Submitted:
24 May 2023
Posted:
30 May 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Neuroendocrine pathways of the stress response and the risk of metabolic syndrome
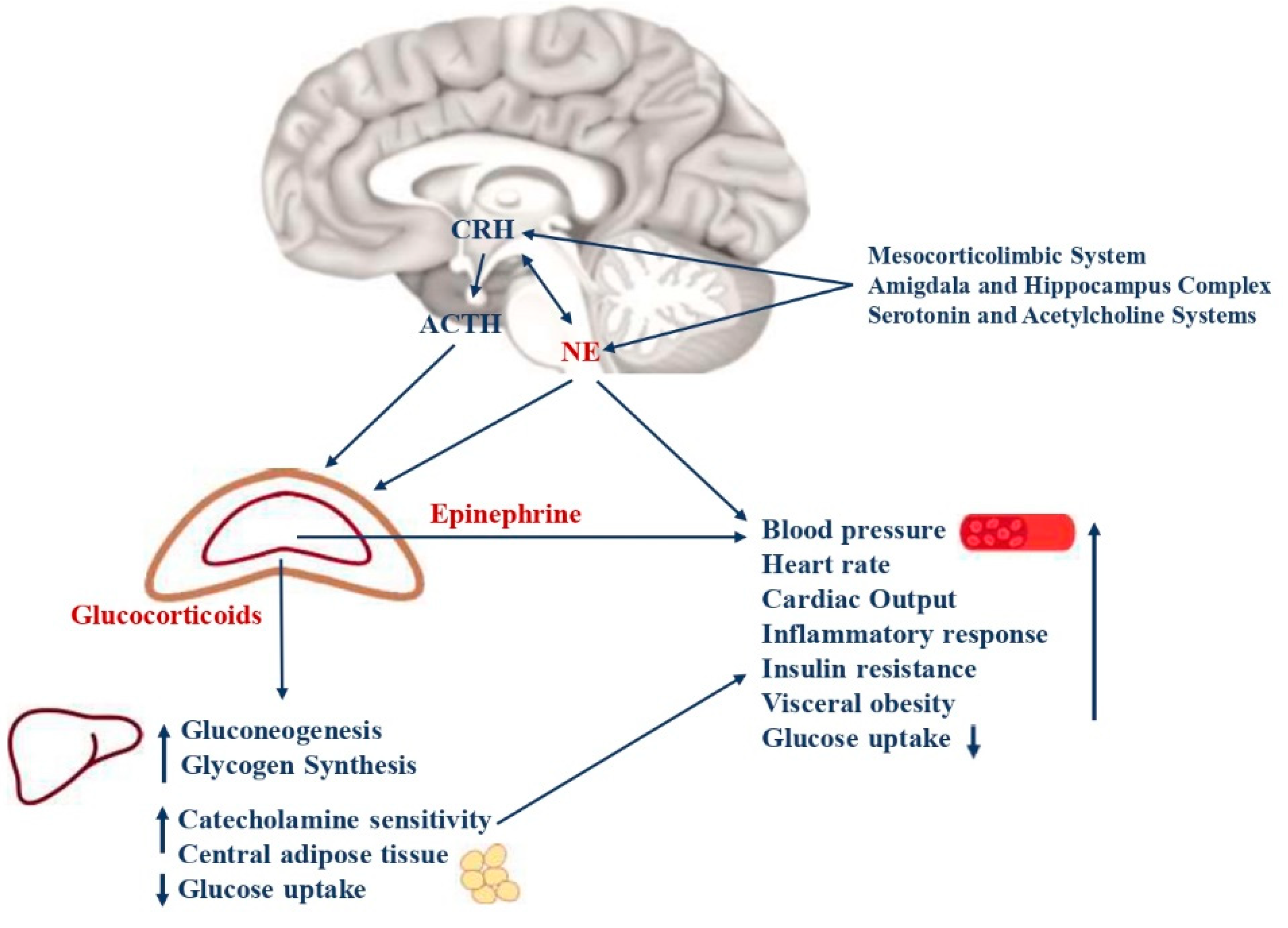
2.1. Hypothalamic-pituitary-adrenal axis
2.2. Arousal and sympathetic nervous systems
2.3. Genetic variants and epigenetic modifications involved in the stress response
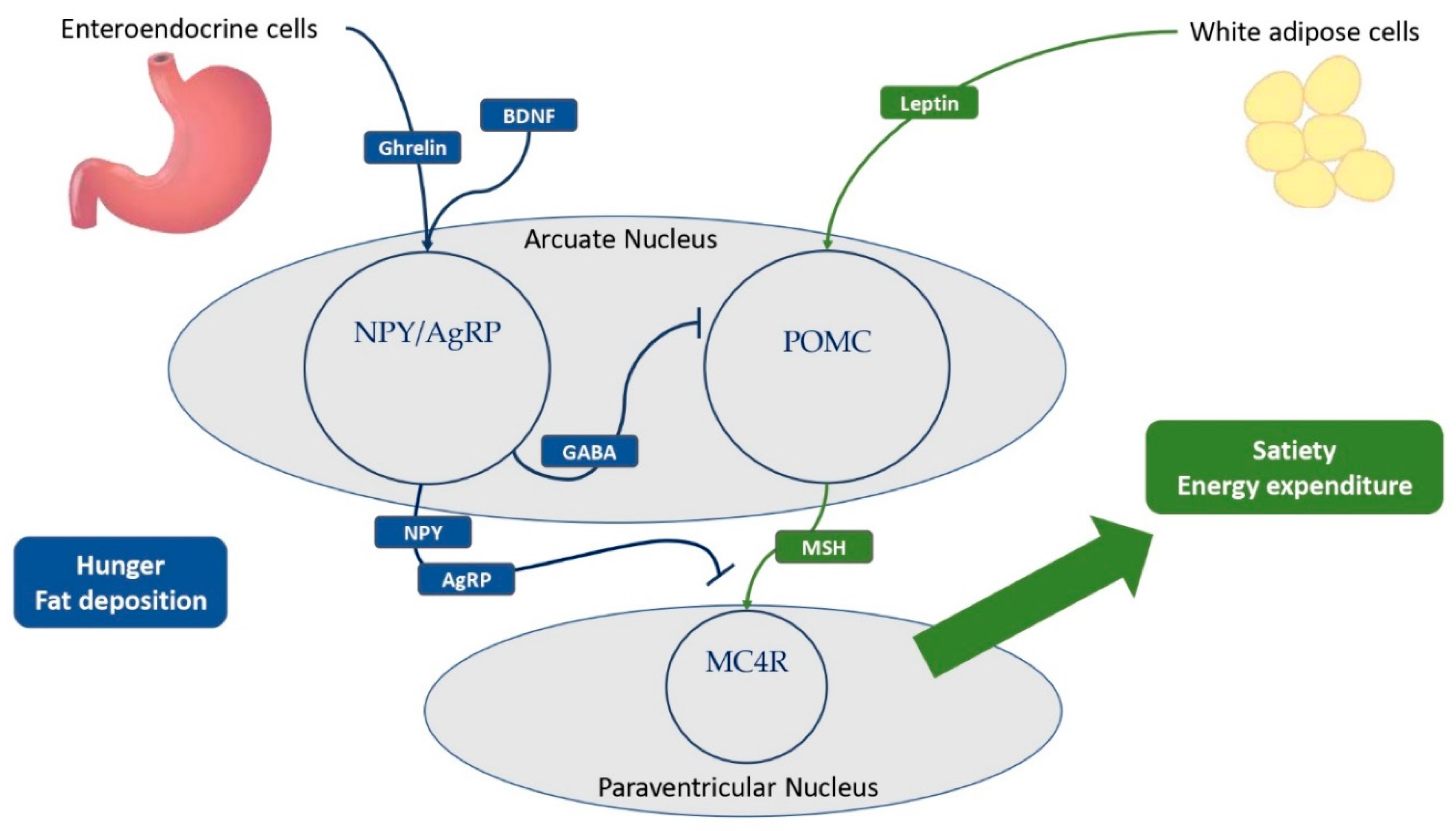
3. The regulation of satiety and hunger in central neuroendocrine pathways
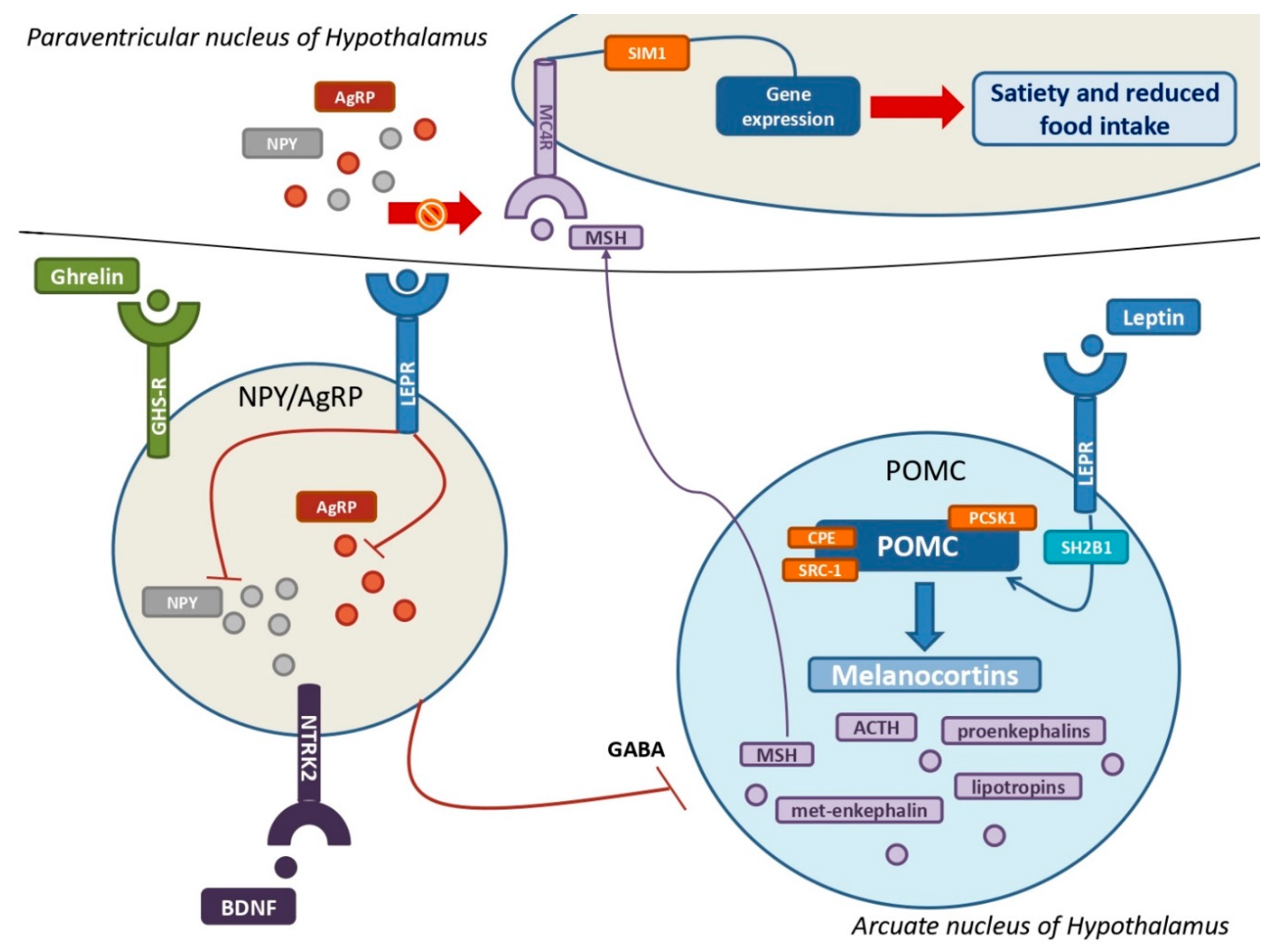
3.1. Leptin and LEPR, a key neuroendocrine tandem with metabolic implications
3.2. The key role of POMC, melanocortins, and related receptors in the development of MetS
3.3. Ghrelin, the NPY-AgRP pathway, and metabolic implications
4. Conclusion
References
- Alberti, K.G.M.M., Zimmet, P., Shaw, J., 2006. Metabolic syndrome-a new world-wide definition. A Consensus Statement from the International Diabetes Federation. Diabet. Med. 23, 469–480. [CrossRef]
- Antunes, H., Santos, C., Carvalho, S., 2009. Serum leptin levels in overweight children and adolescents. Br. J. Nutr. 101, 1262–1266. [CrossRef]
- Balthasar, N., 2006. Genetic dissection of neuronal pathways controlling energy homeostasis. Obes. Silver Spring Md 14 Suppl 5, 222S-227S. [CrossRef]
- Barnes, M.J., McDougal, D.H., 2014. Leptin into the rostral ventral lateral medulla (RVLM) augments renal sympathetic nerve activity and blood pressure. Front. Neurosci. 8, 232. [CrossRef]
- Bartoli, F., Carrà, G., Crocamo, C., Carretta, D., Clerici, M., 2013. Metabolic Syndrome in People Suffering from Posttraumatic Stress Disorder: A Systematic Review and Meta-Analysis. Metab. Syndr. Relat. Disord. 11, 301–308. [CrossRef]
- Bosch, E., Hebebrand, M., Popp, B., Penger, T., Behring, B., Cox, H., Towner, S., Kraus, C., Wilson, W.G., Khan, S., Krumbiegel, M., Ekici, A.B., Uebe, S., Trollmann, R., Woelfle, J., Reis, A., Vasileiou, G., 2021. BDV Syndrome: An Emerging Syndrome With Profound Obesity and Neurodevelopmental Delay Resembling Prader-Willi Syndrome. J. Clin. Endocrinol. Metab. 106, 3413–3427. [CrossRef]
- Cacciottolo, T.M., Henning, E., Keogh, J.M., Bel Lassen, P., Lawler, K., Bounds, R., Ahmed, R., Perdikari, A., Mendes de Oliveira, E., Smith, M., Godfrey, E.M., Johnson, E., Hodson, L., Clément, K., van der Klaauw, A.A., Farooqi, I.S., 2022. Obesity Due to Steroid Receptor Coactivator-1 Deficiency Is Associated With Endocrine and Metabolic Abnormalities. J. Clin. Endocrinol. Metab. 107, e2532–e2544. [CrossRef]
- Calogero, A.E., Norton, J.A., Sheppard, B.C., Listwak, S.J., Cromack, D.T., Wall, R., Jensen, R.T., Chrousos, G.P., 1992. Pulsatile activation of the hypothalamic-pituitary-adrenal axis during major surgery. Metabolism 41, 839–845. [CrossRef]
