Submitted:
29 May 2023
Posted:
30 May 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
1.1. Background on Type 2 Diabetes and Its Treatment Approaches
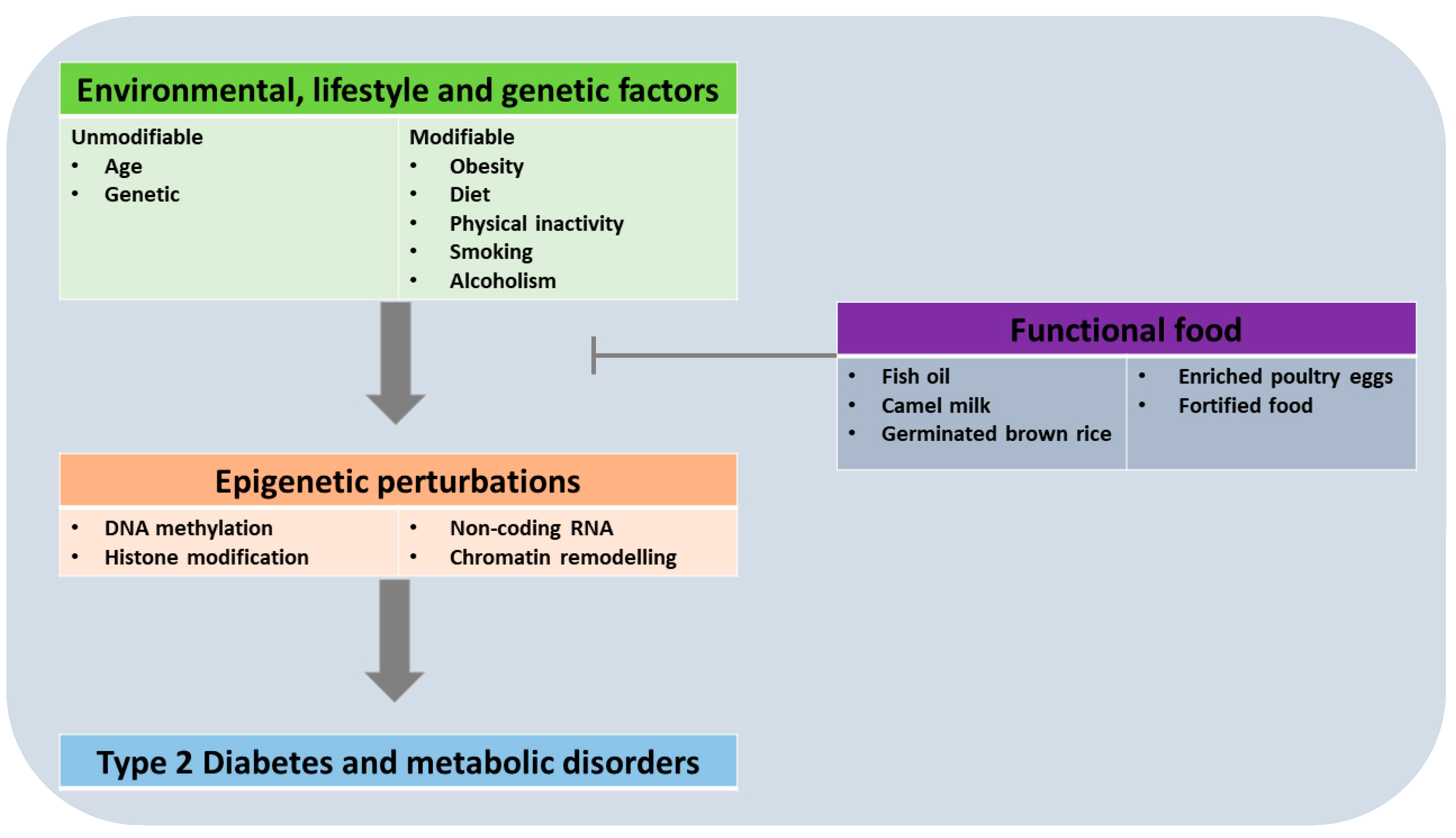
1.2. Dietary Lifestyle as a Risk Factor for Type 2 Diabetes
1.3. The Concept of Epigenetics and Its Role in Chronic Diseases
2. The Role of Epigenetics in T2D
2.1. Overview of Epigenetic Changes Associated with T2D
2.2. Epigenetic Inheritance of T2D
3. Functional Foods and Their Epigenetic Effects
3.1. Health Benefits of Functional Foods against Parentally Triggered Offspring Type 2 Diabetes
3.2. Functional Foods with Preventive Epigenetic Effects in T2D
| Functional Food | Nature of functional food | Bioactive phytochemical/ zoochemical | Study Model | Efficacious levels | Methods | Nature of Antidiabetic Functionality | Mechanistic Basis | Conclusion | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Omega-3 enriched diet | Enriched | Omega-3 (ɷ3) | Offspring of Wistar rat | Efficacy of ɷ3-rich diet extends up to PND90 | Dam; gestation and lactation [control (C: 19% of lipids and ɷ6: ɷ3 = 12), HF (HF: 33% lipids and ɷ6: ɷ3 = 21), or HF enriched with ɷ3 (HFω3: 33% lipids and ɷ6: ɷ3 = 9) diet] | ɷ3 improved glycemic profile (insulin sensitivity, fasting glucose levels) | - | ɷ3-rich diet attenuates or prevents the short-term metabolic disruption elicited by HFD in offspring | [105] |
| Fat diet | Factor out formulation | Perinatal fat restriction | Offspring of mice | Low-fat dietary intervention across gestation and lactation | Dam; gestation or lactation (low and/or high fat diet) Offspring; Glucose tolerance and insulin sensitivity test at 12- and 70-week. |
Perinatal fat restriction provided adequate efficacy to restore insulin sensitivity in aging female progeny. | - | Fat restriction ameliorate glucose dysmetabolism and prevents diabetes | [106] |
| Milk fat | Whole | Milk Fat Globule Membrane (MFGM) | Offspring of C57BL/6 mice | 1,000 mg/kg BW/day MFGM | Dam; 3 weeks pre-gestation + gestation + lactation (high-fat diet (HFD) or a control diet) Pup; lactation (with or without 1,000 mg/kg BW/day MFGM supplementation) |
MFGM ameliorated metabolic disorder and improved glucose tolerance in offspring exposed to maternal HFD in a sex-specific manner. | Sex specific microbiota enrichment in offspring | MFGM is protective against transmitted glucose dysmetabolism | [72] |
| Fish oil food |
Fortified food | n-3 PUFA | Offspring of C57BL6J mice | 30 g FO/kg diet (equivalent to ~ 85–90 mg of FO per day for mice and 10 g per day for humans) | Dam; pre-gestation to lactation [HF diet (45% fat), HF + fish oil (FO - 30 g/kg of diet) and low fat (LF; 10% fat)] F1: after weaning (HF or FO). LF weaned onto LF as control |
FO lowers insulin resistance, reduces glucose intolerance and improves insulin sensitivity. | - | Fish oil improves glucose clearance and insulin sensitivity | [107] |
| Fish oil food |
Fortified food | n-3 PUFA | Offspring of C57BL/6 mice | 12.58 g/kg diet [Fish oil containing 70% (FA) DHA and 10% (FA) EPA in the form of triglyceride (DHA + EPA, 0.68% w/w)] | F0; pregnancy and lactation [control diet (AIN93G), n-3 PUFA-deficient diet and fish oil-contained n-3 PUFA rich diet (DHA + EPA, 0.68%, w/w)] F1; PD21 (high-fat diet or low-fat diet) |
n-3 PUFA deficiency elicits glucose intolerance and insulin resistance in offspring. n-3 PUFA helps ameliorates glucose dysmetabolism. | - | n-3 PUFA could potentially alleviate glucose metabolism disorders by increasing insulin sensitivity, inhibiting gluconeogenesis and promoting glycogenesis | [108] |
| Fish oil food | Fortified food | n-3 PUFA | Offspring of STZ induced GDM Wistar rats | 3rd month to 11th month of age | Dam; 6th day of gestation (STZ 30mk/kg) Offspring of GDM rats: First phase -3 months; standard diet (AIN-93, soybean oil). 2nd phase, 3-11 month; Group1- GDM offspring + AIN-93, soybean oil), Group2- GDM offspring + fish oil (60% n-3 PUFA), and Group3; GDM offspring + safflower oil (n-3 PUFA deficient) all versus control |
n-3 PUFA group had decreased oxidative stress, delayed hepatic telomere lengthening, inflammation with reduced levels of diabetes related metabolites compared to GDM group. | - | n-3 PUFA decreased the long-term risk of developing diabetes | [82] |
