled DMSO and incubation of arm including exposure to natural sunlight
4. Discussion
- Conversion of methylated sulfur compounds to methane
- Oral administration of 13C-labeled DMSO
- Supplementation of 13C-labeled DMSO and methionine to blood samples
- Dermal CH4 emissions after treatment of isotopically labeled DMSO
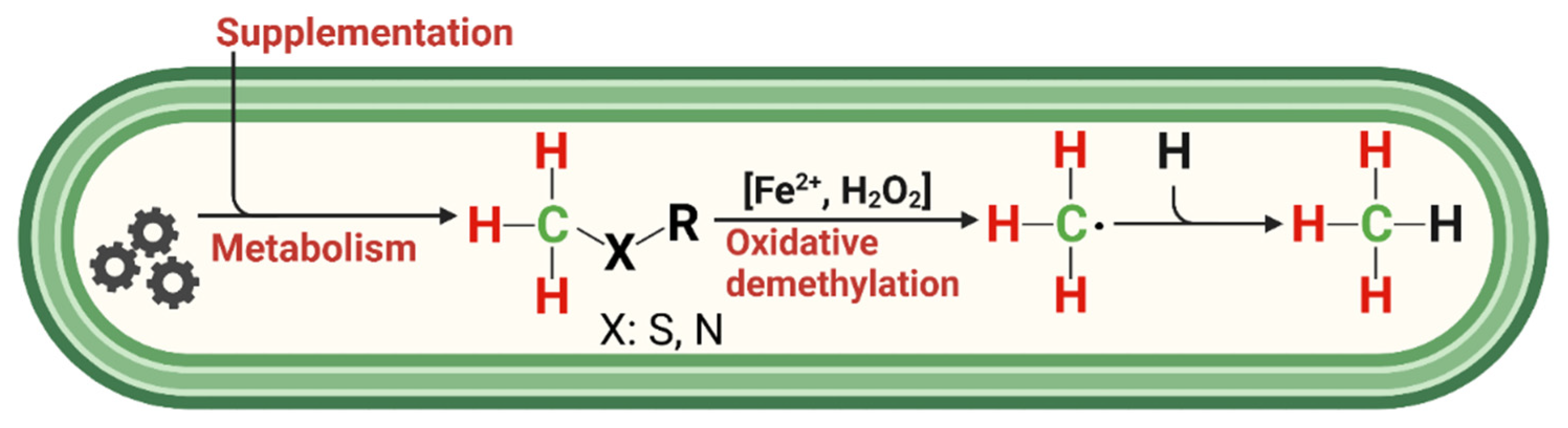
- ROS-induced non-microbial formation of CH4 from methylated S-/N-compounds in humans: A hypothesis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Saunois, M. , et al., The Global Methane Budget 2000–2017. Earth Syst. Sci. Data, 2020. 12(3): p. 1561-1623. [CrossRef]
- Keppler, F. , et al., Methane emissions from terrestrial plants under aerobic conditions. Nature, 2006. 439(7073): p. 187-191. [CrossRef]
- Liu, J. , et al., A novel pathway of direct methane production and emission by eukaryotes including plants, animals and fungi: An overview. Atmospheric Environment, 2015. 115(0): p. 26-35. [CrossRef]
- Martel, A.B. and M.M. Qaderi, Unravelling the effects of blue light on aerobic methane emissions from canola. Journal of Plant Physiology, 2019. 233: p. 12-19. [CrossRef]
- McLeod, A.R. , et al., Ultraviolet radiation drives methane emissions from terrestrial plant pectins. New Phytologist, 2008. 180(1): p. 124-132. [CrossRef]
- Qaderi, M.M. and D.M. Reid, Methane emissions from six crop species exposed to three components of global climate change: temperature, ultraviolet-B radiation and water stress. Physiologia Plantarum, 2009. 137(2): p. 139-147. [CrossRef]
- Wang, Z.-P. , et al., Widespread non-microbial methane production by organic compounds and the impact of environmental stresses. Earth-Science Reviews, 2013. 127(0): p. 193-202. [CrossRef]
- Lenhart, K. , et al., Technical Note: Methionine, a precursor of methane in living plants. Biogeosciences, 2015. 12(6): p. 1907-1914. [CrossRef]
- Tuboly, E. , et al., Methane biogenesis during sodium azide-induced chemical hypoxia in rats. Am J Physiol Cell Physiol, 2013. 304(2): p. C207-14. [CrossRef]
- Ghyczy, M. , et al., Hypoxia-induced generation of methane in mitochondria and eukaryotic cells - An alternative approach to methanogenesis. Cellular Physiology and Biochemistry, 2008. 21(1-3): p. 251-258. [CrossRef]
- Lenhart, K. , et al., Evidence for methane production by saprotrophic fungi. Nat Commun, 2012. 3: p. 1046. [CrossRef]
- Schroll, M. , et al., The stable carbon isotope signature of methane produced by saprotrophic fungi. Biogeosciences, 2020. 17(14): p. 3891-3901. [CrossRef]
- Hartmann, J.F. , et al., High Spatiotemporal Dynamics of Methane Production and Emission in Oxic Surface Water. Environmental Science & Technology, 2020. 54(3): p. 1451-1463. [CrossRef]
- Klintzsch, T. , et al., Effects of Temperature and Light on Methane Production of Widespread Marine Phytoplankton. Journal of Geophysical Research: Biogeosciences, 2020. 125(9): p. e2020JG005793.
