1. Introduction
The sodium-potassium pump, also called Na+/K+ ATPase, is a protein involved in the active transport of ions across the cellular membrane, which ensures the expulsion of three Na+ ions and the internalization of two K+ ions for each molecule of ATP that is enzymatically cleaved. The transmembrane ionic passage mediated by the Na+/K+ pump results in an unequal distribution of cations between the intra- and extracellular sectors, and thus contributes to the maintenance of the relative electronegativity of the intracellular sector, the resting transmembrane electrochemical gradient, as well as the osmolarity of the intracellular and interstitial environment. In addition to the activity of ion transporter, the Na+/K+ ATPase interacts with a number of membrane and intracytoplasmic proteins, intervening in the processes of growth, cell differentiation or apoptosis.
From a structural point of view, the Na
+/K
+ pump is a transmembrane enzyme consisting of an alpha subunit with catalytic role, a glycosylated beta subunit, with the role of assembly and anchoring of polypeptide chains at the membrane level, and a tissue-specific FXYD subunit. Each subunit has a number of isoforms, suggesting differences in the activity and role of isoenzymes across tissues and membrane domains [
1].
It is interesting to note that the Na
+/K
+ pump is also expressed differently in tumor cells. In the case of glioblastoma [
2], clear cell renal cell carcinoma or breast cancer [
3], an alteration of expression was observed both quantitatively- increased expression, and qualitatively-variable expression of isoforms.
Another aspect observed in clear cell carcinoma is the under-expression of the beta-1 subunit [
4]. This results in a defect in the implantation of the pump at the membrane and indirectly in a reduction of the transport activity, similar to a drug blockage. One of the effects of the reduced activity of the pump is the under-expression of E-cadherins, proteins involved in the formation of tight intercellular junctions which favor the process of tumor invasion [
5]. Not only the tumor cells are expressing Na+/K+ pump, but also the cells from the tumor microenvironment, which form a metabolically active component of the tumor stroma and shapes the immune response in cancer [
6,
7,
8,
9].
Cardiotonic glycosides are molecules that block the Na
+/K
+ pump by interacting with the alpha subunit. This effect has been exploited in the development of antiarrhythmic medication or for heart failure. Following the blockade of Na
+/K
+ ATPase, the intracytoplasmic Na+ concentration remains increased, secondarily blocking the activity of the Na
+/Ca
2+ ion exchanger, increasing intracellular Ca2+ concentration and thus, myocardial contraction force [
10,
11].
Ouabain is a cardiotonic glycoside that, by inhibiting the Na
+/K
+ pump, interferes with cell processes mediated directly by the pump, as well as indirectly, by modulating calcium-dependent signaling [
12]. Secondary to intracytoplasmic Ca2+ increase, cascade activation of calmodulin, calmodulin kinase, and Cdc25 protein occurs, which, when translocated to the nuclear level, inhibits cyclin-dependent kinase 1 and cyclin B. Blocking cyclin B results in cell cycle arrest and reduced counts of cells with mitotic potential. In addition, a reduction in cyclin D levels was observed, resulting in a blockade of the cell cycle in the G0 / G1 phase.
The effect of Ouabain on the calmodulin-calcineurin-NFAT-IL-2 pathway resulting in stimulation of calcineurin activity results in increased production of IL-2, a cytokine that stimulates lymphocyte proliferation. Thus, in the tumor microenvironment, Ouabain can promote antitumor immune defense. On the other hand, in Tregs, a subpopulation of CD4 + lymphocytes with an important role in immune tolerance, Da Silva et al. demonstrate that Ouabain reduces the synthesis of IL-2 at the lymphocyte level and consequently decreases the number of T lymphocytes. The beneficial effect of Ouabain, in this case, is that, by reducing the number of regulatory T lymphocytes, it unblocks the immune system [
13].
In addition to ion transporter activity, the Na
+/K
+ pump is also involved in ion-independent cell signaling processes [
14,
15,
16,
17]. One of the most important interactions of the pump is between the alpha-1 chain and the Src protein. This interaction keeps Src in an inactive state and implicitly inhibits any Src-dependent process [
3]. Once ATPase is blocked, Src dissociates and becomes active. Following this activation, Src influences the dimerization of receptors for growth factors, especially EGFR and the activation of Ras and JAK-STAT signaling pathways, involved in the processes of growth, cell differentiation, angiogenesis, migration, adhesion and invasion [
17,
18].
Activation of growth factor receptors (EGFRs) also leads to the generation of reactive oxygen species. Compensator, increases the expression of proteins involved in DNA repair and cell cycle blockade (p53, H2AX, ATM), proteins associated with ER stress (Grp78, ATF6-beta, p-PERK, eIF2A, GADD153) [
17], as well as anti-oxidant (glutathione peroxidase, catalase, superoxide dismutase). NKA inhibits the p38 pathway, a signaling process involved in the rearrangement of the actin cytoskeleton and cell motility. Thus, by inhibiting NKA, Ouabain cancels migration and tumor invasion [
19].
In view of expanding the oncological therapeutic arsenal, this study aims to provide further insights into the effect that blocking the Na+/K+ pump using Ouabain would have in the tumor microenvironment, by investigating changes in the proliferation, adhesion and expression of phenotypic and molecular markers in normal, mesenchymal stem cells (MSCs), tumor-associated fibroblasts (TAFs) and tumor cells (SK-BR-3 breast cancer cell line).
2. Materials and Methods
2.1. Cell Cultures, Reagents and Solutions
SK-BR3 breast cancer cell line was purchased from American Type Culture Collection (ATCC) and further expanded in McCoy’s 5A medium (Gibco BRL, Invitrogen, Carlsbad, CA, USA), supplemented with 10% fetal calf serum (FCS, PromoCell, Heidelberg, Germany) and 1% Penicillin/Streptomycin solution (Pen/Strep, 10,000 IU/ml; PromoCell).
Human mesenchymal stem cells (hMSCs) were purchased from Lonza (Basel, Switzerland), and further cultured and expanded in alpha-minimum essential medium (MEM, Gibco BRL) containing 10% FCS (PromoCell) and 1% Pen/Strep mixture (PromoCell).
Human tumor-associated fibroblasts (TAFs) were accessed from Oncogen biobank, being previously isolated using the method described by Paunescu
et al. [
20]. Briefly, tissue-dissociated cells were washed several times with phosphate-buffered saline (PBS, Gibco) solution, were passed through 0.70/0.40 μm strainer filters, and were plated as single-cell suspension in adherent plastic culture plates, further expanded in Dulbecco’s Modified Eagle Medium (DMEM; Sigma-Aldrich) supplemented with 10% FCS (PromoCell) and 2% Pen/Strep solution (PromoCell).
All cellular types were grown at 37oC, in humid atmosphere containing 5% CO2. Medium replacement was performed every 2-3 days until reaching 80-90% confluence, when cells were detached from the culture flasks using 0.25% Trypsin-EDTA solution (Sigma Aldrich Company, Ayrshire, UK) followed by centrifugation (10 minutes, 300 x g) and re-plated in appropriate culture flasks at a density of 10,000 cells/cm2.
Ouabain octahydrate was purchased from Sigma-Aldrich Company as > 95% (HPLC) powder and a stock solution of 12 x 10-5 M was prepared using PBS (Gibco). The concentrations of 10-5 M, 10-6 M, 10-8 M, 10-9 M were obtained in culture media specific for each cellular type, and were calculated based on the minimum and maximum concentrations reached in biodistribution studies, so that the volume of Ouabain solution (in PBS) will not influence the composition of nutrients in the culture media.
2.2. Immunocytochemistry
Immunocytochemistry was performed for MSCs, TAFs and SK-BR3 cell lines. Cells prepared for these analyses were grown in 4-well chamber slides for 24 hours (control) or for additional 24 hours with different Ouabain concentration in the culture media; cells were washed, fixed with 4% paraformaldehyde and permeabilized with 0.1% Triton X-100 and then investigated for expression of the proteins of interest, by labeling them with the following antibodies: monoclonal mouse anti-swine Vimentin (clone V9), monoclonal anti-human endoglin, CD105 (clone SN6h), monoclonal mouse anti-human vascular endothelial growth factor, VEGF (clone VG1), monoclonal mouse anti-human Ki67 (clone MIB-1), polyclonal rabbit anti-human CD117, polyclonal rabbit anti-human c-erbB-2 oncoprotein. These antibodies were provided by DakoCytomation (Glostrup, Denmark) and tested for human specificity and cross-reactivity. For identification of Na+/K+ pump subunits, we used Sodium Potassium ATP-ase Alpha 1, Alpha 2 and Beta 1 Antibodies (Novus Biological). Staining protocol continued with secondary biotinylated antibody binding, substrate addition, and hematoxylin counterstaining of the nuclei (LSAB2 System-HRP and Envision kit, Dako) following the manufacturer procedures.