| Germinated brown rice | Whole germinated brown rice and oryzanol-rich extract | Oryzanol | Offspring of high-fat diet induced female Sprague Dawley rats | 50% GBR and 100 and 200 mg/kg of oryzanol-rich extract | Pregnant female Sprague Dawley rats were fed with high fat diet (HFD) alone, HFD + 50% germinated brown rice or HFD + oryzanol rich extract (100 or 200 mg/kg/day) throughout pregnancy and lactation. Their offspring were weaned at 4 weeks post-delivery and were followed up until 8 weeks. | GBR and oryzanol produced metabolic outcomes (adiponectin, 8-Iso prostaglandin) that favoured insulin sensitivity better than HFD feeding in the dams and offspring. | Altered global DNA methylation and modulated H3 and H4 acetylation | GBR and oryzanol can ameliorate HFD-induced epigenetically-mediated insulin resistance | [75] |
| Margarine | Fortified | Vitamin A (retinol and β-carotene) | Human offspring (49years) | Increased by 25 % from; 4.2 µg/g of retinol and 3.6 µg/g of β-carotene (equivalent to 0.6 % of the current RDA) to 6 µg/g of retinol and 3 µg/g of β-carotene (equivalent to 0.8 % of the current RDA). β-carotene may be a precursor of retinol. | Longitudinal follow-up on offspring of mothers who had been exposed to the extra vitamin A from margarine fortification (during pregnancy). As aftermath of a mandatory vitamin A fortification (of 25 %) issued by the Denmark government in 1962. | Significant more cases of T2DM in offspring of less-exposed compared to exposed. | - | Foetal exposure to extra vitamin A from fortified margarine may have lowered the risk of developing T2DM in adulthood | [109] |
| Camel milk | Whole | Undenatured camel whey protein (CWP) | Diabetic offspring of STZ diabetic mouse dams (BALB/c) | 100mg/kg for month of parturition | Dam; preconceptionally diabetic (2wks), gestational/ foetal exposure to CWP | Offspring at 3rd month postpartum; CWP restored the expression of ATF-3 and the levels of ROS, pro-inflammatory cytokines. Also normalized glucose and insulin levels compared to diabetic control. Improves survival | - | Mitigates tendency of the offspring to develop diabetes and related complications. | [73] |
| Enriched normo-caloric diets (flaxseed) | Enriched | Alpha-linolenic acid | C57Bl6/J mice | 94g % fat-free chow diet + 6% ALA enrichment (47.9 mole % 18:2n-6) | Dam; 2 weeks pre-conception and during gestation and lactation [diet of 94g % fat-free chow + 6% fat enriched in essential fatty acids (EFA): alpha-linolenic (ALA-18:3, n-3), linoleic (LA-18:2, n-6), or saturated fatty acids (SFA)]. HFD diet = 61.18 g% fat-free chow diet with 38 g% lard. Offspring; a 2-month post-weaning washing-out with normo-caloric diet (regular chow). |
ALA lowers glucose, insulin, HOMA index and stearoyl-CoA desaturase (SCD1) activity. | - | ALA-enriched maternal normo-caloric diets potentially attenuates insulin resistance in adult offspring | [110] |
| Modified Poultry egg | Enriched | Optimized nutrients compared to conventional eggs (Lower; protein, CHO, total lipids, cholesterol, TG, Zn. Higher; Cu, Mg, Vitamin E, C and omega-3 fatty acid (linolenic acid) | Diabetic offspring of diabetic rats induced by synthetic diet rich in high zinc (80mg/kg), high fat and refined sugar | For 30 days before mating | Three parental groups (both males and females); 1) control diet-105D, 2) diabetic diet-105D and 3) diabetic-egg mixed diet -75D+30D (105days before mating). Subset of groups 1&3; offspring (120D of modified egg diet from 9th day after weaning) | Parental intake of modified egg protected from malformations and improved offspring survival Offspring (F1) of diabetic parents fed modified egg had reduced blood glucose, blood pressure and lipid peroxidation. Normal lipid profile and improved enzyme activity. |
- | Though offspring of diabetic had high tendency to become diabetic. Modified egg reverses this risk and ameliorated complications like oxidative stress by normalizing mineral status. | [78] |
| Modified poultry egg | Enriched | Vitamin E and omega-3 fatty acids | Diabetic offspring of NIDDM Wistar rats induced by synthetic diet rich in high zinc (40 or 80mg/kg) | Parental groups (75days before mating); 1) control 20mg Zinc 2) 40mg/kg Zinc 3) 80mg/kg Zinc. Administered EM (4 liquid eggs /kg) to parents (1month before mating -weaning date PND21). Terminal assessment 18days post-weaning. | Enrichment enhanced survival and body weight, restored mineral basal mineral levels, reduced presence of trace elements in urine, prevented offspring abnormalities, | - | Vitamin E and omega-3 fatty acids protect offspring from damaging effects of diabetes | [77] |
4. Implication for the Development of New Prevention and Management Strategies for T2D
5. Conclusions and Future Outlook
Acknowledgments
Conflict of Interest
References
- World Health Organization WHO - The Top 10 Causes of Death. 24 Maggio 2018, 1–7.
