Submitted:
26 May 2023
Posted:
30 May 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Climate change and salmonids distribution
3. In-river habitat conditions preparatory for smolting
4. Smolting

5. Smolt migrations

6. Early post-smolt survival
7. Conclusions
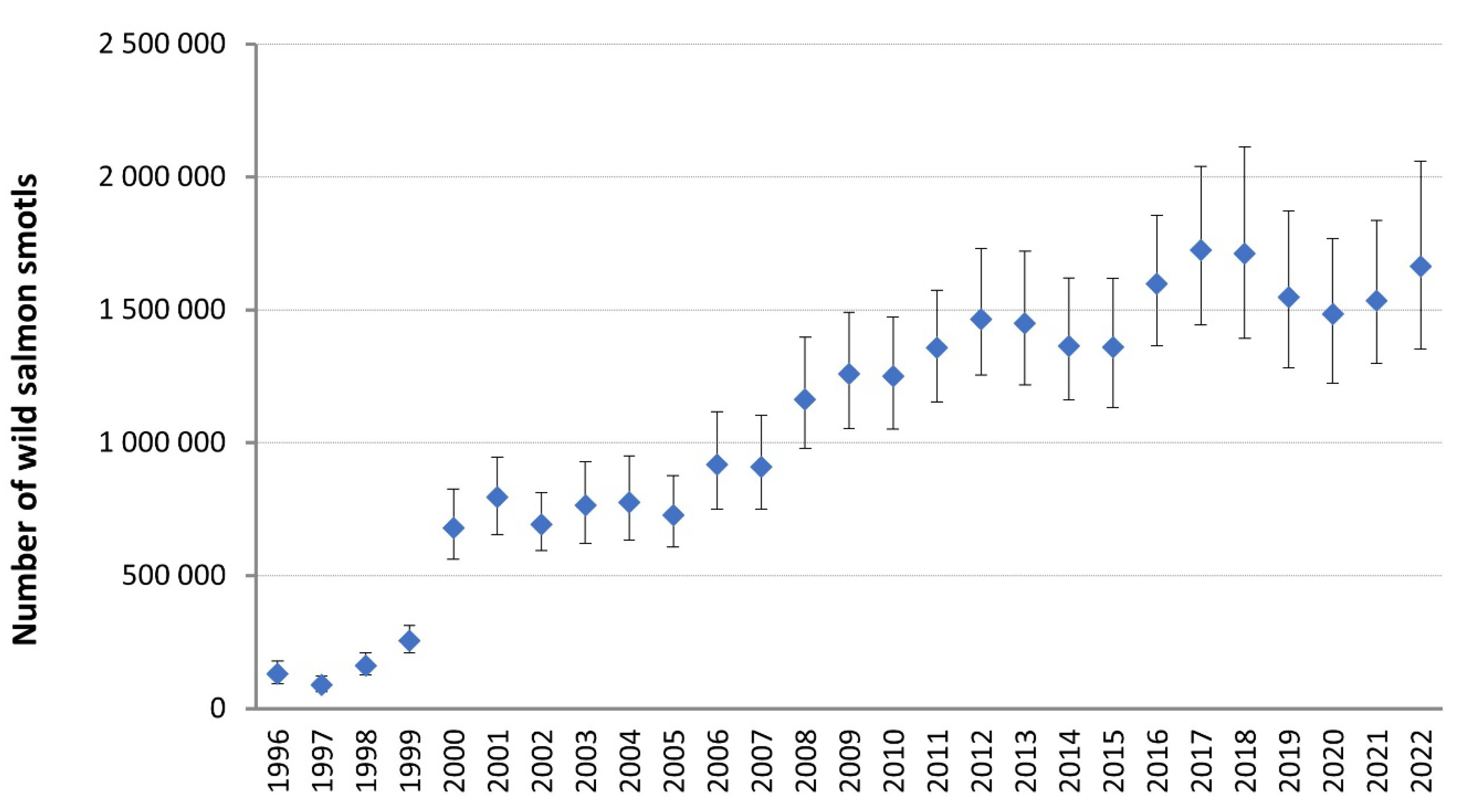
References
- Dittman, A.H.; Quinn, T.P. Homing in Pacific Salmon: Mechanisms and Ecological Basis. J. Exp. Biol. 1996, 199, 83–91. [Google Scholar] [CrossRef]
- McCormick, S.D.; Hansen, L.P.; Quinn, T.P.; Saunders, R.L. 1998. Movement, migration, and smolting of Atlantic salmon (Salmo salar). Can. J. Fish. Aquat. Sci. 1998, 55, 77–92. [Google Scholar] [CrossRef]
- Quinn, T.P.; Myers, K.W. Anadromy and the marine migrations of Pacific salmon and trout: Rounsefell revisited. Rev. Fish. Biol. Fish. 2004, 14, 421–442. [Google Scholar] [CrossRef]
- Lennox, R. J.; Chapman, J. M.; Souliere, C. M.; Tudorache, C.; Wikelski, M.; Metcalfe, J. D.; Cooke, S. J. Conservation physiology of animal migration. Conserv. Physio. 2016, 4, cov072. [Google Scholar] [CrossRef]
- Thorstad, E.B.; Whoriskey, F.; Uglem, I.; Moore, A.; Rikardsen, A.H.; Finstad, B. A critical life stage of the Atlantic salmon Salmo salar: behaviour and survival during the smolt and initial post-smolt migration. J. Fish Biol. 2012, 81, 500–542. [Google Scholar] [CrossRef]
- Lucas, M.C.; Baras, E.; Thom, T.J.; Duncan, D.; Slavík, O. Migration of Freshwater Fish. Blackwell Scientific Publishing. Oxford, UK, 2001, pp. 412.