- Lenhart, K. , et al., Evidence for methane production by the marine algae Emiliania huxleyi. Biogeosciences, 2016. 13(10): p. 3163-3174. [CrossRef]
- Bižić, M. , et al., Aquatic and terrestrial cyanobacteria produce methane. Science Advances, 2020. 6(3): p. eaax5343. [CrossRef]
- Ernst, L. , et al., Methane formation driven by reactive oxygen species across all living organisms. Nature, 2022. 603(7901): p. 482-487. [CrossRef]
- Boros, M. and F. Keppler, Methane Production and Bioactivity-A Link to Oxido-Reductive Stress. Frontiers in Physiology, 2019. 10(1244). [CrossRef]
- Bond, J.H., Jr., R.R. Engel, and M.D. Levitt, Factors influencing pulmonary methane excretion in man. An indirect method of studying the in situ metabolism of the methane-producing colonic bacteria. J Exp Med, 1971. 133(3): p. 572-88.
- Levitt, M.D. , et al., Stability of human methanogenic flora over 35 years and a review of insights obtained from breath methane measurements. Clin Gastroenterol Hepatol, 2006. 4(2): p. 123-9. [CrossRef]
- Peled, Y. , et al., Factors affecting methane production in humans. Digestive Diseases and Sciences, 1987. 32(3): p. 267-271. [CrossRef]
- Keppler, F. , et al., Stable isotope and high precision concentration measurements confirm that all humans produce and exhale methane. Journal of Breath Research, 2016. 10(1): p. 016003. [CrossRef]
- Hopkins, M.J., R. Sharp, and G.T. Macfarlane, Age and disease related changes in intestinal bacterial populations assessed by cell culture, 16S rRNA abundance, and community cellular fatty acid profiles. Gut, 2001. 48(2): p. 198-205. [CrossRef]
- Polag, D., O. Leiß, and F. Keppler, Age dependent breath methane in the German population. Science of The Total Environment, 2014. 481(0): p. 582-587. [CrossRef]
- Mello, C.S. , et al., Methane production and small intestinal bacterial overgrowth in children living in a slum. World J Gastroenterol, 2012. 18(41): p. 5932-9. [CrossRef]
- Pitt, P. , et al., Studies on breath methane: the effect of ethnic origins and lactulose. Gut, 1980. 21(11): p. 951-954. [CrossRef]
- Triantafyllou, K., C. Chang, and M. Pimentel, Methanogens, methane and gastrointestinal motility. J Neurogastroenterol Motil, 2014. 20(1): p. 31-40. [CrossRef]
- Szabó, A. , et al., Exhaled methane concentration profiles during exercise on an ergometer. Journal of Breath Research, 2015. 9(1): p. 016009. [CrossRef]
- Conway de Macario, E. and A.J. Macario, Methanogenic archaea in health and disease: a novel paradigm of microbial pathogenesis. Int J Med Microbiol, 2009. 299(2): p. 99-108.
- Furnari, M. , et al., Reassessment of the role of methane production between irritable bowel syndrome and functional constipation. J Gastrointestin Liver Dis, 2012. 21(2): p. 157-63.
