Submitted:
26 May 2023
Posted:
30 May 2023
You are already at the latest version
Abstract

Keywords:
1. Introduction
2. Nano-materials-based catalysis
2.1. Metal nanoparticle-based catalysis
2.2. Metal chalcogenide nanoparticles-based catalysis
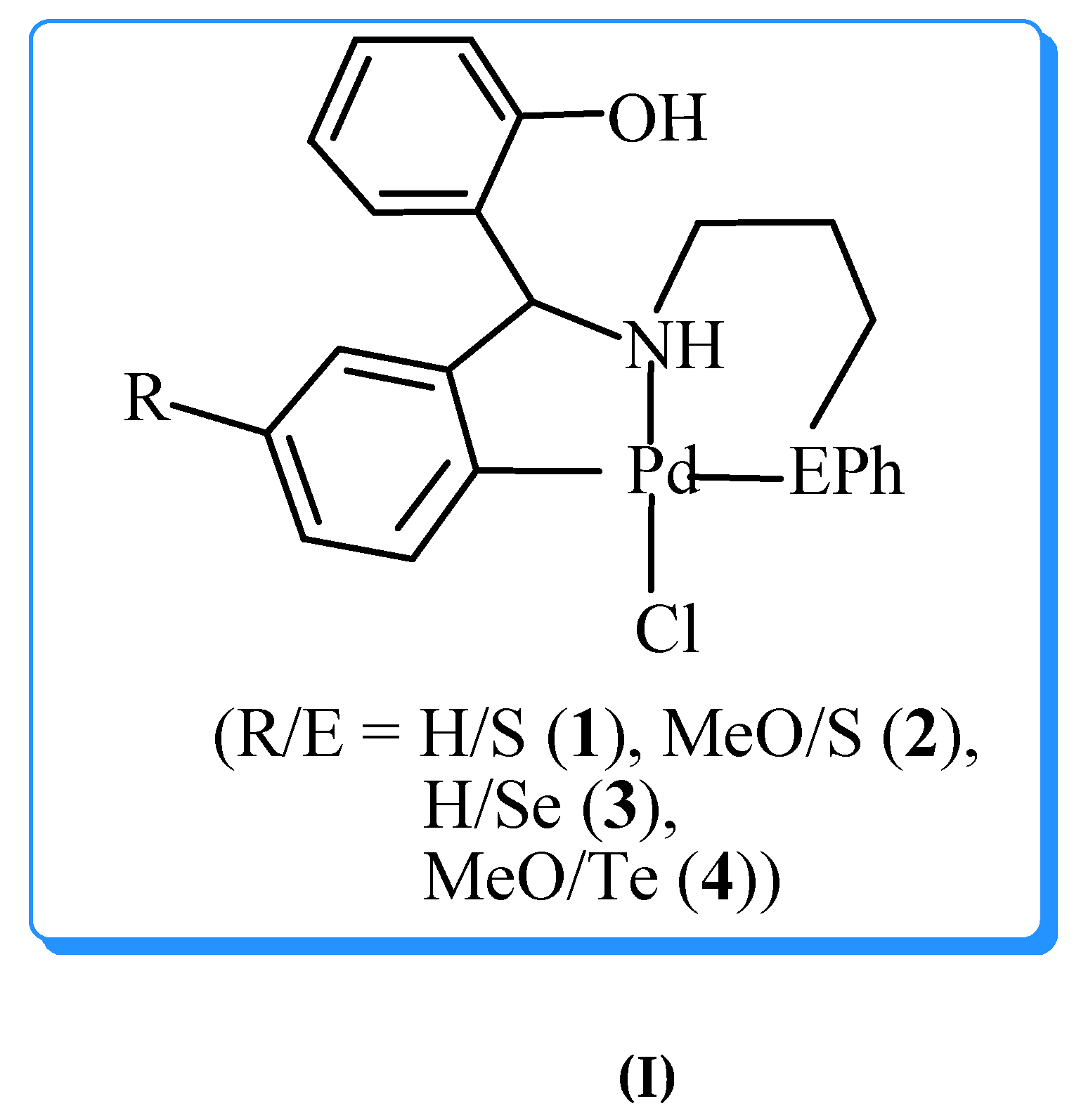
2.3. Metal chalcogenides as photo-/ electro-catalysts
3. Metal Chalcogenide Materials
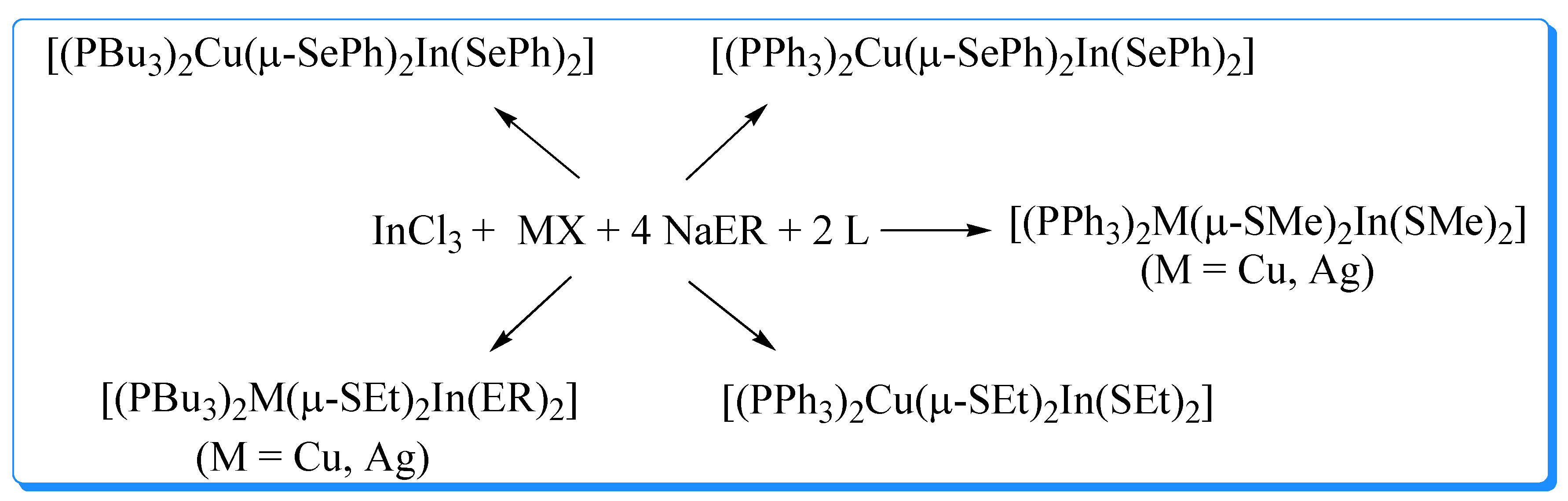
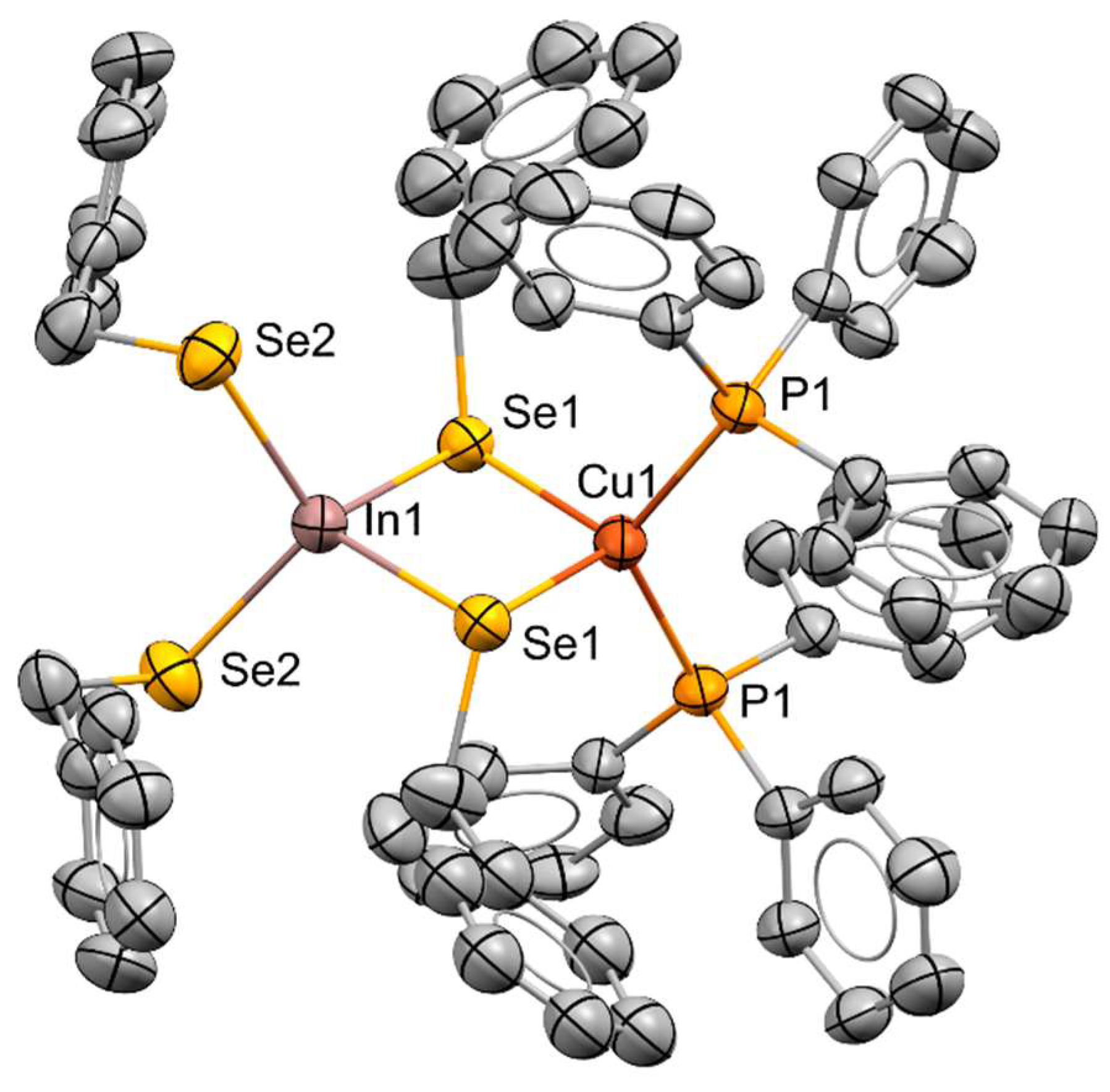
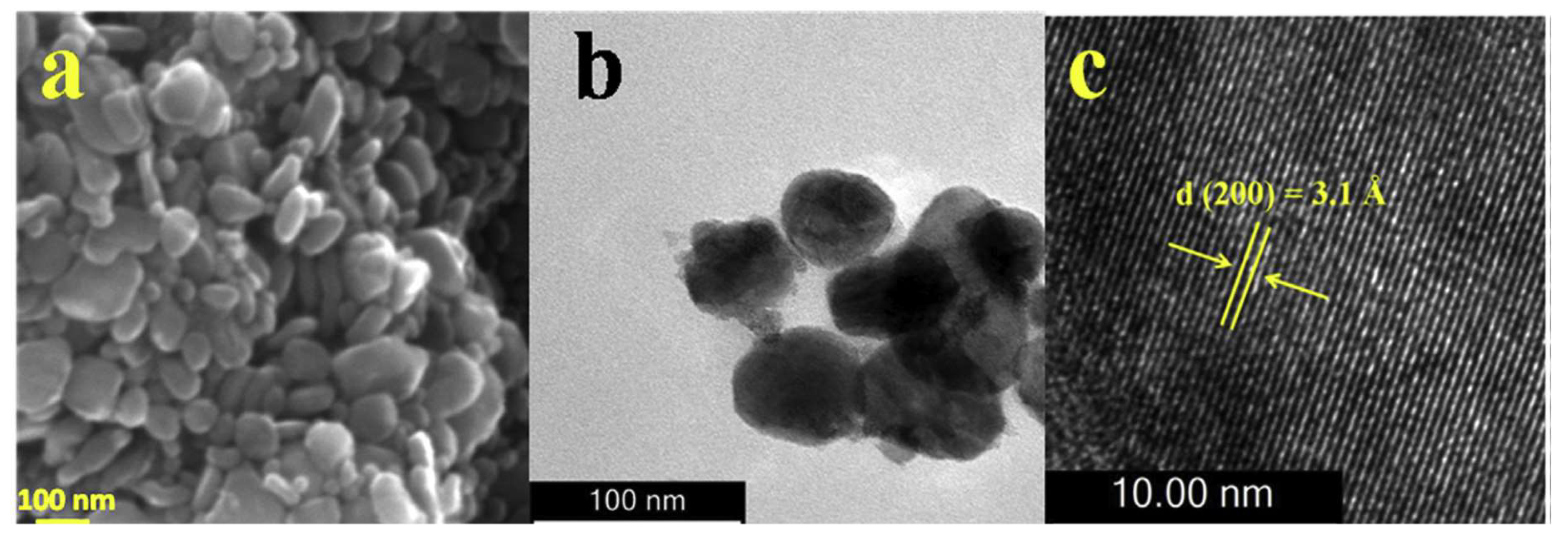
3.1. I-III-VI2 (I = Cu or Ag, III = Ga or In; VI = S or Se) compounds
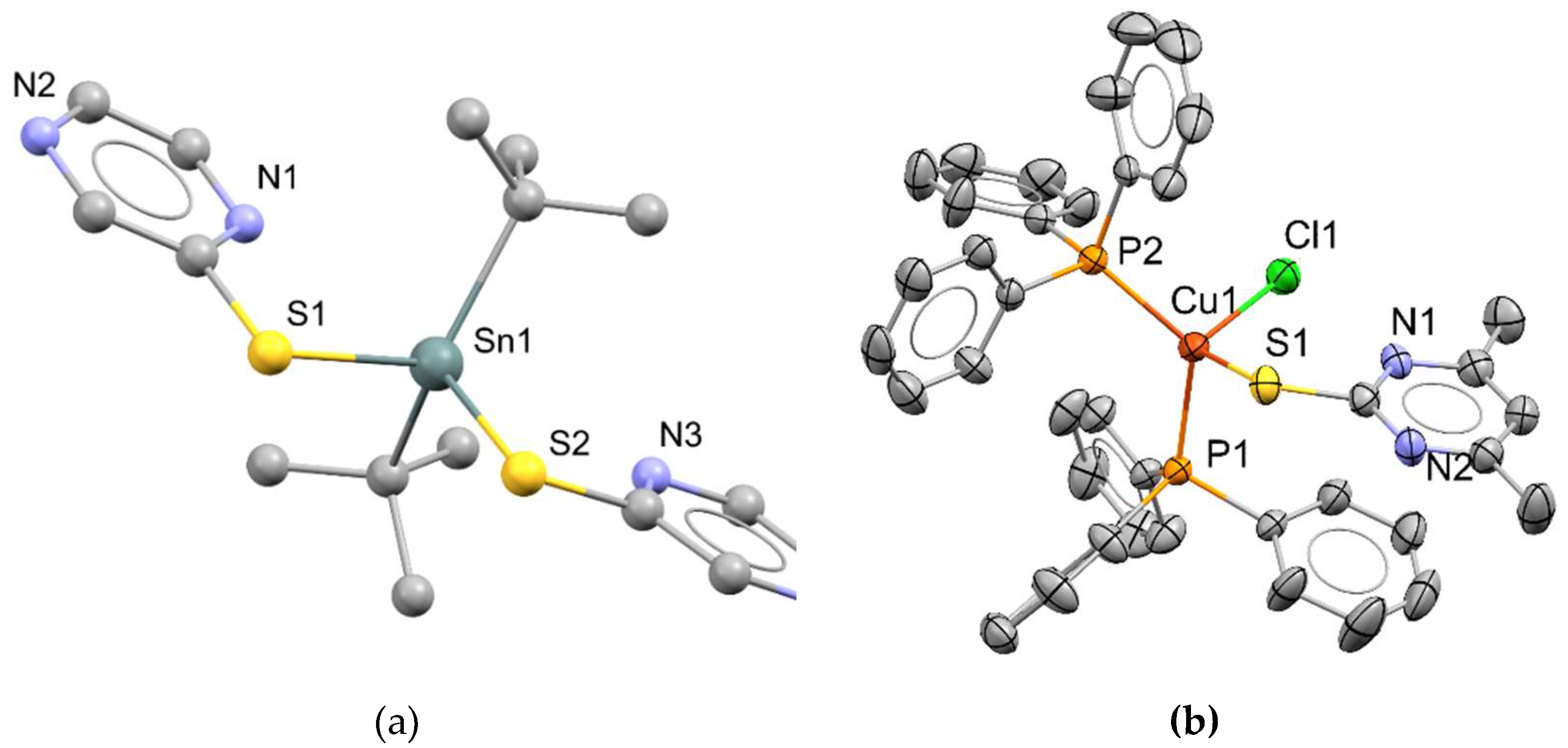
3.2. I-IV-VI (I = Cu, IV = Sn; VI = S or Se) compounds
3.3. I-V-VI (I = Cu or Ag; IV = Sb or Bi; VI = S or Se) compounds
3.4. II-III-VI (II = Zn or Cd; III = In or Ga; VI = S or Se) compounds
3.5. I-IIIx-III′1-x-VI2 (I = Cu; III or III′ = Ga or In; VI = S or Se) quaternary compounds
3.6. I2-II-IV-VI4 (I = Cu; II = Zn or Cd; IV = Sn; VI = S or Se) quaternary compounds
4. Conclusions
Acknowledgments
References
- Banerjea, D. ‘Inorganic chemistry: A modern treatise’, Asian Books Pvt. Ltd., New Delhi, 2012.
- Ray, P.C. ‘A history of Hindu chemistry’, Volume 1, 1902.
- Yam, V.W.W. Angew. Chem. Int. Ed. 2015, 54, 8304-8305.