2.3. Flowcytometry
MSCs and TAFs were detached from the culture flasks using 0.25% Trypsin-EDTA (Sigma), washed twice with PBS, resuspended in 100 µL PBS at a concentration of 105 cells/ml and incubated in the dark at room temperature for 30 minutes with mouse anti-human fluorochrome-conjugated antibody at a dilution specified in manufacturer's protocol. Cells were then washed twice with 1 ml Cell Wash Solution (BD Biosciences, San Jose, CA, USA) each and resuspended in 500 μl of the same solution for further analysis on a four-color capable FACSCalibur (Becton-Dickinson) flow-cytometer. Antibodies panel used for these cellular types included PE-conjugated CD29 and CD73, as well as FITC-conjugated CD90. Acquisition and data analyses were performed using CellQuest software (Becton-Dickinson). SK-BR3 cells were submitted to the same procedures, using an antibodies panel including anti-Her2-FITC, CD29-PE and VEGF-R2 (Kdr)-PE. Flowcytometric analyses were performed for control cells and for cells 24-hours treated with different Ouabain concentrations (10-5 M, 10-6 M, 10-8 M, 10-9 M).
2.4. Annexin V/PI Assay
Annexin V-FITC (Miltenyi Biotec, Gladbach, Germany) was used in cell death flowcytometric studies (apoptosis) combined with Propidium Iodide Staining Solution (BD Biosciences, San Jose, CA, USA) following the manufacturer protocol. Shortly, 106 cells were washed in 1 x Annexin V Binding Buffer (BD Pharmigen) and centrifuged at 300 x g for 10 minutes, resuspended in the same solution and incubated with 10 μl of Annexin V-FITC for 15 minutes in the dark. Washing the cells with 1 ml specific binding buffer and centrifugation were the next steps in the procedure, and the cell pellet was resuspended in 500 μl binding buffer while 1 μg/ml of PI solution was added immediately prior to analysis by flow cytometry. All cellular types were analyzed as control and 24-hours after adding Ouabain solution at concentrations of 10-5 M, 10-6M, 10-8 M, and 10-9 M.
2.5. Cell Cycle Test
We used the CycleTESTTM PLUS DNA Reagent Kit (BD) which provides a set of reagents for nuclear isolation and labeling from cell suspensions. In this study, cell cycle testing was required to identify the phases in which cells are affected by 24 hours exposure to different Ouabain concentrations (10-5 M, 10-6M, 10-8 M, and 10-9 M). This method involves the dissolution of lipids from cell membranes with the help of non-ionic detergents, the elimination of the cytoskeleton and nuclear proteins using trypsin, and chromatin is stabilization with spermine. Propidium iodide (PI) is bound stoichiometrically to the isolated nuclei, the samples being acquired by flowcytometer. Nuclei labeled with PI emit fluorescence at wavelengths between 580 and 650 nm. The FL2 detector of the flow cytometer (FACSCalibur, BD) is used to detect the fluorescence emission at wavelength between 564 and 606 by the labeled cells. Solution A - trypsin/spermine tetrachloride; addition of 250 μl and incubation for 10 minutes at room temperature; Solution B - trypsin inhibitor to prevent RNA digestion; addition of 200 μl and incubation for 10 minutes at room temperature; Solution C – propidium iodide, binds to DNA; addition of 200 μl followed by flowcytometric analysis.
2.6. Flowchamber Assay
6-channels μ-Slide VI ibiTreat flowchamber (Ibidi Integrated BioDiagnostics, Munich, Germany) were coated with VCAM-1 (vascular cell adhesion protein-1), at a concentration of 2 μg/ml, 30 μl/channel, 15 minutes before the experiment. Untreated and 24 hours Ouabain-treated SK-BR3, TAFs, and MSCs were suspended in HBSS medium (Hank’s Balanced Salt Solution, Gibco), 105 cells/100 μl medium/channel in order to test their capacity to adhere at the VCAM-1 substrate, under progressively increasing shear stress of 0.35, 2, 5, 8 and 15 dynes/cm2 generated using ISMATEC pump - IPC High Precision Multichannel Dispenser (IDEX Corporation, Glattburgg, Switzerland). Pictures of the centered microscopic field were taken every 30 seconds, for every value of shear stress, and total cell count was compared with the control. Variations of at least 15% in cell count were considered significant when compared with control cells for the same values of the shear stress.
2.7. RNA Extraction and RT-PCR
Total RNA was extracted with TRIzol reagent (Invitrogen) following the supplier’s instructions. RNA concentration was determined with the ND-1000 spectrophotometer (Wilmington, DE, USA). We used 0.5 μg total RNA for each reverse transcription reaction performed with the AccuScript High Fidelity 1st Strand cDNA Synthesis Kit (Stratagene, Agilent Technologies, USA). cDNA samples were analyzed by quantitative real-time PCR, using the LightCycler 480 SYBR Green I Master (Roche, Florence, SC, USA) and the following primers: Na/K-ATPase alpha 1 forward 5’-AAAAACATGGTCCCTCAGCAA-3’; reverse 5’-CCACAACTTCCTCCGCATTT-3’ (NM_000701.7 (tr. Var 1)) (76 bp); Na/K-ATPase alpha 2 forward 5’-GAATGAGAGGCTCATCAGCATG-3’; reverse 5’-CAAAGTAGGTGAAGAAGCCACCC-3’ (NM_000702.3) (77 bp); Na/K-ATPase alpha 3 forward 5’-AATGCCTACCTTGAGCTCGG-3’; reverse 5’-CTCGGGCAGGTAATAATGGC-3’ (NM_152296.3) (69); Na/K-ATPase alpha 4 forward 5’-GATGATCACAAATTAACCTTGGAAGA-3’; reverse 5’-TTTGCCCTTTGGTGGCTATG-3’ (NM_144699.3 (tr. Var 1) ) (83 bp); Na/K-ATPase beta 1 forward 5’-TCAGTGAATTTAAGCCCACATATCA-3’; reverse 5’-CTTCTGGATCTGAGGAATCTGTGTT-3’ (NM_001677.3) (74 bp); Na/K-ATPase beta 2 forward 5’-CCAGCATGTTCAGAAGCTCAAC-3’; reverse 5’-GCGGCAGACATCATTCTTTTG-3’ (NM_001678.3) (79 bp); Na/K-ATPase beta 3 forward 5’-CTGGCCGAGTGGAAGCTC-3’; reverse 5’-GGTGCGCCCCAGGAA-3’ (NM_001679.2) (60 bp).
HPRT1 was chosen as a suitable reference gene. We performed a relative basic quantitation based on the ΔΔCt method with the LightCycler480 Software (Roche). Quantitative analysis of gene expression was performed for α1, α2 and β1 subunits. For the other subunits, only semi-quantitative analysis was performed by RT-PCR, followed by visualization in 1.5% agarose gel.
2.8. Electron Microscopy
Scanning electron microscopy (SEM) was performed for identification of Ouabain-induced morphological changes in MSCs, TAFs and SK-BR3 cell line. Cells were cultured at 10,000 cells/cm2 in 24 well format cell culture inserts (BD Labware Europe, Le Pont De Claix, France) and Ouabain was added in concentrations of 10-5 M, 10-6M, 10-8 M, and 10-9 M. After 24 hours cells were pre-fixed for 1 hour with 2.5% buffered glutaraldehyde (in PBS), rinsed three times in PBS, and the 0.4 μm pore-sized membranes were detached from the culture inserts. Better image quality was obtained after the cells were sputter-coated with platinum-palladium; SEM analysis was performed with FEI Quanta 3D FEG electron microscope (FEI Company, Eindhoven, The Netherlands).