- Candler, T., Kühnen, P., Prentice, A.M., Silver, M., 2019. Epigenetic regulation of POMC; implications for nutritional programming, obesity and metabolic disease. Front. Neuroendocrinol. 54, 100773. [CrossRef]
- Charmandari, E., Kino, T., Souvatzoglou, E., Chrousos, G.P., 2003. Pediatric Stress: Hormonal Mediators and Human Development. Horm. Res. Paediatr. 59, 161–179. [CrossRef]
- Christian Flemming, G.M., Bussler, S., Körner, A., Kiess, W., 2020. Definition and early diagnosis of metabolic syndrome in children. J. Pediatr. Endocrinol. Metab. 33, 821–833. [CrossRef]
- Chrousos, G.P., 2009. Stress and disorders of the stress system. Nat. Rev. Endocrinol. 5, 374–381. [CrossRef]
- Chrousos, G.P., 2007. Organization and Integration of the Endocrine System: The Arousal and Sleep Perspective. Sleep Med. Clin. 2, 125–145. [CrossRef]
- Dayton, K., Miller, J., 2018. Finding treatable genetic obesity: strategies for success. Curr. Opin. Pediatr. 30, 526–531. [CrossRef]
- Doche, M.E., Bochukova, E.G., Su, H.-W., Pearce, L.R., Keogh, J.M., Henning, E., Cline, J.M., Saeed, S., Dale, A., Cheetham, T., Barroso, I., Argetsinger, L.S., O’Rahilly, S., Rui, L., Carter-Su, C., Farooqi, I.S., 2012. Human SH2B1 mutations are associated with maladaptive behaviors and obesity. J. Clin. Invest. 122, 4732–4736. [CrossRef]
- Durmaz, A., Aykut, A., Atik, T., Özen, S., Ayyıldız Emecen, D., Ata, A., Işık, E., Gökşen, D., Çoğulu, Ö., Özkınay, F., 2021. A New Cause of Obesity Syndrome Associated with a Mutation in the Carboxypeptidase Gene Detected in Three Siblings with Obesity, Intellectual Disability and Hypogonadotropic Hypogonadism. J. Clin. Res. Pediatr. Endocrinol. 13, 52–60. [CrossRef]
- Ericson, M.D., Lensing, C.J., Fleming, K.A., Schlasner, K.N., Doering, S.R., Haskell-Luevano, C., 2017. Bench-top to clinical therapies: A review of melanocortin ligands from 1954 to 2016. Biochim. Biophys. Acta Mol. Basis Dis. 1863, 2414–2435. [CrossRef]
- Farooqi, I.S., Keogh, J.M., Yeo, G.S.H., Lank, E.J., Cheetham, T., O’Rahilly, S., 2003. Clinical spectrum of obesity and mutations in the melanocortin 4 receptor gene. N. Engl. J. Med. 348, 1085–1095. [CrossRef]
- Friedman, J.M., Halaas, J.L., 1998. Leptin and the regulation of body weight in mammals. Nature 395, 763–770. [CrossRef]
- Gao, Y., Lin, Y., Sun, K., Wang, Y., Chen, J., Wang, H., Zhou, X., Fan, X., Hui, R., 2014. Orthostatic Blood Pressure Dysregulation and Polymorphisms of β-Adrenergic Receptor Genes in Hypertensive Patients. J. Clin. Hypertens. 16, 207–213. [CrossRef]