- Jain, V.K.; Kedarnath, G., ‘Applications of metal-selenium and /-tellurium compounds in materials science’ Chapter 11, pp 383-443, in ‘Selenium and tellurium reagents in chemistry and materials science’; Eds. Laitinen, R.; Oilunkaniemi,R., Walther de Gruyter, Germany, 2019.
- Kedarnath, G., ‘Synthesis of advanced inorganic materials through molecular precursors’, Chapter-15, in ‘Handbook on synthesis strategies for advanced materials’ Eds. Tyagi A.K.; Ningthoujam, R.S., Springer Nature Singapore Pte Ltd., 2021.
- ‘Nanomaterials via single source precursors: Synthesis, processing and applications’, Eds. Apblett, A. W.; Barron, A. R.; Hepp, A. F., Elsevier, 2022.
- Jain, V.K.; Chauhan, R.S., ‘Metal chalcogenolates: Synthesis and applications to materials science’, Chapter-3; pp 58-82; in ‘Chalcogen Chemistry: Fundamentals and Applications’, Eds. Lippolis, V.; Santi, C.; Lenardao, E.J.; Braga, A.L., RSC (UK) 2023.
- Gu, C.; Xu, H. M.; Han, S. K.; Gao, M. R.; Yu, S. H., Chem. Soc. Rev. 2021, 50, 6671-6683.
- Steinborn, D. ‘Fundamentals of organometallic catalysis’ Wiley-VCH, Weinheim, Germany, 2012.
- Colacot, T.J. (Ed), ‘New trends in cross-coupling: Theory and applications’, Royal Society of Chemistry, UK, 2015.