3. Results
3.1. Morphological Changes of Ouabain-Treated Cells
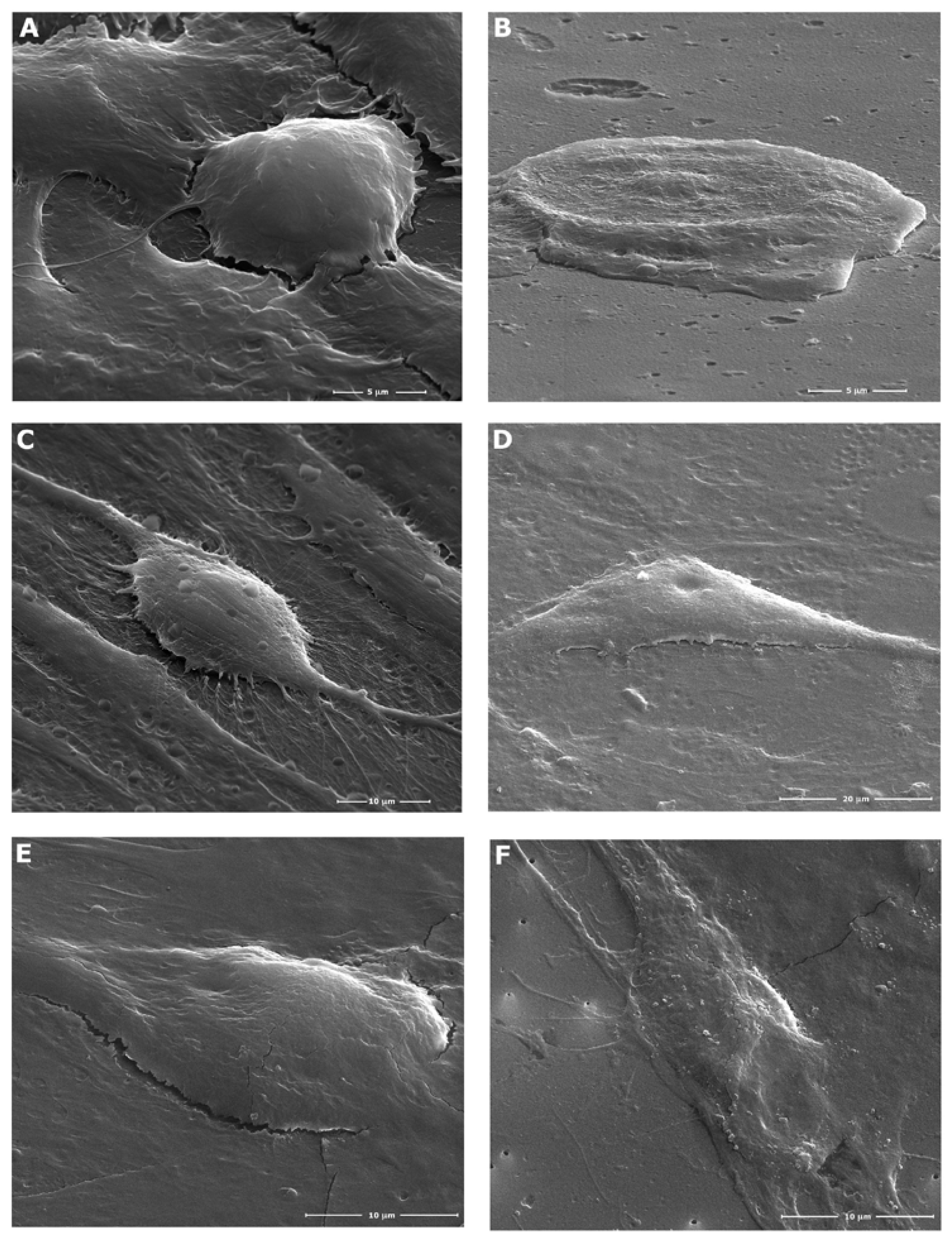
Scanning electron microscopy (SEM) showed that cells that survive the action of Ouabain in a concentration of 10
-5 M or 10
-6 M become more adherent to the culture plate, acquiring characteristics of “ghost cells”, such as SK-BR3 cells (
Figure 1 A and B). MSCs become flattened, enlarge their cytoplasmic content, and the nucleus changes to indented shape (
Figure 1. C and D). TAFs undergo minor morphological changes under the action of 10
-6 M Ouabain solution for 24 hours (
Figure 1. E and F).
3.2. Immunophenotypic Changes Induced by Ouabain in SK-BR-3 Cells, MSCs, and TAFs
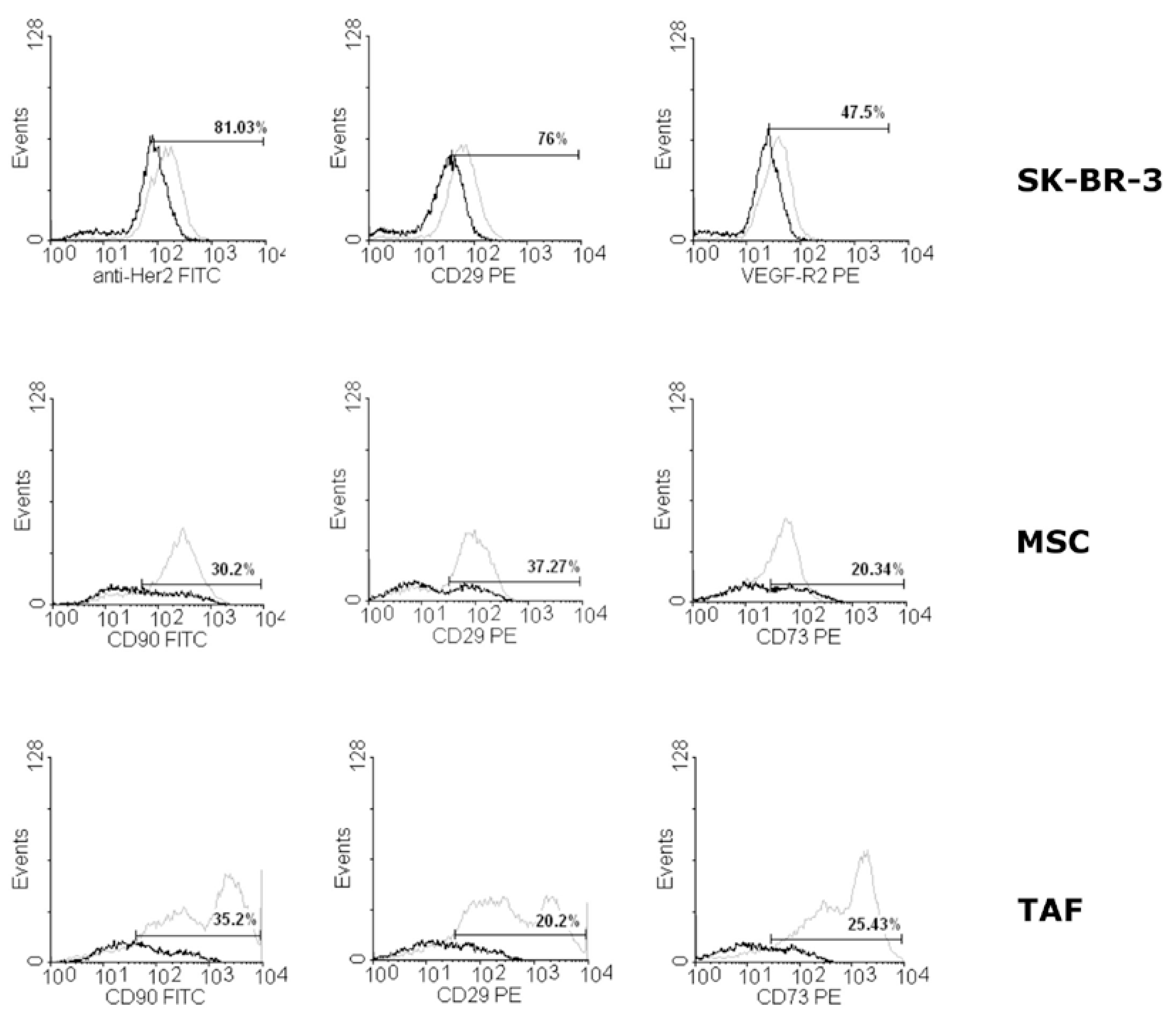
Flowcytometric evaluation of Ouabain-induced changes in surface markers expression was performed for all Ouabain concentrations, 24 hours after addition in the culture media. For SK-BR-3 cells, the decrease in surface markers expression is shown for CD29 adhesion molecule (
Figure 2), while Her2 and VEGF-R2 presented insignificant variation compared to control, untreated cells. The MSCs and TAFs are influenced more profoundly at the level of CD90, CD29 and CD73 surface characteristic markers, the significant decrease being dose-dependent, to the lowest level of 30.2% and 35.2%, 37.27% and 26.2%, and 20.24% and 25.43%, respectively to the markers and cellular type, at the Ouabain concentration of 10
-6 M (
Figure 2).