- Hwang, L. , et al., Evaluating breath methane as a diagnostic test for constipation-predominant IBS. Dig Dis Sci, 2010. 55(2): p. 398-403. [CrossRef]
- Kunkel, D. , et al., Methane on Breath Testing Is Associated with Constipation: A Systematic Review and Meta-analysis. Digestive Diseases and Sciences, 2011. 56(6): p. 1612-1618. [CrossRef]
- Montes, R.G., J. M. Saavedra, and J.A. Perman, Relationship between methane production and breath hydrogen excretion in lactose-malabsorbing individuals. Digestive Diseases and Sciences, 1993. 38(3): p. 445-448. [CrossRef]
- Roccarina, D. , et al., The Role of Methane in Intestinal Diseases. Am J Gastroenterol, 2010. 105(6): p. 1250-1256. [CrossRef]
- Polag, D. and F. Keppler, Global methane emissions from the human body: Past, present and future. Atmospheric Environment, 2019. 214: p. 116823. [CrossRef]
- de Lacy Costello, B.P., M. Ledochowski, and N.M. Ratcliffe, The importance of methane breath testing: a review. J Breath Res, 2013. 7(2): p. 024001. [CrossRef]
- Polag, D. and F. Keppler, Long-term monitoring of breath methane. Science of The Total Environment, 2018. 624: p. 69-77. [CrossRef]
- Polag, D. and F. Keppler, COVID19-vaccination affects breath methane dynamics. bioRxiv, 2022: p. 2022.07.27.501717.
- Boros, M. and F. Keppler, Production and Signaling of Methane. Gasotransmitters, 2018. 12: p. 192.
- Abdulmajeed, A.M. , et al., Interactive effects of temperature and UVB radiation on methane emissions from different organs of pea plants grown in hydroponic system. Journal of Photochemistry and Photobiology B: Biology, 2017. 166: p. 193-201. [CrossRef]
- Abdulmajeed, A.M. and M.M. Qaderi, Intrashoot variation in aerobic methane emissions from pea plants exposed to multiple abiotic stresses. Acta Physiologiae Plantarum, 2017. 39(6): p. 124. [CrossRef]
- Bruhn, D. , et al., Effects of temperature, ultraviolet radiation and pectin methyl esterase on aerobic methane release from plant material. Plant Biology, 2009. 11: p. 43-48. [CrossRef]
- Fraser, W.T. , et al., Emission of methane, carbon monoxide, carbon dioxide and short-chain hydrocarbons from vegetation foliage under ultraviolet irradiation. Plant, Cell & Environment, 2015: p. n/a-n/a.
- Vigano, I. , Röckmann, T., Holzinger, R., van Dijk, A., Keppler, F., Greule, M., Brand, W.A., Geilmann, H., van Weelden, H., The stable isotope signature of methane emitted from plant material under UV irradiation. Atmosheric Environment, 2009. 43: p. 5637-5646. [CrossRef]
- Vigano, I. , et al., Effect of UV radiation and temperature on the emission of methane from plant biomass and structural components. Biogeosciences, 2008. 5(3): p. 937-947. [CrossRef]
- Messenger, D.J., A. R. McLeod, and S.C. Fry, The role of ultraviolet radiation, photosensitizers, reactive oxygen species and ester groups in mechanisms of methane formation from pectin. Plant Cell and Environment, 2009. 32(1): p. 1-9. [CrossRef]
- Althoff, F., A. Jugold, and F. Keppler, Methane formation by oxidation of ascorbic acid using iron minerals and hydrogen peroxide. Chemosphere, 2010. 80(3): p. 286-292. [CrossRef]
- Bruhn, D. , et al., Terrestrial plant methane production and emission. Physiologia Plantarum, 2012. 144(3): p. 201-209. [CrossRef]
- Bruhn, D. , et al., Leaf surface wax is a source of plant methane formation under UV radiation and in the presence of oxygen. Plant Biology, 2014. 16(2): p. 512-516. [CrossRef]
- Keppler, F. , et al., Methane formation in aerobic environments. Environmental Chemistry, 2009. 6(6): p. 459-465. [CrossRef]
- Althoff, F. , et al., Abiotic methanogenesis from organosulphur compounds under ambient conditions. Nat Commun, 2014. 5. [CrossRef]
- Benzing, K. , et al., Nonheme Iron-Oxo-Catalyzed Methane Formation from Methyl Thioethers: Scope, Mechanism, and Relevance for Natural Systems. Chemistry – A European Journal, 2017. 23(43): p. 10465-10472.