- Serp, P.; Philippot, K. (Eds.), ‘Nanomaterials in catalysis’, Weinheim, Germany, 2013.
- Astruc, D., Chem. Rev. 2020, 120, 2, 461–463.
- Lee, K.; Kim, M.; Kim, H. J. Mater. Chem., 2010, 20, 3791-3798.
- Thematic issue of Chemical reviews dedicated to Nanoparticles in Catalysis, Chem. Rev. 2020, 120, issue No. 2.
- Favier, I.; Pla, D.; Gómez, M., Chem. Rev. 2020, 120, 1146−1183; Biffis, A.; Centomo, P.; Zotto, A.D.; Zecca, M., Chem. Rev. 2018, 118, 2249-2295.
- Chahdoura, F.; Favier, I.; Pradel, C.; Mallet-Ladeira, S.; Gómez, M., Catal. Commun. 2015, 63, 47-51.
- Guerrero, M.; Costa, N.J.S.; Vono, L.L.R.; Rossi, L.M.; Gusevskayad, E.V.; Philippot, K., J. Mater. Chem. A, 2013, 1, 1441.
- Ghawale, N.; Dey, S.; Jain, V.K.; Tewari, R., Bull. Mater. Sci. 2009, 32, 15-18.
- De Vries, J.G., Dalton Trans. 2006, 421-429.
- Gaikwad, A.V.; Holuigue, A.; Thathagar, M.B.; ten Elshof J.E.; Rothenberg, G., Chem. Eur. J. 2007, 13, 6908-6913.
- Ganesan, M.; Freemantle, R.G.; Obare, S.O., Chem. Mater. 2007, 19, 3464-3471.
- Arora, A.; Oswal, P.; Rao, G.K.; Kumar, S.; Kumar, A., Dalton Trans., 2021, 50, 8628-8656.
- Arora, A.; Oswal, P.; Datta, A; Kumar, A., Coord. Chem. Rev., 2022, 459, 214406.
- Oswal, P.; Arora, A.; Gairola, S.; Datta, A.; Kumar, A., New J. Chem. 2021, 45, 21449-21487.