- Hameed, I.; Masoodi, S.R.; Mir, S.A.; Nabi, M.; Ghazanfar, K.; Ganai, B.A. Type 2 Diabetes Mellitus: From a Metabolic Disorder to an Inflammatory Condition. World J. Diabetes 2015, 6, 598. [Google Scholar] [CrossRef]
- Deshpande, A.D.; Harris-Hayes, M.; Schootman, M. Epidemiology of Diabetes and Diabetes-Related Complications. Phys. Ther. 2008, 88, 1254–1264. [Google Scholar] [CrossRef] [PubMed]
- Petrie, J.R.; Guzik, T.J.; Touyz, R.M. Diabetes, Hypertension, and Cardiovascular Disease: Clinical Insights and Vascular Mechanisms. Can. J. Cardiol. 2018, 34, 575–584. [Google Scholar] [CrossRef] [PubMed]
- Magkos, F.; Yannakoulia, M.; Chan, J.L.; Mantzoros, C.S. Management of the Metabolic Syndrome and Type 2 Diabetes through Lifestyle Modification. Annu. Rev. Nutr. 2009, 29, 223–256. [Google Scholar] [CrossRef] [PubMed]
- Asif, M. The Prevention and Control the Type-2 Diabetes by Changing Lifestyle and Dietary Pattern. J. Educ. Health Promot. 2014, 3, 1. [Google Scholar] [CrossRef]
- Chaudhury, A.; Duvoor, C.; Reddy Dendi, V.S.; Kraleti, S.; Chada, A.; Ravilla, R.; Marco, A.; Shekhawat, N.S.; Montales, M.T.; Kuriakose, K.; et al. Clinical Review of Antidiabetic Drugs: Implications for Type 2 Diabetes Mellitus Management. Front. Endocrinol. 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Sola, D.; Rossi, L.; Schianca, G.P.C.; Maffioli, P.; Bigliocca, M.; Mella, R.; Corlianò, F.; Paolo Fra, G.; Bartoli, E.; Derosa, G. Sulfonylureas and Their Use in Clinical Practice. Arch. Med. Sci. 2015, 11, 840–848. [Google Scholar] [CrossRef]
- Tuomilehto, J. Functional Foods and Lifestyle Approaches for Diabetes Prevention and Management. Nutrients 2017, 9, 1310. [Google Scholar]
- Kolb, H.; Martin, S. Environmental/lifestyle Factors in the Pathogenesis and Prevention of Type 2 Diabetes. BMC Med. 2017, 15, 131. [Google Scholar] [CrossRef] [PubMed]
- Abubakar, B.; Zawawi, N.; Omar, A.R.; Ismail, M. Predisposition to Insulin Resistance and Obesity due to Staple Consumption of Rice: Amylose Content versus Germination Status. PLoS ONE 2017, 12, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.A. Smoking and Type 2 Diabetes Mellitus. Diabetes Metab. J. 2012, 36, 399–403. [Google Scholar] [CrossRef] [PubMed]
- Toorie, A.M.; Vassoler, F.M.; Qu, F.; Schonhoff, C.M.; Bradburn, S.; Murgatroyd, C.A.; Slonim, D.K.; Byrnes, E.M. A History of Opioid Exposure in Females Increases the Risk of Metabolic Disorders in Their Future Male Offspring. Addict Biol 2021, 26, e12856. [Google Scholar] [CrossRef]
- Uusitupa, M. Lifestyles Matter in the Prevention of Type 2 Diabetes. Diabetes Care 2002, 25, 1650–1651. [Google Scholar] [CrossRef]
- Silva, D.A.S.; Naghavi, M.; Duncan, B.B.; Schmidt, M.I.; de Souza, M. de F.M.; Malta, D.C. Physical Inactivity as Risk Factor for Mortality by Diabetes Mellitus in Brazil in 1990, 2006, and 2016. Diabetol. Metab. Syndr. 2019, 11, 23. [Google Scholar] [CrossRef]
- Al-Yasari, A.; Jabbar, S.; Cabrera, M.A.; Rousseau, B.; Sarkar, D.K. Preconception Alcohol Exposure Increases the Susceptibility to Diabetes in the Offspring. Endocrinology 2021, 162. [Google Scholar] [CrossRef]
- Holst, C.; Becker, U.; Jørgensen, M.E.; Grønbæk, M.; Tolstrup, J.S. Alcohol Drinking Patterns and Risk of Diabetes: A Cohort Study of 70,551 Men and Women from the General Danish Population. Diabetologia 2017, 60, 1941–1950. [Google Scholar] [CrossRef] [PubMed]
- Da Porto, A.; Cavarape, A.; Colussi, G.; Casarsa, V.; Catena, C.; Sechi, L.A. Polyphenols Rich Diets and Risk of Type 2 Diabetes. Nutrients 2021, 13. [Google Scholar] [CrossRef]
- Tertsunen, H.M.; Hantunen, S.; Tuomainen, T.P.; Virtanen, J.K. Adherence to a Healthy Nordic Diet and Risk of Type 2 Diabetes among Men: The Kuopio Ischaemic Heart Disease Risk Factor Study. Eur J Nutr 2021, 60, 3927–3934. [Google Scholar] [CrossRef]
- Shu, L.; Shen, X.M.; Li, C.; Zhang, X.Y.; Zheng, P.F. Dietary Patterns Are Associated with Type 2 Diabetes Mellitus among Middle-Aged Adults in Zhejiang Province, China. Nutr J 2017, 16, 81. [Google Scholar] [CrossRef]
- Imam, M.U.; Ishaka, A.; Ooi, D.J.; Zamri, N.D.M.; Sarega, N.; Ismail, M.; Esa, N.M. Germinated Brown Rice Regulates Hepatic Cholesterol Metabolism and Cardiovascular Disease Risk in Hypercholesterolaemic Rats. J. Funct. Foods 2014, 8, 193–203. [Google Scholar] [CrossRef]