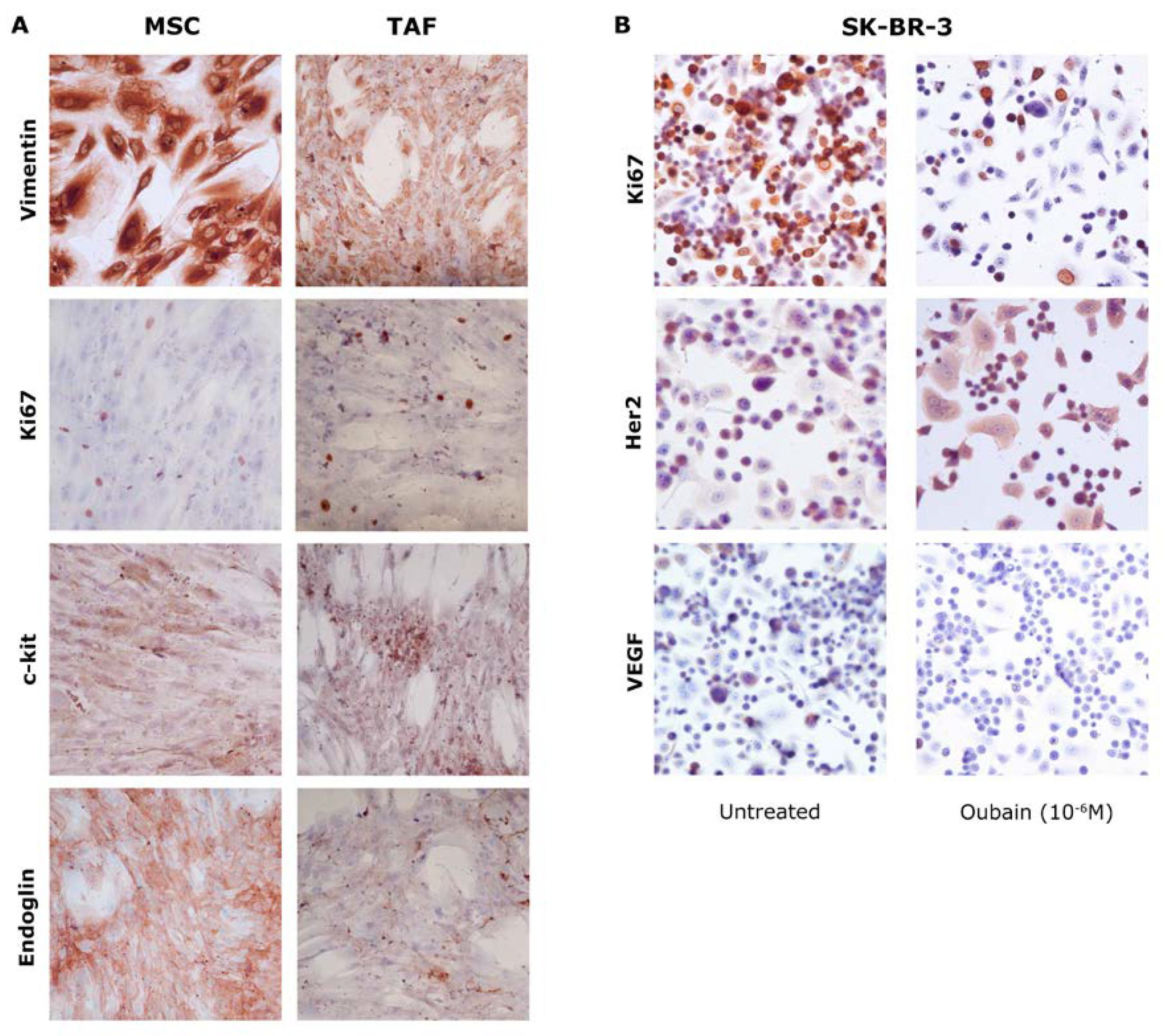
24h after the addition to the culture medium of 10-6 M Ouabain, the modification Vimentin, Ki67, c-kit, Endoglin (CD105) expression in MSCs and TAFs was monitored by immunocytochemistry. A decrease in the proliferation rate of both MSCs and TAFs was found, highlighted by the decrease in the expression of the nuclear proliferation marker Ki67, which was present in only 25% of the cells subjected to the action of the Na+/K+ pump blocker compared to the control. The other markers did not present significant variations in expression levels in MSCs or TAFs.
The average of the Ki67 values was 61.68% for the untreated SK-BR-3 cells, while in the Ouabain treated cells Ki67 was expressed only in 19.31%, thus suggesting that Na+/K+ pump blocker in concentration of 10-6 M has an important antiproliferative effect on SK-BR-3 tumor cells. Also, the specific marker of this cell line, Her2, shows a decrease in expression in cells treated with Ouabain, while the secretion of VEGF in these cells is inhibited.
Immunocytochemistry demonstrated the major influence that Ouabain exerts on all cellular types, depending on the concentration, inducing the decrease in the expression of characteristic cell markers and cell proliferation (
Figure 3).
3.3. Effect of Ouabain on Cellular Viability and Cell Cycle
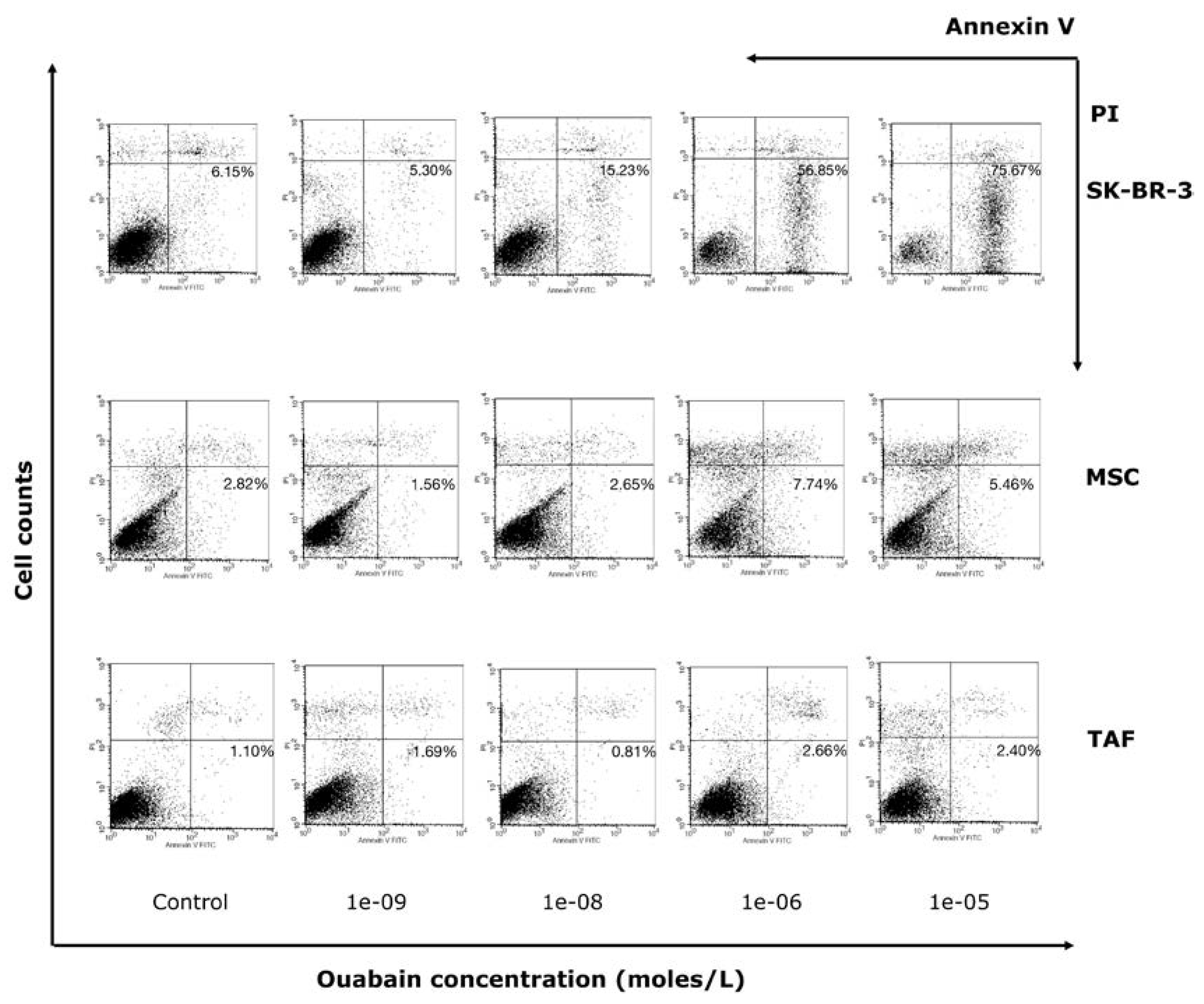
Following flow-cytometric analysis with Annexin V and PI, an increase in the percentage of apoptotic SK-BR-3 cells (positive for Annexin V, negative for propidium iodide) was observed, ranging from 6.15% (control group) to 75 .67% (cells exposed to 10-5 M Ouabain, for 24h). The increase in the number of apoptotic cells occurs in a dose-dependent manner, suggesting that Ouabain exerts a pro-apoptotic effect on SK-BR-3 tumor cells (
Figure 4).