- Baptista, L., E. Clemente da Silva, and G. Arbilla, Oxidation mechanism of dimethyl sulfoxide (DMSO) by OH radical in liquid phase. Physical Chemistry Chemical Physics, 2008. 10(45): p. 6867-6879. [CrossRef]
- Herscu-Kluska, R. , et al., Mechanism of the Reaction of Radicals with Peroxides and Dimethyl Sulfoxide in Aqueous Solution. Chemistry – A European Journal, 2008. 14(19): p. 5880-5889. [CrossRef]
- Illés, E. , et al., Carbonate-radical-anions, and not hydroxyl radicals, are the products of the Fenton reaction in neutral solutions containing bicarbonate. Free Radical Biology and Medicine, 2019. 131: p. 1-6. [CrossRef]
- Kaplan, J. and D.M. Ward, The essential nature of iron usage and regulation. Current Biology, 2013. 23(15): p. R642-R646.
- Mittler, R. , ROS Are Good. Trends in Plant Science, 2017. 22(1): p. 11-19.
- Enami, S., Y. Sakamoto, and A.J. Colussi, Fenton chemistry at aqueous interfaces. Proceedings of the National Academy of Sciences, 2014. 111(2): p. 623-628.
- Dunbar, K.L. , et al., Enzymatic Carbon–Sulfur Bond Formation in Natural Product Biosynthesis. Chemical Reviews, 2017. 117(8): p. 5521-5577. [CrossRef]
- Jacob, S.W. and R. Herschler, Pharmacology of DMSO. Cryobiology, 1986. 23(1): p. 14-27.
- Amemori, S. , et al., Oral dimethyl sulfoxide for systemic amyloid A amyloidosis complication in chronic inflammatory disease: a retrospective patient chart review. J Gastroenterol, 2006. 41(5): p. 444-9. [CrossRef]
- Galvao, J. , et al., Unexpected low-dose toxicity of the universal solvent DMSO. The FASEB Journal, 2014. 28(3): p. 1317-1330. [CrossRef]
- Verheijen, M. , et al., DMSO induces drastic changes in human cellular processes and epigenetic landscape in vitro. Scientific Reports, 2019. 9(1): p. 4641. [CrossRef]
- Hanley, B.P., W. Bains, and G. Church, Review of Scientific Self-Experimentation: Ethics History, Regulation, Scenarios, and Views Among Ethics Committees and Prominent Scientists. Rejuvenation Res, 2019. 22(1): p. 31-42. [CrossRef]
- Einzmann, T. , et al., Application of concentration and 2-dimensional stable isotope measurements of methane to constrain sources and sinks in a seasonally stratified freshwater lake. Frontiers in Environmental Science, in press. [CrossRef]
- Brand, W.A. and T.B. Coplen, Stable isotope deltas: tiny, yet robust signatures in nature. Isotopes in Environmental and Health Studies, 2012. 48(3): p. 393-409.
- Miralles-Robledillo, J.M. , et al., DMSO Reductase Family: Phylogenetics and Applications of Extremophiles. Int J Mol Sci, 2019. 20(13). [CrossRef]
- Le, C. , et al., Emerging Chemical Diversity and Potential Applications of Enzymes in the DMSO Reductase Superfamily. Annual Review of Biochemistry, 2022. 91(1): p. 475-504. [CrossRef]
- Anbar, M. and P. Neta, A compilation of specific bimolecular rate constants for the reactions of hydrated electrons, hydrogen atoms and hydroxyl radicals with inorganic and organic compounds in aqueous solution. The International Journal of Applied Radiation and Isotopes, 1967. 18(7): p. 493-523. [CrossRef]
- Eberhardt, M.K. and R. Colina, The reaction of OH radicals with dimethyl sulfoxide. A comparative study of Fenton's reagent and the radiolysis of aqueous dimethyl sulfoxide solutions. The Journal of Organic Chemistry, 1988. 53(5): p. 1071-1074.
- Lee, Y., C. Lee, and J. Yoon, Kinetics and mechanisms of DMSO (dimethylsulfoxide) degradation by UV/H2O2 process. Water Research, 2004. 38(10): p. 2579-2588.