- Geserick, M., Vogel, M., Gausche, R., Lipek, T., Spielau, U., Keller, E., Pfäffle, R., Kiess, W., Körner, A., 2018. Acceleration of BMI in Early Childhood and Risk of Sustained Obesity. N. Engl. J. Med. 379, 1303–1312. [CrossRef]
- Grassi, G., Quarti-Trevano, F., Seravalle, G., Dell’Oro, R., 2007. Cardiovascular risk and adrenergic overdrive in the metabolic syndrome. Nutr. Metab. Cardiovasc. Dis. 17, 473–481. [CrossRef]
- Graves, L.E., Khouri, J.M., Kristidis, P., Verge, C.F., 2021. Proopiomelanocortin deficiency diagnosed in infancy in two boys and a review of the known cases. J. Paediatr. Child Health 57, 484–490. [CrossRef]
- Gregoric, N., Groselj, U., Bratina, N., Debeljak, M., Zerjav Tansek, M., Suput Omladic, J., Kovac, J., Battelino, T., Kotnik, P., Avbelj Stefanija, M., 2021. Two Cases With an Early Presented Proopiomelanocortin Deficiency-A Long-Term Follow-Up and Systematic Literature Review. Front. Endocrinol. 12, 689387. [CrossRef]
- Guay, S.-P., Brisson, D., Lamarche, B., Biron, S., Lescelleur, O., Biertho, L., Marceau, S., Vohl, M.-C., Gaudet, D., Bouchard, L., 2014. ADRB3 gene promoter DNA methylation in blood and visceral adipose tissue is associated with metabolic disturbances in men. Epigenomics 6, 33–43. [CrossRef]
- Häusl, A.S., Balsevich, G., Gassen, N.C., Schmidt, M.V., 2019. Focus on FKBP51: A molecular link between stress and metabolic disorders. Mol. Metab. 29, 170–181. [CrossRef]
- Hinney, A., Körner, A., Fischer-Posovszky, P., 2022. The promise of new anti-obesity therapies arising from knowledge of genetic obesity traits. Nat. Rev. Endocrinol. 18, 623–637. [CrossRef]
- Hinney, A., Volckmar, A.-L., Knoll, N., 2013. Melanocortin-4 receptor in energy homeostasis and obesity pathogenesis. Prog. Mol. Biol. Transl. Sci. 114, 147–191. [CrossRef]
- Holder, J.L., Butte, N.F., Zinn, A.R., 2000. Profound obesity associated with a balanced translocation that disrupts the SIM1 gene. Hum. Mol. Genet. 9, 101–108. [CrossRef]
- Holmes, M.C., Antoni, F.A., Aguilera, G., Catt, K.J., 1986. Magnocellular axons in passage through the median eminence release vasopressin. Nature 319, 326–329. [CrossRef]
- Ivović, M., Marina, L.V., Šojat, A.S., Tančić-Gajić, M., Arizanović, Z., Kendereški, A., Vujović, S., 2020. Approach to the Patient with Subclinical Cushing’s Syndrome. Curr. Pharm. Des. 26, 5584–5590. [CrossRef]
- Jaakkola, U., Kallio, J., Heine, R.J., Nijpels, G., t’ Hart, L.M., Maassen, J.A., Bouter, L.M., Stehouwer, C.D.A., Dekker, J.M., 2009. Neuropeptide Y polymorphism significantly magnifies diabetes and cardiovascular disease risk in obesity: the Hoorn Study. Eur. J. Clin. Nutr. 63, 150–152. [CrossRef]