- Stegemann, R.; Buchner, D.A. Transgenerational Inheritance of Metabolic Disease. Semin Cell Dev Biol 2015, 43, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Steyn, N.P.; Mann, J.; Bennett, P.H.; Temple, N.; Zimmet, P.; Tuomilehto, J.; Lindström, J.; Louheranta, A. Diet, Nutrition and the Prevention of Type 2 Diabetes. Public Health Nutr 2004, 7, 147–165. [Google Scholar] [CrossRef] [PubMed]
- Abubakar, B.; Yakasai, H.M.; Zawawi, N.; Ismail, M. Compositional Analyses of White, Brown and Germinated Forms of Popular Malaysian Rice to Offer Insight into the Growing Diet-Related Diseases. J. Food Drug Anal. 2018, 26, 706–715. [Google Scholar] [CrossRef]
- Kusuyama, J.; Makarewicz, N.S.; Albertson, B.G.; Alves-Wagner, A.B.; Conlin, R.H.; Prince, N.B.; Alves, C.R.R.; Ramachandran, K.; Kozuka, C.; Xiudong, Y.; et al. Maternal Exercise-Induced SOD3 Reverses the Deleterious Effects of Maternal High-Fat Diet on Offspring Metabolism Through Stabilization of H3K4me3 and Protection Against WDR82 Carbonylation. Diabetes 2022, 71, 1170–1181. [Google Scholar] [CrossRef]
- Liu, S.; Manson, J.E.; Stampfer, M.J.; Hu, F.B.; Giovannucci, E.; Colditz, G.A.; Hennekens, C.H.; Willett, W.C. A Prospective Study of Whole-Grain Intake and Risk of Type 2 Diabetes Mellitus in US Women. Am J Public Health 2000, 90, 1409–1415. [Google Scholar] [CrossRef]
- Maki, K.C.; Phillips, A.K. Dietary Substitutions for Refined Carbohydrate That Show Promise for Reducing Risk of Type 2 Diabetes in Men and Women. J Nutr 2015, 145, 159s–163s. [Google Scholar] [CrossRef]
- Samra, R.A.; Anderson, G.H. Insoluble Cereal Fiber Reduces Appetite and Short-Term Food Intake and Glycemic Response to Food Consumed 75 Min Later by Healthy Men. Am. J. Clin. Nutr. 2007, 86, 972–979. [Google Scholar] [CrossRef]
- Arihara, K. Functional Foods. Encycl. Meat Sci. 2014, 2, 32–36. [Google Scholar] [CrossRef]
- Schwingshackl, L.; Hoffmann, G.; Lampousi, A.M.; Knüppel, S.; Iqbal, K.; Schwedhelm, C.; Bechthold, A.; Schlesinger, S.; Boeing, H. Food Groups and Risk of Type 2 Diabetes Mellitus: A Systematic Review and Meta-Analysis of Prospective Studies. Eur J Epidemiol 2017, 32, 363–375. [Google Scholar] [CrossRef]
- Wu, C.; Morris, J.R. Genes, Genetics, and Epigenetics: A Correspondence. Science 2001, 293, 1103–1105. [Google Scholar] [CrossRef] [PubMed]
- Gibney, E.R.; Nolan, C.M. Epigenetics and Gene Expression. Heredity ( 2010, 105, 4–13. [Google Scholar] [CrossRef] [PubMed]
- Sanusi, K.O.; Uthman, Y.A.; Ooi, D.J.; Ismail, M.; Imam, M.U. Lifestyle and Preventive Medical Epigenetics. Med. Epigenetics 2021, 33–50. [Google Scholar] [CrossRef]
- Ahmed, S.A.H.; Ansari, S.A.; Mensah-Brown, E.P.K.; Emerald, B.S. The Role of DNA Methylation in the Pathogenesis of Type 2 Diabetes Mellitus. Clin. Epigenetics 2020, 12. [Google Scholar] [CrossRef]
- Ling, C.; Rönn, T. Epigenetics in Human Obesity and Type 2 Diabetes. Cell Metab. 2019, 29, 1028–1044. [Google Scholar] [CrossRef]
- Ling, C.; Rönn, T. Epigenetics in Human Obesity and Type 2 Diabetes. Cell Metab. 2019, 29, 1028–1044. [Google Scholar] [CrossRef]
- Daneshpajooh, M.; Bacos, K.; Bysani, M.; Bagge, A.; Ottosson Laakso, E.; Vikman, P.; Eliasson, L.; Mulder, H.; Ling, C. HDAC7 Is Overexpressed in Human Diabetic Islets and Impairs Insulin Secretion in Rat Islets and Clonal Beta Cells. Diabetologia 2017, 60, 116–125. [Google Scholar] [CrossRef]
- Tobi, E.W.; Slieker, R.C.; Luijk, R.; Dekkers, K.F.; Stein, A.D.; Xu, K.M.; Slagboom, P.E.; van Zwet, E.W.; Lumey, L.H.; Heijmans, B.T. DNA Methylation as a Mediator of the Association between Prenatal Adversity and Risk Factors for Metabolic Disease in Adulthood. Sci. Adv. 2018, 4, eaao4364. [Google Scholar] [CrossRef]
- Li, J.; Li, K.; Gao, J.; Guo, X.; Lu, M.; Li, Z.; Li, D. Maternal Exposure to an N-3 Polyunsaturated Fatty Acid Diet Decreases Mammary Cancer Risk of Female Offspring in Adulthood. Food Funct 2018, 9, 5768–5777. [Google Scholar] [CrossRef]
- Sun, Q.; Huang, S.; Wang, X.; Zhu, Y.; Chen, Z.; Chen, D. N6-Methyladenine Functions as a Potential Epigenetic Mark in Eukaryotes. Bioessays 2015, 37, 1155–1162. [Google Scholar] [CrossRef]