- Jonsson, B.; Jonsson, N. A review of the likely effects of climate change on anadromous Atlantic salmon Salmo salar and brown trout Salmo trutta, with particular reference to water temperature and flow. J. Fish Biol. 2009, 75, 2381–447. [Google Scholar] [CrossRef]
- Erkinaro, J.; Czorlich, Y.; Orell, P.; Kuusela, J.; Falkegård, M.; Länsman, M.; Pulkkinen, H.; Primmer, C.R.; Niemelä, E. Life history variation across four decades in a diverse population complex of Atlantic salmon in a large subarctic river. Can. J. Fish. Aquat. Sci. 2018, 76, 42–55. [Google Scholar] [CrossRef]
- Veselov, A.E.; Kazakov, R.V.; Sysoyeva, M.I.; Bahmet, I.N. Ontogenesis of rheotactic and optomotor responses of juvenile Atlantic salmon. Aquaculture 1998, 168, 17–26. [Google Scholar] [CrossRef]
- Björnsson, B.T.; Stefansson, S.O.; McCormick, S.D. Environmental endocrinology of salmon smoltification. Gen. Comp. Endocrinol. 2011, 170, 290–298. [Google Scholar] [CrossRef]
- Piironen, J.; Kiiskinen, P.; Huuskonen, H.; Heikura-Ovaskainen, M.; Vornanen, M. Comparison of smoltification in Atlantic salmon (Salmo salar) from anadromous and landlocked populations under common garden conditions. Ann. Zool. Fenn. 2013, 50, 1–15. [Google Scholar] [CrossRef]
- Nilsen, T.O.; Ebbeson, L.O.E; Stefansson, S.O. Smolting in anadromous and landlocked strains of Atlantic salmon (Salmo salar). Aquaculture 2003, 222, 71–82. [Google Scholar] [CrossRef]
- Crozier, L.G.; Burke, B.J.; Chasco, B.E.; Widener, D.L.; Zabel, R.W. Climate change threatens Chinook salmon throughout their life cycle. Commun. Biol. 2021, 4, 222. [Google Scholar] [CrossRef]
- Olmos, M.; Massiot-Granier, F.; Prévost, E.; Chaput, G.; Bradbury, I.R.; Nevoux, M.; Rivot,E. Evidence for spatial coherence in time trends of marine life history traits of Atlantic salmon in the North Atlantic. Fish Fish. 2019, 20, 322–342. [Google Scholar] [CrossRef]
- Lehnert, S.J.; Kess, T.; Bentzen, P. M.; Kent, P.; Lien, S.; Gilbey, J.; Clément, M.; Jeffery, N.W.; Waples, R.S.; Bradbury, I.R. Genomic signatures and correlates of widespread population declines in salmon. Nat. Commun. 2019, 10, 2996. [Google Scholar] [CrossRef]
- Sobocinski, K.L.; Greene, C.M.; Anderson, J.H.; Kendall, N.W.; Schmidt, M.W.; Zimmerman, M.S.; Kemp, I.M.; Kim, S.; Ruff, C.P. A hypothesis-driven statistical approach for identifying ecosystem indicators of coho and Chinook salmon marine survival. Ecol. Indic. 2021, 124, 107403. [Google Scholar] [CrossRef]
- Dadswell, M.; Spares, A.; Reader, J.; McLean, M.; McDermott, T.; Samways, K.; Lilly, J. The Decline and Impending Collapse of the Atlantic Salmon (Salmo salar) Population in the North Atlantic Ocean: A Review of Possible Causes. Rev. Fish. Sci. Aquac. 2022, 30, 215–258. [Google Scholar] [CrossRef]
- Otero, J.; L'Abée-Lund, J.H.; Castro-Santos, T.; Leonardsson, K.; Storvik, G.O.; Jonsson, B.; Dempson, B.; Russell, I.C.; Jensen, A.J.; Baglinière, J.-L.; Dionne, M.; Armstrong, J.D.; Romakkaniemi, A.; Letcher, B.H.; Kocik, J.F.; Erkinaro, J.; Poole, R.; Rogan, G.; Lundqvist, H.; MacLean, J.C.; Jokikokko, E.; Arnekleiv, J.O.; Kennedy, R.J.; Niemelä, E.; Caballero, P.; Music, P.A.; Antonsson, T.; Gudjonsson, S.; Veselov, A.E.; Lamberg, A.; Groom, S.; Taylor, B.H.; Taberner, M.; Dillane, M.; Arnason, F.; Horton, G.; Hvidsten, N.A.; . Jonsson, I.R.; Jonsson, N.; McKelvey, S.; Næsje, T.F.; Skaala, O.; Smith, G.W.; Sægrov, H.; Stenseth, N.C.; Vøllestad, L.A. Basin-scale phenology and effects of climate variability on global timing of initial seaward migration of Atlantic salmon (Salmo salar). Glob. Chang. Biol. 2014, 20, 61–75. [Google Scholar] [CrossRef]
- Beamish, R.J.; Mahnken, C. A critical size and period hypothesis to explain natural regulation of salmon abundance and the linkage to climate and climate change. Prog. Oceanogr. 2001, 49, 423–437. [Google Scholar] [CrossRef]
- Huusko, A.; Hyvärinen, P. Atlantic salmon abundance and size track climate regimes in the Baltic Sea. Boreal Environ. Res. 2012, 17, 139–149. [Google Scholar]
- Sear, D.; Langdon, P.; Leng, M.; Edwards, M.; Heaton, T.; Langdon, C.; Leyland, J. Climate and human exploitation have regulated Atlantic salmon populations in the River Spey, Scotland, over the last 2000 years. The Holocene 2022, 32, 780–793. [Google Scholar] [CrossRef]
- Spanjer, A.R.; Gendaszek, A.S.; Wulfkuhle, E.J.; Black, R.W.; Jaeger, K.L. Assessing climate change impacts on Pacific salmon and trout using bioenergetics and spatiotemporal explicit river temperature predictions under varying riparian conditions. PLoS One 2022, 17, e0266871. [Google Scholar] [CrossRef]
- Russell, I.C.; Aprahamian, M.W.; Barry, J.; Davidson, I.C.; Fiske, P.; Ibbotson, A.T.; Kennedy, R.J.; Maclean, J.C.; Moore, A.; Otero, J.; Potter, E.C.E.; Todd, C.D. The influence of the freshwater environment and the biological characteristics of Atlantic salmon smolts on their subsequent marine survival. ICES J. Mar. Sci. 2012, 69, 1563–1573. [Google Scholar] [CrossRef]
- Thorstad, E.B.; Bliss, D.; Breau, C.; Damon-Randall, K.; Sundt-Hansen, L.E.; Hatfield, E.M.C.; Horsburgh, G.; Hansen, H.; Maoiléidigh, N. Ó,; Sheehan, T.; Sutton, S.G. 2021 Atlantic salmon in a rapidly changing environment—Facing the challenges of reduced marine survival and climate change. Aquatic Conserv.: Mar. Freshw. Ecosyst. 2021, 3, 2654–2665. [Google Scholar] [CrossRef]
- Jonsson, B.; Jonsson, M.; Jonsson, N. Optimal size at seaward migration in an anadromous salmonid. Mar. Ecol. Prog. Ser. 2016, 559, 193–200. [Google Scholar] [CrossRef]
- Gillis, C.-A.; Ouellet, V.; Breau, C.; Frechette, D.; Bergeron, N. Assessing climate change impacts on North American freshwater habitat of wild Atlantic salmon - urgent needs for collaborative research. Can. Water Resour. J. 2023. [Google Scholar] [CrossRef]
- IPCC. Climate Change 2022: Impacts, Adaptation, and Vulnerability. In Contribution of Working Group II to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Pörtner H-O, Roberts D C, Tignor M, Poloczanska E S, Mintenbeck K, Alegría A, Craig M, Langsdorf S, Löschke S, Möller V, Okem A, Rama R, Eds; Cambridge University Press. Cambridge University Press, Cambridge, UK and New York, NY, USA, 2022, pp 3056.
- Thibeault, J.M.; Seth, A. Changing climate extremes in the Northeast United States: observations and projections from CMIP5. Clim. Change 2014, 127, 273–287. [Google Scholar] [CrossRef]
- Demaria, E. M. C.; Palmer, R.N.; Roundy, J.K. Regional Climate Change Projections of Streamflow in the Northeast and Midwest U.S. J. Hydrol. Reg. Stud. 2016, 5, 309–323. [Google Scholar] [CrossRef]
- Hanssen-Bauer, I.; Førland, E.J. Long-term trends in precipitation and temperature in the Norwegian Arctic: can they be explained by changes in the atmospheric circulation patterns. Clim. Res. 1998, 10, 143–153. [Google Scholar] [CrossRef]
- Dore, M.H.I. Climate change and changes in global precipitation patterns: What do we know? Environ. Int. 2005, 31, 1167–1181. [Google Scholar] [CrossRef]
- Guo, R.; Deser, C.; Terray, L.; Lehner, F. Human Influence on Winter Precipitation Trends (1921–2015) over North America and Eurasia Revealed by Dynamical Adjustment. Geophys. Res. Lett. 2019, 46, 3426–3434. [Google Scholar] [CrossRef]
- Terrier, A.; Martin, P.; Girardin, M.P.; Périé, C.; Legendre, P.; Bergeron, Y. Potential changes in forest composition could reduce impacts of climate change on boreal wildfires. Ecol. Appl. 2013, 23, 21–35. [Google Scholar] [CrossRef] [PubMed]
- Kazakov, R. V. History and Atlantic salmon fishery state in Russia. In Atlanticheskiy losos; Kazakov R.V., Ed.; Nauka, Sankt-Petersburg, Soviet Union, 1998, pp. 335-380.