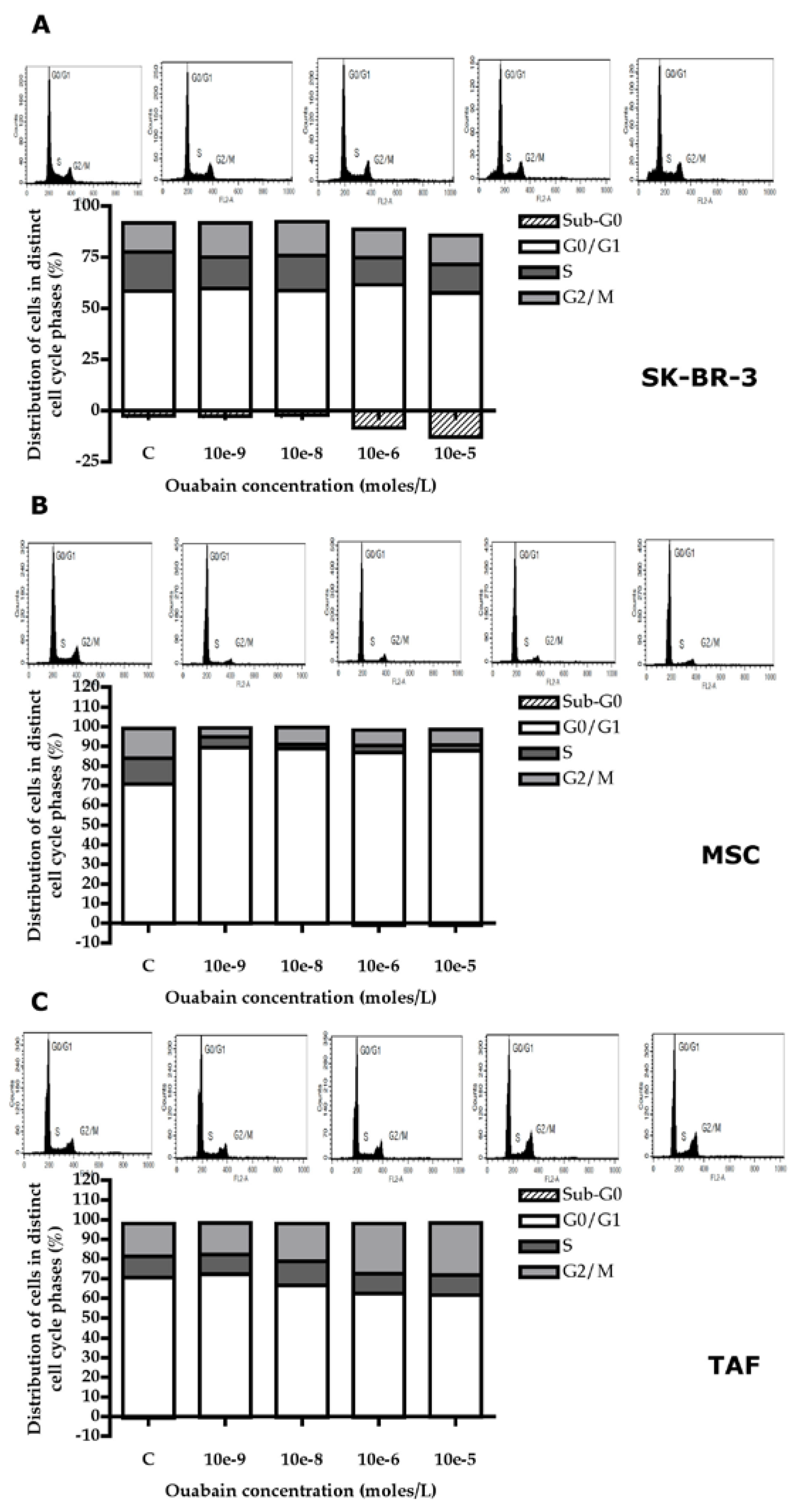
Ouabain induced a concentration-dependent enhancement of the apoptosis of MSCs, with the concentration of 10-6 M having the most significant effect (7.74%). On TAFs, Ouabain induces less significant changes in viability compared to MSCs. Apoptotic cells show an increase depending on the concentration of Ouabain, but the effects are less severe, the maximum percentage of dead cells being in this case only 2.66%, for the concentration of 10-6 M. Following addition of different Ouabain concentrations, an increase in the level of pre-apoptotic SK-BR-3 cells (in the G0 phase) and a reduction in the number of cells in the synthesis phase (S) was detected. The percentage of cells in the G0/G1 and G2/M phases remained relatively constant. An interesting aspect was that 10% - 15% of the SK-BR-3 cells, when treated with high Na+/K+ pump blocker concentrations (10-6 M, 10-5 M), were arrested in a sub-G0 phase, when cells exit the cell cycle.
While MSCs are arrested in the G0/G1 phase of the cell cycle, at different concentrations of Ouabain, Na+/K+ pump blocker exerted minimal influence on TAFs. The data obtained are correlated with the viability/apoptosis test performed under the same working conditions (
Figure 5).
3.4. Functional Studies – Flowchamber Assay
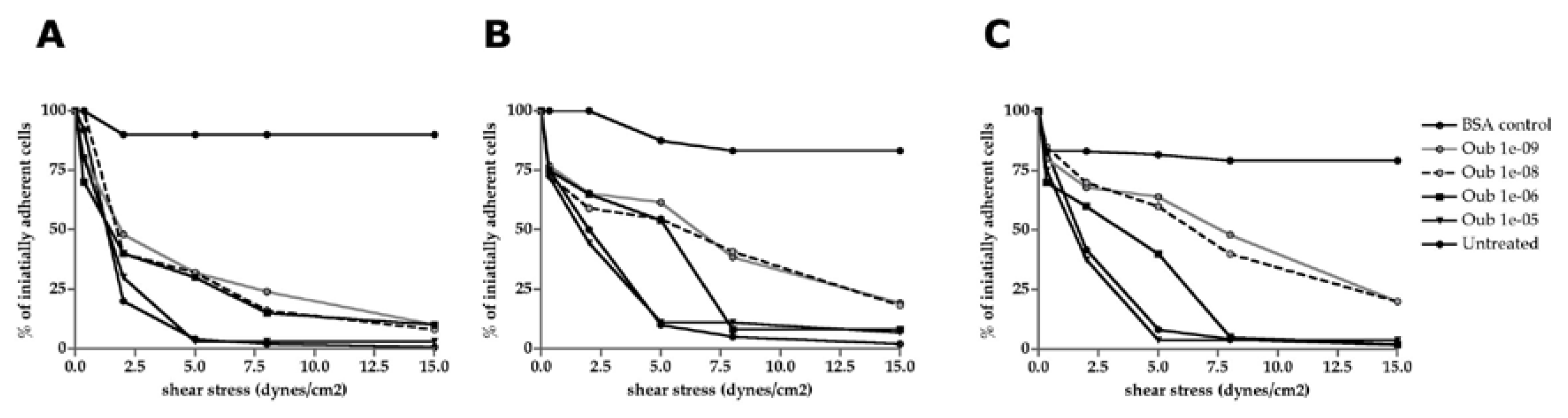
After treating SK-BR-3 cells with different concentrations of Ouabain (10-5 M, 10-6M, 10-8 M, and 10-9 M), placing them in the VCAM-1 coated flowchamber, and applying increasing shear stresses from 0.35 dyne/cm2, there was a significant decrease (>15%) of cell adhesion at a shear stress of 0.35 dyne/cm2, from 98.37% (control) to 41.42%, detected in cells treated with Ouabain in a concentration of 10-8 M. Also, there was significant reduction (>15%) of adherent cells treated with 10-5 M and 10-8 M Ouabain compared to the control, following exposure to shear stresses of 2.5, 8 and 15 dynes/cm2.