- Deaton, A.M.; Bird, A. CpG Islands and the Regulation of Transcription. Genes Dev. 2011, 25, 1010–1022. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Huang, H.; Liu, D.; Cheng, Y.; Liu, X.; Zhang, W.; Yin, R.; Zhang, D.; Zhang, P.; Liu, J.; et al. N6-Methyladenine DNA Modification in Drosophila. Cell 2015, 161, 893–906. [Google Scholar] [CrossRef] [PubMed]
- Pinney, S.E.; Pinney, S.E.O. DNA Methylation and Its Role in the Pathogenesis of Diabetes. Pediatr. Diabetes 2017, 18, 167–177. [Google Scholar]
- Zhang, Q.; Xiao, X.; Zheng, J.; Li, M.; Yu, M.; Ping, F.; Wang, T.; Wang, X. A Maternal High-Fat Diet Induces DNA Methylation Changes That Contribute to Glucose Intolerance in Offspring. Front. Endocrinol. 2019, 10, 871. [Google Scholar] [CrossRef] [PubMed]
- Blake, G.E.T.; Watson, E.D. Unravelling the Complex Mechanisms of Transgenerational Epigenetic Inheritance. Curr. Opin. Chem. Biol. 2016, 33, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Alaskhar Alhamwe, B.; Khalaila, R.; Wolf, J.; von Bülow, V.; Harb, H.; Alhamdan, F.; Hii, C.S.; Prescott, S.L.; Ferrante, A.; Renz, H.; et al. Histone Modifications and Their Role in Epigenetics of Atopy and Allergic Diseases. Allergy Asthma Clin Immunol 2018, 14, 39. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Luan, Y.; Feng, Q.; Chen, X.; Qin, B.; Ren, K. Di; Luan, Y. Epigenetics and Beyond: Targeting Histone Methylation to Treat Type 2 Diabetes Mellitus. Front. Pharmacol. 2022, 12. [Google Scholar] [CrossRef]
- Casas-Agustench, P.; Fernandes, F.S.; Tavares do Carmo, M.G.; Visioli, F.; Herrera, E.; Dávalos, A. Consumption of Distinct Dietary Lipids during Early Pregnancy Differentially Modulates the Expression of microRNAs in Mothers and Offspring. PLoS ONE 2015, 10, e0117858. [Google Scholar] [CrossRef]
- Mantilla-Escalante, D.C.; López de las Hazas, M.-C.; Crespo, M.C.; Martín-Hernández, R.; Tomé-Carneiro, J.; del Pozo-Acebo, L.; Salas-Salvadó, J.; Bulló, M.; Dávalos, A. Mediterranean Diet Enriched in Extra-Virgin Olive Oil or Nuts Modulates Circulating Exosomal Non-Coding RNAs. Eur J Nutr 2021, 60, 4279–4293. [Google Scholar] [CrossRef]
- Núñez-Acuña, G.; Détrée, C.; Gallardo-Escárate, C.; Gonçalves, A.T. Functional Diets Modulate lncRNA-Coding RNAs and Gene Interactions in the Intestine of Rainbow Trout Oncorhynchus Mykiss. Mar. Biotechnol. 2017, 19, 287–300. [Google Scholar] [CrossRef]
- Zhang, Q.; Xiao, X.; Zheng, J.; Li, M.; Yu, M.; Ping, F.; Wang, T.; Wang, X. Improvement in Glucose Metabolism in Adult Male Offspring of Maternal Mice Fed Diets Supplemented with Inulin via Regulation of the Hepatic Long Noncoding RNA Profile. Faseb J 2021, 35, e22003. [Google Scholar] [CrossRef] [PubMed]
- Formichi, C.; Nigi, L.; Grieco, G.E.; Maccora, C.; Fignani, D.; Brusco, N.; Licata, G.; Sebastiani, G.; Dotta, F. Non-Coding Rnas: Novel Players in Insulin Resistance and Related Diseases. Int. J. Mol. Sci. 2021, 22. [Google Scholar] [CrossRef] [PubMed]
- Chi, T.; Lin, J.; Wang, M.; Zhao, Y.; Liao, Z.; Wei, P. Non-Coding RNA as Biomarkers for Type 2 Diabetes Development and Clinical Management. Front. Endocrinol. 2021, 12. [Google Scholar] [CrossRef] [PubMed]
- Perez, M.F.; Lehner, B. Intergenerational and Transgenerational Epigenetic Inheritance in Animals. Nat Cell Biol 2019, 21, 143–151. [Google Scholar] [CrossRef]
- Gill-Randall, R.; Adams, D.; Ollerton, R.L.; Lewis, M.; Alcolado, J.C. Type 2 Diabetes Mellitus--Genes or Intrauterine Environment? An Embryo Transfer Paradigm in Rats. Diabetologia 2004, 47, 1354–1359. [Google Scholar] [CrossRef]
- Dabelea, D.; Hanson, R.L.; Lindsay, R.S.; Pettitt, D.J.; Imperatore, G.; Gabir, M.M.; Roumain, J.; Bennett, P.H.; Knowler, W.C. Intrauterine Exposure to Diabetes Conveys Risks for Type 2 Diabetes and Obesity: A Study of Discordant Sibships. Diabetes 2000, 49, 2208–2211. [Google Scholar] [CrossRef]
- Jimenez-Chillaron, J.C.; Isganaitis, E.; Charalambous, M.; Gesta, S.; Pentinat-Pelegrin, T.; Faucette, R.R.; Otis, J.P.; Chow, A.; Diaz, R.; Ferguson-Smith, A.; et al. Intergenerational Transmission of Glucose Intolerance and Obesity by in Utero Undernutrition in Mice. Diabetes 2009, 58, 460–468. [Google Scholar] [CrossRef]