- Nicola, G.G.; Elvira, B.; Jonsson, B.; Ayllón, D.; Almodóvar, A. Local and global climatic drivers of Atlantic salmon decline in southern Europe. Fish. Res. 2018, 198, 78–85. [Google Scholar] [CrossRef]
- Todd, C.D.; Friedland, K.D.; MacLean, J.C.; Hazon, N.; Jensen, A.J. Getting into hot water? Atlantic salmon responses to climate change in freshwater and marine environments. In Atlantic salmon ecology; Aas, Ø., Klemetsen, A., Einum, S., Skurdal, J. Eds; Blackwell Publishing Ltd, Oxford, UK, 2011, pp 409-443.
- Hedger, R.D.; Sundt-Hansen, L.E.; Forseth, T.; Ugedal, O.; Diserud, O.H.; Kvambekk, Å.S.; Finstad, A.G. Predicting climate change effects on subarctic–Arctic populations of Atlantic salmon (Salmo salar). Can. J. Fish. Aquat. Sci. 2013, 70, 159–168. [Google Scholar] [CrossRef]
- Hastings, R.A.; Rutterford, L.A.; Freer, J.J.; Collin, R.A.; Simpson, S.D.; Genner, M.J. Climate change drives poleward increases and equatorward declines in marine species. Curr. Biol. 2020, 30, 1572–1577. [Google Scholar] [CrossRef] [PubMed]
- Gillson, J.P.; Bašić,T. ; Davison, P.I.; Riley, W.D.; Talks, L.; Walker, A.M.; Russell, I.C. A review of marine stressors impacting Atlantic salmon Salmo salar, with an assessment of the major threats to English stocks. Rev. Fish. Biol. Fisheries 2022, 32, 879–919. [Google Scholar] [CrossRef]
- Jensen, A.J.; Karlsson, S.; Fiske, P.; Hansen, L.P.; Østborg, G.M.; Hindar, K. Origin and life history of Atlantic salmon (Salmo salar) near their northernmost oceanic limit. Can. J. Fish. Aquat. Sci. 2014, 71, 1740–1746. [Google Scholar] [CrossRef]
- Bilous, M.; Dunmall, K. Atlantic salmon in the Canadian Arctic: potential dispersal, establishment, and interaction with Arctic char. Rev. Fish Biol. Fish. 2020, 30, 463–483. [Google Scholar] [CrossRef]
- Parrish, D.L.; Behnke, R.J.; Gephard, S.R.; McCormick, S.D.; Reeves, G.H. Why aren’t there more Atlantic salmon (Salmo salar)? Can. J. Fish. Aquat. Sci. 1998, 55, 281–287. [Google Scholar] [CrossRef]
- Almodóvar, A.; Ayllón, D.; Nicola, G.G.; Jonsson, B.; Elvira, B. Climate-driven biophysical changes in feeding and breeding environments explain the decline of southernmost European Atlantic salmon populations. Can. J. Fish. Aquat. Sci. 2021, 76, 1581–1595. [Google Scholar] [CrossRef]
- Mills, K.E.; Pershing, A.J.; Sheehan, T.F.; Mountain, D. Climate and ecosystem linkages explain widespread declines in North American Atlantic salmon populations. Glob. Chang. Biol. 2013, 19, 3046–3061. [Google Scholar] [CrossRef]
- Berg, O.K. The formation of non-anadromous populations of Atlantic salmon, Salmo salar L., in Europe. J. Fish Biol. 1985; 27, 805–811. [Google Scholar]
- Ozerov, M.Y.; Veselov, A.J. , Lumme, J.; Primmer, C.R. Genetic structure of freshwater Atlantic salmon (Salmo salar L.) populations from the lakes Onega and Ladoga of northwest Russia and implications for conservation. Conserv. Genet. 2010, 11, 1711–1724. [Google Scholar] [CrossRef]
- Lumme, J.; Ozerov, M.Y.; Veselov, A.E.; Primmer, C.R. The Formation of Landlocked Populations of Atlantic Salmon. In Evolutionary Biology of the Atlantic Salmon; Vladic, T., Pettersson, E., Eds.; CRC Press, Boca Raton, USA, 2015, pp. 297.
- Kazakov, R.V. Distribution of Atlantic salmon, Salmo salar L., in freshwater bodies of Europe. Aquaculture Fish. Managem. 1992, 23, 461–475. [Google Scholar] [CrossRef]
- Leinonen, T.; Piironen, J.; Koljonen, M.-L.; Koskiniemi, J.; Kause, A. Restored river habitat provides a natural spawning area for a critically endangered landlocked Atlantic salmon population. PLoS One 2020, 15, e0232723. [Google Scholar] [CrossRef] [PubMed]
- Filipe, A.F.; Markovic, D.; Pletterbauer, F.; Tisseuil, C.; De Wever, A.; Schmutz, S.; Bonada, N.; Freyhof, J. Forecasting fish distribution along stream networks: brown trout (Salmo trutta) in Europe. Divers. Distrib. 2013, 19, 1059–1071. [Google Scholar] [CrossRef]
- Beaupré, J.; Boudreault, J.; Bergeron, N.E.; St-Hilaire, A. Inclusion of water temperature in a fuzzy logic Atlantic salmon (Salmo salar) parr habitat model. J. Therm. Biol. 2020, 87, 102471. [Google Scholar] [CrossRef] [PubMed]
- Corey, E.; Linnansaari, T.; Cunjak, R.A. High temperature events shape the broadscale distribution of juvenile Atlantic salmon (Salmo salar). Freshw. Biol. 2023, 63, 534–545. [Google Scholar] [CrossRef]
- Wilbur, N. M.; O’Sullivan, A.M.; MacQuarrie, K.T.B.; Linnansaari, T.; Curry, R.A. Characterizing physical habitat preferences and thermal refuge occupancy of brook trout (Salvelinus fontinalis) and Atlantic salmon (Salmo salar) at high river temperatures. Riv. Res. Appl. 2020, 36, 769–783. [Google Scholar] [CrossRef]
- Schreck, C.B.; Tort, L. The Concept of Stress in Fish. Fish Physiol. 2016, 35, 1–34. [Google Scholar]
- Zhang, X.; Li, H.-Y.; Deng, Z.D.; Leung, L.R.; Skalski, J.R.; Cooke, S.J. On the variable effects of climate change on Pacific salmon. Ecol. Modell. 2019, 397, 95–106. [Google Scholar] [CrossRef]
- Cunningham, C.J.; Westley, P.A.H.; Adkison, M.D. Signals of large scale climate drivers, hatchery enhancement, and marine factors in Yukon River Chinook salmon survival revealed with a Bayesian life history model. Glob. Chang. Biol. 2018, 24, 4399–4416. [Google Scholar] [CrossRef]
- Hvidsten, N.A.; Diserud, O.H.; Jensen, A.J.; Jensås, J.G.; Johnsen, B.O.; Ugedal, O. Water discharge affects Atlantic salmon Salmo salar smolt production: a 27 year study in the River Orkla, Norway. J. Fish Biol. 2015, 86, 92–104. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, M.J.H.; Tierney, K.B. Warm northern river temperatures increase post-exercise fatigue in an Arctic migratory salmonid but not in a temperate relative. Func. Ecol. 2018, 32, 687–700. [Google Scholar] [CrossRef]
- Kaushal, S.S.; Likens, G.E.; Jaworski, N.A.; Pace, M.L.; Sides, A.M.; Seekell, D.; Belt, K.T.; Secor, D.H.; Wingate, R.L. Rising stream and river temperatures in the United States. Front. Ecol. Environ. 2010, 8, 461–466. [Google Scholar] [CrossRef]
- Isaak, D.J.; Wollrab, S.; Horan, D.; Chandler, G. Climate change effects on stream and river temperatures across the northwest U.S. from 1980–2009 and implications for salmonid fishes. Clim. Change 2012, 113, 499–524. [Google Scholar] [CrossRef]
- Arevalo, E.; Maire, A.; Tétard, S.; Prévost, E.; Lange, F.; Marchand, F.; Josset, Q.; Drouineau, H. Does global change increase the risk of maladaptation of Atlantic salmon migration through joint modifications of river temperature and discharge? Proc. R. Soc. B. 2021, 288, 20211882. [Google Scholar] [CrossRef] [PubMed]
- Smalås, A.; Primicerio, R. ; Kahilainen, Terentyev, P.; Kashulin, N.; Zubova, E.; Amundsen, P-A. Increased importance of cool-water fish at high latitudes emerges from individual level responses to warming. Authorea. 29 January 2023. [Google Scholar] [CrossRef]
- Solomon, D.J.; Lightfoot, G.W. The Thermal Biology of Brown Trout and Atlantic Salmon; Science Reports, Environment Agency, Rio House, Waterside Drive, Aztec West, Almondsbury, Bristol, BS32 4UD, 2008, pp. 42.