- Kaur, Y., de Souza, R.J., Gibson, W.T., Meyre, D., 2017. A systematic review of genetic syndromes with obesity. Obes. Rev. Off. J. Int. Assoc. Study Obes. 18, 603–634. [CrossRef]
- Kesebir, S., 2014. Metabolic syndrome and childhood trauma: Also comorbidity and complication in mood disorder. World J. Clin. Cases 2, 332. [CrossRef]
- Kiss, A., Aguilera, G., 1992. Participation of α1-Adrenergic Receptors in the Secretion of Hypothalamic Corticotropin- Releasing Hormone during Stress. Neuroendocrinology 56, 153–160. [CrossRef]
- Kleinendorst, L., Abawi, O., van der Kamp, H.J., Alders, M., Meijers-Heijboer, H.E.J., van Rossum, E.F.C., van den Akker, E.L.T., van Haelst, M.M., 2020a. Leptin receptor deficiency: a systematic literature review and prevalence estimation based on population genetics. Eur. J. Endocrinol. 182, 47–56. [CrossRef]
- Kleinendorst, L., Abawi, O., van der Voorn, B., Jongejan, M.H.T.M., Brandsma, A.E., Visser, J.A., van Rossum, E.F.C., van der Zwaag, B., Alders, M., Boon, E.M.J., van Haelst, M.M., van den Akker, E.L.T., 2020b. Identifying underlying medical causes of pediatric obesity: Results of a systematic diagnostic approach in a pediatric obesity center. PloS One 15, e0232990. [CrossRef]
- Kleiser, C., Schaffrath Rosario, A., Mensink, G.B.M., Prinz-Langenohl, R., Kurth, B.-M., 2009. Potential determinants of obesity among children and adolescents in Germany: results from the cross-sectional KiGGS Study. BMC Public Health 9, 46. [CrossRef]
- Krude, H., Biebermann, H., Luck, W., Horn, R., Brabant, G., Grüters, A., 1998. Severe early-onset obesity, adrenal insufficiency and red hair pigmentation caused by POMC mutations in humans. Nat. Genet. 19, 155–157. [CrossRef]
- Kühnen, P., Krude, H., Biebermann, H., 2019. Melanocortin-4 Receptor Signalling: Importance for Weight Regulation and Obesity Treatment. Trends Mol. Med. 25, 136–148. [CrossRef]
- Kumar, S., Kelly, A.S., 2017. Review of Childhood Obesity: From Epidemiology, Etiology, and Comorbidities to Clinical Assessment and Treatment. Mayo Clin. Proc. 92, 251–265. [CrossRef]
- Kuo, L.E., Kitlinska, J.B., Tilan, J.U., Li, L., Baker, S.B., Johnson, M.D., Lee, E.W., Burnett, M.S., Fricke, S.T., Kvetnansky, R., Herzog, H., Zukowska, Z., 2007. Neuropeptide Y acts directly in the periphery on fat tissue and mediates stress-induced obesity and metabolic syndrome. Nat. Med. 13, 803–811. [CrossRef]
- Laurila, M., Santaniemi, M., Kesäniemi, Y.A., Ukkola, O., 2014. High plasma ghrelin protects from coronary heart disease and Leu72Leu polymorphism of ghrelin gene from cancer in healthy adults during the 19 years follow-up study. Peptides 61, 122–129. [CrossRef]
- Lemche, E., Chaban, O.S., Lemche, A.V., 2016a. Neuroendocrinological and Epigenetic Mechanisms Subserving Autonomic Imbalance and HPA Dysfunction in the Metabolic Syndrome. Front. Neurosci. 10, 142. [CrossRef]