- Paluru, D.K.; Dey, S.; Wadawale, A.P.; Bhuvanesh, N.; Grupp, A.; Kaim, W.; Jain, V.K., Chem. Asian J. 2016, 11, 401-410.
- Rao, G.K.; Kumar, A.; Kumar, B.; Kumar, D.; Singh, A. K., Dalton Trans. 2012, 41, 1931-1937.
- Saleem, F.; Rao, G.K.; Kumar, A.; Kumar, S.; Singh, M.P.; Singh, A.K., RSC Adv., 2014, 4, 56102-56111 .
- Kumar, S.; Rao, G.K.; Kumar, A.; Singh, M.P.; Singh, A.K., Dalton Trans. 2013, 42, 16939-16948.
- Axet, M. R.; Philippot, K., Chem. Rev. 2020, 120, 1085−1145.
- Luo, L.; Li, H.; Peng, Y.; Feng, C.; Zeng, J., ChemNanoMat, 2018, 4, 451-466.
- Lara, P.; Philippot, K.; Chaudret, B., ChemCatChem 2013, 5, 28-45.
- Taglang, C.; Martínez-Prieto, L.M.; del Rosal, I.; Maron, L.; Poteau, R.; Philippot, K.; Chaudret, B.; Perato, S.; Lone, A.S.; Puente, C.; Dugave, C.; Rousseau, B.; Pieters, G., Angew. Chem. Int. Ed. 2015, 54, 10474-10477.
- Martínez-Prieto, L.M.; Ferry, A.; Lara, P.; Richter, C.; Philippot, K.; Glorius, F.; Chaudret, B., Chem. Eur. J. 2015, 21, 17495-17502.
- Anandaraj, S. J. L.; Kang, L.; DeBeer, S.; Bordet, A.; Leitner, W., Small 2023, 2206806.
- Gopiraman,M.; Saravanamoorthy, S.; Ullah,S.; Ilangovan, A.; Kim, I.S.; Chung, I. M., RSC Adv., 2020, 10, 2545-2559.
- Dey, S.; Jain, V.K., Platinum Metals Rev., 2004, 48, 16-29.
- Joshi, H.; Sharma, K. N.; Sharma, A. K.; Prakash, O.; Singh, A.K., Chem. Commun., 2013, 49, 7483-7485.
- Singh, P.; Singh, A. K., Dalton Trans., 2017, 46, 10037-10049.
- Klauke, K.; Gruber, I.; Knedel, T.O.; Schmolke, L.; Barthel, J.; Breitzke, H.; Janiak, C., Organometallics, 2018, 37, 298-308.
- Arora, A.; Oswal, P., Rao, G. K.; Kumar, S.; Singh, A. K.; Kumar, A., RSC Adv., 2021, 11, 7214-7224.
- Arora, A.; Oswal, P., Rao, G. K.; Kumar, S.; Singh, A. K.; Kumar, A., Catal. Lett., 2022, 152, 1999-2011.
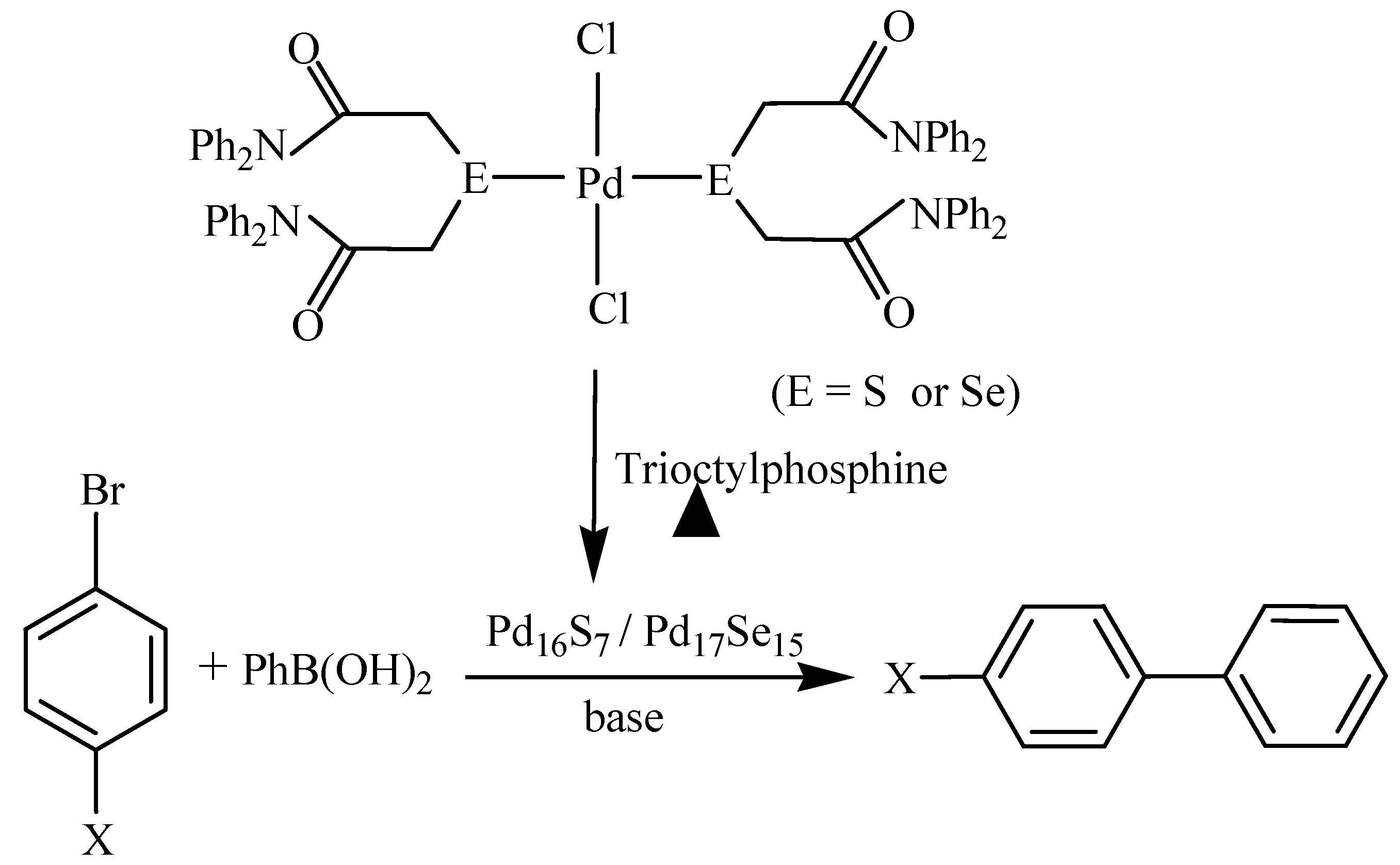
- Rao, G. K., Kumar, A.; Kumar, S.; Dupare, U. B.; Singh, A. K., Organometallics, 2013, 32, 2452-2458.
- Rao, G. K.; Kumar, A.; Ahmed, J.; Singh, A. K., Chem. Commun., 2010, 46, 5954-5956.
- Rao, G. K.; Kumar, A.; Singh, M. P.; Singh, A. K., J. Organomet. Chem., 2014, 749, 1-6.