- McGinnity, P.; Jennings, E.; deEyto, E.; Allott, N.; Samuelsson, P.; Rogan, G.; Whelan, K.; Cross. T. Impact of naturally spawning captive-bred atlantic salmon on wild populations: depressed recruitment and increased risk of climate-mediated extinction. Proc. R. Soc. B. 2009, 276, 3601–3610. [Google Scholar] [CrossRef] [PubMed]
- Piou, C.; Prevost, E. Contrasting effects of climate change in continental vs. oceanic environments on population persistence and microevolution of Atlantic salmon. Glob. Chang. Biol. 2013, 19, 711–723. [Google Scholar] [CrossRef]
- Burton, T.; McKelvey, S.; Stewart, D.C.; Armstrong, J.D.; Metcalfe, N.B. Offspring investment in wild Atlantic salmon (Salmo salar): relationships with smolt age and spawning condition. Ecol. Freshw. Fish 2013, 22, 317–332. [Google Scholar] [CrossRef]
- Burt, J.M.; Hinch, S.G.; Patterson, D.A. The importance of parentage in assessing temperature effects on fish early life history: A review of the experimental literature. Rev. Fish Biol. Fish. 2011, 21, 377–406. [Google Scholar] [CrossRef]
- Jonsson, B.; Jonsson, N. Egg incubation temperature affects the timing of the Atlantic salmon Salmo salar homing migration. J. Fish Biol. 2018, 93, 1016–1020. [Google Scholar] [CrossRef]
- Thompson, J.N.; Beauchamp, D. Size-Selective Mortality of Steelhead during Freshwater and Marine Life Stages Related to Freshwater Growth in the Skagit River, Washington. Trans. Am. Fish. Soc. 2014, 143, 910–925. [Google Scholar] [CrossRef]
- Jonsson, N.; Jonsson, B.; Hansen, L.P. Does climate during embryonic development influence parr growth and age of seaward migration in Atlantic salmon (Salmo salar)? Can. J. Fish. Aquat. Sci. 2005, 62, 2502–2508. [Google Scholar] [CrossRef]
- Crozier, L.G.; Zabel, R.W.; Hockersmith, E.E.; Achord, S. Interacting effects of density and temperature on body size in multiple populations of Chinook salmon. J. Anim. Ecol. 2010, 79, 342–349. [Google Scholar] [CrossRef] [PubMed]
- Cordoleani, F.; Phillis, C.C.; Sturrock, A.M. FitzGerald, A.M.; Malkassian, A.; Whitman, G.E.; Weber, P.K.; Johnson, R.C. Threatened salmon rely on a rare life history strategy in a warming landscape. Nat. Clim. Chang. 2021, 11, 982–988. [Google Scholar] [CrossRef]
- Fullerton, A. H.; Burke, B.J.; Lawler, J.J.; Torgersen, C.E.; Ebersole, J.L.; Leibowitz, S.G. Simulated juvenile salmon growth and phenology respond to altered thermal regimes and stream network shape. Ecosphere 2017, 8, e02052. [Google Scholar] [CrossRef]
- Isaak, D.J.; Young. M.K. Cold-water habitats, climate refugia, and their utility for conserving salmonid fishes. Can. J. Fish. Aquat. Sci. 2023, e–First. [Google Scholar] [CrossRef]
- Dugdale, S.J.; Bergeron, N.E.; St-Hilaire, A. Temporal variability of thermal refuges and water temperature patterns in Atlantic salmon rivers. Remote Sensing of Environment 2013, 136, 358–373. [Google Scholar] [CrossRef]
- Battin, J.; Wiley, M.W.; Ruckelshaus, M.H.; Imaki, H. Projected impacts of climate change on salmon habitat restoration Proc. Natl. Acad. Sci. U.S.A. 2007, 104, 6720–6725. [Google Scholar] [CrossRef]
- Foley, K.M.; Rosenberger, S.A.; Mueter, F.J. Longitudinal Patterns of Juvenile Coho Salmon Distribution and Densities in Headwater Streams of the Little Susitna River, Alaska. Trans. Am. Fish. Soc. 2018, 147, 247–264. [Google Scholar] [CrossRef]
- Jonsson, B.; Jonsson, N.; Brodtkorb, E.; Ingebrigtsen, P.-J. Life-history traits of Brown Trout vary with the size of small streams. Func. Ecol. 2001, 15, 310–317. [Google Scholar] [CrossRef]
- Sutela, T.; Vehanen, T.; Jounela, P. Longitudinal patterns of fish assemblages in European boreal streams. Hydrobiologia 2020, 847, 3277–3290. [Google Scholar] [CrossRef]
- Jespersen, H.; Rasmussen, G.; Pedersen, S. Severity of summer drought as predictor for smolt recruitment in migratory brown trout (Salmo trutta). Ecol. Freshw. Fish 2021, 30, 115–124. [Google Scholar] [CrossRef]
- Mantua, N.; Tohver, I.; Hamlet, A. Climate change impacts on streamflow extremes and summertime stream temperature and their possible consequences for freshwater salmon habitat in Washington State. Clim. Change 2010, 102, 187–223. [Google Scholar] [CrossRef]
- Kang, D.H.; Gao, H.; Shi, X.; ul Islam, S.; Déry, S. J. Impacts of a rapidly declining mountain snowpack on streamflow timing in Canada’s Fraser River basin. Sci. Rep. 2016, 6, 19299. [Google Scholar] [CrossRef]
- Caissie, D. C.; Breau, J. H. ; Cameron,P. Water Temperature Characteristics within the Miramichi and Restigouche Rivers, Miramichi, New Brunswick, Canada. Canadian Science Advisory Secretariat, Research Document 2012/165.