- Pavlinkova, G.; Margaryan, H.; Zatecka, E.; Valaskova, E.; Elzeinova, F.; Kubatova, A.; Bohuslavova, R.; Peknicova, J. Transgenerational Inheritance of Susceptibility to Diabetes-Induced Male Subfertility. Sci. Rep. 2017, 7, 4940. [Google Scholar] [CrossRef]
- Sánchez-Soriano, C.; Pearson, E.R.; Reynolds, R.M. Associations between Parental Type 2 Diabetes Risk and Offspring Birthweight and Placental Weight: A Survival Analysis Using the Walker Cohort. Diabetologia 2022. [Google Scholar] [CrossRef]
- Crudo, A.; Petropoulos, S.; Moisiadis, V.G.; Iqbal, M.; Kostaki, A.; Machnes, Z.; Szyf, M.; Matthews, S.G. Prenatal Synthetic Glucocorticoid Treatment Changes DNA Methylation States in Male Organ Systems: Multigenerational Effects. Endocrinology 2012, 153, 3269–3283. [Google Scholar] [CrossRef]
- Painter, R.C.; Osmond, C.; Gluckman, P.; Hanson, M.; Phillips, D.I.; Roseboom, T.J. Transgenerational Effects of Prenatal Exposure to the Dutch Famine on Neonatal Adiposity and Health in Later Life. Bjog 2008, 115, 1243–1249. [Google Scholar] [CrossRef] [PubMed]
- Barella, L.F.; de Oliveira, J.C.; Mathias, P.C. Pancreatic Islets and Their Roles in Metabolic Programming. Nutrition 2014, 30, 373–379. [Google Scholar] [CrossRef]
- Bouret, S.; Levin, B.E.; Ozanne, S.E. Gene-Environment Interactions Controlling Energy and Glucose Homeostasis and the Developmental Origins of Obesity. Physiol. Rev. 2015, 95, 47–82. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, J.H.; Haase, T.N.; Jaksch, C.; Nalla, A.; Søstrup, B.; Nalla, A.A.; Larsen, L.; Rasmussen, M.; Dalgaard, L.T.; Gaarn, L.W.; et al. Impact of Fetal and Neonatal Environment on Beta Cell Function and Development of Diabetes. Acta Obs. Gynecol Scand 2014, 93, 1109–1122. [Google Scholar] [CrossRef] [PubMed]
- Ockner, R.K.; Manning, J.A. Fatty Acid-Binding Protein in Small Intestine. Identification, Isolation, and Evidence for Its Role in Cellular Fatty Acid Transport. J Clin Invest 1974, 54, 326–338. [Google Scholar] [CrossRef]
- Hasler, C.M. Functional Foods: Benefits, Concerns and Challenges - A Position Paper from the American Council on Science and Health. J. Nutr. 2002, 132, 3772–3781. [Google Scholar] [CrossRef]
- Temple, N.J. A Rational Definition for Functional Foods: A Perspective. Front. Nutr. 2022, 9. [Google Scholar] [CrossRef]
- Gul, K.; Singh, A.K.; Jabeen, R. Nutraceuticals and Functional Foods: The Foods for the Future World. Crit. Rev. Food Sci. Nutr. 2016, 56, 2617–2627. [Google Scholar] [CrossRef]
- Magrone, T.; de Heredia, F.P.; Jirillo, E.; Morabito, G.; Marcos, A.; Serafini, M. Functional Foods and Nutraceuticals as Therapeutic Tools for the Treatment of Diet-Related Diseases. Can. J. Physiol. Pharmacol. 2013, 91, 387–396. [Google Scholar] [CrossRef]
- Bansal, A.; Rashid, C.; Xin, F.; Li, C.; Polyak, E.; Duemler, A.; van der Meer, T.; Stefaniak, M.; Wajid, S.; Doliba, N.; et al. Sex- and Dose-Specific Effects of Maternal Bisphenol A Exposure on Pancreatic Islets of First- and Second-Generation Adult Mice Offspring. Env. Health Perspect 2017, 125, 97022. [Google Scholar] [CrossRef]
- Silva, R.C. da; Colleran, H.L.; Ibrahim, S.A. Milk Fat Globule Membrane in Infant Nutrition: A Dairy Industry Perspective. J. Dairy Res. 2021, 88, 105–116. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Zhang, Q.; Xin, F.; Cao, B.; Qian, L.; Dong, Y. Neonatal Milk Fat Globule Membrane Supplementation During Breastfeeding Ameliorates the Deleterious Effects of Maternal High-Fat Diet on Metabolism and Modulates Gut Microbiota in Adult Mice Offspring in a Sex-Specific Way. Front Cell Infect Microbiol 2021, 11, 621957. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, M.H.; Badr, G.; El Shinnawy, N.A. Camel Whey Protein Improves Lymphocyte Function and Protects against Diabetes in the Offspring of Diabetic Mouse Dams. Int J Immunopathol Pharmacol 2016, 29, 632–646. [Google Scholar] [CrossRef] [PubMed]
- Achari, A.E.; Jain, S.K. Adiponectin, a Therapeutic Target for Obesity, Diabetes, and Endothelial Dysfunction. Int J Mol Sci 2017, 18. [Google Scholar] [CrossRef] [PubMed]
- Adamu, H.A.; Imam, M.U.; Ooi, D.J.; Esa, N.M.; Rosli, R.; Ismail, M. In Utero Exposure to Germinated Brown Rice and Its Oryzanol-Rich Extract Attenuated High Fat Diet-Induced Insulin Resistance in F1 Generation of Rats. BMC Complement Altern Med 2017, 17, 67. [Google Scholar] [CrossRef] [PubMed]