- Rahman, A.; Khan, M.M., New J. Chem. 2021, 45, 19622-19635.
- Khan, M.D.; Amir, M.; Sohail, M.; Sher, M.; Baig, N.; Akhtar, J.; Malik, M.A.; Revaprasadu, N., Dalton Trans. 2018, 47, 5465-5473.
- Kukunuri, S.; Austeria, P.M.; Sampath, S., Chem. Commun. 2016, 52, 206-209.
- Kukunuri, S.; Karthick, S.N..; Sampath, S., J. Mater. Chem. A 2015, 3, 17144-17153.
- Zheng, Y.; Slade, T. J.; Hu, L.; Tan, X. Y.; Luo, Y.; Luo, Z. Z.; Xu, J.; Yan, Q.; Kanatzidis, M. G., Chem. Soc. Rev. 2021, 50, 9022-9054.
- Fan, F. J.; Wu, L.; Yu, S. H., Energy Environ. Sci., 2014, 7, 190-208.
- Coughlan, C.; Ibanez, M.; Dobrozhan, O.; Singh, A.; Cobat, A.; Ryan,K., Chem. Rev., 2017, 17, 5865-6109.
- Zheng, X.; Song, Y.; Liu, Y.; Yang, Y.; Wu, D.; Yang, Y.; Feng, S.; Li, J.; Liu, W.; Shen, Y.; Tian, X., Coord. Chem. Rev. 2023, 475, 214898.
- Stanbery, B. J., Critical Rev. Solid State Mater. Sci., 2002, 27, 73-117.
- Goodman, C. H. L. J. Phys. Chem. Solids, 1958, 6, 305-314.
- Pamplin, B. R. Nature, 1960, 188, 136-137.
- Hirpo, W.; Dhingra, S.; Sutorik, A. C.; Kanatzidis, M. G., J. Am. Chem. Soc., 1993, 115, 1597-1599.
- Banger, K. K.; Jin, M. H. C.; Harris, J. D.; Fanwick, P. E.; Hepp, A.F., Inorg. Chem., 2003, 42, 7713-7715.
- Gardner, J. S.; Shrudha, E.; Wang, C.; Lau, L. D.; Rodriguez, R. G.; Pak, J. J., J. Nanopart. Res., 2008, 10, 633-641.
- Margulieux, K. R.; Sun, C.; Zakharov, L. N.; Holland, A. W.; Pak, J. J., Inorg. Chem., 2010, 49, 3959-3961.
- Margulieux, K. R.; Sun, C.; Kihara, M. T.; Colson, A. C.; Zakharov, L. N.; Whitmire, K. H.; Holland, A. W.; Pak, J. J., Eur. J. Inorg. Chem., 2017, 2068-2077.
- Sun, C.; Westover, R. D.; Margulieux, K. R.; Zakharov, L. N.; Holland, A. W.; Pak, J. J. Inorg. Chem., 2010, 49, 4756-4758.
- Masnovi, J.; Banger, K. K.; Fanwick, P. P.; Hepp, A. F., Polyhedron, 2015, 102, 246-252.
- Pal, M. K.; Dey, S.; Neogy, S.; Kumar, M., RSC Adv., 2019, 9, 18302-18307.
- Banger, K. K.; Cowen, J.; Hepp, A.F., Chem. Mater., 2001, 13, 3827-3829.
- Castro, S. L.; Bailey, S. G.; Raffaelle, R. P.; Banger, K. K.; Hepp, A. F., Chem. Mater., 2003, 15, 3142-3147.
- Castro, S. L.; Bailey, S. G.; Raffaelle, R. P.; Banger, K. K.; Hepp, A.F., J. Phys. Chem. B, 2004, 108, 12429-12435.
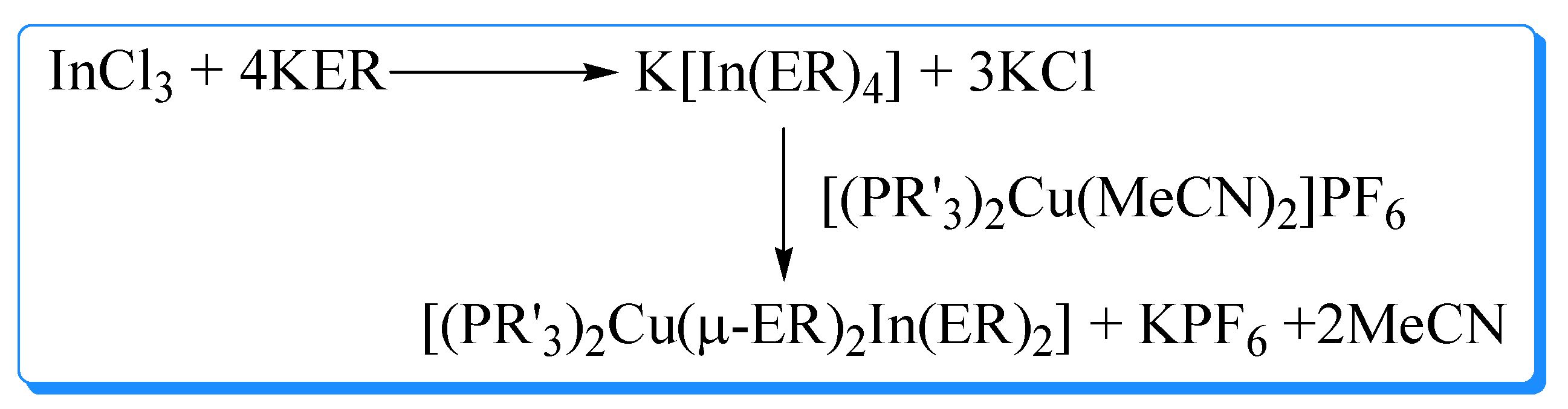
- Zhao, X.; Huang, Y.; Corrigan, J.F., Inorg. Chem. 2016, 55, 10810-10817.
- Nairn, J. J.; Shapiro, P. J.; Twamley, B.; Pounds, T.; Wandruszka, R. V.; Fletcher, T. R.; Williams, M.; Wang, C.; Norton, M.G., Nano Letters, 2006, 6, 1218-1223.
- Sun, C.; Gardner, J. S.; Shrudha, E.; Margulieux, K. R.; Westover, R. D.; Lau, L.; Long, G.; Bajracharya, C.; Wang, C.; Thurber, A.; Punnoose, A.; Rodriguez, R. G.; Pak, J. J., J. Nanomaterials, 2009, 748567.
- Sun, C.; Gardner, J. S.; Long, L.; Bajracharya, C.; Thurber, A.; Punnoose, A.; Rodriguez, R. G.; Pak, J.J., Chem. Mater., 2010, 22, 2699-2701.
- Wooten, A. J.; Werder, D. J.; Williams, D. J.; Casson, J. L.; Hollingsworth, J. A., J. Am. Chem. Soc., 2009, 131, 16177-16188.
- Hollingsworth, J. A.; Hepp, A. F.; Buhro, W. E., Chem. Vap. Deposition, 1999, 5, 105-108.
- Banger, K. K.; Hollingsworth, J. A.; Harris, J. D.; Cowen, J. E.; Buhro, W. E.; Hepp, A. F., Appl. Organomet. Chem., 2002, 16, 617-627.
- Banger, K. K.; Harris, J. D.; Cowen, J. E.; Hepp, A. F., Thin Solid Films, 2002, 403-404, 390-395.