- Hayes, D. S.; Moreira, M.; Boavida, I.; Haslauer, M.; Unfer, G.; Zeiringer, B.; Greimel, F.; Auer, S.; Ferreira, T.; Schmutz, S. Life stage-specific hydropeaking flow rules. Sustainability 2019, 11, 1547. [Google Scholar] [CrossRef]
- Huusko, A.; Greenberg, L.; Stickler, M.; Linnansaari, T.; Nykänen, M.; Vehanen, T.; Koljonen, S.; Louhi,P. ; Alfredsen, K. Life in the Ice Lane: A Review of the Ecology of Salmonids during Winter. River Res. Appl. 2007, 23, 469–491. [Google Scholar] [CrossRef]
- Mäki-Petäys, A.; Muotka, T.; Huusko, A.; Tikkanen, P.; Kreivi, P. Seasonal changes in habitat use and preference by juvenile brown trout, Salmo trutta, in a northern boreal river. Can. J. Fish. Aquat. Sci. 1997, 54, 520–530. [Google Scholar]
- Magnuson, J. J.; Robertson, D.M.; Benson, B.J.; Wynne, R.H.; Livingstone, D.M.; Arai, T.; Assel, R.A.; Barry, R.G.; Card, V.; Kuusisto, K.; Granin, N.G.; Prowse, T.D.; Stewart, K.M.; Vuglinski, V.S. Historical trends in lake and river ice cover in the Northern Hemisphere. Science 2000, 289, 1743–1746. [Google Scholar] [CrossRef] [PubMed]
- Pörtner, H. O.; Peck, M. A. Climate change effects on fishes and fisheries: Towards a cause-and-effect understanding. J. Fish Biol. 2010, 77, 1745–1779. [Google Scholar] [CrossRef] [PubMed]
- van Vliet, M. T. H.; Franssen, W. H. P.; Yearsley, J. R.; Ludwig, F.; Haddeland, I.; Lettenmaier, D. P.; Kabat, P. Global river discharge and water temperature under climate change. Glob. Environ. Change 2013, 23, 450–464. [Google Scholar] [CrossRef]
- Härkönen, L.; Louhi, P.; Huusko, R.; Huusko, A. Wintertime growth in Atlantic salmon under changing climate: the importance of ice cover for individual growth dynamics. Can. J. Fish. Aquat. Sci. 2021, 78, 1479–1485. [Google Scholar] [CrossRef]
- et al. 1996 Graham, W.D.; Thorpe, J.E. ; Metcalfe, N.B. Seasonal current holding performance of juvenile Atlantic salmon in relation to temperature and smolting. Can. J. Fish. Aquat. Sci. 1996, 53, 80–86. [Google Scholar]
- Enders, E.C.; Stickler, M.; Pennell, C.J.; Cote, D.; Alfredsen, K.; Scruton, D.A. Habitat use of Atlantic salmon parr (Salmo salar L. ) during winter. In Proceedings of 14th Workshop on the Hydraulics of Ice Covered Rivers, Quebec City, Canada, 2007; CGU HS Committee on River Ice Processes and the Environment, Canada, 2007, June 19 - 22; pp. 20–22.
- Cunjak, R.A.; Prowse, T.D.; Parrish, D.L. Atlantic salmon (Salmo salar) in winter: “the season of parr discontent”? Can. J. Fish. Aquat. Sci. 1999, 55, 161–180. [Google Scholar] [CrossRef]
- Comte, L.; Olden, J.D.; Tedesco, P.A.; Ruhi, A.; Giam, X. Climate and land-use changes interact to drive long-term reorganization of riverine fish communities globally. Proc. Natl. Acad. Sci. U.S.A. 2021, 118, e2011639118. [Google Scholar] [CrossRef]
- Tabari, H. Climate change impact on flood and extreme precipitation increases with water availability. Sci. Rep. 2020, 10, 13768. [Google Scholar] [CrossRef]
- Harp, R.D.; Horton, D.E. Observed Changes in Daily Precipitation Intensity in the United States. Geophys. Res. Lett. 2022, 49, e2022GL099955. [Google Scholar] [CrossRef]
- Cronin, L.; Regan, F.; Lucy, F.E. Detection of transient pollution events in an Irish river catchment in the context of increasing frequency and intensity of rainfall events due to climate change. EGU General Assembly 2023, EGU23, 15993. [Google Scholar]
- Weyhenmeyer, G.; Prairie, Y.T.; Tranvik, L.J. Browning of Boreal Freshwaters Coupled to Carbon-Iron Interactions along the Aquatic Continuum. PLoS One 2014, 9, e88104. [Google Scholar] [CrossRef]
- Kritzberg, E.S.; Hasselquist, E.M.; Škerlep, M. Stefan Löfgren, S.; Olsson, O.; Stadmark, J.; Valinia, S.; Hansson, L.-A.; Laudon, H. Browning of freshwaters: Consequences to ecosystem services, underlying drivers, and potential mitigation measures. Ambio 2020, 49, 375–390. [Google Scholar] [CrossRef]
- Sethi, S.A.; Carey, M.P.; Gerken, J.; Harris, B.P.; Wolf, N.; Cunningham, C.; Restrepo, F.; Ashline, J. Juvenile salmon habitat use drives variation in growth and highlights vulnerability to river fragmentation. Ecosphere 2022, 13, e4192. [Google Scholar] [CrossRef]
- Vargas-Chacoff, L.; Regish, A.M.; Weinstock, A.; McCormick, S.D. Effects of elevated temperature on osmoregulation and stress responses in Atlantic salmon Salmo salar smolts in fresh water and seawater. J. Fish Biol. 2018, 93, 550–559. [Google Scholar] [CrossRef]
- Thorpe, J.E. Maturation responses of salmonids to changing developmental opportunities. Mar. Ecol. Prog. Ser. 2007, 335, 285–288. [Google Scholar] [CrossRef]
- Copeland, T.; Venditti, D.A. Contribution of three life history types to smolt production in a Chinook salmon (Oncorhynchus tshawytscha) population. Can. J. Fish. Aquat. Sci. 2009, 66, 1658–1665. [Google Scholar] [CrossRef]
- Sturrock, A.M.; Wikert, J.D.; Heyne, T.; Mesick, C.; Hubbard, A.E.; Hinkelman, T.M.; Weber, P.K.; Whitman, G.E.; Glessner, J.G.; Johnson, R.C. Reconstructing the Migratory Behavior and Long-Term Survivorship of Juvenile Chinook Salmon under Contrasting Hydrologic Regimes. PLoS One 2015, 10, e0122380. [Google Scholar] [CrossRef]
- Bassett, M.C.; Patterson, P.A.; Shrimpton, J.M. Temporal and spatial differences in smolting among Oncorhynchus nerka populations throughout fresh and seawater migration J. Fish Biol. 2018, 93, 510–518. [Google Scholar] [CrossRef]
- Elsner, R.A.; Shrimpton, J.M. Is the duration of the smolt window related to migration distance in coho salmon Oncorhynchus kisutch? J. Fish Biol. 2018, 93, 501–509. [Google Scholar] [CrossRef]
- Bohlin, T.; Dellefors, C.; Faremo, U. Optimal Time and Size for Smolt Migration in Wild Sea Trout (Salmo trutta). Can. J. Fish. Aquat. Sci. 2011, 50, 224–232. [Google Scholar] [CrossRef]
- Jutila, E.; Jokikokko, E.; Julkunen. M. Long-term changes in the smolt size and age of Atlantic salmon, Salmo salar L., in a northern Baltic river related to parr density, growth opportunity and postsmolt survival. Ecol. Freshw. Fish 2006, 15, 321–330. [Google Scholar] [CrossRef]
- Gregory, S.D.; Ibbotson, A.T.; Riley, W.D.; Nevoux, M.; Lauridsen, R.B.; Russell, I.A.; Britton, J.R.; Gillingham, P.K.; Simmons, O.M.; Rivot, E. Atlantic salmon return rate increases with smolt length. ICES J. Mar. Sci. 2019, 76, 1702–1712. [Google Scholar] [CrossRef]