- Dubey, P.; Thakur, V.; Chattopadhyay, M. Role of Minerals and Trace Elements in Diabetes and Insulin Resistance. Nutrients 2020, 12, 1864. [Google Scholar] [CrossRef] [PubMed]
- Taneja, S.K.; Mandal, R. Effect of Modified Egg* on Developmental Defects in Neonates of NIDDM Induced Wistar Rats. Indian J Exp Biol 2006, 44, 863–874. [Google Scholar]
- Taneja, S.K.; Singh, K.B. Beneficial Effects of Modified Egg* on Oxidative Stress in F1- Generation of Metabolic Syndrome-X Induced Wistar Rat. Indian J Exp Biol 2009, 47, 104–112. [Google Scholar]
- Asmat, U.; Abad, K.; Ismail, K. Diabetes Mellitus and Oxidative stress—A Concise Review. Saudi Pharm. J. 2016, 24. [Google Scholar] [CrossRef] [PubMed]
- Grunnet, L.G.; Pilgaard, K.; Alibegovic, A.; Jensen, C.B.; Hjort, L.; Ozanne, S.E.; Bennett, M.; Vaag, A.; Brøns, C. Leukocyte Telomere Length Is Associated with Elevated Plasma Glucose and HbA1c in Young Healthy Men Independent of Birth Weight. Sci. Rep. 2019, 9, 7639. [Google Scholar] [CrossRef]
- Wang, J.; Dong, X.; Cao, L.; Sun, Y.; Qiu, Y.; Zhang, Y.; Cao, R.; Covasa, M.; Zhong, L. Association between Telomere Length and Diabetes Mellitus: A Meta-Analysis. J. Int. Med. Res. 2016, 44, 1156–1173. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Xiao, H.; Li, J.; Guo, X.; Cai, W.; Li, D. N-3 Polyunsaturated Fatty Acids Decrease Long-Term Diabetic Risk of Offspring of Gestational Diabetes Rats by Postponing Shortening of Hepatic Telomeres and Modulating Liver Metabolism. Nutrients 2019, 11. [Google Scholar] [CrossRef] [PubMed]
- Bhupathiraju, S.N.; Hu, F.B. Epidemiology of Obesity and Diabetes and Their Cardiovascular Complications. Circ. Res. 2016, 118, 1723–1735. [Google Scholar] [CrossRef]
- Ma, L.; Sun, Z.; Zeng, Y.; Luo, M.; Yang, J. Molecular Mechanism and Health Role of Functional Ingredients in Blueberry for Chronic Disease in Human Beings. Int. J. Mol. Sci. 2018, 19. [Google Scholar] [CrossRef]
- Sulforaphane Protects against Cytokine- and Streptozotocin-Induced β-Cell Damage by Suppressing the NF-κB Pathway. Toxicol. Appl. Pharmacol. 2009, 235, 57–67. [CrossRef] [PubMed]
- Shanak, S.; Saad, B.; Zaid, H.; Carvalho, J.C.T. Metabolic and Epigenetic Action Mechanisms of Antidiabetic Medicinal Plants. Evidence-based Complement. Altern. Med. 2019, 2019. [Google Scholar] [CrossRef]
- Kaur, K.K.; Allahbadia, G.; Singh, M. Therapeutic Potential and Epigenetic Alterations of Plant Phytochemicals (as Epi-Drugs) for the Treatment of Type 2 Diabetes Mellitus: A Systematic Review. Adv Obes Weight Manag. 2021. [Google Scholar]
- Mousavi, A.; Vafa, M.; Neyestani, T.; Khamseh, M.; Hoseini, F. The Effects of Green Tea Consumption on Metabolic and Anthropometric Indices in Patients with Type 2 Diabetes. J. Res. Med. Sci. 2013, 18, 1080–1086. [Google Scholar]
- Ramírez-Alarcón, K.; Victoriano, M.; Mardones, L.; Villagran, M.; Al-Harrasi, A.; Al-Rawahi, A.; Cruz-Martins, N.; Sharifi-Rad, J.; Martorell, M. Phytochemicals as Potential Epidrugs in Type 2 Diabetes Mellitus. Front. Endocrinol. 2021, 12. [Google Scholar] [CrossRef]
- Zhong, J.; Xu, C.; Reece, E.A.; Yang, P. The Green Tea Polyphenol EGCG Alleviates Maternal Diabetes–induced Neural Tube Defects by Inhibiting DNA Hypermethylation. Am. J. Obstet. Gynecol. 2016, 215, 368–e1. [Google Scholar] [CrossRef]
- K. , G.-B.; O., R.-L.; E., B.-C.; J.I., R.-B.; F.I., M.; E., M.-L.; J.A., M. Fatty Acids, Epigenetic Mechanisms and Chronic Diseases: A Systematic Review. Lipids Health Dis. 2019, 18, 178. [Google Scholar] [CrossRef]
- Hussey, B.; Lindley, M.R.; Mastana, S.S.; Mastana, S.S. Omega 3 Fatty Acids, Inflammation and DNA Methylation: An Overview. Clin. Lipidol. 2017, 12, 24–39. [Google Scholar]
- S. , R.; S.C., G.; B., P.; A., G.; B.B., A. Epigenetic Changes Induced by Curcumin and Other Natural Compounds. Genes Nutr. 2011, 6, 93–108. [Google Scholar] [CrossRef] [PubMed]
- Yun, J.M.; Jialal, I.; Devaraj, S. Epigenetic Regulation of High Glucose-Induced Proinflammatory Cytokine Production in Monocytes by Curcumin. J. Nutr. Biochem. 2011, 22, 450–458. [Google Scholar] [CrossRef]