- Li, Z., Zhou, Y., Carter-Su, C., Myers, M.G., Rui, L., 2007. SH2B1 enhances leptin signaling by both Janus kinase 2 Tyr813 phosphorylation-dependent and -independent mechanisms. Mol. Endocrinol. Baltim. Md 21, 2270–2281. [CrossRef]
- Lin, X., Qi, Q., Zheng, Y., Huang, T., Lathrop, M., Zelenika, D., Bray, G.A., Sacks, F.M., Liang, L., Qi, L., 2015. Neuropeptide Y genotype, central obesity, and abdominal fat distribution: the POUNDS LOST trial. Am. J. Clin. Nutr. 102, 514–519. [CrossRef]
- Lotta, L.A., Mokrosiński, J., Mendes de Oliveira, E., Li, C., Sharp, S.J., Luan, J., Brouwers, B., Ayinampudi, V., Bowker, N., Kerrison, N., Kaimakis, V., Hoult, D., Stewart, I.D., Wheeler, E., Day, F.R., Perry, J.R.B., Langenberg, C., Wareham, N.J., Farooqi, I.S., 2019. Human Gain-of-Function MC4R Variants Show Signaling Bias and Protect against Obesity. Cell 177, 597-607.e9. [CrossRef]
- Mahmoud, R., Kimonis, V., Butler, M.G., 2022. Genetics of Obesity in Humans: A Clinical Review. Int. J. Mol. Sci. 23, 11005. [CrossRef]
- McEwen, B.S., 2007. Physiology and Neurobiology of Stress and Adaptation: Central Role of the Brain. Physiol. Rev. 87, 873–904. [CrossRef]
- Michaud, J.L., Boucher, F., Melnyk, A., Gauthier, F., Goshu, E., Lévy, E., Mitchell, G.A., Himms-Hagen, J., Fan, C.M., 2001. Sim1 haploinsufficiency causes hyperphagia, obesity and reduction of the paraventricular nucleus of the hypothalamus. Hum. Mol. Genet. 10, 1465–1473. [CrossRef]
- Morash, B., Li, A., Murphy, P.R., Wilkinson, M., Ur, E., 1999. Leptin gene expression in the brain and pituitary gland. Endocrinology 140, 5995–5998. [CrossRef]
- Napoli, E., Tassone, F., Wong, S., Angkustsiri, K., Simon, T.J., Song, G., Giulivi, C., 2015. Mitochondrial Citrate Transporter-dependent Metabolic Signature in the 22q11.2 Deletion Syndrome. J. Biol. Chem. 290, 23240–23253. [CrossRef]
- Nordang, G.B.N., Busk, Ø.L., Tveten, K., Hanevik, H.I., Fell, A.K.M., Hjelmesæth, J., Holla, Ø.L., Hertel, J.K., 2017. Next-generation sequencing of the monogenic obesity genes LEP, LEPR, MC4R, PCSK1 and POMC in a Norwegian cohort of patients with morbid obesity and normal weight controls. Mol. Genet. Metab. 121, 51–56. [CrossRef]
- Obradovic, M., Sudar-Milovanovic, E., Soskic, S., Essack, M., Arya, S., Stewart, A.J., Gojobori, T., Isenovic, E.R., 2021. Leptin and Obesity: Role and Clinical Implication. Front. Endocrinol. 12, 585887. [CrossRef]
- Pan, W.W., Myers, M.G., 2018. Leptin and the maintenance of elevated body weight. Nat. Rev. Neurosci. 19, 95–105. [CrossRef]