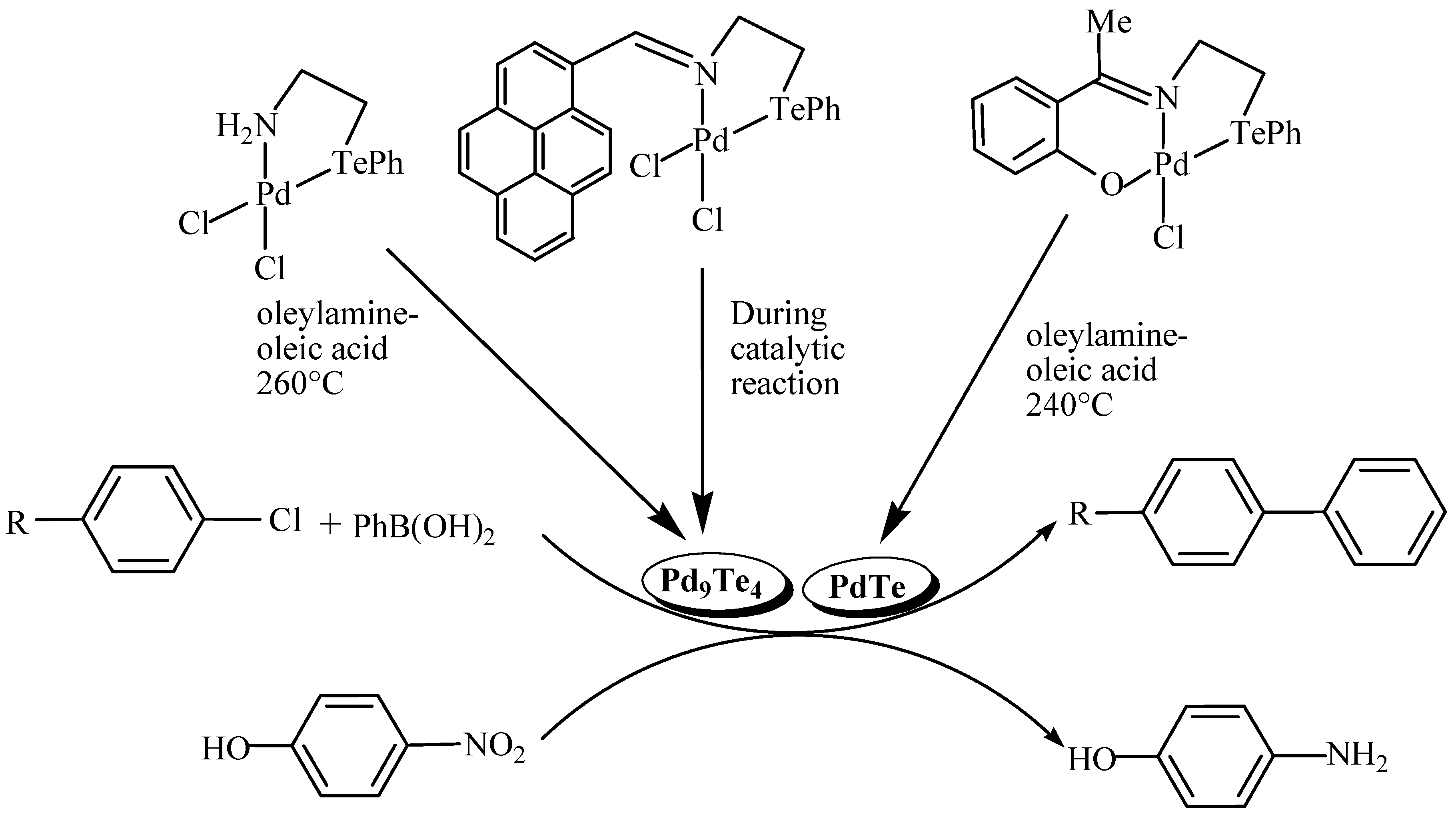
- Pal, M. K.; Dey, S.; Wadawale, A. P.; Kushwah, N.; Kumar , M.; Jain, V. K., Chem. Select, 2018, 3, 8575-8580.
- Sharma, R. K.; Kedarnath, G.; Kushwah, N.; Pal, M. K.; Wadawale, A.; Vishwanath, B.; Paul, B.; Jain, V. K., J. Organomet. Chem., 2013, 747, 113-118.
- Buchmaier, C.; Rath, T.; Pirolt, F.; Knall, A.C.; Kaschnitz, P.; Glatter, O.; Weweska, K.; Hofer, F.; Kunret, B.; Krenn, K.; Trimmer, G., RSC Adv. 2016, 6, 106120-106129.
- Tygai, A.; Kole, G. K.; Shah, A. Y.; Wadawale, A.; Srivastava, A. P.; Kumar, M.; Kedarnath, G.; Jain, V. K., J. Organomet. Chem., 2019, 887, 24-31.
- Tyagi, A.; Shah, A. Y.; Kedarnath, G.; Wadawale, A.; Singh, V.; Tyagi, D.; Betty, C. A.; Lal, C.; Jain, V. K., J. Mater. Sci. Mater. Electronics, 2018, 29, 8937-8946.
- Shah, A.Y.; Karmakar,K.; Tyagi, A; Kedarnath,G. New J. Chem., 2022, 46, 19817-19823.
- Xu, D.; Shen, S.; Zhang, Y.; Gu, H.; Wang, Q., Inorg Chem. 2013, 52, 12958-12962.
- Makin,F.; Alam, F.; Buckingham, M.A.; Lewis, D. J. Scientific Reports, 2022, 12, 5627.
- Rath, T.; MacLachlan, A.J.; Brown, M.D.; Haque, S. A., J. Mater. Chem. A 2015, 3, 24155-24162.
- Kushwah, N.; Kedarnath, G.; Sudarsan, V.; Srivastava, A. P. New J. Chem., 2023, 47, 307-314.
- Sun, C.; Gardner, J.S.; Long, G.; Bajracharya, C.; Thurber, A.; Punnoose, A.; Rodriguez, R. G.; Pak, J. J. Chem. Mater. 2010, 22, 2699–2701.