- Tang, C.; Liu, Y.; Liu, S.; Yang, C.; Chen, L.; Tang, F.; Wang, F.; Zhan, L.; Deng, H.; Zhou, W.; et al. Curcumin and Its Analogs as Potential Epigenetic Modulators: Prevention of Diabetes and Its Complications. Pharmacology 2022, 107, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.C.W.; Popkin, B.M. Intergenerational Diabetes and obesity—A Cycle to Break? PLOS Med. 2017, 14, e1002415. [Google Scholar] [CrossRef]
- Marangoni, F.; Cetin, I.; Verduci, E.; Canzone, G.; Giovannini, M.; Scollo, P.; Corsello, G.; Poli, A. Maternal Diet and Nutrient Requirements in Pregnancy and Breastfeeding. An Italian Consensus Document. Nutrients 2016, 8, 629. [Google Scholar] [CrossRef]
- Marciniak, A.; Patro-Małysza, J.; Kimber-Trojnar, Ż.; Marciniak, B.; Oleszczuk, J.; Leszczyńska-Gorzelak, B. Fetal Programming of the Metabolic Syndrome. Taiwan. J. Obstet. Gynecol. 2017, 56, 133–138. [Google Scholar] [CrossRef]
- Sinclair, K.D.; Allegrucci, C.; Singh, R.; Gardner, D.S.; Sebastian, S.; Bispham, J.; Thurston, A.; Huntley, J.F.; Rees, W.D.; Maloney, C.A.; et al. DNA Methylation, Insulin Resistance, and Blood Pressure in Offspring Determined by Maternal Periconceptional B Vitamin and Methionine Status. Proc Natl Acad Sci U S A 2007, 104, 19351–19356. [Google Scholar] [CrossRef]
- Kazeem, M.I.; Davies, T.C. Anti-Diabetic Functional Foods as Sources of Insulin Secreting, Insulin Sensitizing and Insulin Mimetic Agents. J. Funct. Foods 2016, 20, 122–138. [Google Scholar] [CrossRef]
- Neacsu, M.; Raikos, V.; Benavides-Paz, Y.; Duncan, S.H.; Duncan, G.J.; Christie, J.S.; Johnstone, A.M.; Russell, W.R. Sapogenol Is a Major Microbial Metabolite in Human Plasma Associated with High Protein Soy-Based Diets: The Relevance for Functional Food Formulations. Foods 2020, 9. [Google Scholar] [CrossRef]
- Plat, J.; Baumgartner, S.; Vanmierlo, T.; Lütjohann, D.; Calkins, K.L.; Burrin, D.G.; Guthrie, G.; Thijs, C.; Te Velde, A.A.; Vreugdenhil, A.C.E.; et al. Plant-Based Sterols and Stanols in Health & disease: “Consequences of Human Development in a Plant-Based Environment? ” Prog Lipid Res 2019, 74, 87–102. [Google Scholar] [CrossRef] [PubMed]
- Sang, S.; Chu, Y. Whole Grain Oats, More than Just a Fiber: Role of Unique Phytochemicals. Mol Nutr Food Res 2017, 61. [Google Scholar] [CrossRef] [PubMed]
- Tessari, P.; Lante, A. A Multifunctional Bread Rich in Beta Glucans and Low in Starch Improves Metabolic Control in Type 2 Diabetes: A Controlled Trial. Nutrients 2017, 9. [Google Scholar] [CrossRef] [PubMed]
- Alves-de-Oliveira, D.S.; Bloise, A.; Silva, L.M.L.; Rocha-Junior, R.L.; Lima-Júnior, N.C.; Menezes, L.G.S.; Silva, E.G.S.; De Oliveira, Y.; Wanderley, A.G.; de-Brito-Alves, J.L.; et al. Maternal Consumption of ɷ3 Attenuates Metabolic Disruption Elicited by Saturated Fatty Acids-Enriched Diet in Offspring Rats. Nutr Metab Cardiovasc Dis 2022, 32, 279–289. [Google Scholar] [CrossRef]
- Ueno, M.; Liu, S.; Kiyoi, T.; Sugiyama, T.; Mogi, M. Perinatal Low-Fat Dietary Intervention Affects Glucose Metabolism in Female Adult and Aging Offspring. Geriatr Gerontol Int 2022, 22, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Ramalingam, L.; Menikdiwela, K.R.; Spainhour, S.; Eboh, T.; Moustaid-Moussa, N. Sex Differences in Early Programming by Maternal High Fat Diet Induced-Obesity and Fish Oil Supplementation in Mice. Nutrients 2021, 13. [Google Scholar] [CrossRef]
- Wang, D.D.; Wu, F.; Zhang, L.Y.; Zhao, Y.C.; Wang, C.C.; Xue, C.H.; Yanagita, T.; Zhang, T.T.; Wang, Y.M. Effects of Dietary N-3 PUFA Levels in Early Life on Susceptibility to High-Fat-Diet-Induced Metabolic Syndrome in Adult Mice. J Nutr Biochem 2021, 89, 108578. [Google Scholar] [CrossRef]
- Keller, A.; Ängquist, L.; Jacobsen, R.; Vaag, A.; Heitmann, B.L. A Retrospective Analysis of a Societal Experiment among the Danish Population Suggests That Exposure to Extra Doses of Vitamin A during Fetal Development May Lower Type 2 Diabetes Mellitus (T2DM) Risk Later in Life. Br J Nutr 2017, 117, 731–736. [Google Scholar] [CrossRef]
- Hollander, K.S.; Tempel Brami, C.; Konikoff, F.M.; Fainaru, M.; Leikin-Frenkel, A. Dietary Enrichment with Alpha-Linolenic Acid during Pregnancy Attenuates Insulin Resistance in Adult Offspring in Mice. Arch Physiol Biochem 2014, 120, 99–111. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