- Lerner, A. , et al., Radicals in ‘biologically relevant’ concentrations behave differently: Uncovering new radical reactions following the reaction of hydroxyl radicals with DMSO. Free Radical Biology and Medicine, 2021. 162: p. 555-560. [CrossRef]
- Forman, H.J., A. Bernardo, and K.J.A. Davies, What is the concentration of hydrogen peroxide in blood and plasma? Archives of Biochemistry and Biophysics, 2016. 603: p. 48-53.
- Pathak, M.A. and K. Stratton, Free radicals in human skin before and after exposure to light. Archives of Biochemistry and Biophysics, 1968. 123(3): p. 468-476. [CrossRef]
- Schroeder, P. , et al., Infrared Radiation-Induced Matrix Metalloproteinase in Human Skin: Implications for Protection. Journal of Investigative Dermatology, 2008. 128(10): p. 2491-2497. [CrossRef]
- Li, M. , et al., Human metabolic emissions of carbon dioxide and methane and their implications for carbon emissions. Science of The Total Environment, 2022. 833: p. 155241. [CrossRef]
- Mochalski, P. , et al., Emission rates of selected volatile organic compounds from skin of healthy volunteers. J Chromatogr B Analyt Technol Biomed Life Sci, 2014. 959(100): p. 62-70. [CrossRef]
- Davies, K.J.A. , Oxidative stress: the paradox of aerobic life. Biochemical Society Symposia, 1995. 61: p. 1-31. [CrossRef]
- Milkovic, L. , et al., Short Overview of ROS as Cell Function Regulators and Their Implications in Therapy Concepts. Cells, 2019. 8(8). [CrossRef]
- Zastrow, L. , et al., Free Radical Threshold Value: A New Universal Body Constant. Skin Pharmacology and Physiology, 2015. 28(5): p. 264-268. [CrossRef]
- Sies, H. and D.P. Jones, Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat Rev Mol Cell Biol, 2020. 21(7): p. 363-383. [CrossRef]
- Tuboly, E. , et al., Excessive alcohol consumption induces methane production in humans and rats. Scientific Reports, 2017. 7(1): p. 7329. [CrossRef]
- Pearson, T.W., H. J. Dawson, and H.B. Lackey, Naturally occurring levels of dimethyl sulfoxide in selected fruits, vegetables, grains, and beverages. Journal of Agricultural and Food Chemistry, 1981. 29(5): p. 1089-1091. [CrossRef]
- Ghyczy, M., C. Torday, and M. Boros, Simultaneous generation of methane, carbon dioxide, and carbon monoxide from choline and ascorbic acid - a defensive mechanism against reductive stress? Faseb Journal, 2003. 17(6): p. 1124-+.
- Kell, D.B. , Iron behaving badly: inappropriate iron chelation as a major contributor to the aetiology of vascular and other progressive inflammatory and degenerative diseases. BMC Med Genomics, 2009. 2: p. 2. [CrossRef]
- Chifman, J., R. Laubenbacher, and S.V. Torti, A systems biology approach to iron metabolism. Adv Exp Med Biol, 2014. 844: p. 201-25.
- Basak, T. and R. Kanwar, Iron imbalance in cancer: Intersection of deficiency and overload. Cancer Medicine, 2022: p. 1-17. [CrossRef]
- Boros, M. , et al., The anti-inflammatory effects of methane. Critical care medicine, 2012. 40(4): p. 1269-1278. [CrossRef]
- Juhász, L. , et al., Bioactivity of Inhaled Methane and Interactions With Other Biological Gases. Frontiers in Cell and Developmental Biology, 2022. 9. [CrossRef]
- Jász, D.K. , et al., Reduction in hypoxia-reoxygenation-induced myocardial mitochondrial damage with exogenous methane. J Cell Mol Med, 2021. 25(11): p. 5113-5123. [CrossRef]
- Benke, K. , et al., Methane supplementation improves graft function in experimental heart transplantation. The Journal of Heart and Lung Transplantation, 2021. 40(3): p. 183-192. [CrossRef]
- Keppler, F. , et al., ROS-driven cellular methane formation: Potential implications for health sciences. Clinical and Translational Medicine, 2022. 12(7): p. e905. [CrossRef]
- Liu, C. and J. Zhang, Methane might be made by all living organisms. Nature, 2022. 603(7901): p. 396-397. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