- Debes, P.V.; Piavchenko, N.; Erkinaro, J.; Primmer, C.R. Genetic growth potential, rather than phenotypic size, predicts migration phenotype in Atlantic salmon. Proc. R. Soc. B 2020, 287, 20200867. [Google Scholar] [CrossRef]
- Økland, F.; Jonsson, B.; Jensen, A.J.; Hansen, L.P. Is there a threshold size regulating seaward migration of brown trout and Atlantic salmon? J. Fish Biol. 1993, 42, 541–550. [Google Scholar] [CrossRef]
- Vollset, K.W.; Lennox, R.J.; Lamberg, A.; Skaala, Ø.; Sandvik, A.D.; Sægrov, H.; Kvingedal, E.; Kristensen, T.; Jensen, A.J.; Haraldstad, T.; Barlaup, B.T.; Ugedal, O. Predicting the nationwide outmigration timing of Atlantic salmon (Salmo salar) smolts along 12 degrees of latitude in Norway. Divers. Distrib. 2021, 27, 1383–1392. [Google Scholar] [CrossRef]
- Archer, L.C.; Hutton, S.A.; Harman, L.; Michael, N.; O'Grady, M.N.; Kerry, J.P.; Poole, W.R.; Gargan, P.; McGinnity, P.; Reed, T.E. The Interplay Between Extrinsic and Intrinsic Factors in Determining Migration Decisions in Brown Trout (Salmo trutta): An Experimental Study. Front. Ecol. Evol. 2019, 7, 222. [Google Scholar] [CrossRef]
- Jones, D.A.; Bergman, E.; Greenberg, L. Food availability in spring affects smolting in brown trout (Salmo trutta). Can. J. Fish. Aquat. Sci. 2015, 72, 1694–1699. [Google Scholar] [CrossRef]
- Fängstam, H.; Berglund, I.; Sjöberg, M.; Lundqvist, H. Effects of size and early sexual maturity on downstream migration during smolting in Baltic samon (Salmo salar). J. Fish Biol. 1993, 43, 517–529. [Google Scholar] [CrossRef]
- Nelson, K.C.; Palmer, M.A.; Pizzuto, J.E.; Moglen, G.E.; Angermeier, P.L.; Hilderbrand, R.H.; Dettinger, M.; Katharine Hayhoe, K. Forecasting the combined effects of urbanization and climate change on stream ecosystems: from impacts to management options J. Appl. Ecol. 2009, 46, 154–163. [Google Scholar] [CrossRef]
- Capon, S.J.; Stewart-Koster,B. ; Bunn, S.E. Future of Freshwater Ecosystems in a 1.5°C Warmer World. Front. Environ. Sci. 2021, 9, 784642. [Google Scholar] [CrossRef]
- Woodward, G.; Perkins, D.M.; Brown, L.E. Climate change and freshwater ecosystems: impacts across multiple levels of organization. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2010, 365, 2093–2106. [Google Scholar] [CrossRef]
- Jeppesen, E.; Kronvang, B.; Meerhoff, M.; Søndergaard, M.; Hansen, K.M.; Andersen, H.E.; Lauridsen, T.L.; Liboriussen, L.; Beklioglu, M.; Ozen, A.; Olesen, J.E. Climate change effects on runoff, catchment phosphorus loading and lake ecological state, and potential adaptations. J. Environ. Qual. 2009, 38, 1930–41. [Google Scholar] [CrossRef]
- Pihlainen, P.; Zandersen, M.; Hyytiäinen, K.; Andersen, H.E.; Bartosova, A.; Gustafsson, B.; Jabloun, M.; McCrackin, M.; Meier, H.E.M.; Olesen, J.E.; Saraiva, S.; Swaney, D.; Thodsen, H. Impacts of changing society and climate on nutrient loading to the Baltic Sea. Sci. Total Environ. 2020, 731, 138935. [Google Scholar] [CrossRef]
- Vehanen, T.; Sutela, T.; Aroviita, J.; Karjalainen, S.M.; Riihimäki, J.; Larsson, A.; Vuori, K.-M. Land use in acid sulphate soils degrades river water quality -Do the biological quality metrics respond? Ecol. Indic. 2022, 141, 109085. [Google Scholar] [CrossRef]
- Nilsen, T.O.; Ebbesson, L.O.; Handeland, S.O.; Kroglund, F.; Finstad, B.; Angotzi, A.R.; Stefansson. S.O. Atlantic salmon (Salmo salar L.) smolts require more than two weeks to recover from acidic water and aluminium exposure. Aquat. Toxicol. 2013, 142-143, 33–44. [Google Scholar] [CrossRef]
- Kastl, B.; Obedzinski, M.; Carlson, S. M.; Boucher, W.T.; Grantham, T.E. Migration in drought: Receding streams contract the seaward migration window of endangered salmon. Ecosphere 2022, 13, e4295. [Google Scholar] [CrossRef]
- Bernard, B.; Mandiki, S.N.M.; Duchatel, V.; Rollin, X.; Kestemont, P. A temperature shift on the migratory route similarly impairs hypo-osmoregulatory capacities in two strains of Atlantic salmon (Salmo salar L.) smolts. Fish Physiol. Biochem. 2019, 45, 1245–1260. [Google Scholar] [CrossRef]
- Hansen, L.P.; Jonsson, B. Salmon ranching experiments in the River Imsa: Effect of timing of Atlantic salmon (Salmo salar) smolt migration on survival to adults. Aquaculture 1989, 82, 367–373. [Google Scholar] [CrossRef]
- Bjerck, H.B.; Urke, H.A.; Haugen, T.O.; Alfredsen, J.A.; Ulvund, J.B.; Kristensen, T. Synchrony and multimodality in the timing of Atlantic salmon smolt migration in two Norwegian fjords. Sci. Rep. 2021, 11, 6504. [Google Scholar] [CrossRef]
- Achord, S.; Zabel, R.W.; Sandford, B.P. Migration Timing, Growth, and Estimated Parr-to-Smolt Survival Rates of Wild Snake River Spring–Summer Chinook Salmon from the Salmon River Basin, Idaho, to the Lower Snake River. Trans. Am. Fish. Soc. 2007, 136, 142–154. [Google Scholar] [CrossRef]
- Sykes, G.E.; Johnson, C.J.; Shrimpton, J.M. Temperature and Flow Effects on Migration Timing of Chinook Salmon Smolts. Trans. Am. Fish. Soc. 2009, 138, 1252–1265. [Google Scholar] [CrossRef]
- Frechette, D.M.; Hawkes, J.P.; Kocik, J.F. Managing for Atlantic Salmon Smolt Run Timing Variability in a Changing Climate. N. Am. J. Fish. Manag. 2023, 43, 517–538. [Google Scholar] [CrossRef]
- Antonsson, T.; Gudjonsson, S. Variability in Timing and Characteristics of Atlantic Salmon Smolt in Icelandic Rivers. Trans. Am. Fish. Soc. 2002, 131, 643–655. [Google Scholar] [CrossRef]
- Zydlewski, G.B.; Haro, A.; McCormick, S.D. Evidence for cumulative temperature as an initiating and terminating factor in downstream migratory behavior of Atlantic salmon (Salmo salar) smolts. Can. J. Fish. Aquat. Sci. 2005, 62, 68–78. [Google Scholar] [CrossRef]
- Hvidsten, N. A.; Jensen, A. J.; Vivås, H.; Bakke, Ø. Downstream migration of Atlantic salmon smolts in relation to water flow, water temperature, moon phase and social interaction. Nord. J. Freshw. Res. 1995, 70, 38–48. [Google Scholar]
- Persson, L.; Kagervall, A.; Leonardsson, K.; Royan, M.; Alanärä, A. The effect of physiological and environmental conditions on smolt migration in Atlantic salmon Salmo salar. Ecol. Freshw. Fish 2019, 28, 190–199. [Google Scholar] [CrossRef]
- Harvey, A.C.; Glover, K.A.; Wennevik, V.; Skaala, Ø. Atlantic salmon and sea trout display synchronised smolt migration relative to linked environmental cues. Sci. Rep. 2020, 10, 3529. [Google Scholar] [CrossRef]