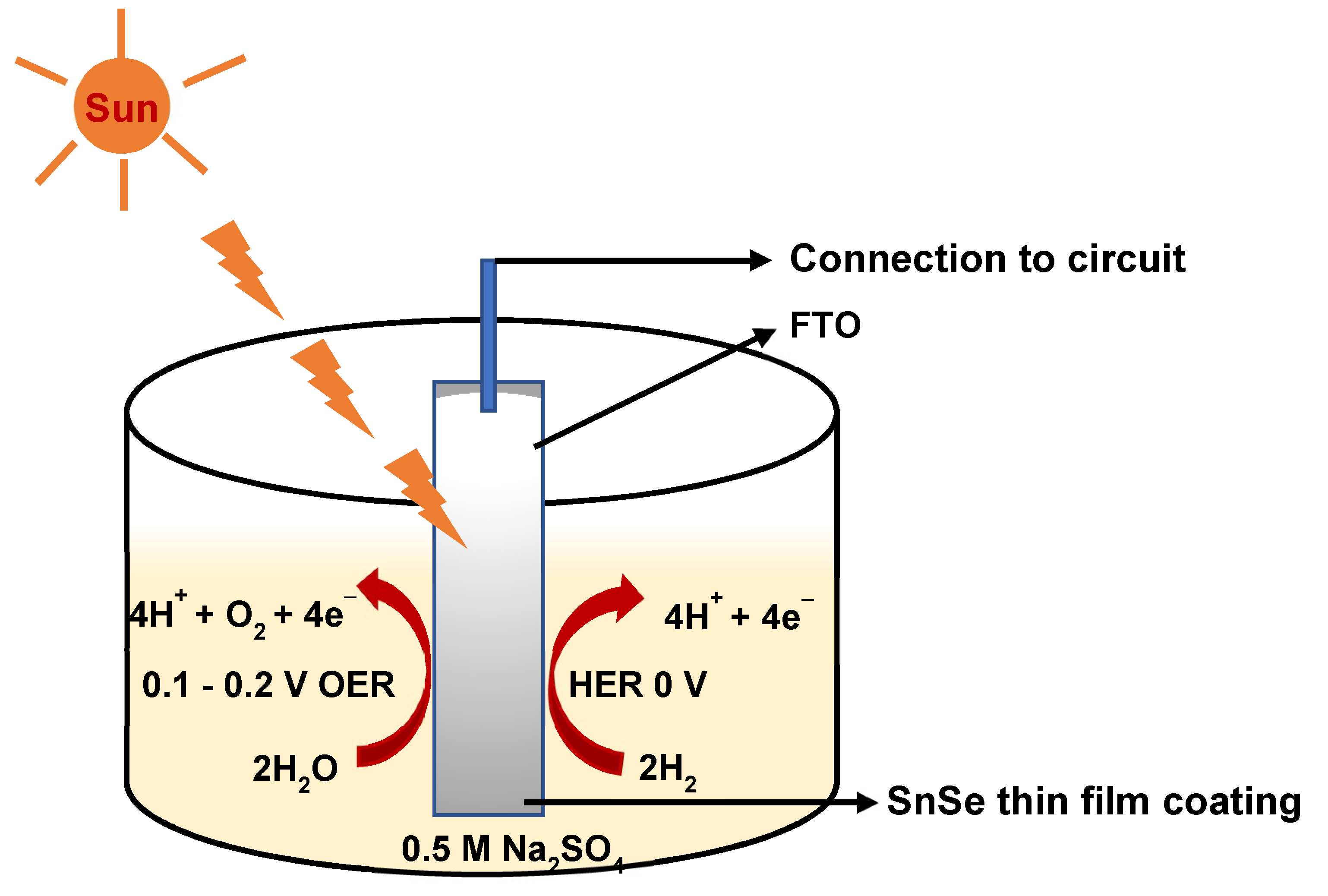
- Murtaza, G.; Alderhami, S.; Alharbi, Y. T.; Zulfiqar, U.; Hossin, M.; Alanazi, A. M.; Almanqur, L.; Onche, E. U.; Venkateswaran, S. P.; Lewis, D. J. ACS Appl. Energy Mater. 2020, 3, 1952−1961.