The ability to adhere to the VCAM-1 substrate under increasing shear stresses is similar for both MSCs and TAFs, decreasing significantly for all shear stresses applied, but only for the cells treated with Ouabain in high concentrations (10-5 M and 10-6 M).
These observations suggest the existence of an ouabain-mediated process by which tumor cells reduce the number of protein interactions with the VCAM-1 molecules lining the flow chamber. Reduced protein interactions with VCAM-1 may be attributed to altered expression of adhesion molecules or a cytoskeletal change with an effect on cell geometry (
Figure 6).
3.5. Effects of Ouabain on Na+/K+ Pump Subunits
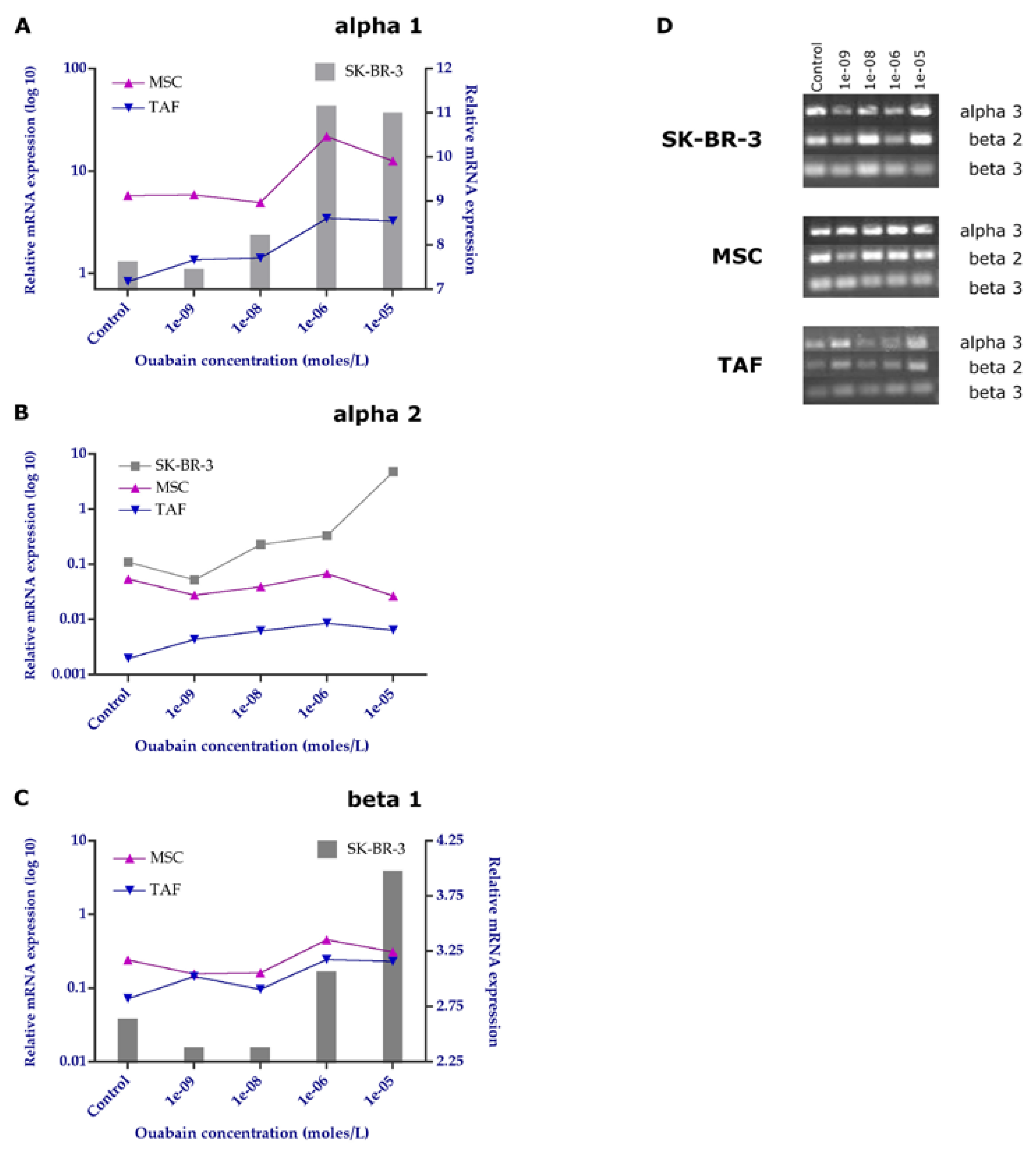
HPRT1 was used as a reference gene and gene expression analysis was performed using relative quantification based on the ΔΔCt method, using LightCycler480 Software (Roche). Quantitative analysis of gene expression was performed for α1, α2 and β1 subunits. The results obtained reveal major changes induced by the addition of Ouabain in the culture medium in gene expression of the Na
+/K
+ pump subunits. Thus, the α1 subunit shows a significant increase at concentrations of 10
-9 and 10
-8 M of Ouabain, after which a constant expression phase appears for concentrations of 10
-6 and 10
-5 M. MSCs show the highest expression for α1 subunit of Na
+/K
+ pump for the, but at very high concentrations of Ouabain (10
-5 M) its expression decreases, compared to the values determined in SK-BR-3 cells (
Figure 7A).
The β1 subunit shows an increased gene expression for Ouabain concentrations between 10-9 and 10-6 M at the level of MSCs and TAFs, but when Ouabain is added to the culture medium at a concentration of 10-5 M, its expression decreases, showing comparable values for all cell types analyzed.
Expression of the α1 subunit of the Na+/K+ pump is basally elevated in SK-BR-3 tumor cells (1.8-fold higher compared to the reference gene HPRT1). The expression plot of the α1 subunit of the Na+/K+ pump describes a parabola with the minimum point located at the level of Ouabain concentration of 10-8 M Expression of the α2 subunit is elevated basally in SK-BR-3 tumor cells compared to HPRT1 (2.3-fold). Different concentrations of ouabain have a different behavior on the expression of the α2 subunit of the Na+/K+ pump. After exposure for 24 h to concentrations of 10-9 M and 10-6 M of Ouabain respectively, a decrease in the expression of the α2 gene is observed 0.66-fold and 0.88-fold over HPRT1, respectively. Exposure of SK-BR3 cells to concentrations of 10-8 M and 10-5 M, respectively, increased α2 subunit expression by 2.23-fold and 4.89-fold, respectively, over HPRT1 expression.
Expression of the β1 subunit is basally elevated 12.17-fold relative to the reference gene HPRT1 and declines steeply following ouabain exposure. There are no significant differences in β1 gene expression between groups treated with increasing concentrations of ouabain. Knowing that the β1 subunit of the Na+/K+ pump ensures the implantation of the Na+/K+ ATPase complex at the membrane level, downregulation of this subunit may result in a reduction in the number of Na+/K+ pumps present at the plasmalemmal level.