- Zydlewski, G.B.; Stich, D.S.; McCormick, S.D. Photoperiod control of downstream movements of Atlantic salmon Salmo salar smolts. J. Fish Biol. 2014, 85, 1023–1041. [Google Scholar] [CrossRef]
- Karppinen, P.; Hynninen, M.; Vehanen, T.; Vähä, J.-P. Variations in migration behaviour and mortality of Atlantic salmon smolts in four different hydroelectric facilities. Fish. Manag. Ecol. 2021, 28, 253–267. [Google Scholar] [CrossRef]
- Aarestrup, K.; Nielsen, C.; Koed, A. Net ground speed of downstream migrating radio-tagged Atlantic salmon (Salmo salar L.) and brown trout (Salmo trutta L.) smolts in relation to environmental factors. Hydrobiologia 2002, 483, 95–102. [Google Scholar] [CrossRef]
- Riley, W.D. Seasonal downstream movements of juvenile Atlantic salmon, Salmo salar L., with evidence of solitary migration of smolts. Aquaculture 2007, 273, 194–199. [Google Scholar] [CrossRef]
- Olsén, K.H.; Petersson, E.; Ragnarsson, B.; Lundqvist, H.; Järvi, T. Downstream migration in Atlantic salmon (Salmo salar) smolt sibling groups. Can. J. Fish. Aquat. Sci. 2011, 61, 328–331. [Google Scholar] [CrossRef]
- Kemp, P.S.; Williams, J.G. Illumination influences the ability of migrating juvenile salmonids to pass a submerged experimental weir. Ecol. Freshw. Fish 2009, 18, 297–304. [Google Scholar] [CrossRef]
- Nielsen, C.; Holdensgaard, G.; Petersen, H.C.; Björnsson, B. T.; Madsen, S.S. Genetic differences in physiology, growth hormone levels and migratory behaviour of Atlantic salmon smolts. J. Fish Biol. 2001, 59, 28–44. [Google Scholar] [CrossRef]
- Stewart, D. C.; Middlemas, S.J.; Youngson, A.F. Population structuring in Atlantic salmon (Salmo salar): Evidence of genetic influence on the timing of smolt migration in sub-catchment stocks. Ecol. Freshw. Fish 2006, 15, 552–558. [Google Scholar] [CrossRef]
- Crozier, L.G.; Hendry, A.P.; Lawson, P.W.; Quinn, T.P; Mantua, N.J.; Battin, J.; Shaw, R.G.; Huey, R.B. Potential responses to climate change in organisms with complex life histories: evolution and plasticity in Pacific salmon. Evol. Appl. 2008, 1, 252–270. [Google Scholar] [CrossRef]
- Kennedy, R.J.; Crozier, W.W. Evidence of changing migratory patterns of wild Atlantic salmon Salmo salar smolts in the River Bush, Northern Ireland, and possible associations with climate change. J. Fish Biol. 2010, 76, 1786–1805. [Google Scholar] [CrossRef]
- Wilson, S.M.; Patterson, D.A.; Moore, J.M. Intra- and inter-population variation in sensitivity of migratory sockeye salmon smolts to phenological mismatch. Mar. Ecol. Prog. Ser. 2022, 692, 119–136. [Google Scholar] [CrossRef]
- Hovel, R.A.; Fresh, K.L.; Schroder, S.L.; Litt, A.H.; Quinn, T.P. A wide window of migration phenology captures inter-annual variability of favourable conditions: Results of a whole-lake experiment with juvenile Pacific salmon. Freshw. Biol. 2019, 64, 46–55. [Google Scholar] [CrossRef]
- Beechie, T.; Buhle, E.; Ruckelshaus, M.; Fullerton, A.; Holsinger, L. Hydrologic regime and the conservation of salmon life history diversity. Biol. Conserv. 2006, 130, 560–572. [Google Scholar] [CrossRef]
- McClure, M. M.; Carlson, S.M.; Beechie, T.J.; Pess, G.R.; Jorgensen, J.C. ; Sogard,, S. M.; Sultan, S.E.; Holzer, D.M.; Travis, J.; Sanderson, B.L.; Power, M.E.; Carmichael, R.W. Evolutionary consequences of habitat loss for Pacific anadromous salmonids: Salmonid habitat loss and evolution. Evol. Appl. 2008; 1, 300–318. [Google Scholar]
- Haraldstad, T.; Haugen, T.O.; Olsen, E.M.; Forseth, T.; Höglund, E. Hydropower-induced selection of behavioural traits in Atlantic salmon (Salmo salar). Sci. Rep. 2021, 11, 16444. [Google Scholar] [CrossRef]
- Gauld, N.R.; Campbell, R.N.; Lucas, M.C. Reduced flow impacts salmonid smolt emigration in a river with low-head weirs. Sci. Total Environ. 2013, 458–460, 435–443. [Google Scholar] [CrossRef]
- Chavarie, L. , Honkanen, M.; Newton, M.; Lilly, J.M.; Greetham, H.R.; Adams, C.A. The benefits of merging passive and active tracking approaches: New insights into riverine migration by salmonid smolts. Ecosphere 2022, 13, e4045. [Google Scholar] [CrossRef]
- Flávio, H.; Kennedy, R.; Ensing, D.; Jepsen, N.; Aarestrup, K. Marine mortality in the river? Atlantic salmon smolts under high predation pressure in the last kilometres of a river monitored for stock assessment. Fish. Manag. Ecol. 2020, 27, 92–101. [Google Scholar] [CrossRef]
- Welch, D.W.; Porter, A.D.; Rechisky, E.L. A synthesis of the coast-wide decline in survival of West Coast Chinook Salmon (Oncorhynchus tshawytscha, Salmonidae). Fish Fish. 2021, 22, 194–211. [Google Scholar] [CrossRef]
- Dukes, J.S.; Mooney, H.A. Does global change increase the success of biological invaders? Trends Ecol. Evol. 1999, 14, 135–139. [Google Scholar] [CrossRef]
- Rahel, F.J.; Olden, J.D. Assessing the Effects of Climate Change on Aquatic Invasive Species. Conserv. Biol. 2008, 22, 521–533. [Google Scholar] [CrossRef]
- Mainka, S.A.; Howard, G.W. Climate change and invasive species: double jeopardy. Integr. Zool. 2010, 5, 102–111. [Google Scholar] [CrossRef]
- Koed, A.; Baktoft, H.; Bak, B. D. Causes of mortality of Atlantic salmon (Salmo salar) and brown trout (Salmo trutta) smolts in a restored river and its estuary. River Res. Appl. 2006, 22, 69–78. [Google Scholar] [CrossRef]
- Jepsen, N. : Flávio, H.; Koed, A. The impact of Cormorant predation on Atlantic salmon and Sea trout smolt survival. Fish. Manag. Ecol. 2019, 26, 183–186. [Google Scholar] [CrossRef]
- Vehanen, T.; Huusko, A.; Bergman, E.; Enefalk, Å.; Louhi, P.; Sutela, T. American mink (Neovison vison) preying on hatchery and wild brown trout (Salmo trutta) juveniles in semi-natural streams. Freshw. Biol. 2022, 67, 433–444. [Google Scholar] [CrossRef]
- Hansen, L.P.; Quinn, T.P. The marine phase of the Atlantic salmon (Salmo salar) life cycle, with comparisons to Pacifc salmon. Can. J. Fish. Aquat. Sci. 1998, 55, 104–118. [Google Scholar] [CrossRef]
- Potter, E.C.E. ; Crozier. W.W. A perspective on the marine survival of Atlantic salmon. In The ocean life of Atlantic salmon: environmental and biological factors influencing survival; Mills, D., Ed.; Fishing News Books, Oxford, UK, 2000, pp 19–36.