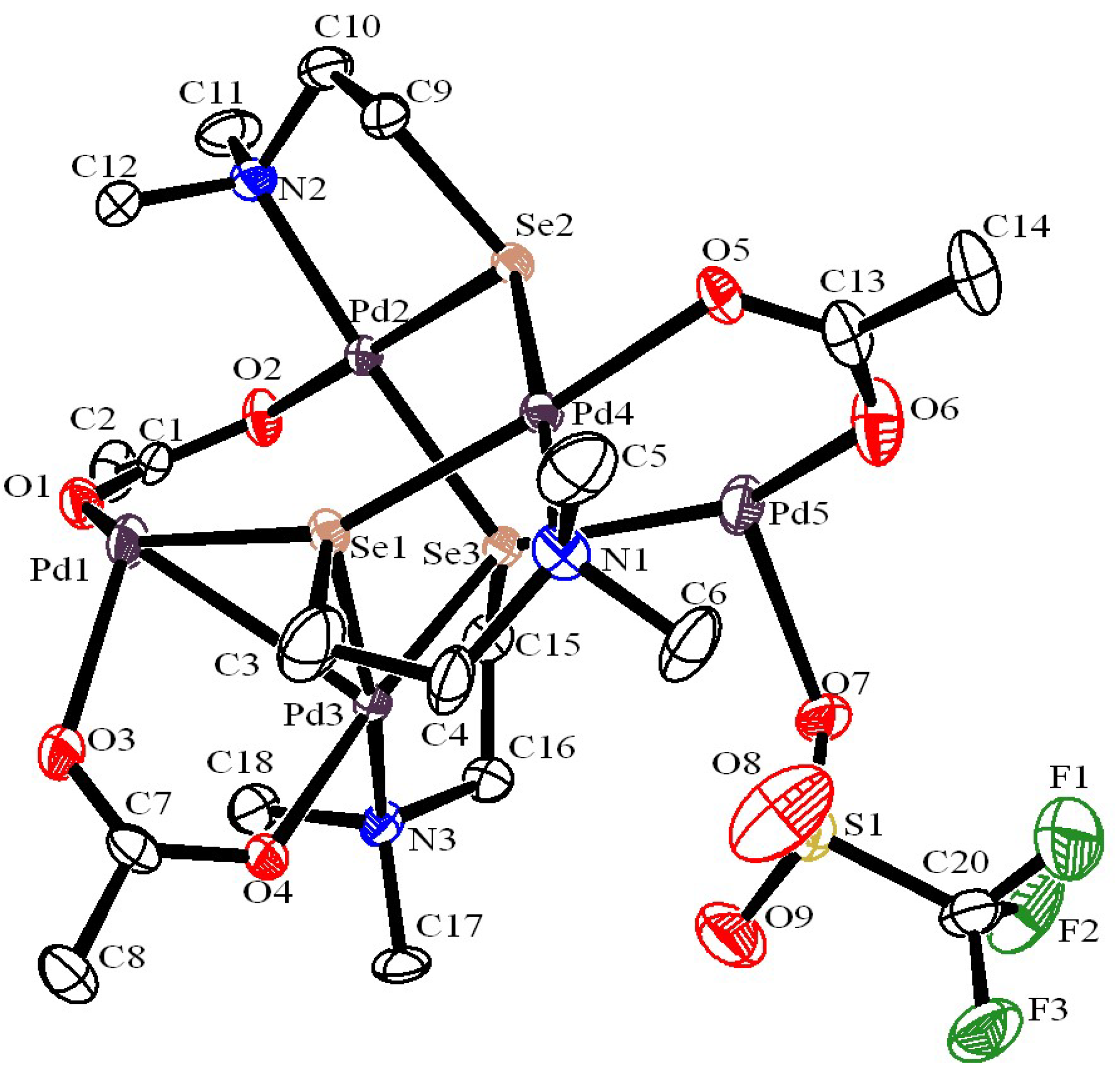
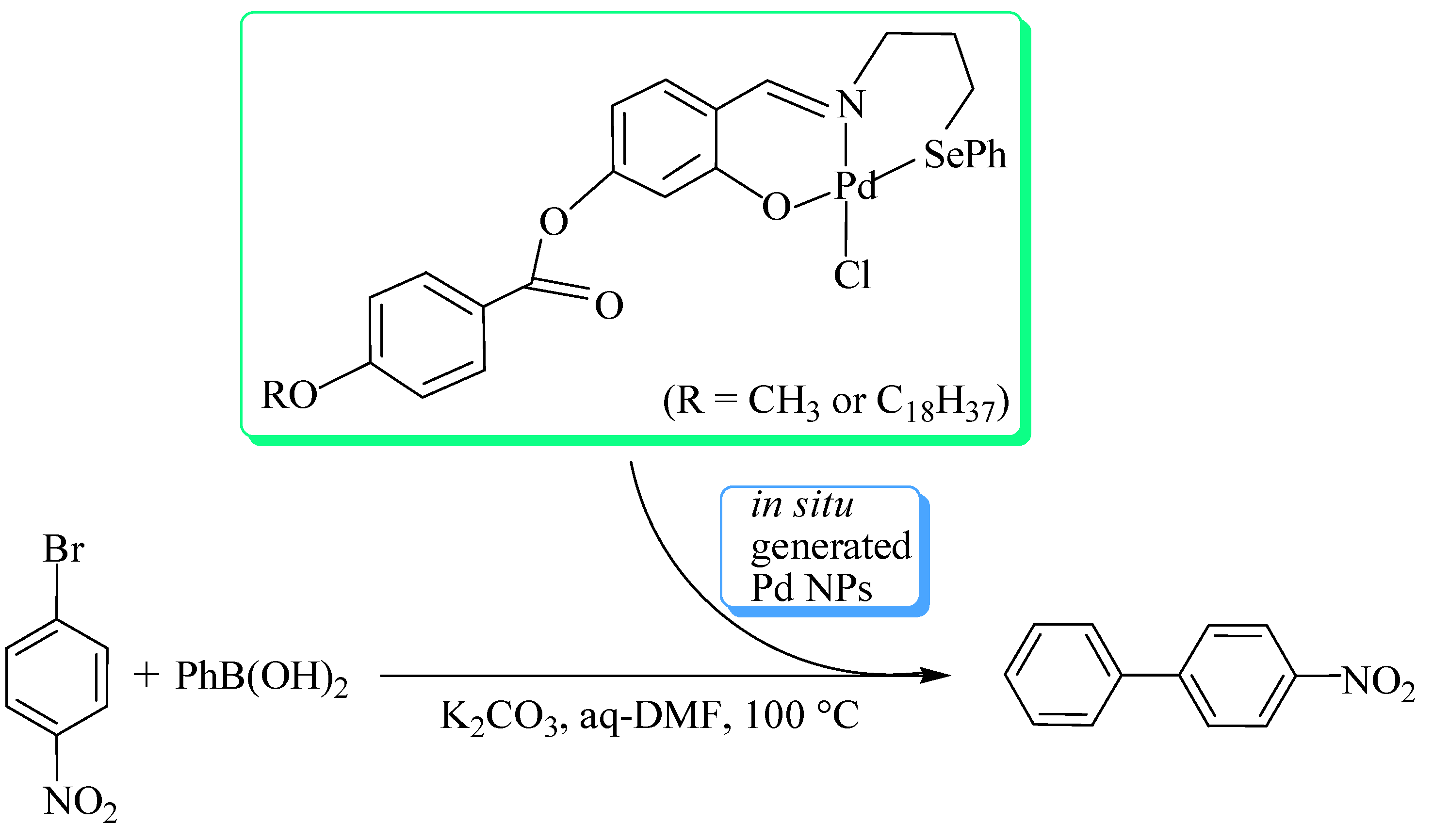
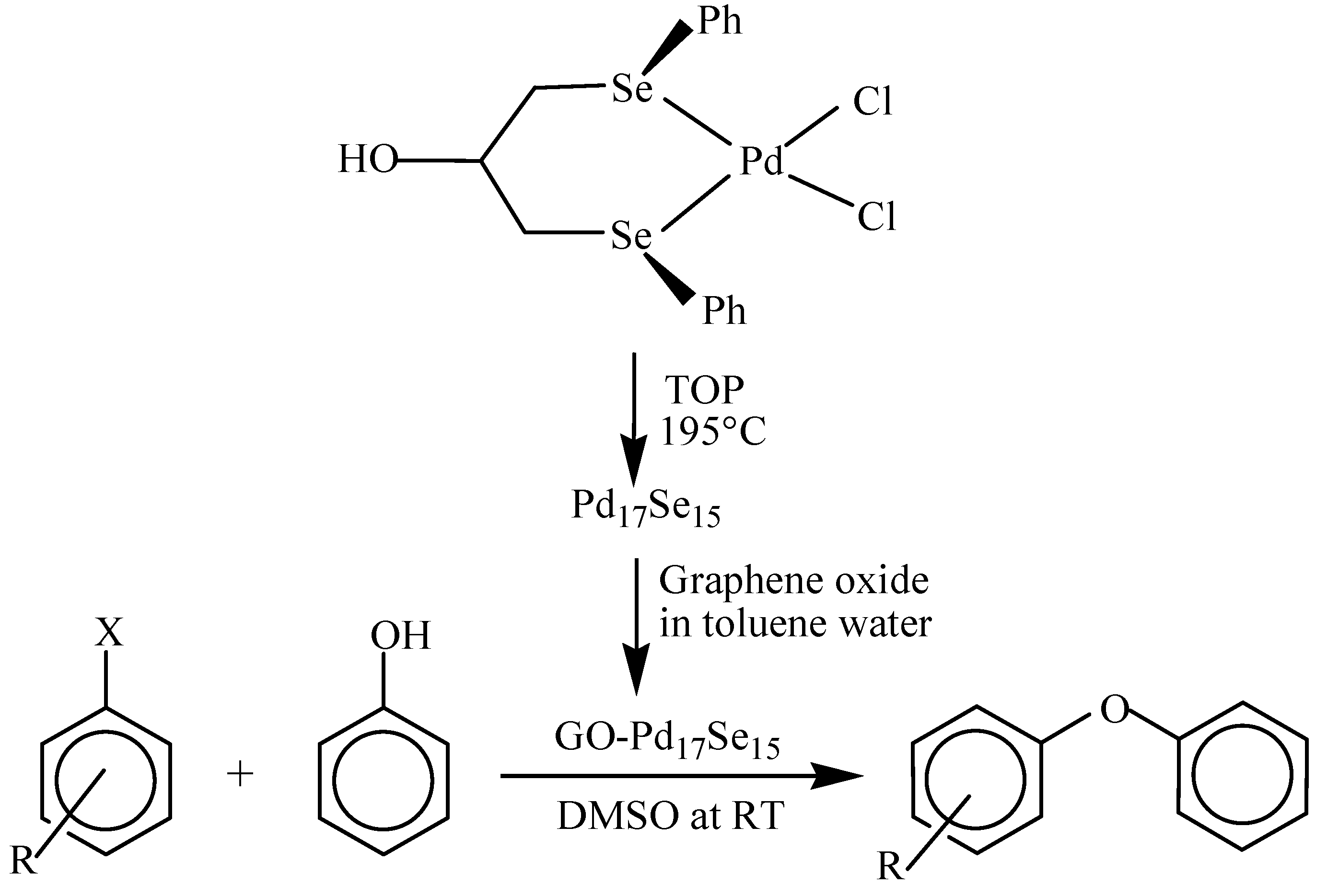
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).