Agarose gel vizualization of the other subunits and semi-quantitative analysis showed that MSCs have the highest stability of gene expression for α3, β2 and β3 subunits, while TAFs express these subunits in low quantity, being also downregulated when Ouabain is added to the culture medium in progressively increasing concentrations (
Figure 7B).
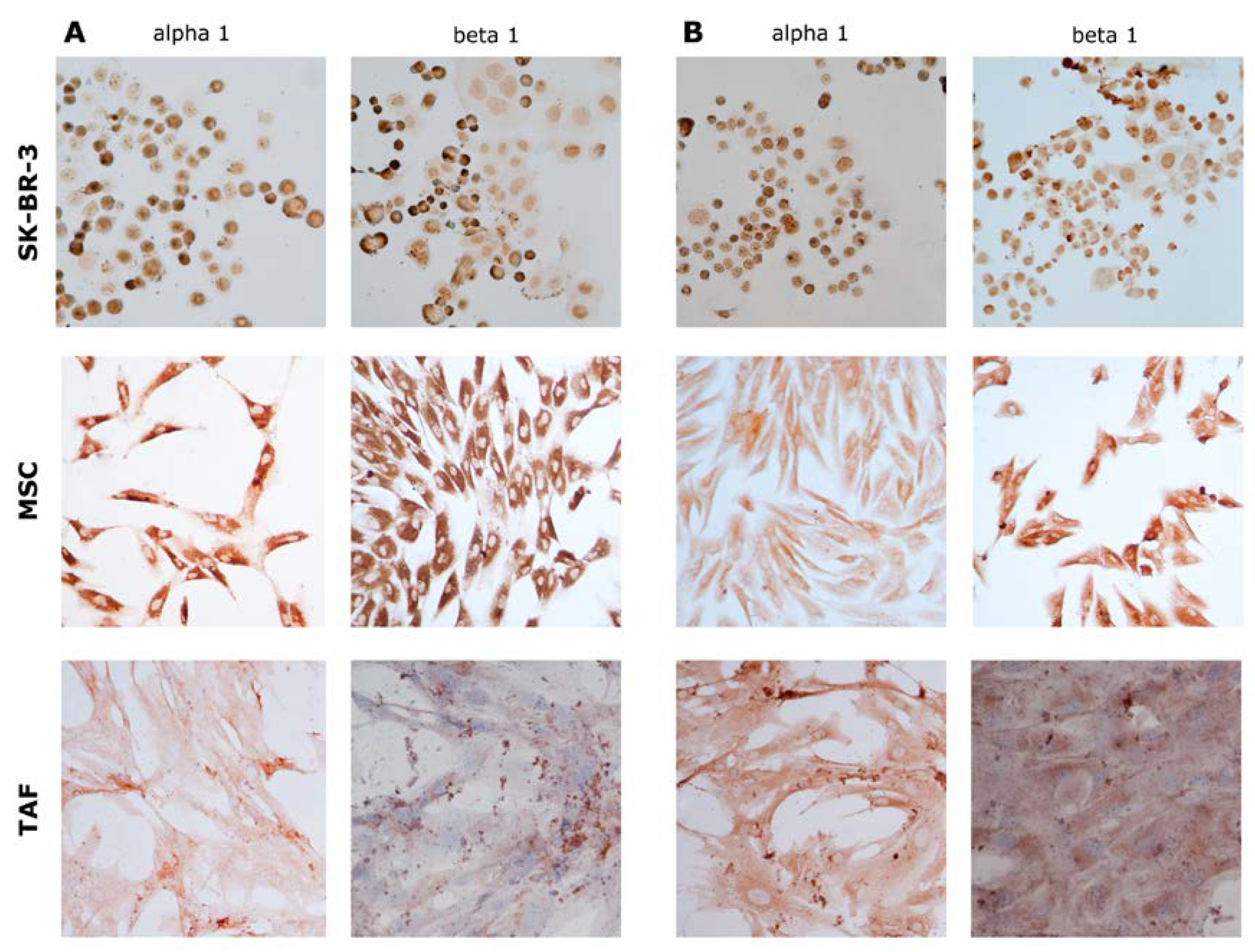
At the protein level, the expression of α1 and beta 1 subunits shows the same expression pattern, highlighted in gene determinations (
Figure 8).
4. Discussion
The oncological therapeutic arsenal is constantly expanding, but still remains incomplete. This present gap is explained by the incomplete knowledge of all the mechanisms underlying tumorigenesis, tumor invasion and metastasis. Cardiotonic glycosides, such as Ouabain, are characterized by the ability to block the ion pump activity of Na
+/K
+ ATP-ase, whose varied roles in cell growth, proliferation, differentiation and adhesion have recently been described, consequently inducing various changes in the cellular phenotype, from the expression of structural proteins, such as adhesion molecules or cytoskeletal proteins, to that of functional proteins involved in cell proliferation and metabolism [
21,
22].
Variable expression of α1 and α2 subunits of the Na+/K+ pump, accompanied by a marked decrease in β1 subunit expression results in an intracytoplasmic increase in the number of NKA α subunits and a reduction in the number of membrane-implanted pumps.
The different patterns of α1 expression we observed can be attributed to a dose-dependent effect of the concentrations of ouabain employed in the study: nanomolar concentrations (10-9 M, 10-8 M) do not inhibit ion pump activity, but may disrupt signaling pathways mediated by the α-1 subunit; micromolar concentrations (10-6 M, 10-5 M) inhibit ion pump activity, generating a low intracellular K+ concentration. This, in turn, will result in the stimulation of the transcription of the gene encoding the α1 subunit, restoring the balance at the cellular level.
The α2 subunit is not normally expressed in mammary epithelial cells – it is preferentially expressed in neurons. This may reflect the ectodermal origin of mammary epithelial cells, as well as the dedifferentiation and aggressiveness of the SK-BR-3 cell line. The β1 subunit is a glycoprotein that ensures the implantation of the Na+/K+ ATP-ase complex in the cell membrane. Its overexpression was observed in the control SK-BR3 cell group. Considering the fact that the Na+/K+ pump present at the membrane level is crucial for the secondary active transport, responsible for the internalization of nutrients, the overexpression of the β1 subunit ensures the supply of nutritional principles necessary for tumor proliferation.
In addition to its metabolic role, the β1 subunit of NKA is also involved in the formation of intercellular junctions. α1-β1 NKA dimers ensure, through the β1 component, the extracellular interaction with adhesion molecules belonging to other cells and through the α1 component the interaction with ankyrin-1, a protein that anchors the NKA complex to the actin cytoskeleton [
23].
From the observations made, the under-expression of the β1 subunit gene results in a reduction in the number of intercellular adhesion molecules. Decreased cell adhesion can be attributed to both reduced levels of β1 integrin (CD29) and reduced levels of the β1 subunit of NKA. In the absence of trophic signals received from the neighboring cells, the ouabain-treated cells will undergo apoptosis even at low concentrations that do not disrupt the ion pump activity. Pongrakhananon
et al. [
24] demonstrated, using the H292 cell line, that cell adhesion and migratory capacity were reduced following exposure to varying concentrations of ouabain (2.5-20 pM) for 24h. These observations were supported by the reduction of the migration index relative to the scratch assay, as well as by the reduction of the expression of integrins β1, β3, β4 and β5 [
24].
Reduction of cell viability following Ouabain treatment, as assessed by the Annexin V/PI assay and by immunocytochemistry using the Ki67 index, was observed in all cells, in a concentration-dependent manner. The suppression of ion pump function by Ouabain results in a decrease in the internalization of nutrients necessary to maintain proper cellular metabolism. The blockage of the Na
+/K
+ pump generates a pathological loop whereby the reduction of internalized glucose levels will lead to a depletion of ATP generated at the mitochondrial level and implicitly, a reduction of the Na
+/K
+ pump activity through ATP deficiency. The results obtained are consistent with the experiments performed by Yang
et al. on U-87MG glioma cells [
2], Xiao
et al. on OS-RC2 renal carcinoma cells [
25], Ninsontia
et al. on H460 lung cancer cells [
26], de Souza
et al. on Caco-2 colorectal cancer [
27], Chang
et al. on DU-145 prostate cancer cells [
17] and Khajah
et al. on MCF-7, YS1.2, pII and YS2.5 breast cancer cells [
3], on osteosarcoma and squamous cell carcinoma [
28,
29].