- Friedland, K.D.; MacLean, J.C.; Hansen, L.P.; Peyronnet, A.J.; Karlsson, L.; Reddin, D.G.; Maoiléidigh, Ó.N.; McCarthy, J.L. The recruitment of Atlantic salmon in Europe. ICES J. Mar. Sci. 2009, 66, 289–304. [Google Scholar] [CrossRef]
- Friedland, K.D.; Shank, B.V.; Todd, C.D.; McGinnity, P.; Nye, J.A. Diferential response of continental stock complexes of Atlantic salmon (Salmo salar) to the Atlantic Multidecadal Oscillation. J. Mar. Syst. 2014, 133, 77–87. [Google Scholar] [CrossRef]
- Salminen, M.; Kuikka, S.; Erkamo, E. Annual variability in survival of sea-ranched Baltic salmon, Salmo salar L.: significance of smolt size and marine conditions. Fish. Manag. Ecol. 1995, 2, 171–184. [Google Scholar] [CrossRef]
- Friedland, K.D.; Reddin, D.G.; McMenemy, J.R.; Drinkwater, K.F. Multidecadal trends in North American Atlantic salmon (Salmo salar) stocks and climate trends relevant to juvenile survival. Can. J. Fish. Aquat. Sci. 2003, 60, 563–583. [Google Scholar] [CrossRef]
- Friedland, K.D.; Hansen, L.P.; Dunkley, D.A.; MacLean, J.C. Linkage between ocean climate, post-smolt growth, and survival of Atlantic salmon (Salmo salar L.) in the North Sea area. ICES J. Mar. Sci. 2000, 57, 419–429. [Google Scholar] [CrossRef]
- Beaugrand, G.; Reid, P.C. Long-term changes in phytoplankton, zooplankton and salmon related to climate. Glob. Chang. Biol. 2003, 9, 801–817. [Google Scholar] [CrossRef]
- Jonsson, B.; Jonsson, N. Migratory timing, marine survival and growth of anadromous brown trout in the River Imsa, Norway. J. Fish Biol. 2009, 74, 621–638. [Google Scholar] [CrossRef]
- Chaput, G. Overview of the status of Atlantic salmon (Salmo salar) in the North Atlantic and trends in marine mortality. ICES J. Mar. Sci. 2012, 69, 1538–1548. [Google Scholar] [CrossRef]
- Olmos, M.; Payne, M.R.; Nevoux, M.; Prévost, E; Chaput, G. ; Du Pontavice, H.; Guitton, J.; Sheehan, T.; Mills, K.; Rivot, E. Spatial synchrony in the response of a long range migratory species (Salmo salar) to climate change in the North Atlantic Ocean. Glob. Chang. Biol. 2020, 26, 1319–1337. [Google Scholar] [CrossRef]
- Friedland, K.D.; Dannewitz, J.; Romakkaniemi, A.; Palm, S.; Pulkkinen, H.; Pakarinen, T.; Oeberst, R. Post-smolt survival of Baltic salmon in context to changing environmental conditions and predators. ICES J. Mar. Sci. 2017, 74, 1344–1355. [Google Scholar] [CrossRef]
- Chaput, G.; Carr, J.; Daniels, J.; Tinker, S.; Jonsen, I.; Whoriskey, F. Atlantic salmon (Salmo salar) smolt and early post-smolt migration and survival inferred from multi-year and multi-stock acoustic telemetry studies in the Gulf of St. Lawrence, northwest Atlantic. ICES J. Mar. Sci. 2019, 76, 1107–1121. [Google Scholar] [CrossRef]
- Jonsson, N.; Jonsson, B. Growth and sexual maturation in Atlantic salmon Salmo salar L. J. Fish Biol. 2007, 71, 245–252. [Google Scholar] [CrossRef]
- Jonsson, B.; Jonsson, N.; Albretsen, J. Environmental change influences the life history of salmon Salmo salar in the North Atlantic Ocean. J. Fish Biol. 2016, 88, 618–637. [Google Scholar] [CrossRef] [PubMed]
- Hvidsten, N.A.; Heggberget, T.G.; Jensen, A.J. Sea water temperatures at Atlantic salmon smolt entrance. Nord. J. Freshw. Res. 1998, 74, 79–86. [Google Scholar]
- Simmons, O.M.; Gregory, S.D.; Gillingham, P.K.; Riley, W.D.; Scott, L.J.; Britton, J.R. Biological and environmental infuences on the migration phenology of Atlantic salmon Salmo salar smolts in a chalk stream in southern England. Freshw. Biol. 2021, 66, 1581–1594. [Google Scholar] [CrossRef]
- IPCC. Climate Change 2007: The Physical Science Basis. In Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change; Solomon, S., Qin, D., Manning, M., Chen, Z., Marquis, M., Averyt, K.B., Tignor, M., Miller, H.L., Eds.; Cambridge University Press, Cambridge, United Kingdom and New York, NY, USA, 2007, pp. 996.
- Peyronnet, A.; Friedland, K.D.; Maoileidigh, N.O.; Manning, M.; Poole, W.R. Links between patterns of marine growth and survival of Atlantic salmon Salmo salar, L. J. Fish Biol. 2007, 71, 684–700. [Google Scholar] [CrossRef]
- Todd, C. D.; Hughes, S. L.; Marshall, T.; MacLean, J. C.; Lonergan, M. E.; Biow, E. M. Detrimental effects of recent ocean surface warming on growth condition of Atlantic salmon. Glob. Chang. Biol. 2008, 14, 1–13. [Google Scholar] [CrossRef]
- Edwards, M.; Beaugrand, G.; Hays, G.C.; Koslow, J.A.; Richardson, A.J. Multi-decadal oceanic ecological datasets and their application in marine policy and management. Trends Ecol. Evol. 2010, 25, 602–610. [Google Scholar] [CrossRef]
- Malick, M.J.; Cox, S.P.; Mueter, F.J.; Peterman, R.M.; Bradford, M. Linking phytoplankton phenology to salmon productivity along a north–south gradient in the Northeast Pacific Ocean. Can. J. Fish. Aquat. Sci. 2015, 72, 697–708. [Google Scholar] [CrossRef]
- Parmesan, C.; Yohe, G. A globally coherent fingerprint of climate change impacts across natural systems. Nature 2003, 421, 37–42. [Google Scholar] [CrossRef]
- Cushing, D.H. Plankton Production and Year-class Strength in Fish Populations: An Update of the Match/Mismatch Hypothesis. Adv. Mar. Biol. 1990, 26, 249–293. [Google Scholar]
- Palm, S.; Romakkaniemi, A.; Dannewitz, J.; Pakarinen, T.; Veneranta, L.; Huusko, R.; Isometsä, K.; Broman, A.; Miettinen, A. Tornionjoen lohi-, meritaimen- ja vaellussiikakannat – yhteinen ruotsalais-suomalainen biologinen selvitys sopivien kalastussääntöjen arvioimiseksi vuodelle 2023. Sveriges lantbruksuniversitet and Luonnonvarakeskus. Helsinki, Finland, 2023, pp. 62.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