- Pépin, L., Colin, E., Tessarech, M., Rouleau, S., Bouhours-Nouet, N., Bonneau, D., Coutant, R., 2019. A New Case of PCSK1 Pathogenic Variant With Congenital Proprotein Convertase 1/3 Deficiency and Literature Review. J. Clin. Endocrinol. Metab. 104, 985–993. [CrossRef]
- Pereira, M.J., Palming, J., Svensson, M.K., Rizell, M., Dalenbäck, J., Hammar, M., Fall, T., Sidibeh, C.O., Svensson, P.-A., Eriksson, J.W., 2014. FKBP5 expression in human adipose tissue increases following dexamethasone exposure and is associated with insulin resistance. Metabolism 63, 1198–1208. [CrossRef]
- Perez-Tilve, D., Hofmann, S.M., Basford, J., Nogueiras, R., Pfluger, P.T., Patterson, J.T., Grant, E., Wilson-Perez, H.E., Granholm, N.A., Arnold, M., Trevaskis, J.L., Butler, A.A., Davidson, W.S., Woods, S.C., Benoit, S.C., Sleeman, M.W., DiMarchi, R.D., Hui, D.Y., Tschöp, M.H., 2010. Melanocortin signaling in the CNS directly regulates circulating cholesterol. Nat. Neurosci. 13, 877–882. [CrossRef]
- Pervanidou, P., 2008. Biology of Post-Traumatic Stress Disorder in Childhood and Adolescence. J. Neuroendocrinol. 20, 632–638. [CrossRef]
- Pervanidou, P., Chrousos, G.P., 2012. Metabolic consequences of stress during childhood and adolescence. Metabolism 61, 611–619. [CrossRef]
- Pervanidou, P., Chrousos, G.P., 2011. Stress and obesity/metabolic syndrome in childhood and adolescence. Int. J. Pediatr. Obes. 6, 21–28. [CrossRef]
- Pervanidou, P., Kolaitis, G., Charitaki, S., Lazaropoulou, C., Papassotiriou, I., Hindmarsh, P., Bakoula, C., Tsiantis, J., Chrousos, G.P., 2007. The Natural History of Neuroendocrine Changes in Pediatric Posttraumatic Stress Disorder (PTSD) After Motor Vehicle Accidents: Progressive Divergence of Noradrenaline and Cortisol Concentrations Over Time. Biol. Psychiatry 62, 1095–1102. [CrossRef]
- Poitou, C., Mosbah, H., Clément, K., 2020. MECHANISMS IN ENDOCRINOLOGY: Update on treatments for patients with genetic obesity. Eur. J. Endocrinol. 183, R149–R166. [CrossRef]
- Qiao, Y., Ma, J., Wang, Y., Li, W., Katzmarzyk, P.T., Chaput, J.-P., Fogelholm, M., Johnson, W.D., Kuriyan, R., Kurpad, A., Lambert, E.V., Maher, C., Maia, J., Matsudo, V., Olds, T., Onywera, V., Sarmiento, O.L., Standage, M., Tremblay, M.S., Tudor-Locke, C., Church, T.S., Zhao, P., Hu, G., ISCOLE Research Group, 2015. Birth weight and childhood obesity: a 12-country study. Int. J. Obes. Suppl. 5, S74-79. [CrossRef]
- Reyes, M., Quintanilla, C., Burrows, R., Blanco, E., Cifuentes, M., Gahagan, S., 2015. Obesity is associated with acute inflammation in a sample of adolescents. Pediatr. Diabetes 16, 109–116. [CrossRef]