The results of the Annexin V/PI investigation suggest that the majority of cells treated with cardiotonic glycosides do not undergo a process of necrosis, but die by apoptosis. These results support the data obtained by Da Silva
et al. [
13] on murine Tregs lymphocytes, by Chang
et al. [
17] on DU-145 cells and Khajah
et al. [
3] on triple-negative breast cancer cells. At micromolar concentrations, the most likely mechanism of Ouabain-induced programmed cell death is due to energy substrate depletion. However, mitochondria-mediated apoptosis by increasing intracellular Ca
2+ concentration remains a mechanism to be discussed, but requires further investigation [
30,
31,
32].
The cell cycle is influenced by the action of Ouabain. We also observed an increase in the level of pre-apoptotic cells (in the sub-G1 phase) and a reduction in the number of cells in the synthesis phase (S) following exposure to different Ouabain concentrations. These results complement the data published by Chang et al. [
17] on prostate cancer cells describing an increase in the number of cells in the G0/G1 phase and in the pre-apoptotic (sub-G1) phase. The number of cells in S phase, respectively G2/M remained constant [
33]. Hiyoshi
et al. [
34] show a reduction in the number of cells in the G0/G1 phase and an increase in those in the S and G2/M phase following exposure to ouabain, 50 nM, for 48 hours [
34]. Contrary to the above, Khajah
et al. found that exposure for 24h to Ouabain does not significantly change the percentage of cells in the G0/G1 phase [
3].
Malignant SK-BR3 cell phenotype alos changes upon exposure to Na
+/K
+ pump inhibitors. Flowcytometry and immunocytochemistry data describe a reduction in the signal intensity of Ouabain-treated cells for Vimentin, Her2, CD29, VEGF-R2. The reduction of Vimentin expression was also observed by Liu et al. on the lung cancer cell line A549 following exposure for 15 h to Ouabain at 25 nM [
35].
The flow chamber test revealed a reduction in cell adhesion on the VCAM substrate coating the flow-chamber. Decreased interactions between cells and VCAM occurred in a dose-dependent manner with respect to Ouabain concentration and shear stress generated by the peristaltic pump. This observation is also supported by the flowcytometric investigations that revealed a reduction of CD29 (β1 integrin), as well as the studies carried out by Pongrakhananon
et al. [
24] on β integrin levels following Ouabain exposure. Translating these observations into a hypothetical situation of a solid tumor treated with Ouabain, the tumor cells will have difficulties invading the adjacent tissues, and even if they manage to reach the circulation, they will lack the ability to form distant solid tumors.
Figure 1.
SEM images of SK-BR3, MSCs and TAFs, control and 24 hours-treated with 10-6 M Ouabain solution. A. Untreated control SK-BR3 cells; B. Ouabain-treated SK-BR3 cells; C. Untreated control MSCs; D. Ouabain-treated MSCs; E. Untreated control TAFs; F. Ouabain-treated TAFs.
Figure 1.
SEM images of SK-BR3, MSCs and TAFs, control and 24 hours-treated with 10-6 M Ouabain solution. A. Untreated control SK-BR3 cells; B. Ouabain-treated SK-BR3 cells; C. Untreated control MSCs; D. Ouabain-treated MSCs; E. Untreated control TAFs; F. Ouabain-treated TAFs.
Figure 2.
Expression of surface markers on SK-BR-3 cells, MSCs and TAFs treated with 10-6 M concentration of Ouabain for 24 hours. The SK-BR-3 cells present decreased variations in Her2, CD29 and VEGF-R2 expression, while MSCs and TAFs characteristic markers are significantly decreased compared to control cells (CD90, CD29, CD73). The control cells’ expression is represented in the histograms in grey lines, while the Ouabain-treated cells graph is in black.
Figure 2.
Expression of surface markers on SK-BR-3 cells, MSCs and TAFs treated with 10-6 M concentration of Ouabain for 24 hours. The SK-BR-3 cells present decreased variations in Her2, CD29 and VEGF-R2 expression, while MSCs and TAFs characteristic markers are significantly decreased compared to control cells (CD90, CD29, CD73). The control cells’ expression is represented in the histograms in grey lines, while the Ouabain-treated cells graph is in black.
Figure 3.
Immunocytochemical evaluation of MSCs, TAFs and SK-BR-3. A. MSCs and TAFs comparative analysis of characteristic markers - Vimentin, Ki67, c-kit, endoglin (CD105) – 24 hours after addition in the culture media of 10-6 M Ouabain; B. SK-BR-3 cells, untreated and 10-6 M Ouabain-treated, showing decreased expression of proliferation rate (Ki67), Her2 and VEGF markers. Ob. 20x.
Figure 3.
Immunocytochemical evaluation of MSCs, TAFs and SK-BR-3. A. MSCs and TAFs comparative analysis of characteristic markers - Vimentin, Ki67, c-kit, endoglin (CD105) – 24 hours after addition in the culture media of 10-6 M Ouabain; B. SK-BR-3 cells, untreated and 10-6 M Ouabain-treated, showing decreased expression of proliferation rate (Ki67), Her2 and VEGF markers. Ob. 20x.
Figure 4.
Annexin V/PI assay showing Ouabain concentration-dependent apoptosis in SK-BR-3 cells, MSCs and TAFs. The most increased pro-apoptotic effect is revealed on SK-BR-3 cells, MSCs and TAFs being less influenced. An augmented population of MSCs and TAFs is positive for PI in a concentration-dependent manner, which indicates necrosis of these cells.
Figure 4.
Annexin V/PI assay showing Ouabain concentration-dependent apoptosis in SK-BR-3 cells, MSCs and TAFs. The most increased pro-apoptotic effect is revealed on SK-BR-3 cells, MSCs and TAFs being less influenced. An augmented population of MSCs and TAFs is positive for PI in a concentration-dependent manner, which indicates necrosis of these cells.
Figure 5.
Evaluation of cell cycle phases in control and Ouabain-treated cells. All cellular types present a decreased proportion of cells in G0/G1 and S phases of the cell cycle, while there is no significant variation for the G2/M phase between control and progressively increasing Ouabain concentrations. A. SK-BR-3 cell line; B. MSCs; C. TAFs.
Figure 5.
Evaluation of cell cycle phases in control and Ouabain-treated cells. All cellular types present a decreased proportion of cells in G0/G1 and S phases of the cell cycle, while there is no significant variation for the G2/M phase between control and progressively increasing Ouabain concentrations. A. SK-BR-3 cell line; B. MSCs; C. TAFs.
Figure 6.
Shear-stress-dependent functional assay of SK-BR-3, MSCs, and TAFs in flowchamber coated with VCAM-1. A. SK-BR-3 cells; B. MSCs; C. TAFs.
Figure 6.
Shear-stress-dependent functional assay of SK-BR-3, MSCs, and TAFs in flowchamber coated with VCAM-1. A. SK-BR-3 cells; B. MSCs; C. TAFs.
Figure 7.
Comparative expression of Na+/K+ pump subunits in control and Ouabain-treated cells (SK-BR-3, MSCs, and TAFs). A. qRT-PCR for α1 subunit expression; B. Expression of α2 subunit; C. expression of β1 subunit of Na+/K+ pump; D. Agarose gel electrophoresis of RT-PCR amplification products for α3, β2 and β3 subunits of Na+/K+ pump.
Figure 7.
Comparative expression of Na+/K+ pump subunits in control and Ouabain-treated cells (SK-BR-3, MSCs, and TAFs). A. qRT-PCR for α1 subunit expression; B. Expression of α2 subunit; C. expression of β1 subunit of Na+/K+ pump; D. Agarose gel electrophoresis of RT-PCR amplification products for α3, β2 and β3 subunits of Na+/K+ pump.
Figure 8.
Immunocytochemical evaluation of Na+/K+ pump subunits α1 and β1. A. Control, untreated cells showing increased basal expression of α1 and β1 subunits, especially in SK-BR-3 cells and MSCs; B. 10-6 M Ouabain-treated cells. Ob. 20x.
Figure 8.
Immunocytochemical evaluation of Na+/K+ pump subunits α1 and β1. A. Control, untreated cells showing increased basal expression of α1 and β1 subunits, especially in SK-BR-3 cells and MSCs; B. 10-6 M Ouabain-treated cells. Ob. 20x.