- Roy, M.S., Roy, A., Gallucci, W.T., Collier, B., Young, K., Kamilaris, T.C., Chrousos, G.P., 1993. The ovine corticotropin-releasing hormone-stimulation test in type I diabetic patients and controls: Suggestion of mild chronic hypercortisolism. Metabolism 42, 696–700. [CrossRef]
- Rubens, M., Bruenig, D., Adams, J.A.M., Suresh, S.M., Sathyanarayanan, A., Haslam, D., Shenk, C.E., Mathews, B., Mehta, D., 2023. Childhood maltreatment and DNA methylation: A systematic review. Neurosci. Biobehav. Rev. 147, 105079. [CrossRef]
- Rui, L., 2014. SH2B1 regulation of energy balance, body weight, and glucose metabolism. World J. Diabetes 5, 511–526. [CrossRef]
- Saeed, S., Arslan, M., Manzoor, J., Din, S.M., Janjua, Q.M., Ayesha, H., Ain, Q.-T., Inam, L., Lobbens, S., Vaillant, E., Durand, E., Derhourhi, M., Amanzougarene, S., Badreddine, A., Berberian, L., Gaget, S., Khan, W.I., Butt, T.A., Bonnefond, A., Froguel, P., 2020. Genetic Causes of Severe Childhood Obesity: A Remarkably High Prevalence in an Inbred Population of Pakistan. Diabetes 69, 1424–1438. [CrossRef]
- Sidibeh, C.O., Pereira, M.J., Abalo, X.M., J. Boersma, G., Skrtic, S., Lundkvist, P., Katsogiannos, P., Hausch, F., Castillejo-López, C., Eriksson, J.W., 2018. FKBP5 expression in human adipose tissue: potential role in glucose and lipid metabolism, adipogenesis and type 2 diabetes. Endocrine 62, 116–128. [CrossRef]
- Singh, R.K., Kumar, P., Mahalingam, K., 2017. Molecular genetics of human obesity: A comprehensive review. C. R. Biol. 340, 87–108. [CrossRef]
- Sitticharoon, C., Chatree, S., Churintaraphan, M., 2013. Expressions of neuropeptide Y and Y1 receptor in subcutaneous and visceral fat tissues in normal weight and obese humans and their correlations with clinical parameters and peripheral metabolic factors. Regul. Pept. 185, 65–72. [CrossRef]
- Sohn, Y.B., 2022. Genetic obesity: an update with emerging therapeutic approaches. Ann. Pediatr. Endocrinol. Metab. 27, 169–175. [CrossRef]
- Straznicky, N.E., Lambert, G.W., Masuo, K., Dawood, T., Eikelis, N., Nestel, P.J., McGrane, M.T., Mariani, J.A., Socratous, F., Chopra, R., Esler, M.D., Schlaich, M.P., Lambert, E.A., 2009. Blunted sympathetic neural response to oral glucose in obese subjects with the insulin-resistant metabolic syndrome. Am. J. Clin. Nutr. 89, 27–36. [CrossRef]
- Styne, D.M., Arslanian, S.A., Connor, E.L., Farooqi, I.S., Murad, M.H., Silverstein, J.H., Yanovski, J.A., 2017. Pediatric Obesity-Assessment, Treatment, and Prevention: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 102, 709–757. [CrossRef]
- Thorsell, A., 2010. Brain neuropeptide Y and corticotropin-releasing hormone in mediating stress and anxiety. Exp. Biol. Med. Maywood NJ 235, 1163–1167. [CrossRef]
- Tsigos, C., Young, R.J., White, A., 1993. Diabetic neuropathy is associated with increased activity of the hypothalamic-pituitary-adrenal axis. J. Clin. Endocrinol. Metab. 76, 554–558. [CrossRef]
- Wabitsch, M., Funcke, J.-B., von Schnurbein, J., Denzer, F., Lahr, G., Mazen, I., El-Gammal, M., Denzer, C., Moss, A., Debatin, K.-M., Gierschik, P., Mistry, V., Keogh, J.M., Farooqi, I.S., Moepps, B., Fischer-Posovszky, P., 2015. Severe Early-Onset Obesity Due to Bioinactive Leptin Caused by a p.N103K Mutation in the Leptin Gene. J. Clin. Endocrinol. Metab. 100, 3227–3230. [CrossRef]
- Wasim, M., Awan, F.R., Najam, S.S., Khan, A.R., Khan, H.N., 2016. Role of Leptin Deficiency, Inefficiency, and Leptin Receptors in Obesity. Biochem. Genet. 54, 565–572. [CrossRef]
- Wei, Z., Zhang, K., Wen, G., Balasubramanian, K., Shih, P.B., Rao, F., Friese, R.S., Miramontes-Gonzalez, J.P., Schmid-Schoenbein, G.W., Kim, H.-S., Mahata, S.K., O’Connor, D.T., 2013. Heredity and cardiometabolic risk: naturally occurring polymorphisms in the human neuropeptide Y(2) receptor promoter disrupt multiple transcriptional response motifs. J. Hypertens. 31, 123–133. [CrossRef]
- Wolf, E.J., Mitchell, K.S., Logue, M.W., Baldwin, C.T., Reardon, A.F., Humphries, D.E., Miller, M.W., 2013. CORTICOTROPIN RELEASING HORMONE RECEPTOR 2 ( CRHR-2 ) GENE IS ASSOCIATED WITH DECREASED RISK AND SEVERITY OF POSTTRAUMATIC STRESS DISORDER IN WOMEN: CRHR-2 and PTSD. Depress. Anxiety 30, 1161–1169. [CrossRef]
- Womersley, J.S., Nothling, J., Toikumo, S., Malan-Müller, S., van den Heuvel, L.L., McGregor, N.W., Seedat, S., Hemmings, S.M.J., 2022. Childhood trauma, the stress response and metabolic syndrome: A focus on DNA methylation. Eur. J. Neurosci. 55, 2253–2296. [CrossRef]
- Xia, Q., Grant, S.F.A., 2013. The genetics of human obesity. Ann. N. Y. Acad. Sci. 1281, 178–190. [CrossRef]
- Yang, Y., van der Klaauw, A.A., Zhu, L., Cacciottolo, T.M., He, Y., Stadler, L.K.J., Wang, C., Xu, P., Saito, K., Hinton, A., Yan, X., Keogh, J.M., Henning, E., Banton, M.C., Hendricks, A.E., Bochukova, E.G., Mistry, V., Lawler, K.L., Liao, L., Xu, J., O’Rahilly, S., Tong, Q., UK10K Consortium, Inês Barroso, null, O’Malley, B.W., Farooqi, I.S., Xu, Y., 2019. Steroid receptor coactivator-1 modulates the function of Pomc neurons and energy homeostasis. Nat. Commun. 10, 1718. [CrossRef]
- Yeo, G.S.H., Chao, D.H.M., Siegert, A.-M., Koerperich, Z.M., Ericson, M.D., Simonds, S.E., Larson, C.M., Luquet, S., Clarke, I., Sharma, S., Clément, K., Cowley, M.A., Haskell-Luevano, C., Van Der Ploeg, L., Adan, R.A.H., 2021. The melanocortin pathway and energy homeostasis: From discovery to obesity therapy. Mol. Metab. 48, 101206. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




